Abstract
Background:
Ketamine’s defining side effects are dissociation and increased blood pressure/heart rate. An oral formulation with delayed absorption could minimize these effects. We recently reported safety and tolerability data for an extended release ketamine tablet in healthy volunteers.
Methods:
To assess safety, tolerability, efficacy, and pharmacokinetics of an extended release oral ketamine tablet in patients with treatment-resistant depression/anxiety. This was a multiple dose open-label flexible dose uncontrolled study in seven patients with treatment-resistant depression/anxiety, who had all previously demonstrated mood improvement to subcutaneous ketamine. Assessments included ratings of anxiety, depression and dissociation, safety and tolerability, and blood samples for ketamine pharmacokinetics and brain-derived neurotrophic factor (BDNF) concentrations.
Results:
Improvements in anxiety and depression ratings occurred gradually over 96 h; all patients had >50% improvements in mood ratings. Ketamine was safe and well tolerated, with no changes in vital signs, and a single brief report of dissociation. Ketamine may induce its own metabolism, as the ratio of norketamine to ketamine increased out to 96 h. Serum BDNF concentrations did not change during the study.
Conclusion:
Ketamine’s safety/tolerability may be improved with an extended release oral formulation. Onset of mood improvement is slightly delayed compared with parenteral dosing. These data support the further development of extended release ketamine tablets for treatment of resistant depression and anxiety disorders.
Keywords: anxiety, depression, dissociation, extended-release ketamine tablet, first-in-patient, pharmacodynamics, pharmacokinetics, safety
Introduction
Ketamine, an N-methyl-d-aspartate (NMDA) antagonist, has fast-onset activity in patients with treatment-resistant depression and anxiety disorders.1,2 Although most published data have been in studies where ketamine is administered as an intravenous infusion, activity has also been reported after alternative routes of administration, including oral, sublingual, intranasal, intramuscular, and subcutaneous routes.3 Evaluation of comparative efficacy is limited by differences in dosing and lack of head-to-head comparative data. A pilot study comparing changes in depressed mood after intravenous (IV), intramuscular (IM), and subcutaneous (SC) dosing reported comparable antidepressant effects, independent of route of administration.4 The most common side effects reported after parenteral administration include dissociation and increases in systolic and diastolic blood pressure, which are associated with peak plasma concentrations of ketamine.4,5 One strategy to improve tolerability might be to administer ketamine orally using an extended release formulation, to delay absorption and thus minimize side effects associated with peak ketamine concentrations. This strategy should not interfere with ketamine’s antidepressant/anxiolytic effects, because norketamine and its hydroxy-metabolites appear to have important roles in ketamine’s antidepressant actions.6–8 We recently reported data from an ascending dose study of a ketamine tablet designed to release drug over 10 h, and found it did not alter blood pressure or heart rate, and had very minor dissociative effects at 240 mg doses.9 This paper reports on the efficacy and safety of this formulation in patients with treatment-resistant depression and/or anxiety, who had previously responded to parenteral ketamine.
Materials and methods
The objectives of this pilot study were to evaluate the efficacy, safety, tolerability, and pharmacokinetics of multiple doses of an extended release ketamine tablet formulation in patients with treatment-resistant depression and/or anxiety. The protocol for this study was approved by the Southern Health and Disability Ethics Committee (16/STH/121) and SCOTT (16/SCOTT/67), and the study was registered with the Australian New Zealand Clinical Trial Registry (ACTRN12616001351404).
This was a multiple dose, open-label, uncontrolled study in up to 12 patients with treatment-resistant DSM-5 major depressive episode (MDE) and/or social anxiety disorder (SAD) and/or generalized anxiety disorder (GAD), performed at the Zenith Technology Clinical Trials Unit. Patient inclusion criteria included having a Hamilton anxiety scale (HAMA10) score of >20, and/or a Liebowitz social anxiety scale (LSAS11) score of >60, and/or a Montgomery-Asberg depression rating scale (MADRS12) score of >20 at screening. All patients had to have previously received subcutaneous ketamine in clinical trials or in clinical settings,5,13 with positive mood responses. All patients provided signed informed consent prior to enrolment, and were assessed as suitable to participate based on review of medical history, physical examination, safety laboratory tests, vital signs, and electrocardiography (ECG0. Patients could remain on established treatments, but could not change these for 4 weeks prior to dosing.
