Abstract
New species of Amanita subgen. Lepidella are described from Guyana. Amanita cyanochlorinosma sp. nov., Amanita fulvoalba sp. nov., and Amanita guyanensis sp. nov. represent the latest additions to the growing body of newly described ectomycorrhizal fungi native to Dicymbe-dominated tropical rainforests. Macro- and micromorphological characters, habitat, and DNA sequence data for the ITS, nrLSU, rpb2, and ef1-α are provided for each taxon, and β-tubulin for most. Distinctive morphological features warrant the recognition of the three new species and a molecular phylogenetic analysis of taxa across Amanita subgen. Lepidella corroborates their infrageneric placements.
Keywords: ectomycorrhizal fungi, Guiana Shield, monodominant forests, Neotropics, new taxa, systematics, taxonomy
INTRODUCTION
Amanita (Amanitaceae, Agaricomycetes, Basidiomycota) is a monophyletic mushroom genus with cosmopolitan distribution (Drehmel et al. 1999, Tulloss 2005). It is estimated that 900–1000 Amanita species exist worldwide, with over 500 currently described (Tulloss 2005, Thongbai et al. 2016, Vargas et al. 2017). Amanita has traditionally been divided into two subgenera based on basidiospore amyloidity (e.g. Singer 1986). Subgenus Amanita includes those species with inamyloid basidiospores, and has traditionally been subdivided into sections Amanita, Vaginatae, and Caesareae based on macromorphology (e.g. Moser 1967). Molecular studies have corroborated these sections by demonstrating the monophyly of each (Weiss et al. 1998, Tang et al. 2015). Subgenus Lepidella includes those species with amyloid basidiospores and has been further subdivided into four sections based on velar morphology: sect. Amidella (marginal appendiculae and saccate volva present), sect. Lepidella (marginal appendiculae present, saccate volva absent), sect. Phalloideae (marginal appendiculae absent, saccate volva present), and sect. Validae (marginal appendiculae and saccate volva absent) (Corner & Bas 1962). Molecular studies have supported the monophyly of sect. Amidella and sect. Validae, whereas sect. Lepidella and sect. Phalloideae may be polyphyletic (Weiss et al. 1998, Drehmel et al. 1999, Moncalvo et al. 2000a, Kim et al. 2013, Tang et al. 2015, Cui et al. 2018).
While a few earliest diverging Amanita species are saprotrophic (Wolfe et al. 2012), the genus is otherwise considered ectomycorrhizal (ECM) and exhibits a wide range of ECM host associations (Tedersoo & Brundrett 2017). Amanita species are prevalent in Fagaceae and Pinaceae dominated higher latitude forests (Tulloss 2005, Truong et al. 2017), some with Holarctic distributions (Geml et al. 2006). Of the ~ 500 validly described species of Amanita, around 130 are known from the tropics (Thongbai et al. 2016). Tropical Amanita species frequently occur in spatially restricted mono- or co-dominant stands of ECM host trees, and thus appear to have smaller geographic ranges. They can, however, be a major component of the local ECM fungal assemblage (Watling & Lee 1995, Henkel et al. 2012, Ebenye et al. 2017). Overall, tropical regions remain mycologically undersampled and many Amanita species remain to be described (Beeli 1935, Bas 1978, Mueller & Halling 1995, Halling 2001, Simmons et al. 2002, Zhang et al. 2004, Tulloss 2005, Henkel et al. 2012, Cui et al. 2018).
Around 35 species of Amanita have been described from lowland tropical South America. Bas (1978) mentioned several poorly documented Brazilian species described in 1937 by Johann Rick, and described eight new central Amazonian species. More recently, 25 species have described from the South American tropics including 11 from Colombia (Tulloss et al. 1992, Tulloss & Franco-Molano 2008), nine from Brazil (Bas & De Meijer 1993, Menolli et al. 2009, Wartchow et al. 2009, Wartchow et al. 2013, Wartchow 2015, Wartchow et al. 2015, Wartchow 2016, Wartchow & Cortez 2016), four from Guyana (Simmons et al. 2002), and one from Ecuador (Wartchow & Gamboa-Trujillo 2012). An additional six species previously described from other regions have been recorded in tropical South America (Tulloss & Halling 1997, Sobestiansky 2005, Wartchow & Tulloss 2007, Lechner & Alberto 2008, Palacio et al. 2015). This steady accrual of new taxa suggests that many South American Amanita species remain to be discovered. The ectotrophic Dicymbe forests of Guyana provide a case in point, where 28 mostly undescribed Amanita species are known to occur (Henkel et al. 2012).
Here three new species of Amanita subgen. Lepidella are described from Guyana. Amanita cyanochlorinosma sp. nov. has a greyish blue pileus, saccate volva, and strong odour of chlorine. Amanita fulvoalba sp. nov. produces robust basidiomata with tawny pilei, marginal appendiculae, and a saccate volva. Amanita guyanensis sp. nov. has a greyish brown pileus with floccose warts and a floccose volva. Each of the new species was compared to previously described Amanita species from the world literature, and their novelty demonstrated by comparison with morphologically similar described species. Molecular data support placement of A. cyanochlorinosma and A. fulvoalba in sect. Lepidella, and A. guyanensis in sect. Validae. Morphological data support these placements with the exception of A. fulvoalba, whose molecular-based placement in sect. Lepidella contrasts with its marginal appendiculae, which typically characterise sect. Amidella. Each of these species has been encountered repeatedly in Guyana’s Dicymbe forests over the past 20 years (Henkel et al. 2012).
MATERIALS AND METHODS
Collections and morphological analyses
Collections were made in Guyana during the May–Jul. rainy seasons of 2000, 2003, 2005, 2007, 2009, 2011, 2012, and 2015 and the Dec.–Jan. rainy seasons of 2004, 2009, and 2016 from the Upper Potaro River Basin, within a 15 km radius of a permanent base camp at 5°18’04.8” N 59°54’40.4” W, 710 m a.s.l. Additional collections were made from the Upper Mazaruni River Basin during Dec. 2010 and Jun. 2012 within a 0.4 km radius of a base camp at 5°26’21.3” N and 60°04’43.1” W, 800 m a.s.l., and Jun. 2011 from the Upper Demerara River Basin at Mabura Ecological Reserve, near a field station located at 5°09′19.0″N 58°41′58.9″W, 100 m a.s.l. At the Potaro sites, basidiomata were collected from monodominant forests of ECM Dicymbe corymbosa (Henkel et al. 2012) and other stands containing ECM Dicymbe altsonii, Aldina insignis and D. corymbosa (Smith et al. 2011). At the Mazaruni site, collections were made from forests co-dominated by ECM Pakaraimaea dipterocarpacea and Dicymbe jenmanii (Smith et al. 2013). At the Mabura site, collections were made in monodominant stands of D. altsonii (Zagt 1997). Macromorphological features of fresh basidiomata were described in the field. Colours were described subjectively and coded according to Kornerup & Wanscher (1978), with colour plates noted in parentheses. Fresh collections were field-dried with silica gel.
Micromorphological features were assessed using an Olympus BX51 microscope with bright field and phase contrast optics. Rehydrated fungal tissues were mounted in H2O, 3 % potassium hydroxide (KOH), and Melzer’s solution. Twenty-five basidiospores were measured from each specimen of each species, including the types. Twenty basidia and hyphal elements of the subhymenium, hymenophoral trama, pileipellis, pileus and stipe trama, and universal veil were measured from each type specimen, and 10 from each additional specimen examined. Length/width Q values for basidiospores are reported as Qr (range of Q values over “n” basidiospores measured) and Qm (mean of Q values ± SD). The notation “[a/b/c]” preceding sets of basidiospore data denotes “‘a’ basidiospores from ‘b’ basidiomata from ‘c’ collections.” Outlying measurements observed in less than 5 % of a given structure are placed in parentheses. Line drawings are freehand composites of microscopic observations. Specimens were deposited in the following herbaria: BRG, University of Guyana; HSC, Humboldt State University; PUL, Kriebel Herbarium, Purdue University.
