Abstract
Background. Due to the effect of pre-heating on the degree of conversion of composite resins and the possible effect on cytotoxicity, the effect of pre-heating of bulk-fill composite resins was investigated on cytotoxicity in this study.
Methods. In this study, three different types of composite resin were used, including Tetric N-Ceram Bulk-Fil, Xtrafil, and Xtrabase. From each composite resin, 10 cylindrical samples (5 mm in diameter and 4 mm in height) were prepared, with five samples preheated to 68°C, and the other five samples polymerized at room temperature (25°C). Twenty-four hours after polymerization, cytotoxicity was assessed by MTT assay on human fibroblasts. Statistical analysis of data was carried out with two-way ANOVA and Sidak Post-Hoc. The significance level of the test was determined at 0.05.
Results. There was no statistically significant difference between the mean percentage of cytotoxicity in terms of pre-heating (P>0.05), but the cytotoxicity of the studied composite resins was significantly different (P<0.001). The cytotoxicity of Tetric N-Ceram Bulk-fil composite resin was higher than that of the two other composite resins.
Conclusion. Pre-heating of bulk-fill composite resin did not affect their cytotoxicity. In addition, the cytotoxicity of different bulk-fill composite resins was not the same.
Keywords: Cell survival, composite resin, tetrazolium salts
Introduction
Composite resins are extensively used as restorative materials in dentistry due to their esthetic, handling characteristics, and controlled working time.1 If the polymerization of composite resins is not adequate, the unreacted monomers remaining in the structure of composite resin might be released into the oral environment after mechanical and chemical degradation during the clinical service.2
Even immediately after placing composite resin restorations, monomers have been found in the saliva, dentin, and pulp.3 These monomers have side effects such as skin, mucous membranes, and eye irritation.4 In the oral cavity, lichenoid reactions have been reported around composite resin restorations.5 Also, Van Dijken et al6 reported that gingival exudate around the healthy enamel was less than that of composite resin restorations in seven days.
Pre-heating of uncured composite resins is popular among dentists as a way to improve the handling features during placement. Pre-heating of composite resins prior to light activation reduces their viscosity,7 and by better wetting of the cavity walls, leads to an increase in marginal adaptation8 and a decrease in microleakage.9
Furthermore, the increase in pre-polymerization temperature will result in better convergence by enhancing the mobility of monomers and radicals.10 Deb et al11 showed that increasing the temperature to 60°C before polymerization in conventional posterior composite resins significantly raises the degree of conversion. Also, improved conversion leads to better physical and mechanical properties, such as enhanced surface hardness, flexural strength, and tensile strength. Furthermore, the remaining unreacted monomers in the composite resin structure will be reduced with further conversion.12
It seems that a reduction in unreacted monomers can affect the cytotoxic properties of composite resins. Knežević et al13 found that, regardless of the chemical composition of the composite resin material, the number of living cells is affected by the pre-heating temperature.13 On the other hand, in another study, the effect of pre-heating on the cytotoxicity of conventional composite resin materials was not significant.11
During the placement of light-activated conventional composite resins, it is recommended to use increments with a thickness of <2 mm (the incremental technique). This method will create a uniform degree of conversion throughout the composite resin thickness and reduce the polymerization stress.14 However, the incremental technique has some disadvantages, such as wasting of time and the probability of creating voids between layers. These voids act as a weak point in the composite resin structure and will compromise its properties.15
Now, a new type of composite resin has been marketed, called bulk-fill, which can be cured in thicknesses of 4, 5, and 6 mm. These materials are made based on more translucent formulations, different types of resins and initiators, and new filler technologies.1 Nevertheless, achieving a sufficient degree of conversion at high depths in this group of composite resins is still challenging. The reason is that by increasing the depth, light efficiency is reduced due to absorption and dispersion.16 Unreacted monomers in the material increase by decreasing the degree of conversion at the depth of the cavity.12
As there is a tendency to use bulk-fill composite resins in deep cavities near the pulp, concerns have been raised about their biocompatibility with the pulp cells.12 Several studies have investigated the cytotoxicity of bulk-fill composite resins.9-17 Toh et al18 investigated the biocompatibility of bulk-fill composite resins with and without pre-polymerized filler in vitro. In their study, some bulk-fill composite resins with a thickness of 4 mm showed a cell viability of <70%.In another study, flowable bulk-fill composite resin showed cytotoxic effects after 24 and 72 hours.19
Considering that pre-heating affects the degree of conversion of composite resin monomers,20 and the degree of conversion might affect cytotoxicity, this study was conducted to investigate the effect of preheating on the cytotoxicity of bulk-fill composite resins. The null hypotheses of this study were as follows: I) Pre-heating does not have any effect on the cytotoxicity of bulk-fill composite resins. II) The cytotoxicity of bulk-fill composite resins will not be different from each other.
