Abstract
Neuropathic pain is one of the most frequently stated complications after spinal cord injury. In post-spinal cord injury, the decrease of gamma aminobutyric acid synthesis within the distal spinal cord is one of the main causes of neuropathic pain. The predominant research question of this study was whether exercise training may promote the expression of glutamic acid decarboxylase-65 and glutamic acid decarboxylase-67, which are key enzymes of gamma aminobutyric acid synthesis, within the distal spinal cord through tropomyosin-related kinase B signaling, as its synthesis assists to relieve neuropathic pain after spinal cord injury. Animal experiment was conducted, and all rats were allocated into five groups: Sham group, SCI/PBS group, SCI-TT/PBS group, SCI/tropomyosin-related kinase B-IgG group, and SCI-TT/tropomyosin-related kinase B-IgG group, and then T10 contusion SCI model was performed as well as the tropomyosin-related kinase B-IgG was used to block the tropomyosin-related kinase B activation. Mechanical withdrawal thresholds and thermal withdrawal latencies were used for assessing pain-related behaviors. Western blot analysis was used to detect the expression of brain-derived neurotrophic factor, tropomyosin-related kinase B, CREB, p-REB, glutamic acid decarboxylase-65, and glutamic acid decarboxylase-67 within the distal spinal cord. Immunohistochemistry was used to analyze the distribution of CREB, p-CREB, glutamic acid decarboxylase-65, and glutamic acid decarboxylase-67 within the distal spinal cord dorsal horn. The results showed that exercise training could significantly mitigate the mechanical allodynia and thermal hyperalgesia in post-spinal cord injury and increase the synthesis of brain-derived neurotrophic factor, tropomyosin-related kinase B, CREB, p-CREB, glutamic acid decarboxylase-65, and glutamic acid decarboxylase-67 within the distal spinal cord. After the tropomyosin-related kinase B signaling was blocked, the analgesic effect of exercise training was inhibited, and in the SCI-TT/tropomyosin-related kinase B-IgG group, the synthesis of CREB, p-CREB, glutamic acid decarboxylase-65, and glutamic acid decarboxylase-67 within the distal spinal cord were also significantly reduced compared with the SCI-TT/PBS group. This study shows that exercise training may increase the glutamic acid decarboxylase-65 and glutamic acid decarboxylase-67 expression within the spinal cord dorsal horn through the tropomyosin-related kinase B signaling, and this mechanism may play a vital role in relieving the neuropathic pain of rats caused by incomplete SCI.
Keywords: Spinal cord injury, neuropathic pain, body-weight-supported treadmill training, glutamic acid decarboxylase 65/67, gamma aminobutyric acid, brain-derived neurotrophic factor, tropomyosin-related kinase B
Introduction
Neuropathic pain (NPP) is one of the common complications after spinal cord injury (SCI). It can be manifested as spontaneous pain, allodynia, or hyperalgesia.1,2 Around 53% of patients with SCI have different degrees of NPP, 76% of which show its typical symptoms one year after SCI.3 The impact of NPP on patients is far more than sensory loss and dyskinesia, and post-SCI pain can also impair the physical and psychological health of patients in numerous ways and may accompany patients throughout their lives.2 Although the current diagnostic methods and treatment techniques are sustained developing, due to the complicated pathogenesis of NPP, the treatment effect on NPP is still not sufficent.4–6
After SCI, the properties of sensory neurons in the spinal cord dorsal horn was manifested by increased responsiveness to peripheral stimulation, prolonged firing time following a stimulus and enhanced spontaneous excitability at rest, which could be the important causes of NPP in the early stage of SCI.5 Currently, it has been reported that the characteristic changes of spinal dorsal horn neurons may be closely related to the imbalance of excitatory and inhibitory neurotransmitter, especially the decrease of inhibitory neurotransmitter (gamma aminobutyric acid, GABA) synthesis, which lead to the abnormal excitation of spinal cord dorsal horn neurons and promote the occurrence of NPP.7–10
GABA, one of the important inhibitory neurotransmitters in the spinal cord, is synthesized by glutamic acid decarboxylase 65/67 (GAD-65/67)11 and plays a central inhibitory and analgesic role by activating its receptor protein GABAA and GABAB.12 Increasing synthesis of the GAD-65/67 (GABA) within the spinal cord through gene transcription or stem cell transplantation can enhance the inhibitory transmission and dramatically relieve NPP after SCI.13–15 In addition, the intrathecal injection of Baclofen, a GABAB receptor agonist, could inhibit the excitatory of neurons and significantly alleviate the NPP in patients with SCI.16 Therefore, the dysfunction of GABAergic inhibition in the spinal cord after SCI could be an important cause of NPP. Additionally, increased synthesis of the GABA or activation of its receptor protein can effectively reduce NPP after SCI.
Both clinical trials and animal experiments have shown that various types of exercise training could relieve NPP caused by SCI or sciatic nerve injury,17–25 which may be associated with exercise training inducing increased synthesis of the brain-derived neurotrophic factor (BDNF), coupled with its high affinity receptor-tropomyosin-related kinase B (TrkB), and the GABA within the spinal cord.20–25 Overexpression of BDNF can stimulate TrkB signaling and promote the GAD-65/67 synthesis within the spinal cord after SCI and within the cerebrum after vascular occlusion.26,27 However, in the SCI rat model, it is unclear whether exercise training can increase the GAD-65/67 synthesis and relieve NPP after SCI through the TrkB signaling. Therefore, in this study, the TrkB antagonist (TrkB-IgG) was used to eliminate the activation effects of BDNF and then explore whether exercise training can increase the GAD-65/67 synthesis and suppress the mechanical allodynia and thermal hyperalgesia through the TrkB signaling in rats with incomplete SCI.
Materials and Methods
Animals and groups
A total of 64 adult female Sprague–Dawley rats (provided by the Laboratory Animal Center of Nantong University, Nantong, Jiangsu, China), weighing around 210–230 g, were divided into sham operation group (Sham group, n = 16), SCI/phosphate buffer solution (PBS) group (SCI/PBS group, n = 16), SCI-treadmill training/PBS group (SCI-TT/PBS group, n = 16), SCI/TrkB-IgG group (n = 8), and SCI-TT/TrkB-IgG group (n = 8). All rats were fed in a specific pathogen free environment which provides constant temperature (22 ±2°C), 50%–60% humidity, free diet, and light/dark cycle of 12/12 h. The current study was reviewed and approved by the Medical Ethics Committee of Nanjing Medical University (2019–792) and conducted in line with the Chinese Laboratory Animal Guide.
