Abstract
The analysis of a combined dataset including 5.8S (ITS) rDNA, 18S rDNA, 28S rDNA, and rpb2 data from species of the Agaricineae (Agaricoid clade) supports a shared monophyletic origin of the monotypic genera Mythicomyces and Stagnicola. The new family Mythicomycetaceae, sister to Psathyrellaceae, is here proposed to name this clade, which is characterised, within the dark-spored agarics, by basidiomata with a mycenoid to phaeocollybioid habit, absence of veils, a cartilaginous-horny, often tapering stipe, which discolours dark brown towards the base, a greyish brown, pale hazel brown spore deposit, smooth or minutely punctate-verruculose spores without a germ pore, cheilocystidia always present, as metuloids (thick-walled inocybe-like elements) or as thin-walled elements, pleurocystidia, when present, as metuloids, pileipellis as a thin ixocutis without cystidioid elements, clamp-connections present everywhere, and growth on wood debris in wet habitats of boreal, subalpine to montane coniferous forests. Simocybe parvispora from Spain (two collections, including the holotype), which clusters with all the sequenced collections of Stagnicola perplexa from Canada, USA, France and Sweden, must be regarded as a later synonym of the latter.
Keywords: Agaricomycetes, Basidiomycota, molecular systematics, new taxa, Phaeocollybia, Psathyrellaceae, taxonomy
INTRODUCTION
The Agaricineae emend. Aime et al. represents one of the seven suborders recently recognised in the Agaricales by Dentinger et al. (2016) using a phylogenomic approach. This corresponds to a previously recognised “Agaricoid” clade, which has been consistently recovered as monophyletic in recent studies (Matheny et al. 2006, 2015, Garnica et al. 2007, Binder et al. 2010, Kohler et al. 2015). Many species in this suborder show pigmented and thick-walled spores (Matheny et al. 2006, 2015, Garnica et al. 2007). Although species producing dark-pigmented spores (dark-pigmented agarics) are present in a few other lineages (e.g. Melanomphalia, Aime et al. 2005 or Ripartites, Walther et al. 2005, Garnica et al. 2007), the overwhelming majority of these have evolved within Agaricineae. The presence of spores with a thickened, dark-pigmented wall is perhaps indicative of adaptations to specialised environments (e.g. dung, burnt sites) (Garnica et al. 2007, Halbwachs et al. 2015).
Within the dark (brown)-spored agarics, the monotypic genera Mythicomyces and Stagnicola were established by Redhead & Smith (1986) based on the two morphologically similar species Agaricus corneipes and Phaeocollybia perplexa, respectively. The two taxa occupy a rather isolated position within the brown-spored agarics and share a complex of characters that make it difficult to place them at the family level: a mycenoid to phaeocollybia-like appearance (i.e. a widely acute umbonate pileus and a dark brown, cartilaginous-horny, tapering stipe with a tawny strigosity at base), a pallid spore deposit, greyish brown, pale hazel brown to milk coffee brown with light purple tones, spores without a germ-pore and almost hyaline or faintly brownish under light microscope, a pileipellis as a thin ixocutis, growth on wood debris, presence of clamp-connections. Mythicomyces differs from Stagnicola mainly by minutely verrucose spores, thick-walled hymenial cystidia and a spore deposit with purple hues. Redhead & Smith (1986) tentatively placed the two genera in the Strophariaceae s. Kuhner (1984) and the Cortinariaceae s.l., respectively, mainly based on spore-print colour. In subsequent years, such family placements were debated and questioned. On morphological basis only, Watling & Gregory (1993) suggested S. perplexa to be probably better placed in Inocybeae within Cortinariaceae. Horak (2005) recognised both in Cortinariaceae and Gulden (2008a, b) in Crepidotaceae (family which includes also Inocybaceae according to the author). Extensive phylogenetic studies based on large datasets by Moncalvo et al. (2002), Matheny et al. (2006, 2015), Padamsee et al. (2008), Nagy et al. (2011) and Zhao et al. (2017) resolved either one or both of these genera in a clade sister to the Psathyrellaceae (Agaricineae, formerly Agaricoid clade s. Matheny et al. 2006, 2015, Garnica et al. 2007, Binder et al. 2010). The molecular work by Matheny & Griffith (2010) based on a dataset limited to Squamanita and allied taxa, indicated M. corneipes as sister (with low support) to a superclade formed by Psathyrellaceae, Cystodermateae and Nidulariaceae. In the phylogenetic analysis by Gulden et al. (2005) based on a dataset of only brown-spored agarics, Stagnicola occupied an incertae sedis position, while Mythicomyces clustered in Strophariaceae s. l. In Broussal & Dumesny (2015), Mythicomyces and Stagnicola were sister to each other (with low support) and were part of a superclade, consisting of Cortinariaceae, Bolbitiaceae, Tubariaceae, Strophariaceae and Hymenogastraceae, which was sister to Psathyrellaceae.
However, in molecular studies where both Mythicomyces and Stagnicola were taken into account at the same time, they were represented at most by two collections each with only 28S rDNA sequences (Moncalvo et al. 2002, Gulden et al. 2005, Padamsee et al. 2008, Broussal & Dumesny 2015).
In accordance with all these molecular studies indicating a sisterhood relationship of the two genera to Psathyrellaceae, Mythicomyces and Stagnicola have recently been classified within the Psathyrellaceae by Gulden (2012a, b), Strittmatter & Obenauer (2013) and Prydiuk (2015, 2018). Inexplicably, and probably because they were unaware of the results of the previous molecular studies, the two most frequently used online registries, Index Fungorum (http://www.indexfungorum.org), Mycobank (http://www.mycobank.org/), and Begerow et al. (2018) assigned the two genera to two different families, namely Mythicomyces to the Psathyrellaceae and Stagnicola to the Strophariaceae.
Currently, whereas at least one collection of Mythicomyces corneipes (AFTOL-ID 972) is represented by multiple markers in GenBank (https://www.ncbi.nlm.nih.gov/genbank/), only a few collections of Stagnicola perplexa are present and only with ribosomal (ITS and 28S) sequences. The aim of the present paper was to provide Stagnicola with a sound phylogenetic placement within the Agaricineae by increasing its taxon sampling and the number of molecular markers. The study of the type collections of Simocybe parvispora, which is part of a research project in progress on the European species of the genus Simocybe, has revealed its conspecificity with Stagnicola perplexa. Morphological and molecular data of these collections were also included because they are central to the main focus of the present work.
MATERIALS AND METHODS
Morphological examination
The microscopic structures were examined from dried material, in different mountants: water, L4 [7.2 g KOH, 160 mL glycerine, 840 mL dH2O, 7.6 g NaCl and 5 mL Invadin (Ciba-Geigy), Clémençon 1972], Melzer’s reagent, ammoniacal Congo red, Phloxine, Cresyl blue and Cotton blue. Cresyl blue and Cotton blue were utilised to highlight the ortho-/metachromatic reactions in the spores. Dried fragments were rehydrated in water and mounted in L4. All microscopic measurements were carried out under oil immersion at ×1 000 with Nikon Eclipse 80i microscope.
Spore measurements were made by photographing all the spores (taken from lamellar squashes of exsiccate material of mature specimens) occurring in the visual field of the microscope using the Mycomètre software (Fannechère 2011). Spore dimensions do not include the hilar appendix, and are reported as follows: average minus standard deviation – average plus standard deviation of length × average minus standard deviation – average plus standard deviation of width; Q = average minus standard deviation – average plus standard deviation of ratio length/width; Qm = average ± standard deviation of ratio length/ width; V = average minus standard deviation – average plus standard deviation of the volume [μm3]; Vm = average ± standard deviation of the volume [μm3]. The approximate spore volume was calculated as that of an ellipsoid (Gross 1972, Meerts 1999). The notation “n/m/p” indicates that measurements were made on “n” randomly selected spores from “m” basidiomes of “p” collections. The width of the basidia was measured at the widest part, and the length was measured from the apex (sterigmata excluded) to the basal septum. Microscopic pictures were taken using a Nikon DS 5M digital connected to a Nikon Eclipse 80i microscope with both brightfield and interferential contrast optics.
DNA extraction, amplification and sequencing
Total DNA was extracted from eight dry specimens (Table 1) employing a modified protocol based on Murray & Thompson (1980). PCR amplification was performed with the primers ITS1F and ITS4 (White et al. 1990, Gardes & Bruns 1993) for the ITS rDNA region, while LR0R and LR5 (Vilgalys & Hester 1990, Cubeta et al. 1991) were used to amplify the 28S (LSU) rDNA region, NS19b and NS41 for the 18S (SSU) rDNA ribosomal region (Hibbett 1996), and bRPB2-6F and bRPB2-7R2 for the RNA polymerase II second largest subunit (rpb2) gene (Liu et al. 1999, Matheny et al. 2007a). PCR reactions were performed under a program consisting of a hot start at 95 °C for 5 min, followed by 35 cycles at 94 °C, 54 °C and 72 °C (45, 30 and 45 s respectively) and a final 72 °C step for 10 min. PCR products were checked in 1 % agarose gels, and positive reactions were sequenced with one or both PCR primers. Chromatograms were checked searching for putative reading errors, and these were corrected. The sequences were submitted to GenBank (http://www.ncbi.nlm.nih.gov/genbank/) and their accession numbers are reported in Table 1.
Table 1.
Samples used for the present phylogenetic studies. Newly sequenced collections are in bold.
