Abstract
Advances in multimodal imaging have significantly contributed to the management of many uveitis diseases in recent years. The most significant developments include the use of optical coherence tomography to obtain a more accurate and reproducible assessment of ocular inflammation, the application of optical coherence tomography angiography in choroiditis and retinal vasculitis, new possibilities for studying vitritis with ultrawide field imaging, and the most recent applications of fundus autofluorescence in uveitis. In this review, we provide an overview of the most significant advances in multimodal imaging of uveitis achieved in recent years.
Keywords: fundus autofluorescence, optical coherence tomography, optical coherence tomography angiography, ultrawide field imaging, uveitis
Introduction
Advances in multimodal imaging have significantly contributed to refining the diagnosis and management of uveitis in the recent years. Different imaging techniques have been applied to ocular inflammatory disorders and the most common indications are illustrated in Table 1. Recently, thanks to the introduction of new imaging modalities and improvements on those already available, significant achievements have been obtained in the field of uveitis. These include the application of optical coherence tomography (OCT) to obtain a more accurate and reproducible assessment of ocular inflammation. OCT devices have also been incorporated into surgical microscopes, gaining new insights into the management of the most complex surgical cases of uveitis. Other major contributions have been obtained with OCT angiography through a non-invasive study of the vascularization of the iris, retina, and choroid, with easier detection of ischemia and neovascularizations. More conventional imaging modalities, such as color fundus photography, have now been implemented into widefield cameras showing almost the entire retina in a single frame. These cameras can also perform fundus autofluorescence (FAF), which has a pivotal role in some posterior uveitis such as serpiginous choroiditis (SC) and white dots syndromes.
Table 1.
Imaging techniques, principal indications and applications in uveitis.
| Imaging technique | Main indications | Common applications in uveitis |
|---|---|---|
| Optical coherence tomography | Macular and optic disk pathology | Macular edema, retinitis, choroiditis, vitreoretinal disorders |
| Optical coherence tomography angiography | Retinal or choroidal vascular pathology | Retinal ischemia, retinitis, choroiditis, retinal and choroidal neovascularizations |
| Ultrawidefield imaging | Vitreoretinal or choroidal pathology involving the periphery | Retinal vasculitis, intermediate and posterior uveitis |
| Fundus autofluorescence | Retinal and retinal pigment epithelium pathology | Choroiditis, white-dot syndromes, masquerade syndromes |
| Fluorescein angiography | Retinal vascular pathology | Retinal ischemia, retinal vasculitis, posterior uveitis, intermediate uveitis, macular edema, retinal and choroidal neovascularizations |
| Indocyanine green angiography | Choroidal vascular pathology | Choroiditis, choroidal neovascularizations |
In this review, we provide the most significant advances in multimodal imaging of uveitis achieved in recent years.
OCT
OCT has become an indispensable ancillary test in the diagnosis and management of uveitis, allowing differential diagnosis and identification of many specific entities.1–7 OCT has rapidly evolved in the last two decades from time-domain OCT to spectral-domain OCT (SD-OCT) and, recently, to Swept Source OCT (SS-OCT).8–10 Although slit-lamp biomicroscopy still remains the standard method for assessment of inflammation of the anterior segment and vitreous, OCT-based methods for quantification of intraocular inflammation are gaining increasing interest as additional diagnostic tools. In a pilot study by Agarwal and colleagues,11 inflammatory cells in the anterior chamber were visualized on OCT scans as hyperreflective dots. The ability of OCT to detect anterior chamber cells, even in eyes with corneal haze or edema, is one of the advantages of this new modality. A more recent study using a SS-OCT found that the objective measurement of anterior chamber flare and cells correlated well with other grading systems.12 In particular, the authors used the optical density ratio between aqueous and air to evaluate the flare on OCT. OCT-based methods have also been applied for an objective assessment of vitreous haze.13,14 These methods offer a better reproducibility and more objective measure of ocular inflammation, which can improve our endpoints for uveitis in future clinical trials.
Secondary complications of uveitis such epiretinal membrane and rhegmatogenous retinal detachment are associated with a high risk of vision loss. Pars plana vitrectomy can improve visual function in selected eyes with chronic uveitis, but surgery in such cases can be complicated. Given the complexity of these patients, intraoperative surgical guidance tools, like intraoperative OCT, have the potential to impact outcomes and intraoperative decision making dramatically. The Determination of Feasibility of Intraoperative Spectral Domain Microscope Combined/Integrated OCT Visualization during En Face Retinal and Ophthalmic Surgery (DISCOVER) study evaluated the role of intraoperative OCT for ophthalmic surgery and concluded that intraoperative OCT is a valuable tool that can impact surgical decision making and may enhance surgical outcomes.15 To date, there are limited studies on the use of intraoperative OCT in patients with uveitis.
OCTA
The technique of optical coherence tomography angiography (OCTA) has completely revolutionized our understanding of the disease pathophysiology, management, and patient outcomes in the subspecialty of uveitis.16–18 The improvement in technological innovations has resulted in better and more efficient OCTA devices, helping in complex differential diagnosis.19,20 Swept-source (SS) OCTA combines the advantages of more than 100,000 A-scans in a short acquisition time with better depth resolution and dyeless angiography that provides vital anatomical alterations in ocular inflammation. In the context of uveitis, OCTA provides valuable information in conditions affecting the choriocapillaris/retinal pigment epithelium (RPE) such as white dot syndromes, choroidal stromal pathologies such as Vogt-Koyanagi-Harada (VKH) syndrome, and other infectious entities such as ocular toxoplasmosis and dengue maculopathy.
One of the most impressive developments due to OCTA is the finding of preserved choriocapillaris flow in eyes with multiple evanescent white dot syndrome (MEWDS; Figure 1).21,22 The lesions in MEWDS, however, appear hypocyanescent on indocyanine green angiography (ICGA). These findings indicate that the pathology of MEWDS may not be related to choriocapillaris ischemia, but rather to a primary RPE disorder, which results in reversible hypocyanescence on ICGA and subsequent ‘photoreceptoritis’ for reasons not clearly elucidated yet.21,23,24 Another possible explanation is that OCTA cannot be sensitive enough to detect subtle choriocapillaris inflammation, explaining the normal appearance observed in MEWDS.25 Further research is needed to clarify the discrepancy between ICGA and OCTA in MEWDS.
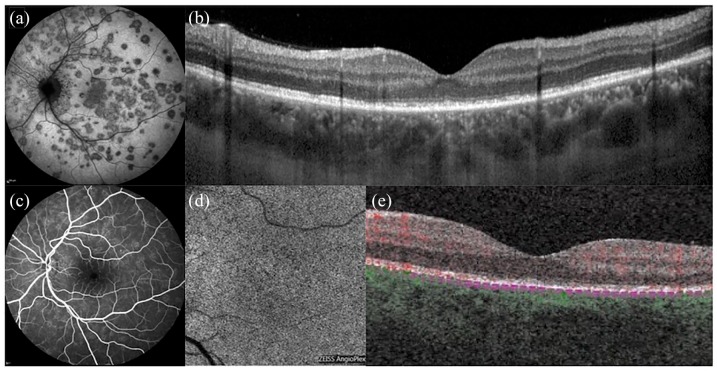
Figure 1.