Intrathecal catheter and SCI
One week before SCI, the L3-4 intrathecal catheter operation was conducted to observe whether this operation injured the spinal cord or nerve.28 Briefly, all rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (0.3 ml/100g). The interspace between the L3-4 spinous process was located and exposed, and the dura mater and subarachnoid cavity were broken through with a 19G puncture needle. A 6 cm long PE-10 catheter (Smiths Medical International Ltd., UK) was inserted into the subarachnoid space for about 2 cm. A 25 μl microinjector was used to inject 20 μl sterile PBS to wash the catheter. Then the external opening of the PE-10 catheter was clamped.
Seven days after intrathecal catheterization, all rats were anesthetized again, and the T10 incomplete SCI model was made by Allen’s method.28,29 The Impactor system was made by New York University (NYU), with a strike end of 2.5 mm diameter, and the dosage of injury was 10 g × 25 mm. The Sham group only exposed the T10 spinal cord. All rats were injected intraperitoneally with 2 ml normal saline. After SCI, all rats were given bladder massage to assist urination twice a day for 5–7 days, until autonomous urination was formed.
Intrathecal administration
After SCI, on the seventh day, recombinant human TrkB-Fc chimera (0.25 µg/µL, TrkB-IgG, R&D Systems, Minneapolis, USA) was used to block the TrkB signaling. TrkB-IgG was solubilized into PBS (0.25 µg/µL) or PBS alone was infused into Alzet osmotic pumps (type 2002, Alzet, Cupertino, USA).20,28 Researchers connected the osmotic pump with the previously placed PE-10 catheter and place the osmotic pump subcutaneously on the back (Sham group, SCI/PBS group and SCI-TT/PBS group implanted with PBS-infused Alzet osmotic pump, SCI/TrkB-IgG group and SCI-TT/TrkB-IgG group using TrkB-IgG solution perfusion Alzet osmotic pump). The working time of the type 2002 osmotic pump was two weeks, so the previous operation was repeated after two weeks. The TrkB signaling was blocked for a total of four weeks.28
Treadmill training
The body weight-supported treadmill training (BWSTT) was performed on the eighth day after SCI.28 Training strategy: SCI-TT/PBS group and SCI-TT/TrkB-IgG group were provided exercise training. Before training, the bladder area massage was for emptying the urine from the bladder. The speed of the treadmill was set at 6 m/min. Training intensity: 20 min for each time, two times per day and five days per week for four consecutive weeks. During training, the weight supported range was set as 20%–40% of rats’ weight based on the functional status of the rat.
Pain-related behaviors assessment
Mechanical withdrawal thresholds (MWTs) assessment
Chaplan et al.30 modified method was used in this assessment. Before operation, rats were placed in transparent cages of a quiet room-temperature environment for 15 min. Then a series of von Frey filaments (0.4 g, 0.6 g, 1.0 g, 1.4 g, 2.0 g, 4.0 g, 6.0 g, 8.0 g, and 15.0 g) were used to vertically to stimulate the skin of the middle part of the hind paw of the rat initially from 2.0 g intensity, and the duration of each stimulation was 6–8 s, according to the up–down method.31 The rat with foot withdrawing or licking reaction was recorded as positive (X), and no response was recorded as negative (O). The assessment time of each rat should be less than 1 min to avoid irritation. The presence of NPP was considered when the stimulation intensity of von Frey monofilament was less than 4 g.32 Assessment time points are preoperative, postoperative day 1, day 7, day 14, day 21, day 28 and day 35 after SCI.
Thermal withdrawal latencies (TWLs) assessment
Thermal withdrawal latency (TWL) assessment references the method provided by Hargreaves et al.33 Before the assessment, the rat was placed in a resin glass evaluation cage to adapt for 15 min. All rats were evaluated three times. The mean value of the foot withdrawing reaction was recorded as the thermal stimulation latencies of each rat. To prevent damage, the interval of each evaluation was set as 10 min, and the intensity of thermal stimulation was set as 20%, and the maximum stimulation time was set as 20 s.20 The evaluation time point was the same as that of MWTs.
Immunohistochemistry
At the end of the experiment, all rats were anesthetized (n = 4 rats per group), fixed with 4% paraformaldehyde, and the targeted spinal cord segment (L4–L5) was separated according to the corresponding nerve root. All the segments were put into 4% paraformaldehyde, were fixed for one night, then dehydrated with alcohol gradient, and finally embedded into paraffin. Next, these spinal cord segments were cut horizontally, and the thickness of each section was 5 μm. One was taken from every six sections and three ones were taken for each sample. After dewaxing and hydration, incubation with H2O2, microwave retrieval and serum blocking, Rabbit anti-CREB monoclonal antibody (ab32515, division 1:500, Abcam, USA), Rabbit anti-CREB (phosphe s133) monoclonal antibody (ab32096, division 1:200, Abcam, USA), Rabbit anti-GAD65 polyclonal antibody (ab203063, division 1:200, Abcam, USA), and mouse anti-GAD67 monoclonal antibody (MAB5406, division 1:1000, Minipore, Germany) were added on each slide and then incubated overnight at 4°C. The next day, after PBS washing of the pieces, the goat anti-rabbit or rabbit anti-mouse antibody labeled with biotin was added and then incubated in a 37°C incubator for 1 h, and the expression was visualized by DAB incubation. Pictures were taken by Olympus DP71 and the Image-Pro Plus 6.0 was used for optical density analysis.
Western blot
After SCI, on the 7th and the 21st day, the rats of Sham group, SCI/PBS group and SCI-TT/PBS group (n = 4 rats per group) were used to analyze the expression of the BDNF and the TrkB. At the end of the fifth week after SCI, the rats of all groups (n = 4 rats per group) were used to analyze the expression of the BDNF, the TrkB, the CREB/p-CREB, and the GAD-65/67. Briefly, the rats were anesthetized as above, and the L4–L5 spinal cord segments of the rats were isolated. After grinded, digested, centrifuged, protein concentration measured, and protein denatured, the sample was loaded in 12% SDS-PAGE gel. Gels were undergone 20 mA constant current electrophoresis, and then membrane transfer with 100 V constant volt for 80 min. The rabbit anti-BDNF monoclonal antibody (dilution 1:1000, Abcam, USA), rabbit anti-TrkB polyclonal antibody (dilution 1:1000, Abcam, USA), rabbit Anti-CREB monoclonal antibody (ab32515, dilution 1:1000, Abcam, USA), rabbit anti-CREB (phospho S133) monoclonal antibody (ab32096, dilution 1:500, Abcam, USA), rabbit anti-GAD65 polyclonal antibody (ab203063, dilution 1:1000, Abcam, USA), mouse anti-GAD67 monoclonal antibody (MAB5406, dilution 1:5000, Minipore, Germany) or rabbit anti-GAPDH monoclonal antibody (ab181602, 1:5000, Abcam, USA) was added and incubated overnight at 4°C. The next day, they were incubated with goat anti-rabbit or rabbit anti-mouse IgG (1:2000) at room temperature for 1 h, and the membrane was washed by the Tris Buffered saline Tween (TBST). The Electrochemiluminescence (ECL) method was used for development, and the Image J software was used for gray analysis after photographing.
