Abstract
Background
Current risk prediction models in acute coronary syndrome (ACS) patients undergoing PCI are mathematically complex. This study was undertaken to assess the accuracy of a modified CHA2DS2-VASc score, comprised of easily accessible clinical factors in predicting adverse events.
Methods
The National Inpatient Sample (NIS) was queried for ACS patients who underwent PCI between 2010 and 2014. We developed a modified CHA2DS2-VASc score for risk prediction in ACS patients. Multivariate mixed effect logistic regression was utilized to study the adjusted risk for adverse outcomes based on the score. The primary outcome evaluated was in-hospital mortality. Secondary outcomes assessed were stroke, respiratory failure, acute kidney injury, all-cause bleeding, pacemaker insertion, vascular complications, length of stay and cost.
Results
There were 252,443 patients admitted with ACS included. Mean age was 62 ± 12 years. The mean CH3A2DS-VASc score was 1.6 ± 1.6. The in-hospital mortality rate was 2.5%. CH3A2DS-VASc score was highly correlated with increased rate of mortality and all secondary outcomes. ROC curve analysis for association of CH3A2DS-VASc score with mortality demonstrates that area under the curve (AUC) = 0.83 (95%C: 0.82–0.84). Stepwise increases in CH3A2DS-VASc score correlated with incremental risk, and total score was an independent predictor of mortality (adjusted OR: 1.99 (95%CI: 1.96–2.03) p < 0.001) and all secondary outcomes.
Conclusion
This study supports the applicability of the CH3A2DS-VASc score as an accurate risk prediction model for ACS patients undergoing PCI and could supplant more complicated models for quality assurance.
Keywords: CHA2DS2-VASC score, Acute coronary syndrome, Percutaneous intervention, Mortality, Risk prediction
1. Introduction
Current risk prediction models in acute coronary syndrome (ACS) patients undergoing percutaneous coronary intervention (PCI) have several purposes, including the assessment of programmatic and operator quality, and providing advice and assistance in clinical decision making [1], [2]. However, they are not often used in practice because they require knowledge of an intricate group of variables and a complex computer-based algorithm to calculate risk. Their computation requires a specialized formula incorporating numerous clinical and laboratory parameters that are not captured in some databases. In fact, the value of the models is determined entirely by the specific variables collected by the sponsoring agency or study [3], [4]. Moreover, some risk prediction models are derived in specific subsets of ACS, and are often quite dependent on cardiogenic shock to dominate the algorithm [1], [2].
The purpose of this study is to investigate the accuracy of applying the CH3A2DS-VASc score as a simple-to-use prediction model applicable to all ACS patients for in-hospital mortality and other adverse events. The modified CHA2DS2-VASC score requires a simple calculation based upon easily accessible clinical factors.
2. Methodology
This study was conducted using The National Inpatient Sample (NIS) of the Health Care Utilization Project (HCUP) sponsored by the Agency for Healthcare Research and Quality. NIS is a publicly available national registry that receives data from all USA community hospital discharges. The basic details of the registry database utilized in this study have previously been published.[5] In brief, the NIS includes administrative as well as demographic data from a 20% sample of inpatient hospitalizations in the United States. NIS provides hospitalization information for over 7 million hospital stays each year with weighted estimate of more than 35 million hospitalizations annually.
2.1. Patient population
The NIS database population is derived from patients throughout the USA in diverse practice settings. All patients aged ≥ 18 who were admitted for ACS (including unstable angina, non-Q myocardial infarction and STEMI) who underwent PCI on that admission between 2010 and 2014 were included. These patients were identified utilizing the International Classification of Diseases—Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes (Suppl. Table 1-2). Patients with atrial fibrillation or flutter were excluded. Patient records with missing values were excluded; accordingly, a complete case analysis was performed.
To optimize the original CHA2DS2-VASc score for application to PCI outcomes in an ACS population, several modifications were made after initial data analysis. At univariate analysis level, all components of the original score were predictive of increased mortality in ACS patients except for hypertension and vascular disease. Exploratory study showed that hypotension and shock correlated with highest risk for mortality and complications. Accordingly, hypertension was replaced with either hypotension or cardiogenic shock and 3 points were assigned to this factor. Similarly, history of previous stroke was less predictive of adverse outcomes, accordingly only one point was assigned for a previous history of stroke. The value of the extra points assigned were adjusted based on the assessment of model fit per each point added. The general use of VASc component of the original score was not predictive of adverse outcomes, as more than 95% of study population had a vascular disease. Accordingly, the VASc component of the original score was limited to the presence of peripheral vascular disease which was predictive of adverse outcomes. The final CH3A2DS-VASc score was calculated by assigning one point each for a prior history of congestive heart failure (CHF), age ≥ 65 years, age ≥ 75 years, diabetes mellitus, peripheral vascular disease, prior history of stroke and three points for hypotension or cardiogenic shock (max 9 points). Female gender was dropped from the model as was not significantly contributing to the score (suppl. Table 3, Suppl. Fig. 3). Patients were divided into three CH3A2DS-VASc score categories; low (0–3), intermediate (4–6) and high (7–9).