Methods
The protocol of the study was approved by the Ethics Committee of Tabriz University of Medical Sciences (No: IR.TBZMED.VCR.REC.1397.207). Teflon cylindrical molds, with a diameter of 5 mm and a height of 4 mm, were used to prepare composite resin samples. Three types of bulk-fill composite resins, Xtrafil (XF), Xtrabase (XB), and Tetric N-Ceram Bulk-fil (TNB), were selected for the study (Table 1). Ten samples were made from each composite resin; five samples were pre-heated prior to polymerization up to 68ºC, while the other five samples were polymerized at room temperature (25ºC).
Table 1. Properties of the tested composite resin materials and their abbreviated codes .
| Material | Type and color | Manufacturer | Lot No. | Organic matrix | Inorganic filler | Inorganic filler content percentage (W) |
| X- tra Fill (XF) | High viscosity bulk- fill universal | Voco, Cuxhaven, Germany | 1618416 | Bis-GMA, UDMA, TEGDMA |
* |
86 |
| X- tra Base (XB) | Low viscosity bulk- fill universal | Voco, Cuxhaven, Germany | 1745721 | Bis-EMA, UDMA, TEGDMA |
* |
75 |
|
Tetric N-Ceram
Bulk-Fill (TNB) |
High viscosity bulk- fill IVA(universal) |
Ivoclar-vivadent, Schaan, Liechtenstein | W30705 |
Bis-GMA, UDMA, Bis-EMA |
Barium glass, ytterbium trifluoride, mixed oxide |
61 |
*Filler composition has not been specified by the manufacturer.
Accordingly, six groups were examined in terms of the type of composite resin and pre-heating (n=5) with a total number of 30 composite resin samples. Pre-heating of composite resins was carried out using a hot water bath with thermostatic control (TELEDYNE HANAU, Buffalo, NY, USA) up to 68°C. Furthermore, the material temperature was measured using a digital temperature microprobe (GBC KDM 350, KON ELCO SpA, Milano, Italy).21
After measuring the temperature of the composite resin, the Teflon mold was completely filled in one step with a single bulk of composite resin with a transparent strip placed on the superior and inferior parts of the cylinders to prevent the formation of the oxygen-inhibited layer. It was then cured by a light-curing device (LITEX 695C Cordless LED Curing Light, Dentamerica, USA) at an intensity of 1100 mW/cm2 for 20 seconds.
A calibrated radiometer (Bisco, IL, USA) was used to ensure the intensity of radiation in each use of the light-curing unit. To simulate the clinical condition, the device was placed in direct contact only with one side of the cylinder.22 The light-cured composite resin samples were sterilized through swabbing with 70% ethanol alcohol. Then, the samples were immersed in DMEM high-glucose culture medium (Dulbecco's Modified Eagle's Medium, Sigma Culture Chemical Co., St. Louis, Mo, USA) with penicillin-streptomycin supplements and fetal bovine serum (10% FBS [Gibco, UK] and 1% P/S [Gibco, UK]) and incubated at 37°C, a relative humidity of 95%, and 5% CO2 for 24 hours. The original solution of the extract of composite resin samples was the same as the culture medium in which the samples had been immersed.18 For the evaluation of the MTT, the original extract solution was diluted with the addition of the fresh medium as follows:23
100%: One part of the original extract
75%: Three parts of the original extract + one part of the fresh medium
50%: One part of the original extract + one part of the fresh medium
25%: One part of the original extract + three parts of the fresh medium
A cell line of human fibroblasts was obtained from the National Cell Bank of Iran (NCBI, Pasteur Institute of Iran). The cells with a density of 2×104 cells/well were incubated in 96-well plates at 37°C and 5% CO2 for 24 hours.18 Each dilution was examined in six wells of plates. In each test, six wells containing human fibroblast cells without composite resin extraction were used as the control group. Finally, the cell vitality was determined by the MTT assay.
