Abstract
Enteroviruses are responsible for a large number of meningoencephalitis cases, especially in children. The objective of this study was to identify modes of diagnosis including the significance of respiratory and cerebrospinal fluid samples, associated clinical characteristics, inpatient management, and outcome of individuals with EV infections of the central nervous system (CNS). Electronic medical records of individuals with enterovirus infections of the central nervous system who presented to the Columbia University Irving Medical Center and Children’s Hospital of New York between January 1, 2012 and December 31, 2017 were reviewed retrospectively for demographic, epidemiological and clinical data. The median age overall was 1.7 months (interquartile range 14 years), and most (62.4%) were male. The majority of CNS infections presented as meningitis (95.7%) and occurred in the summer (45.2%) and fall seasons (37.6%). Eighty-five cases (91.4%) demonstrated enterovirus positivity in cerebrospinal fluid, thirty cases (32.3%) exhibited both cerebrospinal fluid and respiratory positivity and eight cases (8.6%) exhibited respiratory positivity with coinciding neurological findings. Eighty-nine individuals overall (95.7%) received antibiotics and 37 (39.8%) received antiviral treatment. All surviving individuals had favorable Modified Rankin Scores within the zero to two range upon discharge. Testing respiratory samples in addition to cerebrospinal fluid was found to be an important diagnostic tool in enterovirus-associated cases. While clinical outcomes were favorable for an overwhelming majority of cases, etiological understanding of CNS infections is essential for to identify ongoing and changing epidemiological patterns and aid in improving the diagnosis and treatment.
Keywords: enterovirus, acute flaccid myelitis, central nervous system infections, pediatrics, neurodiagnostics
Introduction
In recent years, non-polio enteroviruses (EV) have emerged as pathogens of neurologic significance (Rudolph et al. 2016). EV are an important diagnostic consideration in the evaluation of infectious neurologic disease, particularly meningitis and encephalitis in the pediatric population. EV are responsible for the majority of aseptic meningitis cases, especially in children, and cause more than 10% of encephalitis cases with an identified viral etiology (Rotbart and Hayden 2000; Huang et al. 2004; Soares et al. 2011; Anastasina et al. 2017; Hasbun et al. 2017). Endemic serotypes of EV affect young children each year due to lack of prior exposure and subsequent development of immunity (Centers for Disease Control and Prevention 2018). Children, particularly those less than one year of age, may have prolonged EV viremia duration, which is associated with increased risk of central nervous system (CNS) EV involvement (Huang et al. 2014).
More recently, EV-A71 and EV-D68 have been associated with outbreaks of acute flaccid myelitis (AFM) (Aliabadi et al. 2018) and brainstem encephalitis (Huang et al. 2006). More than 80% of children diagnosed with AFM reported a preceding respiratory or gastrointestinal illness and EV-D68 outbreaks were found to follow similar seasonal and bi-annual patterns as AFM (Messacar et al. 2015). Though the presence of EV in cerebrospinal fluid (CSF) was found in less than 10% of cases (despite 80% displaying CSF pleocytosis), EV-D68 was identified in 47% of respiratory samples collected and was the most commonly identified pathogen across cases (Messacar et al. 2018). In addition, one study of an outbreak of neurological manifestations associated with EV-71 in Denver, Colorado revealed that EV-71 PCR testing of CSF yielded positive results for only 31% of cases and that respiratory testing had higher diagnostic yield than CSF PCR testing in this clinical setting (Perez-Velez et al. 2007). This is supported by similar studies highlighting the limited diagnostic yield of CSF testing for neurological complications associated with EV-71 (Li et al. 2002; Kupila et al. 2005). Persistence of PCR-detectable EV after symptom onset is variable and in respiratory secretions has been shown to last as long as four weeks (Rotbart and Hayden 2000; Han et al. 2010). The variability of detection times of EV across multiple sample sources has prompted a push for widespread testing of not only CSF, but multiple sources including respiratory and fecal, in an effort to delineate the relationship between EV and AFM and inform diagnostic guidelines for coming outbreaks.
As recent EV associated outbreaks such as AFM continue to be a public health concern, understanding the role of respiratory samples in facilitating the diagnosis of enterovirus associated CNS infection, as well as its demographic and clinical features, may provide valuable insights into neurotropic EV infections. In this retrospective study, we review cases of EV infections of the CNS from a major tertiary care center to identify diagnostic workup, associated clinical features, inpatient management, and outcome at hospital discharge.
Methods
Ethics approval
This study was approved by the Columbia University Irving Medical Center (CUMC, New York, New York) Institutional Review Board (IRB). A waiver of consent was granted by the CUMC IRB.