Multimodal imaging of multiple evanescent white dot syndrome (MEWDS). (a) Indocyanine green angiography illustrates scattered hypocyanescent dots. (b) OCT shows attenuation and irregularities of the ellipsoid zone. (c) Fluorescein angiography reveals minimal hyper-fluorescence with ‘wreath-like’ lesions. (d and e) On OCT angiography, the choriocapillaris slab shows a normal flow signal.
OCTA is very useful in autoimmune SC as well as tubercular serpiginous-like choroiditis, acute posterior multifocal placoid pigment epitheliopathy (APMPPE; Figure 2), and multifocal choroiditis (MFC) in determining the area of choriocapillaris flow deficit corresponding to the active choroiditis lesions.26–30 The area of choriocapillaris flow deficit appears predominantly ‘dark’ on en face OCTA and co-localizes with the active lesion on ICGA. OCTA can be also useful in monitoring the healing of the lesions, and the development of paradoxical worsening if the etiology of the choroiditis is tubercular. Once the choroiditis lesions have healed, the OCTA effectively shows choriocapillaris restitution (in case the choroiditis lesions are small in size), or choriocapillaris atrophy (in large choroidal lesions).26,27
Figure 2.
Multimodal imaging of acute posterior multifocal placoid pigment epitheliopathy (APMPPE). In the active phase, (a) fundus photos show multiple yellowish placoid lesions, (b) hypocyanescent on indocyanine green angiography, while (c and d) OCT angiography well delineates the dark areas of choriocapillaris hypoperfusion. Ten days after presentation, (e) fundus autofluorescence shows hyper-autofluorescent lesions.
OCTA is also valuable in detecting both type 1 and type 2 choroidal neovascularization (CNV) in eyes with choroiditis.31–34 Type 1 lesions are extremely rare and have been reported in tubercular serpiginous-like choroiditis.31 On the other hand, type 2 lesions are fairly common and easily detectable on OCTA in choriocapillaritis and other entities such as choroidal granulomas and toxoplasma retinochoroiditis.34,35 Of note, caution must be exercised in distinguishing CNV lesions on OCTA from residual medium-to-large choroidal vessels in case there is an overlying choriocapillaris atrophy.36
Stromal choroiditis such as sarcoidosis, VKH disease, and sympathetic ophthalmia can be evaluated non-invasively using OCTA (Figure 3).37,38 OCTA enables distinction between VKH and central serous chorioretinopathy (CSC) in atypical cases by visualization of hypo-reflective ‘dark dots’ in the choriocapillaris layer in VKH, which are absent in eyes with CSC.38 In addition, OCTA enables determination of the level of inflammation and need for continued immunosuppression in VKH based on the appearance/disappearance of the hypo-reflective ‘dark dots’. Similarly, in sympathetic ophthalmia, widefield OCTA has been shown to assess treatment response. On sequential imaging, flow deficits in the choriocapillaris improved following initiation of steroid and immunosuppressive therapy. Complete resolution was seen after 6-month therapy with a corresponding improvement in visual acuity.37
Figure 3.
Multimodal imaging of stromal choroiditis. (a) Fundus photograph shows yellowish scattered foci of choroiditis, corresponding to hypocyanescent area on (b) indocyanine green angiography (ICGA). On (c) en-face and (d) cross-sectional B-scan OCT angiography, the areas of choroiditis show the absence of decorrelation signal (black spaces), well correlating with ICGA.
OCTA can also be useful in emerging mosquito-borne uveitis. In dengue, maculopathy reveals a number of pathological alterations that include deep retinal plexus flow deficits. Other features include vitreous inflammation, and a yellow-orange foveal lesion (foveolitis). The analyses on OCTA show both ischemic and inflammatory processes that contribute to the development of these changes. This entity has been therefore named as dengue-induced inflammatory, ischemic foveolitis and outer maculopathy (DIII-FOM). The ischemic damage in DIII-FOM is irreversible, and the patients may have persistent scotoma despite healing of the foveolitis.39
Ocular toxoplasmosis has been extensively studied using OCTA including SS-OCTA. OCTA is very sensitive in detecting CNV in ocular toxoplasmosis.35 Retinal vasculitis can also be evaluated using OCTA.40–42 In Behcet’s disease, retinal vasculitis may not be easily assessable over fluorescein angiography due to significant leakage. However, on OCTA, the microvascular changes including area of the foveal avascular zone can be easily assessed and quantified. Parafoveal capillary telangiectasia can also be observed in these eyes.43,44 In other causes of retinal vasculitis, especially occlusive retinal vasculitis due to tuberculosis and other entities, OCTA can delineate the non-perfusion areas, and it may be superior to fluorescein angiography in detecting ischemic changes in conditions such as West Nile virus infection and HIV retinopathy.41,45,46
In summary, OCTA adds significant value in the armamentarium of multimodal imaging in uveitis. There are certain limitations including difficulty in OCTA image acquisition due to small pupil size/synechiae, vitreous haze, complicated cataract, and vitreous floaters in uveitis. With increasing knowledge, the technology of OCTA continues to provide novel valuable information in the assessment of patients with uveitis.
Ultrawide field imaging
Ultrawide field imaging refers to imaging modalities able to capture in a single frame, centered on the fovea, portions of the retina anterior to the vortex veins ampullae.47 This technology can be applied to different imaging modalities, including color pictures, FAF, fluorescein angiography, and ICGA. In uveitis, ultrawide field techniques are progressively gaining a crucial role because of the more comprehensive nature of the information provided, rapid execution, and significant advantages associated.
Being able to provide simultaneous information of both the macula and the retinal periphery, ultrawide field imaging can offer added value when applied to fluorescein angiography. In occlusive retinal vasculitis, it can assess more accurately the entity of retinal non-perfusion and stratify the risk of retinal neovascularization (Figure 4). It can also guide laser photocoagulation, which can be targeted using the information provided by ultrawide field imaging.48–50 On the other hand, users of ultrawide field imaging are often exposed to an excess of information and should be aware that not all the abnormalities shown are clinically significant. In a comprehensive cohort of uveitis patients, peripheral vascular leakage on ultra-widefield fluorescein angiography was seen in more than 50% of eyes. However, this peripheral leakage was not associated with any significant reduction in visual acuity or risk of macular edema, neither at baseline nor at the final follow-up. Also, patients with intermediate uveitis frequently show capillary leakage, which is often more prominent in the retinal periphery. Despite being found also in eyes active clinical inflammation, this peripheral leakage may have a low impact on visual acuity.51
Figure 4.