Statistical analysis
Statistical analysis was performed using SPSS20.0 (IBM, USA) software. All data are expressed as mean ± standard deviation ( ± S). A general linear model was established for the MWTs and TWLs data, and repeated measurement analysis of variance (ANOVA) was performed on the data. Tukey’s post hoc was then used to compare the values of the five groups of rats at the same time point. Immunohistochemistry and Western blot data were analyzed by one-way ANOVA. If the one-way ANOVA was statistically different, the LSD or Bonferroni post hoc analysis was used. P < 0.05 was considered statistically significant.
Results
Dynamic changes of BDNF and TrkB synthesis after SCI with or without BWSTT
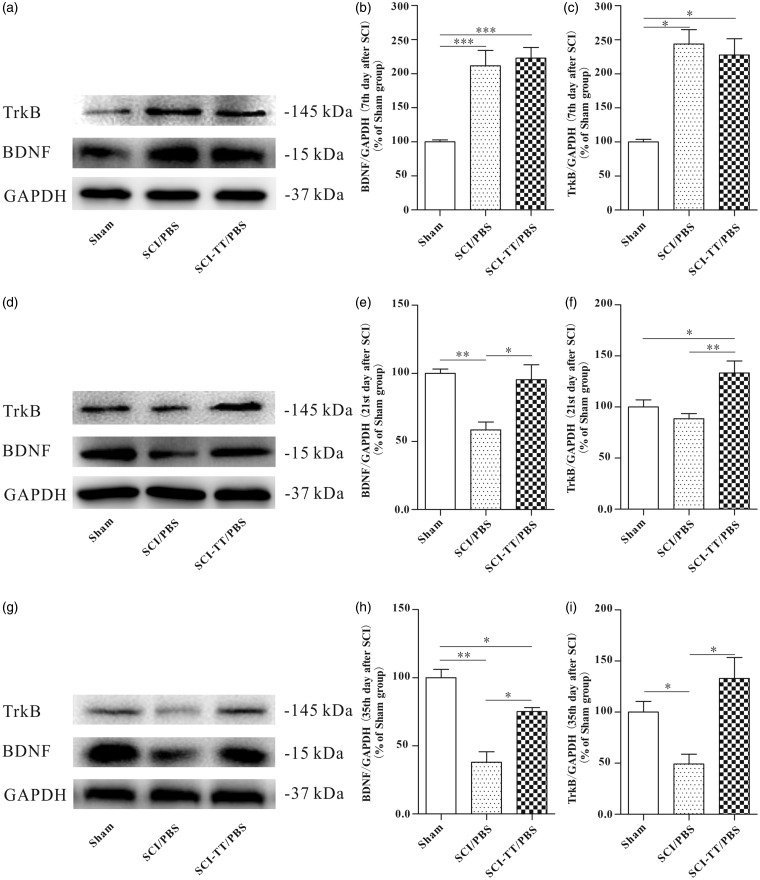
To observe the dynamic changes of the BDNF and TrkB expression in the distal spinal cord of SCI rats after BWSTT, the Western blot analysis was used on the 7th, 21st, and 35th day (Figure 1). The results showed that on the 7th day after SCI, compared with Sham group, the BDNF and TrkB expressions in both the SCI/PBS group and the SCI-TT/PBS group were significantly higher (P < 0.05). However, there was no significant difference between the SCI/PBS group and SCI-TT/PBS group (P > 0.05). On the 21st day after SCI, the BDNF expression in SCI/PBS group was significantly lower than those in the Sham group and the SCI-TT/PBS group (P < 0.05). The difference between the Sham group and the SCI-TT/PBS group did not exit statistical significance (P > 0.05). The TrkB expression in both the Sham group and the SCI/PBS group was significantly lower than those in the SCI-TT/PBS group (P < 0.05), while the difference between the Sham group and the SCI/PBS group was not statistically significant (P > 0.05). At the end of fifth week after SCI, the relative expression levels of the BDNF and the TrkB within the distal spinal cord of the SCI/PBS group were significantly lower than those in the Sham group and the SCI-TT/PBS group (P < 0.05). The expression of the BDNF in the SCI-TT/PBS group was significantly lower than that in the Sham group (P < 0.05). There was no significant difference in the TrkB expression between the SCI-TT/PBS group and the Sham group (P > 0.05).
Figure 1.
The dynamic changes of BDNF and TrkB expression in the distal spinal cord of SCI rats with or without BWSTT. (a), (d), and (g) show the Western blot image of BDNF and TrkB in the Sham group, the SCI/PBS group, and the SCI-TT/PBS group on the 7th day, the 21st day, and the 35th day, respectively. (b), (e), and (h) show the statistical graph of the relative density of BDNF on the 7th day, the 21st day, and 35th day, respectively. (c), (f), and (i) show the statistical graph of the relative density of TrkB on the 7th day, the 21st day, and the 35th day, respectively. n = 4 rats per group. *P < 0.05, **P < 0.01. Statistical analysis was performed using one-way ANOVA followed by the LSD or Bonferroni test.
BWSTT: body weight-supported treadmill training; TT: treadmill training; SCI: spinal cord injury; PBS: phosphate-buffered saline; BDNF: brain-derived neurotrophic factor; TrkB: tropomyosin-related kinase B; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; ANOVA: analysis of variance.
BWSTT improves the MWTs and the TWLs of SCI rats through the TrkB signaling
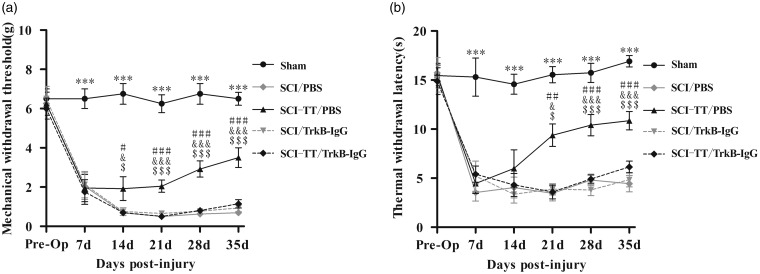
To observe whether the TrkB signaling plays a key role in reducing NPP in SCI rats after BWSTT, the MWTs and TWLs of the hind limbs of SCI rats were evaluated before SCI, first, second, third, fourth, and fifth week after SCI (Figure 2). A general linear model repeated measurement analysis of variance was established for analyzing MWTs and TWLs. The results showed that the MWTs and the TWLs of all five groups of rats were different in time effect and group effect (P < 0.001), and there was an interaction between time effect and group effect (P < 0.001). One week after SCI, all rats showed varying degrees of mechanical allodynia and/or thermal hyperalgesia, and the MWTs and TWLs were significantly lower than that in the Sham group (P < 0.05). And at second, third, fourth, and fifth week after SCI, the MWTs of SCI-TT/PBS group were significantly higher than that of the SCI/PBS group, the SCI/TrkB-IgG group and the SCI-TT/TrkB-IgG group (P < 0.05). At fourth and fifth week after SCI, the TWLs of the SCI-TT/PBS group were significantly higher than that of the SCI/PBS group, the SCI/TrkB-IgG group, and the SCI-TT/TrkB-IgG group (P < 0.05). There was no significant difference in the TWLs between the four groups of SCI rats at the second week after SCI (P > 0.05). And there was no significant difference in the MWTs and TWLs among the SCI/PBS group, the SCI/TrkB-IgG group, and the SCI-TT/TrkB-IgG group at each time point (P > 0.05).