Study design
A retrospective chart review was conducted of individuals with EV infections of the CNS at CUIMC and Children’s Hospital of New York between January 1, 2012 and December 31, 2017. Electronic medical records (EMRs) of individuals discharged with ICD 9 and ICD 10 codes A87.0 (Enteroviral Meningitis), A87.8 (Other Viral Meningitis), A87.9 (Viral Meningitis, Unspecified) 47.8 (Viral Meningitis NEC), 47.9 (Viral Meningitis NOS), 322.9 (Meningitis NOS), A85.0 (Enteroviral Encephalitis), A85.8 (Other Specified Viral Encephalitis), A86 (Unspecified Viral Encephalitis), 49.9 (Viral Encephalitis NOS), 323.82 (Myelitis NEC), G04.89 (Other Myelitis), 341.2 (Acute Myelitis NOS), G04.91 (Myelitis, Unspecified), 323.02 (Myelitis Other Viral), 323.63 and (Post-Infectious Myelitis) were reviewed. Cases of meningitis, encephalitis, and/or myelitis were defined using established clinico-radiographic criteria (Rath et al. 2010; Tapianen et al. 2007; Sejvar et al. 2007). In addition to meeting clinico-radiographic criteria for meningitis, encephalitis, and/or myelitis during the same admission, an etiological diagnosis of EV infection of the CNS was made if individuals tested positive for EV/Rhinovirus in the Filmarray Respiratory Panel (BioFire Diagnostics, Salt Lake City, UT) with evidence of CSF pleocytosis (defined as white cells greater than or equal to five cells/uL) and/or CSF EV PCR using the Cepheid GeneXpert DX CSF EV PCR (Cepheid, Sunnyvale, CA), Filmarray multiplex PCR meningitis/encephalitis panel (BioFire Diagnostics, Salt Lake City, UT), and/or New York State encephalitis panel (New York State Department of Health, Albany, NY).
EMRs were reviewed for demographic, epidemiological, and clinical data. Demographic data (age, sex, race, and ethnicity), presence of immunocompromised state (HIV/AIDS infection, organ transplant, immunosuppressant medications, diabetes mellitus, history of cancer, current cancer, sarcoidosis, asplenia), and epidemiologic factors (neonates born prior to 32 and 37 weeks, sick contacts, recent travel, and season of admission) were gathered. Clinical data including neurological diagnosis, prodromal fever, preceding or coinciding respiratory and/or gastrointestinal illness, initial neurological symptoms, CSF profile (CSF pleocytosis defined by white cells greater than or equal to five cells/uL, CSF protein, CSF glucose), length of hospital and ICU stay, need for intubation, neuroimaging features, EEG findings, Modified Rankin Scores (MRS) at discharge as well as treatment data were gathered for analysis.
Statistical analysis
Descriptive analyses, including mean, median, and standard deviation, were calculated for each continuous variable. Frequencies of each categorical variable were calculated to compare differences between groups. Sub-analyses were conducted for individuals exhibiting EV positivity in a respiratory panel with no corresponding positivity in CSF as well as immunocompromised and premature individuals. All analyses were conducted using RStudio version 3.4.3 (RStudio, Boston, MA).
Results
Clinical characteristics
