Abstract
Computer-generated characters, so-called avatars, are widely used in advertising, entertainment, human–computer interaction or as research tools to investigate human emotion perception. However, brain responses to avatar and human faces have scarcely been studied to date. As such, it remains unclear whether dynamic facial expressions of avatars evoke different brain responses than dynamic facial expressions of humans. In this study, we designed anthropomorphic avatars animated with motion tracking and tested whether the human brain processes fearful and neutral expressions in human and avatar faces differently. Our fMRI results showed that fearful human expressions evoked stronger responses than fearful avatar expressions in the ventral anterior and posterior cingulate gyrus, the anterior insula, the anterior and posterior superior temporal sulcus, and the inferior frontal gyrus. Fearful expressions in human and avatar faces evoked similar responses in the amygdala. We did not find different responses to neutral human and avatar expressions. Our results highlight differences, but also similarities in the processing of fearful human expressions and fearful avatar expressions even if they are designed to be highly anthropomorphic and animated with motion tracking. This has important consequences for research using dynamic avatars, especially when processes are investigated that involve cortical and subcortical regions.
Keywords: face, emotion, avatar, computer-generated character, fMRI
Introduction
While we are becoming more experienced with computer-generated characters, or avatars, that are used in animated films, social media or as human–computer interfaces, we do not know how we react and adapt to interactions with our virtual counterparts (Kätsyri et al., 2017; Hsu, 2019). The use of avatars in entertainment and commercial settings is accompanied by an increasing use of avatars to investigate emotion perception since avatars enable highly standardized experiments that resemble real-life social situations (Zaki and Ochsner, 2009; Crookes et al., 2015; de Borst and de Gelder, 2015). As a result, facial expressions of avatars have been shown to influence human decision making and cooperative behavior, which is crucial for the commercial use of avatars (Choi et al., 2012; de Melo et al., 2011a, 2011b; Scherer and Von Wangenheim, 2014). Hence, it seems vital to investigate the underpinnings of human behavior and associated brain processes during interactions with human-like avatars (Cross, Hortensius, et al., 2019; Epley et al., 2008). In the present study, we investigate whether brain activation differs in response to dynamic human facial expressions and dynamic avatar facial expressions, and if so, which brain regions show activation differences.
Central to the processing of facial expressions and facial identity is a distributed network of brain regions that responds more strongly to human faces than to other visual information (Dricu and Frühholz, 2016; Fernández Dols and Russell, 2017). In this neural face perception network, the posterior and anterior superior temporal sulcus (STS) as well as the inferior frontal gyrus (IFG) form a dorsal pathway sensitive to dynamic features of faces like facial motion and gaze. Conversely, the inferior occipital gyrus, the fusiform gyrus (FG) and the anterior temporal lobe comprise the ventral pathway where invariant features of faces like form and configuration are processed (Haxby et al., 2000; Duchaine and Yovel, 2015).
Based on the functional characterization of these brain regions for face perception, one may hypothesize that differences in brain responses to dynamic human and avatar expressions depend on the specific functions of these pathways. Thus, the ventral pathway, which is tuned to invariant facial features, may respond equally to anthropomorphic avatar faces and their human counterparts. Especially the FG may show equal responses to human and avatar faces, given its importance in the holistic processing of the facial form, independent of motions and emotions. On the other hand, the dorsal pathway may be activated differently by dynamic human and avatar facial expressions. Computer-generated faces often lack subtle dynamic features, such as expression-related wrinkles. Since dorsal regions are mainly engaged in the processing of facial motion, it is plausible that the STS and the IFG show stronger responses to dynamic human expressions compared to dynamic avatar expressions.
In addition to the cortical pathways discussed above, previous research has identified a subcortical route that is particularly involved in the processing of emotional facial expressions (Haxby et al., 2000; Vuilleumier, 2005). This subcortical face processing route is formed by the amygdala, together with the pulvinar and the superior colliculus and may precede responses to dynamic human expressions in the ventral temporal cortex (Johnson, 2005; Méndez-Bértolo et al., 2016). It has been proposed that this rapid subcortical processing is made possible by a magnocellular channel to the amygdala that is tuned to low-spatial frequency input. Typically, low-spatial frequency input provides information about coarse stimulus features like the configuration or form of a face. Conversely, a slower parvocellular channel to face-sensitive cortical regions is attuned to high-spatial frequency information in faces. This fine-grained parvocellular input thus provides slow but high-resolution information about local features of faces like expression-related wrinkles (Vuilleumier et al., 2003; Kumar and Srinivasan, 2011; Dima et al., 2018).
Although the exact functional role of the subcortical route in face perception remains controversial (Pessoa and Adolphs, 2010; McFadyen et al., 2017), it is assumed that it enables the fast detection of fear- or threat-related environmental signals in the absence of slower cortical processing (LeDoux, 2000; Adolphs, 2001; Vuilleumier et al., 2003). To date, no study has investigated whether the subcortical route that conveys low-spatial frequency information to the amygdala also mediates the processing of dynamic human and avatar expressions. It is thus not known whether amygdala responses to dynamic human and avatar expressions differ from cortical responses. In general, we would assume that human and avatar faces both entail coarse low-spatial frequency information about face configuration and form activating the amygdala. However, the composition and the range of the spatial frequency spectrum of avatar faces may depend on their level of elaboration (e.g. detectable wrinkles or not) and thus may differ from human faces with a broad spatial frequency spectrum.
Given the increasing use of avatars, several behavioral and imaging studies have investigated processing differences between human and avatar facial expressions. On a behavioral level, previous studies have shown that facial expressions are reliably recognized in both static and dynamic human and avatar faces (Dyck et al., 2008; Gutiérrez-Maldonado et al., 2014). On a neural basis, however, ventral and dorsal regions of the face perception network (Haxby et al., 2000; Duchaine and Yovel, 2015) showed stronger responses to static human expressions than to static avatar expressions (Moser et al., 2007; James et al., 2015; Kätsyri et al., 2020). More precisely, the FG, the STS and the IFG were more activated by static human than avatar expressions (Moser et al., 2007; James et al., 2015; Kätsyri et al., 2020). Former results on amygdala responses to human and avatar facial expressions are mixed. Whereas two studies comparing static emotional expressions in human and avatar faces found no significant differences in amygdalar responses (Moser et al., 2007; Kätsyri et al., 2020), another study using neutral pictures of human and cartoon faces showed a stronger response of the amygdala to human faces (James et al., 2015).