Figure 2.
Effects of BWSTT on mechanical withdrawal thresholds (MWTs) and thermal withdrawal latencies (TWLs) in rats with or without blocking TrkB signaling. (a) Variation trend of MWTs of rats in each group at different time points. (b) Variation trend of TWLs of rats in each group at different time points. n = 8 rats per group. Statistical analysis used repeated measures ANOVA, and Tukey’s post hoc was then used to compare the values of the five groups of rats at the same time point. Compared with Sham group after 1 week of SCI, ***P < 0.001. SCI-TT/PBS group vs SCI/PBS group, #P < 0.05, ##P < 0.01, ###P < 0.001. SCI-TT/PBS group v.s. SCI/TrkB-IgG group, &P < 0.05, &&P < 0.001. SCI-TT/PBS group vs SCI-TT/TrkB-IgG group, $P < 0.05, $$P < 0.01, $$$P < 0.001. There was no significant difference in the MWTs and TWLs among the SCI/PBS group, the SCI/TrkB-IgG group, and the SCI-TT/TrkB-IgG group at each time point.
BWSTT: body weight-supported treadmill training; TT: treadmill training; SCI: spinal cord injury; PBS: phosphate-buffered saline; BDNF: brain-derived neurotrophic factor; TrkB: tropomyosin-related kinase B; Pro-Op: pre-operation.
Blocking the TrkB signaling weaken the promoting effect of BWSTT on CREB and p-CREB synthesis
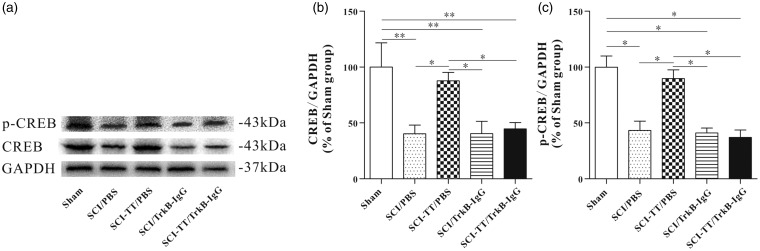
To further verify whether BWSTT can increase the synthesis of CREB and p-CREB within the distal spinal cord, and whether blocking the TrkB signaling can affect the synthesis of CREB and p-CREB within spinal cord, both immunohistochemistry and Western blot analysis were applied to manage the two proteins (Figures 3 and 4).
Figure 3.
Effect of BWSTT on CREB and p-CREB synthesis in the distal spinal cord of SCI rats with or without blocking TrkB signaling. (a) Western blot image of CREB and p-CREB in the Sham group, the SCI/PBS group, the SCI-TT/PBS group, the SCI/TrkB-IgG group, and the SCI-TT/TrkB-IgG group at the end of the experiment. (b) The statistical graph of the relative density of CREB. (c) The statistical graph of the relative density of p-CREB. n = 4 rats per group. *P < 0.05, **P < 0.01. Statistical analysis was performed using one-way ANOVA followed by the LSD or Bonferroni test.
BWSTT: body weight-supported treadmill training; TT: treadmill training; SCI: spinal cord injury; PBS: phosphate-buffered saline; TrkB: tropomyosin-related kinase B; CREB: cAMP-response element binding protein; p-CREB: Phospho-CREB; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; ANOVA: analysis of variance.
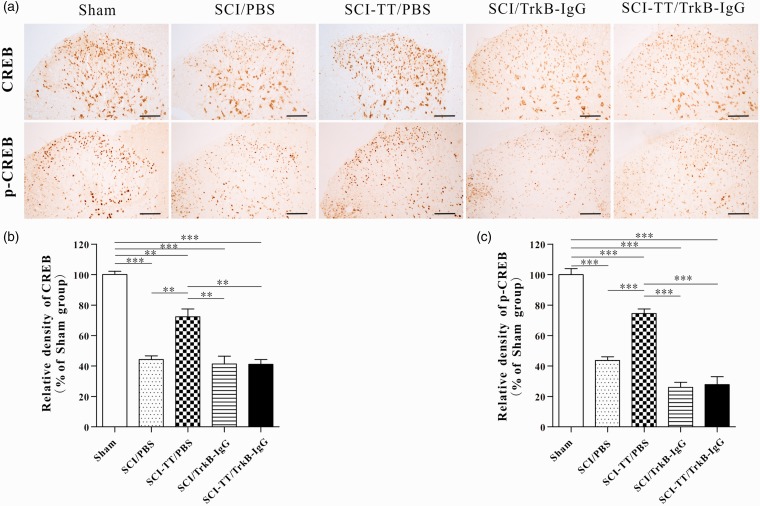
Figure 4.
Immunohistochemical analysis of the synthesis and distribution of CREB and p-CREB within the spinal cord dorsal horn in five groups of rats. (a) Immunohistochemical results of CREB and p-CREB showed that the two proteins were widely expressed in the gray matter of the spinal cord dorsal horn, CREB was mainly expressed in the neuronal cytoplasm, and p-CREB was mainly expressed in the neuron nucleus. (b) and (c) the statistical graph of the relative density of CREB and p-CREB. **P < 0.01, ***P < 0.001. Scale bar: 100 µm. Statistical analysis was performed using one-way ANOVA followed by the Bonferroni test.
TT: treadmill training; SCI: spinal cord injury; PBS: phosphate-buffered saline; TrkB: tropomyosin-related kinase B; CREB: cAMP-response element binding protein; p-CREB: Phospho-CREB; ANOVA: analysis of variance.
Western blot and immunohistochemistry results of CREB showed that there was no significant difference between the Sham group and the SCI-TT/PBS group (P > 0.05). Compared with the Sham group and the SCI-TT/PBS group, the CREB expression levels of the SCI/PBS group, the SCI/TrkB-IgG group and the SCI-TT/TrkB-IgG group were significantly decreased (P < 0.05). There was no significant difference among the SCI/PBS group, the SCI/TrkB-IgG group and the SCI-TT/TrkB-IgG group (P > 0.05).