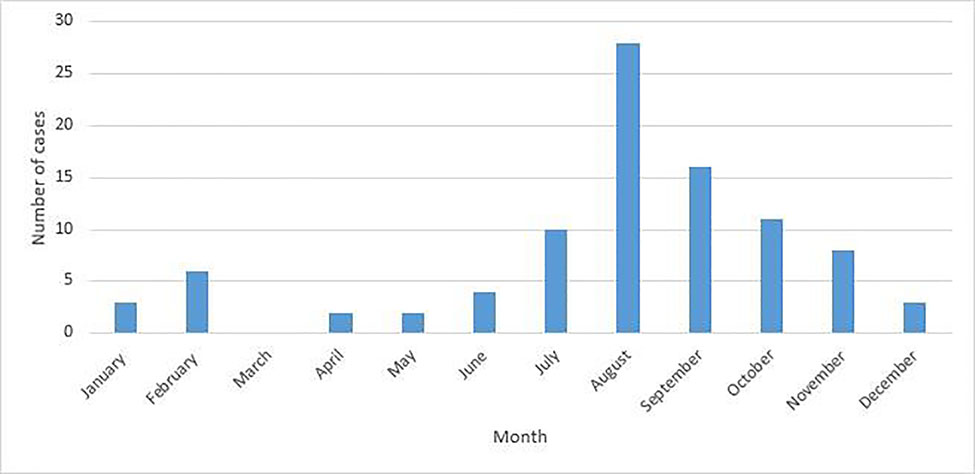
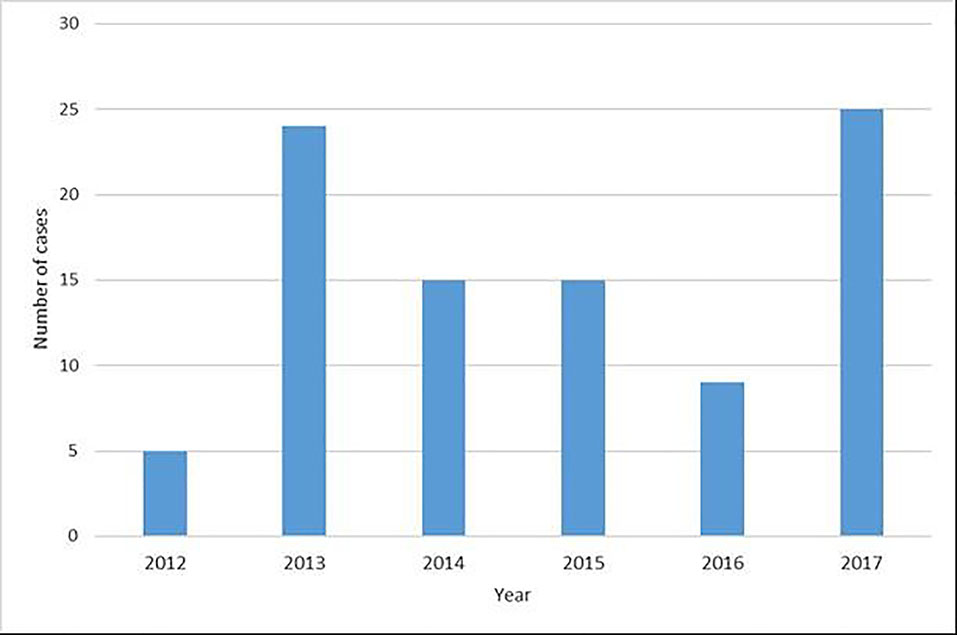
Ninety-three individuals presented with EV infections of the CNS during the study period. The median age overall was 1.7 months (interquartile range 14 years), 82 (88.2 %) were under eighteen years of age, and 58 (62.4 %) were male (see Table 1). Eighty-nine individuals (95.7%) presented with meningitis and three (3.2%) with meningoencephalitis/encephalitis. Forty-two individuals overall (45.2%) presented in the summer, 35 (37.6%) in the fall, twelve (12.9%) in the winter, and four (4.3%) in the spring (Figure 1). The highest monthly caseload was recorded in August (28 cases, 30.1%), and caseloads analyzed by year (Figure 2) revealed spikes in 2013 (24, 25.8 %) and 2017 (25, 26.9 %). Of those five years and older who reported neurological symptoms, the most common symptom was headache reported by 23 individuals (88.5%), followed by neck pain or stiffness in five individuals (19.23%) and photophobia in three individuals (11.5%). In children less than five years old, the most common symptom reported by caregivers was increased agitation and fussiness, reported in 29 individuals (43.3%). Twenty-eight individuals (41.8%) and their caregivers reported no neurological symptoms, and 75 (80.7%) had non-focal neurological exams. Data delineating specific serotypes of EV were not available, as this is not routinely performed in our clinical setting.
Table 1.
Characteristics of the study population
| All Cases | RVP+, negative CSF Cohort | Immunocompromised/Premature | |
|---|---|---|---|
| N=93 | N=8 | N=13 | |
| Demographics | |||
| Age (years) | |||
| Median (SD) | 0.14 (13.9) | 0.19 (14.63) | 0.14 (13.9) |
| Sex | |||
| Male | 58 (62.37%) | 4 (50.00%) | 6 (46.15%) |
| Female | 35 (37.63%) | 4 (50.00%) | 7 (53.85%) |
| Epidemiological Factors | |||
| Neonates | |||
| Born <37 weeks gestation | 7 (7.53%) | 0 (0.00%) | 7 (53.85%) |
| Born <32 weeks gestation | 1 (1.08%) | 1 (12.50%) | 1 (7.69%) |
| Sick exposures | |||
| No exposure to sick contacts | 48 (51.61%) | 6 (75.00%) | 7 (53.85%) |
| Exposure to sick contacts | 44 (47.31%) | 2 (25.00%) | 6 (46.15%) |
| Unknown | 1 (1.08%) | 0 (0%) | 0 (0.00%) |
| Travel outside of North America | |||
| No recent travel | 83 (89.25%) | 7 (87.50%) | 11 (84.62%) |
| Recent travel | 6 (6.45%) | 0 (0.00%) | 2 (15.38%) |
| Unknown | 4 (4.30%) | 1 (12.50%) | 0 (0.00%) |
| Immune Factors | |||
| Total immunocompromised | 5 (5.38%) | 2 (25.00%) | 13 (100.00%) |
| Total non-immunocompromised | 88 (94.62%) | 6 (75.00%) | 0 (0.00%) |
| Transplant | 1 (1.08%) | 1 (12.50%) | 1 (7.69%) |
| Other Immunosuppressant Medications | 2 (2.15%) | 1 (12.50%) | 2 (15.38%) |
| Diabetes | 1 (2.15%) | 1 (12.50%) | 1 (7.69%) |
| History of cancer | 3 (3.23%) | 0 (0.00%) | 3 (23.08%) |
| Clinical Factors | |||
| Diagnosis | |||
| Encephalitis | 1 (1.08 %) | 0 (0.00%) | 1 (7.69%) |
| Meningitis | 89 (95.70%) | 7 (87.50%) | 11 (84.62%) |
| Meningoencephalitis | 3 (3.23 %) | 1 (12.50%) | 1 (7.69%) |
| Enterovirus Positivity | |||
| Respiratory panel | 38 (40.86%) | 8 (100.00%) | 6 (46.15%) |
