Abstract
Due to various challenges in diagnosing chlamydiosis in pigs, antibiotic treatment is usually performed before any molecular or antibiotic susceptibility testing. This could increase the occurrence of tetracycline-resistant Chlamydia (C.) suis isolates in the affected pig population and potentiate the reoccurrence of clinical signs. Here, we present a case of an Austrian pig farm, where tetracycline resistant and sensitive C. suis isolates were isolated from four finishers with conjunctivitis. On herd-level, 10% of the finishers suffered from severe conjunctivitis and sows showed a high percentage of irregular return to estrus. Subsequent treatment of whole-herd using oxytetracycline led to a significant reduction of clinical signs. Retrospective antibiotic susceptibility testing revealed tetracycline resistance and decreased susceptibility to doxycycline in half of the ocular C. suis isolates, and all isolates were able to partially recover following a single-dose tetracycline treatment in vitro. These findings were later confirmed in vivo, when all former clinical signs recurred three months later. This case report raises awareness of tetracycline resistance in C. suis and emphasizes the importance of preventative selection of tetracycline resistant C. suis isolates.
Keywords: Chlamydia suis, fertility problems, conjunctivitis, minimal inhibition concentration, multilocus sequence typing, recovery testing, tetracycline resistance
1. Introduction
Chlamydial infections have been associated with a variety of diseases in pigs [1], including conjunctivitis [2,3], pneumonia [4], enteritis [5,6], and polyarthritis [7]. Additionally, Chlamydia spp. can cause a wide range of reproductive disorders such as abortions [8], perinatal mortality [9], vaginal discharge, repeated breeding [10,11], as well as poor reproductive performances in sows [12]. Chlamydia (C.) suis is the most prevalent chlamydial species in pigs [1,13]. The diagnosis of Chlamydiaceae infections, in particular, antibiotic susceptibility testing, is time-consuming and laborious due to their obligate intracellular nature. For diagnosing chlamydial infections in veterinary medicine, open questions containing the sampling type and timing, depend on the animal host species, clinical signs, or anatomical localization to test. Moreover, only few laboratories can offer the cultivation of these bacteria, restricting diagnosis to molecular methods, and thus any statement regarding their growth characteristics, virulence, and antibiotic resistance patterns will be missing [14]. Detecting evidence for the involvement of C. suis in the pathogenesis of fertility problems in sows is especially challenging. Therefore, C. suis infections are often diagnosed clinically following the exclusion of other well-known pathogens, but without the detection of C. suis using either nucleic acid amplification tests (NAATs) or serological methods, and without the identification of chlamydial inclusions using immunohistochemical or immunofluorescence staining [15]. Cultivation of these obligate intracellular bacteria is very laborious and expensive. Consequently, antibiotic susceptibility testing, which requires cell culture systems, is not performed on a routine basis.
Following the diagnosis of chlamydial infections in veterinary medicine, in the absence of an effective anti-C. suis vaccine, tetracyclines are usually the treatment of choice [15]. Tetracyclines are easy to apply via food or water. They are not on the WHO list of critically important antimicrobials [16] in contrast to macrolides, which are the treatment of choice in human chlamydial infections. In pigs, this could be a cause for concern considering that C. suis is the only chlamydial species known to have naturally acquired genes that encode tetracycline resistance [17,18]. Moreover, there is evidence for intra- and interspecies recombination upon co-infection in vitro such as tetA(C) transfer among C. suis isolates, as well as from C. suis to C. trachomatis [19,20]. All of these factors might have severe consequences for human health, considering that both C. suis and C. trachomatis DNA have been detected simultaneously in the eyes of trachoma patients in Nepal. Moreover, C. suis was isolated from ocular and rectal samples originating from slaughterhouse workers and pig farmers [20,21,22,23]. The use of sub-inhibitory concentrations of tetracycline, especially in the presence of tetracycline-resistant C. suis isolates, could lead to treatment failure and the selection of tetracycline resistance on herd-level with the potential recurrence of clinical signs [24,25]. However, despite the evidence for C. suis tetracycline resistance, the treatment of chlamydiosis in pigs is still limited to tetracyclines [15].
2. Case Study
The need for ethics approval in this case is deemed unnecessary according to national Austrian regulations (Tiergesundheitsdienstverordnung 2009, BGBl. II Nr. 434/2009), because data had been collected during routine diagnostic measures within the herd health management.
The case herd was located in Lower Austria in a family-owned farrow-to-finish farm housing 60 sows and 350 fattening pigs. In 2017, an increase of the irregular return to estrus rate over the last year, from 10% to more than 25% on average, was recorded. Sows of all parities were involved. About 20% of sows in estrus had a yellowish mucous vaginal discharge. Since then, oxytetracycline at an inconsistent concentration was fed during each estrus period for around five days, without any improvement. Abortions were not recorded. Conjunctivitis was not observed in sows, but in the fattening unit approximately 10% of the oldest finishers (19 and 22 weeks of age) showed severe reddening of the conjunctiva, prolapse of the third eyelid, and seromucous ocular discharge. No other clinical signs (e.g., fever, coughing, wasting, or diarrhea) were noted. Disinfection of the barns was not performed on a routine basis.