Details of the 60 mg tablet formulation are provided elsewhere.14 On Day 1, after an overnight fast, patients received a 60 mg dose at 8 a.m., and 60–120 mg at 8 p.m. On Days 2–4, patients could receive 60–240 mg doses 12-hourly, with the final dose being given at 72 h. The actual dose administered to each participant was based on whether or not their symptoms had improved, along with assessments of safety and tolerability. The selected dose range was based on safety and tolerability data from an earlier healthy volunteer study.9
Safety assessments included vital signs, ECGs, safety laboratory tests pre-dose through to 96 h post-dose. Tolerability assessments included reported adverse events throughout the study. Suicidality was assessed using the Columbia Suicide Rating Scale (CSSRS) and dissociation monitored using the Clinician Administered Dissociative States Scale (CADSS) pre-dose and throughout the study.15,16 Anxiety symptoms were evaluated using the HAMA and Fear Questionnaire (FQ) scales and depression using the MADRS.17
Blood samples to determine plasma ketamine and norketamine concentrations were taken pre-dose and then at 2, 12, 14, 24, 26, 36, 38, 48, 50, 60, 62, 72, 74, and 96 h post-dose (i.e. pre-dose and 2 h post-dose). Blood samples for serum brain-derived neurotrophic factor (BDNF) concentrations were taken pre-dose and then at 2, 12, 14, 24, 72, 74, 84, 86, and 96 h post-dose. Samples were centrifuged and serum stored at –80°C until analyzed. Details of the ketamine, norketamine, and BDNF assays are reported elsewhere.9
Summary statistics (means, standard deviations, and coefficients of variation) were determined for safety laboratory test data, ECG, and ketamine, norketamine, and BDNF concentration data. Categorical variables were analyzed using counts and percentages. The relationship between norketamine:ketamine ratios and time was evaluated by regression analysis.
Results
Nine patients were screened, and a single cohort of seven patients enrolled in and completed this study. All patients had failed to respond to multiple prior medication and psychotherapy trials, and had extensive comorbidity (see Supplementary table for details). There were three females and four males. Mean age was 27.0 years (range 22–33). All seven met criteria for SAD, and 6/7 had current MDE of moderate or greater severity (MADRS scores >20). Mean HAMA at screening was 22.9 (indicative of severe anxiety). All patients were currently taking antidepressants and had not responded to prior trials of antidepressants and group or individual psychotherapy, including cognitive behavioral therapy (CBT). All patients titrated up to 240 mg by 60 h. Mean doses were 60 mg at 0 h; 120 mg at 12 h; 180 mg at 24 h; 223 mg at 36 h; 231 mg at 48 h; 240 mg at 60 h and 240 mg at 72 h.
Changes in anxiety rating scales
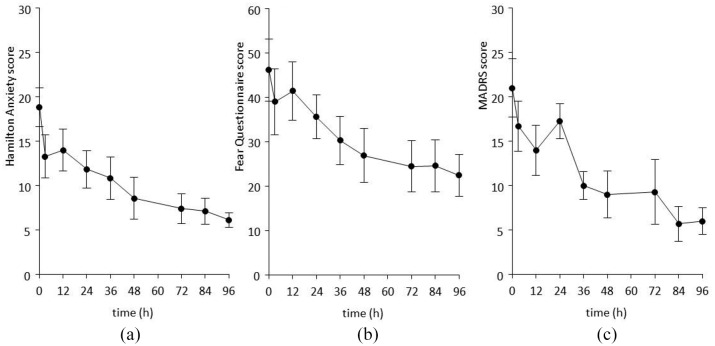
Mean HAMA rating-time profiles are shown in Figure 1A. There was an initial rapid decrease in scores at 12 h, with a more gradual reduction out to 96 h (final mean score = 6.1). All seven patients had 50% or greater reductions in HAMA compared with baseline. Mean FQ rating-time profiles are shown in Figure 1B. There was gradual reduction in scores out to 96 h (final mean score = 22.4). Six of seven patients had 50% or greater reductions in FQ compared with baseline. Mean MADRS rating-time profiles are shown in Figure 1C. There was a gradual reduction in scores out to 96 h (final mean score = 5.7). All seven patients had 50% or greater reductions in MADRS compared with baseline.
Figure 1.
Effect of extended release ketamine tablets on mean (SEM) HAMA (A), FQ (B), and MADRS (C) scores.
HAMA, Hamilton anxiety scale; FQ, fear questionnaire; MADRS, Montgomery-Asberg depression rating scale; SEM, standard error of the mean.
Safety and tolerability
A total of five adverse events, rated as mild in intensity, were reported: dizziness (n = 1), dissociation (n = 1), and headache (n = 3). Dissociation, with onset 30 min after dosing and which lasted 2 h, was reported by one patient whose dose increased from 120 mg to 180 mg. No dissociation was reported at 240 mg. There were no changes in CADSS scores. There were no changes in safety laboratory tests or vital signs during the study. There were no ECG changes of note: no patients had QTcF values >500 ms or increases from baseline >60 ms. CSSRS assessments showed no evidence of increased suicidal ideation at any time during the study by any patient.