MTT dye (3-4.5-dimethylthiazol-2-yl-2, 5-diphenyltetrazolium bromide) (Sigma, USA) was dissolved in a solution of PBS (phosphate-buffered saline) (Gibco, UK) at a concentration of 5 mg/mL. Then, 10 mL of the MTT solution was added to each well containing 100 mL of culture medium. The yellow MTT solution was converted to an insoluble purple formazan over 5-hour incubation with mitochondrial dehydrogenase enzyme. Finally, the medium on the cells was completely removed and replaced with 200 μL of DMSO (dimethyl sulfoxide) (Merck, German) to dissolve the formazan produced by the cells. The optical density of the formazan solution was determined using an ELISA reader (Organon Teknika, Netherlands) at a wavelength of 540 nm, and the percentage of cytotoxicity was calculated as follows:23
Cytotoxicity percentage = 1 – the mean absorbance of toxicant-treated cells/mean absorbance of control ×100
Statistical analysis
At each of the culture medium concentrations (100%, 75%, 50%, and 25%) separately, two-way ANOVA was used to evaluate the effect of pre-heating on the cytotoxicity in terms of the composite resin type. The utilized post hoc test was Sidak. Data were analyzed using SPSS 17, and the significance level of the test was determined at 0.05.
Results
Table 2 shows the means and standard deviations of cytotoxicity percentages in terms of pre-heating and the type of composite resin (in the cell culture medium with the studied concentrations). Also, the error-bar diagram is presented in Figure 1.
Table 2. Means and standard deviations of cytotoxicity, in terms of pre-heating and type of composite resin (in cellular culture medium with different concentrations) .
| Concentration | |||||||||
| Composite type | Preheating | 100% | 75% | 50% | 25% | ||||
| SD | Mean | SD | Mean | SD | Mean | SD | Mean | ||
| XB | No | 1.51 | 23.01 | .630 | 23.42 | 3.36 | 17.54 | 1.99 | 15.69 |
| Yes | 1.94 | 23.23 | 2.04 | 22.59 | 1.93 | 16.77 | 1.23 | 15.04 | |
| XF | No | 1.43 | 23.72 | 2.50 | 23.24 | 3.34 | 17.68 | 0.21 | 15.04 |
| Yes | 1.58 | 23.34 | 2.90 | 23.43 | 4.28 | 17.49 | 0.22 | 15.34 | |
| TNB | No | 3.88 | 29.87 | 1.89 | 22.63 | 3.59 | 17.58 | .610 | 15.00 |
| Yes | 4.03 | 28.77 | 1.25 | 21.77 | 2.78 | 17.50 | 0.88 | 15.20 | |
Figure 1.
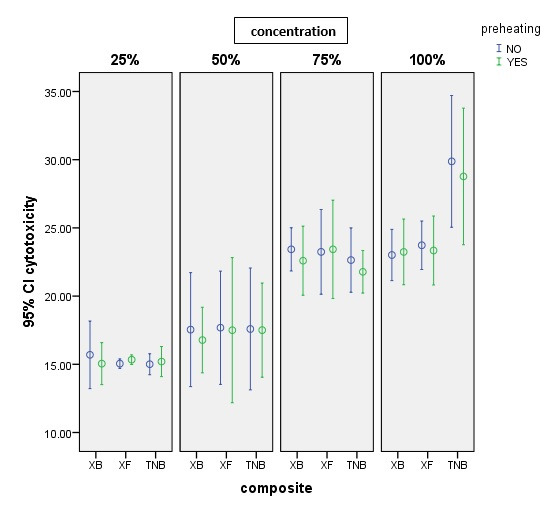
Error-bar cytotoxicity diagram in terms of the type of composite resin and the pre-heating variable.
Two-way ANOVA for each concentration showed no statistically significant difference between the mean cytotoxicity percentages in terms of pre-heating at any of the four concentrations (P>0.05). At a concentration of 100%, there was a significant difference between the mean percentages of cytotoxicity in the three types of studied composite resins (P<0.001). However, at three concentrations of 25%, 50%, and 75%, this difference was not statistically significant (P>0.05). There was not a significant interaction between pre-heating and composite resin type at any of the four concentrations (P>0.05).
At a concentration of 100%, the results of post hoc Sidak test (α=0.05) revealed a statistically significant difference between the mean cytotoxicity percentage of the TNB composite resin and its mean in both XB and XF composite resins; the mean percentage of cytotoxicity in TNB composite resin was higher than that of the two other composite resins (P<0.001). However, there was no statistically significant difference between the mean cytotoxicity percentages of XF and XB composite resins (P>0.05).