Due to the clinical signs, the veterinarian suspected Chlamydia spp. to be the causative agent of conjunctivitis in the finishers, which was confirmed by molecular and culture methods: in four out of five conjunctival swabs, a Chlamydiales-specific real-time PCR targeting a fragment of about 207–215 bp of the 16S rRNA region developed by Lienard [26], yielded positive results. Subsequent Sanger sequencing of purified PCR products (200 bp amplicon of the 16S gene) [26] was performed, and the sequences were compared against the National Center for Biotechnology Information (NCBI) database using BLAST-n, categorized according to the first 16S rRNA BLAST-hit identification, and the closest known organism found was C. suis (100% nucleotide identity). Furthermore, C. suis was successfully isolated from all four swabs following inoculation onto LLC-MK2 cells (rhesus monkey kidney cells). The species identity was then confirmed using established NAATs, a Chlamydiaceae-specific real-time PCR targeting a 111 bp sequence of the 23S rRNA [27], followed by species-identification using an Arraymate microarray [28,29], a method that can detect mixed infections with other chlamydiae [27,30]. Despite these findings, C. suis investigation of vaginal and cervical swabs taken from sows with vaginal discharge remained negative, but C. suis DNA was identified by PCR in the urogenital tract of one sow slaughtered due to irregular return to estrus [31], though isolation attempts remained unsuccessful.
Subsequently, the herd veterinarian started whole-herd treatment with oxytetracycline (40 mg/kg body weight/q 24 h) over 21 days, as in-feed medication. Additional improvements of biosecurity measures, mainly focusing on cleaning and disinfection, were put in place. Signs of conjunctivitis disappeared and fertility problems were reduced (less than 10 percent return to estrus rates, no vaginal discharge). Three months later, the farmer reported new cases of conjunctivitis in six pigs. At the same time, the fertility problems insidiously reoccurred. This time, fattening pigs were no longer treated, while sow treatment over the insemination time was continued.
Due to the recurrence of clinical signs, retrospectively, further investigations regarding the molecular characterization of the retained isolates taken at the first herd visit, prior to the antimicrobial treatment by C. suis-specific multilocus sequence typing (MLST) [32] and antimicrobial susceptibility testing [19], were performed (Table 1).
Table 1.
Summary of characteristics of ocular C. suis isolates 1–4.
| Isolate 1 | Isolate 2 | Isolate 3 | Isolate 4 | |
|---|---|---|---|---|
| fattening ID | 494 MS | 490 MS | 329 MS | 330 MS |
| sequence type | ST279 | ST278 | ST277 | ST276 |
| phylogenetic clade | 1. clade | 2. clade | 2. clade | 2. clade |
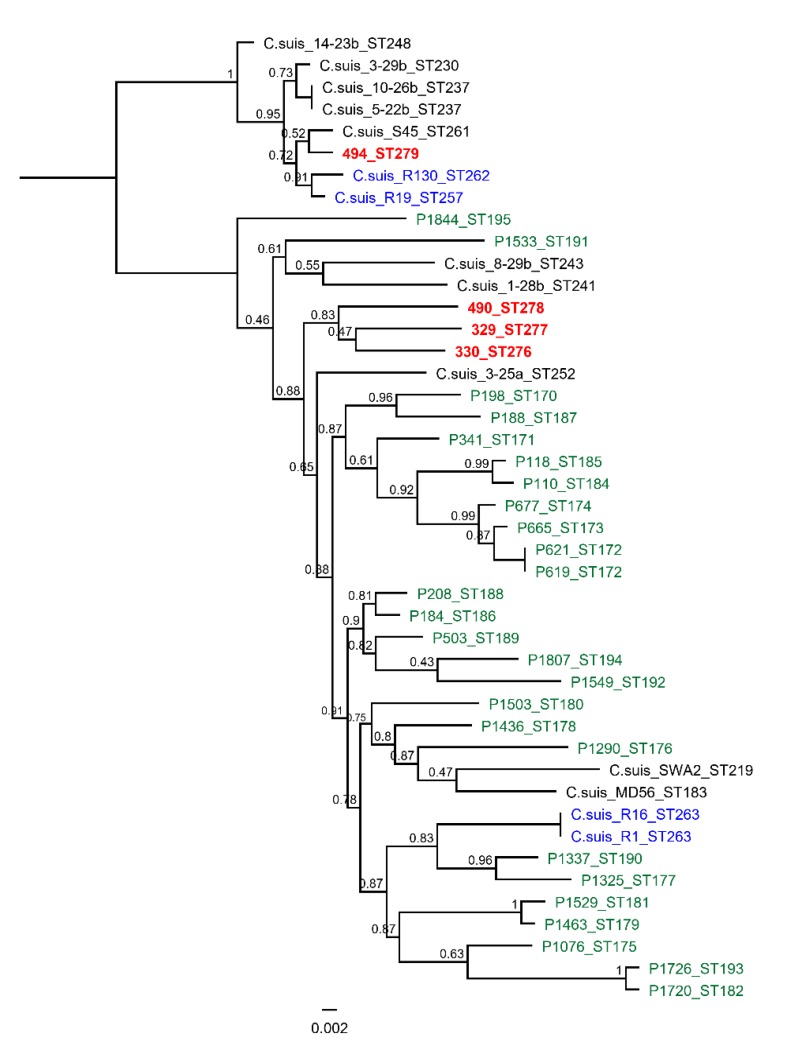
Following MLST, the isolates were denoted with new distinct sequence types (STs: ST 276 for isolate 330 MS, ST 277 for isolate 329 MS, ST278 for isolate 490 MS, and ST279 for isolate 494 MS), and the phylogenetic analyses clustered the isolates into two genetically distinct clades (Figure 1). The 494 MS isolate clustered in the first major clade together with other Swiss and US isolates [33,34], along with type strain S45, which was isolated in Austria in the 1960s [33]. The other three isolates clustered in the genetically diverse second major clade, which included other European, US, and Chinese C. suis pig isolates. The 490 MS, 329 MS, and 330 MS isolates also formed a distinct well-supported subclade. MS is the abbreviation for “fattening pig” in german.
Figure 1.
