Abstract
The histamine H4 receptor, belonging to the family of G-protein coupled receptors, is an increasingly attractive drug target. It plays an indispensable role in many cellular pathways, and numerous H4R ligands are being studied for the treatment of several inflammatory, allergic, and autoimmune disorders, including pulmonary fibrosis. Activation of H4R is involved in cytokine production and mediates mast cell activation and eosinophil chemotaxis. The importance of this receptor has also been shown in inflammatory models: peritonitis, respiratory tract inflammation, colitis, osteoarthritis, and rheumatoid arthritis. Recent studies suggest that H4R acts as a modulator in cancer, neuropathic pain, vestibular disorders, and type-2 diabetes, however, its role is still not fully understood.
Keywords: histamine H4 receptor, G protein-coupled receptors, allergic diseases, inflammatory diseases, autoimmune disorders, neuropathic pain, cancer
1. Introduction
Histamine action via distinct receptors (H1R–H4R) modulates diverse physiological as well as pathological processes. Due to their differential receptor pharmacology and signal transduction properties, histamine has characteristic effects dependent upon the histamine receptor subtype it is bound to. Histamine receptors H1–H4 are widespread throughout the body but there is limited knowledge about the H4R. The role of H4R in neuropathic pain transmission and other diseases is still controversial after nearly 20 years since its discovery. This may be due to biased signaling of histamine and H4 receptor agonists and differential effects on multiple signaling pathways in central and peripheral parts of the sensory nervous system. However, in the last two decades, there was a particular increment in evidence supporting participation of H3R and H4R in neuropathic pain modulation [1]. Histamine has also been identified to be responsible for a vascular type headache, e.g., migraine, hence the antihistamines are regarded as a possible treatment [2]. The proper action of particular subtypes of histamine receptors is of special importance as it has been shown for instance for the delirium syndrome in which H1R and H2R antagonists have pro-delirium potential, while H3R antagonists have proved to be beneficial in combating delirium. The H4R may also play an indirect role requiring further intensive exploration [3].
Pulmonary fibrosis is the most frequent form of interstitial lung disease. Unavailability of effective therapies has led to the urge of exploiting novel curative approaches. Histamine receptor H4 has been recognized as a new target for inflammatory and immune diseases, and H4R ligands reduced inflammation and oxidative stress in lung tissue. It has been shown that poly(ADP-ribose) polymerase (PARP-1) and H4R are both involved in inflammatory and fibrotic responses. Treatment with H4R antagonist JNJ7777120 ((5-chloro-1H-indol-2-yl)(4-methyl-1-piperazinyl)-methanone; CAS Number 459168-41-3; Molecular Weight: 277.8) in a condition of PARP-1 inhibition, provides anti-inflammatory and anti-fibrotic effects, causing reduction in airway remodeling and bronchoconstriction. Its synergistic effect with selective PARP-1 inhibitors could be of potential use for the treatment of pulmonary fibrosis [4]. Viral infections can be important contributors to development of asthma and chronic obstructive pulmonary disease. Pulmonary fibrosis is the main factor leading to pulmonary dysfunction and quality of life decline in SARS survivors. Gaining a deeper understanding of the interaction between Coronaviruses and the innate immune system of the host may shed light on the development and persistence of inflammation in the lungs and can possibly reduce the risk of lung inflammation caused by CoVs [5].
2. The Histamine Receptors—Localization and Function
Histamine receptors, numbered in the order of their discovery H1R-H4R, are G protein-coupled receptors (GPCRs) that constitute the largest family of cell surface receptors in humans and play a key role in cellular signaling. In the central nervous system (CNS), the histaminergic system is mainly modulated by histamine, an inflammatory biogenic amine involved in wide range of pathophysiological effects through interaction with histamine GPCRs which belong to class A (rhodopsin-like) GPCRs. These GPCRs differ in localization and cellular signaling mechanisms and they even differ in the level of constitutive activity, i.e., the ability to adopt an active conformation independent of ligand binding [6,7]. H1R and H2R are found in the brain and periphery, H3R is abundant in the CNS, while H4R has low expression, if any, in the CNS and is predominantly expressed on a variety of peripheral immune cells such as eosinophils, dendritic cells, mast cells (HMC-1, LAD-2, and primary cord blood derived CD34+ human mast cells), leukocytes, and T-cells (including γδT, T helper 1, 2, Th17, and CD8 cells) [6,8,9,10,11,12]. The presence and role of H4R in brain nervous tissue is yet elusive and not fully known but the presence of H4R in non-neuronal cells in the brain has been confirmed [13,14]. Functional H4 receptors that increase [35S]-GTPγS binding and/or decrease noradrenaline release have not been identified in human, guinea pig, and mouse cortex [15]. In human mast cells, H4R mediates release of cytokines, leukotrienes, and chemokines (TGF-β1, TNF-α, TNF-β, PDGF-BB, TIMP-2, M-CSF, IP-10, IL-16, IL-6, IL-3, IL-10, MIP-1α, IL-1α, ICAM-1, Eotaxin-2, RANTES, IL-8, MCP-1, and IL-6sR) [10].
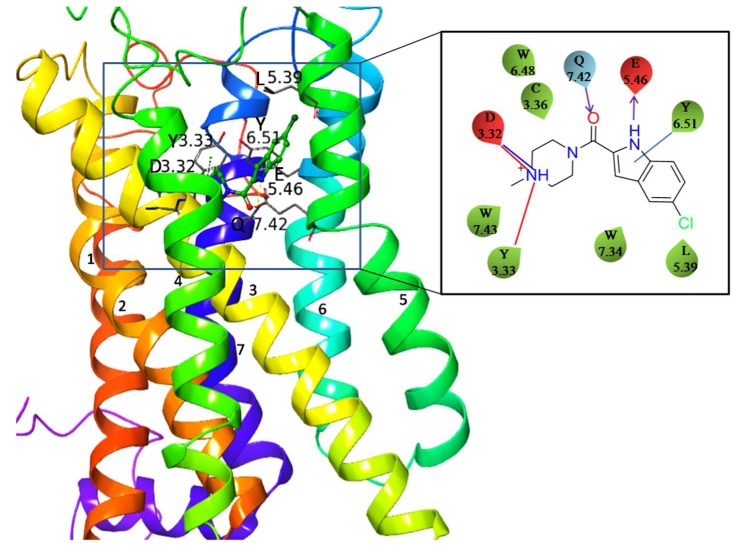
Being a member of the most populated class A of the GPCR superfamily, human H4R also contains seven transmembrane helices and a short amphipathic helix that possibly runs parallel to the cytosolic membrane surface. It consists of 390 amino acid residues possessing all of the highly conserved sequence motifs [16,17] of the class A GPCRs including the most evolutionary conserved residues in each of the transmembrane helices: N1.50, D2.50, R3.50, W4.50, P5.50, P6.50, and P7.50 (Ballesteros–Weinstein numbering [18]) indicating the same activation mechanism of H4R as that of the other receptors in class A GPCRs [19]. The Ballesteros–Weinstein numbering scheme of GPCRs provides information about the relative positions of amino acids present in seven transmembrane helices. Each residue of the receptor is recognized by two numbers separated by a dot; the first number (1–7) indicates the number of the transmembrane helix where the residue is located while the second number indicates its position in relation to the most conserved residue, assigned number 50, of the same helix. The prominent residues such as D3.32 and W7.40, specific for amine-activated GPCRs, are also present in the H4R [20]. It has been observed that the two agonists (histamine and OUP-16) exhibit complementary interactions with residues D3.32, E5.46, and T6.55, while the reference antagonist JNJ7777120 exhibits interactions with D3.32 and E5.46 only (Figure 1), implicating a differentiating role of T6.55 in ligand binding and receptor activation [21,22]. There are also striking complementarities between the H4R binding pocket and the structural properties of most H4R antagonists. They consist of a minimum of one, or preferably two, positively charged groups complementary to two negatively charged residues in the binding pocket, namely D3.32 and E5.46, and such double interaction is crucial for the interaction of high affinity ligands with H4R [21].
Figure 1.
The homology model of H4R with docked JNJ7777120 antagonist. The specific ligand–receptor interactions are shown on the right panel. D3.32 forms both a hydrogen bond and an ionic interaction with the charged amine group of the ligand.
Among the histamine receptors, H1R and H4R possess 40% amino acid identity in the transmembrane region and they recognize the same endogenous ligand that is histamine. Due to such similarity the crystal structure of H1R has been used by many researchers for building the homology models of H4R. However, there are substantial differences in histamine receptor binding sites. For instance, N4.57 in H4R is equivalent to W4.56, L5.39 to K5.39, E5.46 to N5.46, and Q7.42 to G7.42 in H1R. Additionally, the mutations of residues N4.57 and E5.46 resulted in significant alteration of inhibition constants of JNJ7777120 which was the first reported H4R antagonist [23] and the homology model of H4R featured two specific hydrogen bonds and ionic interactions of JNJ7777120 to D3.32 and E5.46 [24]. H4R has the highest sequence homology with H3R as it possesses 37% amino acid identity in protein sequence and 58% identity in the transmembrane region. It is evident that a number of ligands of H4R also have a high affinity for H3R due to the identical amino acids within the binding site of both receptors, including E5.46, Y3.33, and Y6.51, involved in ligand binding [25]. These amino acids residues contribute to the similarity between the binding sites of hH3R and hH4R forcing similar conformations of ligands. This explains the number of ligands which are antagonists of both receptors. Additionally, various substituted histamine derivatives such as R-(α)-methylhistamine have significant H4R binding in addition to H3R [6]. Istyastono et al. have shown that the E5.46Q mutation impaired the binding strength of clobenpropit and its derivatives in both those receptors [26]. Moreover, the L5.39V and E5.46Q mutations resulted in a decrease of binding of the reported ligands to H4R. This finding emphasized the importance of the E5.46 residue which provides a crucial interaction with antagonists [27].