DNA extraction, amplification, sequencing and phylogenetic analyses
DNA was extracted from dried basidioma tissue of types and additional specimens using the Wizard® Genomic DNA Purification kit (Promega Co., WI, USA). Five DNA gene fragments were sequenced, including those coding for the second-largest subunit of RNA polymerase II (rpb2), translation elongation factor 1-alpha (ef1-α) and beta-tubulin (β-tub), along with two non-protein coding regions, the internal transcribed spacer (ITS) and nuclear ribosomal large subunit (nrLSU). Primer pairs ITS1F/ITS4B (Gardes & Bruns 1993), LROR/LR6 (Vilgalys & Hester 1990, Moncalvo et al. 2000b), EF1-983F/EF1-2218R (Rehner & Buckley 2005), Am-β-tubulin F/Am-β-tubulin R (Cai et al. 2014) and Am-6F/Am-7R (Cai et al. 2014) were used to amplify ITS, nrLSU, ef1-α, β-tub and rpb2, respectively. Amplification reactions included 12.5 μL of Promega PCR Mastermix (Promega Co., WI, USA), 1.25 μL of each primer (at 10 μM) and approximately 100 ng of DNA. The final PCR reaction volume was 25 μL. The recommended cycling conditions for each primer pair were followed. PCR products were sequenced by GeneWiz® (South Plainfield, NJ, USA). To get readable ITS and nrLSU sequences for specimens MCA 3962 and TH 9172, these fragments were cloned using the pGem®-T Easy Vector System (Promega Co., WI, USA) following manufacturer’s protocols. Ten colonies for each specimen for each locus were suspended in 30 μL of sterile water. PCR reactions were done as described above, using the original primers for each gene fragment and 10 μL of clone/sterile water mixture. All amplicons were sequenced. Sequences were edited using Sequencher v. 5.2.3 software (Gene Codes Corporation, MI, USA) and deposited in GenBank.
Initial BLAST searches with the ITS and nrLSU sequences for each new species confirmed their affinity with Amanita subgen. Lepidella. Infrageneric relationships of the three new species was assessed with phylogenetic analyses using nrLSU, ef1-α, β-tub and rpb2 (Cai et al. 2014). Sequences for the final dataset were downloaded from GenBank and included exemplars from 76 specimens from all four sections of Amanita subgen. Lepidella. Eight species from Amanita subgen. Amanita were used as outgroup taxa. Table 1 gives all taxa, collection information, GenBank numbers, and references for specimens used in the phylogenetic analysis.
Table 1.
Taxa, voucher information and GenBank accession numbers for specimens used in the phylogenetic analysis. Taxa described here and type specimens are indicated in bold.
| Species | Voucher | Locality | nrLSU | rpb2 | ef1-a | β-tub |
|---|---|---|---|---|---|---|
| Amanita abrupta | BW_HP_101 | Massachusetts, USA | HQ539660a | -- | -- | -- |
| Amanita asteropus | RET 730-2 | France | KY274804b | -- | -- | -- |
| Amanita aurantiobrunnea | MCA 4420 | Guyana | MK105506c | MK092931c | -- | MK092938c |
| Amanita ballerina | OR1026 | Thailand | MH157079d | KY656884e | -- | KY656865e |
| Amanita bisporigera | RET 377-9 | Tennessee, USA | KJ466434f | -- | KJ481936f | KJ466501f |
| Amanita brunneolocularis | ANDES_F313 NVE57 | Colombia | FJ890044g | -- | -- | -- |
| Amanita brunnescens | PBM 2429 | AY631902h | AY780936h | AY881021h | -- | |
| Amanita calochroa | MCA 3927 | Guyana | KC155375i | -- | -- | -- |
| Amanita aff. campinaranae | MCA 5878 | Guyana | MK105507c | MK092934c | MK092947c | MK092940c |
| Amanita castanea | MFLU:15-01424 | Thailand | KU877539j | -- | -- | -- |
| Amanita chlorinosma | RET 328-6 | New York, USA | HQ539676a | -- | -- | -- |
| Amanita citrina | ANDES_F2117_ NVE616 | Colombia | KT008032k | -- | -- | -- |
| Amanita cokeri | BW_STF 090506-19 | Massachusetts, USA | HQ593113a | -- | -- | -- |
| Amanita congolensis | RET 346-6 | Gambia | HQ539736a | -- | -- | -- |
| Amanita clarisquamosa | HKAS29514 | AF024448l | -- | -- | -- | |
| Amanita clelandii | PSC 2524 | Australia | HQ539680a | -- | -- | -- |
| Amanita cf. cruetilemurum | RET 600-3 | California, USA | KP711840m | -- | -- | -- |
| Amanita cyanochlorinosma | MCA 3962 | Guyana | MK105495c | MK092931c | MK092943c | MK092936c |
| MK105496c | ||||||
| Amanita cyanochlorinosma | TH 9172 | Guyana | MK105493c | MK092933c | MK092945c | MK092939c |
| MK105494c | ||||||
| Amanita cyanopus | TH 8912 | Guyana | KT339210n | -- | -- | -- |
| Amanita daucipes | RET 386-8 | Pennsylvania, USA | HQ539688a | -- | -- | -- |
| Amanita eriophora | RET 350-4 | Cambodia | HQ539672a | -- | -- | -- |
| Amanita excelsa | HKAS31510 | China | AY436491o | -- | -- | -- |
| Amanita exitialis | HKAS75775 | China | JX998053p | KJ466592f | JX998002p | KJ466504f |
| Amanita farinacea | PSC 2529 | Australia | HQ539692a | -- | -- | -- |
| Amanita flavipes | ASIS26281 | KU139456q | -- | -- | -- | |
| Amanita franchetii | JM 96/27 | North Carolina, USA | AF097381r | -- | -- | -- |
| Amanita aff. fritillaria | HKAS56832 | China | KJ466479f | KJ466644f | KJ481979f | KJ466558f |
| Amanita fuliginea | HKAS75782 | China | JX998049p | KJ466597f | JX997996p | KJ466509f |
| Amanita fuligineoides | HKAS52727 | China | JX998047p | KJ466599f | -- | KJ466511f |
| Amanita fulvoalba | MCA 6920 | Guyana | MK105498c | -- | -- | -- |
| TH 8056 | Guyana | MK105499c | MK092925c | -- | -- | |
| TH 8455 | Guyana | MK105500c | MK092927c | -- | -- | |
| TH 9043 | Guyana | MK105501c | MK092928c | MK092946c | -- | |
| TH 10395 | Guyana | MK105497c | MK092926c | MK092942c | -- | |
| Amanita guyanensis | MCA 3155 | Guyana | MK105504c | -- | -- | MK092935c |
| TH 9767 | Guyana | MK105502c | MK092929c | MK092948c | MK092937c | |
| TH 9772 | Guyana | MK105503c | MK092930c | MK092944c | MK092941c | |
| Amanita lanivolva | TH 9190 | Guyana | KT339292n | -- | -- | -- |
| Amanita lavendula | RET 639-7 | Ontario, Canada | KR865979m | -- | -- | -- |
| Amanita luteofusca | PSC 1093b | Australia | HQ539705a | -- | -- | -- |
| Amanita luteolovelata | PSC 2187 | Australia | HQ539706a | -- | -- | -- |
| Amanita manginiana | HKAS56933 | China | KJ466438f | KJ466603f | KJ481943f | KJ466515f |
| Amanita modesta | HKAS79688 | China | KJ466440f | KJ466605f | KJ481944f | KJ466516f |
| Amanita morrisii | RET 672-6 | New Jersey, USA | KR919770m | -- | -- | -- |
| Amanita novinupta | RET 60-2 | Oregon, USA | KU248118m | -- | -- | -- |
| Amanita oberwinkleriana | HKAS77330 | China | KJ466441f | KJ466606f | KJ481946f | -- |
| Amanita ocreata | HKAS79686 | California, USA | KJ466442f | KJ466607f | KJ481947f | KJ466518f |
| Amanita aff. odorata | KM 70 | Cameroon | MK105505c | -- | -- | -- |
| Amanita orsonii | RET 717-8 | India | KX270345b | -- | -- | -- |
| Amanita pallidorosea | HKAS75786 | China | JX998054p | KJ466627f | JX998011p | KJ466539f |
| Amanita parvipantherina | HKAS56822 | China | JN941163s | JQ031115s | KJ482005f | KJ466566f |
| Amanita peckiana | RET 320-3 | New York, USA | HQ539720a | -- | -- | -- |
| Amanita phalloides | HKAS75773 | California, USA | JX998060p | KJ466612f | JX998000p | KJ466523f |