These results indicate that human and avatar facial expressions are not processed in the same way in both dorsal and ventral regions of the face perception network. Moreover, there is also a processing difference between cortical and subcortical regions that may be attributed to their differential sensitivity to certain ranges of spatial frequency. Yet, those results have been obtained using static facial expressions. So, virtually nothing is known about the differential processing of dynamic human and avatar facial expressions. To help closing this gap, we assessed brain responses to dynamic facial expressions of actors and their customized avatar look-alikes, which have been developed for this study. During the acquisition of functional MRI data, participants watched short videos of fearful and neutral expressions of the actors and avatars. By asking our participants to rate the intensity of the presented expressions within 2 weeks after the scanning session, we were able to investigate whether the intensity of the expressions also influences brain activation.
Based on previous results with static avatar expressions (Moser et al., 2007; James et al., 2015; Kätsyri et al., 2020) and the role of the dorsal pathway for dynamic information in face perception (Haxby et al., 2000; Duchaine and Yovel, 2015), we expected the STS and the IFG to show stronger responses to dynamic human facial expressions than to dynamic avatar facial expressions. Furthermore, we presumed that the processing difference between dynamic human and avatar faces should be larger for fearful expressions than for neutral expressions. We expected such an interaction effect to be present in the STS and the IFG because those regions are sensitive to dynamic features of facial expressions, which characterize fearful more than neutral expressions.
Materials and methods
Participants
We recruited 30 healthy participants aged between 18 and 62 years (16 female; Mage = 39.98 years; SDage = 12.38 years) who reported no history of psychiatric or neurological disorders. During data collection, we had to exclude four participants from the final analyses for various reasons including excessive movement, vigilance problems and discomfort (see Supplementary material). This resulted in a final sample of 26 participants aged between 18 and 62 years (13 female; Mage = 40.64 years; SDage = 12.24 years). Ethical approval for this study was obtained by the local ethics committee and subjects were only tested after given their written informed consent in line with the Declaration of Helsinki.
Stimuli
We used a set of videos that have been developed for the study in a three-step process in cooperation with the Zurich University of the Arts: (i) fearful and neutral human facial expressions were recorded from four actors, (ii) for each actor, a customized avatar was created by a graphic artist (Pinterac SARL, France) to match their appearance (see Figure 1), (iii) by motion tracking with 66 tracking points (FaceRig©, Holotech Studios SRL, Romania), the actors’ recorded expressions were conveyed onto the avatar faces (see Supplementary material). For each actor and each avatar, eight fearful and eight neutral videos were shown during the scanning session, resulting in a total of 128 videos, each lasting 3 s.
Fig. 1.

Depiction of the female actors on the upper left and the male actors on the lower left. Their respective avatars are shown on the right.
In addition, we constructed 128 scrambled versions of these videos by using a 40 × 45 grid and randomly scrambling the grid squares with MATLAB 2017a. Thus, we obtained a set of videos containing dynamic information equivalent to the videos with fearful expressions, but without emotional or facial attributes. This condition enabled us to test whether BOLD responses associated with dynamic fearful expressions can be ascribed to the mere processing of low-level dynamic visual information. The set of scrambled videos was divided into two subsets of 64 videos each and one of the two subsets was assigned pseudo-randomly (in the sequence of scanning) to each participant.
Procedure
Before scanning, participants were familiarized with the fMRI task. They were instructed that they would see videos of human and avatar faces showing fearful or neutral expressions and videos with scrambled patterns. They were told to watch the displayed faces or scrambled patterns. Occasionally, a video with a red square centered on the displayed face or scrambled pattern would appear to which they would have to respond with a button press. This task was used to ensure participants’ attention. The simplicity of the task, however, also ensured that the participants could naturally track the videos. Participants completed two functional runs while passively viewing 32 videos of fearful expressions (16 human, 16 avatar), 32 videos of neutral expressions (16 human, 16 avatar), 32 scrambled videos and 8 videos with a red square (control trials). This resulted in a total of 104 trials per run and 208 trials per session. The total number of button presses and the response times were recorded. Trials were randomly presented in an event-related design with jittered intertrial intervals (see Figure 2). After scanning, participants were reimbursed with 30 Swiss Francs and reminded that they were asked to rate the videos of human and avatar expressions within 2 weeks in an online rating survey according to their intensity. Each survey contained on average 16 videos showing fearful human expressions, 16 videos showing fearful avatar expressions and two videos showing neutral human expressions, as well as two videos showing neutral avatar expressions as a control condition (see Supplementary material). After each video, the intensity of the facial expression had to be rated on a scale from 1 (not very intense) to 6 (extremely intense).
Fig. 2.

Exemplary depiction of the procedure during the two functional runs. After a fixation cross, a video showing either a human or an avatar facial expression, a scrambled video or a control video was displayed. Participants were instructed to passively watch the videos and to respond with a button press when a video with a red square appeared. After each video a black screen was shown.
MRI acquisition and processing
Apparatus and acquisition parameters
Stimuli were presented using Cogent 2000 (version: 1.32) under MATLAB 2015a. Videos were displayed via a back-projection that was visible by a mirror on the head coil (visual angle of the faces: 8.5° vertical, 7° horizontal). MRI scans were obtained on a 3 Tesla Philips Achieva scanner (Philips Medical Systems, Best, The Netherlands) using a 32-channel head coil. We acquired structural images covering the whole brain using a T1-weighted MPRAGE sequence with the following parameters: TR = 8.1 ms, TE = 3.7 ms, slices = 176 sagittal slices, voxel size = 1 × 1 × 1 mm, matrix size = 240 × 164 mm, FOV = 240 × 240 mm, flip angle = 8°, no fat suppression, total acquisition time = 05:37. To track BOLD responses in the whole brain, we used an EPI sequence with 32 sequential ascending axial slices co-planar to the AC–PC line and an interslice gap of 0.4 mm (TR = 1800 ms, TE = 30 ms, voxel size = 2.75 × 2.75 × 3.5 mm, matrix size = 80 × 82 mm, FOV = 222 × 222 mm, flip angle = 75°, total acquisition time = 14:22). The first 10 volumes of each run were discarded by the scanner to allow the equilibration of T1 saturation effects so that in total 467 volumes per run were acquired.
MRI preprocessing
We analyzed functional data using SPM12 (version 6906; Wellcome Trust Centre for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm/) on MATLAB 2017a. Functional images were realigned to the first image in the series, corrected for slice timing to the middle slice using sinc interpolation, and the mean functional image was co-registered to the individual anatomical image. Next, the anatomical scans were segmented into different tissue types and normalized to the Montreal Neurological Institute template using DARTEL (Ashburner, 2007). During this step, a mean anatomical template for the whole group was generated. Functional images were resampled to a voxel size of 2 × 2 × 2 mm and spatially smoothed (8 mm full-width half-maximum Gaussian kernel). After pre-processing, the realignment parameters were reviewed and participants moving >2 mm in either x-, y- or z-direction were excluded from further analyses.