The mid-point rooted approximately-maximum-likelihood phylogenetic tree constructed using an alignment of concatenated multilocus sequence typing (MLST) sequences from the four isolates from this study, and an additional ten European, four US, and twenty-six Chinese C. suis isolates. The support values are displayed on the nodes. The isolates from this study are denoted in bold and red letters, other European strains in black, US strains in blue, and Chinese strains in green. ST for each is denoted at the end of the strain name. The figure was created in Geneious Prime v.2019.1, Biomatters (www.geneious.com).
For tetracycline and doxycycline susceptibility testing, all isolates were further tested for the presence of the tetA(C) resistance gene by a PCR assay established by Dugan et al. using the primer pair CS43/CS47 [17]. Two isolates (329 MS and 494 MS) were tetA(C)-positive, whereas the other two were (330 MS, 490 MS) negative. To verify the resistance of C. suis to tetracycline and doxycycline, the minimum inhibitory concentration (MIC) of tetracycline and doxycycline for all four isolates was determined as previously described [19]. The resulting consensus MIC was based on three values: First, the MIC value resulting from “initial phenotype” testing, which was based on a fast screening method developed by Marti et al. [19], where 96-well plates were simultaneously seeded and infected onto serial dilutions of the antibiotic of choice, and where the MIC was defined as the first concentration where the number of inclusions was strongly reduced compared to the control. Second, the value obtained through the method described by Suchland et al. [20], who defined the MIC as, “two times the concentration where over 90% of all inclusions were altered in size and morphology” compared to the control, and third, the MIC was determined according to Donati et al. [35], who defined the MIC as, “the lowest concentration that reduced the number of inclusions more than 90% compared to the level of drug-free controls” (Table 2a,b). A consensus MIC is necessary, because small discrepancies (2-fold differences) between assays are considered within the expected variations of these in vitro assays [19].
Table 2.
(a) Susceptibility testing of tetracycline in vitro according to Marti et al. [19], and (b) susceptibility testing of doxycycline in vitro according to Marti et al. [19]. MIC: Minimum inhibitory concnetration.
| (a) | ||||
| MIC (µg/mL) | 329 MS | 330 MS | 490 MS | 494 MS |
| Initial phenotype | 2 to 4 | 0.125 | 0.125 | 4 |
| MIC (Suchland) | 4 | 0.06 | 0.125 | 4 |
| MIC (Donati) | 4 | 0.06 | 0.125 | 4 |
| MIC (consensus) | 4 | 0.06–0.125 | 0.125 | 4 |
| Interpretation | resistant | sensitive | sensitive | Resistant |
| (b) | ||||
| MIC (µg/mL) | 329 MS | 330 MS | 490 MS | 494 MS |
| Initial phenotype | 0.25 | 0.03–0.06 | 0.06 | 0.125 |
| MIC (Suchland) | 0.5 | 0.06 | 0.06 | 0.25 |
| MIC (Donati) | 0.25 | 0.06 | 0.06–0.125 | 0.25 |
| MIC (consensus) | 0.25–0.5 | 0.06 | 0.06 | 0.125–0.25 |
| Interpretation | Reduced susceptibility | sensitive | sensitive | Reduced susceptibility |
TetA(C)-positive isolates (TcR), 329 MS and 494 MS, had high MIC values (4 µg/mL) against tetracycline (resistant if ≥4 µg/mL [35,36]) and were therefore confirmed to be resistant to tetracycline in vitro, while the MICs of tetA(C)-negative isolates (TcS), 330 MS and 490 MS, had MICs ranging from 0.06 to 0.125 µg/mL and were thus considered to be tetracycline sensitive. In contrast, no isolate showed in vitro resistance to doxycycline, although the MICs of TcR isolates were two- to eight-fold higher than those of TcS isolates (0.125–0.5 µg/mL compared to 0.06 µg/mL).