2.2. Statistical analysis
Categorical variables were compared using Pearson's chi-square (χ2) test and presented as percentages. Continuous variables with normal distribution are expressed as mean ± standard deviation and compared using ANOVA tests. Continuous variables with skewed distribution are expressed as median with inter-quartile ranges and compared by using the Wilcoxon rank-sum test.
The cost for each inpatient hospitalization was measured for each patient by multiplying the total hospital charge with cost-to-charge ratio. To obtain a standardized cost over the study period, adjusted cost for each hospitalization year was calculated in terms of the December 2014 cost after adjusting for the inflation using the Consumer Price Index inflation calculator provided by the United States Department of Labor [22]. Given the nested observations in the NIS database, binary outcomes were modeled using a mixed-effect logistic model to account for the potential correlation of observation within each hospital. A hierarchical model was formed with unique hospital identification number as random effects in the model. Potential patient-level factors including demographics, insurance type and comorbid conditions as well as hospital level factors including hospital size, teaching status and ownership were adjusted in the multivariate analysis. The length of stay and total charges for each hospitalization were modelled with mixed effect generalized linear regression model with a negative binomial function to account for over dispersion in length of stay and with gamma function to account for positive skewness of the total charges. Odd ratios with 95% confidence intervals were reported for binary outcomes and incidence rate ratio referred to as (mean ratios) with 95% confidence intervals for the numeric outcomes. Calculated mean ratio, represents the increase or decrease in percent of LOS and cost. [6], [7] For example, IRR of 1.17 for LOS represents a 17% increase in mean LOS for a given variable compared to the reference.
To test model discrimination, receiver-operating characteristic (ROC) analysis and the area under the curve (AUC) were used to identify the sensitivity and specificity of the cut-off points for mortality. The optimal cut-off value was defined by Youden’s index which provides the maximum vertical distance from the line of equality to the point [x, y] were × represents (1- specificity) and y represents sensitivity.
Internal validation of the score was evaluated by bootstrapping with 100 iterations. The differences between the mean of AUCs for the bootstrap samples and the full dataset were used to calculate the optimism of the of the original AUCs calculated for both models. The optimism is a measure for the ability of the model to perform in the original data from which it was derived versus a new test population. A low optimism score correlates with higher model performance.
Given the large sample, traditional tests would have huge power to detect minuscule deviations from the perfect fit and tend to reject the hypothesis of perfect fit, even if the model if the calibration is acceptable [8]. Accordingly, a graphical method for calibration was performed by plotting observed vs predicted rates of mortality. The calibration plot yielded an intercept that measures the extent to which predictions are either too high or too low (calibration-in-the-large) by comparing the mean of all predicted risks with the mean observed risk. An intercept value of 0 would be considered as an ideal. The slope of the calibration plot would be equal to 1.0 in the case of a perfect model. The model external application was evaluated by measuring the AUC and calibration plot in a random sample of 2000 ACS patients derived from NIS database of the year 2015. All analyses were performed using STATA 15 (Stata Corp); a p value < 0.05 was considered significant.
2.3. Outcomes
The primary outcome was all cause in-hospital mortality. Secondary outcomes were in-hospital complications including stroke, pacemaker insertion, all-cause bleeding, acute kidney injury, respiratory failure, vascular complications, length of stay and cost.
3. Results
3.1. Participants