Imaging analysis
Statistical analyses at the first level were performed with a general linear model across the whole brain. High-pass temporal filtering was set to a cut off of 128 s to filter out low-frequency noise. Individual trials were modeled with the SPM12 default canonical hemodynamic response function defined by the onset and duration of the videos. We modeled the following conditions as regressors of interest: condition face type (Human > Avatar), condition facial expression (Fear > Neutral), as well as condition scramble (non-scrambled > scrambled). Control trials were also modeled as regressors of interest but excluded for group-level analyses. To minimize false-positive findings related to task-correlated motion, realignment parameters were included as regressors of no interest.
To obtain results at group level, we analyzed first-level contrast images with one-sample t-tests. Median rating differences per participant were included as covariates to account for potential BOLD response differences due to dissimilar intensity levels of the videos (see section behavioral analysis). As one participant had not completed the rating survey, the median rating difference of the other participants was used as a substitute. Brain activation patterns bigger than a cluster extent of k = 5 and remaining under a voxel-wise FWE corrected P-value of <0.05 will be presented in the results section.
Defining regions of interest
In addition to the whole-brain analysis, a region-of-interest (ROI) analysis was performed in order to limit the analysis to groups of voxels that have been shown to be functionally coherent in previous studies. The following independent ROIs were a priori defined based on previous literature and using the Neuromorphometrics atlas implemented in SPM12 (http://www.neuromorphometrics.com/): the FG, the STS, the IFG and the amygdala. Based on the group-level statistical parametric maps, average ROI signals were extracted and compared for the different conditions using MarsBaR (Brett et al., 2002). The resulting t-test outcomes were considered significant if they were below a Bonferroni-corrected statistical threshold of P < 0.05.
Behavioral analysis
We examined the intensity ratings of human and avatar facial expressions with the exact Wilcoxon test using SPSS (Version 23). First, we analyzed median differences between ratings of fearful and neutral facial expressions separately for human and avatar videos. Second, we investigated median differences between ratings of fearful human and fearful avatar facial expressions as well as median differences between ratings of neutral human and neutral avatar facial expressions. To test for rating differences associated with the gender of the displayed character, we computed median differences between videos with male and female characters separately for human and avatar videos (see Supplementary material).
Results
Behavioral responses
To control subject’s task engagement during the scanning session, participants were required to respond with a button press to infrequent presented control videos with a red square centered on the displayed face or scrambled pattern. Average detection rate of control videos was nearly perfect (99%) with a median response time of 754 ± 246 ms.
The statistical analysis of the rating of fearful and neutral videos showed that fearful human expressions were rated as more intense (Mdn = 5) than neutral human expressions (Mdn = 2; exact Wilcoxon test: z = −4.47, P < 0.001). The same difference was observable for avatar faces, with fearful avatar expressions rated as more intense (Mdn = 3) in comparison to neutral avatar expressions (Mdn = 2; exact Wilcoxon test: z = −3.16, P < 0.001). Fearful human expressions were judged as more intense (Mdn = 5) than fearful avatar expressions (Mdn = 3; exact Wilcoxon test: z = −4.31, P < 0.001). No significant difference was apparent for neutral human and neutral avatar expressions (exact Wilcoxon test: z = −1.19, P = 0.244). Analysis results of the rating of female versus male faces are shown in Supplementary Table S1.
BOLD response to human and avatar facial expressions
First, we contrasted fearful human and avatar facial expressions across the whole brain. Fearful human expressions elicited stronger responses in the posterior and anterior STS, the anterior insular cortex (aIC), as well as in the posterior (PCC) and ventral anterior cingulate cortex (vACC; see Table 1 and Figure 3). The analysis of the a priori defined independent ROIs revealed that fearful human expressions elicited stronger responses than fearful avatar expressions in bilateral STS (left: T = 4.75, pcorr < 0.001, right: T = 5.36, pcorr < 0.001) and bilateral IFG (left: T = 3.14, pcorr = 0.022, right: T = 2.81, pcorr = 0.047). Second, we contrasted neutral human and avatar expressions with one another. They did not evoke significantly different responses in either the whole-brain analysis or the ROI analysis. The interaction contrast face type (human versus avatar) × facial expression (fearful versus neutral) revealed that the right STS showed a larger response difference between human and avatar faces for fearful expressions than for neutral expressions (ROI analysis: T = 2.8, pcorr = 0.050, see Figure 4 for distribution of beta estimates per condition and Figure 5 for a direct comparison of the whole-brain activation cluster in bilateral STS).
Table 1.
Clusters showing a significant difference in BOLD response to fearful human expressions compared to fearful avatar expressions in the second level whole-brain analysis
| Brain area | Side | k | MNI coordinates | T-value | P-FWE | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| STS, posterior | R | 50 | 60 | −36 | 6 | 6.69 | 0.009 |
| 64 | −46 | 8 | 6.29 | 0.020 | |||
| STS, anterior | R | 23 | 56 | 2 | −20 | 7.27 | 0.003 |
| 52 | 8 | −24 | 6.49 | 0.013 | |||
| STS, anterior | L | 21 | −58 | −10 | −12 | 6.87 | 0.006 |
| −58 | −2 | −14 | 5.93 | 0.040 | |||
| Cingulate gyrus, posterior | R | 5 | 2 | −48 | 32 | 6.02 | 0.033 |
| Cingulate gyrus, ventral anterior | R | 10 | 4 | 10 | −8 | 6.42 | 0.015 |
| Insular cortex, anterior | R | 14 | 30 | 16 | −12 | 6.90 | 0.006 |
Notes. Voxel-wise FWE corrected p-value is shown. Missing values under k indicate that the activation peak of the respective brain area pertains to the above-mentioned cluster. In cases where several activation peaks belonged to one cluster, the brain areas were listed according to the size of their T-value. STS = superior temporal sulcus.
Fig. 3.