| Amanita porphyria | RET 370-10 | Newfoundland, Canada | KP866187t | -- | -- | -- |
| Amanita proxima | RET 290-10 | France | HQ539728a | -- | -- | -- |
| Amanita pseudoporphyria | HKAS56984 | China | KJ466451f | KJ466613f | KJ481952f | KJ466524f |
| Amanita rhoadsii | DD97/13 | North Carolina, USA | AF097391r | -- | -- | -- |
| Amanita rhopalopus | BW_RET 386-3 | West Virginia, USA | HQ539733a | -- | -- | -- |
| Amanita rimosa | HKAS77335 | China | KJ466455f | KJ466393f | KJ481957f | KJ466532f |
| Amanita rubrovolvata | HKAS56744 | China | JN941156u | JQ031117s | -- | -- |
| Amanita sepiacea | ASIS26353 | KU139443q | -- | -- | -- | |
| HKAS38716 | China | AY436501o | -- | -- | -- | |
| Amanita sp. | HKAS77321 | China | KJ466481f | KJ466646f | -- | KJ466560f |
| HKAS77322 | Ohio, USA | KJ466470f | KJ466650f | KJ481984f | KJ466564f | |
| HKAS77339 | South Korea | KJ466482f | KJ466647f | KJ481981f | KJ466561f | |
| HKAS77340 | China | KJ466483f | KJ466648f | KJ481982f | KJ466562f | |
| HKAS77344 | China | KJ466465f | KJ466634f | KJ481969f | KJ466548f | |
| Amanita sp. 12 | TH 9128 | Guyana | JN168681v | -- | -- | -- |
| Amanita sp. 14 | TH 8247 | Guyana | KT339281n | -- | -- | -- |
| Amanita cf. spissacea | OR1214 | Thailand | KY747478e | KY656886e | -- | KY656867e |
| Amanita suballiacea | RET 491-7 | Michigan, USA | KJ466486f | KJ466602f | KJ481942f | KJ466514f |
| Amanita cf. subcokeri | RET 97-3 | New Jersey, USA | HQ539747a | -- | -- | -- |
| Amanita subfrostiana | HKAS57042 | China | JN941162s | JQ031118s | KJ482003f | KJ466565f |
| Amanita subglobosa | HKAS58837 | China | JN941152s | JQ031121s | KJ482004f | KJ466567f |
| Amanita subjunquillea | HKAS77325 | China | KJ466490f | KJ466656f | KJ481988f | KJ466574f |
| Amanita cf. tephrea | RET 378-9 | New York, USA | HQ539751a | -- | -- | -- |
| Amanita vestita | HKAS79687 | China | KJ466494f | KJ466662f | KJ481995f | KJ466581f |
| Amanita virgineoides | HKAS79691 | China | KJ466495f | KJ466663f | KJ481996f | KJ466582f |
| Amanita virosa | HKAS56694 | Finland | JX998058p | KJ466664f | JX998007p | KJ466583f |
| Amanita volvata | RV97/24 | Virginia, USA | AF097388r | -- | -- | -- |
| Amanita westii | BW_SH26 | Texas, USA | HQ539759a | -- | -- | -- |
| Amanita xerocybe | TH 8930 | Guyana | KC155384i | -- | -- | -- |
| Amanita zangii | HKAS77331 | China | KJ466500f | KJ466669f | KJ482001f | KJ466589f |
Wolfe et al. 2012, Mycologia 104: 22–33;
Tulloss et al., unpubl. data;
this study;
Raspe, unpubl. data;
Thongbai et al. 2017, PloS One 12: e0182131;
Cai et al. 2014, Mycol. Prog. 13: 1008;
Vargas et al., unpubl. data;
Matheny et al., unpubl. data;
Smith et al. 2013, PLoS One 8: e55160.;
Thongbai et al. 2016, Phytotaxa 286: 211–231;
Vargas et al., unpubl. data;
Weiss et al. 1998, Canad. J. Bot. 76: 1170–1179;
Tulloss et al., unpubl. data;
Smith et al. 2017, New Phytol. 215: 443–453;
Zhang et al. 2004, Fungal Diversity 17: 219–238;
Cai et al. 2012, Plant Diversity and Resources 34: 614–622;
Seok, unpubl. data;
Drehmel et al. 1999, Mycologia 91: 610–618;
Schoch et al. 2012, Proc. Nat. Acad. Sci. USA 109: 6241–6246;
Hughes, unpubl. data;
Weiss, unpubl. data;
Smith et al. 2011, New Phytol. 192: 699–712.
Sequences were aligned in MEGA v. 7 (Kumar et al. 2016) using the MUSCLE algorithm (Edgar 2004) with refinements to the alignment done manually. Individual gene alignments were concatenated manually after inspection for intergene conflict. Phylogenies were reconstructed using maximum likelihood (ML) and Bayesian methods. PartitionFinder v. 1.1.0 (Lanfear et al. 2012) was used to determine the best partitioning strategy and model of molecular evolution for each partition for both the ML and Bayesian analyses. Maximum likelihood bootstrap analysis for phylogeny and assessment of branch support by bootstrap percentages (% BS) was performed using RAxML (Stamatakis 2014). One-thousand bootstrap replicates were produced. Bayesian analyses for the reporting of Bayesian posterior probability (BPP) support for branches were conducted using the program MrBayes v. 3.2.6 (Ronquist et al. 2012). Four simultaneous, independent runs, each with four Markov chain Monte Carlo (MCMC) chains, were initiated and run at a temperature of 0.20 for 50 M generations, sampling trees every 1000 generations until the standard deviation of the split frequencies reached a final stop value of 0.01. The initial 20 % of trees were discarded as burn-in and a maximum clade credibility tree from the remaining trees was produced using TreeAnnotator. The final alignment and phylogeny can be accessed in TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S23533).
RESULTS
Nine ITS (GenBank accessions: MK064186–MK064193, MK097470), 15 nrLSU, seven ef1-α, ten rpb2 and seven β-tub sequences were generated in this study, ranging from 444–761, 535–1075, 804–1120, 482–1174 and 289–380 bp, respectively. For specimens MCA 3962 and TH 9172, one ITS amplicon of Amanita origin was recovered for both specimens, while two nrLSU amplicons of Amanita origin were recovered from each specimen, one with a 15 bp intron relative to the other; both nrLSU sequences were used in the phylogenetic analysis. After the ends of the individual alignments were trimmed, the size of the aligned dataset was as follows: nrLSU was 941 bp, ef1-α was 536, rpb2 was 669 and β-tub was 235. A 598 bp intron in the rpb2 sequence that was present in specimens MCA 6920, TH 8056, TH 8455, TH 9043 and TH 10395 was removed from the alignment.
Amanita subgen. Lepidella was resolved as monophyletic with strong statistical support (99 % BS/0.98 BPP), along with each of the four sections in subgen. Lepidella: Amidella (100 % BS/0.99 BPP), Lepidella (88 % BS/0.91 BPP), Phalloideae (99 % BS/0.98 BPP) and Validae (99 % BS/0.98 BPP) (Fig. 1). Specimens MCA 3155, TH 9767 and TH 9772 were conspecific and formed a well-supported lineage (98 % BS/1.00 BPP) resolved to sect. Validae and represent A. guyanensis (Fig. 1). Specimens MCA 3962 and TH 9172 were conspecific and formed a well-supported lineage (100 % BS/0.99 BPP), representing A. cyanochlorinosma, and specimens MCA 6290, TH 8056, TH 8455, TH 9043 and TH 10395 were conspecific and formed another well-supported lineage (100 % BS/1.00 BPP), representing A. fulvoalba. The two latter species were resolved to sect. Lepidella (Fig. 1).
Fig. 1.

Maximum-likelihood phylogeny generated from the analysis of four gene fragments (nrLSU, ef1-α, β-tub and rpb2) from 76 specimens in Amanita subgen. Lepidella and eight outgroup specimens in Amanita subgen. Amanita. The new species A. cyanochlorinosma and A. fulvoalba are resolved in sect. Lepidella and A. guyanensis in sect. Validae. Thickened black bars represent nodes with greater than 90 % BS and 0.95 BPP; support values shown above branches represent % BS/BPP; -- represents no support. New species are in bold, and all species from Guyana are in blue.
TAXONOMY
Amanita cyanochlorinosma Mighell & T.W. Henkel, sp. nov. MycoBank MB827394. Figs 2, 3.
Fig. 2.