Pharmacokinetic data
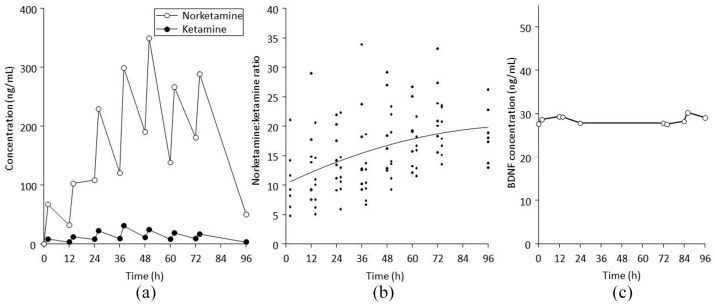
Mean ketamine and norketamine concentration-time profiles are shown in Figure 2A. Norketamine concentrations were substantially higher than ketamine at all timepoints. The increasing concentrations of both analytes over time reflects increasing doses (all patients were receiving 240 mg 12 hourly by 60 h). Norketamine:ketamine ratios increased over time, from 10.8 at 2 h to 18.6 at 96 h (Figure 2B). Data variability also decreased over time, from 51.7% at 2 h to 25.2% at 96 h. Serum BDNF concentrations remained unchanged over 96 h (Figure 2C).
Figure 2.
(A) Mean concentration-time profiles for ketamine and norketamine after 12 hourly dosing of extended release ketamine tablets out to 72 h. (B) Individual norketamine:ketamine ratios over time, with a fitted regression line. (C) Mean serum BDNF concentration-time profiles after 12 hourly dosing of extended release ketamine tablets out to 72 h.
BDNF, brain-derived neurotrophic factor.
Discussion
The main findings of this pilot study are that ketamine had broad anxiolytic and antidepressant effects in patients with severe treatment-resistant SAD/GAD/MDE. Ketamine was safe and well tolerated in this population, with only one report of dissociation, and no evidence of sympathomimetic changes in blood pressure or heart rate. Most drug present after oral dosing was norketamine. Changes in norketamine:ketamine ratios over 96 h may indicate autoinduction. Serum BDNF concentrations were not changed by ketamine.
We observed anxiolytic and antidepressant effects in all patients; however, the speed of onset with the extended release tablet formulation appeared to be modestly slower than after subcutaneous dosing. Six of the seven patients had participated in subcutaneous dosing in earlier studies for resistant anxiety.5,13 The reductions in HAMA and FQ scores at 48 h after oral dosing in this study were comparable to changes 24 h after subcutaneous dosing (Table 1).
Table 1.
Mean and post-dose FQ, HAMA and CADSS scores after oral and SC dosing in six patients with both sets of data (subcutaneous data are from references).5,13 The time at which CADSS data were obtained postdose corresponds to time of peak ketamine concentration.5,9
| Formulation | SC/injectable | Oral/ext-release tablet | ||
|---|---|---|---|---|
| Time | pre | 24 h | pre | 48 h |
| HAMA | 21.7 | 6.8 | 18.3 | 7.7 |
| FQ | 47.8 | 28 | 43.7 | 22.7 |
| Time | pre | 0.5 h | pre | 3 h |
| CADSS | 2.4 | 22.3 | 5.6 | 3.3 |
CADSS, clinician administered dissociative states scale; HAMA, Hamilton anxiety scale; FQ, fear questionnaire; SC, subcutaneous.
Oral ketamine was well tolerated by all participants. There were no changes in blood pressure or heart rate at any dose. There was one report of dissociation of mild intensity at a dose of 180 mg, but not at higher doses. In contrast, in our earlier ascending dose study in healthy volunteers, there were 11 reports of dissociation at 240 mg but not at lower doses.9 It is possible that the lower rates of dissociation in this study are due to tolerance development, and that dissociation might be minimized in future studies by dose titration. CADSS data were available for six of the seven patients who had prior subcutaneous dosing in earlier studies for treatment-resistant anxiety.5,13 At the time when ketamine concentrations were maximal, CADSS scores increased by 20 points after subcutaneous dosing, whereas scores fell by three points after oral dosing (Table 1). We previously reported that dissociation scores correlated with peak ketamine concentrations after subcutaneous dosing,4,13 and the decline in dissociation ratings after oral dosing may reflect relatively lower peak concentrations, which occur some hours after dosing.9
Although the modestly slower (~24 h) onset of anxiolytic/antidepressant activity after oral dosing compared with subcutaneous dosing could be one reason to favour parenteral over oral dosing, the improved tolerability (no changes in blood pressure or heart rate, and minimal dissociation) are important advantages for oral dosing. Presumably, these changes would shorten the time patients would need to be observed after initial dosing, could allow patients to dose themselves at home, and might improve compliance.