| Taxon | Voucher | Country | GenBank acc. numbers | |||
|---|---|---|---|---|---|---|
| ITS | 28S | 18S | rpb2 | |||
| Agaricaceae sp. | RC_Mart06_016 | Martinique | HQ839742 | HQ839743 | HQ839744 | HQ839745 |
| Agaricus aff. campestris | PBM 2580 | Massachusetts, USA | DQ486682 | DQ110871 | DQ113914 | ── |
| Agaricus bisporus | AFTOL-ID 448/strain OMF | Denmark/USA | DQ404388 | AY635775 | AY787216 | AF107785 |
| Agaricus sylvaticus | JFM-AS | Taiwan | ── | AJ244523 | AJ012405 | ── |
| Agrocybe pediades | AFTOL-ID 1493 | California, USA | DQ484057 | DQ110872 | DQ113915 | ── |
| Agrocybe praecox | AFTOL-ID 728 | Washington, USA | AY818348 | AY646101 | AY705956 | DQ385876 |
| Agrocybe rivulosa | strain CCB160 | Tennessee, USA | KF830098 | KF830090 | KF830078 | KF830069 |
| Agrocybe smithii | AFTOL-ID 1494 | Washington, USA | DQ484058 | DQ110873 | DQ115779 | ── |
| Bogbodia uda (“Nematoloma longisporum”) | AFTOL-ID 1893 | Massachusetts, USA | DQ490634 | DQ457681 | DQ444863 | ── |
| Bolbitius viscosus | PBM 3032 | Tennessee, USA | HQ840656 | HQ840657 | KJ137269 | HQ840658 |
| Bolbitius vitellinus | AFTOL-ID 1730 | Washington, USA | DQ200920 | AY691807 | AY705955 | DQ385878 |
| Chlorophyllum agaricoides | AFTOL-ID 440 | Greece | DQ200928 | AY700187 | AY657010 | ── |
| Conocybe lactea | PBM 2706 | Massachusetts, USA | DQ486693 | DQ457660 | DQ437683 | DQ470834 |
| Conocybe smithii | CCB 185 | Oregon, USA | KF830097 | KF830088 | ── | KF830068 |
| Coprinellus disseminatus | SFSU MRK18/strain 24.3/strain C345.1 | Various | AY461838 | AF056456 | ── | DQ056143 |
| Coprinopsis atramentaria | PBM 992 | Washington, USA | DQ486694 | DQ457661 | DQ115781 | ── |
| Coprinopsis cinerea | KACC49356/C13/okayama 7#130 | Various | AF345819 | AF041494 | M92991 | XM_001829088 |
| Coprinus comatus | AFTOL-ID 626 | California, USA | AY854066 | AY635772 | AY665772 | AY780934 |
| Cortinarius aurilicis | TSJ1998-101 | France | DQ083772 | AY684152 | AY705957 | DQ083880 |
| Cortinarius bolaris | IB19990199 /strain REG MB 96-086/ | Germany | AF389169 | AY293173 | AY293125 | ── |
| Cortinarius iodes | IB19850061/AFTOL-ID 285 | Massachusetts, USA | AF389133 | AY702013 | AY771605 | AY536285 |
| Cortinarius sodagnitus | TF2001-094/AFTOL-ID 811 | Denmark | DQ083812 | AY684151 | AY752975 | DQ083920 |
| Cortinarius violaceus | MTS 4854/AFTOL-ID 814 | Washington, USA | DQ486695 | DQ457662 | AY705950 | DQ470835 |
| Crassisporium funariophylum (“Pachylepyrium carbonicola”) | TENN 028784/AHS44809 | Idaho, USA | HQ222013 | HQ832460 | HQ832427 | ── |
| TENN 028785/AHS65056 | Idaho, USA | HQ222014 | HQ222015 | HQ832428 | ── | |
| PBM 2293/PBM1411 | Washington, USA | ── | DQ986294 | ── | HQ832438 | |
| strain Moser 49/22 | Austria | KF830095 | KF830085 | ── | ── | |
| strain Moser 49/8 | Austria | KF830096 | KF830086 | ── | ── | |
| Crepidotus cf. applanatus | PBM717 (WTU) | Washington, USA | DQ202273 | AY380406 | AY705951 | AY333311 |
| Crepidotus sp. | PBM3463 | Western Australia, AU | HQ728537 | HQ728538 | HQ728539 | HQ728540 |
| Crepidotus variabilis | REG JE 5.3 | Unknown | ── | AY293174 | AY293126 | ── |
| Crucibulum laeve | REG Crul1/DSH 96-02 | Unknown | DQ486696 | AF336246 | AF026624 | DQ470836 |
| Cyathus striatus | DSH 96-028/Cyst1/DSH 96-001 | Unknown | DQ486697 | AF336247 | AF026617 | DQ472711 |
| Cystoderma amianthinum | TENN063549/AFTOL-ID 1553 | Wales, UK | GU296098 | DQ154108 | GU296097 | ── |
| Descolea maculata | AFTOL-ID 1521 | Western Australia, AU | DQ192181 | DQ457664 | DQ440633 | ── |
| Descolea phlebophora | TENN 063626/PBM 3108 | New Zealand | HQ728543 | HQ728544 | KJ137258 | HQ728545 |
| Descolea recedens | TENN 063870/PBM 3211 | Tasmania, AU | HQ728546 | HQ827174 | ── | HQ827175 |
| Descolea tenuipes | TENN 063871/PBM 3212 | Tasmania, AU | HQ832453 | HQ832466 | HQ832432 | HQ832443 |
| Flammula alnicola | PBM 2608/AFTOL-ID 1501 | Tennessee, USA | DQ486703 | DQ457666 | DQ113916 | DQ472714 |
| Flammulaster sp. | PBM 1871 | Washington, USA | ── | AY380408 | ── | AY333315 |
| PBM 3449 | Tasmania, AU | HQ827176 | HQ827177 | HQ827178 | ── | |
| Galerina atkinsoniana | PBM 2719/AFTOL-ID 1760 | Colorado, USA | DQ486705 | DQ457668 | DQ440634 | ── |
| Galerina clavus | Contu_15122007 | Italy | ── | HQ832461 | HQ832429 | ── |
| Galerina marginata | AFTOL-ID 465 | Massachusetts, USA | DQ192182 | DQ457669 | DQ440635 | ── |
| Galerina semilanceata | PBM 1398/AFTOL-ID 1497 | Washington, USA | DQ486706 | AY038309 | DQ440639 | AY337357 |
| Galerina sp. | PR 6574 | USA, Puerto Rico | HQ827182 | HQ827183 | HQ827184 | HQ839737 |
| Hebeloma affine | NI 270904 | Ontario, Canada | FJ436320 | EF561632 | HQ832422 | FJ436321 |
| Hebeloma angustilamellatum (“Anamika angustilamellata”) | AFTOL-ID 543 | China | AY575919 | AY575919 | DQ092918 | ── |
| Hebeloma olympianum | BK 21-Nov-98-20 | Washington, USA | ── | AY038310 | ── | AY337359 |
| Hebeloma velutipes | AFTOL-ID 980 PBM2277 | California, USA | AY818351 | AY745703 | AY752972 | DQ472718 |
| Hydnangium carneum | TENN 063868/PBM 3209 | Tasmania, AU | HQ832445 | HQ832455 | HQ832423 | HQ832433 |
| Hymenagaricus taiwanensis | AFTOL-ID 1383 | Taiwan | DQ490633 | DQ457680 | DQ089016 | ── |
| Hypholoma australianum (“Hypholoma australe”) | PBM 3481 | Western Australia, AU | HQ832446 | HQ832456 | KJ137259 | HQ832434 |
| Hypholoma fasciculare | PBM 1844 | Washington, USA | ── | AY380409 | ── | AY337413 |
| Hypholoma sublateritium | AFTOL-ID 597 | Massachusetts, USA | AY818349 | AY635774 | AY787215 | ── |
| Hypholoma subviride | TENN 062712/TJB10226 | Tennessee, USA/Belize | HQ222020 | HQ832457 | HQ832424 | HQ832435 |
| Inocybe aff. asterospora | PBM 2014/PBM 2453 | New York, USA | DQ404390 | AY702015 | AY654889 | ── |
| Inocybe mutata | PBM 2953/PBM 2542/AFTOL-ID 1632 | Tennesse/Massachusetts, USA | JQ801410 | AY732212 | DQ457623 | DQ472729 |
| Inocybe myriadophylla | AFTOL-ID 482 | Finland | DQ221106 | AY700196 | AY657016 | AY803751 |
| Inocybe pallidicremea | PBM2448 /PBM2039 | Washington, USA | HQ201357 | AY380385 | ── | AY337388 |
| Inocybe rimosoides | AFTOL-ID 520 | New York, USA | DQ404391 | AY702014 | AY752967 | DQ385884 |
| Inocybe unicolor | PBM 2589/PBM1841/DUKE RV7/4 | Tennessee/Missouri, USA | EU523554 | AY380403 | AF287836 | AY337409 |
| Laccaria amethystina | DSH s.n. | Unknown | ── | AF393062 | AF287837 | ── |
| Laccaria bicolor | TWO 752 (MONT)/Cham3/S238N-H82 | Montana, USA/France | DQ149869 | AF042588 | ── | XM_001873347 |
| Laccaria ochropurpurea | AFTOL-ID 1477 | France/New York, USA | AF006598 | AY700200 | AY654886 | DQ472731 |
| Laccaria pumila | DSH s.n. | Unknown | ── | AF287869 | AF287838 | ── |
| Lacrymaria velutina | AFTOL-ID 478 | Massachusetts, USA | DQ490639 | AY700198 | AY654885 | DQ472733 |
| Langermannia gigantea | DSH96-032 | Unknown | ── | AF518603 | AF026622 | ── |
| Lepiota cristata | ECV2449/AFTOL-ID 1625 | Michigan, USA | AF391041 | DQ457685 | DQ457627 | ── |
| Lepiota maculans | JMB 080509_18 | Tennessee, USA | HM222939 | HQ832458 | HQ832425 | HQ832436 |
| Leucoagaricus barssii | AFTOL-ID 1899 | California, USA | DQ911600 | DQ911601 | GU187658 | DQ911602 |
| Lycoperdon pyriforme | AFTOL-ID 480/DSH96-054 | Unknown | AY854075 | AF287873 | AF026619 | AY218495 |
| Macrolepiota dolichaula | AFTOL-ID 481/AFTOL-ID 529 | China | DQ221111 | DQ411537 | AY771602 | DQ385886 |
| Macrolepiota procera | 18-X-1990, R.P.J. de Kok/DSH 96-038 | Netherlands | AY243589 | AF518628 | ── | ── |
| Mycocalia denudata | AFTOL-ID 2018/CBS 494.85 | Canada | DQ911596 | DQ911597 | DQ911598 | KJ137274 |
| Mythicomyces corneipes | AFTOL-ID 972 | Washington, USA | DQ404393 | AY745707 | DQ092917 | DQ408110 |
| DAOM 178138 | Canada | ── | AF261381 | ── | ── | |
| strain KB51 | Pakistan | KY648897 | ── | ── | ── | |
| ES11.10.2.A | Germany | KC964108 | ── | ── | ── | |
| Naucoria escharioides | PAM03/99/PBM 1719 | France/Washington, USA | AY900086 | AY380405 | ── | AY337411 |
| Nidula niveotomentosa | AFTOL-ID 1945/CBS250.84 | Canada | DQ917654 | DQ986295 | GU296099 | KJ137275 |
| Phaeocollybia festiva | AFTOL-ID 1489/PBM 2366 | Norway | DQ494682 | AY509119 | DQ462516 | AY509118 |
| Phaeomarasmius fulvidulus | Okada 170163 | Argentina | KF830092 | KF830087 | ── | ── |