| CSF Enterovirus PCR | 55 (59.14%) | 0 (0.00%) | 7 (53.85%) |
| Biofire panel | 30 (32.26%) | 0 (0.00%) | 4 (30.77%) |
| New York City (NYC) Encephalitis Panel | 3 (3.23%) | 0 (0.00%) | 1 (7.69%) |
| Respiratory illness | |||
| Recent respiratory illness | 45 (48.39%) | 8 (100.00%) | 8 (61.54%) |
| Gastrointestinal illness | |||
| Recent gastrointestinal illness | 13 (13.98 %) | 3 (37.50%) | 2 (15.38%) |
| Fever | |||
| Fever | 80 (86.02%) | 7 (87.50%) | 9 (69.23%) |
| Initial neurological symptom(s) | |||
| Headache | 24 (25.81%) | 2 (25.00%) | 2 (15.38%) |
| Neck pain | 3 (3.23%) | 0 (0.00%) | 0 (0.00%) |
| Neck stiffness | 6 (6.45%) | 0 (0.00%) | 2 (15.38%) |
| Lethargy | 4 (4.30%) | 0 (0.00%) | 1 (7.69%) |
| Altered mental status | 3 (3.23%) | 1 (12.50%) | 2 (15.38%) |
| Photophobia | 3 (3.23%) | 0 (0.00%) | 0 (0.00%) |
| Agitation | 29 (31.18%) | 2 (25.00%) | 3 (23.08%) |
| None | 28 (30.11%) | 2 (25.00%) | 5 (38.46%) |
| Other | 7 (7.53%) | 2 (25.00%) | 2 (15.38%) |
| Intubation | |||
| Intubated | 2 (2.15%) | 1 (12.50%) | 2 (15.38%) |
| Length of hospital stay (days) | |||
| Mean | 3 | 11 | 5 |
| Median | 2 | 2 | 3 |
| SD | 7 | 23 | 5 |
| Length of ICU stay (days) | |||
| Mean | 1 | 3 | 3 |
| Median | 0 | 0 | 0 |
| SD | 3 | 8 | 6 |
Abbreviations: Respiratory panel positive cohort (RVP+ Cohort)
Fig. 1.
Monthly caseloads
Fig. 2.
Yearly caseloads
Clinical course
The median hospital stay was two days (SD 8), and three (3.1%) were discharged from the emergency room. Of those discharged from the emergency room, all exhibited EV positivity in the CSF FilmArray meningitis/encephalitis panel. Overall, eight out of 93 recruited patients (8.6%) were admitted to the ICU with a mean ICU stay of one day (SD 4), and two (25%) were intubated. Eighty-five cases (91.4%) demonstrated EV positivity in the CSF, and eight out of 85 cases (8.3%) exhibited positivity in respiratory panels with coinciding neurological findings (Table 2). Thirteen cases (14.9%) of those demonstrating positivity in CSF did not have respiratory samples tested. Thirty cases (32.3%) exhibited both CSF and respiratory sample positivity by PCR. Thirty-eight (40.9%) exhibited EV/rhinovirus positivity in a respiratory panel, 55 (59.1%) in a CSF EV PCR, 30 (32.2%) in the meningitis/encephalitis panel, and three (3.2%) in the New York State Encephalitis Panel. Adjusting for age, seventy-seven in total (82.8%) had evidence of CSF pleocytosis with a median WBC of 78/uL (IQR 237/uL) in individuals over two months old and 267/uL (IQR 805/uL) for those 2 months and younger. Forty-one (44.1%) of the 74 with corresponding serum glucose levels recorded exhibited CSF glucose less than two-thirds that of corresponding serum glucose levels, and 64 (68.8%) had CSF protein greater than 50 mg/dL. Of the eight testing positive in a respiratory panel exclusively, all had evidence of CSF pleocytosis with median WBC of 44/uL (IQR 314/uL), 4 (50%) had CSF glucose less than two-thirds that of corresponding serum glucose levels and seven (87.5%) had elevated protein greater than 50 mg/dL.