Formal pharmacokinetic analysis was not possible in this study due to the limited number of pharmacokinetic samples collected (pre-dose and 2 h post-dose), and a study design that permitted flexible dosing. As we reported previously, after five doses of oral ketamine given 12-hourly, AUC0-12 values were smaller than the corresponding single dose mean area under the curve (AUC0-∞) values for higher (120 and 240 mg) dose groups.9 Lin et al. reported that daily oral dosing of ketamine to rats for 2 weeks increased activity of CYP1A2, -3A, and -2B6, based on metabolism of probe substrates.18 CYP3A and -2B6 are involved in the metabolism of ketamine to norketamine, and CYP2B6 in the metabolism of norketamine to hydroxy-metabolites. Our finding of increasing norketamine:ketamine ratios over 96 h would be consistent with autoinduction. From a drug interaction perspective, if ketamine dosing in depression occurs twice weekly, induction may be minimal. This should be evaluated in future studies.
Changes in central BDNF levels have been identified as one component of ketamine’s antidepressant mechanism of action.7 Haile et al. reported a negative association between plasma BDNF concentrations and decrease in depression rating scores 4 h after ketamine dosing in depressed patients.19 We did not identify any changes in this study, and we have previously reported negative findings for BDNF changes after subcutaneous dosing of ketamine in patients with resistant anxiety and in healthy volunteers after oral ketamine.5,9 Additionally, we have reported on acute changes in plasma and serum BDNF in rodents given ketamine that are unrelated to brain BDNF concentrations.20 In summary, peripheral BDNF does not appear to be a useful biomarker for brain BDNF changes.
The limitations of this study should be acknowledged. This was a brief (96 h) phase Ib study in a small number of anxious/depressed patients, who had extensive comorbidities, and who had previously shown positive mood responses to ketamine. Based on the small number of subjects, and their selection based on prior positive mood responses to ketamine, it is not possible to compare efficacy data with unselected populations. Definitive safety and tolerability information and establishing appropriate dose ranges for patients with resistant depression and anxiety disorders will require further testing in larger clinical populations. The extended release oral formulation used in this study has slow dissolution across the physiological pH range and in ethanolic solution.14 Taken together with the fact that the tablet is very hard, and potentially difficult to crush, this could limit abuse potential. Additional studies are needed to further examine the impact of formulation on abuse liability.
In conclusion, multiple doses of extended release ketamine tablets up to 240 mg 12-hourly were safe and well tolerated in patients with treatment-resistant depression and anxiety. There was only one report of dissociation, and no evidence of sympathomimetic changes in blood pressure or heart rate. Oral ketamine had broad anxiolytic and antidepressant effects, with onset delayed (~24 h) compared with parenteral ketamine dosing. The results of this study support the further development of extended release ketamine tablets for treatment resistant depression and anxiety disorders.
Supplemental Material
Supplemental material, Supplemental_Table for Safety and efficacy of extended release ketamine tablets in patients with treatment-resistant depression and anxiety: open label pilot study by Paul Glue, Natalie J. Medlicott, Shona Neehoff, Peter Surman, Fred Lam, Noelyn Hung and Cheung-tak Hung in Therapeutic Advances in Psychopharmacology
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Douglas Pharmaceuticals Ltd.
Conflict of interest statement: Paul Glue and Natalie Medlicott have a contract with Douglas Pharmaceuticals Ltd to develop novel ketamine formulations. Within the last 3 years, Paul Glue has attended an advisory board for Janssen Pharmaceuticals. Peter Surman is an employee of Douglas Pharmaceuticals Ltd. No other authors have disclosures.
ORCID iD: Paul Glue  https://orcid.org/0000-0002-7305-2800
https://orcid.org/0000-0002-7305-2800
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Paul Glue, Hazel Buckland Chair of Psychological Medicine, School of Medical Sciences, University of Otago, PO Box 913, Dunedin, 9054, New Zealand.
Natalie J. Medlicott, School of Pharmacy, University of Otago, Dunedin, New Zealand
Shona Neehoff, Psychological Medicine, University of Otago, Dunedin, New Zealand.
Peter Surman, Douglas Pharmaceuticals Ltd, Auckland, New Zealand.
Fred Lam, Zenith Technology Ltd, Dunedin, New Zealand.