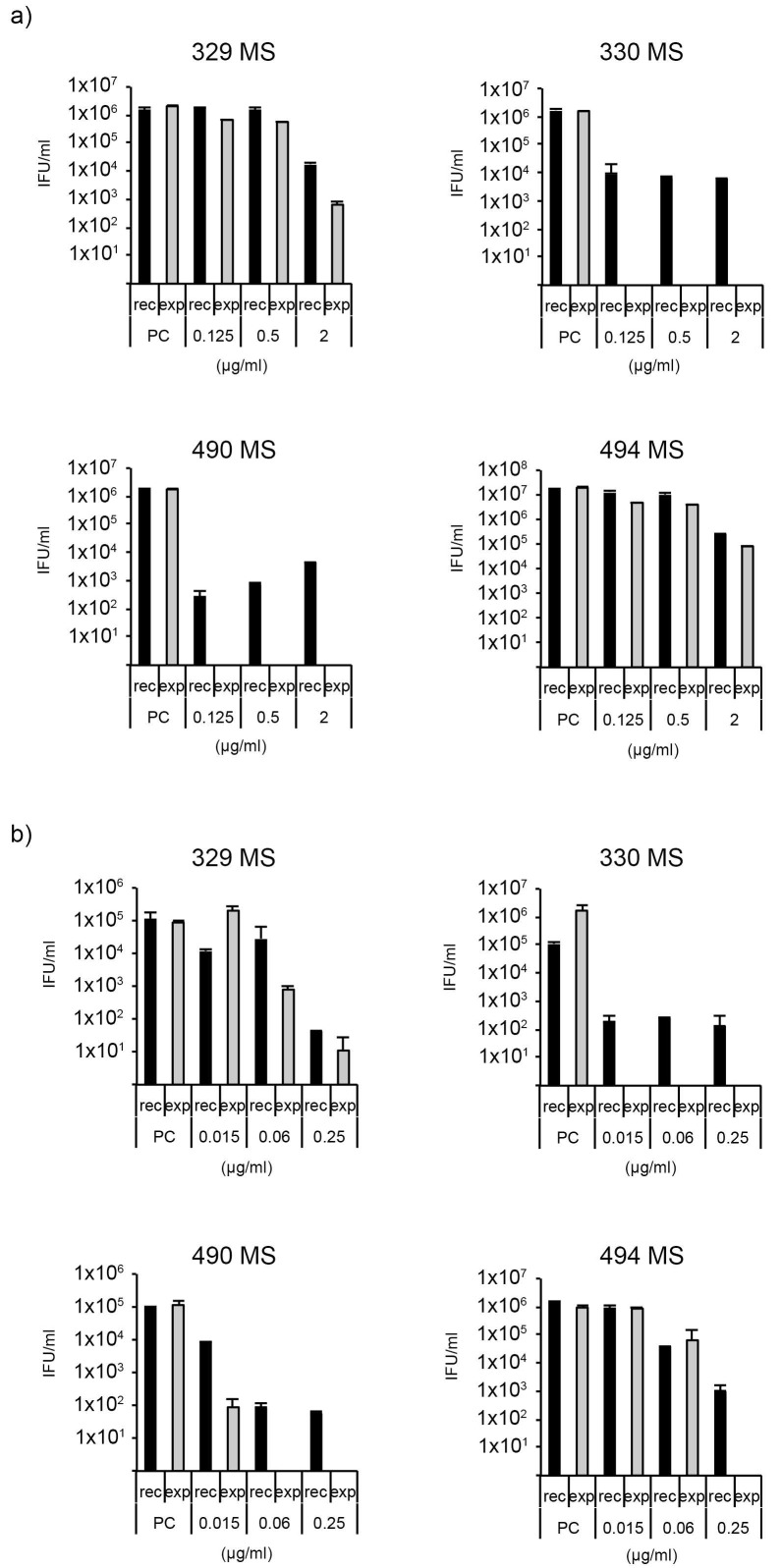
A recovery assay according to Marti et al. [18] was performed to determine the recovery from single-dose treatment with either tetracycline or doxycycline (Figure 2a,b). Briefly, all four isolates were exposed to low, moderate, and high concentrations of tetracycline (0.125, 0.5, and 2 µg/mL) or doxycycline (0.015, 0.06, and 0.25 µg/mL). After 48 h, the supernatant was either replaced with antibiotic-free (recovery, rec) or antibiotic-containing medium (continued exposure, exp), and incubated for another 48 h before samples were collected to infect fresh monolayers to measure the inclusion forming units per mL (IFU/mL).