A plethora of studies have related the heterogenic and complex pharmacology of histamine receptors to various diseases: H1R to the allergic inflammation, anaphylaxis, and motion sickness [28,29], H2R to the stimulation of gastric acid secretion leading to peptic ulcer, GERD and aspiration pneumonitis [30,31], H3R to the neurotransmission controlling sleep, cognitive processes, schizophrenia, epilepsy, and pain [32,33,34,35,36,37], and H4R to the immune responses (cancers, myocarditis) and inflammation [38,39,40,41,42] (Figure 2). The H3 and H4 receptors have relatively high affinity for histamine (5–10 nM) compared to the low affinity of H1R and H2R which is in the μM range [6,43]. Hence, the biological response has been linked directly with the local tissue histamine concentration and functional expression of different receptors [6].
Figure 2.
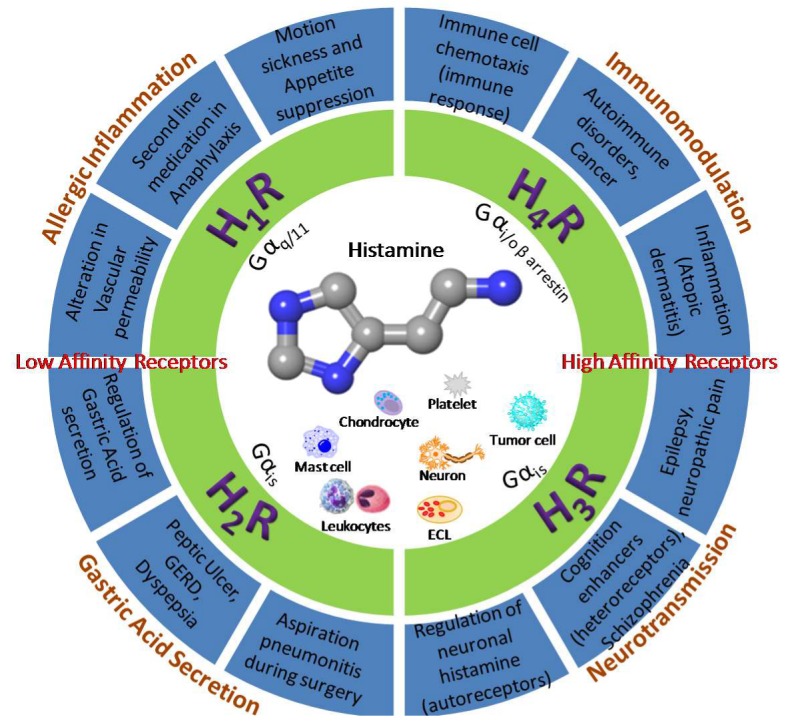
Classification of histamine receptors (H1R–H4R) in relation to their functions. H1R–H3R transduce extracellular signals via Gαq/11, Gαis, and Gαi/o, respectively, while H4R acts through Gαi/o and β-arrestin. H1R and H2R are low-affinity receptors while H3R and H4R are high-affinity receptors towards histamine. Ligands of H1R–H4R have therapeutic applications in allergic inflammation, gastric acid secretion, neurotransmission, and immunomodulation, respectively. The information in the figure is partially based on [44].
3. Species Differences of H4R
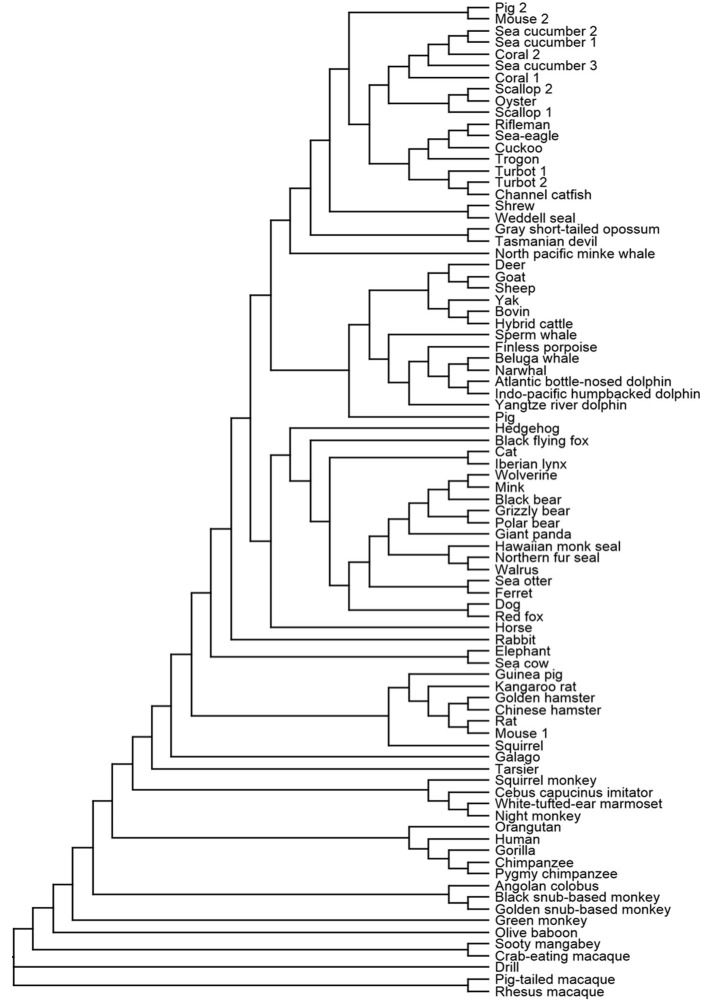
Following the identification of the human H4R (UniProt id: Q9H3N8), various sequences of mouse, rat, guinea pig, pig, dog, and monkey H4R have been reported and functionally expressed [38]. Eighty-five protein sequences of H4R orthologues from different species have been extracted from the UniProt database and aligned to draw the phylogenetic relationship between H4R orthologues (Scheme 1). The H4 receptors of the chimpanzee, gorilla, and orangutan show the highest sequence homology (98–99%) with the human orthologue (hH4R). H4 receptors of some species are highly homologous to hH4R with sequence homology between 78% and 94%, specifically those of macaques, baboon, drill, Angolan colobus, mangabey, Cebus capucinus imitator, marmoset, and Philippine tarsier (Table 1). Orthologues in some species were only moderately homologous to hH4R with sequence homology between 54% and 73% while the least homologous showed homology ranging from 10% to 47%. Pig, mouse, smooth cauliflower coral, Japanese scallop, turbot, and pig have each two H4R orthologues while sea cucumber has three orthologues. However, these orthologues, show only 10–36% homology to hH4R while all others show a substantially higher homology (>50%). As some of the sequences are still incomplete, changes in the phylogenetic tree are to be expected. Within these GPCR sequences, the typical aminergic GPCR features (D3.32 in TM3 and E5.46 in TM5) can often be found. Detailed analysis of most of these species variants is however lacking even though it could provide useful tools to dissect receptor–ligand binding. Using site-directed mutagenesis Wifling et al. have proved that the F169, located in the second extracellular loop ECL2, is a crucial amino acid for differential interactions, affinities, and potencies of certain agonists with the human and mouse H4R orthologues [45]. Receptor sequence differences have implications even for ligand function as the JNJ7777120 ligand acts as a partial inverse agonist at the human H4R, but as a partial agonist at the rat and mouse H4R which possess lower constitutive activity than their human counterpart. Therefore, differences in pharmacological activities of H4R ligands between different species might hamper preclinical development of future H4R drugs [46].
Scheme 1.
Phylogenetic tree of H4R orthologues. The sequences were obtained from UniProt [47] and the sequences were aligned with ClustalW and the cladogram was created with Clustal Omega service [48].
Table 1.
Sequence similarities of species specific H4R to the human orthologue.