The results of Western blot and immunohistochemistry of p-CREB showed that the expression in the SCI-TT/PBS group was significantly higher than that of the SCI/PBS group, the SCI/TrkB-IgG group and the SCI-TT/TrkB IgG group (P < 0.05). In addition, there was no significant difference in p-CREB expression among the SCI/PBS group, the SCI/TrkB-IgG group and the SCI-TT/TrkB-IgG group (P > 0.05). The optical density (immunohistochemistry) of the SCI/PBS group was significantly higher than that of the SCI/TrkB-IgG group and the SCI-TT/TrkB-IgG group (P < 0.05), but there was no significant difference between the SCI/TrkB-IgG group and the SCI-TT/TrkB-IgG group (P > 0.05).
Blocking the TrkB signaling reduced the promotion effect of exercise training on GAD-65/67 expression
To further verify whether BWSTT can increase the synthesis of the GAD-65/67 within the distal spinal cord, and whether blocking the TrkB signaling affects the synthesis of the GAD-65/67 within spinal cord, the above two proteins were also analyzed by immunohistochemistry and Western blot (Figures 5 and 6).
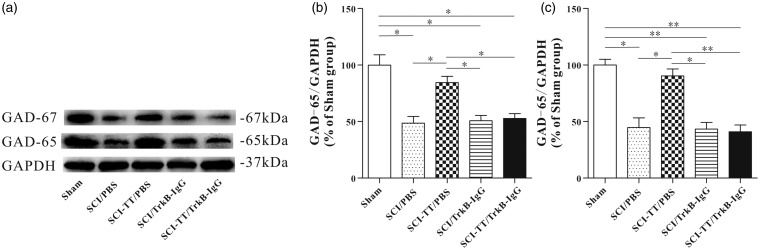
Figure 5.
Effect of BWSTT on GAD65 and GAD-67 synthesis in the distal spinal cord of SCI rats with or without blocking TrkB signaling. (a) Western blot of GAD65 and GAD-67 in the Sham group, the SCI/PBS group, the SCI-TT/PBS group, the SCI/TrkB-IgG group and the SCI-TT/TrkB-IgG group at the end of the experiment. (b) The statistical graph of the relative density of GAD-65. (c) The statistical graph of the relative density of GAD-67. n = 4 rats per group. *P < 0.05, **P<0.01. Statistical analysis was performed using one-way ANOVA followed by the Bonferroni test.
BWSTT: body weight-supported treadmill training; TT: treadmill training; SCI: spinal cord injury; PBS: phosphate-buffered saline; TrkB: tropomyosin-related kinase B; GAD: glutamic acid decarboxylase; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; ANOVA: analysis of variance.
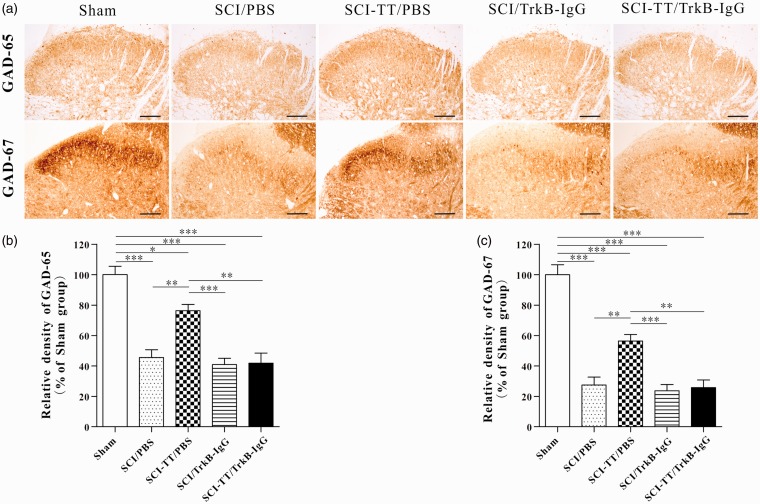
Figure 6.
Immunohistochemical analysis of the synthesis and distribution of GAD-65 and GAD-67 within the spinal cord dorsal horn in five groups of rats. (a) The immunohistochemical results of GAD-65 and GAD-67 showed that the two proteins are widely expressed in the dorsal horn of spinal cord. (b) and (c) The statistical graph of the relative density of GAD-65 and GAD-67. *P < 0.05, **P < 0.01, ***P < 0.001. Scale bar: 100 µm. Statistical analysis was performed using one-way ANOVA followed by the Bonferroni test.
TT: treadmill training; SCI: spinal cord injury; PBS: phosphate-buffered saline; TrkB: tropomyosin-related kinase B; GAD: glutamic acid decarboxylase; ANOVA: analysis of variance.
Western blot and immunohistochemical results showed that the expression levels of the GAD-65 and the GAD-67 in the four SCI groups were significantly reduced compared with the Sham group (P < 0.05). Compared with the SCI-TT/PBS group, the expression levels of GAD-65 and GAD-67 in the SCI/PBS group, the SCI/TrkB-IgG group and the SCI-TT/TrkB-IgG group were significantly reduced (P < 0.05). There was no significant difference in the relative expression levels of the two proteins among the SCI/PBS group, the SCI/TrkB-IgG group and the SCI-TT/TrkB-IgG group (P> 0.05).
Discussion
The current study observed the effect of exercise training on NPP after SCI. The results showed that (a) SCI lead to mechanical allodynia and thermal hyperalgesia, increased the expression of BDNF in the early stage of injury, but reduce it in later stage within the distal spinal cord of rats, (b) in the later stage of SCI, activity-based exercise training increased the synthesis of BDNF, TrkB, CREB, p-CREB, GAD-65, and GAD-67 within the distal spinal cord of rats with SCI, and improve the MWTs and TWLs, and (c) after blocking of the TrkB signaling, the synthesis of CREB, p-CREB, GAD-65, and GAD-67 was significantly reduced in SCI-TT/TrkB-IgG group compared with SCI-TT/PBS group, and the analgesic effect of exercise training was significantly inhibited.