Discussion
In this study, the cytotoxic effects of pre-heated and non-pre-heated bulk-fill composite resins were investigated in a human fibroblast cell line. In general, cell culture is used to evaluate the cytotoxicity potential of dental composite resins. There are several in vitro test models for the evaluation of cytotoxicity of dental biomaterials. They include the direct contact method, where the materials directly contact the cellular layer, the indirect contact method, where a barrier is placed between the cell and the biomaterial layer, and the extract method, where the extracts of the materials are placed in contact with cells. The best in vitro test is a test that can better simulate in vivo conditions. In a study by Lim et al,24 these three test methods for composite resin materials were compared, and the results indicated significant and strong correlations between all the three methods. Also, the extract test was more sensitive than contact tests. After the test model, the next factor is cell type selection. Studies have employed different techniques and primary or permanent cells.25,26 Most researchers prefer permanent cell lines due to their morphology and homogeneous growth characteristics. The reason is that primary cells are taken from different donors, which will differ in their developmental and cultural characteristics. Nevertheless, permanent cell lines have a poor simulation of the oral environment compared with primary cells.27
Previous studies have reported that the pre-heating of composite resins can enhance the rate of polymerization and the degree of conversion, and reduce the amount of uncured monomers by temporarily reducing the viscosity.28,29 However, Marigo et al30 showed that different degrees of conversion of composite resins do not necessarily lead to different cytotoxic effects. Therefore, pre-heating does not change the degree of conversion of composite resin to the extent that it affects its cytotoxicity.
The results of this study also indicated that pre-heating of the bulk-fill composite resins used did not have a statistically significant effect on their cytotoxicity, and the study was unable to reject the first hypothesis. Thus, the pre-heating of the composite resins did not cause adverse reactions, and the cytocompatibility of the components released from the composite resin was not affected. This result was consistent with those of a study by Deb et al.11 In their reports, the pre-heating of composite resins did not significantly influence their cytotoxicity. However, the study by Knežević et al13 suggested that cell viability was affected by the pre-heating temperature.
In their study, the highest cell viability was reported in composite resins pre-heated up to 54°C with 40 seconds of curing time and at 37°C with 20 seconds of curing time. They used lymphocytes to investigate cell viability by direct contact with composite resin samples (not their extracts), and staining with different techniques and materials. However, in the mentioned study, similar to ours, there was no statistically significant difference at 68°C.13
The results in the present study also indicated that different composite resins had different cytotoxicity levels. Therefore, the second hypothesis was rejected. This result was consistent with the results of other studies.11,19,31 The cytotoxicity of TNB composite resin was higher than that of XB and XF composite resins at 100% concentration, and no statistically significant difference was observed between XF and XB composite resins.
In a study by Nascimento et al,31 the results of cytotoxicity with the MTT assay were similar for XB and XF composite resins. Kamalak et al19 found different cytotoxic results for bulk-fill composite resins. The differences in the cytotoxicity of XB and XF composite resins with TNB composite resins seem to be related to their chemical composition.
For example, a higher filler percentage might reduce the solubility of the composite resin. There are many reports suggesting that composite resins with a higher filler content offer a lower solvent adsorption rate compared to composite resins with low filler content.32,33 In addition, lower solvent adsorption might result in the lower release of components.34 According to the manufacturer’s information, the filler percentages of TNB bulk-fill composite resins, XF, and XB are 61%, 86%, and 75%, respectively.
Therefore, the higher cytotoxicity of the TNB is probably related to a lower filler content of this composite resin and higher release of components compared to other composite resins. On the other hand, TEGDMA monomer has a synergistic effect on the extent of polymerization in the composite resin structure.34 According to the manufacturer’s information, there is no TEGDMA monomer in the chemical structure of TNB composite resins. This molecule is present in the structure of XB and XF composite resins and might cause enhanced polymerization in them and reduce their monomer elution compared to TNB.
Furthermore, the TNB composite resin contains pre-polymerized fillers. Unreacted residual or pendant double bands of C-C in these fillers might increase the leachable monomers.35 In addition to the leaching of unreacted monomers, the release of initiators, other organic matrix additives, and metallic ions from mineral fillers might also cause cytotoxicity of the composite material. Fluoride in the chemical structure of some composite resins can be cytotoxic for tissues through several mechanisms, such as inhibiting the activity of enzymes, producing reactive oxygen species, destroying the antioxidant defense system, as well as inducing inflammation and apoptosis.18 Among the studied composites, TNB composite resin had fluoride in its chemical composition, and this ion might lead to the more significant cytotoxic results for this composite resin.