| Species | Scientific Name | UniProt ID | Similarity to hH4R | |
|---|---|---|---|---|
| 1 | Human | Homo sapiens | Q9H3N8 | - |
| 2 | Chimpanzee | Pan troglodytes | H2QED2 | 99% |
| 3 | Gorilla | Gorilla | G3QS38 | 98% |
| 4 | Pygmy chimpanzee | Pan paniscus | A0A2R9BQY6 | 98% |
| 5 | Orangutan | Pongo abelii | H2NW27 | 98% |
| 6 | Crab-eating macaque | Macaca fascicularis | Q3V8G8 | 94% |
| 7 | Pig-tailed macaque | Macaca nemestrina | A0A2K6D1G7 | 94% |
| 8 | Rhesus macaque | Macaca mulatta | G7NKH9 | 94% |
| 9 | Olive baboon | Papio anubis | A0A096NGN9 | 94% |
| 10 | Drill | Mandrillus leucophaeus | A0A2K5YBZ5 | 94% |
| 11 | Angolan colobus | Colobus angolensis palliatus | A0A2K5HHL6 | 93% |
| 12 | Sooty mangabey | Cercocebus atys | A0A2K5LQL7 | 93% |
| 13 | Black snub-based monkey | Rhinopithecus bieti | A0A2K6MXG3 | 93% |
| 14 | Golden snub-based monkey | Rhinopithecus roxellana | A0A2K6RWF0 | 93% |
| 15 | Green monkey | Chlorocebus sabaeus | A0A0D9RYY4 | 90% |
| 16 | Ma’s Night monkey | Aotus nancymaae | A0A2K5CHI5 | 90% |
| 17 | Cebus capucinus imitator | Cebus capucinus imitator | A0A2K5RKQ4 | 90% |
| 18 | White-tufted-ear marmoset | Callithrix jacchus | F7IT43 | 89% |
| 19 | Squirrel monkey | Saimiri boliviensis | A0A2K6TG45 | 88% |
| 20 | Philippine tarsier | Tarsius syrichta | A0A1U7UM57 | 78% |
| 21 | Small-eared galago | Otolemur garnettii | H0WYC8 | 73% |
| 22 | Thirteen-lined ground squirrel | Ictidomys tridecemlineatus | I3MG71 | 72% |
| 23 | Dog | Canis lupus familiaris | J9P1C3 | 71% |
| 24 | Golden hamster | Mesocricetus auratus | A0A1U7Q7T1 | 71% |
| 25 | Grizzly bear | Ursus arctos horribilis | A0A3Q7WBT8 | 70% |
| 26 | Polar bear | Ursus maritimus | A0A384C2G0 | 70% |
| 27 | Pig | Sus scrofa | Q8WNV9 (Pig 1) | 70% |
| 28 | A0A5G2QV28 (Pig 2) | 10% | ||
| 29 | Red fox | Vulpes vulpes | A0A3Q7SYT7 | 70% |
| 30 | Black flying fox | Pteropus alecto | L5K5C7 | 69% |
| 31 | African elephant | Loxodonta africana | G3STF1 | 69% |
| 32 | Giant panda | Ailuropoda melanoleuca | G1M6D3 | 69% |
| 33 | Chinese hamster | Cricetulus griseus | A0A3L7I1V9 | 69% |
| 34 | Horse | Equus caballus | F6Z8L3 | 69% |
| 35 | Sea cow | Trichechus manatus latirostris | A0A2Y9E7N3 | 69% |
| 36 | Rabbit | Oryctolagus cuniculus | G1TKW6 | 68% |
| 37 | Iberian lynx | Lynx pardinus | A0A485N8M7 | 68% |
| 38 | Cat | Felis catus | M3WE71 | 68% |
| 39 | Pacific walrus | Odobenus rosmarus divergens | A0A2U3WW63 | 68% |
| 40 | Rat | Rattus norvegicus | Q91ZY1 | 68% |
| 41 | Kangaroo rat | Dipodomys ordii | A0A1S3F272 | 68% |
| 42 | Hawaiian monk seal | Neomonachus schauinslandi | A0A2Y9GRV4 | 68% |
| 43 | Northern fur seal | Callorhinus ursinus | A0A3Q7Q9W4 | 67% |
| 44 | Sea otter | Enhydra lutris kenyoni | A0A2Y9ITU9 | 67% |
| 45 | Hedgehog | Erinaceus europaeus | A0A1S3A2Y6 | 67% |
| 46 | European domestic ferret | Mustela putorius furo | M3Y4H4 | 67% |
| 47 | Mouse | Mus musculus | Q91ZY2 (Mouse 1) | 67% |
| 48 | B2ZGH2 (Mouse 2) | 66% | ||
| 49 | Goat | Capra hircus | A0A452DKI0 | 65% |
| 50 | Sheep | Ovis aries | W5PBL0 | 65% |
| 51 | Sperm whale | Physeter macrocephalus | A0A2Y9F727 | 65% |
| 52 | Hybrid cattle | Bos indicus*Bos taurus | A0A4W2DVG0 | 64% |
| 53 | Yak | Bos mutus | L8IEJ5 | 64% |
| 54 | Bovine | Bos taurus | E1BBS2 | 64% |
| 55 | Guinea pig | Cavia porcellus | Q91ZY3 | 63% |
| 56 | Black bear | Ursus americanus | A0A452QKW6 | 62% |
| 57 | Yangtze river dolphin | Lipotes vexillifer | A0A340YGS9 | 61% |
| 58 | American mink | Neovison vison | U6CNR7 | 61% |
| 59 | Beluga whale | Delphinapterus leucas | A0A2Y9PB56 | 59% |
| 60 | Yangtze finless porpoise | Neophocaena asiaeorientalis | A0A341CIF8 | 59% |
| 61 | European red deer | Cervus elaphus hippelaphus | A0A212C702 | 59% |
| 62 | Indo-pacific humpbacked dolphin | Sousa chinensis | A0A484GQ08 | 57% |
| 63 | Narwhal | Monodon monoceros | A0A4U1FGC1 | 56% |
| 64 | Wolverine | Gulo gulo | A0A3P4RYS2 | 55% |
| 65 | Atlantic bottle-nosed dolphin | Tursiops truncatus | A0A2U3V3K5 | 54% |
| 66 | Gray short-tailed opossum | Monodelphis domestica | F6QB56 | 47% |
| 67 | North-Pacific minke whale | Balaenoptera acutorostrata scammoni | A0A452C640 | 46% |
| 68 | Tasmanian devil | Sarcophilus harrisii | G3X3P1 | 45% |
| 69 | Weddell seal | Leptonychotes weddellii | A0A2U3YB28 | 42% |
| 70 | White-tailed sea-eagle | Haliaeetus albicilla | A0A091PX74 | 42% |
| 71 | Trogon | Apaloderma vittatum | A0A091NQC4 | 41% |
| 72 | Cuckoo | Cuculus canorus | A0A091G9T7 | 40% |
| 73 | Turbot | Scophthalmus maximus | A0A2U9BJT1 (Turbot 1) | 36% |
| 74 | A0A2U9C3Q1 (Turbot 2) | 36% | ||
| 75 | Channel catfish | Ictalurus punctatus | A0A2D0RQW6 | 36% |
| 76 | Chinese tree shrew | Tupaia chinensis | L8YD15 | 35% |
| 77 | Rifleman | Acanthisitta chloris | A0A091MN56 | 31% |
| 78 | Scallop | Mizuhopecten yessoensis | A0A210PRL2 (Scallop 1) | 26% |
| 79 | A0A210PS14 (Scallop 2) | 22% | ||
| 80 | Oyster | Crassostrea gigas | K1PU39 | 24% |
| 81 | Coral | Stylophora pistillata | A0A2B4RTL0 (Coral 1) | 17% |
| 82 | A0A2B4RX53 (Coral 2) | 14% | ||
| 83 | Sea cucumber | Stichopus japonicus | A0A2G8KHM7 (Sea cucumber 1) |
15% |
| 84 | A0A2G8L2L5 (Sea cucumber 2) |
13% | ||
| 85 | A0A2G8JXR8 (Sea cucumber 3) |
20% |
4. The Pharmacological Effects of H4R Ligands
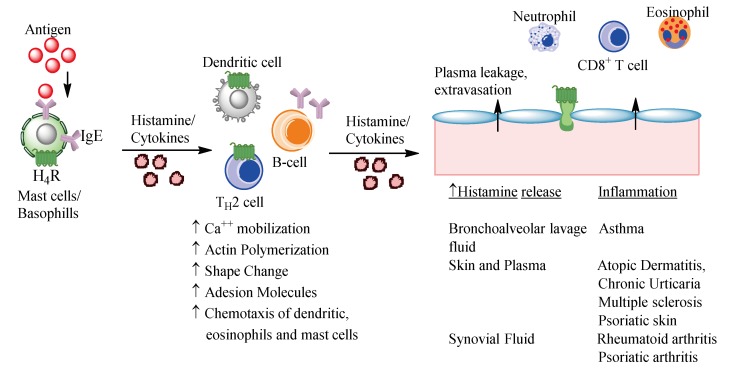
Although the pharmacology of H4R ligands is yet not fully elucidated H4R has been widely studied and reviewed since its characterization and cloning in 2000 [25,49]. The vast body of accumulating knowledge on physiological and pathophysiological functions associated with H4R modulation can be exploited for therapeutic purposes [11]. The properties of H4R make this amine receptor and its ligands of interest to specialists in the field of allergology, neurobiology, gastroenterology, endocrinology, and also to researchers of cardiovascular functions [6,50]. The results of research on the role of H4R in various pathophysiological and immunological processes indicate its association with the development and course of many diseases including a crucial role of H4R in airway and dermal inflammation (Figure 3), pruritus, ocular inflammation, arthritis, systemic lupus erythematosus, Sjogren’s syndrome, multiple sclerosis, gastric ulcer, cancer, and pain [12,51].
Figure 3.
Potential role of histamine and histamine H4R-induced recruitment of eosinophils and mast cells in chronic allergic inflammation. Histamine has been known to be a major mediator of inflammation. Histamine H4 receptors are expressed on the surface of both eosinophils and mast cells. Allergen may crosslink immunoglobulin E (IgE) on mast cells to release histamine, lipid mediators, and cytokines. Antigen is also processed by dendritic cells and macrophages for presentation to T-helper cells. During this process a local release of histamine and cytokines may occur. Histamine can act on a variety of cells and at different levels. In asthma histamine can facilitate the recruitment of inflammatory cells by regulating the chemotaxis of additional dendritic cells, eosinophils, and mast cells to the airways via the action at H4R. Histamine may additionally affect cytokine release from CD8+ cells via binding to H4R and from eosinophils, neutrophils, and mast cells through multiple histamine receptors.
4.1. Allergic Diseases
Inflammatory conditions were for a long time thought to be mediated by activation of the histamine receptor subtype 1. However, the discovery and pharmacological characterization of H4R ligands especially antagonists, (and, to a lesser extent H3R and even H2R ligands) on mast cells, eosinophils, and T cells demonstrates the possibility of its involvement in inflammatory conditions/symptoms such as atopic dermatitis (AD), asthma, allergic rhinitis, rheumatoid arthritis (RA), and pruritus in humans. This is evident from the results obtained in diverse experimental models of inflammation including hepatic ischemia-reperfusion, colitis, atopic dermatitis, in which H4R antagonists (JNJ7777120, JNJ10191584, thioperamide) proved to be efficient anti-inflammatory agents with reduced neutrophil recruitment and release of cytokines [51,52]. Preclinical and clinical data strongly suggest the regulatory involvement of H4R in the calcium influx and cellular chemotaxis [53,54], hence establishing a link between the potential therapeutic application of selectively acting H4R ligands to inflammatory conditions while also indicating involvement of H4R in diseases accompanied by itch and pain [55]. The investigations of histamine in the inflammation process have led to a development of the first highly potent and selective non-imidazole H4R antagonist JNJ7777120, followed by reexamination and synthesis of a plethora of H4R-targeted compounds [50,51].