Suppl. Fig. 1 illustrates the algorithm used in assessing inclusion of ACS patient records from the NIS database. There were 252,443 patients included. Mean age was 62.0 ± 12.7 years. Table 1 summarizes the baseline characteristics of study population including demographic information, comorbidities and hospital characteristics according to their CH3A2DS-VASc score category.
Table 1.
Comparison of baseline characteristics among ACS with PCI population as per CH3A2DS-VASc score.
| Total | Low score | Medium score | High score | p-value | |
|---|---|---|---|---|---|
| N = 252,443 | N = 221,462 | N = 28,968 | N = 2013 | ||
| CH3A2DS-Vasc score | 1.6 (1.6) | 1.1 (1.0) | 4.6 (0.7) | 7.2 (0.4) | <0.001 |
| Age in years | 62.0 (12.7) | 60.7 (12.3) | 70.7 (11.9) | 79.1 (6.7) | <0.001 |
| Indicator of sex | <0.001 | ||||
| Male | 68.9% | 70.5% | 57.9% | 50.9% | |
| Female | 31.1% | 29.5% | 42.1% | 49.1% | |
| Race | <0.001 | ||||
| White | 76.3% | 76.4% | 75.3% | 74.5% | |
| Other | 23.7% | 23.6% | 24.7% | 25.5% | |
| Diabetes Mellitus | 32.5% | 29.3% | 53.6% | 78.1% | <0.001 |
| Hypertension | 69.2% | 68.9% | 70.5% | 76.9% | <0.001 |
| Hyperlipidemia | 68.7% | 69.5% | 63.2% | 63.6% | <0.001 |
| Hypothyroidism | 8.1% | 7.6% | 11.8% | 15.1% | <0.001 |
| Obstructive sleep apnea | 5.8% | 5.7% | 6.4% | 5.0% | <0.001 |
| Obesity | 15.7% | 15.9% | 14.0% | 11.9% | <0.001 |
| History of smoking | 48.0% | 49.6% | 37.2% | 26.8% | <0.001 |
| Drug abuse | 2.6% | 2.8% | 1.5% | 0.5% | <0.001 |
| Chronic pulmonary disease | 15.4% | 14.3% | 22.7% | 26.2% | <0.001 |
| Congestive heart failure | 16.3% | 11.0% | 52.1% | 84.6% | <0.001 |
| Coronary artery disease | 90.4% | 90.4% | 89.9% | 91.4% | 0.004 |
| Peripheral vascular disorders | 8.6% | 6.2% | 24.4% | 47.5% | <0.001 |
| Priory CABG | 10.2% | 9.4% | 15.5% | 19.3% | <0.001 |
| Prior PCI | 14.9% | 14.8% | 15.9% | 15.0% | <0.001 |
| Renal failure | 10.9% | 8.8% | 24.9% | 40.4% | <0.001 |
| History of stroke | 5.4% | 3.9% | 15.5% | 28.5% | <0.001 |
| Deficiency anemias | 9.8% | 7.9% | 22.6% | 35.7% | <0.001 |
| Chronic blood loss anemia | 0.4% | 0.3% | 1.1% | 1.7% | <0.001 |
| Alcohol abuse | 3.2% | 3.3% | 2.6% | 1.0% | <0.001 |
| Liver disease | 1.1% | 1.1% | 1.4% | 1.1% | <0.001 |
| Rheumatoid arthritis/collagen vascular diseases | 2.1% | 2.0% | 2.6% | 2.7% | <0.001 |
| Solid tumor without metastasis | 0.9% | 0.8% | 1.4% | 2.1% | <0.001 |
| Metastatic cancer | 0.4% | 0.3% | 0.6% | 1.0% | <0.001 |
| Psychoses | 1.9% | 1.8% | 2.3% | 2.4% | <0.001 |
| Depression | 6.4% | 6.3% | 7.4% | 7.3% | <0.001 |
| Number of chronic conditions | 6.0 (5.0–8.0) | 6.0 (5.0–7.0) | 8.0 (6.0–10.0) | 10.0 (8.0–12.0) | <0.001 |
| Hospital: Location/teaching status | <0.001 | ||||
| Rural | 6.9% | 7.0% | 6.2% | 5.3% | |
| Urban nonteaching | 37.7% | 37.8% | 37.2% | 37.3% | |
| Urban teaching | 55.3% | 55.2% | 56.6% | 57.4% | |
| Hospital: Bed size | 0.49 | ||||
| Small | 9.0% | 9.0% | 8.9% | 8.3% | |
| Medium | 23.6% | 23.6% | 23.2% | 23.4% | |
| Large | 67.5% | 67.4% | 67.8% | 68.3% |
Data are presented as mean (SD) and median (IQR) for continuous measures, and % for categorical measures.
The mean CH3A2DS-VASc score was 1.6 ± 1.6 (range 1 to 9) (Suppl. Fig. 2). Diabetes mellitus was the most common individual component of the CH3A2DS-VASc score in the study population, and was present in 32.5% of the total population.
Peripheral vascular disease and history of stroke were the least prevalent components (8.6% and 5.4% of the total population respectively) (Table 1). Patients with CH3A2DS-VASc score ≤ 3 represented the majority of the study population and accounted for 87.7% of the total. Those with CH3A2DS-VASc score ≥ 7 were less frequent, comprising 0.8% of the total.
3.2. Primary outcome
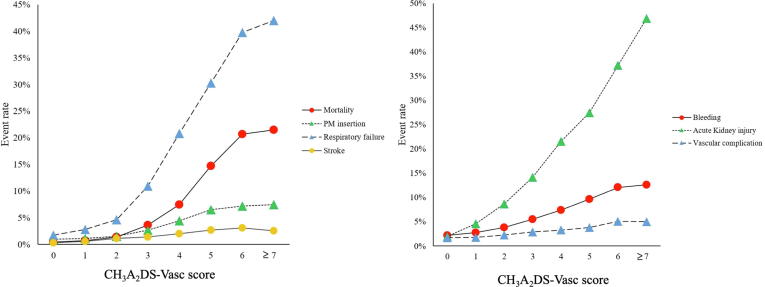
CH3A2DS-VASc score correlated significantly with increased mortality risk (Fig. 1). All cause in-hospital mortality was 21.5% among patients with high CH3A2DS-vasc score compared to 11.6% and 1.1% for medium and low scores respectively (p < 0.001) (Table 2). Using CH3A2DS-VASc score as a continuous variable, multivariate analysis showed that higher CH3A2DS-VASc score was a strong independent predictor of mortality (adjusted OR: 1.99 (95%CI: 1.96–2.03) p < 0.001) (Table 3). In subgroup analysis, similar results were observed in both STEMI and NSTEMI groups (Supp. Table 4).
Fig. 1.
Mortality and complication rates according to CH3A2DS-vasc score.
Table 2.