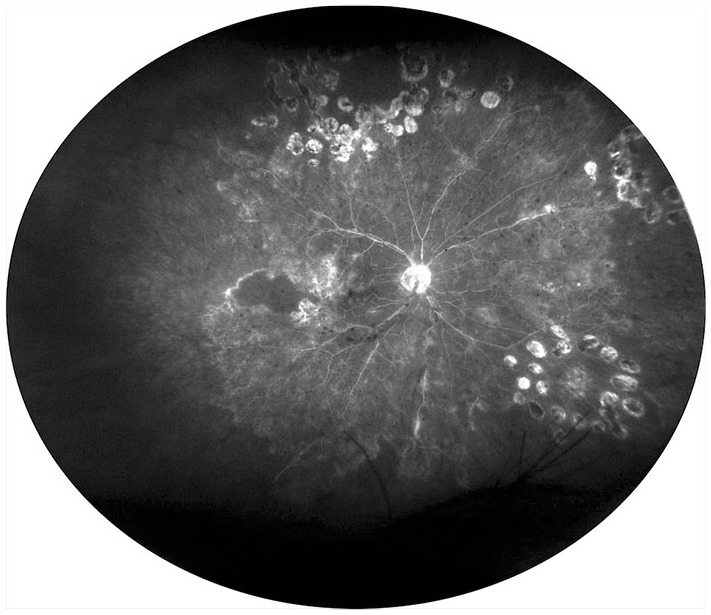
Ultrawide field fluorescein angiography of occlusive retinal vasculitis showing extensive areas of capillary nonperfusion, laser scars, and diffused vascular leakage.
Another key capability of devices using parabolic mirrors is their ability to provide information on the vitreous, which has traditionally been difficult to study because of its transparency and liquid nature. For instance, by applying this ultrawide field technique, we recently reported a peculiar pattern of vitritis along the vitreous fibrils in cases of vitreoretinal lymphoma (Figure 5).52 In another paper, the assessment of vitritis with this technique was found to well correlate with the grade of vitritis observed clinically.53 This application may provide an additional tool for monitoring the grade of vitreous haze, which is one of the main end-points in clinical trials for uveitis.
Figure 5.
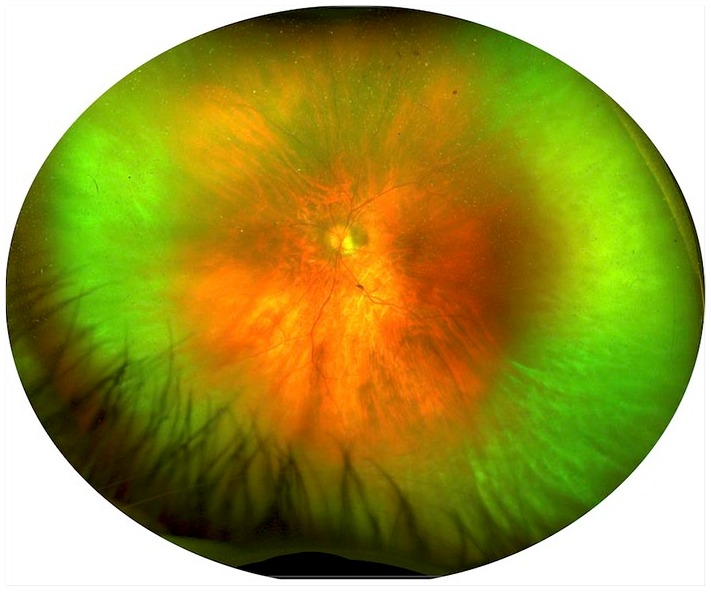
Ultrawide field imaging of biopsy-proven vitreoretinal lymphoma. The pseudocolor image shows the presence of vitritis along the most peripheral vitreous fibrils with an ‘Aurora Borealis’ appearance, as previously reported.
Another difficulty observed in uveitis patients is poor pupillary dilatation for posterior synechiae or iris inflammation. The possibility to obtain images of the retinal periphery with ultrawide field imaging even with undilated pupils offers a major advantage for the examiner.54
Other advantages of ultrawide field imaging in uveitis include a better staging of many ocular inflammatory disorders. The frequency of paradoxical worsening in tubercular serpiginous-like choroiditis has been found to be significantly higher when using an ultrawide field device as compared to conventional fundus cameras, which have a more limited field of view of the retina.55 In that study, the information provided by ultrawide field imaging changed the management of more than one-third of patients. Also in cytomegalovirus retinitis, this technique proved to be helpful, with a better delineation of lesion borders and more comfort perceived by patients because of the rapid acquisition of the images.56,57
Ultrawide field imaging demonstrated clinical usefulness also in non-infectious uveitis. Based on this imaging modality, a simple way to assess and grade sunset glow fundus complicating VKH has been described.58 Sunset glow fundus was classified into three progressive stages based on the severity of choroidal depigmentation, which could be better appreciated on ultrawide field images (Figure 6). Eyes presenting with more advanced depigmentation were also those that experience a higher number of recurrences and complications, like cataract and glaucoma. The more comprehensive assessment provided can also be helpful to identify disorders masquerading as uveitis, including retinal dystrophies and ocular tumors.59,60
Figure 6.
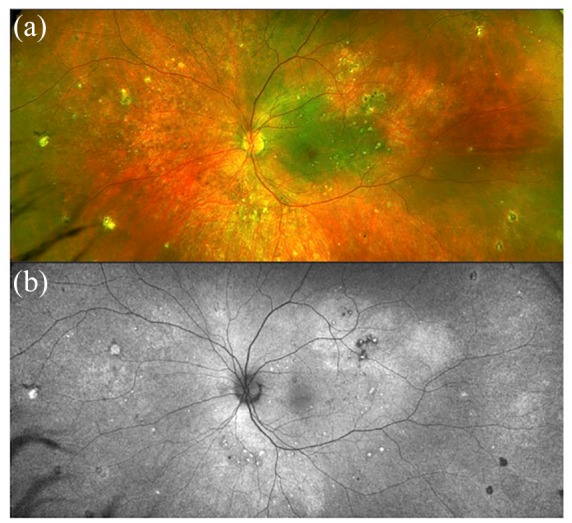
(a) Multimodal imaging of Vogt-Koyanagi-Harada (VKH) syndrome showing mild depigmentation of the retinal pigment epithelium on fundus photograph. (b) Fundus autofluorescence reveals scattered hypo and hyper autofluorescent areas.
Future goals of ultrawide field imaging should focus on the improvement of resolution and incorporation of additional imaging techniques, like OCTA. With the most recent machines, OCTA can already scan a significant amount of the retinal periphery, reaching wide field dimensions.61 This will be particularly important in uveitis, where repeated examinations to monitor the treatment response are often required and should include the assessment of retinal periphery.