Currently, many H4R ligands are known, synthesized, and evaluated [56,57]. Studies using selective H4R ligands in animal models of pruritus revealed a role for H4R in mediating chronic pruritus associated with conditions such as atopic dermatitis [51,58]. Antagonists of H4R (JNJ7777120, JNJ39758979, INCB38579, and others) reduced pruritus in a number of animal studies [59] as well as itching sensation in different conditions in human patients. Alcaftadine, a topical ophthalmic drug indicated for the prevention of itching associated with allergic conjunctivitis, is a potent H1R and H2R antagonist (in fact, inverse agonist) with weak inverse agonistic activity also towards H4R [60]. Administration of H1R/H4R antagonists or co-administration of H1R and H4R antagonists will probably be effective also in humans. Such antagonists are more efficacious as compared to olopatadine (H1R antagonist without H4R activity) [61]. Consequently, these studies indicate that H4R is involved in mediating pruritic responses in humans, and that H4R antagonists are ought to be effective in the treatment of pruritic histamine-mediated conditions, such as AD, acute urticaria, allergic rhinitis, or allergic conjunctivitis.
The histamine receptor H4R was also found on cartilage cells–chondrocytes [62,63]. As the presence of the histamine triggering protein (HRF) has been identified in the joints of people with RA, it seems very likely that H4R antagonists will be used in the future in the treatment of RA [64]. This receptor may also be important in the pathogenesis of Sjörgen’s syndrome, erythematous lupus erythematosus, and atopic dermatitis [65]. H4R activation not only results in phosphorylation of ERK and PI3K in a time dependent manner but it also leads to enhanced synthesis of inflammatory mediators associated with allergic reactions. It leads to inflammatory conditions as well as contributes to postinflammatory visceral hypersensitivity, thus, making H4R antagonists important for reducing inflammation and normalizing postinflammatory visceral hypersensitivity [66].
4.2. Asthma
H4R seems to be an interesting pharmacological target in the treatment of asthma [6]. Asthma is a condition typically characterized by involvement of eosinophils and mast cells [67,68,69]. Extensive studies have provided evidence detailing the functional profile of H4R in eosinophil biology [70] and in the chemotaxis and differentiation of other immune cell types. In experiments carried out on animal models of inflammation of the airways, it was observed that in mice lacking the H4R gene, there was a significant reduction in the allergic reaction caused by the administration of a chicken protein-ovalbumin [71]. Chemotaxis of eosinophils was shown to be blocked by H4R selective antagonists (JNJ7777120, JNJ39758979, or JNJ10191584) in animal asthma models due to priming and T cell activation [51,72] while induced by histamine and selective H4R agonists (e.g., 4-methylhistamine) [72]. Some selective H4R antagonists in animal models of asthma proved beneficial by mediating lung function and inflammation [51,73]. In asthma animal models, H4R antagonists act either directly by reducing the number of T cells at the site of inflammation [74] or indirectly when it is involved in dendritic cell function driving the response [51]. However, none of the H4R antagonists have been introduced to treat the above disorders.
4.3. Diabetes
The histamine receptor H4 may also be a therapeutic target in diseases not directly related to inflammation. For instance, H4R is suggested to be important in the pathogenesis of diabetes. In streptozotocin-induced diabetic rats H4R is overexpressed in tubular epithelial cells [75], and administration of a H4R antagonist resulted in a decreased blood sugar [76]. H4R participates in diabetic nephropathy progression through both a direct effect on tubular reabsorption and an indirect action on renal tissue architecture via inflammatory cell recruitment. Therefore, H4R antagonism emerges as a possible new multi-mechanism therapeutic approach to counteract development of diabetic nephropathy [77].
4.4. Parkinson’s and Alzheimer’s Diseases
Evidence about the H4R antagonist JNJ7777120 inhibiting propagation of microglial inflammation by attenuating the release of M1 microglial cells and largely preventing the pathological progression of Parkinson’s disease-like pathology and motor dysfunction has been provided by the latest research [78]. These findings support H4R as a promising novel therapeutic target for Parkinson’s disease. For Alzheimer’s disease the precise mechanism of histamine-induced Alzheimer’s pathology is not well known although the increased levels of histamine in plasma and in some areas of the brain are seen in Alzheimer’s dementia brain [79]. It is known that H3R can regulate cognitive and memory functions in the hippocampus so it could be involved in Alzheimer’s pathology [80]. Since H4R is also present in the brain and its stimulation regulates neuronal functions, then stimulating H4 receptors may have some beneficial effects in the brain of Alzheimer’s disease patients. Recently, it has been found that clobenpropit, a selective H3R antagonist with partial H4R agonist property, caused a significant reduction in amyloid-β deposits in a rat model of Alzheimer-like brain pathology. This effect was accompanied by marked reduction in neuronal or glial reactions so such dual-action compounds may have neuroprotective properties [81].
High similarity between H3R and H4R entails considerable similarity in ligand affinities and facilitates simultaneous activation of both receptors. Dual-acting H3R/H4R ligands may exhibit therapeutic potential in diverse pathological conditions, such as neuropathic pain, cancer, Parkinson’s, and inflammatory diseases [7,82]. Dual H3R/H4R imidazole containing ligands used so far includes compounds such as imetit, immepip, clobenpropit, and thioperamide [7].
4.5. Autoimmune Diseases
The characterization of a histamine receptor H4R with putative immunomodulating properties encouraged new hopes for the translational exploitation of this new therapeutic target for the still unmet medical needs, specifically asthma, autoimmune diseases, and a host defense. Rheumatoid arthritis (RA), which is a systemic autoimmune disorder, is characterized by chronic synovitis of peripheral joints, cartilage and bone destruction followed by joint disability. It was found that histamine and Th17 cytokines induced osteoclast differentiation from monocytes and JNJ7777120 decreased the osteoclastogenesis and the osteoclastogenic role of H4R has been evident in patients with RA [83]. Studies in the animal model of RA have shown that the H4R antagonist JNJ7777120 reduces the degree and severity of joint damage and reduces the number of cells producing IL-17 in the joint, thus, significantly inhibiting the inflammatory process in joints [84]. H4R involvement has been also confirmed in several types of cancers: melanoma [85], breast cancer [86], pancreatic cancer [87], and colorectal cancer [88]. H4R can regulate the aging and apoptosis of cancer cells and blocking H4R by antagonists inhibits tumor cell proliferation [86]. Histamine receptors play also an important role in the pathogenesis of multiple sclerosis. It turned out that H1R and H2R play a propathogenic role while H3R and H4R may reduce the risk of the disease [89].
5. Clinical Trials of Drug Candidates Targeting H4R
Recently, H4R research has been gaining a lot of importance and the clinical studies were initiated for the putative therapeutic exploitation in inflammatory and allergic disorders [38] such as atopic dermatitis (AD) [59,90], pruritus, asthma, rheumatoid arthritis (RA), as well as in vestibular disease (Table 2) [91]. Toreforant (JNJ38518168), the first oral H4R antagonist, has been explored for the treatment of RA patients with active disease despite concomitant methotrexate therapy (phase 2 trials, ClinicalTrials.gov database entry NCT01862224 and dose range finding study NCT01679951) [92,93]. Both studies were prematurely terminated in 2014 because of the lack of efficacy. The similar phase 2 clinical studies for the same compound evaluating efficacy and safety of toreforant in patients with symptomatic uncontrolled, persistent eosinophilic asthma (NCT01823016) [94], and in patients with moderate to severe plaque-type psoriasis (NCT02295865) [95] were completed in 2015 and 2016. In the former study toreforant (at the dose tested) failed to provide any therapeutic benefit [96]. Preclinical toxicity studies of another H4R antagonist, JNJ39758979, provided sufficient evidence of an excellent safe profile encouraging the clinical level testing [72]. JNJ39758979 was observed to mitigate RA in the collagen-induced arthritis models (CIAM) [59]. The completed phase 2 clinical trial demonstrating its safety and effectiveness in human volunteers with persistent asthma (NCT00946569) whereas several phase 1 studies stating its safety and pharmacokinetics, as well as its effect on histamine-induced itch (pruritus) (NCT01068223) in healthy male volunteers have successfully been accomplished [97,98]. Simultaneously, the two phase 2 clinical studies were initiated to find a dose range of JNJ39758979 in patients with RA despite concomitant methotrexate therapy (NCT01480388) and patients with uncontrolled asthma (NCT01493882) but they were withdrawn in 2014 and 2015, respectively, due to the same reasons [99,100]. This adverse effect was predicted to be related with reactive metabolites of JNJ39758979 and not with H4R antagonism. Hence, the significant reduction in the pruritus after JNJ39758979 administration can be concluded in the way that drug-induced agranulocytosis can be most likely an off-target effect and other H4R antagonists could be beneficial in the treatment of AD, particularly pruritus, without serious adverse effects [101]. In the similar clinical studies, another oral, potent, and selective H4R antagonist ZPL3893787 has completed phase 2 clinical trials determining its safety, efficacy, and tolerability on pruritus in adult subjects with moderate to severe AD (NCT02424253) [102] and in patients with plaque psoriasis (NCT02618616) [103] in 2016 but no results for both these studies were posted on ClinicalTrials.gov. Results showed that ZPL3893787 improved inflammatory skin lesions in patients with AD, confirming H4R antagonism as a novel therapeutic option [90]. Additionally, in two different phase 2 trials, there is an evaluation safety and efficacy of ZPL3893787 in patients with moderate to severe AD (NCT03517566) [104] and the impact of its concomitant use along with topical corticosteroids (TCS) and/or topical calcineurin inhibitors (TCI) in patients with AD (NCT03948334) [105]. The efficacy of Seliforant (SENS-111) in patients suffering from acute unilateral vestibulopathy is currently under evaluation in Phase 2 trial (NCT03110458) [106]. The above-mentioned observations indicate a wide range of potential clinical applications of H4R ligands.