Rates of Mortality and complications, total charges and mean length of stay among ACS with PCI population as per CH3A2DS-VASc score.
| Total | Low score | Medium score | High score | p-value | |
|---|---|---|---|---|---|
| N = 252,443 | N = 221,462 | N = 28,968 | N = 2013 | ||
| All cause In-hospital Mortality | 2.5% | 1.1% | 11.6% | 21.5% | <0.001 |
| PM insertion | 1.9% | 1.4% | 5.4% | 7.5% | <0.001 |
| ICD defibrillator placement | 0.4% | 0.3% | 1.1% | 1.5% | <0.001 |
| All cause bleeding | 3.9% | 3.1% | 8.8% | 12.6% | <0.001 |
| Post-procedural bleeding | 0.5% | 0.4% | 1.1% | 1.7% | <0.001 |
| Post-procedural hematoma | 1.9% | 1.8% | 3.1% | 4.2% | <0.001 |
| Acute post-hemorrhagic anemia | 1.9% | 1.3% | 5.8% | 8.9% | <0.001 |
| Acute Kidney injury | 8.4% | 5.8% | 25.7% | 46.9% | <0.001 |
| Stroke | 0.9% | 0.7% | 2.3% | 2.5% | <0.001 |
| Respiratory failure | 6.7% | 3.8% | 26.5% | 42.0% | <0.001 |
| Vascular complication | 2.2% | 2.0% | 3.7% | 5.0% | <0.001 |
| Cost (USD, IQR) | 18,238 (13,787–25,327) |
17,683 (13,497–24,024) |
24,084 (16,931–37,400) |
31,146 (21,321–48,002) |
<0.001 |
| Length of stay (Days, IQR) | 3 (2–4) | 2 (2–3) | 4 (3–8) | 7 (4–11) | <0.001 |
Data are Presented as median (IQR) for continuous measures, and % for categorical measures.
Table 3.
Multivariate analysis shows CH3A2DS-Vasc score correlation with study outcomes among study population (A) and among external random sample of 2000 patients from NIS 2015 (B).
| A- Study population |
B- External sample |
|||||
|---|---|---|---|---|---|---|
| Adjusted OR / MR | 95% CI | P value | Adjusted OR / MR | 95% CI | P value | |
| Mortality | 1.99 | [1.96–2.03] | <0.001 | 2.17 | [1.74–2.71] | <0.001 |
| Stroke | 1.34 | [1.30–1.37] | <0.001 | 1.31 | [1.01–1.69] | 0.041 |
| PMI | 1.48 | [1.46–1.51] | <0.001 | 1.36 | [1.10–1.67] | <0.001 |
| ARF | 1.91 | [1.89–1.93] | <0.001 | 1.96 | [1.73–2.21] | <0.001 |
| AKI | 1.60 | [1.58–1.61] | <0.001 | 1.58 | [1.38–1.80] | <0.001 |
| Bleeding | 1.32 | [1.30–1.33] | <0.001 | 1.43 | [1.21–1.70] | <0.001 |
| Vascular | 1.15 | [1.13–1.17] | <0.001 | 1.14 | [0.93–1.40] | 0.213 |
| LOS | 1.17 | [1.17–1.18] | <0.001 | 1.15 | [1.11–1.19] | <0.001 |
| Cost | 1.11 | [1.10–1.11] | <0.001 | 1.08 | [1.05–1.10] | <0.001 |
* Calculated odd ratio was adjusted for race, gender, components of Elixhauser comorbidity index that had significant association with each outcome at univariate level, history of prior CABG or PCI, insurance type, year of admission, hospital location, bed-size and teaching status and hospital ownership.
Each component of CH3A2DS-VASC score was independently predictive of mortality. The strongest predictive factors for increased mortality risk were Hypotension/Cardiogenic shock (AOR: 17.71 (95%CI: 16.78-18.70) p < 0.001, Age ≥ 75 (AOR: 3.43 (95%CI: 3.23-3.64) p < 0.001) and congestive heart failure (AOR 3.12 (95%CI: 2.96–3.29) p < 0.001) (Suppl. Table 3).
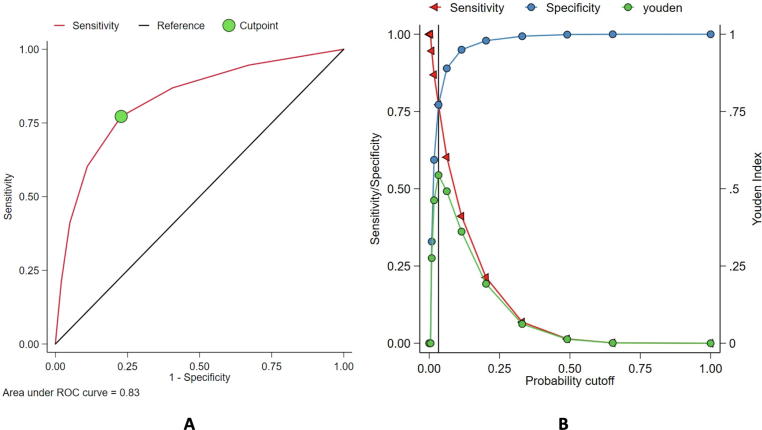
ROC curve was constructed to determine the predictive accuracy of the CH3A2DS-VASc score for in-hospital mortality. The model had excellent discrimination, with AUC = 0.83 (95%C: 0.82–0.84]) and the optimal cutoff of CH3A2DS -VASc score to predict increase in mortality was ≥ 2 where the Youden Index (J) was highest (0.54) with a sensitivity of 77% and specificity of 77% (Fig. 2).
