Abstract
Right ventricular (RV) dyssynchrony has been related to outcome in pulmonary arterial hypertension. Prospectively, we performed echocardiography with measurement of right ventricular dyssynchrony and pressure–volume loop catheterization in 27 pulmonary arterial hypertension patients. Afterload and diastolic function emerged as determinates of wall stress, which results in dyssynchrony.
Keywords: pulmonary arterial hypertension, right ventricular dyssynchrony, pressure–volume loop catheter, coupling
To the Editor,
Coupling of the right ventricle to its load is considered to be one of the main determinants of symptomatology and outcome in pulmonary arterial hypertension (PAH).1,2 To maintain right ventricular (RV)–pulmonary arterial coupling, the right ventricle reacts with adaptation and eventually maladaptation as disease progresses.3 Among various maladaptive mechanism, RV dyssynchrony has been described as a relevant determinant of RV function,4,5 exercise capacity6 and outcome7 in PAH. RV dyssynchrony is measured in echocardiography by two-dimensional (2D) speckle-tracking with the assessment of the four mid-basal RV segments,4 since the apical segments have been shown to have high variability in normal subjects.5 The gold standard method for evaluation of load independent RV function requires the acquisition of pressure–volume (PV) loops with the measurement of end-systolic elastance (Ees; defines contractility), arterial elastance (Ea; defines afterload) and the ratio of Ees/Ea (defines RV–pulmonary arterial coupling)2 either derived from the single beat method or across multiple cardiac cycles with reduction of venous return.8 However, the association of load-independent RV function and dyssynchrony remains unknown.
We analyzed PAH patients (diagnosis made according updated recommendations9) from the Right Heart I study10 (ClinicalTrials.gov identifier: NCT03403868) and the Giessen Pulmonary Hypertension Registry11 with available 2D speckle-tracking echocardiography between January 2016 and March 2019. Patients with chronic thromboembolic pulmonary hypertension, insufficient echocardiographic quality (frame rate, basal segments or electrocardiogram), lack of RV wall stress data or bundle branch block were excluded. All patients underwent echocardiography (and cardiac magnetic resonance imaging for calibrating the PV catheter) on day 1 and PV catheterization on day 2. All participating patients gave written informed consent and the study was approved by the ethics committee of the Faculty of Medicine at the University of Giessen (approval number: 108/15). Briefly, as recommended, the four-chamber apical view was obtained12 and offline analysis of six standard segments and corresponding time–strain longitudinal curves of the RV were performed (EchoPac version 201, GE Healthcare, Wauwatosa, WI, USA). RV dyssynchrony was defined as the standard deviation of the times to peak-systolic strain for the four mid-basal RV segments corrected to Bazett's formula.4 PV loop catheterization (CA-Nr 41063, CD Leycom, Zoetermeer, The Netherlands) including the assessment of RV diastolic stiffness (end-diastolic elastance, Eed) was performed as described previously.8,10,13–15 When the PV loops showed a clear overlap, a single beat was chosen for calculation of Ees and Ea. A theoretical isovolumetric maximum pressure (Pmax) was calculated from the sinusoidal extrapolation of first derivative-determined isovolumetric portions of the RV pressure curve. Ees is calculated as (Pmax – end-systolic pressure (ESP))/stroke volume, and Ea is calculated as ESP/stroke volume.2 RV wall stress was calculated from cardiac magnetic resonance imaging (cMRI) and PV-loop-pressure-data using the following equation: 0.5 × RV systolic pressure × RV end-systolic radius/RV end-systolic wall thickness according the Laplace's law.
Association between variables was evaluated with Pearson correlation and multivariate linear regression analysis (multicollinearity was assessed by variance inflation factor, indices were ln- or log-transformed).
Twenty-seven patients with PAH (idiopathic (n = 20; 74.1%)) were enrolled. RV dyssynchrony was 74 ± 38 ms, RV wall stress was 176 ± 100 mmHg, Ees was 0.50 mmHg/ml (interquartile range (IQR): 0.30–0.76 mmHg/ml), Ea was 0.80 mm Hg/ml (IQR: 0.46–1.10 mmHg/ml), Eed was 0.18 mm Hg/ml (IQR: 0.07–0.27 mmHg/ml, n = 25) and Ees/Ea was 0.70 (IQR: 0.38–1.03).