Table 2.
Details of compounds which are/have been in clinical trial studies which started/ended/terminated in the 2014–2019 period.
| Compound | Clinical Indications | Phase | Status | ClinicalTrials.Gov Database Entry |
Ref. |
|---|---|---|---|---|---|
JNJ38518168 (Toreforant)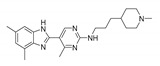
|
RA | 2 | T | NCT01862224 | [92] |
| RA | 2 | T | NCT01679951 | [93] | |
| Asthma | 2 | C | NCT01823016 | [94] | |
| Psoriasis | 2 | C | NCT02295865 | [95] | |
JNJ39758979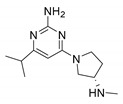
|
RA | 2 | W | NCT01480388 | [99] |
| Asthma | 2 | W | NCT01493882 | [100] | |
| ZPL3893787 (Adriforant/PF3893787/ZPL389) 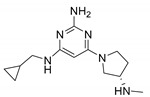
|
AD | 2 | C | NCT02424253 | [102] |
| Psoriasis | 2 | C | NCT02618616 | [103] | |
| AD | 2 | R | NCT03517566 | [104] | |
| AD | 2 | R | NCT03948334 | [105] | |
SENS-111 (Seliforant)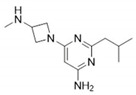
|
Unilateral Vestibulopathy | 2 | R | NCT03110458 | [106] |
Status: T: terminated; C: completed; R: recruiting; W: withdrawn.
6. Challenges and Perspectives
The H4R research triggered serious concern as to the role of histamine in the regulation of immune (patho)physiology. It has been established that JNJ7777120 acts as an antagonist in respect to G protein-dependent signaling, but it also recruits β-arrestin to the receptor in a non-G protein-dependent manner [107]. Moreover, JNJ7777120 acts as a partial inverse agonist at the human H4R but as a partial agonist at the rat and mouse H4 receptors [46], which show a lower constitutive activity than their human counterpart [45,46,108,109]. Frequently generated controversies and even in vivo misleading results in a variety of experimental models have been the repercussions of these problems [109]. The clinical development of JNJ7777120 as a prototype experimental tool was hampered due to several setbacks that surfaced over the past two decades including: localized concerns over the receptor subtypes, ligand binding and functional selectivity, constitutive and intrinsic activity and the biased signaling [6,46,50,51,95,110], its short half-life in vivo, and the hypoadrenocorticism toxicity concerns [50]. Therefore, the experimental findings on the role of H4R cannot be relied upon and need thorough investigation with caution.
Although GPCR biased signaling significantly complicates drug discovery attempts, it makes a great promise to design specific ligands with minor side effects [95,111]. The precise drugs have rapidly become the center of research for therapeutic exploitation in immunopharmacology as well as clinical immunology [90,112,113]. However, in addition to H4R, significant evidence attributes some immunomodulatory properties to H2R [90,110], thus, dissection of histamine functions in the immune system becomes indispensable. Although there are many problems in H4R research, a significant number of studies focusing on H4R provide an optimistic research perspective for this new drug target.
Author Contributions
Conceptualization, P.M. (Pakhuri Mehta) and S.F.; writing—original draft preparation, P.M. (Pakhuri Mehta); writing—review and editing, P.M. (Pakhuri Mehta), P.M. (Przemysław Miszta), P.R., O.M., P.K. and S.F.; visualization, P.M. (Pakhuri Mehta); supervision, S.F.; funding acquisition, S.F., P.R. and O.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NATIONAL SCIENCE CENTRE, POLAND, grant OPUS 2017/25/B/NZ7/02788.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Obara I., Telezhkin V., Alrashdi I., Chazot P.L. Histamine, histamine receptors, and neuropathic pain relief. Br. J. Pharmacol. 2020;177:580–599. doi: 10.1111/bph.14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Worm J., Falkenberg K., Olesen J. Histamine and migraine revisited: Mechanisms and possible drug targets. J. Headache Pain. 2019;20:30. doi: 10.1186/s10194-019-0984-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chazot P.L., Johnston L., McAuley E., Bonner S. Histamine and Delirium: Current Opinion. Front. Pharmacol. 2019;10:299. doi: 10.3389/fphar.2019.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durante M., Sgambellone S., Lanzi C., Nardini P., Pini A., Moroni F., Masini E., Lucarini L. Effects of PARP-1 Deficiency and Histamine H4 Receptor Inhibition in an Inflammatory Model of Lung Fibrosis in Mice. Front. Pharmacol. 2019;10:525. doi: 10.3389/fphar.2019.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., Pan P., Wang W., Hu D., Liu X., et al. Coronavirus infections and immune responses. J. Med. Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panula P., Chazot P.L., Cowart M., Gutzmer R., Leurs R., Liu W.L., Stark H., Thurmond R.L., Haas H.L. International union of basic and clinical pharmacology. XCVIII. Histamine receptors. Pharmacol. Rev. 2015;67:601–655. doi: 10.1124/pr.114.010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corrêa M.F., Fernandes J.P.d.S. Histamine H4 receptor ligands: Future applications and state of art. Chem. Biol. Drug Des. 2015;85:461–480. doi: 10.1111/cbdd.12431. [DOI] [PubMed] [Google Scholar]
- 8.Cataldi M., Borriello F., Granata F., Annunziato L., Marone G. Histamine receptors and antihistamines: From discovery to clinical applications. Chem. Immunol. Allergy. 2014;100:214–226. doi: 10.1159/000358740. [DOI] [PubMed] [Google Scholar]
- 9.Cowden J.M., Yu F., Banie H., Farahani M., Ling P., Nguyen S., Riley J.P., Zhang M., Zhu J., Dunford P.J., et al. The histamine H4 receptor mediates inflammation and Th17 responses in preclinical models of arthritis. Ann. Rheum. Dis. 2014;73:600–608. doi: 10.1136/annrheumdis-2013-203832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jemima E.A., Prema A., Thangam E.B. Functional characterization of histamine H4 receptor on human mast cells. Mol. Immunol. 2014;62:19–28. doi: 10.1016/j.molimm.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Schneider E.H., Seifert R. The histamine H4-receptor and the central and peripheral nervous system: A critical analysis of the literature. Neuropharmacology. 2016;106:116–128. doi: 10.1016/j.neuropharm.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Sadek B., Stark H. Cherry-picked ligands at histamine receptor subtypes. Neuropharmacology. 2016;106:56–73. doi: 10.1016/j.neuropharm.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Haas H.L., Panula P.P. Histamine receptors. Neuropharmacology. 2016;106:1–2. doi: 10.1016/j.neuropharm.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Zhu J., Qu C., Lu X., Zhang S. Activation of microglia by histamine and substance P. Cell. Physiol. Biochem. 2014;34:768–780. doi: 10.1159/000363041. [DOI] [PubMed] [Google Scholar]
- 15.Feliszek M., Speckmann V., Schacht D., von Lehe M., Stark H., Schlicker E. A search for functional histamine H4 receptors in the human, guinea pig and mouse brain. Naunyn Schmiedebergs Arch. Pharmacol. 2015;388:11–17. doi: 10.1007/s00210-014-1053-6. [DOI] [PubMed] [Google Scholar]
- 16.Filipek S. Molecular switches in GPCRs. Curr. Opin. Struct. Biol. 2019;55:114–120. doi: 10.1016/j.sbi.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Q., Yang D., Wu M., Guo Y., Guo W., Zhong L., Cai X., Dai A., Jang W., Shakhnovich E.I., et al. Common activation mechanism of class A GPCRs. eLife. 2019;8:e50279. doi: 10.7554/eLife.50279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ballesteros J.A., Weinstein H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 1995;25:366–428. [Google Scholar]
- 19.Zhu Y., Michalovich D., Wu H.-L., Tan K.B., Dytko G.M., Mannan I.J., Boyce R., Alston J., Tierney L.A., Li X., et al. Cloning, Expression, and Pharmacological Characterization of a Novel Human Histamine Receptor. Mol. Pharmacol. 2001;59:434–441. doi: 10.1124/mol.59.3.434. [DOI] [PubMed] [Google Scholar]
- 20.Huang E.S. Construction of a sequence motif characteristic of aminergic G protein–coupled receptors. Protein Sci. 2003;12:1360–1367. doi: 10.1110/ps.0305603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pappalardo M., Shachaf N., Basile L., Milardi D., Zeidan M., Raiyn J., Guccione S., Rayan A. Sequential application of ligand and structure based modeling approaches to index chemicals for their hH4R antagonism. PLoS ONE. 2014;9:e109340. doi: 10.1371/journal.pone.0109340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiss R., Noszal B., Racz A., Falus A., Eros D., Keseru G.M. Binding mode analysis and enrichment studies on homology models of the human histamine H4 receptor. Eur. J. Med. Chem. 2008;43:1059–1070. doi: 10.1016/j.ejmech.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Lim H.D., de Graaf C., Jiang W., Sadek P., McGovern P.M., Istyastono E.P., Bakker R.A., de Esch I.J., Thurmond R.L., Leurs R. Molecular determinants of ligand binding to H4R species variants. Mol. Pharmacol. 2010;77:734–743. doi: 10.1124/mol.109.063040. [DOI] [PubMed] [Google Scholar]