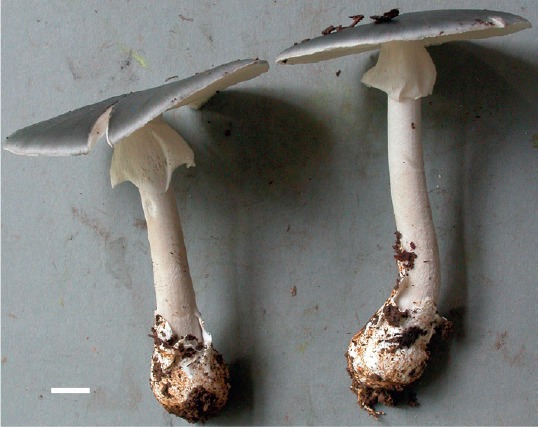
Amanita cyanochlorinosma (Henkel 9172, type). Scale bar = 1 cm.
Fig. 3.

Amanita cyanochlorinosma. A. Basidia and subhymenium. B. Basidiospores. C. Marginal tissue of lamellae. D. Slightly crushed tissue from volval limb. Scale bar =10 µm.
Etymology: cyano- (Gk. comp.) = blue; chlorinosma, referring to the pileus colour and chlorine odour reminiscent of Amanita chlorinosma.
Diagnosis: Similar to Amanita modesta but differs in its strong chlorine odour, larger basidiospores, and greyish blue pileus colour.
Description: Pileus 55–78(–114) mm broad, 3–8 mm tall, planate with slightly downturned margin, with age slightly upturned with a broad, shallow central depression, overall greyish blue (22F4), lighter concolorous (22D3) toward margin, progressively darker brownish grey (10F3–10F4, 11F3–11F4) over disc; surface subviscid, finely appressed radially fibrillose over outer 2/3, appressed matted fibrillose over disc; margin entire, under hand lens finely crenulate; volval elements absent; trama 1 mm thick at margin, 1 mm over lamellae, 3 mm at stipe, white, solid, unchanging. Lamellae finely and abruptly adnexed, thin, crowded, off-white to faintly pinkish cream (5A1–5A2), unchanging; edges concolourous, under hand lens very finely roughened-eroded; lamellulae numerous, usually 2–3, 2–21 mm long. Stipe 55–89(–136) × 6–13 mm, equal to slightly tapering upward from basal bulb, white to faint grey (10A1–10B1), subglabrous macroscopically, under hand lens finely matted fibrillose-floccose, darkening slightly with pressure; bulb 22–30 × 16–31 mm, subglobose, subabrupt apically, narrowing toward base and subradicate; trama white, solid, unchanging. Volva membranous, tightly adhering to bulb with 1–2 ascending limbs loosely appressed to stipe, off-white (2A1–2A2), discoloured brown from adhering soil, with white hyphal cords descending from base. Annulus initially superior, subsuperior with age, white to off-white (2A1–2A2 KW) throughout, thin-membranous, appressed to stipe at apex, lower margin outflaring and pendant, occasionally finely perforate. Odour strongly of chlorine; taste not obtained. Basidiospores white in medium deposit, [200/8/8] (6.0–)7.0–9.0(–10.0) × (4.0–)4.5–6.0(–8.0) μm, Qr = (1.0–)1.16–1.6(–1.66), Qm = 1.32, broadly ellipsoid, smooth, hyaline, amyloid; wall slightly thickened; hilar appendix truncate, up to 1 μm long; contents usually granular-guttulate. Basidia 23–41 × 5.5–8.0 μm, clavate, 4-sterigmate, rarely 3-sterigmate; sterigmata 1.0–5.0 μm long. Subhymenium up to 35 μm thick, composed of globose to ovoid, angular elements up to 44 μm wide. Marginal tissue of lamellae composed of abundant, easily dislodged subglobose to pyriform elements, these 16–27 × 10–19 μm. Lamellar trama bilateral; mediostratum 15–35 μm wide, composed of branched, interwoven, occasionally inflated hyphae, 2–17 μm wide; lateral stratum diverging obtusely from the mediostratum, composed of uninflated hyphae terminating in 1–3 inflated cylindrical to ovoid elements, these 10–49 × 8–18 μm. Pileipellis an ixomixtocutis with two distinct layers; suprapellis 20–115 μm thick; hyphae 2–11 μm wide, partially gelatinised, hyaline, thin-walled, loosely interwoven; subpellis 40–75 μm thick; hyphae 2–8 μm wide, non-gelatinised, hyaline, thin-walled, densely interwoven. Pileus trama with abundant acrophysalides, these 23–122 × 10–44 μm, ellipsoidal to clavate, usually with an abruptly tapered base; contents occasionally granular-guttulate; uninflated tramal hyphae 2–10 μm wide, frequently branching. Stipe trama composed of longitudinally arranged, ovoid, ellipsoid, or clavate acrophysalides, these 25–203 × 12–60 μm, occasionally with granular contents; uninflated, non-conductive tramal hyphae 2–15 μm; conductive hyphae absent to moderately frequent in localized clusters, up to 23 μm wide. Volva at stipe base composed of densely interwoven, often branching, uninflated hyphae 36–174 × 2–10.5 μm; terminal cells cylindrical or clavate, 25–158 × 6–43 μm. Partial veil composed of uninflated hyphae 1–6.5 μm wide, highly branched, thin-walled, densely interwoven; terminal cells 18–50 × 6–16 μm, mostly clavate, occasionally cylindrical or subfusiform; all elements occasionally containing diffuse or clustered granules. Clamp connections absent on hyphae of all tissues.
Habit, habitat and distribution: Solitary, or rarely in pairs, on humic mats of forest floor under D. corymbosa; also found in stands containing D. corymbosa, D. altsonii and A. insignis or P. dipterocarpaceae and D. jenmanii; known from the type locality in the Upper Potaro River Basin and ~25 km to the west in the Upper Mazaruni Basin.
Typus: Guyana, Region 8 Potato-Siparuni, Pakaraima Mountains, Upper Potaro River Basin, ~20 km east of Mt. Ayanganna, Tadang Base Camp 2 km south of Potaro River at 5°16′14.5″N 59°50′39.1″W, elevation 710–750 m; ~ 0.3 km ESE of base camp, on root mat in Dicymbe corymbosa and Dicymbe altsonii co-dominant forest, 30 Dec. 2009, Henkel 9172 (holotype BRG 41298; isotype HSC G1229); GenBank accessions: ITS MK064187; nrLSU MK105493, MK105494; rpb2 MK092933; ef1-α MK092956; β-tub MK092939.
Additional specimens examined: Guyana, Region 8 Potato-Siparuni, Pakaraima Mountains, Upper Potaro River Basin, ~15 km east of Mt. Ayanganna, Potaro base camp at 5°18′04.8″N 59°54′40.4″W, 710–750 m a.s.l.; under D. corymbosa, 3 km E of base camp, 7 May 2001, Henkel 8057 (BRG 41299, HSC G1230); 3.5 km SE of base camp, 19 May 2001, Henkel 8182 (BRG 41300, HSC G1230); 1 km SW of base camp, 25 Jun. 2001, Henkel 8375 (BRG 41301, HSC G1231); 3 km SE of base camp, 11 Jun. 2004, Henkel 8669 (BRG 41302, HSC G1232); 0.4 km SW of base camp near Blackwater Creek, 1 Jul. 2006, Aime 3147 (BRG 41303, PUL F24395); 14 Jun. 2015, Henkel 10083 (BRG 41304, HSC G1233); vicinity of base camp, 18 May 2010, Aime 3962 (BRG 41305, PUL F24396); ~20 km east of Mt. Ayanganna, Tadang base camp at 5°16′14.5″N 59°50′39.1″W, 710–750 m a.s.l.; 100 m E of base camp, under D. corymbosa and D. altsonii, 4 Jun. 2013, Henkel 9737 (BRG 41306, HSC G1234).
Notes: Amanita cyanochlorinosma is a distinctive species recognised in the field by its medium-sized, solitary basidiomata, glabrous, viscid, greyish blue pileus contrasting with the white hymenophore, stipe, and veils, strong odour of chlorine, superior annulus, and saccate-limbate volva. The species is best placed in sect. Lepidella due to its amyloid, broadly ellipsoid basidiospores, saccate volva, membranous annulus, chlorine odour, and gills that do not darken with desiccation (Corner & Bas 1962, Bas 1969). Although the absence of pileal appendiculae suggests placement in sect. Phalloideae, the strong chlorine odour, while known from a handful of sect. Phalloideae species, was emphasized by Bas (1969) as an important character of section Lepidella, occurring in about half of the known species. Additionally, the phylogenetic analysis indicated that A. cyanochlorinosma is nested within sect. Lepidella (Fig. 1).