Table 2.
Clinical characteristics of patients with positive respiratory panel for EV/rhinovirus and negative CSF testing for enteroviruses:
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
|---|---|---|---|---|---|---|---|---|
| Age | 40 years | 9 years | 1 month | 12 days | 21 years | 2 months | 1 month | 2 months |
| Gender | M | F | M | M | F | F | F | M |
| Symptoms on presentation | Altered mental status, dry cough, poor PO intake, clonus, intermittent tremor, hyperreflexivity, chest pain, slow speech, abdominal pain. | Headache, NBNB emesis, chest pain, general weakness, blurry vision with and without headaches. | Maculopapular rash on neck and abdomen. | Pallor, delayed capillary refill, hypoxia. | Headache, nausea/emesis, diarrhea, photophobia, phonophobia, rhinorrhea, mild abdominal pain, neck stiffness. | Nasal congestion, diarrhea, decreased PO, less urinary output, injected pharynx, lethargy. | Fussiness. | Brief episode of face and lip shaking, eyes rolling back, no limb movement for 4 minutes. |
| Fever | Y | Y | Y | N | Y | Y | Y | Y |
| Diagnosis | Meningoencephalitis | Meningitis | Meningitis | Meningitis | Meningitis | Meningitis | Meningitis | Meningitis |
| CSF profile | WBC: 12 Neutrophils: 1 Lymphocytes: 95 Monocytes: 4 Eosinophils: 0 Glucose: 58 Protein: 70 |
WBC: 300 Neutrophils: 20 Lymphocytes: 72 Monocytes: 7 Eosinophils: 0 Basophils: 1 Glucose: 73 Protein: 85 |
WBC: 55 Neutrophils: 9 Lymphocytes: 14 Monocytes: 77 Eosinophils: 0 Glucose: 56 Protein: 58 |
WBC: 13 Neutrophils: 1 Lymphocytes: 76 Monocytes: 23 Eosinophils: 0 Glucose: 53 Protein: 142 |
WBC: 579 Neutrophils: 0 Lymphocytes: 86 Atyp lymph: 2 Monocytes: 0 Eosinophils: 0 Glucose: 82 Protein: 56 |
WBC: 446 Neutrophils: 74 Lymphocytes: 2 Monocytes: 24 Eosinophils: 0 Glucose: 62 Protein: 64 |
WBC: 26 Neutrophils: 2 Lymphocytes: 59 Bands: 1 Monocytes: 38 Macrophages: 10 Eosinophils: 0 Glucose: 67 Protein: 48 |
WBC: 33 Neutrophils: 0 Lymphocytes: 37 Monocytes: 60 Macrophages: 3 Eosinophils: 3 Glucose: 67 Protein: 39 |
| Neuroimaging findings | Patchy foci of subinsular, superior frontal subcortical, and periventricular white matter signal abnormality without associated mass effect, no abnormal enhancement | Normal | N/A | Normal | Normal | N/A | N/A | N/A |
| EEG findings | Moderate diffuse slowing, no epileptiform discharges | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| MRS score | 6 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
Overall, 23 individuals (24.7%) underwent neuroimaging, and one (1.1%) underwent EEG studies. Four (17.4%) individuals displayed structural abnormalities on neuroimaging; two individuals (50%) exhibited hydrocephalus, and two (50.0%) demonstrated multifocal subcortical and periventricular white matter signal abnormalities. Of the eight who tested positive in a respiratory panel exclusively, four (50%) had neuroimaging completed. Of those four, three (75.0%) had normal MRI scans, and one (25.0%) displayed patchy subcortical and periventricular white matter hyperintensities. Overall, two (8.3%) of the 24 individuals with neuroimaging studies had spinal cord imaging and one demonstrated diffuse T2 hyperintensity within the ventral and lateral cord extending from brainstem to the T1 spinal level with sparing of the dorsal columns.