In the central nervous system, GAD-65 is mainly expressed in inhibitory neuronal axon terminals, while GAD-67 is mainly expressed in cell bodies and nerve terminals, the both of them are key enzymes of GABA synthesis.11,34 In the spinal cord of rats, GAD-65 and GAD-67 are widely expressed in the spinal cord gray matter and presented with immunoreactive punctate structures especially in the spinal cord dorsal horn.8,11
After SCI, the expression of GAD-65 and GAD-67 in the spinal cord dorsal horn is significantly decreased, which may reduce the GABAergic inhibition and lead to the occurrence of NPP.8,9 This may be due to the loss of GABAergic neurons in the spinal cord dorsal horn of rats after SCI, or the lack of inhibition of primary access fibers by GABAergic neurons.7
Meanwhile, in the early stage of SCI, due to the large amount of BDNF synthesis in the spinal cord (mainly secreted by activated glial cells) and the lack of effective neural regulatory mechanisms, the excessive BDNF in the spinal cord could potentially contribute the development of maladaptive plasticity, promote over-excitation (central sensitization) and strengthen circuits through activating of TrkB signaling, then contribute to the maintenance of chronic pain.35–37 In the later stages of SCI, the expression of BDNF in the distal spinal cord will gradually decrease, thereby reducing the remodeling and repairing of the spinal cord neural circuit.20,28
BDNF, as one of the important neurotrophic factors in the central nervous system, activates the high-affinity receptor TrkB to exert the vital effects of neuroprotection, neuroregulation, synaptic regeneration, neuronal activities, and so on.38,39 In the cerebral cortex,40–42 cerebellum,43 hippocampus,44,45 midbrain,46 and spinal cord47 of rodent, BDNF/TrkB may be a critical factor in the formation and regulation of GABAergic inhibitory neural circuits.
Exercise training as one of the functional rehabilitation training programs after SCI, it can promote the expression of BDNF and TrkB within the injured spinal cord by increasing the activation of spinal sensory-motor neural circuits, enhancing neural plasticity and neural function recovery.20,28,48 Also, it can adjust spinal neuron excitability, reduce spasticity and allodynia after SCI.20,49 Studies have shown that exercise training can regulate spinal neuron excitability by increasing synthesis of GAD-65 and GAD-67 in the spinal cord dorsal horn as reducing NPP after SCI and peripheral nerve injury.23–25 This may be related to the exercise-induced increase of BDNF and the promotion of nerve system functional remodeling.7,20,49
CREB is one of the core transcription factors that mediate gene transcription downstream of the TrkB signaling and exerts an important role in neuromodulation.38,50 Sánchez-Huertas et al.40 found that BDNF/TrkB could promote p-CREB activating of the GAD65 promoter (5.5-kb 5’ GAD65-luc construct) and regulatory regions through Ras/ERK signaling (one of the TrkB signaling cascades) in cortical inhibitory interneurons. Yin et al.51 found that the GAD-67 level may be regulated by p-CREB in olfactory bulb cells. However, the mechanism of the p-CREB promoting the GAD-67 synthesis needs further study.
Besides, most studies suggest that early exercise training can reduce NPP after SCI by reducing calcitonin gene-related peptide,19 increasing glial-derived neurotrophic factor,21 and potassium-chloride transporter expression20 in the spinal cord dorsal horn. However, some studies have found that early exercise training can increase the excitability of spinal cord dorsal horn neurons through the TrkB signaling and then promote the occurrence of NPP after SCI.22 This phenomenon may be due to the different exercise methods (the latter used passive robotic-assisted stepping exercise), because the activity-based training is mostly used to reduce NPP. Grau et al.52 suggested that controlled exercise training (activity-based exercise) may promote neurological recovery in SCI rats, while uncontrolled training may have the opposite effect. Additionally, Almeida et al.53 found that exercise training could promote the normalization of BDNF expression in the dorsal root ganglion (DRG) and reduce sciatic nerve injury induced NPP in a mouse model. However, it is still unclear whether exercise training can regulate BDNF expression in DRG and ameliorate NPP in rats with SCI.
It has been discovered that the muscle spindle feedback could direct locomotor recovery and neural circuit reorganization after SCI.54 In addition, blocking the sensory afferents of the spinal cord in neonatal mice can significantly reduce the plasticity of GABAergic neurons in the dorsal horn of the spinal cord then may promote the occurrence of NPP.55 Also, activity-based exercise training can increase motor sensory input, reduce or inhibit neuronal apoptosis, promote neural network functional remodeling, and improve motor and sensory dysfunction after SCI.7,56 Therefore, we thought that activity-based exercise therapy may promote the remodeling of the neural circuit in the injured spinal cord through sensory feedback then reduce NPP after SCI. However, the specific mechanism of exercise training in reducing NPP still needs further study.
In summary, the results of the current study show that early activity-based exercise training can increase the MWTs and TWLs in rats with incomplete SCI by increasing the synthesis of GAD-65/67 within the spinal cord dorsal horn, and this mechanism may be related to exercise-induced BDNF synthesis and TrkB signaling activation. Explanatorily, activity-based exercise training can regulate the GABAergic inhibition in the spinal cord dorsal horn through the TrkB signaling, and ameliorate mechanical allodynia and thermal hyperalgesia in rats with incomplete SCI. There are several limitations to this study. First, in the neurotrophic factors (NTs) family, the current study only observed the effect of exercise-induced BDNF and TrkB expression and the blockage of the TrkB signaling but did not explore other NTs and their receptors. Second, within the spinal cord, the current study observed the effect of exercise training on only one neurotransmitter synthesis, GABA, and did not observe other neurotransmitters. Finally, this research only observed the regularity of pain changes within five weeks after SCI and did not conduct follow-up or long-term observation. Therefore, further research should explore the effects of activity-based exercise training on the expression of other neurotransmitters and proteins in rats with SCI and conduct long-term segmentation observation studies to explore the mechanism of exercise training on relieving NPP in rats with SCI.
Acknowledgments
The authors acknowledge Zhiwei Yang (the Affiliated Suzhou Science & Technology Town Hospital of Nanjing Medical University) for valuable advice. The authors thank the staff who kindly helped in facilitating the experiment.
Authors Contributions
XL, QHW, and JD were responsible for designing and performing the experiments, extracting and analyzing data, preparing the figures, interpreting the results, updating the reference lists, and writing the drafts of the manuscript. SW performed the experiments, screened the potentially eligible studies, and extracted data. QFW and CD conceived the experimental design and reviewed the drafts of the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by Suzhou “Kejiaoxingwei” Youth Science and Technology Project (KJXW2018082), People’s Livelihood Science and Technology Project of Suzhou Science and Technology Bureau (SYS201785; SYS2019016), the Application Research Project of Nantong city (JC2019020), and National Natural Science Foundation of China (81672258). The views expressed in this article are those of the authors and not an official position of the institution or funder.