In a study by Marigo et al,30 there was no statistically significant difference in the cytotoxicity of composite resins. In their study, the effect of cytotoxicity of flowable composite resin was investigated on human pulp cells. The utilized cells were not cell lines, where the dimensions of the examined samples were smaller than those of our study samples. Also, Rodríguez-Lozano et al17 examined the effects of cytotoxicity of two flowable bulk-fill composite resins on pulp and PDL stem cells. They found no significant difference between the two composite resins. The cells employed in that study were not cell lines and were extracted from the impacted third molars of the patients. Additionally, the composite resins studied by them were different from those of the present study.
In this study, only a cell line was used, which was a limitation of the study. These cells had a weaker clinical simulation condition. Also, in the clinical use of dental materials, there are barriers, such as dentin. In future studies, a combination of different curing methods, other cell lines, or primary cells, comparison of the direct and indirect contact methods, and greater clinical simulation conditions can be addressed. Recently, in vivo and in vitro studies have indicated that monomers have interactions with the immune system, genotoxicity, estrogenicity,36 hypersensitivity, and the production of active oxygen species.19 These parameters can also be examined to gain deeper insights in this regard.
Conclusion
This study suggested that pre-heating of bulk-fill composite resins up to 68°C did not affect the toxicity of human fibroblasts. Furthermore, the cytotoxicity of different bulk-fill composite resins varied depending on their chemical composition. Nevertheless, future studies are required to further investigate the cytotoxicity of these materials.
Authors’ Contributions
The concept and the design of the study were developed by MEEC, HS, EJN, and LS. The MTT method was carried out by HSH. Data entry and statistical analyses were carried out by MB and AAA. The manuscript was written by HS, LS, PAO, and MAK. All the authors participated in the literature review. All authors have read and approved the final manuscript.
Acknowledgments
The authors would like to thank the Dental and Periodontal Research Center of Tabriz University of Medical Sciences.
Funding
This research was carried out by financial support of the Dental and Periodontal Research Center, Tabriz University of Medical Sciences.
Competing Interests
The authors declare no competing interests with regards to the authorship and/or publication of this article.
Ethics approval
This research was approved by Research Ethics Committee of Tabriz University of Medical Sciences in 2018.
References
- 1.Karacolak G, Turkun LS, Boyacioglu H, Ferracane JL. Influence of increment thickness on radiant energy and microhardness of bulk-fill resin composites. Dent Mater J. 2018;37(2):206–13. doi: 10.4012/dmj.2017-032. [DOI] [PubMed] [Google Scholar]
- 2.Freund M, Munksgaard EC. Enzymatic degradation of BISGMA/TEGDMA‐polymers causing decreased microhardness and greater wear in vitro. Eur J Oral Sci. 1990;98(4):351–5. doi: 10.1111/j.1600-0722.1990.tb00984.x. [DOI] [PubMed] [Google Scholar]
- 3.Pulgar R, Olea-Serrano MF, Novillo-Fertrell A, Rivas A, Pazos P, Pedraza V. et al. Determination of bisphenol A and related aromatic compounds released from bis-GMA-based composites and sealants by high performance liquid chromatography. Environ Health Perspect. 2000;108(1):21–7. doi: 10.1289/ehp.0010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lönnroth E-C, Shahnavaz H. Use of polymer materials in dental clinics, case study. Swed Dent J. 1997;21(4):149–59. [PubMed] [Google Scholar]