| T 1495 | Argentina | KF830091 | KF830080 | KF830072 | KF830063 | |
| Phaeomarasmius proximans | AFTOL-ID 979/PBM1936 (WTU) | Vermont, USA | DQ404381 | AY380410 | AY752970 | AY333314 |
| Phaeomyces dubiosus strain | PAM06110301 | France | KF830099 | KF830089 | KF830077 | KF830070 |
| Pholiota aff. astragalina | PBM 2975 | Tennessee, USA | HQ832448 | HQ832462 | KJ137263/KJ137264 | HQ832439 |
| Pholiota multicingulata | TENN 063875 | New Zealand | HQ832449 | HQ832463 | HQ832430 | HQ832440 |
| Pholiota nubigena(“Nivatogastrium nubigenum”) | AFTOL-ID 1500 | California, USA | DQ494679 | DQ470815 | DQ459373 | ── |
| Pholiota squarrosa | AFTOL-ID 1627 | Colorado, USA | DQ494683 | DQ470818 | DQ465337 | ── |
| Pholiotina filaris | AFTOL-ID 1498 | Massachusetts, USA | DQ494684 | DQ470819 | DQ465338 | ── |
| Pleuroflammula flammea | AFTOL-ID 1381/MCA 339 | Unknown | DQ494685 | AF367962 | DQ089021 | DQ474124 |
| Pleuroflammula praestans | PBM3461 | Western Australia, AU | HQ832450 | HQ832464 | HQ832431 | HQ832441 |
| Pleuroflammula tuberculosa | PAM 02072903 | France | HQ832452 | HQ832465 | KJ137265 | HQ832442 |
| Psathyloma catervatim | PBM 3420 | Tasmania, AU | HQ840663 | HQ840664 | HQ840665 | HQ840666 |
| Psathyloma leucocarpum | PBM 3116 | New Zealand | HQ840659 | HQ840660 | HQ840661 | HQ840662 |
| Psathyrella candolleana | AFTOL-ID 1507 | Massachusetts, USA | DQ494689 | DQ110874 | DQ465339 | ── |
| Psathyrella gracilis | J 130 | Unknown | ── | AF041533 | DQ851582 | ── |
| Psathyrella rhodospora | AFTOL-ID 723 MP133 (MIN) | Minnesota, USA | DQ267129 | AY645058 | DQ089018 | ── |
| Psathyrella spadicea | AFTOL-ID 1628 | Colorado, USA | DQ494690 | DQ470822 | DQ465340 | ── |
| Psilocybe caerulipes | T SAT09-216-06 | Tennessee, USA | KC669282 | KF830084 | KF830075 | KF830067 |
| Psilocybe cubensis | strain DNA2052 | Unknown | KF830094 | KF830083 | KF830074 | KF830066 |
| Psilocybe cyanescens | PSMICSY-200 | Unknown | KJ137276 | KJ137277 | KJ137266 | KJ137278 |
| Psilocybe sp. (“Pachylepyrium funariophilum”) | strain TENN 6030 | Washington, USA | ── | AF261513 | ── | ── |
| Psilocybe stuntzii | VT1263 | Unknown | ── | AF042567 | DQ851584 | ── |
| Psilocybe subaeruginosa | PBM 3218 TENN065481 | Tasmania, AU | KC669278 | KF830079 | KF830071 | KF830062 |
| Romagnesiella clavus | AMB 15091 | Italy | ── | MK353795 | MK353799 | MK359092 |
| Romagnesiella clavus (“Tubaria minima”) | PAM 06090110 | France | EF051060 | EF051055 | ── | ── |
| Simocybe serrulata | AFTOL-ID 970 | Massachusetts, USA | DQ494696 | AY745706 | DQ465343 | DQ484053 |
| Simocybe sp. | PBM 3031 | Tennessee, USA | GQ893023 | GQ892979 | KJ137267 | HQ832444 |
| Squamanita paradoxa | TENN 063549/GG_BM05B | Wales, UK | GU296096 | EF535266 | GU296095 | ── |
| Stagnicola perplexa | DAOM 191293 | British Columbia, Canada | ── | AF261509 | ── | ── |
| Stagnicola perplexa | Broussal 20160928_909MB | France | MK351604 | MK353788 | MK353797 | MK359087 |
| DAOM 191292 | British Columbia, Canada | MK351605 | MK353789 | ── | MK359088 | |
| DAOM 191296 | Newfoundland Labrador, Canada | MK351606 | MK353790 | ── | MK359089 | |
| DAOM 191295 | British Columbia, Canada | MK351607 | MK353791 | ── | ── | |
| SFSU F-032462 | California, USA | MK351608 | MK353792 | MK353798 | MK359090 | |
| Stagnicola perplexa (“Simocybe parvispora”) | AH 25260 holotype | Spain | MK351609 | MK353793 | ── | MK359091 |
| AH 25282 paratype | Spain | MK351610 | MK353794 | ── | ── | |
| KS-BR126 | Sweden | MK045203 | ── | ── | ── | |
| Stropharia ambigua | AFTOL-ID 726 | Washington, USA | AY818350 | AY646102 | DQ092924 | DQ484054 |
| Stropharia rugosoannulata | Hopple D258 | Unknown | DQ494697 | AF518654 | AF026635 | ── |
| Tricholoma myomyces | KMS 589 | Unknown | DQ825428 | U76459 | DQ367422 | DQ367436 |
| Tricholoma palustre | AFTOL-ID 1497 | Massachusetts, USA | DQ494699 | AY700197 | AY757267 | DQ484055 |
| Tubaria confragosa | AFTOL-ID 498 | Washington, USA | DQ267126 | AY700190 | AY665776 | DQ408113 |
| Tubaria furfuracea | MCA 391 | California, USA | ── | AF205710 | DQ851587 | ── |
| Tubaria serrulata | AFTOL-ID 1528 | Western Australia, AU | DQ182507 | DQ156128 | DQ462517 | ── |
| Tubaria sp. | PBM 3355 | Tasmania, AU | HQ839739 | HQ839740 | HQ839741 | ── |
| BM378_17 | Washington, USA | HQ832454 | HQ832467 | KJ137268 | HQ839738 | |
| Tubaria vinicolor | AFTOL-ID 499 | Washington, USA | DQ536417 | DQ536415 | DQ536416 | DQ536418 |
| Tulostoma macrocephala | strain Long 10111 | Unknown | ── | AF518663 | AF026625 | ── |
| Verrucospora flavofusca | AFTOL-ID 655 | China | DQ241779 | DQ470825 | AY665783 | ── |
Phylogenetic analysis
BLAST (Altschul et al. 1997) was used to select the most closely related sequences from INSD public databases (www.insd.org). Two distinct alignments were built. 1) A multigenic alignment including 5.8S rDNA, 28S (LSU) rDNA, 18S (SSU) rDNA and rpb2 sequences from representative species of the major lineages in the Agaricineae based mainly on Matheny et al. (2015). Two species of Tricholoma (T. myomyces, T. palustre) (Tricholomatineae), were used as outgroups to root the tree, because of their phylogenetic position external to the Agaricineae (Matheny et al. 2015, Dentinger et al. 2016). 2) A ITS rDNA data alignment of Mythicomyces and Stagnicola sequences, using Psathyrella candolleana as outgroup taxon. Sequences were aligned in MEGA v. 6.0 (Tamura et al. 2013) software with its Muscle application and then corrected manually. GTR+G models were chosen for both the alignments. The datasets were analysed using Bayesian inference (BI) and Maximum likelihood (ML) criteria. The Bayesian analysis was performed through the CIPRES Science Gateway platform (Miller et al. 2010) by using the MrBayes v. 3.2.6 algorithm with 28S rDNA-18S rDNA-5.8S rDNA-rpb2 data partitioned, two simultaneous runs, four chains, temperature fixed at 0.2 and sampling every 1 000 generations until reaching the convergence parameters (standard deviation less than 0.01) after about 5 M generations. The first 25 % trees were discarded as burn-in. Finally, a full search for the best-scoring Maximum Likelihood tree was performed in RAxML v.7.0.4 (Stamatakis 2006) using the standard search algorithm (data partitioned, GTRMIX model, 2 000 bootstrap replications). Significance threshold was set above 0.95 for posterior probability (BPP) and 70 % for bootstrap proportions (MLBP).
RESULTS
Phylogenetic analyses
The final multigenic alignment is composed of 129 OTU and contained 4 100 total sites: 1 443 sites from 28S, 1 800 sites from 18S, 158 sites from 5.8S and 699 sites from rpb2. The ITS alignment consisted of 12 collections and contained 664 sites.
As both Bayesian and Maximum likelihood analyses produced similar topologies, only the Bayesian trees with both BPP and MBP values are shown (Figs 1, 2). The concatenated analysis (Fig. 1) supported the existence of at least 14 major lineages (families) within the Agaricineae. Nine of these (Agaricaceae, Bolbitiaceae, Cortinariaceae, Crassisporiaceae, Hydnangiaceae, Inocybaceae, Mythicomycetaceae, Psathyrellaceae and Tubariaceae) received strong statistical support (BPP ≥ 0.95 and MLBP ≥ 70 %); Crepidotaceae, Nidulariaceae, Squamanitaceae and Strophariaceae showed high BPP values (≥ 0.95) but only poor maximum likelyhood bootstrap support (< 70 %). For the first time a significant sister relationship (BPP = 0.96) was obtained between Crassisporiaceae and Cortinariaceae based on Bayesian inference. Mythicomyces and Stagnicola clustered as sister (BPP = 100 and MLBP = 100 %) in a strongly supported clade (BPP = 100 and MLBP = 100 %), the Mythicomycetaceae. The family is sister with strong statistical support (BPP = 100 and MLBP = 100 %) to the Psathyrellaceae.
Fig. 1.
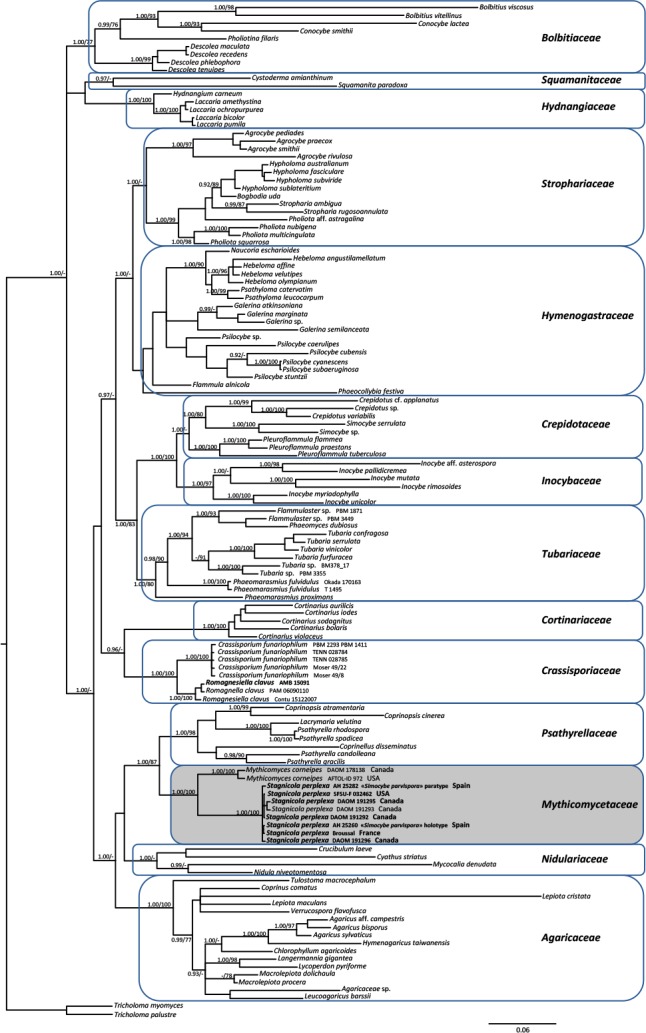
Phylogeny of the Agaricineae based on Bayesian Inference and Maximum Likelihood analysis of a dataset of four nuclear gene regions (5.8S-rDNA, 28S-rDNA, 18S-rDNA and rpb2). Tricholoma myomyces and T. palustre were used as outgroup taxa. Only BPP ≥ 0.95 and ≥ MLBP 70 % are indicated above branches. The newly sequenced collections are in bold. Clade nomenclature follows mainly Matheny et al. (2015).
Fig. 2.