ORCID iD
Xiangzhe Li https://orcid.org/0000-0003-0979-0160
References
- 1.Sezer N, Akkuş S, Uğurlu FG. Chronic complications of spinal cord injury. World J Orthop 2015; 6: 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakipoglu-Yuzer GF, Atçı N, Ozgirgin N. Neuropathic pain in spinal cord injury. Pain Phys 2013; 16: 259–264. [PubMed] [Google Scholar]
- 3.Burke D, Fullen BM, Stokes D, Lennon O. Neuropathic pain prevalence following spinal cord injury: a systematic review and meta-analysis. Eur J Pain 2017; 21: 29–44. [DOI] [PubMed] [Google Scholar]
- 4.Widerström-Noga E. Neuropathic pain and spinal cord injury: phenotypes and pharmacological management. Drugs 2017; 77: 967–984. [DOI] [PubMed] [Google Scholar]
- 5.Siddall PJ, Middleton JW. Spinal cord injury-induced pain: mechanisms and treatments. Pain Manag 2015; 5: 493–507. [DOI] [PubMed] [Google Scholar]
- 6.Alles SRA, Smith PA. Etiology and pharmacology of neuropathic pain. Pharmacol Rev 2018; 70: 315–347. [DOI] [PubMed] [Google Scholar]
- 7.Gwak YS, Hulsebosch CE. GABA and central neuropathic pain following spinal cord injury. Neuropharmacology 2011; 60: 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meisner JG, Marsh AD, Marsh DR. Loss of GABAergic interneurons in laminae I–III of the spinal cord dorsal horn contributes to reduced GABAergic tone and neuropathic pain after spinal cord injury. J Neurotrauma 2010; 27: 729–737. [DOI] [PubMed] [Google Scholar]
- 9.Deumens R, Mazzone GL, Taccola G. Early spread of hyperexcitability to caudal dorsal horn networks after a chemically-induced lesion of the rat spinal cord in vitro. Neuroscience 2013; 229: 155–163. [DOI] [PubMed] [Google Scholar]
- 10.Berrocal YA, Almeida VW, Puentes R, Knott EP, Hechtman JF, Garland M, Pearse DD. Loss of central inhibition: implications for behavioral hypersensitivity after contusive spinal cord injury in rats. Pain Res Treat 2014; 2014: 178278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackie M, Hughes DI, Maxwell DJ, Tillakaratne NJK, Todd AJ. Distribution and colocalisation of glutamate decarboxylase isoforms in the rat spinal cord. Neuroscience 2003; 119: 461–472. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Lei Y, Tian Y, Xu S, Shen X, Wu H, Bao S, Wang F. The etiological contribution of GABAergic plasticity to the pathogenesis of neuropathic pain. Mol Pain 2019; 15: 1744806919847366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Wolfe D, Hao S, Huang S, Glorioso J, Mata M, Fink D. Peripherally delivered glutamic acid decarboxylase gene therapy for spinal cord injury pain. Mol Ther 2004; 10: 57–66. [DOI] [PubMed] [Google Scholar]
- 14.Liu W, Liu Z, Liu L, Xiao Z, Cao X, Cao Z, Xue L, Miao L, He X, Li W. A novel human foamy virus mediated gene transfer of GAD67 reduces neuropathic pain following spinal cord injury. Neurosci Lett 2008; 432: 13–18. [DOI] [PubMed] [Google Scholar]
- 15.Hwang I, Hahm SC, Choi KA, Park SH, Jeong H, Yea JH, Kim J, Hong S. Intrathecal transplantation of embryonic stem cell-derived spinal GABAergic neural precursor cells attenuates neuropathic pain in a spinal cord injury rat model. Cell Transplant 2016; 25: 593–607. [DOI] [PubMed] [Google Scholar]
- 16.Kumru H, Benito-Penalva J, Kofler M, Vidal J. Analgesic effect of intrathecal baclofen bolus on neuropathic pain in spinal cord injury patients. Brain Res Bull 2018; 140: 205–211. [DOI] [PubMed] [Google Scholar]
- 17.Dugan EA, Sagen J. An intensive locomotor training paradigm improves neuropathic pain following spinal cord compression injury in rats. J Neurotrauma 2015; 32: 622–632. [DOI] [PubMed] [Google Scholar]
- 18.Sato G, Osumi M, Morioka S. Effects of wheelchair propulsion on neuropathic pain and resting electroencephalography after spinal cord injury. J Rehabil Med 2017; 49: 136–143. [DOI] [PubMed] [Google Scholar]
- 19.Nees TA, Tappe-Theodor A, Sliwinski C, Motsch M, Rupp R, Kuner R, Weidner N, Blesch A. Early-onset treadmill training reduces mechanical allodynia and modulates calcitonin gene-related peptide fiber density in lamina III/IV in a mouse model of spinal cord contusion injury. Pain 2016; 157: 687–697. [DOI] [PubMed] [Google Scholar]
- 20.Tashiro S, Shinozaki M, Mukaino M, Renault-Mihara F, Toyama Y, Liu M, Nakamura M, Okano H. BDNF induced by treadmill training contributes to the suppression of spasticity and allodynia after spinal cord injury via upregulation of KCC2. Neurorehabil Neural Repair 2015; 29: 677–689. [DOI] [PubMed] [Google Scholar]
- 21.Detloff MR, Smith EJ, Quiros Molina D, Ganzer PD, Houlé JD. Acute exercise prevents the development of neuropathic pain and the sprouting of non-peptidergic (GDNF- and artemin-responsive) c-fibers after spinal cord injury. Exp Neurol 2014; 255: 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Endo T, Ajiki T, Inoue H, Kikuchi M, Yashiro T, Nakama S, Hoshino Y, Murakami T, Kobayashi E. Early exercise in spinal cord injured rats induces allodynia through TrkB signaling. Biochem Biophys Res Commun 2009; 381: 339–344. [DOI] [PubMed] [Google Scholar]
- 23.Tashiro S, Nishimura S, Shinozaki M, Takano M, Konomi T, Tsuji O, Nagoshi N, Toyama Y, Liu M, Okano H, Nakamura M. The amelioration of pain-related behavior in mice with chronic spinal cord injury treated with neural stem/progenitor cell transplantation combined with treadmill training. J Neurotrauma 2018; 35: 2561–2571. [DOI] [PubMed] [Google Scholar]
- 24.Farzad B, Rajabi H, Gharakhanlou R, Allison DJ, Hayat P, Jameie SB. Swimming training attenuates allodynia and hyperalgesia induced by peripheral nerve injury in an adult male rat neuropathic model: effects on Irisin and GAD65. Pain Med 2018; 19: 2236–2245. [DOI] [PubMed] [Google Scholar]
- 25.Kami K, Taguchi MS, Tajima F, Senba E. Improvements in impaired GABA and GAD65/67 production in the spinal dorsal horn contribute to exercise-induced hypoalgesia in a mouse model of neuropathic pain. Mol Pain 2016; 12: 174480691662905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziemlińska E, Kügler S, Schachner M, Wewiór I, Czarkowska-Bauch J, Skup M. Overexpression of BDNF increases excitability of the lumbar spinal network and leads to robust early locomotor recovery in completely spinalized rats. Plos One 2014; 9: e88833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang DJ, Lee N, Choi C, Jeon I, Oh S, Shin DA, Hwang T, Lee HJ, Kim SU, Moon H, Hong KS, Kang KS, Song J. Therapeutic effect of BDNF-overexpressing human neural stem cells (HB1.F3.BDNF) in a rodent model of middle cerebral artery occlusion. Cell Transplant 2013; 22: 1441–1452. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Wu Q, Xie C, Wang C, Wang Q, Dong C, Fang L, Ding J, Wang T. Blocking of BDNF-TrkB signaling inhibits the promotion effect of neurological function recovery after treadmill training in rats with spinal cord injury. Spinal Cord 2019; 57: 65–74. [DOI] [PubMed] [Google Scholar]