Figure 2.
(a) Tetracycline recovery assay, and (b) doxycycline recovery assay according to Marti et al. [19]. X-axis: concentration of antimicrobials, y-axis: bars showing the number of viable elementary bodies EBs. Each recovery assay was performed once with two technical replicates. PC: positive control, rec: recovery group, exp: continuously exposed group.
The recovery assay confirmed the MIC results of tetracycline with markedly better recovery for the two TcR isolates (329 MS and 494 MS) compared to the TcS isolates (330 MS and 490 MS). Moreover, while doxycycline resistance could not be confirmed for certain isolates (MIC ≤ 4 µg/mL [37]), both MIC determination and the recovery assay indicate that the susceptibility of the TcR isolates was reduced compared to the TcS isolates.
3. Conclusions
We detected genetically diverse C. suis isolates in the herd described in this case report. Genetic diversity is consistent with previous studies [38,39,40], depicting the unprecedented diversity of the C. suis genome compared to other chlamydial species, which is strongly influenced by recombination and plasmid exchange. A broad diversity of isolates circulates within Europe, even within individual farms or within the same animal [39]. Together with studies from the USA, Switzerland, Japan, and China, this present case report on Austrian fattening pigs further illustrates a consistent diversity on a global level rather than regional clustering, even though C. suis is genetically quite diverse. This diversity has also been observed for other veterinary chlamydial species such as C. pecorum and C. psittaci [30,39,40].
While C. suis DNA was detected in the uterus of a slaughtered sow, fecal contamination during the slaughtering process could not be excluded. Difficulties in detecting Chlamydia in sows with reproductive failure are a common issue for veterinarians, who often decide to treat the sows with antibiotics regardless of the molecular findings, a strategy that was also employed in this case.
The ocular C. suis isolates were not only genetically different but also either resistant or sensitive to tetracyclines. Interestingly, both tetracycline sensitive and resistant isolates were isolated from animals that lived in the same pen, and were therefore in regular physical contact with each other during the study period, which coincides with the findings of a study investigating Swiss fattening pigs [36,39]. It could be hypothesized that resistance originates from the continued short-time application of oxytetracycline in sows at the time of insemination. The necessity of antimicrobial treatment as performed here, especially before valid susceptibility testing, is questionable, and should be viewed critically. However, it is performed in many herds where Chlamydia spp. are suspected to be involved in the fertility problems and are often the only possible choice due to the lack of anti-C. suis vaccines on the market.
A striking observation was the fact that both tetA(C)-positive isolates (329 MS and 494 MS) were tetracycline-resistant, but they only displayed decreased susceptibility to doxycycline in vitro compared to the two tetA(C)-negative, tetracycline and doxycycline sensitive isolates (330 MS and 490 MS). These findings stand in contrast to published results of Di Francesco et al., who concluded that the presence of a tetA(C)-containing genomic island is linked to both tetracycline and doxycycline resistance, but the findings are in line with another study from Germany [41]. The contrasting resistance in these two antibiotics could be explained by differences in terms of pharmacodynamics and pharmacokinetics between tetracyclines as a natural tetracycline, and doxycycline as a synthetic tetracycline [42]. The regular use of oxytetracycline, which is closer related to tetracycline than to doxycycline, in this herd over years could be a possible explanation for the differences in resistance between tetracycline and doxycycline. However, further studies are needed to investigate this phenomenon.
To estimate the resistance situation for Chlamydia, i.e., to confirm tetracycline resistance in C. suis, isolation and antibiotic susceptibility testing must be performed in addition to the detection of the tetA(C) gene by PCR, as there can be discrepancies between tetA(C) PCR results of clinical swab samples and in vitro testing for tetracycline resistance [36]. In this study, however, such discrepancies were not observed, giving a clear indication that the identified tetA(C) gene was part of C. suis and did not originate from another bacterial species.
As a final conclusion to this study, we propose to establish chlamydial cultivation as part of routine diagnostics in pigs for three reasons: First, infections with TcR C. suis isolates in the pork industry are rising [43,44,45,46], which could complicate the treatment of porcine chlamydiosis, and might even pose a threat for public health considering that transmission of C. suis to humans has been reported [36]. Second, TcR isolates cannot be conclusively identified without cultivation, because, while current molecular techniques may identify the tetA(C) gene, they cannot determine whether it originates from C. suis or another bacterial species. Finally, taking together the increasing number of TcR C. suis isolates and the inability to characterize them outside of in vitro assays, it is crucial to establish routine cultivation procedures in order to predict the clinical impact that TcR C. suis isolates may have on porcine health, a highly relevant question that has gained little attention so far, apart from case studies [44].
Acknowledgments
The authors thank the responsible farm veterinarian and the farmer for the excellent cooperation in this case. We thank Theresa Pesch and Barbara Prähauser from the Institute of Veterinary Pathology, University of Zurich, for technical help.