FAF
FAF provides a map of lipofuscin’s distribution in the retina and different autofluorescence patterns have been found helpful in the differential diagnosis of many infectious and non-infectious uveitis, as well as masquerade syndromes. In most studies and ongoing clinical trials, FAF is performed using a confocal scanning laser ophthalmoscope (cSLO) with 488 nm blue-light excitation, but also other systems with different excitation light and barrier filters have been developed. Since FAF is the result of a cellular functional status, many studies have shown how this imaging modality can provide insights about the pathogenesis of posterior uveitis and be a useful prognostic tool.62
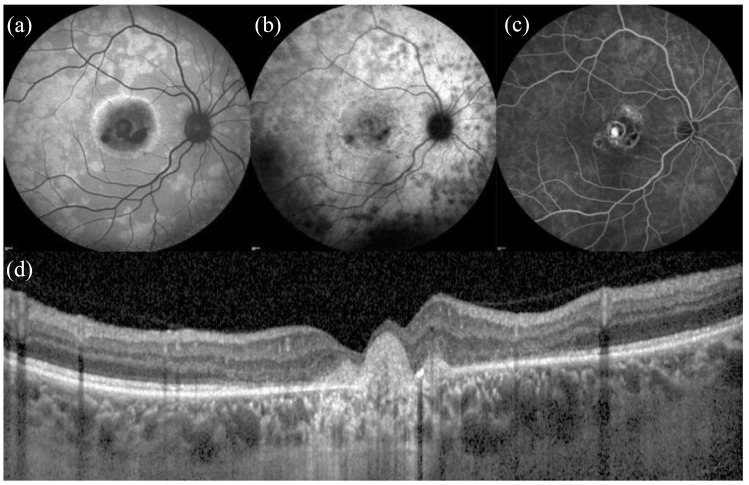
Multifocal choroiditis and punctate inner choroidopathy (PIC), once believed to be distinct pathologies, are now considered different spectrums of the same entity. Both MFC and PIC are mostly bilateral diseases characterized by development of circular scars with a high incidence of CNV and visual loss without treatment. A study showed that FAF detected more hypo-autofluorescent spots than chorioretinal scars seen clinically, in particular when smaller lesions (<125 µm) are considered.62 In another study, FAF revealed hyperautofluorescence surrounding active lesions with associated CNV and hypoautofluorescence when lesions responded to treatment. The persistence of hyperautofluorescence raised the risk of persistent activity and recurrences.63 For these reasons, FAF might be a sensitive measure of disease activity in MFC and PIC.64 Recently, with FAF characterization, a new possible variant of MFC and PIC has been described in patients complaining acute vision loss or paracentral scotomas. This subset of patients presented large hyper-autofluorescent areas on FAF, not clinically visible, surrounding chorioretinal lesions (Figure 7).65 These areas corresponded to attenuation of photoreceptor complex found on OCT and visual field defects detected on perimetry.
Figure 7.
Multimodal imaging of punctate inner choroidopathy (PIC) complicated by light sensations and paracentral scotomas. (a) Fundus autofluorescence reveals scattered paracentral hyper autofluorescent areas next to a macular hypo autofluorescent scar. These paracentral areas appear hypocyanescent on (b) indocyanine green angiography and mildly hyperfluorescent on (c) fluorescein angiography. (d) OCT illustrates the hyper-reflective macular scar and mild disorganization of outer retinal layers.
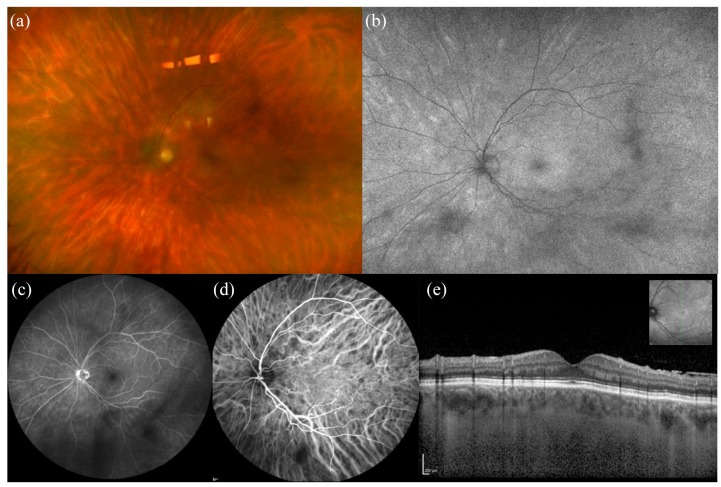
Another uveitis where FAF can reveal more lesions than ophthalmoscopic examination is birdshot chorioretinopathy (BSCR; Figure 8).66 BSCR is a stromal choroiditis associated with HLA-A29 characterized by the development of scattered hypopigmented yellow lesions with persistent chorioretinal inflammation.67 FAF lesions mostly correspond to those detected on ICGA, but they may appear hyper-autofluorescent at first (Figure 8) and turn hypo-autofluorescent over time if the disease is not controlled. The hypo-autofluorescent spots seen on FAF correlate with persistent visual field defects.66 These observations support the concept that an early and aggressive immunomodulatory therapy, before the appearance of permanent damage (e.g. hypo-autofluorescent spots on FAF), may improve visual prognosis.68
Figure 8.
Multimodal imaging of birdshot chorioretinopathy. (a) Fundus photograph shows yellowish scattered foci of choroiditis, corresponding to hyper autofluorescent lesions on (b) fundus autofluorescence. (c) Fluorescein angiography shows some hypo fluorescent areas corresponding to vitreous opacities and (d) indocyanine green angiography reveals the presence of hypocyanescent foci of choroiditis. (e) OCT does not show significant changes in the macular scan.
Similarly, in MEWDS, FAF can identify the characteristic multiple hyper-autofluorescent spots during the acute phase, which correspond to the white dots observed clinically and ellipsoid disruptions on OCT. These dots fade away with the resolution of inflammation, but FAF can confirm the diagnosis of MEWDS even in the subacute phase.69 Indeed, after the resolution of the patient’s symptoms, FAF can detect new transient hyper-autofluorescent lesions in the previously affected areas, corresponding to subretinal deposits observed on OCT.70 These secondary lesions, unlike the ones of acute MEWDS, remained hyper-autofluorescent after photobleaching on quantitative autofluorescence. This finding supported the theory that the acute hyperautofluorescence dots derived by a sort of window-effect toward RPE, while the subacute hyperautofluorescence was at least partially due to an intrinsic fluorescence of subretinal deposits.70
In VKH, two patterns were observed on FAF in the acute phase and, interestingly, depending on the timing of therapy. Patients treated with early intensive immunosuppression showed mild hyperautofluorescence which diminished up to the norm in disease remission. Conversely, patients either not treated or receiving a delayed treatment showed diffused zones of hyperautofluorescence which resolved within 6 months into patchy hypo- and hyperautofluorescence areas. Thus, prompt treatment could prevent permanent damage, as demonstrated by FAF.71
In acute zonal occult outer retinopathy (AZOOR; Figure 9), a rare syndrome presenting with acute loss of function of some retinal areas, photopsia, and minimal fundus changes, FAF has a pivotal role in diagnosis, because it depicts very well the typical trizonal pattern. In particular, normal autofluorescence area is observed outside a demarcating line (zone 1), a speckled hyper-autofluorescence is seen within the active lesion (zone 2), and hypoautofluorescence is detected where chorioretinal atrophy is developed (zone 3).72 Furthermore, FAF allowed to discover other similar lesions, not clear on clinical ophthalmoscopy, also in periphery, suggesting that AZOOR may be a multifocal disease.73
Figure 9.
Multimodal imaging of acute zonal occult outer retinopathy (AZOOR). (a) Fundus photography shows minimal outer retina and retinal pigment epithelium changes, while (b) FAF depicts very well the typical trizonal pattern. In particular, normal autofluorescence is observed outside a demarcating line (zone 1), speckled hyper-autofluorescence is seen within the active lesion (zone 2), and hypo-autofluorescence is detected where chorioretinal atrophy is developed (zone 3). Panels on the bottom row show (c) ICGA in the late phase, (d) infrared fundus image and (e) OCT.