Group-level statistical parametric maps showing stronger responses to fearful human than to fearful avatar facial expressions (voxel-wise P-FWE < 0.05). For better illustration, the clusters are shown on the mean anatomical template of the study population (top and middle row) and a part of them also on the ‘mni152_2009bet’ template from MRIcroGL (bottom row). Small clusters (k < 15) are highlighted with dashed lines (middle row) indicating the corresponding sagittal or coronal section (bottom line), where the clusters are highlighted with red circles. r, right; l, left; pSTS, posterior superior temporal sulcus; aSTS, anterior superior temporal sulcus; PCC, posterior cingulate cortex; vACC, ventral anterior cingulate cortex; aIC, anterior insular cortex.
Fig. 4.

Distribution of beta estimates per condition showing both main effects (face type and face expression) and the interaction effect in the right STS. Whiskers indicate the 25th and the 75th percentile. *P < 0.05 Bonferroni corrected.
Fig. 5.

Group-level statistical parametric maps showing the response difference between human and avatar faces for fearful expressions versus neutral expressions in bilateral posterior STS (voxel-wise P-FWE < 0.05). Note that the direct comparison of the clusters was significant only for the right posterior STS in the ROI analysis and not in the whole-brain analysis. Therefore, the whole-brain clusters shown serve descriptive purposes only.
BOLD response to fearful and neutral facial expressions
Next, we contrasted fearful and neutral expressions displayed in human faces across the whole brain. Fearful human expressions evoked stronger responses in bilateral inferior occipital cortex, ventral and dorsal temporal cortex, the left aIC and bilateral amygdalae (see Table 2 and Figure 6). The ROI analysis revealed that fearful human expressions evoked stronger responses than neutral human expressions in bilateral amygdala (left: T = 4.12, pcorr = 0.002, right: T = 5, pcorr < 0.001), right occipital FG (T = 3.2, pcorr = 0.019), bilateral STS (left: T = 4.97, pcorr < 0.001, right: T = 6.32, pcorr < 0.001) and bilateral IFG (left: T = 3.62, pcorr = 0.007, right: T = 3.58, pcorr < 0.007). We then contrasted fearful and neutral avatar expression across the whole brain. Fearful avatar expressions elicited stronger responses in bilateral inferior occipital cortex as well as ventral and dorsal temporal cortex (see Table 3 and Figure 7). The ROI analysis showed that fearful avatar expressions elicited stronger responses than neutral avatar expressions in the left amygdala (T = 3.27, pcorr = 0.016) and right STS (T = 3.35, pcorr = 0.013).
Table 2.
Clusters showing a significant difference in BOLD response to fearful human expressions compared to neutral human expressions in the second level whole-brain analysis
| Brain area | Side | k | MNI coordinates | T-value | P-FWE | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Inferior occipital gyrus | R | 816 | 48 | −64 | 6 | 10.06 | <0.001 |
| Middle temporal gyrus, posterior | R | 54 | −50 | 4 | 9.48 | <0.001 | |
| STS, posterior | R | 52 | −36 | 8 | 7.77 | <0.001 | |
| FG, middle | R | 24 | 46 | −42 | −20 | 6.35 | 0.015 |
| Inferior occipital gyrus | L | 5 | −30 | −88 | 6 | 6.39 | 0.014 |
| Inferior occipital gyrus | L | 413 | −42 | −68 | 2 | 10.09 | <0.001 |
| STS, posterior | L | −48 | −46 | 8 | 8.07 | <0.001 | |
| Insular cortex, anterior | L | 6 | −32 | 6 | −16 | 5.96 | 0.033 |
| Amygdala | R | 27 | 24 | 2 | −16 | 6.18 | 0.021 |
| Amygdala | L | 6 | −18 | −2 | −12 | 6.03 | 0.028 |
Notes. Voxel-wise FWE corrected p-value is shown. Missing values under k indicate that the activation peak of the respective brain area pertains to the above-mentioned cluster. In cases where several activation peaks belonged to one cluster, the brain areas were listed according to the size of their T-value. STS = superior temporal sulcus; FG = fusiform gyrus.
Fig. 6.

Group-level statistical parametric maps showing stronger responses to fearful human than to neutral human expressions (voxel-wise P-FEW < 0.05). For better illustration, the clusters are shown on the mean anatomical template of the study population (top left and bottom row) and on the ‘mni152_2009bet’ template from MRIcroGL (top right). Images in the top row show coronal and axial sections of the clusters in bilateral inferior occipital cortex (see TEMP and lOCG) as well as in ventral and dorsal temporal cortex (see TEMP and rFG). Images in the bottom row show clusters in bilateral amygdala and left anterior insular cortex. TEMP = bilateral dorsal temporal cortex; rFG = right fusiform gyrus; lOCG = left inferior occipital gyrus; AMY = bilateral amygdala; laic = left anterior insular cortex.
Table 3.
Clusters showing a significant difference in BOLD response to fearful avatar expressions compared to neutral avatar expressions in the second level whole-brain analysis
| Brain area | Side | k | MNI coordinates | T-value | P-FWE | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Middle temporal gyrus, posterior | R | 105 | 56 | −60 | 0 | 6.98 | 0.004 |
| Inferior occipital gyrus | R | 46 | −64 | 2 | 6.15 | 0.023 | |
| STS, posterior | R | 53 | 48 | −42 | 6 | 7.65 | 0.001 |
| Inferior occipital gyrus | L | 35 | −48 | −70 | 4 | 6.70 | 0.007 |
| STS, posterior | L | 59 | −56 | −54 | 6 | 7.33 | 0.002 |
| FG, middle | L | 38 | −42 | −46 | −18 | 6.89 | 0.005 |
Notes. Voxel-wise FWE corrected P-value is shown. Missing values under k indicate that the activation peak of the respective brain area pertains to the above-mentioned cluster. In cases where several activation peaks belonged to one cluster, the brain areas were listed according to the size of their T-value.
Fig. 7.
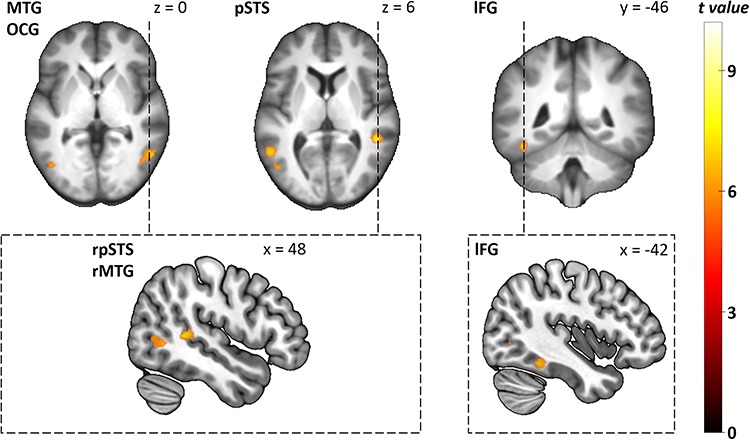
Group-level statistical parametric maps showing stronger responses to fearful avatar than to neutral avatar expressions (voxel-wise P-FEW < 0.05). For better illustration, the clusters are shown on the mean anatomical template of the study population (top row) and on the ‘mni152_2009bet’ template from MRIcroGL (bottom row). Images on the top left, top middle and bottom left show axial and sagittal sections of the clusters in bilateral inferior occipital gyrus and dorsal temporal cortex. Images on the top right and bottom right show coronal and sagittal sections of the cluster in the ventral temporal cortex. r, right; l, left; MTG, middle temporal gyurs; OCG, inferior occipital gyrus; pSTS, posterior superior sulcus; FG, fusiform gyrus.