Noelyn Hung, Zenith Technology Ltd, Dunedin, New Zealand.
Cheung-tak Hung, Zenith Technology Ltd, Dunedin, New Zealand.
References
- 1. Sartori SB, Singewald N. Novel pharmacological targets in drug development for the treatment of anxiety and anxiety-related disorders. Pharmacol Ther 2019; 204: 107402. [DOI] [PubMed] [Google Scholar]
- 2. Xu Y, Hackett M, Carter G, Loo C, et al. Effects of low-dose and very low-dose ketamine among patients with major depression: a systematic review and meta-analysis. Int J Neuropsychopharmacol 2016; 19: pii: pyv124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andrade C. Ketamine for depression, 4: in what dose, at what rate, by what route, for how long, and at what frequency? J Clin Psychiatry 2017; 78: e852–e857. [DOI] [PubMed] [Google Scholar]
- 4. Loo CK, Gálvez V, O’Keefe E, et al. Placebo-controlled pilot trial testing dose titration and intravenous, intramuscular and subcutaneous routes for ketamine in depression. Acta Psychiatr Scand 2016; 134: 48–56. [DOI] [PubMed] [Google Scholar]
- 5. Glue P, Neehoff S, Sabadel A, et al. Effects of ketamine in patients with treatment-refractory generalized anxiety and social anxiety disorders: exploratory double-blind psychoactive-controlled replication study. J Psychopharmacol. Epub ahead of print 17 September 2019. DOI: 10.1177/0269881119874457. [DOI] [PubMed] [Google Scholar]
- 6. Paul RK, Singh NS, Khadeer M, et al. (R,S)-Ketamine metabolites (R,S)-norketamine and (2S,6S)-hydroxynorketamine increase the mammalian target of rapamycin function. Anesthesiology 2014; 121: 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry 2018; 23: 801–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zanos P, Moaddel R, Morris PJ, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016; 533: 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glue P, Medlicott NJ, Surman P, et al. Ascending-dose study of controlled-release ketamine tablets in healthy volunteers: pharmacokinetics, pharmacodynamics, safety, and tolerability. J Clin Pharmacol. Epub ahead of print 17 February 2020. DOI: 10.1002/jcph.1573. [DOI] [PubMed] [Google Scholar]
- 10. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol 1959; 32: 50–55 [DOI] [PubMed] [Google Scholar]
- 11. Liebowitz MR. Social phobia. Mod Probl Pharmacopsychiatry 1987; 22: 141–173. [DOI] [PubMed] [Google Scholar]
- 12. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134: 382–389. [DOI] [PubMed] [Google Scholar]
- 13. Glue P, Medlicott NJ, Harland S, et al. Ketamine’s dose-related effects on anxiety symptoms in patients with treatment refractory anxiety disorders. J Psychopharmacology 2017; 31: 1302–1305. [DOI] [PubMed] [Google Scholar]
- 14. Glue P, Medlicott NJ, Surman PW. Extended release pharmaceutical formulation. US Patent Office, 10,441,544 B2, 2019. [Google Scholar]
- 15. Posner K, Brent D, Lucas C, et al. Columbia-suicide severity rating scale (C-SSRS). New York: Columbia University Medical Center, 2008. [Google Scholar]
- 16. Bremner JD, Krystal JH, Putnam F, et al. Measurement of dissociative states with the Clinician Administered Dissociative States Scale (CADSS). J Trauma Stress 1998; 11: 125–136. [DOI] [PubMed] [Google Scholar]
- 17. Marks IM, Mathews AM. Brief standard self-rating for phobic patients. Behav Res Ther 1979; 17: 263–267. [DOI] [PubMed] [Google Scholar]
- 18. Lin F, He Y, Zhang L, et al. Assessment of the effect of ketamine on cytochrome P450 isoforms activity in rats by cocktail method. Int J Clin Exper Med 2015; 8: 4335–4341. [PMC free article] [PubMed] [Google Scholar]
- 19. Haile CN, Murrough JW, Iosifescu DV, et al. Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int J Neuropsychopharmacology 2014; 17: 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Le Nedelec M, Glue P, Winter H, et al. Acute low-dose ketamine produces a rapid and robust increase in plasma BDNF without altering brain BDNF concentrations. Drug Deliv Trans Res 2018; 8: 780–786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Table for Safety and efficacy of extended release ketamine tablets in patients with treatment-resistant depression and anxiety: open label pilot study by Paul Glue, Natalie J. Medlicott, Shona Neehoff, Peter Surman, Fred Lam, Noelyn Hung and Cheung-tak Hung in Therapeutic Advances in Psychopharmacology