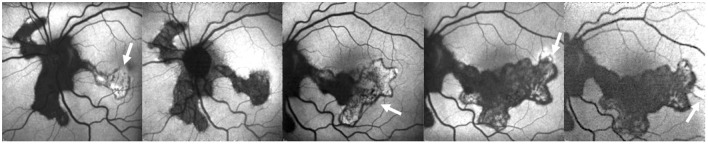
In SC, FAF demonstrated to be more accurate than other exams in showing the extent and progression of RPE involvement (Figure 10). The area of hyperautofluorescence, marking active inflammation, corresponded to the final hypo-autofluorescent scar. Moreover, hyperautofluorescence at the scars’ borders was a useful tool to identify early a disease’s relapse.73 FAF allowed also to distinguish active and resolved stages of tubercular serpiginous-like choroiditis, a distinct entity included in the spectrum of ocular tuberculosis. During the acute phase, FAF shows hyperautofluorescence of the lesions. As the disease begins to heal, it shows a stippled pattern of predominant hyperautofluorescence with a hypo-autofluorescent border surrounding the lesions. Gradually, the hypoautofluorescence border progresses and the lesions became mostly hypo-autofluorescent, still with a stippled pattern. On complete healing, the lesions become homogenously hypoautofluorescent.74
Figure 10.
Fundus autofluorescence of serpiginous choroiditis showing the annual progression of the disease in the areas of activity (arrows). The active borders of choroiditis appear hyper-autofluorescent and the inactive areas show hypo-autofluorescence.
FAF could also help to monitor ocular syphilis since a hyper-autofluorescent pattern overlying retinal lesions and resolving with effective antibiotic treatment was described.75
FAF was also studied in the context of masquerade syndromes. In the majority of eyes with active vitreoretinal lymphoma (VRL), a subset of primary central nervous system lymphoma, a granular autofluorescence pattern could be observed and, interestingly, also in some patients where the classic leopard spot appearance was absent on fluorescein angiography (FA). Hyperautofluorescence could be the sign of sub-RPE infiltrate by lymphomatous cells, while hypoautofluorescence could be caused either by lymphomatous infiltrates’ masking effect or by resulting RPE atrophy.76 Taking together all these findings, abnormal autofluorescence could be a useful addition to monitor and detect possible relapses in patients with VRL. Hereditary retinal disorders which can masquerade as uveitis can also be easily recognized with FAF because they are characterized by specific patterns.59
Recently, a new confocal 450 nm blue-light FAF device has been introduced. This wavelength could excite a different range of fluorophores. Moreover, this new confocal light-emitting diode (LED) FAF system detects the full emission spectrum on a color sensor, providing so-called ‘color’ FAF and allowing to separate the emission spectrum into long-wave and short-wave components (‘red’ and ‘green’ component).77 Studies are necessary to evaluate the potential role of this new imaging modality in uveitis.
Conclusion
In conclusion, many recent imaging findings and new applications demonstrated the potential to change our clinical approach in the diagnosis and management of uveitis. In these patients, multimodal imaging may provide significant advantages because of the complex nature of many inflammatory entities, especially when guided by clinical presentation, personal and familial history. The treating physician should be familiar with the current advantages and limitations of each imaging technique to distinguish between specific uveitis entities. Implementation of imaging-based methods to quantify intraocular inflammation, either in the anterior segment or in the vitreous chamber, seems a promising strategy to obtain more objective and reliable endpoints for future clinical trials in uveitis. Future of ocular imaging in patients with chronic uveitis would be directed toward the implementation of non-invasive and wide field techniques.
Footnotes
Conflict of interest statement: AM, AA, AGM, SH, GM, VG and EM have no disclosures. GQ has the following disclosures: Allergan (S), Bayer (S); Novartis (S), Zeiss (S), Allergan (C), Alimera (C), Bausch and Lomb (C), Novartis (C), Bayer (C), Heidelberg (C), and Zeiss (C). F.B. has the following disclosures: Allergan (S), Alimera (S), Bayer (S), Farmila-Thea (S), Schering Pharma (S), Sanofi-Aventis (S), Novagali (S), Pharma (S), Hoffmann-La Roche (S), Genetech (S), and Novartis (S).
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Alessandro Marchese  https://orcid.org/0000-0001-7716-7261
https://orcid.org/0000-0001-7716-7261
Giuseppe Querques  https://orcid.org/0000-0002-3292-9581
https://orcid.org/0000-0002-3292-9581
Contributor Information
Alessandro Marchese, Department of Ophthalmology, San Raffaele Scientific Institute, IRCCS Ospedale San Raffaele, Vita-Salute San Raffaele University, Via Olgettina 60, 20132 Milan, Italy.
Aniruddha Agarwal, Department of Ophthalmology, Postgraduate Institute of Medical Education & Research (PGIMER), Chandigarh, India.
Alessio Grazioli Moretti, Department of Ophthalmology, San Raffaele Scientific Institute, IRCCS Ospedale San Raffaele, Vita-Salute San Raffaele University, Milan, Italy.
Sabia Handa, Department of Ophthalmology, Postgraduate Institute of Medical Education & Research (PGIMER), Chandigarh, India.
Giulio Modorati, Department of Ophthalmology, San Raffaele Scientific Institute, IRCCS Ospedale San Raffaele, Vita-Salute San Raffaele University, Milan, Italy.
Giuseppe Querques, Department of Ophthalmology, San Raffaele Scientific Institute, IRCCS Ospedale San Raffaele, Vita-Salute San Raffaele University, Milan, Italy.
Francesco Bandello, Department of Ophthalmology, San Raffaele Scientific Institute, IRCCS Ospedale San Raffaele, Vita-Salute San Raffaele University, Milan, Italy.
Vishali Gupta, Department of Ophthalmology, Postgraduate Institute of Medical Education & Research (PGIMER), Chandigarh, India.
Elisabetta Miserocchi, Department of Ophthalmology, San Raffaele Scientific Institute, IRCCS Ospedale San Raffaele, Vita-Salute San Raffaele University, Milan, Italy.