Fig. 2.
A) Receiver operating characteristics (ROC) curve showing the relationship between sensitivity (Y-axis) and 1-specificity (X-axis) in determining the ability of CH3A2DS-vasc for predicting mortality. The area under the ROC curve (AUC) for CH3A2DS-VASc score is 0.83 (95%C: 0.82–0.84). The highest Youden index (indicated by the circle symbol) was observed for CH3A2DS-VASc score of ≥ 2, B) Intersection between specificity and sensitivity with Youden index point equal to 0.54 with a sensitivity of 77% and specificity of 77%.
3.3. Secondary outcomes
Overall, higher CH3A2DS-VASc score significantly correlated with increased risk for all secondary outcomes including, pacemaker insertion, stroke, all-cause bleeding, acute kidney injury, respiratory failure, vascular complications, length of stay and cost (Fig. 1, Suppl. Fig. 4). Table 2 summarizes the rate of each complication according to CH3A2DS-VASc score group category. On multivariate analysis after accounting for potential confounders, CH3A2DS-VASc score was an independent predictor for increased risk for respiratory failure with adjusted odd ratio (AOR): 1.91 (95%CI: 1.89–1.93), p < 0.001, AKI AOR: 1.60 (95%CI: 1.58–1.61), p < 0.001, bleeding AOR: 1.32 (95%CI: 1.30–1.33), p < 0.001, Pacemaker insertion with AOR 1.48 (95%CI: 1.46–1.51), p < 0.001, stroke with AOR 1.34 (95%CI: 1.30–1.37), p < 0.001, vascular complications AOR: 1.15 (95%CI: 1.13–1.18), p < 0.001, increased length of stay with adjusted MR 1.17 (95%CI: 1.17–1.18), p < 0.001 and increased cost with adjusted MR 1.11 (95%CI: 1.10–1.11) < 0.001. (Table 3). In subgroup analysis, similar results were seen in both STEMI and NSTEMI groups (Supp. Table 4).
4. Model validation
The model was internally validated by means of bootstrapping. After 100 iterations, the mean AUC for the boot strapped samples was 0.83 (95%CI: 0.82–0.84). The model optimism was estimated as 0.00 indicating minimal overfitting of the model to the data. The calibration analysis was on average correct with a calibration-in-the-large coefficient equal to 0, and the calibration slope was equal to 1 which correlate with a perfect model (Suppl. Fig. 5-A). The model was externally validated in a random sample of 2000 patients derived from NIS database of the year 2015. The CH3A2DS-VASc score significantly correlated with mortality with AOR 2.17 (95%CI: 1.74–2.71), p < 0.001 and had high discrimination ability with AUC of 0.84 (95%CI: 0.77–0.91). In addition, the score correlated significantly with all other study outcomes except for vascular complications (Table 3). The calibration analysis was on average correct with a calibration-in-the-large coefficient equal to < 0.01, and the calibration slope was equal to 1, which closely correlates with a “perfect” model. (Suppl. Fig. 5-B)
5. Discussion
In this study, a modified CHA2DS2-VASc score independently predicted in-hospital mortality and procedural adverse outcomes in a linear fashion. Its predictive accuracy is as accurate as more complex registry models but does not require a computerized algorithm to calculate.
The clinical applicability of this simple-to-calculate risk prediction model allows for early risk stratification of ACS patients undergoing PCI relying on readily accessible clinical factors to compute a risk score without need for complicated formulas or laboratory findings. One of the most important advantages of this score over other models is its strong predictive power in non-mortality outcomes, including bleeding, respiratory failure and acute kidney injury.
Physicians and patients can accurately estimate procedural outcomes and predict adverse events using easily obtainable clinical variables with minimal interpretation prior to any invasive procedure. Additionally, the score may be useful as an initial appraisal of clinical quality, using variables available in non-clinical databases. Moreover, since these variables are not easily “gamed”, the score will have some degree of independence from biased observation. For these reasons, this CH3A2DS-VASc score provides a simple-to-calculate algorithm in clinical practice which therefore provides advantages over more complicated and similarly precise algorithms [1], [2].
The CHA2DS2-VASc score was originally validated to predict the risk of stroke in atrial fibrillation (AF) population, but is actually a simple calculation predictive of vascular disease. In general, CHA2DS2-VASc has been utilized with various success rates as a predictive tool for adverse outcomes in different clinical settings [9], [10], [11]. Despite the high success of CHA2DS2-VASc score in predicting vascular complications and overall adverse effects, its use among the PCI population has been with limited study populations. Abugroun et al. investigated the applicability of the CHA2DS2-VASc score in ACS population in a wide sample derived from the NIS and concluded that the score correlated with adverse outcomes. However, when individually analyzed, the original score components were not all predictive of adverse outcomes. For instance, hypertension was predictive of lower odds for mortality and vascular disease had no significant association with adverse outcomes. Accordingly, the original score had lower discriminative power with AUC 0.58 [12].