Few other Amanita species resemble A. cyanochlorinosma. Amanita modesta from lowland tropical rainforests of Malaysia is similar to A. cyanochlorinosma in its small to medium size, membranous annulus, saccate volva, and bluish pileus lacking volval remnants (Corner & Bas 1962). Amanita cyanochlorinosma differs from A. modesta by its chlorine odour, longer basidiospores (7.0–9.0 µm versus 5.9–7.8 µm), and greyish blue as opposed to mouse grey to purplish umber pileus. Amanita cyanochlorinosma resembles the Japanese A. griseoturcosa in its similarly-sized basidiomata, greyish blue pileus, and velar structures, but differs in its chlorine odour and shorter basidiospores (7.0–9.0 µm versus 8.4–12.0 µm) (Tulloss & Yang 2018).
Amanita fulvoalba Mighell & T.W. Henkel, sp. nov. MycoBank MB827395. Figs 4, 5.
Fig. 4.

Amanita fulvoalba (Henkel 10395, type). Scale bar = 1 cm.
Fig. 5.

Amanita fulvoalba. A. Basidia and subhymenium. B. Basidiospores. C. Marginal tissue of lamellae. D. Slightly crushed tissue from volval limb. Scale bars = 10 µm.
Etymology: fulvo- (L. comp.) = yellowish brown; alba- (L. adj. A) = white, referring to the pileus and stipe colours, respectively.
Diagnosis: Similar to A. aurantiobrunnea but differs in its yellowish brown pileus and gelatinised inner volva layer.
Description: Pileus 85–110 mm in broad, 11–22 mm tall, broadly convex to planate with low, broad umbo, yellowish brown (5C8) over disc, lighter concolorous (5B5) toward margin, tacky to moist, shiny and glabrous macroscopically, under hand lens finely appressed radially fibrillose, toward margin minutely rivulose; margin entire, bearing fugacious, fibrillose, irregular white appendiculae, these occasionally triangular, 1–5 mm long; volval remnants lacking; trama 0.5–2 mm thick at margin, 3 mm over lamellae, 6–8 mm over stipe, solid, white, unchanging. Lamellae finely adnexed to adnate, thin, subcrowded, off-white, unchanging; edges concolourous, under hand lens minutely eroded; lamellulae one, 1–40 mm long. Stipe 55–120 × 14–28 mm, sub-cylindrical, white, bruising light orange, longitudinally striate and floccose basally, downy-woolly centrally, finely pulverulent with short striations at apex; bulb 37–47 × 25–40 mm, subglobose to ellipsoid, subabrupt to abrupt, radicating slightly; trama white, solid, unchanging. Volva two layered; inner layer a white, erect to outcurved flange extending 2–3 mm above bulb apex; outer layer enclosing basal bulb, densely membranous, with 1–3 non-clasping limbs ascending 10–19 mm above bulb; exterior off-white, dry, glabrous; interior off-white, moist, shiny, glabrous. Annulus superior, descending with age to central, pendant, fugacious-membranous; exterior off-white, floccose; interior concolourous, striate, floccose. Odour mildly fruity-fungoid. Taste fungoid, indistinct. Basidiospores white in medium deposit, [250/8/8] (6.0–)7.0–9.0(–11) × (5.0–) 5.5–8.0 μm, Qr = (1.1–)1.14–1.43(–1.5), Qm = 1.29, subglobose to broadly ellipsoid, thin-walled, smooth, hyaline, opaque, amyloid; hilar appendix cylindric to conic, truncate, up to 1 mm long; contents granular-guttulate. Basidia 32–53.5 × 7.75–10 μm, clavate, 4-sterigmate; sterigmata 1.5–4.5 μm long; contents granular-guttulate. Subhymenium 18–35 μm thick, composed of 3–4 layers of globose to elliptical, irregularly polygonal elements, these 8–42 μm wide. Marginal tissue of lamellae sterile, composed of easily dislodged globose to pyriform elements, these 16–25 μm wide. Lamellar trama bilateral; mediostratum 20–45 μm wide, composed of interwoven, frequently branched uninflated hyphae with scattered ovoid to ellipsoid inflated elements, these 26–110 × 2–15 μm; lateral stratum diverging obtusely from the mediostratum, composed of branched uninflated hyphae with clavate to ovoid, inflated elements, these 26–106 × 7–28 μm. Pileipellis an ixtomixtocutis with two distinct layers; suprapellis 150–260 μm thick; hyphae 1.5–8 μm wide, strongly gelatinised, periclinal, hyaline, thin-walled, loosely interwoven; subpellis 170–340 μm thick; hyphae 1.5–4 μm wide, slightly gelatinised, mostly periclinal, hyaline, thin-walled, densely interwoven. Pileus trama with abundant acrophysalides, these 25–244 × 11–49 μm, cylindrical, clavate, ellipsoid, or ovoid, often with irregular swollen protrusions; contents frequently granular; uninflated tramal hyphae 2–14 μm wide, branching, often swollen at branch nodes and near acrophysalides; conductive hyphae rare to moderately abundant. Stipe trama with abundant acrophysalides, these 19–97(–223) × 9–44 μm, longitudinally oriented, cylindrical, clavate, ovoid, or ellipsoid; uninflated, non-conductive tramal hyphae 3–12 μm wide; conductive hyphae rare, up to 25 μm wide. Volva at stipe base composed of densely interwoven, uninflated, highly branched hyphae 2–10 μm wide, and abundant inflated cells, these 24–154 × 10–75 μm, limoniform, clavate, globose, or ellipsoid; contents often with granular clusters; inner layer partly gelatinised. Partial veil composed of spherical or rarely limoniform, ellipsoid, or subclavate elements, these 8–45 μm wide, with one or rarely two protruding lateral bulges that occasionally extend into short filaments; uninflated hyphae 2–8 μm wide, thin-walled, attached to inflated cells or in short, detached fragments; all elements diffuse granular or with granular clusters. Clamp connections absent on hyphae of all tissues.
Habit, habitat, and distribution: Solitary or rarely in pairs on humic mat of forest floor under D. corymbosa; known only from the type locality in the Upper Potaro River Basin.
Typus: Guyana, Region 8 Potato-Siparuni, Pakaraima Mountains, Upper Potaro River Basin, ~15 km east of Mt. Ayanganna, Potaro base camp located at 5°18′04.8″N 59°54′40.4″W, 710–750 m, 1 km SW of base camp, on root mat in Dicymbe corymbosa monodominant forest, 29 Dec. 2016, Henkel 10395 (holotype BRG 41307; isotype HSC G1235); GenBank accessions: ITS MK064190; nrLSU MK105497; rpb2 MK092926; ef1-α MK092942.
Additional specimens examined: Guyana, Region 8 Potato-Siparuni, Pakaraima Mountains, Upper Potaro River Basin, ~15 km east of Mt. Ayanganna, Potaro base camp at 5°18′04.8″N 59°54′40.4″W, 710–750 m a.s.l., under D. corymbosa; vicinity of base camp, 7 May 2001, Henkel 8056 (BRG 41308, HSC G1236); 3 km SE of base camp, 10 Jun. 2002, Henkel 8455 (BRG 41309, HSC G1237); 28 Jun. 2004, Henkel 8720 (BRG 41310, HSC G1238); 1.5 km SE of base camp, 30 Jun. 2006, Henkel 8863 (BRG 43311, HSC G1239); 2 km SE of base camp, 13 July 2009, Henkel 9043 (BRG 41312, HSC G1240); 1 km SW of base camp, 25 Jun. 2016, Aime 6290 (BRG 41313, PUL F24397); 28 Dec. 2016, Henkel 10394 (BRG 41314, HSC G1241).
Notes: Amanita fulvoalba is recognised in the field by its medium to large, solitary or paired basidiomata, glabrous yellowish brown pileus often bearing fugacious marginal appendiculae, white hymenophore, stipe, and veils, fugacious-membranous annulus, and robust, saccate-limbate volva. Amanita fulvoalba is best placed in sect. Lepidella due to its amyloid, subglobose to broadly ellipsoid basidiospores, basal bulb, limbate volva, pileal appendiculae, and lamellae that do not darken with desiccation (Corner & Bas 1962, Bas 1969). Several features of A. fulvoalba, however, suggest an affinity for sect. Amidella, including the saccate volva, friable partial veil, and marginal appendiculae. However, given the gelatinised inner volva layer of A. fulvoalba, its marginal appendiculae are likely remains of the friable partial veil, not of the universal veil as in members of sect. Amidella. Additionally, the phylogenetic analysis shows that A. fulvoalba is nested within sect. Lepidella (Fig. 1).