References
- 1. Giuffre C, Miserocchi E, Modorati G, et al. Central serous chorioretinopathylike mimicking multifocal vitelliform macular dystrophy: an ocular side effect of mitogen/extracellular signal-regulated kinase inhibitors. Retin Cases Brief Rep 2018; 12: 172–176. DOI: 10.1097/ICB.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 2. Marchese A, Romano F, Cicinelli MV, et al. Chorioretinal punched-out lesions in pseudoxanthoma elasticum. Retina 2018; 38: e43–e44. DOI: 10.1097/IAE.0000000000002052. [DOI] [PubMed] [Google Scholar]
- 3. Corbelli E, Miserocchi E, Marchese A, et al. Ocular toxicity of mirvetuximab. Cornea 2019; 38: 229–232. DOI: 10.1097/ICO.0000000000001805. [DOI] [PubMed] [Google Scholar]
- 4. Giuffre C, Miserocchi E, Marchese A, et al. Widefield OCT angiography and ultra-widefield multimodal imaging of Susac syndrome. Eur J Ophthalmol. Epub ahead of print 10 April 2019. DOI: 10.1177/1120672119843281. [DOI] [PubMed] [Google Scholar]
- 5. Cicinelli MV, Marchese A, Miserocchi E, et al. Retinal and choroidal changes of vitreoretinal lymphoma from active to remission phase after intravitreal rituximab. Ocul Immunol Inflamm. Epub ahead of print 8 August 2019. DOI: 10.1080/09273948.2019.1616769. [DOI] [PubMed] [Google Scholar]
- 6. Giuffre C, Marchese A, Cicinelli MV, et al. Multimodal imaging and treatment of syphilitic choroidal neovascularization. Retin Cases Brief Rep. Epub ahead of print 13 August 2019. DOI: 10.1097/ICB.0000000000000912. [DOI] [PubMed] [Google Scholar]
- 7. Marchese A, Agarwal A, Miserocchi E, et al. Features of retinitis-like lesions in vitreoretinal lymphoma. Ocul Immunol Inflamm. Epub ahead of print 30 September 2019. DOI: 10.1080/09273948.2019.1648835. [DOI] [PubMed] [Google Scholar]
- 8. Gallagher MJ, Yilmaz T, Cervantes-Castaneda RA, et al. The characteristic features of optical coherence tomography in posterior uveitis. Br J Ophthalmol 2007; 91: 1680–1685. DOI: 10.1136/bjo.2007.124099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rabiolo A, Zucchiatti I, Marchese A, et al. Multimodal retinal imaging in central serous chorioretinopathy treated with oral eplerenone or photodynamic therapy. Eye (Lond) 2018; 32: 55–66. DOI: 10.1038/eye.2017.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baghdasaryan E, Tepelus TC, Marion KM, et al. Analysis of ocular inflammation in anterior chamber-involving uveitis using swept-source anterior segment OCT. Int Ophthalmol 2019; 39: 1793–1801. DOI: 10.1007/s10792-018-1005-0. [DOI] [PubMed] [Google Scholar]
- 11. Agarwal A, Ashokkumar D, Jacob S, et al. High-speed optical coherence tomography for imaging anterior chamber inflammatory reaction in uveitis: clinical correlation and grading. Am J Ophthalmol 2009; 147: 413–416.e3. DOI: 10.1016/j.ajo.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 12. Invernizzi A, Marchi S, Aldigeri R, et al. Objective quantification of anterior chamber inflammation: measuring cells and flare by anterior segment optical coherence tomography. Ophthalmology 2017; 124: 1670–1677. DOI: 10.1016/j.ophtha.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 13. Montesano G, Way CM, Ometto G, et al. Optimizing OCT acquisition parameters for assessments of vitreous haze for application in uveitis. Sci Rep 2018; 8: 1648. DOI: 10.1038/s41598-018-20092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keane PA, Karampelas M, Sim DA, et al. Objective measurement of vitreous inflammation using optical coherence tomography. Ophthalmology 2014; 121: 1706–1714. DOI: 10.1016/j.ophtha.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ehlers JP, Goshe J, Dupps WJ, et al. Determination of feasibility and utility of microscope-integrated optical coherence tomography during ophthalmic surgery: the DISCOVER Study RESCAN Results. JAMA Ophthalmol 2015; 133: 1124–1132. DOI: 10.1001/jamaophthalmol.2015.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pichi F, Sarraf D, Morara M, et al. Pearls and pitfalls of optical coherence tomography angiography in the multimodal evaluation of uveitis. J Ophthalmic Inflamm Infect 2017; 7: 20. DOI: 10.1186/s12348-017-0138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pichi F, Sarraf D, Arepalli S, et al. The application of optical coherence tomography angiography in uveitis and inflammatory eye diseases. Prog Retin Eye Res 2017; 59: 178–201. DOI: 10.1016/j.preteyeres.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 18. Hassan M, Agarwal A, Afridi R, et al. The role of optical coherence tomography angiography in the management of uveitis. Int Ophthalmol Clin 2016; 56: 1–24. DOI: 10.1097/IIO.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 19. Pierro L, Marchese A, Gagliardi M, et al. Optical coherence tomography angiography of retinal cavernous hemangioma. Ophthalmic Surg Lasers Imaging Retina 2017; 48: 684–685. DOI: 10.3928/23258160-20170802. [DOI] [PubMed] [Google Scholar]
- 20. Pierro L, Marchese A, Gagliardi M, et al. Choroidal excavation in choroidal osteoma complicated by choroidal neovascularization. Eye (Lond) 2017; 31: 1740–1743. DOI: 10.1038/eye.2017.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yannuzzi NA, Swaminathan SS, Zheng F, et al. Swept-source OCT angiography shows sparing of the choriocapillaris in multiple evanescent white dot syndrome. Ophthalmic Surg Lasers Imaging Retina 2017; 48: 69–74. DOI: 10.3928/23258160-20161219-10. [DOI] [PubMed] [Google Scholar]
- 22. Pichi F, Srvivastava SK, Chexal S, et al. En face optical coherence tomography and optical coherence tomography angiography of multiple evanescent white dot syndrome: new insights into pathogenesis. Retina 2016; 36(Suppl. 1): S178–S188. DOI: 10.1097/IAE.0000000000001255. [DOI] [PubMed] [Google Scholar]
- 23. Gaudric A, Mrejen S. Why the dots are black only in the late phase of the indocyanine green angiography in multiple evanescent white dot syndrome. Retin Cases Brief Rep 2017; 11(Suppl. 1): S81–S85. DOI: 10.1097/ICB.0000000000000422. [DOI] [PubMed] [Google Scholar]
- 24. Zicarelli F, Mantovani A, Preziosa C, et al. Multimodal imaging of multiple evanescent white dot syndrome: a new interpretation. Ocul Immunol Inflamm. Epub ahead of print 15 August 2019. DOI: 10.1080/09273948.2019.1635169. [DOI] [PubMed] [Google Scholar]