- 5.Blomgren J, Axell T, Sandahl O, Jontell M. Adverse reactions in the oral mucosa associated with anterior composite restorations. J Oral Pathol Med. 1996;25(6):311–3. doi: 10.1111/j.1600-0714.1996.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 6.Van Dijken J, Sjöström S. Development of gingivitis around aged restorations of resin-modified glass ionomer cement, polyacid-modified resin composite (compomer) and resin composite. Clin Oral Investig. 1998;2(4):180–3. doi: 10.1007/s007840050067. [DOI] [PubMed] [Google Scholar]
- 7.Lucey S, Lynch C, Ray N, Burke F, Hannigan A. Effect of pre‐heating on the viscosity and microhardness of a resin composite. J Oral Rehabil. 2010;37(4):278–82. doi: 10.1111/j.1365-2842.2009.02045.x. [DOI] [PubMed] [Google Scholar]
- 8.Fróes-Salgado NR, Silva LM, Kawano Y, Francci C, Reis A, Loguercio AD. Composite pre-heating: effects on marginal adaptation, degree of conversion and mechanical properties. Dent Mater. 2010;26(9):908–14. doi: 10.1016/j.dental.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Wagner WC, Aksu M, Neme A, Linger J, Pink FE, Walker S. Effect of pre-heating resin composite on restoration microleakage. Oper Dent. 2008;33(1):72–8. doi: 10.2341/07-41. [DOI] [PubMed] [Google Scholar]
- 10.Daronch M, Rueggeberg F, De Goes M, Giudici R. Polymerization kinetics of pre-heated composite. J Dent Res. 2006;85(1):38–43. doi: 10.1177/154405910608500106. [DOI] [PubMed] [Google Scholar]
- 11.Deb S, Di Silvio L, Mackler HE, Millar BJ. Pre-warming of dental composites. Dent Mater. 2011;27(4):e51–e9. doi: 10.1016/j.dental.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Pongprueksa P, De Munck J, Duca RC, Poels K, Covaci A, Hoet P. et al. Monomer elution in relation to degree of conversion for different types of composite. J Dent. 2015;43(12):1448–55. doi: 10.1016/j.jdent.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Knežević A, Želježić D, Kopjar N, Duarte Jr S, Tarle Z. In Vitro Biocompatibility of Preheated Giomer and Microfilled-Hybrid Composite. Acta Stomatol Croat. 2018;52(4):286–97. doi: 10.15644/asc52/4/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.HIRABAYASHI S, HOOD JA, HIRASAWA T. The Extent of Polymerization of Class II Light-cured Composite Resin Restorations Effects of Incremental Placement Technique, Exposure Time and Heating for Resin Inlays. Dent Mater J. 1993;12(2):159–70,273. doi: 10.4012/dmj.12.159. [DOI] [PubMed] [Google Scholar]
- 15.Alqudaihi F, Cook N, Diefenderfer K, Bottino M, Platt J. Comparison of Internal Adaptation of Bulk-fill and Increment-fill Resin Composite Materials. Oper Dent. 2019;44(1):E32–E44. doi: 10.2341/17-269-L. [DOI] [PubMed] [Google Scholar]
- 16.Lee S-K, Kim T-W, Son S-A, Park J-K, Kim J-H, KIM H-I. et al. Influence of light-curing units on the polymerization of low-shrinkage composite resins. Dent Mater J. 2013;32(5):688–94. doi: 10.4012/dmj.2013-027. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-Lozano F, Serrano-Belmonte I, Calvo JP, Coronado-Parra M, Bernabeu-Esclapez A, Moraleda J. Effects of two low-shrinkage composites on dental stem cells (viability, cell damaged or apoptosis and mesenchymal markers expression) J Mater Sci Mater Med. 2013;24(4):979–88. doi: 10.1007/s10856-013-4849-x. [DOI] [PubMed] [Google Scholar]
- 18.Toh W, Yap A, Lim S. In vitro biocompatibility of contemporary bulk-fill composites. Oper Dent. 2015;40(6):644–52. doi: 10.2341/15-059-L. [DOI] [PubMed] [Google Scholar]
- 19.Kamalak H, Kamalak A, Taghizadehghalehjoughi A, Hacımüftüoğlu A, Nalcı KA. Cytotoxic and biological effects of bulk fill composites on rat cortical neuron cells. Odontology. 2018:1–12. doi: 10.1007/s10266-018-0354-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Korashy D. Post-gel shrinkage strain and degree of conversion of preheated resin composite cured using different regimens. Oper Dent. 2010;35(2):172–9. doi: 10.2341/09-072-L. [DOI] [PubMed] [Google Scholar]