Among the very few described Amanita species worldwide that resemble A. fulvoalba, the sympatric A. aurantiobrunnea is most similar in pileus color, its white stipe, delicate membranous annulus, saccate, two-layered volva, and similar basidiospore dimensions (Simmons et al. 2002). Amanita aurantiobrunnea can be separated from A. fulvoalba by its deeper orange (vs. yellowish brown) pileus and orange, friable (vs. gelatinised) inner volva layer.
The European Amanita proxima has a saccate volva, basal bulb, and similar basidioma dimensions as A. fulvoalba. However, the former has an ochraceous to reddish brown volva, whitish to ivory pileus, and a more persistent annulus than A. fulvoalba. Amanita gayana, a species known only from the description from Chile, loosely resembles A. fulvoalba in its orange pileus, limbate, membranous volva, and white stipe. The species is, however, much smaller than A. fulvoalba, with a pileus < 54 mm wide and a stipe < 13.5 mm tall, and has pale yellow as opposed to white lamellae (Tulloss & Yang 2018).
Amanita guyanensis Mighell & T.W. Henkel, sp. nov. MycoBank MB827396. Figs 6, 7.
Fig. 6.

Amanita guyanensis (Henkel 9767, type). Scale bar = 1 cm.
Fig. 7.

Amanita guyanensis. A. Basidia and subhymenium. B. Basidiospores. C. Slightly crushed tissue of volval wart on pileus. Scale bars = 10 µm.
Etymology: Guyana, and -ensis (L. adj. B), referring to the known distribution of the species across central Guyana.
Diagnosis: Similar to A. brunnescens but differs in its smaller, unclefted basal bulb.
Description: Pileus 10–94 mm broad, 4–34 mm tall, broadly convex, with age upturned, dark grey-brown or greyish brown (6F2, 7F4) throughout or darker over disc; surface dry to subviscid, glabrous macroscopically, under hand lens minutely appressed radially fibrillose, finely felted over disc; marginal fibrils separating with age revealing pale brown ground; margin entire, splitting slightly with age; volval warts with uniform, occasionally concentric arrangement, flattened to pyramidal, 1–10 mm wide, up to 1 mm tall, light grey-brown (6E3–6F3) with lighter concolourous to nearly off-white (4A1–4A2) apices, detersile; trama 0.5 mm at margin, 2 mm over lamellae, 3 mm over stipe, solid, white, unchanging. Lamellae finely adnexed to subfree, thin, close to crowded, white, becoming greyish or slightly orange-tinted (5C3–5C4) with age; edges concolourous, finely roughened, unchanging or browning slightly with pressure; lamellulae 1–2, 2–5 mm long. Stipe 35–105 × 5–14 mm, equal to slightly tapering upward from basal bulb, flaring slightly at extreme apex to 8–22 mm; apical portion above annulus usually white, occasionally grey (6D3–6D4), subscabrous; lower portion with greyish brown (6F4) appressed fibrils over white ground, these more concentrated toward base; bulb 8–20 × 11–30 mm, subglobose, subabrupt and occasionally flattened at apex, angled slightly from stipe axis, greyish over apex, lower portion progressively lighter concolourous and finely tomentose; volva of light grey-brown matted fibrillose to floccose scales adhering to bulb apex; trama white, subsolid, unchanging. Annulus subsuperior, 20–30 mm below stipe apex, membranous, pendant; interior white to off-white; exterior whitish marginally, grey near stipe; extreme margin an eroded band of grey, fine, floccose scales. Odour minimal, fungoid. Taste slightly sweet, occasionally with bitter overtones. Basidiospores white in medium deposit, [225/9/9] 5.0–9.0(–11.0) × (4.0–)5.0–9.0(–10.0) µm, Qr = (0.94–)1.0–1.27(–1.33), Qm = 1.09, subglobose to globose, thin-walled, smooth, hyaline, amyloid; hilar appendix subpyramidal, truncate, up to 1 mm long; contents usually one dark brown guttule, rarely of smaller guttules or granules. Basidia 22–38 × 5.5–13 µm, clavate, 4-sterigmate; contents granular or guttulate, sometimes both; sterigmata 2–5 µm long, lanceolate to slightly incurved. Subhymenium 16–30 µm thick, composed of 3–4 layers of subglobose to irregularly polygonal elements, these 3–19 µm wide. Marginal tissue of lamellae partially gelatinised, composed of easily dislodged, mostly globose, occasionally ellipsoid, fusiform or clavate elements, these 9–65 × 8–22 µm. Lamellar trama bilateral; mediostratum 20–40 µm wide, of interwoven, branching hyphae; inflated elements up to 17 µm wide, cylindrical or clavate; lateral stratum contiguous with mediostratum and diverging at an obtuse angle; hyphae 4–19 µm wide; conductive hyphae rare. Volval pileal warts composed of inflated, brownish globose cells often with a small, protruding filament. Pileipellis an ixtomixtocutis, two-layered with indistinct boundary; suprapellis 140–240 µm thick, hyphae heavily gelatinised, thin-walled, densely interwoven; subpellis 140–250 µm thick, hyphae brownish, densely interwoven, periclinal. Pileus trama with frequent acrophysalides, these cylindric, fusiform, ellipsoid, or ovoid, 22–190 × 8–50 µm; uninflated tramal hyphae densely interwoven, highly branching, 2–6 µm; conductive hyphae not observed; contents of most elements of small (≤ 1 µm), globose, hyaline guttules and/or opaque granules, often in clusters. Stipe trama longitudinally oriented, with clavate or occasionally subglobose to fusiform acrophysalides, these 32–315 × 17–58 µm; uninflated hyphae highly branched, 2–6 µm wide; conductive hyphae not observed.Volva at stipe base composed mostly of inflated, globose or rarely ellipsoid to limoniform elements, 22–49 µm wide; filamentous hyphae sparse, present as branched fragments or attached to inflated elements. Partial veil composed of densely interwoven, branching, highly serpentine uninflated hyphae 1–7 µm wide, most with opaque granular contents. Clamp connections absent on hyphae of all tissues.
Habit, habitat, and distribution: Solitary to scattered on humic mat of forest floor under D. corymbosa; also found in stands containing D. corymbosa and D. altsonii or Pakaraimaea dipterocarpacea and Dicymbe jenmanii on a variety of soil types; known from the type locality in the Upper Potaro River Basin, ~25 km to the west in the Upper Mazaruni Basin, and ~100 km east in the Mabura Ecological Reserve.
Typus: Guyana, Region 8 Potato-Siparuni, Pakaraima Mountains, Upper Potaro River Basin, ~20 km east of Mt. Ayanganna, Tadang base camp 2 km south of Potaro River at 5°16′14.5″N 59°50′39.1″W, elevation 710–750 m; 0.3 km NE of base camp in Dicymbe corymbosa and Dicymbe altsonii co-dominant forest, 7 Jun. 2013, Henkel 9767 (holotype BRG 41315; isotype HSC); GenBank accessions: ITS MK064192; nrLSU MK105502; rpb2 MK092929; ef1-α MK092948; β-tub MK092937.