Author Contributions
Conceptualization, C.U. and H.M.; Investigation, C.U., L.S., A.I.-K., M.J., and R.B.; Writing—original draft preparation, C.U.; Writing—review and editing, M.J., H.M., and N.B.; Visualization, H.M. and M.J.; Supervision, H.M. and N.B.; Project Administration, C.U. All authors have read and agreed to the published version of the manuscript.
Funding
Open Access Funding by the University of Veterinary Medicine Vienna. This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Schautteet K., Vanrompay D. Chlamydiaceae infections in pig. Vet. Res. 2011;42:29. doi: 10.1186/1297-9716-42-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers D.G., Andersen A.A. Conjunctivitis caused by a swine Chlamydia trachomatis-like organism in gnotobiotic pigs. J. Vet. Diagn. Investig. 1999;11:341–344. doi: 10.1177/104063879901100408. [DOI] [PubMed] [Google Scholar]
- 3.Becker A., Lutz-Wohlgroth L., Brugnera E., Lu Z.H., Zimmermann D.R., Grimm F., Grosse Beilage E., Kaps S., Spiess B., Pospischil A. Intensively kept pigs pre-disposed to chlamydial associated conjunctivitis. J. Vet. Med. Ser. A. 2007;54:307–313. doi: 10.1111/j.1439-0442.2007.00963.x. [DOI] [PubMed] [Google Scholar]
- 4.Reinhold P., Kirschvink N., Theegarten D., Berndt A. An experimentally induced Chlamydia suis infection in pigs results in severe lung function disorders and pulmonary inflammation. Vet. Res. 2008;39:35. doi: 10.1051/vetres:2008012. [DOI] [PubMed] [Google Scholar]
- 5.Guscetti F., Schiller I., Sydler T., Heinen E., Pospischil A. Experimental enteric infection of gnotobiotic piglets with Chlamydia suis strain S45. Vet. Microbiol. 2009;135:157–168. doi: 10.1016/j.vetmic.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 6.Rogers D.G., Andersen A.A. Intestinal lesions caused by a strain of Chlamydia suis in weanling pigs infected at 21 days of age. J. Vet. Diagn. Investig. 2000;12:233–239. doi: 10.1177/104063870001200306. [DOI] [PubMed] [Google Scholar]
- 7.Turner G.V.S. A microbiological study of polyarthritis in slaughter pigs. J. S. Afr. Vet. Assoc. 1982;53:99–101. [PubMed] [Google Scholar]
- 8.Thoma R., Guscetti F., Schiller I., Schmeer N., Corboz L., Pospischil A. Chlamydiae in porcine abortion. Vet. Pathol. 1997;34:467–469. doi: 10.1177/030098589703400512. [DOI] [PubMed] [Google Scholar]
- 9.Woollen N., Daniels E.K., Yeary T., Leipold H.W., Phillips R.M. Chlamydial infection and perinatal mortality in a swine herd. J. Am. Vet. Med. Assoc. 1990;197:600–601. [PubMed] [Google Scholar]
- 10.Kauffold J., Melzer F., Berndt A., Hoffmann G., Hotzel H., Sachse K. Chlamydiae in oviducts and uteri of repeat breeder pigs. Theriogenology. 2006;66:1816–1823. doi: 10.1016/j.theriogenology.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 11.Camenisch U., Lu Z.H., Vaughan L., Corboz L., Zimmermann D.R., Wittenbrink M.M., Pospischil A., Sydler T. Diagnostic investigation into the role of Chlamydiae in cases of increased rates of return to oestrus in pigs. Vet. Rec. 2004;155:593–596. doi: 10.1136/vr.155.19.593. [DOI] [PubMed] [Google Scholar]
- 12.Eggemann G., Wendt M., Hoelzle L.E., Jäger C., Weiss R., Failing K. Zum vorkommen von chlamydien-infektionen in zuchtsauenbeständen und deren bedeutung für das fruchtbarkeitsgeschehen. DTW Dtsch. Tierarztl. Wochenschr. 2000;107:3–10. [PubMed] [Google Scholar]
- 13.Hoffmann K., Schott F., Donati M., Di Francesco A., Hässig M., Wanninger S., Sidler X., Borel N. Prevalence of chlamydial infections in fattening pigs and their influencing factors. PLoS ONE. 2015;10:e0143576. doi: 10.1371/journal.pone.0143576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borel N., Polkinghorne A., Pospischil A. A review on chlamydial diseases in animals: Still a challenge for pathologists? Vet. Pathol. 2018;55:374–390. doi: 10.1177/0300985817751218. [DOI] [PubMed] [Google Scholar]
- 15.Broes A., Taylor D.J., Martineau G.-P. Miscellaneous Bacterial Infections. In: Zimmerman J.J., Karriker L.A., Ramirez A., Schwartz K.J., Stevenson G.W., Zhang J., editors. Diseases of Swine. 11th ed. Wiley-Blackwell; Hoboken, NJ, USA: 2019. pp. 981–1001. [Google Scholar]