- 21.Oskoee PA, Azar FP, Navimipour EJ. The effect of repeated preheating of dimethacrylate and silorane-based composite resins on marginal gap of class V restorations. J Dent Res Dent Clin Dent Prospects. 2017;11(1):36. doi: 10.15171/joddd.2017.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Łagocka R, Jakubowska K, Chlubek D, Buczkowska-Radlińska J. The Influence of Irradiation Time and Layer Thickness on Elution of Triethylene Glycol Dimethacrylate from SDR® Bulk-Fill Composite. Biomed Res Int. 2016;2016 doi: 10.1155/2016/3481723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koohpeima F, Mokhtari MJ, Doozandeh M, Jowkar Z, Yazdanshenas F. Comparison of Cytotoxicity of New Nanohybrid Composite, Giomer, Glass Ionomer and Silver Reinforced Glass Ionomer using Human Gingival Fibroblast Cell Line. J Clin Pediatr Dent. 2017;41(5):368–73. doi: 10.17796/1053-4628-41.5.368. [DOI] [PubMed] [Google Scholar]
- 24.Lim S, Yap A, Loo C, Ng J, Goh C, Hong C. et al. Comparison of cytotoxicity test models for evaluating resin-based composites. Hum Exp Toxicol. 2017;36(4):339–48. doi: 10.1177/0960327116650007. [DOI] [PubMed] [Google Scholar]
- 25.Hensten‐pettersen A, Helgelan K. Sensitivity of different human cell lines in the biologic evaluation of dental resin‐based restorative materials. Eur J Oral Sci. 1981;89(1):102–7. doi: 10.1111/j.1600-0722.1981.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 26.Schmalz G. Use of cell cultures for toxicity testing of dental materials—advantages and limitations. J Dent. 1994;22:S6–S11. doi: 10.1016/0300-5712(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 27.Geurtsen W, Lehmann F, Spahl W, Leyhausen G. Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3 and three human primary fibroblast cultures. J Biomed Mater Res A. 1998;41(3):474–80. doi: 10.1002/(SICI)1097-4636(19980905)41:3<474::AID-JBM18>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 28.Alshali RZ, Salim NA, Satterthwaite JD, Silikas N. Post-irradiation hardness development, chemical softening, and thermal stability of bulk-fill and conventional resin-composites. J Dent. 2015;43(2):209–18. doi: 10.1016/j.jdent.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 29.El-Korashy D. Post-gel shrinkage strain and degree of conversion of preheated resin composite cured using different regimens. Oper Dent. 2010;35(2):172–9. doi: 10.2341/09-072-L. [DOI] [PubMed] [Google Scholar]
- 30.Marigo L, Spagnuolo G, Malara F, Martorana GE, Cordaro M, Lupi A. et al. Relation between conversion degree and cytotoxicity of a flowable bulk-fill and three conventional flowable resin-composites. Eur Rev Med Pharmacol Sci. 2015;19(23):4469–80. [PubMed] [Google Scholar]
- 31.Nascimento AS, Lima DB, Fook MVL, Albuquerque MSd, Sanino MA, Borges SMP. et al. Physicomechanical characterization and biological evaluation of bulk-fill composite resin. Braz Oral Res. 2018;32 doi: 10.1590/1807-3107bor-2018.vol32.0107. [DOI] [PubMed] [Google Scholar]
- 32.Örtengren U, Wellendorf H, Karlsson S, Ruyter I. Water sorption and solubility of dental composites and identification of monomers released in an aqueous environment. J Oral Rehabil. 2001;28(12):1106–15. doi: 10.1046/j.1365-2842.2001.00802.x. [DOI] [PubMed] [Google Scholar]
- 33.Braden M, Clarke R. Water absorption characteristics of dental microfine composite filling materials: I Proprietary materials. Biomaterials. 1984;5(6):369–72. doi: 10.1016/0142-9612(84)90038-3. [DOI] [PubMed] [Google Scholar]
- 34.Lempel E, Czibulya Z, Kovács B, Szalma J, Tóth Á, Kunsági-Máté S. et al. Degree of conversion and BisGMA, TEGDMA, UDMA elution from flowable bulk fill composites. Int J Mol Sci. 2016;17(5):732. doi: 10.3390/ijms17050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manojlovic D, Radisic M, Vasiljevic T, Zivkovic S, Lausevic M, Miletic V. Monomer elution from nanohybrid and ormocer-based composites cured with different light sources. Dent Mater. 2011;27(4):371–8. doi: 10.1016/j.dental.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 36.Longo DL, PAULA-SILVA FWG, Faccioli LH, Gaton-Hernandez PM, QUEIROZ AMd, SILVA LABd. Cytotoxicity and cytokine expression induced by silorane and methacrylate-based composite resins. J Appl Oral Sci. 2016;24(4):338–43. doi: 10.1590/1678-775720150449. [DOI] [PMC free article] [PubMed] [Google Scholar]