Additional specimens examined: Guyana, Region 8 Potato-Siparuni, Pakaraima Mountains, Upper Potaro River Basin, ~15 km east of Mt. Ayanganna, Potaro base camp at 5°18′04.8″N 59°54′40.4″W, 710–750 m a.s.l., under D. corymbosa; 4 km SW of base camp, 5 May 2001, Henkel 8034 (BRG 41316, HSC G1244); 3 km SE of base camp, 11 May 2001, Henkel 8083 (BRG 41317, HSC G1245); 28 Jun. 2004, Henkel 8712 (BRG 41318, HSC G1246); 0.4 km SW of base camp near Blackwater Creek, 1 Jul. 2006, Aime 3155 (BRG 41319, PUL F24398); vicinity of base camp, 11 Jul. 2008, Henkel 8931 (BRG 41320, HSC G1243); 4 km SW of base camp, 20 May 2010, Aime 3991 (BRG 41321, PUL F24399); 0.5 km E of base camp, 15 Jun. 2015, Henkel 10081 (BRG 41322, HSC G1247). ~20 km east of Mt. Ayanganna, Tadang Base Camp 2 km south of Potaro River at 5°16′14.5″N 59°50′39.1″W, 710–750 m a.s.l., under D. corymbosa and D. altsonii; 2 km SW of base camp, 8 Jun. 2013, Henkel 9772 (BRG 41323, HSC G1248). Region 10 Upper Demerara-Berbice, Mabura Ecological Reserve, field station at 5°09′19.0″N 58°41′58.9″W, ~100 m a.s.l.; 0.4 km WNW of field station in Dicymbe altsonii plot #1, 1 Jun. 2011, Henkel 9624 (BRG 41324, HSC G1249). Region 7 Cuyuni-Mazaruni, Pakaraima Mountains. Mazaruni River Basin, ~20 km NW of Mt. Ayanganna summit, base camp located at 5°26′21.3"N 60°04′43.1"W, 760 m a.s.l., in savanna fringing forest dominated by P. dipterocarpacea and D. jenmanii; ~0.4 km NW of base camp, 28 Dec. 2010, Henkel 9563 (BRG 41325, HSC G1250); 0.2 km ESE of base camp, 4 Jun. 2012, Henkel 9674 (BRG 41326, HSC G1251).
Notes: Amanita guyanensis is recognised in the field by its dark grey-brown pileus bearing off-white to greyish volval patches, white stipe, subglobose, angled basal bulb with a apical ring of grey floccose scales, and white, pendant annulus with a grey margin. Amanita guyanensis is best placed in sect. Validae due its amyloid, subglobose to globose basidiospores, deeply pigmented pileus, pulverulent volva, and the absence of marginal appendiculae (Corner & Bas 1962, Bas 1969, Cui et al. 2018). Additionally, the phylogenetic analysis shows that A. guyanensis is allied with other species in a monophyletic sect. Validae, including the type species of the section, A. excelsa (Fig. 1).
Worldwide there are numerous species of sect. Validae which resemble A. guyanensis in stature and colour. In the Neotropics, A. campinaranae from the central Amazon closely resembles A. guyanensis in stature, pigmentation, and velar characteristics. However A. campinaranae differs from A. guyanensis in its pallid, white to greyish pileus, forked lamellae, and smaller basidiospores (5.6–6.7 × 5.5–6.5 µm vs. 5–9 × 5–9 µm) (Bas 1978). The sympatric and loosely similar A. perphaea can be separated by its more fragile partial veil, sulcate-striate pileus margin, distinct encrusting pigments in the pileipellis, and overall darker grey colour of the pileus, volva, and stipe (Simmons et al. 2002).
Among tropical African Amanita species A. echinulata is similar to A. guyanensis in stature and both may have a bitter taste, but A. echinulata can be separated by its grey annulus, dark sooty brown pileus and stipe, and smaller basidiospores (Beeli 1935; Mighell & Henkel pers. obs.). An as yet undescribed species (Amanita morphospecies #19) collected in Cameroon’s Dja Biosphere Reserve is macroscopically very similar to A. guyanensis (Mighell & Henkel unpubl. data). This species exhibits similar basidioma size ranges, pigmentation, and velar characteristics as those of A. guyanensis, but differs by its less defined volval remnants on the stipe and odour of raw potato.
Amanita innatifibrilla from subtropical China has a dark grey-brown pileus bearing concolorous to grey warts and floccose volval remnants at its bulb apex, but differs from A. guyanensis in its smaller stature, innate pileal fibrils, regularly central annulus, fusiform bulb, and smaller basidiospores (Cui et al. 2018). Species from Singapore described by Corner & Bas (1962) include A. squamosa which resembles A. guyanensis in its sepia-fuscous pileus, pale brownish warts, and white annulus, but differs in its spindle-shaped bulb with recurved scales and smaller basidiospores. Amanita tristis is similar in basidioma size, colours, and volval remnants to A. guyanensis, but its ellipsoid basidiospores are smaller (4.9–6.1 × 4.3–4.6 µm vs. 5–9 × 5–9 µm) than the subglobose to globose spores of A. guyanensis.
Among north temperate species similar to A. guyanensis, A. brunnescens and A. sepiacea each have similarly sized basidiomata, a dark grey to brown pileus, white, superior annulus, basal bulb with apically adhering volval remnants, and basidiospores of similar size. Amanita brunnescens is separated by its large, marginate, clefted basal bulb, as opposed to the smaller, unclefted bulb of A. guyanensis. Amanita sepiacea has grey squamules on the stipe base, which contrast with the longitudinal fibrils of A. guyanensis (Tulloss & Yang 2018). Amanita porphyria and A. submaculata loosely resemble A. guyanensis, but the former is differentiated by its violaceous pileus and volva, and the latter by its reddening of bruised lamella and exposed stipe trama.
Finally, A. xanthomargaros, A. pausiaca, A. walpolei, A. luteolovelata, and A. luteofusca all have a grey to brown pileus and similar basidioma size, but can be differentiated from A. guyanensis by their yellow pigments in either the universal veil, annulus, or stipe tissues.
ACKNOWLEDGEMENTS
We thank the following funding sources: National Science Foundation (NSF) DEB-0918591, DEB-1556338, National Geographic Society’s Committee for Research and Exploration grants 6679-99, 7435-03 and 8481-08 to T.W.H., and NSF DEB-0732968 to M.C.A. Field assistance in Guyana was provided by M. Chin, P. Henkel, D. Husbands, J. Uehling, C. Andrew, V. Joseph, P. Joseph, F. Edmund, and L. Edmund. Research permits were granted by the Guyana Environmental Protection Agency. This paper is number 229 in the Smithsonian Institution’s Biological Diversity of the Guiana Shield Program publication series.
REFERENCES
- Bas C. (1969). Morphology and subdivision of Amanita and a monograph of its section Lepidella. Persoonia 5: 285–573. [Google Scholar]
- Bas C. (1978). Studies in Amanita - I: some species from Amazonia. Persoonia 10: 1–22. [Google Scholar]
- Bas C, de Meijer AAR. (1993). Amanita grallipes, a new species of Amanita subsection Vittadiniae from Southern Brazil. Persoonia 15: 345–350. [Google Scholar]
- Beeli M. (1935). Flore iconographique des champignons du Congo. Fascicle I. Amanita, Amanitopsis, Volvaria. Jardin botanique de l’Etat, Belgium. [Google Scholar]
- Cai Q, Tulloss RE, Tang LP, et al. (2014). Multi-locus phylogeny of lethal amanitas: Implications for species diversity and historical biogeography. BMC Evolutionary Biology 14: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corner EJH, Bas C. (1962). The genus Amanita in Singapore and Malaya. Persoonia 2: 241–304. [Google Scholar]
- Cui YY, Cai Q, Tang LP, et al. (2018). The family Amanitaceae: molecular phylogeny, higher-rank taxonomy and the species in China. Fungal Diversity 91: 5–230. [Google Scholar]
- Drehmel D, Moncalvo JM, Vilgalys R. (1999). Molecular phylogeny of Amanita based on large-subunit ribosomal DNA sequences: implications for taxonomy and character evolution. Mycologia 91: 610–618. [Google Scholar]
- Ebenye MCH, Taudière A, Niang N, et al. (2017). Ectomycorrhizal fungi are shared between seedlings and adults in a monodominant Gilbertiodendron dewevrei rain forest in Cameroon. Biotropica 49: 256–267. [Google Scholar]
- Edgar RC. (2004). MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardes M, Bruns TD. (1993). ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118. [DOI] [PubMed] [Google Scholar]
- Geml J, Laursen GA, O’Neill K, et al. (2006). Beringian origins and cryptic speciation events in the fly agaric (Amanita muscaria). Molecular Ecology 15: 225–239. [DOI] [PubMed] [Google Scholar]