Prediction of in-hospital mortality post PCI has been the subject of many risk prediction models, including Mayo Clinic Risk Score (MCRS) and New York State Risk Score (NYSRS). Such models were to a large extent based on multiple clinical, laboratory and angiographic factors. [13], [14], [15]. The published AUC of all of these models are in the 0.75–0.80 ranges. Despite the lack of laboratory variables including creatinine in the CH3A2DS-VASc score, the constellation of variables available in the score seems to be at least equivalent to the sum of most variables in currently validated risk models. In addition, CH3A2DS-VASc score is based only on risk factor variables, which makes it more generalizable and less variable. In particular, prediction is made without reference to angiographic or PCI procedural variables, which of course is only known once the procedure is undertaken. Suppl. Table 5 summarizes the components and applicability of commonly used risk prediction models in ACS population. Each component of CH3A2DS-VASc was independently predictive of mortality.
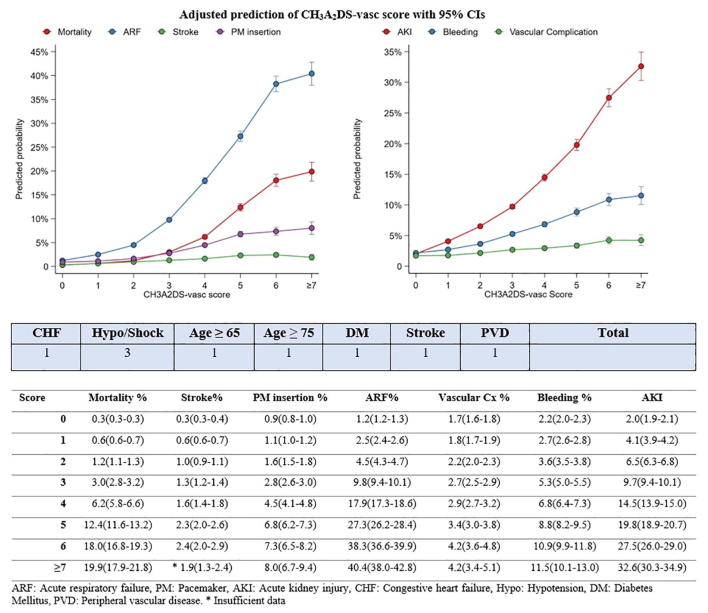
Based on the CH3A2DS-VASc we developed a nomogram that can easily be used on bedside to calculate the adjusted predicted risk for mortality and adverse outcomes for all ACS patients undergoing PCI. (Fig. 3) To demonstrate the easy applicability of the score, a 56-year-old male with history of diabetes mellitus, and heart failure who developed acute coronary event complicated with hypotension and underwent PCI would have a total CH3A2DS-VASc score of 5 which would correlate with adjusted predicted risk of mortality equal to 12.4%. Risk for ARF 27.3%, stroke 2.3%, need for PM insertion 6.8%, AKI 19.8%, bleeding 8.8% and vascular complication of 3.4%. In contrast, a young 40-year-old female with no prior medical history if admitted for ACS event and underwent PCI, will have a CH3A2DS-VASc score of only 0 with predicted adjusted all-cause mortality is 0.3%, ARF 1.2%, Stroke 0.3%, PMI 0.9%, AKI 2%, bleeding event 2.2% and vascular complication 1.7%. These examples demonstrate the easy applicability of the score for risk stratification for ACS patients undergoing PCI.
Fig. 3.
Nomogram for calculation of adjusted predicted risk for each complication based on each patient calculated CH3A2DS-VASc score.
The NYSRS is basically a three-structured model that identified cardiogenic shock, index hospitalization congestive heart failure (CHF) and age as important prognostic factors for in-hospital mortality [14]. In the current study, age and CHF are independent predictors of post PCI in hospital mortality. Since pre-PCI cardiogenic shock is a well-validated poor outcome predictor, it was similarly incorporated in the computation of the model. In addition, this study shows that CH3A2DS-VASc score also predicts bleeding risk, which was not tested as an outcome in any other risk prediction models.
Eagle et al (11) tested a simple risk prediction model (GRACE-6-month) for predicting 6 months risk for mortality in patients with ACS. Although GRACE-6-month model is generalizable since it was based on a large population group within GRACE registry, however, the model was focused towards prediction of long-term mortality and was not tested against other ACS complications. In addition, the model used variables including degree of heart failure and cardiac arrest on admission unlike CH3A2DS-VASc score, which is mainly based on age and baseline comorbidities and development of hypotension or cardiogenic shock. Inclusion of cardiac arrest and new onset heart failure of course raises predictive accuracy of survival but depends on subjective interpretation and adds mathematical complexity to the calculation of risk.
The ACUITY-PCI (Acute Catheterization and Urgent Intervention Triage Strategy-Percutaneous Coronary Intervention) Risk Score is another relatively recent score was developed and validated by Palmerini et al. in 2012. [16] Factors contributed to the ACUITY-PCI score included insulin-treated diabetes, renal insufficiency; degree of ST segment elevation, level of cardiac biomarker elevation as well as the extent of coronary artery disease. While ACUITY-PCI score predicted adverse events, however, it was only applicable to patients with unstable angina or NSTEMI. Similar to MCRS, it included angiographic variables and kidney function as an important predictor. The absence of these two variables might be a limitation to the CH3A2DS-VASc score, but it allows the score to be more useful pre-PCI in order to risk stratify all patients planned for intervention regardless of the clinical setting or coronary anatomy, which can be taken into consideration post procedure.