Eighty-nine individuals (95.7%) received empiric antibiotics with CNS coverage for bacterial meningitis for an average of 3.3 days (IQR 1.5). Thirty-seven (39.8%) were administered antivirals for an average of 1.5 days (IQR 1) and all were administered acyclovir with the exception of one individual (1.1%) who was also treated with valganciclovir out of concern for cytomegalovirus associated with recent heart transplant. Four (4.3%) individuals were administered corticosteroids, and one (1.1%) intravenous immunoglobulin (IVIg) for a T2 hyperintense intramedullary lesion found on spinal imaging. Of those discharged without admission, all received one dose of antibiotics and none received antivirals. All surviving individuals had favorable MRS scores within the zero to two range upon discharge. One individual expired in the setting of cardiac arrest secondary to hypoxemic respiratory failure, and an autopsy revealed enterovirus infection of the transplanted heart.
Discussion
In this single center retrospective study, we demonstrated the utility of respiratory sample testing in the diagnosis of EV infections of the CNS. Some individuals in this cohort displayed EV positivity in respiratory samples with no corresponding EV positivity in CSF despite CSF pleocytosis. These findings are in accordance with past studies that demonstrate the limited diagnostic yield of CSF testing for certain EV strains, such as EV-71 (Li et al. 2002; Kupila et al. 2005). Though an important diagnostic test in patients with possible enterovirus associated neurological syndromes, virus testing positive in the respiratory tracts does not provide direct evidence for the CNS infection but may support an etiological diagnosis. In addition, more than half of the 74 with recorded corresponding serum glucose levels exhibited CSF glucose less than two-thirds that of corresponding serum glucose levels. This further emphasizes the importance of interpretation of CSF results in the context of the full clinical picture, as some individuals may have atypical CSF findings. Diagnosis of EV CNS disease for those with EV strains more likely to be detectable in CSF is facilitated by CSF PCR testing, including rapid result multiplex panels like the FilmArray ME panel. Rapid diagnostic tests are especially important in the diagnosis of enterovirus infections of the CNS given the possibility of discharge from the emergency department without admission in mild cases as well as early antimicrobial discontinuation. As not all individuals who present with EV infection of the CNS may show EV positivity in CSF or may display atypical CSF profiles, testing of additional samples, especially respiratory, at the time of initial presentation is crucial.
The challenges of diagnosing EV infections of the CNS mirror similar challenges in confirming non-polio EV associated with AFM, such as EV71 and EV-D68. Since 2014, EV has been the most commonly detected virus in respiratory samples from children with AFM (Aliabadi et al. 2018). This furthers the need for more comprehensive testing from multiple sample sources when EV infections of the CNS is suspected. Such testing may help avoid the administration of unnecessary treatments, such as antibiotics, in cases of EV infections. This is especially relevant as the majority of individuals in this cohort were administered antibiotics for the entirety of their hospital stay. According to recommendations from the Centers for Disease Control (CDC), treatment of enteroviral disease is supportive, as no specific treatments have been found to significantly improve recovery (Centers for Disease Control and Prevention 2018). Additionally, many were administered antivirals, and no significant differences in MRS scores upon discharge or in length of stay were seen between those who did and those who did not receive antivirals.
Most findings are consistent with previous data revealing that EV are most prevalent in the summer and fall seasons and that young age and male sex are known risk factors for EV meningitis (Centers for Disease Control and Prevention 2018; Rudolph et al. 2016; Chen et al. 2018). Most cases occurred in children with no additional pre-disposing factors, such as an immunocompromised state or pre-mature birth. Despite evidence that CNS infection can result in more severe clinical presentation in immunocompromised individuals, there were no notable differences in outcome and clinical course between the immunocompromised and non-immunocompromised cohorts with the exception of IVIg administration and intubation (Sonneville et al. 2017). Fever was the most common symptom across all age groups, and the most common neurological symptoms reported by individuals five years of age and older was headache. The most commonly reported symptoms in those younger than five years was agitation, presenting a challenge to clinicians given that such non-specific symptoms may indicate a wide variety of clinical syndromes. In such cases, CSF studies, as well as testing of non-CSF samples such as respiratory samples, may be especially useful.
There are several limitations of this study. The FilmArray Respiratory Panel used for all respiratory testing in this cohort does not distinguish between rhinovirus and EV. However, as many respiratory panels utilized in a clinical setting do not distinguish between EV and rhinovirus, this method better reflects and informs diagnostic procedures in the typical clinical setting, where serological testing is not routinely conducted. Secondly, since cases were identified retrospectively, there is a possibility that the ICD codes used to identify potential cases are not comprehensive. For this reason, cases with ICD codes for unknown and unspecified cases of CNS infection were also reviewed. Similarly, retrospective analysis of EMRs may be hampered by incomplete or subjective information. Despite this, the comprehensive reporting of laboratory testing and imaging studies in electronic records is a strength of this study. Young children could also have been overrepresented in this cohort as older individuals may not receive the same diagnostic work up as those less than two months who typically have a complete infectious workup, including lumbar puncture. Finally, this was a single-center study, and clinical presentations and practices could vary by region and year. Thus, caution should be used when generalizing results.