- 24.Kuhne S., Kooistra A.J., Bosma R., Bortolato A., Wijtmans M., Vischer H.F., Mason J.S., de Graaf C., de Esch I.J., Leurs R. Identification of Ligand Binding Hot Spots of the Histamine H1 Receptor following Structure-Based Fragment Optimization. J. Med. Chem. 2016;59:9047–9061. doi: 10.1021/acs.jmedchem.6b00981. [DOI] [PubMed] [Google Scholar]
- 25.Liu C., Ma X.-J., Jiang X., Wilson S.J., Hofstra C.L., Blevitt J., Pyati J., Li X., Chai W., Carruthers N. Cloning and pharmacological characterization of a fourth histamine receptor (H4) expressed in bone marrow. Mol. Pharmacol. 2001;59:420–426. doi: 10.1124/mol.59.3.420. [DOI] [PubMed] [Google Scholar]
- 26.Istyastono E.P., Kooistra A.J., Vischer H.F., Kuijer M., Roumen L., Nijmeijer S., Smits R.A., de Esch I.J.P., Leurs R., de Graaf C. Structure-based virtual screening for fragment-like ligands of the G protein-coupled histamine H4 receptor. Med. Chem. Comm. 2015;6:1003–1017. doi: 10.1039/C5MD00022J. [DOI] [Google Scholar]
- 27.Schultes S., Kooistra A.J., Vischer H.F., Nijmeijer S., Haaksma E.E.J., Leurs R., de Esch I.J.P., de Graaf C. Combinatorial Consensus Scoring for Ligand-Based Virtual Fragment Screening: A Comparative Case Study for Serotonin 5-HT3A, Histamine H1, and Histamine H4 Receptors. J. Chem. Inf. Model. 2015;55:1030–1044. doi: 10.1021/ci500694c. [DOI] [PubMed] [Google Scholar]
- 28.Jie Q., Kodithuwakku N.D., Yuan X., He G., Chen M., Xu S., Wu Y. Anti-allergic and anti-inflammatory properties of a potent histamine H1 receptor antagonist, desloratadine citrate disodium injection, and its anti-inflammatory mechanism on EA.hy926 endothelial cells. Eur. J. Pharmacol. 2015;754:1–10. doi: 10.1016/j.ejphar.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 29.Thangam E.B., Jemima E.A., Singh H., Baig M.S., Khan M., Mathias C.B., Church M.K., Saluja R. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front. Immunol. 2018;9:1873. doi: 10.3389/fimmu.2018.01873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shim Y.K., Kim N. The Effect of H2 Receptor Antagonist in Acid Inhibition and Its Clinical Efficacy. Korean J. Gastroenterol. Taehan Sohwagi Hakhoe Chi. 2017;70:4–12. doi: 10.4166/kjg.2017.70.1.4. [DOI] [PubMed] [Google Scholar]
- 31.van Wering H.M., Benninga M.A. Histamine-2 Receptor Antagonist in the Treatment of Gastroesophageal Reflux Disease. Springer; Berlin/Heidelberg, Germany: 2017. pp. 987–994. [DOI] [Google Scholar]
- 32.Mohsen A., Yoshikawa T., Miura Y., Nakamura T., Naganuma F., Shibuya K., Iida T., Harada R., Okamura N., Watanabe T., et al. Mechanism of the histamine H(3) receptor-mediated increase in exploratory locomotor activity and anxiety-like behaviours in mice. Neuropharmacology. 2014;81:188–194. doi: 10.1016/j.neuropharm.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Jarskog L.F., Lowy M.T., Grove R.A., Keefe R.S., Horrigan J.P., Ball M.P., Breier A., Buchanan R.W., Carter C.S., Csernansky J.G., et al. A Phase II study of a histamine H (3) receptor antagonist GSK239512 for cognitive impairment in stable schizophrenia subjects on antipsychotic therapy. Schizophr. Res. 2015;164:136–142. doi: 10.1016/j.schres.2015.01.041. [DOI] [PubMed] [Google Scholar]
- 34.Sadek B., Saad A., Latacz G., Kuder K., Olejarz A., Karcz T., Stark H., Kiec-Kononowicz K. Non-imidazole-based histamine H3 receptor antagonists with anticnvulsant activity in different seizure models in male adult rats. Drug Des. Dev. Ther. 2016;10:3879–3898. doi: 10.2147/DDDT.S116192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sadek B., Saad A., Subramanian D., Shafiullah M., Lazewska D., Kiec-Kononowiczc K. Anticonvulsant and procognitive properties of the non-imidazole histamine H3 receptor antagonist DL77 in male adult rats. Neuropharmacology. 2016;106:46–55. doi: 10.1016/j.neuropharm.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 36.Sadek B., Schwed J.S., Subramanian D., Weizel L., Walter M., Adem A., Stark H. Non-imidazole histamine H3 receptor ligands incorporating antiepileptic moieties. Eur. J. Med. Chem. 2014;77:269–279. doi: 10.1016/j.ejmech.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Eissa N., Khan N., Ojha S.K., Lazewska D., Kiec-Kononowicz K., Sadek B. The Histamine H3 Receptor Antagonist DL77 Ameliorates MK801-Induced Memory Deficits in Rats. Front. Neurosci. 2018;12:42. doi: 10.3389/fnins.2018.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu W.L. Histamine H4 receptor antagonists for the treatment of inflammatory disorders. Drug Discov. Today. 2014;19:1222–1225. doi: 10.1016/j.drudis.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Corrêa M.F., Varela M.T., Balbino A.M., Torrecilhas A.C., Landgraf R.G., Troncone L.R.P., Fernandes J.P.d.S. 1-[(2,3-Dihydro-1-benzofuran-2-yl) methyl] piperazines as novel anti-inflammatory compounds: Synthesis and evaluation on H3R/H4R. Chem. Biol. Drug Des. 2017;90:317–322. doi: 10.1111/cbdd.12947. [DOI] [PubMed] [Google Scholar]
- 40.Sterle H.A., Nicoud M.B., Massari N.A., Táquez Delgado M.A., Herrero Ducloux M.V., Cremaschi G.A., Medina V.A. Immunomodulatory role of histamine H4 receptor in breast cancer. Br. J. Cancer. 2019;120:128–138. doi: 10.1038/s41416-018-0173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lazewska D., Mogilski S., Hagenow S., Kuder K., Gluch-Lutwin M., Siwek A., Wiecek M., Kaleta M., Seibel U., Buschauer A., et al. Alkyl derivatives of 1,3,5-triazine as histamine H4 receptor ligands. Bioorg. Med. Chem. 2019;27:1254–1262. doi: 10.1016/j.bmc.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 42.Stasiak A., Gola J., Kraszewska K., Mussur M., Kobos J., Mazurek U., Stark H., Fogel W.A. Experimental autoimmune myocarditis in rats and therapeutic histamine H1–H4 receptor inhibition. J. Physiol. Pharmacol. 2018;69:889–900. doi: 10.26402/jpp.2018.6.13. [DOI] [PubMed] [Google Scholar]
- 43.Alexander S.P., Christopoulos A., Davenport A.P., Kelly E., Marrion N.V., Peters J.A., Faccenda E., Harding S.D., Pawson A.J., Sharman J.L. The Concise Guide to PHARMACOLOGY 2017/18: G protein-coupled receptors. Br. J. Pharmacol. 2017;174:S17–S129. doi: 10.1111/bph.13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tiligada E., Ennis M. Histamine pharmacology: From Sir Henry Dale to the 21st century. Br. J. Pharmacol. 2020;177:469–489. doi: 10.1111/bph.14524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wifling D., Bernhardt G., Dove S., Buschauer A. The Extracellular Loop 2 (ECL2) of the Human Histamine H4 Receptor Substantially Contributes to Ligand Binding and Constitutive Activity. PLoS ONE. 2015;10:e0117185. doi: 10.1371/journal.pone.0117185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wifling D., Löffel K., Nordemann U., Strasser A., Bernhardt G., Dove S., Seifert R., Buschauer A. Molecular determinants for the high constitutive activity of the human histamine H4 receptor: Functional studies on orthologues and mutants. Br. J. Pharmacol. 2015;172:785–798. doi: 10.1111/bph.12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.UniProt Database. [(accessed on 23 April 2020)]; Available online: https://www.uniprot.org/
- 48.Clustal Omega Service. [(accessed on 23 April 2020)]; Available online: https://www.ebi.ac.uk/Tools/msa/clustalo/
- 49.Nakamura T., Itadani H., Hidaka Y., Ohta M., Tanaka K. Molecular Cloning and Characterization of a New Human Histamine Receptor, HH4R. Biochem. Biophys. Res. Commun. 2000;279:615–620. doi: 10.1006/bbrc.2000.4008. [DOI] [PubMed] [Google Scholar]
- 50.Thurmond R.L., Venable J., Savall B., La D., Snook S., Dunford P.J., Edwards J.P. Clinical Development of Histamine H4 Receptor Antagonists. In: Hattori Y., Seifert R., editors. Histamine and Histamine Receptors in Health and Disease. Springer International Publishing; Cham, Switzerland: 2017. pp. 301–320. [DOI] [PubMed] [Google Scholar]