Most of the other validated risk score for the evaluation of clinical outcome after PCI (clinical SYNTAX, SYNTAX II, National Cardiovascular Database Registry CathPCI, and the ACEF [age, creatinine, ejection fraction] model) are less widely used in clinical daily practice because of the necessity of complex calculation and substantial inter-observer and intra-observer variability [17], [18], [2], [19]. Similarly, while TIMI score has proven efficiency on risk stratification of patients with chest pain with potential ACS, the score requires a combination of history information together with EKG and laboratory data, which adds to its complexity.
Orvin et al. demonstrated that the CH3A2DS-VASc score calculated at the time of the index PCI was accurate in predicting adverse outcomes, including all-cause mortality and mortality or myocardial infarction after PCI. [20] However, short-term in-hospital outcomes were not assessed, and its predictive capability would be different in each acuity level presentation. In this study, the modified CHA2DS2-VASc score was demonstrated not just to predict mortality but also to have high predictive ability for various short-term adverse outcomes in all ACS patients. It can be used prospectively, allowing for its wider use and implication to predict both short and long-term post-PCI outcomes.
The impact of CHA2DS2-VASC score on the likelihood of developing acute kidney injury has not been well explored. Kurtul et all described the significant association between the CHA2DS2-VASC score and contrast-induced nephropathy (CIN) in patients with ACS who underwent emergent PCI [21]. In this study, a significant correlation and a linear relation between modified CHA2DS2-VASc and post PCI acute kidney injury was demonstrated regardless of the kidney injury etiology or the urgency of the intervention.
6. Limitations
The conduct of this analysis has several limitations. The study was retrospective. The use of ICD codes may have led to inaccuracies in estimating diagnosis of certain comorbidities and complications. To improve the accuracy, a set of ICD codes that were previously validated in previous studies was utilized. In addition, the accuracy of the analysis is dependent on the accuracy of the data collected in the database. Given the limitation of the NIS database and lack of laboratory, radiological information, it was not possible to compare the predictive results obtained by our model to other validated risk scores. It is highly recommended to externally validate this risk model in a patient population and compare obtained results from the CH3A2DS-VASc score to other widely used models.
7. Conclusion
CH3A2DS-VASc score is a highly significant predictor of adverse events, including in-hospital mortality and several other procedural-related complications, in ACS patients undergoing PCI. This study supports its applicability as an accurate risk prediction model for ACS patients undergoing PCI, and could supplant more complicated models for purposes of quality assurance and clinical application. One of the most important advantages of this score is its strong predictive power in non-mortality outcomes, including bleeding, vascular complications, and acute kidney injury.
Disclosure
None of the investigators have any conflicts of interest to declare.
CRediT authorship contribution statement
Ashraf Abugroun: Conceptualization, Methodology, Resources, Formal analysis, Visualization, Writing - original draft. Abdalla Hassan: Methodology, Writing - review & editing. Safwan Gaznabi: Writing - review & editing. Hakeem Ayinde: Writing - review & editing. Ahmed Subahi: Writing - review & editing. Mohammed Samee: Methodology, writing - review & editing. Adhir Shroff: Methodology, Writing - review & editing. Lloyd W. Klein: Supervision, Methodology, Writing - review & editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100532.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Risk adjustment for in-hospital mortality of contemporary patients with acute myocardial infarction: The Acute Coronary Treatment and Intervention Outcomes Network (ACTION) Registry®–Get With The Guidelines (GWTG)TM acute myocardial infarction mortality model and risk score - ScienceDirect. https://www.sciencedirect.com/science/article/pii/S0002870310009038. Accessed November 16, 2018. [DOI] [PubMed]
- 2.Peterson E.D., Dai D., DeLong E.R. Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the National Cardiovascular Data Registry. J. Am. Coll. Cardiol. 2010;55(18):1923–1932. doi: 10.1016/j.jacc.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein L.W., Maroney J. Risk-Adjusted Models of 30-Day Mortality Following Coronary Intervention. JACC Cardiovasc. Interv. 2013;6(6):623–624. doi: 10.1016/j.jcin.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Klein L.W., Ho K.K.L., Singh M. Quality assessment and improvement in interventional cardiology: A position statement of the society of cardiovascular angiography and interventions, Part II: Public reporting and risk adjustment. Catheter. Cardiovasc. Interv. 2011;78(4):493–502. doi: 10.1002/ccd.23153. [DOI] [PubMed] [Google Scholar]
- 5.HCUPnet. HCUPnet. https://hcupnet.ahrq.gov. Accessed November 13, 2018.