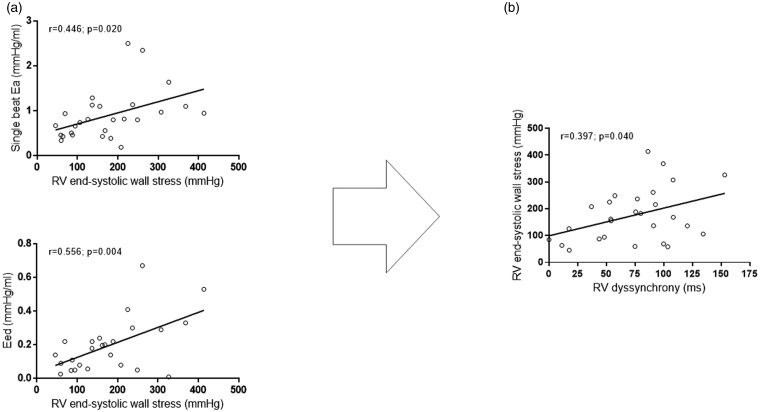
Ea and Eed showed a significant correlation with RV wall stress (Fig. 1), while Ees or Ees/Ea did not. In addition, we observed a weak association of RV dilatation (end-diastolic volume) with RV dyssynchrony (rho = 0.382, p = 0.050). We observed no direct association of Eed with RV dyssynchrony (rho = 0.169, p = 0.419). In bivariate linear regression (including Ea and Eed), Ea remained significantly associated with RV wall stress (unstandardized B coefficient: 91.5; p = 0.026; standard error of estimate (SEE): 38.2; 95% confidence interval (CI) for B 12.1 to 170.1). In turn, RV end-systolic wall stress was a significant determinant of RV dyssynchrony (unstandardized B coefficient: 0.15; p = 0.040; SEE: 0.07; 95% CI for B 0.01 to 0.30).
Fig. 1.
(a) Correlation of Ea and Eed with RV end-systolic wall stress and (b) correlation of RV end-systolic wall stress and with RV dyssynchrony. Pearson correlation.
Ea: arterial elastance; RV: right ventricular; Eed: end-diastolic elastance.
Our study identified the effective Ea (as a measure of afterload) to be the major determinant of RV wall stress which in turn influences RV dyssynchrony in severe PAH. Noteworthy, previous work showed an association of PVR as measure of afterload with RV dyssynchrony.5 As nicely demonstrated, lowering PVR also resulted in a substantially improvement in RV dyssynchrony even below the prognostic relevant cut-off of 23 ms. In fact, a combination of various maladaptive processes, such as RV dilatation and RV eccentric hypertrophy, leads to high RV wall stress and concomitant RV dyssynchrony.5 The current study is the first to evaluate the association of RV wall stress and Ea with RV dyssynchrony; both measured with gold standard PV loops and through Laplace's law, respectively. We observed no direct association of Eed with RV dyssynchrony, although our data indicate that impaired RV diastolic function contributes to RV dyssynchrony through high RV wall stress. The discussion about right ventricular stiffening, its association with fibrosis and the impact of fibrosis on RV adaptation and maladaptation is a matter of ongoing and controversial discussion and needs further evaluation.16 Studies exploring the longitudinal evolution of RV wall stress, dyssynchrony and the impact of RV–pulmonary arterial coupling in early states of the disease are warranted.
In conclusion, effective Ea and Eed as a measure of RV diastolic function emerged as a determinate of RV wall stress, which in turn results in RV dyssynchrony in advanced and severe PAH.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Projektnummer 268555672 – SFB 1213, Project B08. For the article, editorial assistance was provided by Dr Claire Mulligan (Beacon Medical Communications Ltd, Brighton, UK), funded by the University of Giessen.
Ethical approval
The study was approved by the ethics committee of the Faculty of Medicine at the University of Giessen (approval number 108/15).
Guarantor
M.J.R., K.T.
Contributorship
K.T., H.A.G., W.S., H.G. and M.J.R.: study design; K.T., H.A.G., W.S., H.G. and M.J.R.: patient recruitment; K.T., R.B., J.W., H.A.G., R.N., W.S., H.G., R.V. and M.J.R.: data collection and analysis; K.T., R.B., H.G. and M.J.R.: statistical analyses; K.T., R.B., J.W., H.A.G., R.N., W.S., H.G., S.H., R.V. and M.J.R.: drafting of the manuscript; K.T., R.B., J.W., H.A.G., R.N., W.S., H.G., S.H., R.V. and M.J.R.: critical revision of the manuscript for important intellectual content. K.T. was the principal investigator, had access to all the data in the study and takes full responsibility for the integrity and accuracy of the data analysis.