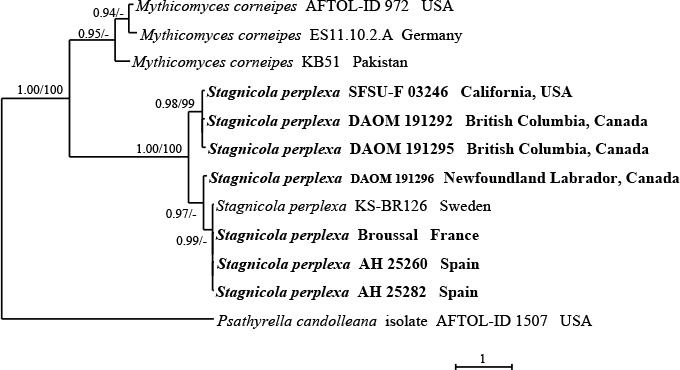
Phylogeny of the Mythicomycetaceae based on Bayesian Inference and Maximum likelihood analysis of ITS rDNA sequences with Psathyrella candolleana as outgroup taxon. Only BPP ≥ 0.95 and ≥ MLBP 70 % are indicated above branches. The newly sequenced collections are in bold.
The ITS analysis (Fig. 2) highlighted the presence of three subclades in Stagnicola perplexa which would seem to be quite related to a different geographic origin (North America vs. Europe, but see below the notes about the species).
Taxonomy
Mythicomycetaceae Vizzini, Consiglio & M. Marchetti, fam. nov. MycoBank MB829479.
Habit mycenoid to phaeocollybioid (phaeocollybia-like). Veils absent (gymnocarpic development). Pileus 5–30 mm, hemispherical-conical, obtusely to acutely conical, bell-shaped, umbonate or papillate. Lamellae adnexed to narrowly adnate. Stipe 15–70 × 0.5–2 mm, cylindrical, often tapering towards the base (but without pseudorhiza), typically cartilagineous-elastic, tough, corneus (horny) (marasmius cohaerens-like), shiny, gradually darkening (reddish-brown to blackish) from base upwards, with tawny basal strigosity. Spore deposit greyishbrown, pale hazel brown to milky coffee brown with light purple hues. Spores ovoid to ellipsoid, often somewhat inequilateral, smooth or minutely punctate-verruculose, without a germ pore, thin-to thick-walled, almost hyaline or faintly brownish under light microscope, binucleate, walls cyanophilous, inamyloid or dextrinoid. Basidia clavate, usually 4-spored. Cheilocystidia present, thick-walled, inocybe-like, often with hyaline crystals at apex and slightly amyloid at apex, or thin-walled and inamyloid. Pleurocystidia absent, if present then only as thick-walled elements. Hymenophoral trama regular, consisting of parallel hyphae. Pileipellis a thin ixocutis with parietal pigment. Clamp-connections present.
Type genus: Mythicomyces Redhead & A.H. Sm., Canad. J. Bot. 64: 643. 1986.
Habit: Saprotrophic on wood debris, Northern Hemisphere, mostly temperate to boreal.
Genera included: Mythicomyces and Stagnicola.
Notes: The genus Mythicomyces and not the genus Stagnicola was chosen as type of the family because Stagnicola Jeffreys (1830) is also a genus of snails (aquatic pulmonate gastropod mollusks) and there is a larger body of literature on Stagnicola Jeffreys (e.g. searches in GenBank®, Scopus®, Biosis®) than there is on Stagnicola Redhead & Smith, which can cause confusion.
Mythicomyces Redhead & A.H. Sm., Canad. J. Bot. 64: 643. 1986.
Etymology: from Greek mythikòs = mythical and mykes = fungus.
Development gymnocarpic. Habit mycenoid/collybioid to phaeocollybia-like. Pileus obtusely conical later convex, with a broad umbo, up to 4/5-striate, hygrophanous, greasy-shiny, slightly viscid, smooth, reddish brown, orange brown, at margin yellowish brown. Lamellae adnate to narrowly adnate, crowded, straw yellow, then cinnamon to greyish-brown. Stipe central, smooth, cartilaginous, rigid, glossy, shiny, flexuous, red brown at apex, darker and discolouring brown to blackish towards the base, with a tawny basal mycelial tomentum. Smell indistinct, taste indistinct or slightly bitterish. Spore print greyish brown to yellowish brown, pale purplish brown. Spores ovoid to ellipsoid, often somewhat inequilateral, minutely roughened, punctate-verruculose, with a small plage, lacking a germ-pore, thick-walled; pale greyish to yellowish brown in water (practically hyaline) under the microscope, cyanophilous, dextrinoid, inamyloid, slightly metachromatic in Cresyl blue, binucleate. Basidia usually 4-spored. Cheilocystidia and Pleurocystidia metuloid (thick-walled), thin-walled at the pedicel, abundant, ventricose, utriform to lageniform or fusiform, someones with hyaline crystals at apex, moderately amyloid in the apical part. Pileipellis a thin ixocutis. Caulocystidia present. Clamp-connections present. Tissues non-sarcodimitic.
Type species: Mythicomyces corneipes (Fr.) Redhead & A.H. Sm.
Ecology and distribution: Saprotrophic on plant debris, mainly wood, in wet, mossy areas, usually hemiboreal to boreal, Europe, North America and Asia.
Mythicomyces corneipes (Fr.) Redhead & A.H. Sm., Mycotaxon 118: 456. 2011.
Basionym: Agaricus corneipes Fr., Öfvers. K. Vetensk Akad. Förh. 18: 25. 1861.
Synonyms: Psilocybe corneipes (Fr.) P. Karst., Bidr. Känn. Finl. Nat. Folk 32: 504. 1879.
Geophila corneipes (Fr.) Quél., Enchiridion Fungorum in Europa media et praesertim in Gallia Vigentium: 114. 1886.
Mythicomyces corneipes (Fr.) Redhead & A.H. Sm., Canad. J. Bot. 64: 643. 1986 (Nom. inval., Art. 33.5, 33.7, 33.8).
Gruber P-88 (neotype, MICH).
Selected descriptions: Smith (1938: 26, fig. 2b, d, f, as Psilocybe corneipes); (Smith (1949: 518–520, as Psilocybe corneipes); Redhead & Smith (1986: 643–645); Moser & Jülich (1987: III Mythicomyces 1); Huhtinen & Vauras (1992: 7–10); Stålberg (1991: 64–67); Ludwig (2001a: 397–398, 2001b: plate 107, 51.1); Strittmatter & Obenhauer (2013: 338–340); Prydiuk (2015: 56–58).
Ecology and distribution: Rare. Gregarious, in autumn, saprotrophic on plant debris, among mosses in moist habitats, such as edges of bogs, brook ravines, or under conifers or birch in soil wet from spring flooding. Found throughout the Northern Hemisphere, Europe (mainly northern part), North America (most common in the Pacific Northwest region) and Asia (Pakistan). So far known from Finland, Norway, Sweden (Fries 1861, Stålberg 1991, Huhtinen & Vauras 1992, Gulden 2008a, 2012a), Estonia (see locked ITS sequence UDB024379 in UNITE, https://unite.ut.ee, specimen TU109530, 11.09.2015, Valga maakond, Otepää vald, leg. I. Kytövuori), Spain (Mythicomyces sp. environmental 18S sequence DQ304712T4B-S13. L. Laiz et al.), Germany (Gminder & Saar 2012, Strittmatter & Obenhauer 2013), Russia (Palamarchuk 2009), Ukraine (Prydiuk 2015, 2018), USA, Canada (Morgan 1917, Smith 1938, 1975, Redhead & Smith 1986, Castellano et al. 2003), and Pakistan (ITS sequence KY648897, strain KB51, 06.09.2013, leg. A.N. Khalid & K. Bakht).
Notes: The species was originally named Agaricus corneipes by Fries (1861), who described it from collections made in a fir forest near Alsike, Sweden, as mainly characterised by a glossy, shiny, very rigid horny stipe darkening towards the base and similar to that of Agaricus cohaerens. The species was placed in Psilocybe by Karsten (1879) and in Geophila by Quélet (1886). It was subsequently recorded in North America (northwestern USA) by Morgan (1907, as Psilocybe corneipes), who again underlined its resemblance to Marasmius cohaerens and by Smith (1938, 1975, as Psilocybe corneipes), who also provided photos. These last two authors described the spores as smooth and with a hyaline germ-pore.
Subsequently, in his monographic treatment of the genus Psilocybe worldwide, Guzmán (1983), after examining Smith’s collections, excluded the taxon from Psilocybe, because of its roughened spores lacking a germ pore, presence of metuloids, a pale spore print, stipe texture, and the tawny basal mycelium. Guzmán suggested that probably the best placement for the species would be in Galerina.
Redhead & Smith (1986) pointed out that some of the features of the species did not fully fit Galerina, in particular the colour of the spore in mass (not ochre to rusty brown), the spores lacking a plage, presence of metuloids, stipe texture and tawny basal mycelium, and established Mythicomyces for accommodate this puzzling species.
Redhead & Smith (1986) proposed the genus Mythicomyces citing as type “Mythicomyces corneipes (Fries) comb. nov.” and listing Fries (1863) (and not the earlier Fries 1861) for the basionym Agaricus corneipes. They listed also as obligate synonym the validly published name Psilocybe corneipes (Fr.) P. Karst. (Karsten 1879: 504). While the indication of the type fulfilled the requirements for a valid publication of the generic name (Art. 37.2), the incorrect citation of the basionym did not meet the requirements for a valid publication of the binomial. Therefore, they published the correct, valid combination later (Redhead et al. 2010).
Redhead & Smith (1986) placed the genus provisionally in the Strophariaceae, mainly because the habit of the basidiomes and spore print colour fit the broad concept of that family as circumscribed by Kühner (1980, 1984), which included all the non-ectomycorrhizal taxa with a cinnamon-brown, rusty-brown to lilac-brown spore deposit. They noted, however, that the genus did not fit a more restricted concept of Strophariaceae (Singer 1986) due to the lack of a germ-pore and the roughened spore wall.
Later, Huhtinen & Vauras 1992, after studying several collections from Fennoscandia, Canada and USA, discovered features never reported by previous authors. In particular, an amyloid reaction in cystidial walls, the dextrinoid reaction of the spores and the presence of a small plage (visible in light microscopy). The latter spore character, detected by scanning electron microscopy also by Prydiuk (2015) in Ukrainian collections, is typical of most Galerina species (Smith 1964, Bon 1992, Wood 2001, Gulden et al. 2005, Haan & Walleyn 2009, Gulden 2012c). Subsequent molecular works demonstrated, however, that Galerina is phylogenetically unrelated to Mythicomyces and had to be placed in the family Hymenogastraceae (Matheny et al. 2006, 2015).
Stagnicola Redhead & A.H. Sm., Canad. J. Bot. 64: 645. 1986.
Development gymnocarpic. Habit mycenoid/collybioid to phaeocollybia-like. Pileus conical to convex, umbonate, hygrophanous, smooth, lubricous-viscid to greasy, striate, tawny, fulvous to sienna, orange yellowish at margin. Lamellae adnexed-ventricose, crowded with olivaceous tints and concolorous edge. Stipe central, smooth, bay or purplish reddish, dark brown to blackish towards the base, horny, cartilaginous, shiny, often deeply tapering towards base, marasmius cohaerens-like, xeromphalina-like (but without forming a true pseudorhiza), with a saffron to ochre basal mycelial tomentum. Smell and taste indistinct or astringent, bitterish. Spore print deep olive buff to pale hazel brown. Spores ellipsoid to amygdaliform-reniform, smooth, without a germ-pore, pale hazel, yellowish brown in water (practically hyaline) under the microscope, non-dextrinoid, inamyloid, cyanophilous, non-metachromatic, binucleate. Basidia usually 4-spored, rarely 1–2-spored. Cheilocystidia thin-walled, cylindrical to fusiform. Pleurocystidia absent. Hymenophoral trama regular, consisting of parallel hyphae. Pileipellis a thin ixocutis of encrusted hyphae with yellow brown parietal pigment. Caulocystidia present. Clamp-connections present. Tissues non-sarcodimitic.