- 51.Thurmond R.L. The histamine H4 receptor: From orphan to the clinic. Front. Pharmacol. 2015;6:65. doi: 10.3389/fphar.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deiteren A., De Man J.G., Pelckmans P.A., De Winter B.Y. Histamine H(4) receptors in the gastrointestinal tract. Br. J. Pharmacol. 2015;172:1165–1178. doi: 10.1111/bph.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ehling S., Rossbach K., Dunston S.M., Stark H., Baumer W. Allergic inflammation is augmented via histamine H4 receptor activation: The role of natural killer cells in vitro and in vivo. J. Dermatol. Sci. 2016;83:106–115. doi: 10.1016/j.jdermsci.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 54.Grosicki M., Kieć-Kononowicz K. Human eosinophils potential pharmacological model applied in human histamine H4 receptor research. Curr. Med. Chem. 2015;22:2087–2099. doi: 10.2174/0929867322666150311151905. [DOI] [PubMed] [Google Scholar]
- 55.De Benedetto A., Yoshida T., Fridy S., Park J.E., Kuo I.H., Beck L.A. Histamine and Skin Barrier: Are Histamine Antagonists Useful for the Prevention or Treatment of Atopic Dermatitis? J. Clin. Med. 2015;4:741–755. doi: 10.3390/jcm4040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levoin N., Labeeuw O., Billot X., Calmels T., Danvy D., Krief S., Berrebi-Bertrand I., Lecomte J.M., Schwartz J.C., Capet M. Discovery of nanomolar ligands with novel scaffolds for the histamine H4 receptor by virtual screening. Eur. J. Med. Chem. 2017;125:565–572. doi: 10.1016/j.ejmech.2016.09.074. [DOI] [PubMed] [Google Scholar]
- 57.Labeeuw O., Levoin N., Billot X., Danvy D., Calmels T., Krief S., Ligneau X., Berrebi-Bertrand I., Robert P., Lecomte J.M., et al. Synthesis and evaluation of a 2-benzothiazolylphenylmethyl ether class of histamine H4 receptor antagonists. Bioorg. Med. Chem. Lett. 2016;26:5263–5266. doi: 10.1016/j.bmcl.2016.09.049. [DOI] [PubMed] [Google Scholar]
- 58.Ohsawa Y., Hirasawa N. The Role of Histamine H1 and H4 Receptors in Atopic Dermatitis: From Basic Research to Clinical Study. Allergol. Int. 2014;63:533–542. doi: 10.2332/allergolint.13-RA-0675. [DOI] [PubMed] [Google Scholar]
- 59.Savall B.M., Chavez F., Tays K., Dunford P.J., Cowden J.M., Hack M.D., Wolin R.L., Thurmond R.L., Edwards J.P. Discovery and SAR of 6-alkyl-2,4-diaminopyrimidines as histamine H(4) receptor antagonists. J. Med. Chem. 2014;57:2429–2439. doi: 10.1021/jm401727m. [DOI] [PubMed] [Google Scholar]
- 60.Chigbu D.I., Coyne A.M. Update and clinical utility of alcaftadine ophthalmic solution 0.25% in the treatment of allergic conjunctivitis. Clin. Ophthalmol. 2015;9:1215–1225. doi: 10.2147/OPTH.S63790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McLaurin E.B., Marsico N.P., Ackerman S.L., Ciolino J.B., Williams J.M., Villanueva L., Hollander D.A. Ocular itch relief with alcaftadine 0.25% versus olopatadine 0.2% in allergic conjunctivitis: Pooled analysis of two multicenter randomized clinical trials. Adv. Ther. 2014;31:1059–1071. doi: 10.1007/s12325-014-0155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grzybowska-Kowalczyk A., Maslinska D., Wojciechowska M., Szukiewicz D., Wojtecka-Lukasik E., Paradowska A., Maldyk P., Maslinski S. Expression of histamine H4 receptor in human osteoarthritic synovial tissue. Inflamm. Res. 2008;57(Suppl. 1):S63–S64. doi: 10.1007/s00011-007-0631-1. [DOI] [PubMed] [Google Scholar]
- 63.Grzybowska-Kowalczyk A., Wojtecka-Lukasik E., Maslinska D., Gujski M., Maslinski S. Human and clinical aspects of histamine. Inflamm. Res. 2007;56(Suppl. 1):S59–S60. doi: 10.1007/s00011-006-0529-3. [DOI] [PubMed] [Google Scholar]
- 64.Rzodkiewicz P., Wojtecka-Łukasik E., Maśliński S. Role of histamine in rheumatoid diseases. Reumatol. Rheumatol. 2010;48:49–53. [Google Scholar]
- 65.Yu B., Shao Y., Li P., Zhang J., Zhong Q., Yang H., Hu X., Chen B., Peng X., Wu Q., et al. Copy number variations of the human histamine H4 receptor gene are associated with systemic lupus erythematosus. Br. J. Dermatol. 2010;163:935–940. doi: 10.1111/j.1365-2133.2010.09928.x. [DOI] [PubMed] [Google Scholar]
- 66.Deiteren A., De Man J.G., Ruyssers N.E., Moreels T.G., Pelckmans P.A., De Winter B.Y. Histamine H4 and H1 receptors contribute to postinflammatory visceral hypersensitivity. Gut. 2014;63:1873–1882. doi: 10.1136/gutjnl-2013-305870. [DOI] [PubMed] [Google Scholar]
- 67.Andersson C., Tufvesson E., Diamant Z., Bjermer L. Revisiting the role of the mast cell in asthma. Curr. Opin. Pulm. Med. 2016;22:10–17. doi: 10.1097/MCP.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 68.George L., Brightling C.E. Eosinophilic airway inflammation: Role in asthma and chronic obstructive pulmonary disease. Ther. Adv. Chronic Dis. 2016;7:34–51. doi: 10.1177/2040622315609251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Metcalfe D.D., Pawankar R., Ackerman S.J., Akin C., Clayton F., Falcone F.H., Gleich G.J., Irani A.M., Johansson M.W., Klion A.D., et al. Biomarkers of the involvement of mast cells, basophils and eosinophils in asthma and allergic diseases. World Allergy Organ. J. 2016;9:7. doi: 10.1186/s40413-016-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grosicki M., Wójcik T., Chlopicki S., Kieć-Kononowicz K. In vitro study of histamine and histamine receptor ligands influence on the adhesion of purified human eosinophils to endothelium. Eur. J. Pharmacol. 2016;777:49–59. doi: 10.1016/j.ejphar.2016.02.061. [DOI] [PubMed] [Google Scholar]
- 71.Rosa A.C., Pini A., Lucarini L., Lanzi C., Veglia E., Thurmond R.L., Stark H., Masini E. Prevention of bleomycin-induced lung inflammation and fibrosis in mice by naproxen and JNJ7777120 treatment. J. Pharmacol. Exp. Ther. 2014;351:308–316. doi: 10.1124/jpet.114.215152. [DOI] [PubMed] [Google Scholar]
- 72.Thurmond R.L., Chen B., Dunford P.J., Greenspan A.J., Karlsson L., La D., Ward P., Xu X.L. Clinical and preclinical characterization of the histamine H(4) receptor antagonist JNJ-39758979. J. Pharmacol. Exp. Ther. 2014;349:176–184. doi: 10.1124/jpet.113.211714. [DOI] [PubMed] [Google Scholar]
- 73.Hartwig C., Munder A., Glage S., Wedekind D., Schenk H., Seifert R., Neumann D. The histamine H4-receptor (H4R) regulates eosinophilic inflammation in ovalbumin-induced experimental allergic asthma in mice. Eur. J. Immunol. 2015;45:1129–1140. doi: 10.1002/eji.201445179. [DOI] [PubMed] [Google Scholar]
- 74.Mahapatra S., Albrecht M., Behrens B., Jirmo A., Behrens G., Hartwig C., Neumann D., Raap U., Bahre H., Herrick C., et al. Delineating the role of histamine-1- and -4-receptors in a mouse model of Th2-dependent antigen-specific skin inflammation. PLoS ONE. 2014;9:e87296. doi: 10.1371/journal.pone.0087296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Veglia E., Grange C., Pini A., Moggio A., Lanzi C., Camussi G., Chazot P.L., Rosa A.C. Histamine receptor expression in human renal tubules: A comparative pharmacological evaluation. Inflamm. Res. 2015;64:261–270. doi: 10.1007/s00011-015-0807-z. [DOI] [PubMed] [Google Scholar]
- 76.Rosa A.C., Grange C., Pini A., Katebe M., Benetti E., Collino M., Miglio G., Bani D., Camussi G., Chazot P. Overexpression of histamine H4 receptors in the kidney of diabetic rat. Inflamm. Res. 2013;62:357–365. doi: 10.1007/s00011-012-0587-7. [DOI] [PubMed] [Google Scholar]
- 77.Pini A., Grange C., Veglia E., Argenziano M., Cavalli R., Guasti D., Calosi L., Ghè C., Solarino R., Thurmond R.L., et al. Histamine H4 receptor antagonism prevents the progression of diabetic nephropathy in male DBA2/J mice. Pharmacol. Res. 2018;128:18–28. doi: 10.1016/j.phrs.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 78.Zhou P., Homberg J.R., Fang Q., Wang J., Li W., Meng X., Shen J., Luan Y., Liao P., Swaab D.F., et al. Histamine-4 receptor antagonist JNJ7777120 inhibits pro-inflammatory microglia and prevents the progression of Parkinson-like pathology and behaviour in a rat model. Brain. Behav. Immun. 2019;76:61–73. doi: 10.1016/j.bbi.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 79.Nuutinen S., Panula P. Histamine in neurotransmission and brain diseases. Adv. Exp. Med. Biol. 2010;709:95–107. doi: 10.1007/978-1-4419-8056-4_10. [DOI] [PubMed] [Google Scholar]
- 80.Medhurst A.D., Roberts J.C., Lee J., Chen C.P., Brown S.H., Roman S., Lai M.K. Characterization of histamine H3 receptors in Alzheimer’s Disease brain and amyloid over-expressing TASTPM mice. Br. J. Pharmacol. 2009;157:130–138. doi: 10.1111/j.1476-5381.2008.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patnaik R., Sharma A., Skaper S.D., Muresanu D.F., Lafuente J.V., Castellani R.J., Nozari A., Sharma H.S. Histamine H3 Inverse Agonist BF 2649 or Antagonist with Partial H4 Agonist Activity Clobenpropit Reduces Amyloid Beta Peptide-Induced Brain Pathology in Alzheimer’s Disease. Mol. Neurobiol. 2018;55:312–321. doi: 10.1007/s12035-017-0743-8. [DOI] [PubMed] [Google Scholar]