In favor of a concise presentation of our main results, whole-brain analyses concerning the scrambled videos are summarized in Supplementary Tables S2–S5. In short, contrasts between non-scrambled videos and their scrambled versions generally showed stronger responses in the inferior occipital cortex as well as in the ventral and dorsal temporal cortex to non-scrambled videos. Non-scrambled videos of human and avatar fearful expressions also evoked stronger responses in the amygdala than their scrambled versions.
Discussion
Summary
We set out to investigate whether dynamic facial expressions displayed by avatar faces are processed within the same cortical and subcortical brain regions known to be involved in the processing of dynamic human facial expressions. To this aim, we assessed the brain topography of BOLD responses to dynamic human and avatar faces displaying either fearful or neutral expressions. Using videos of actors and their customized avatars derived from the same videos by motion tracking, we were able to demonstrate that the topography and the magnitude of brain response differed between human and avatar faces, yet only when fearful expressions were presented. Our imaging analysis revealed predominantly right-sided brain areas that showed stronger responses to fearful human expressions compared to fearful avatar expressions. This cluster entailed cortical areas such as bilateral posterior and anterior parts of the superior temporal region, the IFG, the right PCC and vACC, as well as the right aIC. For the amygdala, a subcortical core region in face and emotion perception, no response difference was found when comparing fearful expressions between human and avatar faces. Furthermore, our analysis revealed an interaction effect in the right superior temporal region. That is, the response difference between human and avatar faces was larger for fearful than for neutral expressions in the right superior temporal region.
When comparing fearful and neutral expressions displayed by human faces, we were able to show stronger BOLD responses to fearful human expressions in dorsal and ventral regions of the face perception network as well as in the left aIC and bilateral amygdala. A qualitatively similar response pattern, but with fewer and smaller activation peaks, was found when comparing fearful and neutral expressions displayed by avatar faces. This cluster entailed posterior dorsal temporal regions, inferior occipital and fusiform regions, as well as the left amygdala.
Modulation of brain activity by facial motion
In line with our hypothesis, we found stronger BOLD responses to fearful human expressions than to fearful avatar expressions in posterior and anterior parts of the superior temporal region and the IFG. These differential responses also appeared in comparison to scrambled videos, which indicates that the processing difference between fearful human and avatar expressions is not only associated with dynamic low-level visual features. This is in line with contemporary models of face perception, in which the superior temporal region and the IFG belong to the dorsal face processing pathway specialized for the processing of dynamic features of faces (Haxby et al., 2000; Duchaine and Yovel, 2015). Since subtle motions like that of wrinkles were not detectable in our avatar faces for technical reasons, fearful avatar expressions may have evoked weaker BOLD responses in these regions compared to human expressions. However, the weaker BOLD responses to avatar expressions were also present when we modeled the intensity of facial expressions as covariate. Hence, it is more plausible that even when using motion tracking, avatar facial expressions resemble artificial facial motion that does not activate these regions in the same way as biological facial motion (Sarkheil et al., 2013). Support for this hypothesis comes from a study investigating BOLD responses to biological motion in natural movie scenes and animated versions of these scenes. This study showed that areas implicated in the processing of biological motion such as the STS exhibit stronger responses to the same biological motion in natural movie scenes than in animated scenes (Mar et al., 2007).
Furthermore, we observed at a descriptive level that the stronger responses to fearful human in contrast to fearful avatar faces were more apparent in the right brain hemisphere. This is in line with a recognition advantage for facial expressions containing high-spatial frequency information presented to the left visual field and thus processed in the right brain hemisphere (as opposed to the presentation in the right visual field and processing in the left brain hemisphere; Kumar and Srinivasan, 2011). Hence, the primarily right-sided responses we found may be associated with differences in the high-spatial frequency range between fearful human and avatar expressions.
In contrast to previous studies applying static avatar stimuli (Moser et al., 2007; Kätsyri et al., 2020), we found a similar BOLD response in the FG to fearful human and avatar expressions. Face perception models propose the FG to represent invariant face information such as form or identity (Haxby et al., 2000; Duchaine and Yovel, 2015). Invariant facial information was present in both the human and avatar faces, which may be associated with the comparable activation pattern. Further support comes from studies showing that the FG was sensitive only to faces differing in typicality relative to an average face shape and that already a coarse representation of a face is sufficient to correctly identify it as a generic face (Said et al., 2010; Goffaux et al., 2011). Because our avatars were customized to the human face of their respective actor as closely as possible, such typicality is present in both the human faces and the avatar faces and may thus have equally activated the FG.
Modulation of brain activity by emotion
To interpret our results, it is necessary to emphasize that we only found different BOLD responses to human and avatar faces when comparing fearful but not neutral expressions. Thus, emotional expressions exert an important influence on the processing of human and avatar faces. They activate the amygdala via a fast magnocellular channel conveying low-spatial frequency information and thus enabling rapid evaluation of general stimulus features (Vuilleumier et al., 2003; de Gelder et al., 2005; Vuilleumier and Pourtois, 2007; Adolphs, 2010). In line with this crude appraisal function of the amygdala, only coarse, typically blurred information is processed over this subcortical route (Vuilleumier, 2005). The presence of such coarse low-spatial frequency information in human and avatar faces may explain the comparable amygdala responses.
In addition, it is plausible that the amygdala has a modulatory effect on subsequent face perception via backward connections to cortical areas, such as the IFG and the STS (Furl et al., 2013; Schirmer and Adolphs, 2017). Correspondingly, evidence was found for amygdala feedback loops to ventral and dorsal temporal areas during the processing of emotional facial expressions. These results indicate a modulatory effect of the amygdala on visual attention and successive processes underlying human face perception (Vuilleumier et al., 2001; Furl et al., 2013). Thus, our finding regarding stronger responses to fearful human than to fearful avatar expressions in dorsal temporal regions and the IFG may indicate complex visual coding of motion or gaze information present in fearful expressions and enhanced via feedback connections from the amygdala.