Habit: Saprotrophic, usually on rotten plant debris (buried needles, leaves, twigs), in damp places, in moist to wet sites, coniferous forests, acid soils, often among Sphagnum, montane-boreal, Europe and North America.
Type species: Stagnicola perplexa (P.D. Orton) Redhead & A.H. Sm.
Stagnicola perplexa (P.D. Orton) Redhead & A.H. Sm., Canad. J. Bot. 64: 645. 1986.
Basionym: Phaeocollybia perplexa P.D. Orton, Kew Bull. 31: 713. 1977.
Synonyms: Agaricus cidaris var. minor Fr., Icon. select. Hymenomyc. t. 123: 2. 1878.
Naucoria cidaris var. minor (Fr.) Sacc., Syll. Fung. 5: 831. 1887.
Simocybe parvispora Bandala et al., Sydowia 60: 183. 2008.
Selected descriptions: Orton (1977: 713, as Phaeocollybia perplexa); Redhead & Smith (1986: 645, 646); Laber & Marklund (1992: 54–56); Watling & Gregory (1993: 93–95; figs 136–138, p. 128, 129); Ludwig (2001a: 659, 660, 2001b: plate 173, 82.1); Bandala et al. (2008: 183–185, fig. 1 p. 188, fig. 2 p. 189; as Simocybe parvispora); Broussal & Dumesny (2015: 238–240).
Microscopy (based mainly on Broussal 20160928_909MB, SFSU-F-032462 and DAOM 191295). Fig. 3.
Fig. 3.
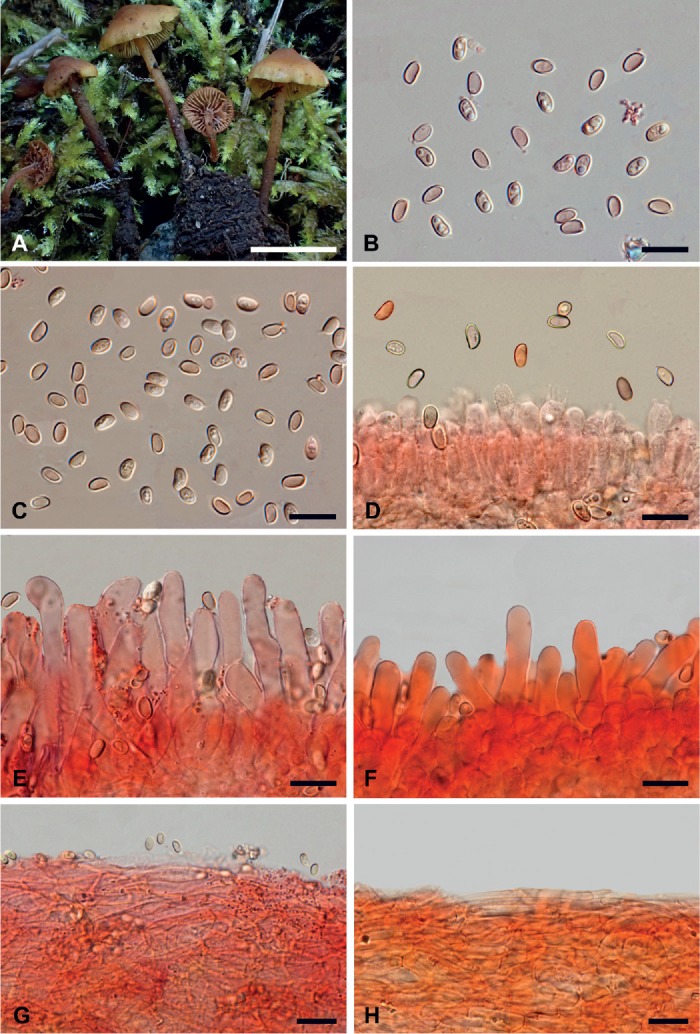
Macro- and micromorphological features of Stagnicola perplexa. A. Basidiomata in the field (Broussal 20160928_909MB). B. Spores (Broussal 20160928_909MB). C. Spores (DAOM 191295). D. Spores and hymenium (SFSU-F-032462). E. Cheilocystidia (DAOM 191295). F. Cheilocystidia (SFSU-F-032462). G. Pileipellis (DAOM 191295). H. Pileipellis (SFSU-F-032462). B–H in ammoniacal Congo red. Scale bars: A = 10 mm; B–H = 10 μm. Photographs: A, Hélène Dumesny; B–H, Mauro Marchetti.
Spores 5.1–6.1 × 3.0–3.5 µm (145/3/3) (on average 5.6 × 3.3 µm), Q = 1.57–1.85 (Qm 1.71), V = 24.3–38.8 μm3 (Vm = 31.5 μm3), subellipsoid with a flat to depressed adaxial side in lateral view, mainly ellipsoid in front view, hilar appendix visible, smooth, wall up to 0.3–0.5 µm thick, pale yellowish in water, slightly darker at wall level, often mono- to multiguttulate, cyanophilic, inamyloid, non-dextrinoid, non-metachromatic in Cresyl blue. Basidia 18–28 × 5–8 µm, mainly tetra-spored, subcylindrical to clavate, even subcapitate, with up to 6 µm long sterigmata, content mostly smooth, at times guttulate. Hymenophoral trama regular, consisting of thin-walled, hyaline to yellowish cylindrical hyphae, 4–8 µm wide, having a parallel arrangement. Occasionally, it was observed the occurrence of crystalline particles either free or sticking to the hyphal walls. Subhymenium hardly differentiated. Cheilocystidia 25–40(–45) × 4.5–7(–8) µm, thin-walled, sub-cylindrical, at times flexuous or slightly ventricose or clavate, with a rounded, occasionally subogival or lobate apex, other times with a tapered base; edge heteromorphous. Pleurocystidia not found. Pileipellis a regular thin ixocutis, consisting of cylindrical, yellowish, thin-walled hyphae, 3–8(–10) µm wide, smooth but with occasional crystalline deposits, at times with clavate terminal elements; subcutis well differentiated, composed of short articles, l6–12(–15) µm wide, subvesicular or allantoid. Pileocystidia not found. Stipe hyphae 2–8(–10) µm wide, mostly cylindraceous, at times fusiform, parallel, often short-celled, hyaline to yellowish, thin-walled, occasionally it can be noted the presence of polymorphous, refractive, small-sized crystalline deposits. Caulocystidia present in the apical portion of the stipe, similar to the hymenial ones but more irregular in shape. Clamp-connections common everywhere. Tissues non-sarcodimitic.
Specimens examined: France: Haute-Auvergne: Condat, Cantal, Maubert et Gaulis forest, alt. 872 m, coniferous forest (Picea abies, Abies alba), on debris in mossy area, 28 Sep. 2016, H. Dumesny [det. M. Broussal] (Broussal 20160928_909MB). Canada: British Columbia: Queen Charlotte Is., Graham I., Kliki Damen Cr. mouth, in a drying temporary pool amongst Carex, 16 Sep. 1982, S.A. Redhead (DAOM 191292); Queen Charlotte Is., Graham I., Yakoun R. near Port Clements, on reed bed, along river, 15 Sep. 1982, S.A. Redhead (DAOM 191295). Newfoundland-Labrador: Gros Morne Natl. Park, on debris in wet depression by alders, Bakers Brook Pond trail, 19 Sep. 1983, S.A. Redhead (DAOM 191296). USA: California: Siskiyou County, Shasta-Trinity National Forest, alt. 485 m, Abies magnifica litter with an understory of Symphoricarpos sp., 8 Nov. 2012, C. Schwarz [det. S. Davison] (SFSU-F-032462).
Microscopy (based on Simocybe parvispora AH 25282 (paratype). Fig. 4.
Fig. 4.
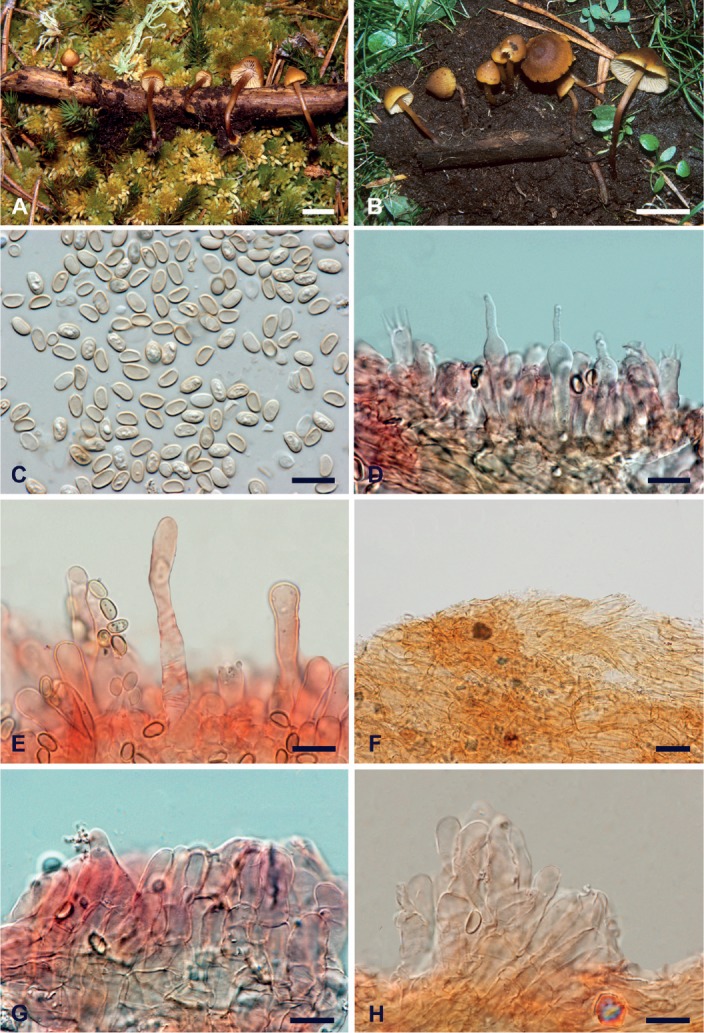
Macro- and micromorphological features of Simocybe parvispora. A. Basidiomata in the field (AH 25260, holotype). B. Basidiomata in the field (AH 25282, paratype). C. Spores (AH 25282). D. Mono- and tetrasporic basidia (AH 25282). E. Cheilocystidia (AH 25282). F. Pileipellis (AH 25282). G. Caulocystidia (AH 25282). H. Caulocystidia (AH 25282). C–H in ammoniacal Congo red. Scale bars: A, B = 10 mm; C–H = 10 μm. Photographs: A, B, Fernando Esteve-Raventós; C–H, Mauro Marchetti.