EV infections remain a common pathogen with a diverse clinical spectrum. Monitoring and management requires close coordination between those in the fields of neurology, infectious disease, emergency medicine, internal medicine, and pediatrics. Regarding diagnosis, this study illustrates the utility of testing respiratory samples and rapid diagnostic assays in individuals presenting with neurological symptoms, especially children. Future studies would benefit from incorporating tests that identify specific strains of EV and their respective clinical features.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Rudolph H, Schroten H, Tenenbaum T (2016) Enterovirus Infections of the Central Nervous System in Children: An Update. Pediatr Infect Dis J. 35(5):567–569. doi: 10.1097/INF.0000000000001090. [DOI] [PubMed] [Google Scholar]
- 2.Huang C, Morse D, Slater B, Anand M, Tobin E, Smith P, Dupuis M, Hull R, Ferrera R, Rosen B, Grady L (2004) Multiple-year experience in the diagnosis of viral central nervous system infections with a panel of polymerase chain reaction assays for detection of 11 viruses. Clin Infect Dis. 39(5): 630–635. doi: 10.1086/422650. [DOI] [PubMed] [Google Scholar]
- 3.Soares CN, Cabral-Castro MJ, Peralta JM, de Freitas MR, Zalis M, Puccioni-Sohler M (2011) Review of the etiologies of viral meningitis and encephalitis in a dengue endemic region. J Neurol Sci 303(1–2): 75–79. doi: 10.1016/j.jns.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Anastasina M, Domanska A, Palm K, Butcher S (2017) Human picornaviruses associated with neurological diseases and their neutralization by antibodies. J Gen Virol 98(6): 1145–1158. doi: 10.1099/jgv.0.000780. [DOI] [PubMed] [Google Scholar]
- 5.Hasbun R, Rosenthal N, Balada-Llasat JM, Chung J, Duff S, Bozzette S, Zimmer L, Ginocchio CC (2017) Epidemiology of Meningitis and Encephalitis in the United States, 2011–2014. Clin Infect Dis 65(3): 359–363. doi: 10.1093/cid/cix319. [DOI] [PubMed] [Google Scholar]
- 6.Aliabadi N, Messacar K, Pastula DM, Robinson CC, Leshem E, Sejvar J, Nix WA, Oberste MS, Feikin DR, Dominguez SR (2016) Enterovirus D68 Infection in Children with Acute Flaccid Myelitis, Colorado, USA, 2014. Emerg Infect Dis 22(8):1387–94 doi: 10.3201/eid2208.151949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messacar K, Asturias EJ, Hixon AM, Van Leer-Buter C, Niesters HGM, Tyler KL, Abzug MJ, Dominguez SR (2018) Enterovirus D68 and acute flaccid myelitis-evaluating the evidence for causality. Lancet Infect Dis 18(8):e239–e247. doi: 10.1016/S1473-3099(18)30094-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pérez-Vélez CM, Anderson MS, Robinson CC, McFarland EJ, Nix WA, Pallansch MA, Oberste MS, Glodé MP (2007) Outbreak of Neurologic Enterovirus Type 71 Disease: A Diagnostic Challenge. Clin Infect Dis 45(8): 950–957.doi: 10.1086/521895 [DOI] [PubMed] [Google Scholar]
- 9.Li C, Yang M, Chen R, Lin TY, Tsao KC, Ning HC, Liu HC, Lin SF, Yeh WT, Chu YT, Yang KD (2002) Clinical manifestations and laboratory assessment in an enterovirus 71 outbreak in southern Taiwan. Scand J Infect Dis 34(2):104–9. Doi: 10.1080/00365540110077119 [DOI] [PubMed] [Google Scholar]
- 10.Kupila L, Vuorinen T, Vainionpä R, Marttila RJ, Kotilainen P (2005) Diagnosis of enteroviral meningitis by use of polymerase chain reaction of cerebrospinal fluid, stool, and serum specimens. Clin Infect Dis 40(7):982–7. Doi: 10.1086/428581 [DOI] [PubMed] [Google Scholar]
- 11.Rotbart HA, Hayden FG (2000) Picornavirus infections: a primer for the practitioner. Arch Fam Med. 9(9):913–920. https://www.ncbi.nlm.nih.gov/pubmed/11031400. [DOI] [PubMed] [Google Scholar]
- 12.Han J, Ma XJ, Wan JF, Liu YH, Han YL, Chen C, Tian C, Gao C, Wang M and Dong XP (2010) Long persistence of EV71 specific nucleotides in respiratory and feces samples of the patients with Hand-Foot-Mouth Disease after recovery. BMC Infect Dis 10: 178 Doi: 10.1186/1471-2334-10-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang MC, Wang SM, Hsu YW, Lin HC, Chi CY, Liu CC (2006) Long-term cognitive and motor deficits after enterovirus 71 brainstem encephalitis in children. Pediatrics. 118(6):e1785–1788. https://pediatrics.aappublications.org/content/118/6/e1785. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (2018) What Parents Need to Know about Enterovirus D68. https://www.cdc.gov/features/evd68/index.html. Accessed February 2, 2019.