- 16.Angulo F.J., Collignon P., Powers J.H., Chiller T.M., Aidara-Kane A., Aarestrup F.M. World Health Organization ranking of antimicrobials according to their importance in human medicine: A critical step for developing risk management strategies for the use of antimicrobials in food production animals. Clin. Infect. Dis. 2009;49:132–141. doi: 10.1086/599374. [DOI] [PubMed] [Google Scholar]
- 17.Dugan J., Rockey D.D., Jones L., Andersen A.A. Tetracycline resistance in Chlamydia suis mediated by genomic islands inserted into the chlamydial inv-like gene. Antimicrob. Agents Chemother. 2004;48:3989–3995. doi: 10.1128/AAC.48.10.3989-3995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenart J., Andersen A.A., Rockey D.D. Growth and development of tetracycline-resistant Chlamydia suis. Antimicrob. Agents Chemother. 2001;45:2198–2203. doi: 10.1128/AAC.45.8.2198-2203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marti H., Borel N., Dean D., Leonard C.A. Evaluating the antibiotic susceptibility of Chlamydia—New approaches for in vitro assays. Front. Microbiol. 2018;9:1414. doi: 10.3389/fmicb.2018.01414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suchland R.J., Sandoz K.M., Jeffrey B.M., Stamm W.E., Rockey D.D. Horizontal transfer of tetracycline resistance among Chlamydia spp. in vitro. Antimicrob. Agents Chemother. 2009;53:4604. doi: 10.1128/AAC.00477-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dean D., Rothschild J., Ruettger A., Kandel R.P., Sachse K. Zoonotic Chlamydiaceae species associated with trachoma, Nepal. Emerg. Infect. Dis. 2013;19:1948. doi: 10.3201/eid1912.130656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Puysseleyr K., de Puysseleyr L., Dhondt H., Geens T., Braeckman L., Morré S.A., Cox E., Vanrompay D. Evaluation of the presence and zoonotic transmission of Chlamydia suis in a pig slaughterhouse. BMC Infect. Dis. 2014;14:560. doi: 10.1186/s12879-014-0560-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Puysseleyr L., de Puysseleyr K., Braeckman L., Morré S.A., Cox E., Vanrompay D. Assessment of Chlamydia suis infection in pig farmers. Transbound. Emerg. Dis. 2017;64:826–833. doi: 10.1111/tbed.12446. [DOI] [PubMed] [Google Scholar]
- 24.Reinhold P., Liebler-Tenorio E., Sattler S., Sachse K. Recurrence of Chlamydia suis infection in pigs after short-term antimicrobial treatment. Vet. J. 2011;187:405–407. doi: 10.1016/j.tvjl.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Hogan R.J., Mathews S.A., Mukhopadhyay S., Summersgill J.T., Timms P. Chlamydial persistence: Beyond the biphasic paradigm. Infect. Immun. 2004;72:1843–1855. doi: 10.1128/IAI.72.4.1843-1855.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lienard J., Croxatto A., Aeby S., Jaton K., Posfay-Barbe K., Gervaix A., Greub G. Development of a new Chlamydiales-specific real-time PCR and its application to respiratory clinical samples. J. Clin. Microbiol. 2011;49:2637–2642. doi: 10.1128/JCM.00114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blumer S., Greub G., Waldvogel A., Hässig M., Thoma R., Tschuor A., Pospischil A., Borel N. Waddlia, Parachlamydia and Chlamydiaceae in bovine abortion. Vet. Microbiol. 2011;152:385–393. doi: 10.1016/j.vetmic.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 28.Li M., Jelocnik M., Yang F., Gong J., Kaltenboeck B., Polkinghorne A., Feng Z., Pannekoek Y., Borel N., Song C., et al. Asymptomatic infections with highly polymorphic Chlamydia suis are ubiquitous in pigs. BMC Vet. Res. 2017;13:370. doi: 10.1186/s12917-017-1295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borel N., Kempf E., Hotzel H., Schubert E., Torgerson P., Slickers P., Ehricht R., Tasara T., Pospischil A., Sachse K. Direct identification of Chlamydiae from clinical samples using a DNA microarray assay: A validation study. Mol. Cell. Probes. 2008;22:55–64. doi: 10.1016/j.mcp.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Schnee C., Sachse K. DNA microarray-based detection of multiple pathogens: Mycoplasma spp. and Chlamydia spp. Methods Mol. Biol. 2015;1247:193–208. doi: 10.1007/978-1-4939-2004-4_15. [DOI] [PubMed] [Google Scholar]
- 31.Schiller I., Schifferli A., Gysling P., Pospischil A. Growth characteristics of porcine chlamydial strains in different cell culture systems and comparison with ovine and avian chlamydial strains. Vet. J. 2004;168:74–80. doi: 10.1016/S1090-0233(03)00039-X. [DOI] [PubMed] [Google Scholar]