Spores 5.2–6 × 3–3.5 µm (44/1/1) (av. 5.6 × 3.2 µm), Q = 1.57–1.90 (Qm 1,74), V = 25.2–36.6 μm3 (Vm = 30.9 μm3), ellipsoid in side-view, often with an almost flat adaxial side, occasionally with central constriction, mostly ellipsoid in front-view, smooth. Lacking a germ pore, wall up to 0.3–0.5 µm thick, hilar appendix visible, pale yellowish in water, often mono- or multi-guttulate, cyanophilic, iodine-negative, non-metachromatic in Cresyl blue. Basidia 18–28 × 5–7 µm, tetrasporic, sterigmata up to 6 µm long, often even monosporic with a sterigm up to 10 µm long, subcylindrical to clavate or even subcapitate, content mostly smooth, at times guttulate. Hymenophoral trama regular, made up by cylindrical, hyaline to yellowish, thin-walled hyphae, 4–8 µm wide, occasionally it is possible to observe minute crystalline formations. Subhymeniun hardly differentiated. Cheilocystidia 25–55(–60) × 4.5–7(–8) µm, thin-walled, hyaline, subcylindrical, often flexuous or slightly ventricose or clavate, with rounded or ogival apex, plentiful to scarce, at times completely absent. Pleurocistidia not found. Pileipellis a regular thin ixocutis, with long to short hyphae, at times with clavate terminal elements, 3–7 µm wide, thin-walled, yellowish, with occasional crystalline deposits; subcutis and underlying layer well differentiated, consisting of short hyphae, 8–20 µm wide, subvesicular or allantoid. Pileocystidia not found. Stipe hyphae 2–8(–10) µm wide, mostly cylindrical, at times fusiform, parallel, often short-celled, with yellowish content, thin-walled, refractive polymorphous crystalline deposits present. Caulocystidia numerous in the apical portion, tufted, at times multi-septate, subcylindrical-clavate or utricular, even lageniform, 20–40 × 3–8 µm. Clamp-connections common everywhere. Tissues non-sarcodimitic.
Ecology and distribution: Rare. Gregarious, in autumn, saprotrophic on plant debris, in moist to wet areas, usually in acid coniferous forests among mosses. Found throughout the Northern Hemisphere (Europe and North America) and so far known from Sweden and Finland (Fries 1878 as Agaricus cidaris var. minor, Stridvall & Stridvall 1996, Gulden 2008b, 2012b), Great Britain (Scotland, Orton 1977 as Phaeocollybia perplexa; Watling & Gregory 1993), Germany (Laber & Marklund 1992), France (Broussal & Dumesny 2015, Spain (Bandala et al. 2008 as Simocybe parvispora), Moldova (Manic 2015), USA and Canada (Smith 1937, Redhead & Smith 1986). In Index Fungorum and MycoBank, Panaeolus sphinctrinus var. minor, described by Singer (1960, 1969) based on Mexican and Argentine collections, is (mistakenly) considered as a posterior synonym of A. corneipes and consequently Niveiro & Albertó (2012), Coimbra (2015) and Begerow et al. (2018) reported in their checklists S. perplexa (= P. sphinctrinus var. minor) as present in Argentine. In comparison with M. corneipes, however, P. sphinctrinus var. minor has a non-corneous stipe, lacks a tawny mycelial tomentum at stipe base and its spores are darker, hexagonal to citriform in shape, have a with germ-pore and measure 12–13.3 × 9–9.3 µm (Singer 1960, Guzmán & Pérez-Patraca 1972, Gerhardt 1996).
Specimens examined: Spain: Castilla-La Mancha: Guadalajara, road from Aldeanueva de Atienza to Condemios de Arriba, river Pelagallinas, on decaying branches of Pinus sylvestris, alt. 1380 m, 2 Oct. 1999, Villarreal et coll. (AH 25282-paratype/topotype).
Notes: The species was clearly first reported from Sweden by Fries (1878) as Agaricus (Naucoria) cidaris var. minor (plate 123, fig. 2). Fries differentiated the variety from the type (to date considered a true Phaeocollybia), mainly on the lack of a rooting stipe. Saccardo (1887) then combined this variety in Naucoria. Smith (1937) signalled it from North America, providing also a photo (plate 23, fig. c). When the species was collected for the first time by Orton (1977), the English mycologist, unaware of Fries’s taxon, was not able, at first, to place it in a known genus (hence the specific epithet of “perplexa”, i.e. puzzled). Subsequently, after additional collections, in spite of the smooth, pale-coloured spores and the pileus not strongly viscid, he became convinced that the new taxon had to be placed in the genus Phaeocollybia [traditionally included in Cortinariaceae due to the presence of a viscid pileus surface, a pseudorhiza, rusty-brown, ornamented spores, and the absence of a veil (Heim 1931, Horak 1977, Laber 1982, 1991)]. It was accommodated near P. jennyae, since other features such as the conical umbonate pileus, horny cartilaginous rooting stipe, absence of veils, absence of pleurocystidia, bitter astringent taste and yellowish olive lamellae fitted neatly into the genus. Horak (1977) considered the inclusion of the species in Phaeocollybia doubtful and questionable. Redhead & Smith (1986) removed P. perplexa from Phaeocollybia mainly because of its pale smooth spores, a not truly rooting stipe and presence of a tawny tomentum at stipe base, transferring it to the monotypic genus Stagnicola. Subsequent accurate morpho-ecological works by Redhead & Malloch (1986), Norvell (1998a, b, 2000, 2004) and Norvell & Exeter (2008) allowed a better circumscription of Phaeocollybia, which, when additional distinguishing features were discovered, led to a better delimitation of P. stagnicola and the other brown-spored agarics. Noteworthy among the new features are the (pileo)stipitocarpic-monovelangiocarpic development revealed by the presence of a thin pellicular veil (primordial envelope sheath) sheathing the subterranean primordium, but tearing during basidiome elongation and easily overlooked in mature basidiomes (where velar remnants are only observable as fibrillose patches on the aerial stipe); the presence of a rhizomorphic pseudorhiza (a pseudorhiza forming several thread-like myceliar cords that make contact with the plant root tips); tibiiform diverticula on the hyphae of the mycelium, a pellicular veil and sarcodimitic pseudotissues in the pseudorhizal trama, often present also in the stipititrama, pileitrama, and hymenophoral trama. Last but not least, evidence that Phaeocollybia is an ectomycorrhizal genus (trophic lifestyle confirmed also by the stable isotopes analysis by Trudell et al. 2004), even though some species are possibly parasitic. Molecular phylogeny placed Phaeocollybia in Hymenogastraceae (Matheny et al. 2006, 2015).
As first suggested by Redhead & Smith (1985), species of the genus Tubaria (Tubariaceae) may resemble S. perplexa, but they possess a non-umbonate pileus, veils, broadly attached, adnate to subdecurrent lamellae, a non-tapering stipe which is fibrous and fleshy and with white basal mycelium, and thin-walled easily collapsing spores (Singer 1986, Bon 1992, Volders 2002, Matheny et al. 2007b).
Stagnicola perplexa could be confused with the central-stemmed species of Simocybe (e.g. S. centunculus, S. sumptuosa) (Crepidotaceae), but the latter differ in having olivaceous tinges on pileus surface, more pigmented, distinctly ovoid-reniform spores and a trichodermic-hymenidermic pileipellis with well-developed pileocystidia (Romagnesi 1962, Senn-Irlet 1995, Aime et al. 2005, Horak & Ronikier 2011). The morphological affinities between Stagnicola and Simocybe are such that, according to our phylogenetic analyses (Figs 1, 2), the recently described Simocybe parvispora from Spain (Bandala et al. 2008) is to be regarded as identical to S. perplexa. Moreover, also the morphological study of the two sequenced Simocybe parvispora collections (holotype and paratype) showed characters that, based on the descriptions in the literature (Orton 1977, Redhead & Smith 1986, Laber & Marklund 1992, Watling & Gregory 1993, Broussal & Dumesny 2015) and our personal observations, match perfectly those of S. perplexa.
Finally, also the two recently described sister genera Crassisporium and Romagnesiella (Crassisporiaceae) have some characters in common with Stagnicola. In particular, they share a collybioid habit, a filamentous pileipellis, pale-coloured, smooth spores and presence of clamp-connections. Nonetheless, they differ in the non-umbonate pileus, fleshy, non-rooting stipe, which does not progressively darken towards the base and lacks a tawny basal tomentum and the non-dextrinoid spores with walls becoming rusty brown to reddish brown or reddish cinnamon in KOH. Additionally, Crassisporium, typified by Pholiotina funariophila, a taxon traditionally placed in the polyphyletic genus Pachylepirium (Matheny et al. 2015), is distinguished by a fugacious veil on pileus and stipe surface, thick-walled spores (> 0.5 µm thick) with a broad and conspicuous germ pore (often > 0.5 µm wide) and carbonicolous habitat (Matheny et al. 2015), while Romagnesiella, typified by Galerina clavus, may be differentiated by a dry, non-hygrophanous pileus (Matheny et al. 2015).
The phylogenetic analysis based on ITS sequences (Fig. 2) showed that Stagnicola perplexa collections from Europe and from North America form slightly different subclades, but, in the multigene analysis (Fig. 1), these small differences are no longer perceptible.
DISCUSSION
In the field, Mythicomyces corneipes and Stagnicola perplexa can be easily confused due to a series of shared characters such as a similar habit, absence of veils, pale-coloured lamellae, pale spore deposit, a tapering corneus-rigid stipe, gradually blackening from base upward and with a tawny basal mycelium and occurrence in the same habitats. Microscopically, however, Mythicomyces can be easily distinguished by the minutely roughened, verrucose spores and the presence of thick-walled, encrusted hymenial cystidia.
The presence in both Agaricus corneipes and Phaeocollybia perplexa of this unusual combination of characters, which is anomalous in dark-spored agarics, caused uncertainty as to their intergeneric relationships and family placement, which remained controversial and debated (Redhead & Smith 1986, Huhtinen & Vauras 1992) until the application of molecular techniques. However, while the molecular works provided a definitive answer regarding both the validity and independence of these two genera within the dark-spored agarics (Gulden et al. 2005) and their sister relationships (Moncalvo et al. 2002, Padamsee et al. 2008, Broussal & Dumesny 2015), the data on their definitive family placement have remained inconclusive, because of the poor taxon sampling and the few sequences available for these two taxa, for S. perplexa in particular.
Our analysis, which includes seven new Stagnicola collections with four molecular markers, clearly indicates that, in agreement with previous works (Moncalvo et al. 2002, Padamsee et al. 2008, Broussal & Dumesny 2015), the two genera are sister (with high support, BPP = 1, MLBP = 87%) to the Psathyrellaceae. The family Psathyrellaceae includes all the taxa formerly treated under the name Coprinaceae, but with the exclusion of Coprinus comatus (type of the genus) and allied species, which were found to be more closely related to Agaricaceae (Redhead et al. 2001). They have a saprotrophic nutritional mode or, rarely, parasite other agarics (e.g. Psathyrella epimyces) and are characterized by a dark brown, purplish brown to black spore deposit, non-cyanophilous thick-walled spores, usually with a distinct germ-pore and, in the psathyrelloid taxa, with pigment in the walls bleaching in concentrated sulfuric acid, without iodine reactions, hymenial cystidia often present, sterile pseudoparaphyses surrounding the basidia present in the coprinoid taxa, a non-radicating, fleshy, fibrous, non-corneous stipe, lamellae deliquescing in the coprinoid genera [ability to digest themselves by means of autodigestive chitinases (Kües 2000)], pileipellis a cutis or more commonly an ephitelium/hymeniderm, often covered with velar structures (Singer 1986, as Coprinaceae, Redhead et al. 2001, Noordeloos 2005, as Coprinaceae partim; Knudsen & Vesterholt 2012).