- 25. Lages V, Mantovani A, Papadia M, et al. MEWDS is a true primary choriocapillaritis and basic mechanisms do not seem to differ from other choriocapillaritis entities. J Curr Ophthalmol 2018; 30: 281–286. DOI: 10.1016/j.joco.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mandadi SKR, Agarwal A, Aggarwal K, et al. Novel findings on optical coherence tomography angiography in patients with tubercular serpiginous-like choroiditis. Retina 2017; 37: 1647–1659. DOI: 10.1097/IAE.0000000000001412. [DOI] [PubMed] [Google Scholar]
- 27. Klufas MA, Phasukkijwatana N, Iafe NA, et al. Optical coherence tomography angiography reveals choriocapillaris flow reduction in placoid chorioretinitis. Ophthalmol Retina 2017; 1: 77–91. DOI: 10.1016/j.oret.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 28. Burke TR, Chu CJ, Salvatore S, et al. Application of OCT-angiography to characterise the evolution of chorioretinal lesions in acute posterior multifocal placoid pigment epitheliopathy. Eye (Lond) 2017; 31: 1399–1408. DOI: 10.1038/eye.2017.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mangeon M, Zett C, Amaral C, et al. Multimodal evaluation of patients with acute posterior multifocal placoid pigment epitheliopathy and serpiginous choroiditis. Ocul Immunol Inflamm 2018; 26: 1212–1218. DOI: 10.1080/09273948.2017.1335757. [DOI] [PubMed] [Google Scholar]
- 30. Montorio D, Giuffre C, Miserocchi E, et al. Swept-source optical coherence tomography angiography in serpiginous choroiditis. Br J Ophthalmol 2018; 102: 991–995. DOI: 10.1136/bjophthalmol-2017-310989. [DOI] [PubMed] [Google Scholar]
- 31. Aggarwal K, Agarwal A, Sharma A, et al. Detection of type 1 choroidal neovascular membranes using optical coherence tomography angiography in tubercular posterior uveitis. Retina 2019; 39: 1595–1606. DOI: 10.1097/IAE.0000000000002176. [DOI] [PubMed] [Google Scholar]
- 32. Klufas MA, O’Hearn T, Sarraf D. Optical coherence tomography angiography and widefield fundus autofluorescence in punctate inner choroidopathy. Retin Cases Brief Rep 2015; 9: 323–326. DOI: 10.1097/ICB.0000000000000203. [DOI] [PubMed] [Google Scholar]
- 33. Nakao S, Kaizu Y, Oshima Y, et al. Optical coherence tomography angiography for detecting choroidal neovascularization secondary to punctate inner choroidopathy. Ophthalmic Surg Lasers Imaging Retina 2016; 47: 1157–1161. DOI: 10.3928/23258160-20161130-13. [DOI] [PubMed] [Google Scholar]
- 34. Aggarwal K, Agarwal A, Gupta V. Type 2 choroidal neovascularization in a choroidal granuloma detected using swept-source optical coherence tomography angiography. Ophthalmic Surg Lasers Imaging Retina 2018; 49: 534–539. DOI: 10.3928/23258160-20180628-11. [DOI] [PubMed] [Google Scholar]
- 35. Turkcu FM, Sahin A, Yuksel H, et al. Octa imaging of choroidal neovascular membrane secondary to toxoplasma retinochoroiditis. Ophthalmic Surg Lasers Imaging Retina 2017; 48: 509–511. DOI: 10.3928/23258160-20170601-11. [DOI] [PubMed] [Google Scholar]
- 36. Nesper PL, Lutty GA, Fawzi AA. Residual choroidal vessels in atrophy can masquerade as choroidal neovascularization on optical coherence tomography angiography: introducing a clinical and software approach. Retina 2018; 38: 1289–1300. DOI: 10.1097/IAE.0000000000001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brar M, Sharma M, Grewal SPS, et al. Treatment response in sympathetic ophthalmia as assessed by widefield OCT angiography. Ophthalmic Surg Lasers Imaging Retina 2018; 49: 726–730. DOI: 10.3928/23258160-20180831-13. [DOI] [PubMed] [Google Scholar]
- 38. Aggarwal K, Agarwal A, Mahajan S, et al. The role of optical coherence tomography angiography in the diagnosis and management of acute Vogt-Koyanagi-Harada disease. Ocul Immunol Inflamm 2018; 26: 142–153. DOI: 10.1080/09273948.2016.1195001. [DOI] [PubMed] [Google Scholar]
- 39. Agarwal A, Aggarwal K, Dogra M, et al. Dengue-induced inflammatory, ischemic foveolitis and outer maculopathy: a swept-source imaging evaluation. Ophthalmol Retina 2019; 3: 170–177. DOI: 10.1016/j.oret.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 40. Tian M, Tappeiner C, Zinkernagel MS, et al. Swept-source optical coherence tomography angiography reveals vascular changes in intermediate uveitis. Acta Ophthalmol 2019; 97: e785–e791. DOI: 10.1111/aos.14024. [DOI] [PubMed] [Google Scholar]
- 41. Khairallah M, Kahloun R, Gargouri S, et al. Swept-source optical coherence tomography angiography in West Nile virus chorioretinitis and associated occlusive retinal vasculitis. Ophthalmic Surg Lasers Imaging Retina 2017; 48: 672–675. DOI: 10.3928/23258160-20170802-11. [DOI] [PubMed] [Google Scholar]
- 42. Agarwal A, Afridi R, Agrawal R, et al. Multimodal imaging in retinal vasculitis. Ocul Immunol Inflamm 2017; 25: 424–433. DOI: 10.1080/09273948.2017.1319494. [DOI] [PubMed] [Google Scholar]
- 43. Emre S, Guven-Yilmaz S, Ulusoy MO, et al. Optical coherence tomography angiography findings in Behcet patients. Int Ophthalmol 2019; 39: 2391–2399. DOI: 10.1007/s10792-019-01080-1. [DOI] [PubMed] [Google Scholar]
- 44. Khairallah M, Abroug N, Khochtali S, et al. Optical coherence tomography angiography in patients with behcet uveitis. Retina 2017; 37: 1678–1691. DOI: 10.1097/IAE.0000000000001418. [DOI] [PubMed] [Google Scholar]
- 45. Agarwal A, Mahajan S, Khairallah M, et al. Multimodal imaging in ocular tuberculosis. Ocul Immunol Inflamm 2017; 25: 134–145. DOI: 10.1080/09273948.2016.1231332. [DOI] [PubMed] [Google Scholar]
- 46. Agarwal A, Invernizzi A, Acquistapace A, et al. Analysis of retinochoroidal vasculature in human immunodeficiency virus infection using spectral-domain OCT angiography. Ophthalmol Retina 2017; 1: 545–554. DOI: 10.1016/j.oret.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 47. Choudhry N, Duker JS, Freund KB, et al. Classification and guidelines for widefield imaging: recommendations from the International Widefield Imaging Study Group. Ophthalmol Retina 2019; 3: 843–849. DOI: 10.1016/j.oret.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 48. Sheemar A, Temkar S, Takkar B, et al. Ultra-wide field imaging characteristics of primary retinal vasculitis: risk factors for retinal neovascularization. Ocul Immunol Inflamm 2019; 27: 383–388. DOI: 10.1080/09273948.2018.1508729. [DOI] [PubMed] [Google Scholar]
- 49. Cicinelli MV, Marchese A, Aragona E, et al. Ultra-widefield imaging of vasoocclusive retinopathy secondary to antiphospholipid syndrome. Retina 2019; 39: e32–e33. DOI: 10.1097/IAE.0000000000002593. [DOI] [PubMed] [Google Scholar]