- 6.Kumar A.J., Henzer T., Rodday A.M., Parsons S.K. Risk factors for length of stay and charge per day differ between older and younger hospitalized patients with AML. Cancer Med. 2018;7(6):2744–2752. doi: 10.1002/cam4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adejumo A.C., Akanbi O., Pani L. Among inpatients, ischemic bowel disease predisposes to Clostridium difficile infection with concomitant higher mortality and worse outcomes. Eur. J. Gastroenterol. Hepatol. 2019;31(1):109–115. doi: 10.1097/MEG.0000000000001273. [DOI] [PubMed] [Google Scholar]
- 8.Paul P., Pennell M.L., Lemeshow S. Standardizing the power of the Hosmer-Lemeshow goodness of fit test in large data sets. Stat Med. 2013;32(1):67–80. doi: 10.1002/sim.5525. [DOI] [PubMed] [Google Scholar]
- 9.Conrotto F., D’Ascenzo F., D’Onofrio A. Predictive ability of the CHADS2 and CHA2DS2-VASc scores for stroke after transcatheter aortic balloon-expandable valve implantation: an Italian Transcatheter Balloon-Expandable Valve Implantation Registry (ITER) sub-analysis. Eur. J. Cardiothorac. Surg. 2016;50:867–873. doi: 10.1093/ejcts/ezw199. [DOI] [PubMed] [Google Scholar]
- 10.Chua S.-K., Lo H.-M., Chiu C.-Z., Shyu K.-G. Use of CHADS2 and CHA2DS2-VASc Scores to Predict Subsequent Myocardial Infarction, Stroke, and Death in Patients with Acute Coronary Syndrome: Data from Taiwan Acute Coronary Syndrome Full Spectrum Registry. PLOS ONE. 2014;9(10) doi: 10.1371/journal.pone.0111167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CHA2 DS2 -VASc score and adverse outcomes in patients with heart failure with reduced ejection fraction and sinus rhythm. [DOI] [PMC free article] [PubMed]
- 12.Ashraf Abugroun, Abdalla Hassan, Safwan Gaznabi. Abstract 12425: CHA2DS2-VASc Score is Highly Predictive of In-Hospital Mortality and Procedural Complications in Acute Coronary Syndrome (ACS) Undergoing Percutaneous Coronary Intervention (PCI) Circulation. 2018;138(Suppl_1) doi: 10.1161/circ.138.suppl_1.12425. A12425–A12425. [DOI] [Google Scholar]
- 13.Singh M., Rihal C.S., Lennon R.J., Spertus J., Rumsfeld J.S., Holmes D.R. Bedside estimation of risk from percutaneous coronary intervention: the new Mayo Clinic risk scores. Mayo Clin. Proc. 2007;82(6):701–708. doi: 10.4065/82.6.701. [DOI] [PubMed] [Google Scholar]
- 14.Negassa A., Monrad E.S., Bang J.Y., Srinivas V.S. Tree-structured risk stratification of in-hospital mortality after percutaneous coronary intervention for acute myocardial infarction: a report from the New York State percutaneous coronary intervention database. Am. Heart J. 2007;154(2):322–329. doi: 10.1016/j.ahj.2007.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amin L.Z., Amin H.Z., Nasution S.A., Panggabean M., Shatri H. The New Mayo Clinic Risk Score Characteristics in Acute Coronary Syndrome in Patients Following Percutaneous Coronary Intervention. J. Tehran. Heart Cent. 2017;12(4):149–154. [PMC free article] [PubMed] [Google Scholar]
- 16.Palmerini T., Genereux P., Caixeta A. A New Score for Risk Stratification of Patients With Acute Coronary Syndromes Undergoing Percutaneous Coronary Intervention: The ACUITY-PCI (Acute Catheterization and Urgent Intervention Triage Strategy-Percutaneous Coronary Intervention) Risk Score. JACC Cardiovasc. Interv. 2012;5(11):1108–1116. doi: 10.1016/j.jcin.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Farooq V., Vergouwe Y., Räber L. Combined anatomical and clinical factors for the long-term risk stratification of patients undergoing percutaneous coronary intervention: the Logistic Clinical SYNTAX score. Eur. Heart J. 2012;33(24):3098–3104. doi: 10.1093/eurheartj/ehs295. [DOI] [PubMed] [Google Scholar]
- 18.Farooq V., van Klaveren D., Steyerberg E.W. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet Lond. Engl. 2013;381(9867):639–650. doi: 10.1016/S0140-6736(13)60108-7. [DOI] [PubMed] [Google Scholar]
- 19.Wykrzykowska J.J., Garg S., Onuma Y. Value of age, creatinine, and ejection fraction (ACEF score) in assessing risk in patients undergoing percutaneous coronary interventions in the “All-Comers” LEADERS trial. Circ. Cardiovasc. Interv. 2011;4(1):47–56. doi: 10.1161/CIRCINTERVENTIONS.110.958389. [DOI] [PubMed] [Google Scholar]
- 20.Orvin K., Bental T., Assali A., Lev E.I., Vaknin-Assa H., Kornowski R. Usefulness of the CHA2DS2-VASC Score to Predict Adverse Outcomes in Patients Having Percutaneous Coronary Intervention. Am. J. Cardiol. 2016;117(9):1433–1438. doi: 10.1016/j.amjcard.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Kurtul A., Yarlioglues M., Duran M. Predictive Value of CHA2DS2-VASC Score for Contrast-Induced Nephropathy After Percutaneous Coronary Intervention for Acute Coronary Syndrome. Am. J. Cardiol. 2017;119(6):819–825. doi: 10.1016/j.amjcard.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 22.CPI Home : U.S. Bureau of Labor Statistics [Internet]. [cited 2018 Nov 18]. Available from: https://www.bls.gov/cpi/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.