- 15.Cheng HY, Huang YC, Yen TY, Hsia SH, Hsieh YC, Li CC, Chang LY, Huang LM (2014) The correlation between the presence of viremia and clinical severity in patients with enterovirus 71 infection: a multi-center cohort study. BMC Infect Dis 14:417. doi: 10.1186/1471-2334-14-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rathe B, Magnus M, Heininger U (2010) Evaluating the Brighton Collaboration case definitions, aseptic meningitis, encephalitis, myelitis, and acute disseminated encephalomyelitis, by systematic analysis of 255 clinical cases. Vaccine 28(19):3488–95. doi: 10.1016/j.vaccine.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 17.Tapiainen T, Prevots R, Izurieta HS, Abramson J, Bilynsky R, Bonhoeffer J, Bonnet MC, Center K, Galama J, Gillard P, Griot M, Hartmann K, Heininger U, Hudson M, Koller A, Khetsuriani N, Khuri-Bulos N, Marcy SM, Matulionyte R, Schöndorf (2007) Aseptic meningitis: case definition and guidelines for collection, analysis and presentation of immunization safety data. Vaccine. 25(31):5793–802. doi: 10.1016/j.vaccine.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 18.Sevjar JJ, Kohl KS, Bilynsky R, Blumberg D, Cvetkovich T, Galama J, Gidudu J, Katikaneni L, Khuri-Bulos N, Oleske J, Tapiainen T, Wiznitzer M (2007) Encephalitis, myelitis, and acute disseminated encephalomyelitis (ADEM): case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. (31):5771–92. doi: 10.1016/j.vaccine.2007.04.060 [DOI] [PubMed] [Google Scholar]
- 19.Filmarray Respiratory Panel. BioFire Diagnostics, Salt Lake City, UT. [Google Scholar]
- 20.Cepheid GeneXpert DX CSF enterovirus PCR. Cepheid, Sunnyvale, CA. [Google Scholar]
- 21.Filmarray multiplex PCR meningitis/encephalitis panel. BioFire Diagnostics, Salt Lake City, UT. [Google Scholar]
- 22.New York State encephalitis panel. New York State Department of Health, Albany, NY. [Google Scholar]
- 23.RStudio Team. RStudio: Integrated Development for R 2015. RStudio Inc., Boston, Massachusets. [Google Scholar]
- 24.Centers for Disease Control (2018) Viral Meningitis https://www.cdc.gov/meningitis/viral.html. Accessed February 2, 2019.
- 25.Chen P, Lin X, Liu G, Wang S, Song L, Tao Z, Xu A4 (2018) Analysis of enterovirus types in patients with symptoms of aseptic meningitis in 2014 in Shandong, China. Virology. 516:196–201. doi: 10.1016/j.virol.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 26.Lyons JL (2018) Viral Meningitis and Encephalitis. Neuro Inf Dis 24(5)1284–1297. [DOI] [PubMed] [Google Scholar]
- 27.McGill F, Griffiths MJ, Solomon T (2017) Viral meningitis: current issues in diagnosis and treatment. Curr Opin Infect Dis (2): 248–256. doi 10.1097/QCO.0000000000000355. [DOI] [PubMed] [Google Scholar]
- 28.Weil M, Mandelboim M, Mendelson E, Manor Y, Shulman L, Ram D, Barkai G, Shemer Y, Wolf D, Kra-Oz Z, Weiss L, Pando R, Hindiyeh M, Sofer D (2017) Human Enterovirus D68in clinical and sewage samples in India. J Clin Virol. 86:52–55. doi: 10.1016/j.jcv.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Carrion Martin AI, Pebody RG, Danis K, Ellis J, Niazi S, DE Lusignan S, Brown KE, Zambon M, Allen DJ (2017) The emergence of enterovirus D68 in England in autumn 2014 and the necessity for reinforcing enterovirus respiratory screening. Epidemiol Infect 145(9):1855:1864. doi: 10.1017/S0950268817000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (2019) Acute Flaccid Myelitis https://www.cdc.gov/acute-flaccid-myelitis/afm-surveillance.html. Accessed February 2, 2019.