- 82.Shan L., Bao A.M., Swaab D.F. The human histaminergic system in neuropsychiatric disorders. Trends Neurosci. 2015;38:167–177. doi: 10.1016/j.tins.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 83.Kim K.W., Kim B.M., Lee K.A., Lee S.H., Firestein G.S., Kim H.R. Histamine and Histamine H4 Receptor Promotes Osteoclastogenesis in Rheumatoid Arthritis. Sci. Rep. 2017;7:1197. doi: 10.1038/s41598-017-01101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abd-Allah A.R., Ahmad S.F., Alrashidi I., Abdel-Hamied H.E., Zoheir K.M., Ashour A.E., Bakheet S.A., Attia S.M. Involvement of histamine 4 receptor in the pathogenesis and progression of rheumatoid arthritis. Int. Immunol. 2014;26:325–340. doi: 10.1093/intimm/dxt075. [DOI] [PubMed] [Google Scholar]
- 85.Massari N.A., Medina V.A., Lamas D.J.M., Cricco G.P., Croci M., Sambuco L., Bergoc R.M., Rivera E.S. Role of H4 receptor in histamine-mediated responses in human melanoma. Melanoma Res. 2011;21:395–404. doi: 10.1097/CMR.0b013e328347ee53. [DOI] [PubMed] [Google Scholar]
- 86.Medina V.A., Brenzoni P.G., Lamas D.J., Massari N., Mondillo C., Nunez M.A., Pignataro O., Rivera E.S. Role of histamine H4 receptor in breast cancer cell proliferation. Front. Biosci. 2011;3:1042–1060. doi: 10.2741/310. [DOI] [PubMed] [Google Scholar]
- 87.Cricco G.P., Mohamad N.A., Sambuco L.A., Genre F., Croci M., Gutiérrez A.S., Medina V., Bergoc R., Rivera E., Martin G. Histamine regulates pancreatic carcinoma cell growth through H3 and H4 receptors. Inflamm. Res. 2008;57:23–24. doi: 10.1007/s00011-007-0611-5. [DOI] [PubMed] [Google Scholar]
- 88.Boer K., Helinger E., Helinger A., Pocza P., Pos Z., Demeter P., Baranyai Z., Dede K., Darvas Z., Falus A. Decreased expression of histamine H1 and H4 receptors suggests disturbance of local regulation in human colorectal tumours by histamine. Eur. J. Cell Biol. 2008;87:227–236. doi: 10.1016/j.ejcb.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 89.Saligrama N., Noubade R., Case L.K., del Rio R., Teuscher C. Combinatorial roles for histamine H1-H2 and H3-H4 receptors in autoimmune inflammatory disease of the central nervous system. Eur. J. Immunol. 2012;42:1536–1546. doi: 10.1002/eji.201141859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Werfel T. Novel systemic drugs in treatment of atopic dermatitis: Results from phase II and phase III studies published in 2017/2018. Curr. Opin. Allergy Clin. Immunol. 2018;18:432–437. doi: 10.1097/ACI.0000000000000477. [DOI] [PubMed] [Google Scholar]
- 91.Attali P., Gomeni R., Wersinger E., Poli S., Venail F. The effects of SENS-111, a new H4R antagonist, on vertigo induced by caloric test in healthy volunteers (HV) is related to plasma concentrations. Clin. Ther. 2016;38:e4. doi: 10.1016/j.clinthera.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 92.A Synovial Biopsy Study of JNJ-38518168 in Participants with Active Rheumatoid Arthritis Despite Methotrexate Therapy (TERMINATED), Database Entry NCT01862224. [(accessed on 24 May 2013)]; Available online: https://www.clinicaltrials.gov/
- 93.A Dose Range Finding Study of JNJ-38518168 in Patients with Active Rheumatoid Arthritis in Spite of Treatment with Methotrexate (TERMINATED), Database Entry NCT01679951. [(accessed on 6 September 2012)]; Available online: https://www.clinicaltrials.gov/
- 94.A Study of JNJ-38518168 in Symptomatic Adult Participants with Uncontrolled, Persistent Asthma (COMPLETED), Database Entry NCT01823016. [(accessed on 4 April 2013)]; Available online: https://www.clinicaltrials.gov/
- 95.Riddy D.M., Cook A.E., Diepenhorst N.A., Bosnyak S., Brady R., la Cour C.M., Mocaer E., Summers R.J., Charman W.N., Sexton P.M. Isoform-specific biased agonism of histamine H3 receptor agonists. Mol. Pharmacol. 2017;91:87–99. doi: 10.1124/mol.116.106153. [DOI] [PubMed] [Google Scholar]
- 96.Kollmeier A., Greenspan A., Xu X., Silkoff P., Barnathan E., Loza M., Jiang J., Zhou B., Chen B., Thurmond R. Phase 2a, randomized, double-blind, placebo-controlled, multicentre, parallel-group study of an H4R-antagonist (JNJ-39758979) in adults with uncontrolled asthma. Clin. Exp. Allergy. 2018;48:957–969. doi: 10.1111/cea.13154. [DOI] [PubMed] [Google Scholar]
- 97.Kollmeier A., Francke K., Chen B., Dunford P.J., Greenspan A.J., Xia Y., Xu X.L., Zhou B., Thurmond R.L. The histamine H(4) receptor antagonist, JNJ 39758979, is effective in reducing histamine-induced pruritus in a randomized clinical study in healthy subjects. J. Pharmacol. Exp. Ther. 2014;350:181–187. doi: 10.1124/jpet.114.215749. [DOI] [PubMed] [Google Scholar]
- 98.Kiss R., Keseru G.M. Novel histamine H4 receptor ligands and their potential therapeutic applications: An update. Expert Opin. Ther. Pat. 2014;24:1185–1197. doi: 10.1517/13543776.2014.959494. [DOI] [PubMed] [Google Scholar]
- 99.A Dose Range Finding Study of JNJ-39758979 in Patients with Active Rheumatoid Arthritis Currently Treated with Methotrexate (WITHDRAWN), Database Entry NCT01480388. [(accessed on 28 November 2011)]; Available online: https://www.clinicaltrials.gov/
- 100.Study of JNJ-39758979 in Symptomatic Adult Patients with Uncontrolled Asthma (WITHDRAWN), Database Entry NCT01493882. [(accessed on 16 December 2011)]; Available online: https://www.clinicaltrials.gov/
- 101.Murata Y., Song M., Kikuchi H., Hisamichi K., Xu X.L., Greenspan A., Kato M., Chiou C.F., Kato T., Guzzo C. Phase 2a, randomized, double-blind, placebo-controlled, multicenter, parallel-group study of a H4R-antagonist (JNJ-39758979) in Japanese adults with moderate atopic dermatitis. J. Dermatol. 2015;42:129–139. doi: 10.1111/1346-8138.12726. [DOI] [PubMed] [Google Scholar]
- 102.A Study to Determine the Efficacy of ZPL-3893787 in Subjects with Atopic Dermatitis (COMPLETED), Database Entry NCT02424253. [(accessed on 23 April 2015)]; Available online: https://www.clinicaltrials.gov/
- 103.A Study to Determine the Efficacy of ZPL-3893787 in Subjects with Plaque Psoriasis (COMPLETED), Database Entry NCT02618616. [(accessed on 1 December 2015)]; Available online: https://www.clinicaltrials.gov/
- 104.A Study to Assess the Safety and Efficacy of ZPL389 in Patients with Moderate to Severe Atopic Dermatitis (RECRUITING), Database Entry NCT03517566. [(accessed on 7 May 2018)]; Available online: https://www.clinicaltrials.gov/
- 105.A Study to Assess the Safety and Efficacy of ZPL389 with TCS/TCI in Atopic Dermatitis Patients (ZESTExt) (RECRUITING), Database Entry NCT03948334. [(accessed on 13 May 2019)]; Available online: https://www.clinicaltrials.gov/
- 106.Efficacy of SENS-111 in Patients Suffering From Acute Unilateral Vestibulopathy (RECRUITING), Database Entry NCT03110458. [(accessed on 12 April 2017)]; Available online: https://www.clinicaltrials.gov/
- 107.Rosethorne E.M., Charlton S.J. Agonist-biased signaling at the histamine H4 receptor: JNJ7777120 recruits β-arrestin without activating G proteins. Mol. Pharmacol. 2011;79:749–757. doi: 10.1124/mol.110.068395. [DOI] [PubMed] [Google Scholar]
- 108.Strasser A., Wittmann H.J., Buschauer A., Schneider E.H., Seifert R. Species-dependent activities of G-protein-coupled receptor ligands: Lessons from histamine receptor orthologs. Trends Pharmacol. Sci. 2013;34:13–32. doi: 10.1016/j.tips.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 109.Seifert R., Strasser A., Schneider E.H., Neumann D., Dove S., Buschauer A. Molecular and cellular analysis of human histamine receptor subtypes. Trends Pharmacol. Sci. 2013;34:33–58. doi: 10.1016/j.tips.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Monczor F., Fernandez N. Current knowledge and perspectives on histamine H1 and H2 receptor pharmacology: Functional selectivity, receptor crosstalk, and repositioning of classic histaminergic ligands. Mol. Pharmacol. 2016;90:640–648. doi: 10.1124/mol.116.105981. [DOI] [PubMed] [Google Scholar]
- 111.Kenakin T. Signaling bias in drug discovery. Expert. Opin. Drug Discov. 2017;12:321–333. doi: 10.1080/17460441.2017.1297417. [DOI] [PubMed] [Google Scholar]
- 112.Tiligada E., Ishii M., Riccardi C., Spedding M., Simon H.U., Teixeira M.M., Landys Chovel Cuervo M., Holgate S.T., Levi-Schaffer F. The expanding role of immunopharmacology: IUPHAR Review 16. Br. J. Pharmacol. 2015;172:4217–4227. doi: 10.1111/bph.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Riccardi C., Levi-Schaffer F., Tiligada E. Immunopharmacology and Inflammation. Springer; Berlin/Heidelberg, Germany: 2018. [Google Scholar]