- Halling RE. (2001). Ectomycorrhizae: co-evolution, significance, and biogeography. Annals of the Missouri Botanical Garden 88: 5–13. [Google Scholar]
- Henkel TW, Aime MC, Chin MML, et al. (2012). Ectomycorrhizal fungal sporocarp diversity and discovery of new taxa in Dicymbe monodominant forests of the Guiana Shield. Biodiversity and Conservation 21: 2195–2220. [Google Scholar]
- Kim CS, Jo JW, Kwag Y-N, et al. (2013). Taxonomic study of Amanita subgenus Lepidella and three unrecorded Amanita species in Korea. Mycobiology 41: 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornerup A, Wanscher JH. (1978). Methuen handbook of colour. 3rd edn Eyre Methuen, London. [Google Scholar]
- Kumar S, Stecher G, Tamura K. (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfear R, Calcott B, Ho SY, et al. (2012). PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analysis. Molecular Biology and Evolution 29: 1695–1701. [DOI] [PubMed] [Google Scholar]
- Lechner BE, Alberto E. (2008). Poisonous species of Agaricales found in Argentina: new record of Amanita pantherina and revaluation of the edibility of Tricholoma equestre. Boletin de la Sociedad Argentina de Botanica 43: 227–35. [Google Scholar]
- Menolli N, Capelari M, Baseia IG. (2009). Amanita viscidolutea, a new species from Brazil with a key to Central and South American species of Amanita section Amanita. Mycologia 101: 395–400. [DOI] [PubMed] [Google Scholar]
- Moncalvo J-M, Drehmel D, Vilgalys R. (2000a). Variation in modes and rates of evolution in nuclear and mitochondrial ribosomal DNA in the mushroom genus Amanita (Agaricales, Basidiomycota): phylogenetic implications. Molecular Phylogenetics and Evolution 16: 48–63. [DOI] [PubMed] [Google Scholar]
- Moncalvo J-M, Lutzoni FM, Rehner SA, et al. (2000b). Phylogenetic relationships of agaric fungi based on nuclear large subunit ribosomal DNA sequences. Systematic Biology 49: 278–305. [DOI] [PubMed] [Google Scholar]
- Moser M. (1967). Die Röhrlinge und Blätterpilze (Agaricales). Gustav Fischer Verlag, Germany. [Google Scholar]
- Mueller GM, Halling RE. (1995). Evidence for high biodiversity of Agaricales (Fungi) in Neotropical montane Quercus forests. In: Biodiversity and conservation of neotropical montane forests (Churchill S, ed). The New York Botanical Garden, USA: 303–312. [Google Scholar]
- Palacio M, Gutierrez Y, Franco-Molano AE, et al. (2015). New records of macrofungi (Basidiomycota) for Colombia from a tropical dry forest. Actualidades Biologicas 37: 79–99. [Google Scholar]
- Rehner SA, Buckley E. (2005). A Beauveria phylogeny inferred from nuclear ITS and EF1-αsequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97: 84–98. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, et al. (2012). MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch CL, Seifert KA, Huhndorf S, et al. (2012). Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Sciences, USA 109: 6241–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons C, Henkel T, Bas C. (2002). The genus Amanita in the Pakaraima Mountains of Guyana. Persoonia 17: 563–582. [Google Scholar]
- Singer R. (1986). The Agaricales in modern taxonomy. 4th edn Koeltz Scientific Books, Germany. [Google Scholar]
- Smith ME, Henkel TW, Aime MC, et al. (2011). Ectomycorrhizal fungal diversity and community structure on three co-occurring leguminous canopy tree species in a Neotropical rainforest. New Phytologist 192: 699–712. [DOI] [PubMed] [Google Scholar]
- Smith ME, Henkel TW, Uehling JK, et al. (2013). The ectomycorrhizal fungal community in a neotropical forest dominated by the endemic Dipterocarp Pakaraimaea dipterocarpacea. PLoS One 8: e55160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobestiansky G. (2005). Contribution to a macromycete survey of the states of Rio Grande do Sul and Santa Catarina in Brazil. Brazilian Archives of Biology and Technology 48: 437–57. [Google Scholar]
- Stamatakis A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang LP, Cai Q, Lee SS, et al. (2015). Taxonomy and phylogenetic position of species of Amanita sect. Vaginatae s.l. from tropical Africa. Mycological Progress 14: 39–54. [Google Scholar]
- Tedersoo L, Brundrett MC. (2017). Evolution of ectomycorrhizal symbiosis in plants. In: Biogeography of mycorrhizal symbiosis (Tedersoo L, ed). Springer, USA: 407–467. [Google Scholar]
- Thongbai B, Tulloss RE, Miller SL, et al. (2016). A new species and four new records of Amanita (Amanitaceae; Basidiomycota) from Northern Thailand. Phytotaxa 286: 211–231. [Google Scholar]
- Truong C, Sánchez-Ramírez S, Kuhar F, et al. (2017). The Gondwanan connection – Southern temperate Amanita lineages and the description of the first sequestrate species from the Americas. Fungal Biology 121: 638–51. [DOI] [PubMed] [Google Scholar]
- Tulloss RE, Ovrebo CL, Halling RE. (1992). Studies on Amanita (Amanitaceae) from Andean Colombia. Memoirs of the New York Botanical Garden 66: 1–46. [Google Scholar]
- Tulloss RE, Halling RE. (1997). Type studies of Amanita morenoi and Amanita pseudospreta and a reinterpretation of crassospores in Amanita. Mycologia 89: 278–288. [Google Scholar]
- Tulloss RE. (2005). Amanita - distribution in the Americas with comparison to eastern and southern Asia and notes on spore character variation with latitude and ecology. Mycotaxon 93: 189–231. [Google Scholar]
- Tulloss RE, Franco-Molano AE. (2008). Studies in Amanita subsection Vittadiniae 1 – A new species from Colombian savanna. Mycotaxon 105: 317–323. [Google Scholar]
- Tulloss RE, Yang ZL. (2018). Subgenus Lepidella in Amanitaceae studies. [http://www.amanitaceae.org/?subgenus%20Lepidella], accessed 12 April 2018.
- Vargas N, Pardo-de La Hoz CJ, Danies G, et al. (2017). Defining the phylogenetic position of Amanita species from Andean Colombia. Mycologia 109: 261–76. [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartchow F. (2. 015). Amanita tenacipulvis, a new species from Amazonian campinarana. Sydowia 67: 75–9. [Google Scholar]
- Wartchow F. (2016). Amanitaviridissima (Amanitaceae, Basidiomycota), a striking new species from highlands of the semiarid region of Bahia, Brazil. Plant Ecology and Evolution 149: 241–248. [Google Scholar]
- Wartchow F, Cortez VG. (2016). A new species of Amanita growing under Eucalyptus is discovered in South Brazil. Mycosphere 7: 262–267. [Google Scholar]
- Wartchow F, Gamboa-Trujillo JP. (2012). Amanita chocoana - a new species from Ecuador. Mycotaxon 121: 405–12. [Google Scholar]
- Wartchow F, Maia LC, Cavalcanti MAQ. (2013). Studies on Amanita (Agaricomycetidae, Amanitaceae) in Brazil: two yellow gemmatoid taxa. Nova Hedwigia 96: 61–71. [Google Scholar]
- Wartchow F, Sulzbacher MA, Baseia IG. (2015). Amanita psammolimbata, a new species from Northeastern Brazilian sand dunes. Mycosphere 6: 260–5. [Google Scholar]
- Wartchow F, Tulloss RE, Cavalcanti MA. (2007). The discovery of Amanita lilloi in Brazil. Mycotaxon 99: 167–174. [Google Scholar]
- Wartchow F, Tulloss R, Cavalcanti MAQ. (2009). Amanita lippiae: a new species from the semi-arid caatinga region of Brazil. Mycologia 101: 864–870. [DOI] [PubMed] [Google Scholar]
- Watling R, Lee LS. (1995). Ectomycorrhizal fungi associated with members of the Dipterocarpaceae in peninsular Malaysia. Journal of Tropical Forest Science 7: 647–669. [Google Scholar]
- Weiss M, Yang ZL, Oberwinkler F. (1998). Molecular phylogenetic studies in the genus Amanita. Canadian Journal of Botany 76: 1170–1179. [Google Scholar]
- Wolfe BE, Tulloss RE, Pringle A. (2012). The irreversible loss of a decomposition pathway marks the single origin of an ectomycorrhizal symbiosis. PLoS One 7: e39597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagt RJ. (1997). Pre-dispersal and early post-dispersal demography, and reproductive litter production, in the tropical tree Dicymbe altsonii in Guyana. Journal of Tropical Ecology 13: 511–526. [Google Scholar]
- Zhang LF, Yang JB, Yang ZL. (2004). Molecular phylogeny of eastern Asian species of Amanita (Agaricales, Basidiomycota): taxonomic and biogeographic implications. Fungal Diversity 17: 219–238. [Google Scholar]