In contrast to our results, James et al. (2015) compared brain responses to neutral human and neutral cartoon faces and found stronger responses to human faces in the amygdala, the FG, the STS and the IFG. Possible explanations for these divergent findings are the use of static faces and bold differences between human faces and artificial cartoon faces. Accordingly, it has been shown that different levels of face realism elicit different neural responses as measured by EEG. Thus, the artificial appearance of computer-generated characters may change their processing (Schindler et al., 2017). However, the avatars in the present study were more realistic and tailored to the individual actors’ faces.
Are we empathic toward avatars?
Perceiving emotions in others almost inevitably elicites empathy in us, i.e. the attempt to understand and share the emotional state of another (Preston and de Waal, 2002). It is not yet known whether this can also be achieved by the perception of avatars. Although we did not assess empathy directly in our study, it may be possible to address this question cautiously based on the observed brain responses. We found stronger BOLD responses to fearful human expressions than to avatar expressions in the aIC, the vACC and the PCC. While the insula represents an integration hub for interoceptive processes and thus plays an important role in the perception of physiological signals within one’s own body (Craig, 2002; Garfinkel and Critchley, 2013), the PCC is linked (among other functions) with body ownership (Guterstam et al., 2015). The vACC is further associated with implicit emotion regulation (Etkin et al., 2015). Thus, different responses to fearful human and avatar facial expressions in the aforementioned brain regions may be explained by their association with empathy processes (Decety and Lamm, 2006; Bernhardt and Singer, 2012).
It is assumed that mirror neurons, which typically discharge when performing a certain motoric action as well as when the same motoric action is observed, are a potential basis for empathy-related brain responses (Gallese et al., 1996; Rizzolatti and Sinigaglia, 2016). This so-called mirroring is also transferable to brain responses associated with experiencing an emotional state as well as observing the same emotional state in another individual (Rizzolatti and Sinigaglia, 2016). It may be hypothesized that observing fear in avatars evokes less mirroring in associated brain regions since they are easily identified as artificial or non-human and therefore less important for human interaction (Gu and Han, 2007; Fan and Han, 2008; Bernhardt and Singer, 2012; Rizzolatti and Sinigaglia, 2016). Empirical studies on empathy-related brain responses to computer-generated characters, however, are still rare. Most results stem from studies using robots and show that people empathize with robots and exhibit corresponding neural responses, albeit to a lesser extent compared to responses to humans.
Equivocally, we should consider that not empathy but rather processes related to emotional intelligence may account for the observed variance in brain responses and rating differences between human and avatar faces. It is possible that our lack of expertise with computer-generated faces is associated with lower measures of emotional intelligence regarding facial expressions of avatars. In line with this, previous studies have shown that measures of emotional intelligence are positively associated with anterior insula activity during face processing (Quarto et al., 2016) and gray matter volume of the insula (Killgore et al., 2012; Karle et al., 2018). This must be considered when using avatars for research concerning social interactions (Bombari et al., 2015; Cross, Riddoch, et al., 2019; Hortensius et al., 2018; Rosenthal-von der Pütten et al., 2014; Suzuki et al., 2015).
Limitations and future directions
Although our study contributes new findings, it has necessary limitations. First, we only used fearful and neutral facial expressions. Consequently, our results cannot be generalized to other facial expressions, as valence-dependent effects are possible (Dyck et al., 2008; Fusar-Poli et al., 2009). Second, differences to other studies may in part be due to the software applied to create and animate avatars. The quality of motion tracking of the employed software (FaceRig©) may be limited compared to future advances in software development. Another limitation is that the intensity rating was performed within 2 weeks after the scanning session. This may have affected the participants’ rating and thus our results are not comparable to studies in which participants provide their rating during the scanning session.
Future studies may clarify whether the complexity of computer-generated faces influences brain responses and recognition profiles of different emotional expressions. It is plausible that the absence of high-spatial frequency information in computer-generated faces represents a missing cue for emotion recognition. On the other hand, this absence could be advantageous because the facial expression may be less ambiguous. By using computer-generated faces showing only major features of facial expressions, we are able to generate tasks for emotion recognition with a low level of complexity (Dyck et al., 2008). Such tasks may be especially beneficial for the training of emotion recognition in clinical populations considering the degree of their impairment (Dyck et al., 2010; Arsalidou et al., 2011; Szemere and Jokeit, 2015; Steiger and Jokeit, 2017). With increasing performance, the task may also be adjusted in difficulty by directly manipulating the perceptual features of the facial expressions (Macedo et al., 2015). This possibility only applies to computer-generated characters and thus represents an important alternative to picture-based training.
Conclusion
There are both differences and similarities in the patterns of brain responses to humans and avatars depending on the expressions shown. While amygdalar and cortical responses in ventral temporal regions do not differ in response to fearful human and avatar expressions, dorsal temporal and inferior frontal regions sensitive to dynamic facial information exhibit distinct responses. Together, this means that although avatar expressions evoke amygdala responses associated with the fast processing of emotions similar to human expressions, the artificial facial motion of avatars is likely not to be processed in the same way as human facial motion. Furthermore, empathy-related processes or processes associated with emotional intelligence may interplay with this. These interpretations may have important consequences for the implementation of avatars in various commercial and public applications and research. Nevertheless, we do not believe that the current findings imply that computer-generated faces are of no use considering their methodological benefits. Instead, we consider it necessary that researchers acknowledge these potential limitations and implement avatars according to the overall goal of the study.
Supplementary Material
Acknowledgements
We thank Dr Andrew Gasbarro for his valuable remarks regarding the improvement of the manuscript, and also to Dr Roger Luechinger for providing the technical equipment required to perform an event-related study as well as to Dr Torsten Straube, Prof. Dr Bernhard Schuknecht and his colleagues from the Medizinisch Radiologisches Institut (MRI) Zurich.
Funding
This work was supported by the Swiss National Science Foundation (project number 166416).
Conflict of Interest
None declared.