- 29.Lindsey AE, Loverso RL, Tovar CA, Hill CE, Beattie MS, Bresnahan JC. An analysis of changes in sensory thresholds to mild tactile and cold stimuli after experimental spinal cord injury in the rat. Neurorehabil Neural Repair 2000; 14: 287–300. [DOI] [PubMed] [Google Scholar]
- 30.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 31.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 1980; 20: 441–462. [DOI] [PubMed] [Google Scholar]
- 32.Nirogi R, Goura V, Shanmuganathan D, Jayarajan P, Abraham R. Comparison of manual and automated filaments for evaluation of neuropathic pain behavior in rats. J Pharmacol Toxicol Methods 2012; 66: 8–13. [DOI] [PubMed] [Google Scholar]
- 33.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988; 32: 77–88. [DOI] [PubMed] [Google Scholar]
- 34.McLaughlin BJ, Barber R, Saito K, Roberts E, Wu JY. Immunocytochemical localization of glutamate decarboxylase in rat spinal cord. J Comp Neurol 1975; 164: 305–321. [DOI] [PubMed] [Google Scholar]
- 35.Coull JAM, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 2005; 438: 1017–1021. [DOI] [PubMed] [Google Scholar]
- 36.Huang Y, Lee KH, Grau JW. Complete spinal cord injury (SCI) transforms how brain derived neurotrophic factor (BDNF) affects nociceptive sensitization. Exp Neurol 2017; 288: 38–50. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Mei X, Zhang L, Lv G. The correlation among the dynamic change of Zn2+, ZnT-1, and brain-derived neurotrophic factor after acute spinal cord injury in rats. Biol Trace Elem Res 2011; 143: 351–358. [DOI] [PubMed] [Google Scholar]
- 38.Keefe KM, Sheikh IS, Smith GM. Targeting neurotrophins to specific populations of neurons: NGF, BDNF, and NT-3 and their relevance for treatment of spinal cord injury. IJMS 2017; 18: 548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gómez-Pinilla F, Huie JR, Ying Z, Ferguson AR, Crown ED, Baumbauer KM, Edgerton VR, Grau JW. BDNF and learning: evidence that instrumental training promotes learning within the spinal cord by up-regulating BDNF expression. Neuroscience 2007; 148: 893–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez-Huertas C, Rico B. CREB-dependent regulation of GAD65 transcription by BDNF/TrkB in cortical interneurons. Cereb Cortex 2011; 21: 777–788. [DOI] [PubMed] [Google Scholar]
- 41.Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 1999; 98: 739–755. [DOI] [PubMed] [Google Scholar]
- 42.Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron 2008; 60: 610–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rico B, Xu B, Reichardt F. TrkB receptor signaling is required for establishment of GABAergic synapses in the cerebellum. Nat Neurosci 2002; 5: 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Canas N, Pereira IT, Ribeiro JA, Sebastião AM. Brain-derived neurotrophic factor facilitates glutamate and inhibits GABA release from hippocampal synaptosomes through different mechanisms. Brain Res 2004; 1016: 72–78. [DOI] [PubMed] [Google Scholar]
- 45.Ohba S, Ikeda T, Ikegaya Y, Nishiyama N, Matsuki N, Yamada M. BDNF locally potentiates GABAergic presynaptic machineries: target-selective circuit inhibition. Cereb Cortex 2005; 15: 291–298. [DOI] [PubMed] [Google Scholar]
- 46.Ko MY, Jang EY, Lee JY, Kim SP, Whang SH, Lee BH, Kim HY, Yang CH, Cho HJ, Gwak YS. The role of ventral tegmental area gamma-aminobutyric acid in the chronic neuropathic pain after spinal cord injury in rats. J Neurotrauma 2018; 35: 1755–1764. [DOI] [PubMed] [Google Scholar]
- 47.Betley JN, Wright CVE, Kawaguchi Y, Erdélyi F, Szabó G, Jessell TM, Kaltschmidt JA. Stringent specificity in the construction of a GABAergic presynaptic inhibitory circuit. Cell 2009; 139: 161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gomez-Pinilla F, Ying Z, Roy R, Molteni R, Edgerton V. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol 2002; 88: 2187–2195. [DOI] [PubMed] [Google Scholar]
- 49.Hou J, Nelson R, Nissim N, Parmer R, Thompson FJ, Bose P. Effect of combined treadmill training and magnetic stimulation on spasticity and gait impairments after cervical spinal cord injury. J Neurotrauma 2014; 31: 1088–1106. [DOI] [PubMed] [Google Scholar]
- 50.Herold S, Jagasia R, Merz K, Wassmer K, Lie DC. CREB signalling regulates early survival, neuronal gene expression and morphological development in adult subventricular zone neurogenesis. Mol Cell Neurosci 2011; 46: 79–88. [DOI] [PubMed] [Google Scholar]
- 51.Yin H, Chen K, Shih JC, Tien T. Down-regulated GABAergic expression in the olfactory bulb layers of the mouse deficient in monoamine oxidase B and administered with amphetamine. Cell Mol Neurobiol 2010; 30: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grau JW, Huang Y. Metaplasticity within the spinal cord: evidence brain-derived neurotrophic factor (BDNF), tumor necrosis factor (TNF), and alterations in GABA function (ionic plasticity) modulate pain and the capacity to learn. Neurobiol Learn Mem 2018; 154: 121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Almeida C, DeMaman A, Kusuda R, Cadetti F, Ravanelli MI, Queiroz AL, Sousa TA, Zanon S, Silveira LR, Lucas G. Exercise therapy normalizes BDNF upregulation and glial hyperactivity in a mouse model of neuropathic pain. Pain 2015; 156: 504–513. [DOI] [PubMed] [Google Scholar]
- 54.Takeoka A, Vollenweider I, Courtine G, Arber S. Muscle spindle feedback directs locomotor recovery and circuit reorganization after spinal cord injury. Cell 2014; 159: 1626–1639. [DOI] [PubMed] [Google Scholar]
- 55.Li J, Baccei ML. Neonatal injury alters sensory input and synaptic plasticity in GABAergic interneurons of the adult mouse dorsal horn. J Neurosci 2019; 39: 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ozdemir RA, Perez MA. Afferent input and sensory function after human spinal cord injury. J Neurophysiol 2018; 119: 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]