There is an evident morphological hiatus between Mythicomyces/Stagnicola with their pale-coloured spores without germ-pore, corneous, tapering stipe and absence of veils, pileipellis as a thin ixocutis, and the members of the Psathyrellaceae. No genus within the coprinoid taxa Coprinellus, Coprinopsis, Parasola (Redhead et al. 2001, Nagy et al. 2009, 2010, 2011, 2012, 2013a, b) as well as in the polyphyletic Psathyrella s.l. (Kits van Waveren 1985), including the recently segregated genera Homophron, Kauffmania, Typhrasa (Örstadius & Knudsen 2012, Örstadius et al. 2015), shows clear morphological affinities with Mythicomyces and Stagnicola.
It stands to reason that forcing the two genera into Psathyrellaceae s.l., as proposed by Gulden 2012a, b, Strittmatter & Obenauer 2013, Prydiuk 2015, 2018), makes this family heterogeneous and non-natural, hence the necessity to establish the new family, Mythicomycetaceae proposed in this paper.
ACKNOWLEDGEMENTS
The authors are grateful to staff at Herbaria AH (F. Javier Rejos), AMB (Gianfranco Medardi), and DAOM (Jennifer Wilkinson) for loan of collections. Gabriele Cacialli (Livorno, Italy) is acknowledged for his bibliographic expertise. Micheline Broussal (Cournonsec, France), Fernando Esteve-Raventós (Madrid, Spain), Brandon Matheny (Knoxville, USA), Gabriel Moreno (Madrid, Spain), Mark Miller (San Diego, USA), and Scott Redhead (Ottawa, Canada) are also acknowledged for their valuable collaboration.
REFERENCES
- Aime MC, Vilgalys R, Miller OK. (2005). The Crepidotaceae (Basidiomycota, Agaricales): phylogeny and taxonomy of the genera and revision of the family based on molecular evidence. American Journal of Botany 92: 74–82. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, et al. (1990). Basic local alignment search tool. Journal of Molecular Biology 215: 403–410. [DOI] [PubMed] [Google Scholar]
- Bandala VM, Esteve-Raventós F, Montoya L. (2008). Two remarkable brown-spored agarics from Spain: Simocybe parvispora sp. nov. and Crepidotus ibericus comb. nov. Sydowia 60: 181–196. [Google Scholar]
- Begerow D, McTaggart A, Agerer R. (2018). Syllabus of Plant Families. Part 1/3. Basidiomycota and Entorrhizomycota. Borntraeger Science Publishers, Stuttgart, Germany. [Google Scholar]
- Binder M, Larsson KH, Matheny PB, et al. (2010). Amylocorticiales ord. nov. and Jaapiales ord. nov.: early diverging clades of agaricomycetidae dominated by corticioid forms. Mycologia 201: 865–888. [DOI] [PubMed] [Google Scholar]
- Bon M. (1992). Clé monographique des espèces galéro-naucorioides. Documents Mycologiques 21: 1–89. [Google Scholar]
- Broussal M, Dumesny E. (2015). Une récolte française de Stagnicola perplexa. Bulletin de la Société Mycologique de France 131: 237–243. [Google Scholar]
- Castellano MA, Cázares E, Fondrick B, et al. (2003). Handbook to Additional Fungal Species of Special Concern in the Northwest Forest Plan. General Technical Report PNW-GTR-572. Part 7: Species Gyromitra montana to Phaeocollybia fallax (PDF) (Report). United States Department of Agriculture, Forest Service: S3–81. [Google Scholar]
- Clémençon H. (1972). Zwei verbesserte Präparierlösungen für die microskopische Untersuchung von Pilze. Zeitschrift für Pilzkunde 38: 49–53. [Google Scholar]
- Coimbra VRM. (2015). Checklist of Central and South American Agaricales (Basidiomycota) II: Strophariaceae. Mycosphere 6: 441–458. [Google Scholar]
- Cubeta MA, Echandi E, Abernethy T, et al. (1991). Characterization of anastomosis groups of binucleate Rhizoctonia species using restriction analysis of an amplified ribosomal RNA gene. Phytopathology 81: 1395–1400. [Google Scholar]
- de Haan A, Walleyn R. (2009). Studies in Galerina. Galerinae Flandriae (3). Fungi non Delineati 46: 1–84. [Google Scholar]
- Dentinger BTM, Gaya E, O’Brien H, et al. (2016). Tales from the crypt: genome mining from fungarium specimens improves resolution of the mushroom tree of life. Biological Journal of the Linnean Society 117: 11–32. [Google Scholar]
- Fannechére G. (2011). Mycomètre, logiciel d’aide à la mesure et de traitement statistique. http://mycolim.free.fr/DOC_SML/ mycm202/Charg_Mycm202.htm
- Fries EM. (1861). Hymenomycetes novi vel minus cogniti, in Suecia 1852–1860 observati. Öfversigt af Kongliga Vetenskaps-Akademiens förhandlingar 18: 19–34. [Google Scholar]
- Fries EM. (1863). Monographia Hymenomycetum Sueciae 2: [147]–355. CA Leffler, Uppsala, Sweden. [Google Scholar]
- Fries EM. (1878). Icones selectae Hymenomycetum nondum delineatorum / sub auspiciis Regiae Academiae Scientiarum Holmiensis editae ab Elia Fries; Volumen secundum, Fasc. II-III. Typographia centralis & Ed. Berling, Holmiae et Upsaliae, Sweden. [Google Scholar]
- Gardes M, Bruns TD. (1993). ITS primers with enhanced specificity for Basidiomycetes—application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118. [DOI] [PubMed] [Google Scholar]
- Garnica S, Weiss M, Walther G, et al. (2007). Reconstructing the evolution of agarics from nuclear gene sequences and basidiospore ultrastructure. Mycological Research 9: 1019–1029. [DOI] [PubMed] [Google Scholar]
- Gerhardt E. (1996). Taxonomische revision der Gattungen Panaeolus und Panaeolina (Fungi, Agaricales, Coprinaceae). Bibliotheca Botanica 147: 1–150. [Google Scholar]
- Gminder A, Saar G. (2012). Ergänzungen zur Großpilzflora von Baden-Württemberg. Andrias 19: 185–223. [Google Scholar]
- Gross G. (1972). Kernzahl und sporenvolumen bei einigen Hymeno-gasterarten. Zeitschrift für Pilzkunde 38: 109–158. [Google Scholar]
- Gulden G, Stensrud O, Shalchian-Tabrizi K, et al. (2005). Galerina Earle: A polyphyletic genus in the consortium of dark-spored agarics. Mycologia 97(4): 823–837. [DOI] [PubMed] [Google Scholar]
- Gulden G. (2008a). Mythicomyces Redheаd & A.H. Sm. In: Fungа Nordicа. Agаricoid, boletoid and cyphelloid genera (Knudsen H, Vesterholt J, eds). Nordsvаmp, Copenhаgen, Denmark: 907. [Google Scholar]
- Gulden G. (2008b). Stagnicola Redheаd & A.H. Sm. In: Fungа Nordicа. Agаricoid, boletoid and cyphelloid genera (Knudsen H, Vesterholt J, eds). Nordsvаmp, Copenhаgen, Denmark: 910. [Google Scholar]
- Gulden G. (2012a). Mythicomyces Redheаd & A.H. Sm. In: Fungа Nordicа. Agаricoid, boletoid, clаvаrioid, cyphelloid аnd gаstroid generа (Knudsen H, Vesterholt J, eds). 2nd edn Nordsvаmp, Copenhаgen, Denmark: 688. [Google Scholar]
- Gulden G. (2012b). Stagnicola Redheаd & A.H. Sm. In: Fungа Nordicа. Agаricoid, boletoid, clаvаrioid, cyphelloid аnd gаstroid generа (Knudsen H, Vesterholt J, eds). 2nd edn Nordsvаmp, Copenhаgen, Denmark: 728. [Google Scholar]
- Gulden G. (2012c). Galerina Earle. In: Fungа Nordicа. Agаricoid, boletoid, clаvаrioid, cyphelloid аnd gаstroid generа (Knudsen H, Vesterholt J, eds). 2nd edn Nordsvаmp, Copenhаgen, Denmark: 886–903. [Google Scholar]
- Guzmán G, Pérez-Patraca AM. (1972). Las especies conocidas del género Panaeolus en México. Boletín de la Sociedad Mexicana de Micologia 6: 17–53. [Google Scholar]
- Guzmán G. (1983). The genus Psilocybe. A systematic revision of the known species including the history, distribution and chemistry of the hallucinogenic species. Beihefte Nova Hedwigia 74: 1–439. [Google Scholar]
- Jeffreys JG. (1830). A synopsis on the testaceous pneumonobranchous Mollusca of Great Britain. Transactions of the Linnean Society of London 16: 323–392. [Google Scholar]
- Halbwachs H, Brandl R, Bässler C. (2015). Spore wall traits of ectomycorrhizal and saprotrophic agarics may mirror their distinct lifestyles. Fungal Ecology 17: 197–204. [Google Scholar]
- Heim R. (1931). Le genre Inocybe: Précédé d’une introduction générale à l’étude des agarics ochrosporés. Paul Lechevalier & Fils, Paris, France. [Google Scholar]
- Hibbett DS. (1996). Phylogenetic evidence for horizontal transmission of group I introns in the nuclear ribosomal DNA of mushroom-forming fungi. Molecular Biology and Evolution 13: 903–917. [DOI] [PubMed] [Google Scholar]
- Horak E. (1977). Further additions towards a monograph of Phaeocollybia. Sydowia 29: 28–70. [Google Scholar]
- Horak E. (2005). Röhrlinge und Blätterpilze in Europa. Elsevier GmbH, Spectrum Akademischer Verlag, München, Germany. [Google Scholar]
- Horak E, Ronikier A. (2011). Simocybe montana (Crepidotaceae, Agaricales), a new species from the alpine belt in the Swiss Alps and the Romanian Carpathians. Mycological Progress 10: 439–443. [Google Scholar]
- Huhtinen S, Vauras J. (1992). Mythicomyces corneipes, a rare agaric, in Fennoscandia. Karstenia 32: 7–12. [Google Scholar]
- Karsten PA. (1879). Rysslands, Finlands och den Skandinaviska halföns Hattsvampar. Förra Delen: Skifsvampar. Bidrag till Kännedom av Finlands Natur och Folk 32: 1–571. [Google Scholar]
- Kits van Waveren E. (1985). The Dutch, French and British species of Psathyrella. Persoonia Suppl. 2: 1–300. [Google Scholar]
- Knudsen H, Vesterholt J. (eds) (2012). Psathyrellaceae Redheаd, Vilgalys & Hopple. In: Fungа Nordicа. Agаricoid, boletoid, clаvаrioid, cyphelloid аnd gаstroid generа. 2nd edn Nordsvаmp, Copenhаgen, Denmark: 661–728. [Google Scholar]
- Kohler A, Kuo A, Nagy LG, et al. (2015). Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nature Genetics 47: 410–415. [DOI] [PubMed] [Google Scholar]
- Kües U. (2000). Life history and developmental processes in the basidiomycete Coprinus cinereus. Microbiology and Molecular Biology Reviews 64: 316–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühner R. (1980). Les Hyménomycètes agaricoïdes (Agaricales, Tricholomatales, Pluteales, Russulales). Etude générale et classification. Bulletin mensuel de la Société linnéenne de Lyon 49 Numero special: 1–1027. [Google Scholar]