References
- Adolphs R. (2001). The neurobiology of social cognition. Current Opinion in Neurobiology, 11(2), 231–9. doi: 10.1016/S0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Adolphs R. (2010). What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences, 1191, 42–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsalidou M., Morris D., Taylor M.J. (2011). Converging evidence for the advantage of dynamic facial expressions. Brain Topography, 24(2), 149–63. [DOI] [PubMed] [Google Scholar]
- Ashburner J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38(1), 95–113. [DOI] [PubMed] [Google Scholar]
- Bernhardt B.C., Singer T. (2012). The neural basis of empathy. Annual Review of Neuroscience, 35(1), 1–23. [DOI] [PubMed] [Google Scholar]
- Bombari D., Schmid Mast M., Canadas E., Bachmann M. (2015). Studying social interactions through immersive virtual environment technology: virtues, pitfalls, and future challenges. Frontiers in Psychology, 6, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst A.W., Gelder B. (2015). Is it the real deal? Perception of virtual characters versus humans: an affective cognitive neuroscience perspective. Frontiers in Psychology, 6(576), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M., Anton J.-L., Valabregue R., Poline J.-B (2002). Region of interest analysis using an SPM toolbox [abstract] .Presented at the 8th International Conference of Functional Mapping of the Human Brain, Sendai, Japan. [Google Scholar]
- Choi A., Melo C.De, Woo W., Gratch J. (2012). Affective engagement to emotional facial expressions of embodied social agents in a decision-making game. Computer Animation and Virtual Worlds, 23(3–4), 331–42. [Google Scholar]
- Cogent 2000 [Computer software]. (n.d.). London, UK: Wellcome Laboratory of Neurology. Retrieved from http://www.vislab.ucl.ac.uk/cogent.php.
- Craig A.D. (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience, 3, 655–66. [DOI] [PubMed] [Google Scholar]
- Crookes K., Ewing L., Gildenhuys J.D., et al. (2015). How well do computer-generated faces tap face expertise? PLoS One, 10(11), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross E.S., Hortensius R., Wykowska A. (2019). From social brains to social robots: applying neurocognitive insights to human-robot interaction. Philosophical Transactions of the Royal Society, B: Biological Sciences, 374, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross E.S., Riddoch K.A., Pratts J., Titone S., Chaudhury B., Hortensius R. (2019). A neurocognitive investigation of the impact of socializing with a robot on empathy for pain. Philosophical Transactions of the Royal Society, B: Biological Sciences, 374, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J., Lamm C. (2006). Human empathy through the lens of social neuroscience. The Scientific World Journal, 6, 1146–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dima D.C., Perry G., Messaritaki E., Zhang J., Singh K.D. (2018). Spatiotemporal dynamics in human visual cortex rapidly encode the emotional content of faces. Human Brain Mapping, 39, 3993–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dricu M., Frühholz S. (2016). Perceiving emotional expressions in others: activation likelihood estimation meta-analyses of explicit evaluation, passive perception and incidental perception of emotions. Neuroscience and Biobehavioral Reviews, 71, 810–28. [DOI] [PubMed] [Google Scholar]
- Duchaine B., Yovel G. (2015). A revised neural framework for face processing. Annual Review of Vision Science, 1, 393–416. [DOI] [PubMed] [Google Scholar]
- Dyck M., Winbeck M., Leiberg S., Chen Y., Gur R.C., Mathiak K. (2008). Recognition profile of emotions in natural and virtual faces. PLoS One, 3(11), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck M., Winbeck M., Leiberg S., Chen Y., Mathiak K. (2010). Virtual faces as a tool to study emotion recognition deficits in schizophrenia. Psychiatry Research, 179(3), 247–52. [DOI] [PubMed] [Google Scholar]
- Epley N., Waytz A., Akalis S., Cacioppo J.T. (2008). When we need a human: motivational determinants of anthropomorphism. Social Cognition, 26(2), 143–55. [Google Scholar]
- Etkin A., Büchel C., Gross J.J. (2015). The neural bases of emotion regulation. Nature Reviews Neuroscience, 16, 693–700. [DOI] [PubMed] [Google Scholar]
- Fan Y., Han S. (2008). Temporal dynamic of neural mechanisms involved in empathy for pain: an event-related brain potential study. Neuropsychologia, 46(1), 160–73. [DOI] [PubMed] [Google Scholar]
- Fernández Dols J.-M., Russell J.A. (2017). In: Russell J.A., Fernández Dols J.M., editors. The Science of Facial expression, Vol. 1, New York, NY: Oxford University Press. [Google Scholar]
- Furl N., Henson R.N., Friston K.J., Calder A.J. (2013). Top-down control of visual responses to fear by the amygdala. Journal of Neuroscience, 33(44), 17435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Placentino A., Carletti F., et al. (2009). Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry and Neuroscience, 34(6), 418–32. [PMC free article] [PubMed] [Google Scholar]
- Gallese V., Fadiga L., Fogassi L., Rizzolatti G. (1996). Action recognition in the premotor cortex. Brain, 119(2), 593–609. [DOI] [PubMed] [Google Scholar]
- Garfinkel S.N., Critchley H.D. (2013). Interoception, emotion and brain: new insights link internal physiology to social behaviour. Commentary on: “anterior insular cortex mediates bodily sensibility and social anxiety” by Terasawa et al. (2012). Social Cognitive and Affective Neuroscience, 8(3), 231–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelder B., Morris J.S., Dolan R.J. (2005). Unconscious fear influences emotional awareness of faces and voices. Proceedings of the National Academy of Sciences of the United States of America, 102(51), 18682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffaux V., Peters J., Haubrechts J., Schiltz C., Jansma B., Goebel R. (2011). From coarse to fine? Spatial and temporal dynamics of cortical face processing. Cerebral Cortex, 21(2), 467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X., Han S. (2007). Attention and reality constraints on the neural processes of empathy for pain. NeuroImage, 36(1), 256–67. [DOI] [PubMed] [Google Scholar]
- Guterstam A., Björnsdotter M., Gentile G., Ehrsson H.H. (2015). Posterior cingulate cortex integrates the senses of self-location and body ownership. Current Biology, 25(11), 1416–25. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Maldonado J., Rus-Calafell M., González-Conde J. (2014). Creation of a new set of dynamic virtual reality faces for the assessment and training of facial emotion recognition ability. Virtual Reality, 18(1), 61–71. [Google Scholar]
- Haxby J.V., Hoffman E.A., Gobbini M.I. (2000). The distributed human neural system for face perception. Trends in Cognitive Sciences, 4(6), 223–33. [DOI] [PubMed] [Google Scholar]