ORCID iDs
Rebecca Vanderpool https://orcid.org/0000-0001-6038-0568 Antonia Dalmer https://orcid.org/0000-0001-7440-1171 Henning Gall https://orcid.org/0000-0001-7016-7373
References
- 1.Ren X, Johns RA and Gao WD. EXPRESS: Right Heart in Pulmonary Hypertension: From Adaptation to Failure. Pulmonary circulation 2019. DOI: 10.1177/2045894019845611. [DOI] [PMC free article] [PubMed]
- 2.Tello K, Seeger W, Naeije R, et al. Right heart failure in pulmonary hypertension: Diagnosis and new perspectives on vascular and direct right ventricular treatment. Br J Pharmacol 2019. DOI: 10.1111/bph.14866. [DOI] [PubMed]
- 3.Vonk Noordegraaf A, Chin KM, Haddad F, et al. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. The European respiratory journal 2019; 53. DOI: 10.1183/13993003.01900-2018. [DOI] [PMC free article] [PubMed]
- 4.Badagliacca R, Poscia R, Pezzuto B, et al. Right ventricular dyssynchrony in idiopathic pulmonary arterial hypertension: determinants and impact on pump function. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation 2015; 34: 381–389. [DOI] [PubMed]
- 5.Badagliacca R, Reali M, Poscia R, et al. Right Intraventricular Dyssynchrony in Idiopathic, Heritable, and Anorexigen-Induced Pulmonary Arterial Hypertension: Clinical Impact and Reversibility. JACC Cardiovascular imaging 2015; 8: 642–652. [DOI] [PubMed]
- 6.Badagliacca R, Papa S, Valli G, et al. Right ventricular dyssynchrony and exercise capacity in idiopathic pulmonary arterial hypertension. The European respiratory journal 2017; 49. DOI: 10.1183/13993003.01419-2016. [DOI] [PubMed]
- 7.Cheng XL, Liu BY, Wu WC, et al. Impact of right ventricular dyssynchrony on prognosis of patients with idiopathic pulmonary arterial hypertension. Pulmonary circulation 2019; 9. DOI: 10.1177/2045894019883609. [DOI] [PMC free article] [PubMed]
- 8.Richter MJ, Peters D, Ghofrani HA, et al. Evaluation and Prognostic Relevance of Right Ventricular-Arterial Coupling in Pulmonary Hypertension. American journal of respiratory and critical care medicine 2020; 201: 116–119. [DOI] [PubMed]
- 9.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. The European respiratory journal 2019; 53. DOI: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed]
- 10.Tello K, Dalmer A, Axmann J, et al. Reserve of Right Ventricular-Arterial Coupling in the Setting of Chronic Overload. Circulation Heart failure 2019; 12: e005512. [DOI] [PubMed]
- 11.Gall H, Felix JF, Schneck FK, et al. The Giessen Pulmonary Hypertension Registry: Survival in pulmonary hypertension subgroups. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation 2017; 36: 957–967. [DOI] [PubMed]
- 12.Kiely DG, Levin D, Hassoun P, et al. EXPRESS: Statement on imaging and pulmonary hypertension from the Pulmonary Vascular Research Institute (PVRI). Pulmonary circulation 2019. DOI: 10.1177/2045894019841990. [DOI] [PMC free article] [PubMed]
- 13.Tello K, Dalmer A, Vanderpool R, et al. Impaired right ventricular lusitropy is associated with ventilatory inefficiency in pulmonary arterial hypertension. The European respiratory journal 2019; 54. DOI: 10.1183/13993003.00342-2019. [DOI] [PubMed]
- 14.Tello K, Wan J, Dalmer A, et al. Validation of the Tricuspid Annular Plane Systolic Excursion/Systolic Pulmonary Artery Pressure Ratio for the Assessment of Right Ventricular-Arterial Coupling in Severe Pulmonary Hypertension. Circ Cardiovasc Imaging 2019; 12: e009047. [DOI] [PMC free article] [PubMed]
- 15.Tello K, Dalmer A, Vanderpool R, et al. Cardiac Magnetic Resonance Imaging-Based Right Ventricular Strain Analysis for Assessment of Coupling and Diastolic Function in Pulmonary Hypertension. JACC Cardiovascular imaging 2019; 12: 2155-2164. [DOI] [PubMed]
- 16.Andersen S, Nielsen-Kudsk JE, Vonk Noordegraaf A, et al. Right Ventricular Fibrosis. Circulation 2019; 139: 269–285. [DOI] [PubMed]