- 32.Hartley J.C., Kaye S., Stevenson S., Bennett J., Ridgway G. PCR detection and molecular identification of Chlamydiaceae Species. J. Clin. Microbiol. 2001;39:3072–3079. doi: 10.1128/JCM.39.9.3072-3079.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sachse K., Grossmann E., Jäger C., Diller R., Hotzel H. Detection of Chlamydia suis from clinical specimens: Comparison of PCR, antigen ELISA, and culture. J. Microbiol. Methods. 2003;54:233–238. doi: 10.1016/S0167-7012(03)00040-X. [DOI] [PubMed] [Google Scholar]
- 34.Busch M., Thoma R., Schiller I., Corboz L., Pospischil A. Occurrence of Chlamydiae in the genital tracts of sows at slaughter and their possible significance for reproductive failure. J. Vet. Med. Ser. B. 2000;47:471–480. doi: 10.1046/j.1439-0450.2000.00415.x. [DOI] [PubMed] [Google Scholar]
- 35.Donati M., Balboni A., Laroucau K., Aaziz R., Vorimore F., Borel N., Morandi F., Nepita E.V., Di Francesco A. Tetracycline susceptibility in Chlamydia suis pig isolates. PLoS ONE. 2016;11:e0149914. doi: 10.1371/journal.pone.0149914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wanninger S., Donati M., Di Francesco A., Hässig M., Hoffmann K., Seth-Smith H.M.B., Marti H., Borel N. Selective pressure promotes tetracycline resistance of Chlamydia suis in fattening pigs. PLoS ONE. 2016;11:e0166917. doi: 10.1371/journal.pone.0166917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Francesco A., Donati M., Rossi M., Pignanelli S., Shurdhi A., Baldelli R., Cevenini R. Tetracycline-Resistant Chlamydia suis Isolates in Italy. Vet. Rec. 2008;163:251–252. doi: 10.1136/vr.163.8.251. [DOI] [PubMed] [Google Scholar]
- 38.Chahota R., Ogawa H., Ohya K., Yamaguchi T., Everett K.D.E., Fukushi H. Involvement of multiple Chlamydia suis genotypes in porcine conjunctivitis. Transbound. Emerg. Dis. 2018;65:272–277. doi: 10.1111/tbed.12645. [DOI] [PubMed] [Google Scholar]
- 39.Seth-Smith H.M.B., Wanninger S., Bachmann N., Marti H., Qi W., Donati M., Di Francesco A., Polkinghorne A., Borel N. The Chlamydia suis genome exhibits high levels of diversity, plasticity, and mobile antibiotic resistance: Comparative genomics of a recent livestock cohort shows influence of treatment regimes. Genome Biol. Evol. 2017;9:750–760. doi: 10.1093/gbe/evx043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joseph S.J., Marti H., Didelot X., Read T.D., Dean D. Tetracycline selective pressure and homologous recombination shape the evolution of Chlamydia suis: A recently identified zoonotic pathogen. Genome Biol. Evol. 2016;8:2613–2623. doi: 10.1093/gbe/evw182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peisker M., Berens C., Schnee C. Understanding tetracycline resistance in Chlamydia suis; Proceedings of the 5th European Meeting on Animal Chlamydiosis and Zoonotic Implications (EMAC 5); Odessa, Ukraine. 3–5 October 2018; p. 23. [Google Scholar]
- 42.Bryskier A. Tetracyclines. In: Bryskier A., editor. Antimicrobial Agents: Antibacterials and Antifungals. ASM Press; Washington, DC, USA: 2005. pp. 642–651. [Google Scholar]
- 43.Di Francesco A., Baldelli R., Cevenini R., Magnino S., Pignanelli S., Salvatore D., Galuppi R., Donati M. Seroprevalence to Chlamydiae in Pigs in Italy. Vet. Res. 2006;42:29–38. [PubMed] [Google Scholar]
- 44.Borel N., Regenscheit N., Di Francesco A., Donati M., Markov J., Masserey Y., Pospischil A. Selection for tetracycline-resistant Chlamydia suis in treated pigs. Vet. Microbiol. 2012;156:143–146. doi: 10.1016/j.vetmic.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 45.Schautteet K., de Clercq E., Miry C., van Groenweghe F., Delava P., Kalmar I., Vanrompay D. Tetracycline-resistant Chlamydia suis in cases of reproductive failure on Belgian, Cypriote and Israeli pig production farms. J. Med. Microbiol. 2013;62:331–334. doi: 10.1099/jmm.0.042861-0. [DOI] [PubMed] [Google Scholar]
- 46.Borel N., Leonard C., Slade J., Schoborg R.V. Chlamydial antibiotic resistance and treatment failure in veterinary and human medicine. Curr. Clin. Microbiol. Rep. 2016;3:10–18. doi: 10.1007/s40588-016-0028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]