- 50. Sharief L, Lightman S, Blum-Hareuveni T, et al. Clinical outcome of retinal vasculitis and predictors for prognosis of ischemic retinal vasculitis. Am J Ophthalmol 2017; 177: 206–212. DOI: 10.1016/j.ajo.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 51. Laovirojjanakul W, Acharya N, Gonzales JA. Ultra-widefield fluorescein angiography in intermediate uveitis. Ocul Immunol Inflamm 2019; 27: 356–361. DOI: 10.1080/09273948.2017.1371764. [DOI] [PubMed] [Google Scholar]
- 52. Marchese A, Miserocchi E, Giuffre C, et al. Aurora borealis and string of pearls in vitreoretinal lymphoma: patterns of vitreous haze. Br J Ophthalmol 2019; 103: 1656–1659. DOI: 10.1136/bjophthalmol-2018-313491. [DOI] [PubMed] [Google Scholar]
- 53. Dickson D, Agarwal A, Sadiq MA, et al. Assessment of vitreous haze using ultra-wide field retinal imaging. J Ophthalmic Inflamm Infect 2016; 6: 35. DOI: 10.1186/s12348-016-0105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tripathy K, Chawla R, Venkatesh P, et al. Ultrawide field imaging in uveitic non-dilating pupils. J Ophthalmic Vis Res 2017; 12: 232–233. DOI: 10.4103/2008-322X.205360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Aggarwal K, Agarwal A, Deokar A, et al. Ultra-wide field imaging in paradoxical worsening of tubercular multifocal serpiginoid choroiditis after the initiation of anti-tubercular therapy. Ocul Immunol Inflamm 2019; 27: 365–370. DOI: 10.1080/09273948.2017.1373829. [DOI] [PubMed] [Google Scholar]
- 56. Tadepalli S, Bajgai P, Dogra M, et al. Ultra-widefield fundus autofluorescence in cytomegalovirus retinitis. Ocul Immunol Inflamm. Epub ahead of print 28 May 2019. DOI: 10.1080/09273948.2019.1595671. [DOI] [PubMed] [Google Scholar]
- 57. Mudvari SS, Virasch VV, Singa RM, et al. Ultra-wide-field imaging for cytomegalovirus retinitis. Ophthalmic Surg Lasers Imaging 2010; 41: 311–315. DOI: 10.3928/15428877-20100430-03. [DOI] [PubMed] [Google Scholar]
- 58. Lee EK, Lee SY, Yu HG. A clinical grading system based on ultra-wide field retinal imaging for sunset glow fundus in Vogt-Koyanagi-Harada disease. Graefes Arch Clin Exp Ophthalmol 2015; 253: 359–368. DOI: 10.1007/s00417-014-2710-7. [DOI] [PubMed] [Google Scholar]
- 59. Marchese A, Rabiolo A, Corbelli E, et al. Ultra-widefield imaging in patients with angioid streaks secondary to pseudoxanthoma elasticum. Ophthalmol Retina 2017; 1: 137–144. DOI: 10.1016/j.oret.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 60. Lavine JA, Singh AD, Sharma S, et al. Ultra-widefield multimodal imaging of primary vitreoretinal lymphoma. Retina 2019; 39: 1861–1871. DOI: 10.1097/IAE.0000000000002260. [DOI] [PubMed] [Google Scholar]
- 61. Marchese A, Miserocchi E, Modorati G, et al. Widefield OCT angiography of idiopathic retinal vasculitis, aneurysms, and neuroretinitis. Ophthalmol Retina 2017; 1: 567–569. DOI: 10.1016/j.oret.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 62. Samy A, Lightman S, Ismetova F, et al. Role of autofluorescence in inflammatory/infective diseases of the retina and choroid. J Ophthalmol 2014; 2014: 418193. DOI: 10.1155/2014/418193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Turkcuoglu P, Chang PY, Rentiya ZS, et al. Mycophenolate mofetil and fundus autofluorescence in the management of recurrent punctate inner choroidopathy. Ocul Immunol Inflamm 2011; 19: 286–292. DOI: 10.3109/09273948.2011.580072. [DOI] [PubMed] [Google Scholar]
- 64. Haen SP, Spaide RF. Fundus autofluorescence in multifocal choroiditis and panuveitis. Am J Ophthalmol 2008; 145: 847–853. DOI: 10.1016/j.ajo.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 65. Munk MR, Jung JJ, Biggee K, et al. Idiopathic multifocal choroiditis/punctate inner choroidopathy with acute photoreceptor loss or dysfunction out of proportion to clinically visible lesions. Retina 2015; 35: 334–343. DOI: 10.1097/IAE.0000000000000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Giuliari G, Hinkle DM, Foster CS. The spectrum of fundus autofluorescence findings in birdshot chorioretinopathy. J Ophthalmol 2009; 2009: 567693. DOI: 10.1155/2009/567693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Herbort CP, Jr, Pavesio C, LeHoang P, et al. Why birdshot retinochoroiditis should rather be called ‘HLA-A29 uveitis’? Br J Ophthalmol 2017; 101: 851–855. DOI: 10.1136/bjophthalmol-2016-309764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tomkins-Netzer O, Taylor SR, Lightman S. Long-term clinical and anatomic outcome of birdshot chorioretinopathy. JAMA Ophthalmol 2014; 132: 57–62. DOI: 10.1001/jamaophthalmol.2013.6235. [DOI] [PubMed] [Google Scholar]
- 69. Furino C, Boscia F, Cardascia N, et al. Fundus autofluorescence and multiple evanescent white dot syndrome. Retina 2009; 29: 60–63. DOI: 10.1097/IAE.0b013e31818c5e04. [DOI] [PubMed] [Google Scholar]
- 70. Gal-Or O, Sorenson JA, Gattoussi S, et al. Multiple evanescent white dot syndrome with subretinal deposits. Retin Cases Brief Rep 2019; 13: 314–319. DOI: 10.1097/ICB.0000000000000602. [DOI] [PubMed] [Google Scholar]
- 71. Ayata A, Dogru S, Senol MG, et al. Autofluorescence findings in Vogt-Koyanagi-Harada disease. Eur J Ophthalmol 2009; 19: 1094–1097. [DOI] [PubMed] [Google Scholar]
- 72. Mrejen S, Khan S, Gallego-Pinazo R, et al. Acute zonal occult outer retinopathy: a classification based on multimodal imaging. JAMA Ophthalmol 2014; 132: 1089–1098. DOI: 10.1001/jamaophthalmol.2014.1683. [DOI] [PubMed] [Google Scholar]
- 73. Yeh S, Forooghian F, Wong WT, et al. Fundus autofluorescence imaging of the white dot syndromes. Arch Ophthalmol 2010; 128: 46–56. DOI: 10.1001/archophthalmol.2009.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gupta A, Bansal R, Gupta V, et al. Fundus autofluorescence in serpiginouslike choroiditis. Retina 2012; 32: 814–825. DOI: 10.1097/IAE.0b013e3182278c41. [DOI] [PubMed] [Google Scholar]
- 75. Eandi CM, Neri P, Adelman RA, et al. Acute syphilitic posterior placoid chorioretinitis: report of a case series and comprehensive review of the literature. Retina 2012; 32: 1915–1941. DOI: 10.1097/IAE.0b013e31825f3851. [DOI] [PubMed] [Google Scholar]
- 76. Ishida T, Ohno-Matsui K, Kaneko Y, et al. Fundus autofluorescence patterns in eyes with primary intraocular lymphoma. Retina 2010; 30: 23–32. DOI: 10.1097/IAE.0b013e3181b408a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Borrelli E, Nittala MG, Abdelfattah NS, et al. Comparison of short-wavelength blue-light autofluorescence and conventional blue-light autofluorescence in geographic atrophy. Br J Ophthalmol 2018; 103: 610–616. DOI: 10.1136/bjophthalmol-2018-311849. [DOI] [PMC free article] [PubMed] [Google Scholar]