- Hortensius R., Hekele F., Cross E.S. (2018). The perception of emotion in artificial agents. IEEE Transactions on Cognitive and Developmental Systems, 10(4), 852–64. [Google Scholar]
- Hsu T. (2019). These influencers arenʼt flesh and blood, yet millions follow them. New York Times, Available:https://www.nytimes.com/2019/06/17/business/media/miquela-virtual-influencer.html [2019, June 18, 2019]
- James T.W., Potter R.F., Lee S., Kim S., Stevenson R.A., Lang A. (2015). How realistic should avatars be? An initial fMRI investigation of activation of the face perception network by real and animated faces. Journal of Media Psychology, 27(3), 109–17. [Google Scholar]
- Johnson M.H. (2005). Subcortical face processing. Nature Reviews Neuroscience, 6, 766–74. [DOI] [PubMed] [Google Scholar]
- Karle K.N., Ethofer T., Jacob H., et al. (2018). Neurobiological correlates of emotional intelligence in voice and face perception networks. Social Cognitive and Affective Neuroscience, 13(2), 233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kätsyri J., Mäkäräinen M., Takala T. (2017). Testing the ‘uncanny valley’ hypothesis in semirealistic computer-animated film characters: an empirical evaluation of natural film stimuli. International Journal of Human Computer Studies, 97, 149–61. [Google Scholar]
- Kätsyri J., Gelder B., Borst A. (2020). Amygdala responds to direct gaze in real but not in computer-generated faces. NeuroImage, 204, 1–12. [DOI] [PubMed] [Google Scholar]
- Killgore W.D.S., Weber M., Schwab Z.J., et al. (2012). Gray matter correlates of trait and ability models of emotional intelligence. Neuroreport, 23(9), 551–5. [DOI] [PubMed] [Google Scholar]
- Kumar D., Srinivasan N. (2011). Emotion perception is mediated by spatial frequency content. Emotion, 11(5), 1144–51. [DOI] [PubMed] [Google Scholar]
- LeDoux J. (2000). Emotion circuits in the brain. Demystifying the Brain, 23, 155–84. [DOI] [PubMed] [Google Scholar]
- Macedo M., Marques A., Queirós C. (2015). Virtual reality in assessment and treatment of schizophrenia: a systematic review. Jornal Brasileiro de Psiquiatria, 64(1), 70–81. [Google Scholar]
- Mar R.A., Kelley W.M., Heatherton T.F., Macrae N.C. (2007). Detecting agency from the biological motion of veridical vs animated agents. Social Cognitive and Affective Neuroscience, 2(3), 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadyen J., Mermillod M., Mattingley J.B., Halász V., Garrido M.I. (2017). A rapid subcortical amygdala route for faces irrespective of spatial frequency and emotion. The Journal of Neuroscience, 37(14), 3864–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo C.M., Carnevale P., Gratch J. (2011a). The effect of expression of anger and happiness in computer agents on negotiations with humans. The 10th International Conference on Autonomous Agents and Multiagent Systems, 3, 937–44. [Google Scholar]
- Melo C.M., Carnevale P., Gratch J. (2011b). The impact of emotion displays in embodied agents on emergence of cooperation with people. Presence Teleoperators and Virtual Environments, 20(5), 449–65. [Google Scholar]
- Méndez-Bértolo C., Moratti S., Toledano R., et al. (2016). A fast pathway for fear in human amygdala. Nature Neuroscience, 19(8), 1041–9. [DOI] [PubMed] [Google Scholar]
- Moser E., Derntl B., Robinson S., Fink B., Gur R.C., Grammer K. (2007). Amygdala activation at 3T in response to human and avatar facial expressions of emotions. Journal of Neuroscience Methods, 161(1), 126–33. [DOI] [PubMed] [Google Scholar]
- Pessoa L., Adolphs R. (2010). Emotion processing and the amygdala: from a “low road” to “many roads” of evaluating biological significance. Nature Reviews Neuroscience, 11, 773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston S.D., Waal F.B.M. (2002). Empathy: its ultimate and proximate bases. The Behavioral and Brain Sciences, 25(1), 1–20. [DOI] [PubMed] [Google Scholar]
- Quarto T., Blasi G., Maddalena C., et al. (2016). Association between ability emotional intelligence and left insula during social judgment of facial emotions. PLoS One, 11(2), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G., Sinigaglia C. (2016). The mirror mechanism: a basic principle of brain function. Nature Reviews Neuroscience, 17, 757–65. [DOI] [PubMed] [Google Scholar]
- Rosenthal-von der Pütten A.M., Schulte F.P., Eimler S.C., et al. (2014). Investigations on empathy towards humans and robots using fMRI. Computers in Human Behavior, 33, 201–12. [Google Scholar]
- Said C.P., Dotsch R., Todorov A. (2010). The amygdala and FFA track both social and non-social face dimensions. Neuropsychologia, 48(12), 3596–605. [DOI] [PubMed] [Google Scholar]
- Sarkheil P., Goebe R., Schneider F., Mathiak K. (2013). Emotion unfolded by motion: a role for parietal lobe in decoding dynamic facial expressions. Social Cognitive and Affective Neuroscience, 8(8), 950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer A., Von Wangenheim F. (2014). Service with a smile or screen? How replacing personnel with machines affects customers’ satisfaction with a service. Advances in Consumer Research, 42, 662–3. [Google Scholar]
- Schindler S., Zell E., Botsch M., Kissler J. (2017). Differential effects of face-realism and emotion on event-related brain potentials and their implications for the uncanny valley theory. Scientific Reports, 7, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer A., Adolphs R. (2017). Emotion perception from face, voice, and touch: comparisons and convergence. Trends in Cognitive Sciences, 21(3), 216–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger B.K., Jokeit H. (2017). Why epilepsy challenges social life. Seizure, 44, 194–8. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Galli L., Ikeda A., Itakura S., Kitazaki M. (2015). Measuring empathy for human and robot hand pain using electroencephalography. Scientific Reports, 5, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szemere E., Jokeit H. (2015). Quality of life is social - towards an improvement of social abilities in patients with epilepsy. Seizure, 26, 12–21. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. (2005). How brains beware: neural mechanisms of emotional attention. Trends in Cognitive Sciences, 9(12), 585–94. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Pourtois G. (2007). Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia, 45(1), 174–94. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Armony J.L., Driver J., Dolan R.J. (2001). Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron, 30(3), 829–41. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Armony J.L., Driver J., Dolan R.J. (2003). Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nature Neuroscience, 6(6), 624–31. [DOI] [PubMed] [Google Scholar]
- Zaki J., Ochsner K. (2009). The need for a cognitive neuroscience of naturalistic social cognition. Annals of the New York Academy of Sciences, 1167(1), 16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