- Kühner R. (1984). Some mainlines of classification in the gill fungi. Mycologia 76: 1059–1074. [Google Scholar]
- Laber D. (1982). Die europäischen Arten der Gattung Phaeocollybia (Wurzelschnitzlinge) und ihr Vorkommen in südliches Schwarzwald. Zeitschrift für Mykologie 48: 89–98. [Google Scholar]
- Laber D. (1991). Erganzung zu ‘Die europäischen Arten der Gattung Phaeocollybia und ihr Vorkommen in südliches Schwarzwald.’ Zeitschrift für Mykologie 57: 109–116. [Google Scholar]
- Laber D, Marklund H. (1992). Stagnicola perplexa (Orton) Redhead & Smith = Agaricus cidaris var. minor Fries, eine sehr seltene Art in Europa? Zeitschrift für Mykologie 58: 53–56. [Google Scholar]
- Liu YJ, Whelen S, Hall BD. (1999). Phylogenetic relationships among Ascomycetes: evidence from an RNA Polymerase II Subunit. Molecular Biology and Evolution 16: 1799–1808. [DOI] [PubMed] [Google Scholar]
- Ludwig E. (2001a). Pilzkompendium Band 1: Beschreibungen. IHW Verlag, Eching, Germany. [Google Scholar]
- Ludwig E. (2001b). Pilzkompendium Band 1: Abbildulgen. IHW Verlag, Eching, Germany. [Google Scholar]
- Manic S. (2015). Macromicetele din ecosistemele Republicii Moldova (taxonomie, bioecologie, corologie). PhD Dissertation, Biological Sciences, Academia de Ştiinţe a Moldovei Grădina Botanică (Institut), Chişinău, Moldova. [Google Scholar]
- Matheny PB, Curtis JM, Hofstetter V, et al. (2006). Major clades of Agaricales: a multilocus phylogenetic overview. Mycologia 98: 982–995. [DOI] [PubMed] [Google Scholar]
- Matheny PB, Griffith JW. (2010). Mycoparasitism between Squamanita paradoxa and Cystoderma amianthinum (Cystodermateae, Agaricales). Mycoscience 51: 456–461. [Google Scholar]
- Matheny PB, Wang Z, Binder M, et al. (2007a). Contributions of rpb2 and tef1 to the phylogeny of mushrooms and allies (Basidiomycota, Fungi). Molecular Phylogenetics and Evolution 43: 430–451. [DOI] [PubMed] [Google Scholar]
- Matheny P B, Vellinga EC, Bougher N, et al. (2007b). Taxonomy of displaced species of Tubaria. Mycologia 99: 569–585. [DOI] [PubMed] [Google Scholar]
- Matheny PB, Moreau PA, Vizzini A, et al. (2015). Crassisporium and Romagnesiella: two new genera of dark-spored Agaricales. Systematics and Biodiversity 13: 28–41. [Google Scholar]
- Meerts P. (1999). The evolution of spores in agarics: do big mushrooms have big spores? Journal of Evolutionary Biology 12: 161–165. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. (2010). Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), 14 November 2010, New Orleans, LA: 1–8. [Google Scholar]
- Moncalvo JM, Vilgalys R, Redhead SA, et al. (2002). One hundred and seventeen clades of euagarics. Molecular Phylogenetics and Evolution 23: 357–400. [DOI] [PubMed] [Google Scholar]
- Morgan AP. (1907). North American species of Agaricaceae. The Journal of Mycology 13: 246–255. [Google Scholar]
- Moser M, Jülich W. (1987). Farbatlas der Basidiomyceten. 3. Gustav Fischer, Stuttgart, Germany. [Google Scholar]
- Murray HG, Thompson WF. (1980). Rapid isolation of high molecular weight DNA. Nucleic Acids Research 8: 4321–4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy LG, Kocsubé S, Papp T, et al. (2009). Phylogeny and character evolution of the coprinoid mushroom genus Parasola as inferred from LSU and ITS nrDNA sequence data. Persoonia 22: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy GL, Urban A, Örstadius L, et al. (2010). The evolution of autodigestion in the mushroom family Psathyrellaceae (Agaricales) inferred from Maximum Likelihood and Bayesian methods. Molecular Phylogenetics and Evolution 57: 1037–1048. [DOI] [PubMed] [Google Scholar]
- Nagy GL, Walther G, Házi J, et al. (2011). Understanding the evolutionary processes of fungal fruiting bodies: correlated evolution and divergence times in the Psathyrellaceae. Systematic Biology 60: 303–331. [DOI] [PubMed] [Google Scholar]
- Nagy LG, Házi J, Vágvölgyi C, et al. (2012). Phylogeny and species delimitation in the genus Coprinellus with special emphasis on the haired species. Mycologia 104: 254–275. [DOI] [PubMed] [Google Scholar]
- Nagy LG, Desjardin ED, Vágvölgyi C, et al. (2013a). Phylogenetic analyses of Coprinopsis section Lanatuli and Atramentarii identify multiple species within morphologically defined taxa. Mycologia 105: 112–114. [DOI] [PubMed] [Google Scholar]
- Nagy LG, Vágvölgyi C, Papp T. (2013b). Morphological characterization of clades of the Psathyrellaceae (Agaricales) inferred from a multigene phylogeny. Mycological Progress 12: 505–517. [Google Scholar]
- Niveiro N, Albertó EO. (2012). Checklist of the Argentine Agaricales 2. Coprinaceae and Strophariaceae. Mycotaxon 120: 505, http://dx.doi.org/10.5248/119.505. [Google Scholar]
- Noordeloos ME. (2005). Coprinaceae Overeem. In: Flora Agaricina Neerlandica 6 (Noordeloos ME, Kuyper THW, Vellinga EC, eds). CRC Press, Taylor & Francis Group, Boca Raton, USA: 21. [Google Scholar]
- Norvell LL. (1998a). The biology and taxonomy of Pacific Northwest species of Phaeocollybia Heim (Agaricales, Cortinariaceae). Ph.D. dissertation (Dept. Botany), University of Washington, Seattle, USA. [Google Scholar]
- Norvell LL. (1998b). Observations on development, morphology and biology in Phaeocollybia. Mycological Research 102: 615–630. [Google Scholar]
- Norvell LL. (2000). Phaeocollybia in Western North America 1: The Phaeocollybia kauffmanii complex. Canadian Journal of Botany 78: 1055–1076. [Google Scholar]
- Norvell LL. (2004). Phaeocollybia in western North America 4: Two new species with tibiiform cheilocystidia and section Versicolores reconsidered. Mycotaxon 90: 241–260. [Google Scholar]
- Norvell LL, Exeter RL. (2008). Phaeocollybia of Pacific Northwest North America. United States Department of Interior Bureau of Land Management Salem District, Salem, USA. [Google Scholar]
- Örstadius L, Knudsen H. (2012). Psathyrella (Fr.) Quél. In: Funga Nordica. Agaricoid, boletoid, cyphelloid and gasteroid genera (Knudsen H, Vesterholt J, eds). 2nd edn Nordsvаmp, Copenhаgen, Denmark: 692–728. [Google Scholar]
- Örstadius L, Ryberg M, Larsson E. (2015). Molecular phylogenetics and taxonomy in Psathyrellaceae (Agaricales) with focus on psathyrelloid species: introduction of three new genera and 18 new species. Mycological Progress 14: 25. [Google Scholar]
- Orton PD. (1977). Notes on British Agarics: V. Kew Bulletin 31: 709–721. [Google Scholar]
- Padamsee M, Matheny PB, Dentinger BT, et al. (2008). The mushroom family Psathyrellaceae: evidence for large-scale polyphyly of the genus Psathyrella. Molecular Phylogenetics and Evolution 46: 415–429. [DOI] [PubMed] [Google Scholar]
- Palamarchuk MA. (2009). The xylotrophic agaricoid Basidiomycetes of Pechoro-Ilych reserve (North Urals). Conifers of the Boreal Area 26: 67–72. (in Russian) [Google Scholar]
- Prydiuk MP. (2015). Mythicomyces (Psathyrellaceae), a new for Ukraine genus of mushrooms. Ukrainian Botanical Journal 72: 55–60. [Google Scholar]
- Prydiuk MP. (2018). Mushrooms of the families Bolbitiaceae and Psathyrellaceae of Ukraine: species composition, distribution, evolution. Ph.D. dissertation for Doctor of Science degree in Biology (Dr. Sci. Biol.), specialty 03.00.21 – Mycology. M.G. Kholodny Institute of Botany of National Academy of Sciences of Ukraine, Kyiv, Ukraine. [Google Scholar]
- Quélet L. (1886). Enchiridion fungorum in Europа mediа et prаsertim in Gаlliа vigentium. O. Doin, Pаris, France. [Google Scholar]
- Redhead SA, Malloch DW. (1986). The genus Phaeocollybia (Agaricales) in eastern Canada and its biological status. Canadian Journal of Botany 64: 1249–1254. [Google Scholar]
- Redhead SA, Smith AH. (1986). Two new genera of agarics based on Psilocybe comeipes and Phaeocollybia perplexa. Canadian Journal of Botany 64: 643–647. [Google Scholar]
- Redhead SA, Vilgalys R, Moncalvo JM, et al. (2001). Coprinus Persoon and the disposition of Coprinus species sensu lato. Taxon 50: 203–241. [Google Scholar]
- Redhead SA, Ammirati JF, Norvell LL, et al. (2011). Validation of combinations with basionyms published by Fries in 1861. Mycotaxon 118: 455–458. [Google Scholar]
- Romagnesi H. (1962). Les Naucoria du groupe Centunculus (Ramicola Velen.). Bulletin Trimestriel de la Société Mycologique de France 78: 337–358. [Google Scholar]
- Saccardo PA. (1887). ylloge Hymenomycetum, Vol. I. Agaricineae. Sylloge Fungorum 5: 1–1146. [Google Scholar]
- Senn-Irlet B. (1995). Die Gattung Simocybe Karsten in Europa. Mycologia Helvetica 7: 27–61. [Google Scholar]
- Singer R. (1960). Sobre algunas especies de hongos presumiblemente psicotropicos. Lilloa 30: 117–127. [Google Scholar]
- Singer R. (1969). Mycoflora australis. Beihefte zur Nova Hedwigia 29: 1–405. [Google Scholar]
- Singer R. (1986). The Agaricales in modern taxonomy, 4th ed Koeltz Scientific Books, Koenigstein, Germany. [Google Scholar]
- Smith AH. (1937). Unusual agarics from Michigan. IV. Papers from the Michigan Academy of Science, Arts and Letters 22: 215–223. [Google Scholar]
- Smith AH. (1938). New and unusual Agarics from North America: I. Mycologia 30: 20–41. [Google Scholar]
- Smith AH. (1949). Mushrooms in their natural habitats. Sawyer’s Inc, Portland, USA. [Google Scholar]
- Smith AH. (1975). A field guide to Western Mushrooms. Univ. Mich. Press, Ann Arbor, USA. [Google Scholar]
- Smith AH, Singer R. (1964). A Monograph of the Genus Galerina Earle. Hafner Publishing, New York, USA. [Google Scholar]
- Stålberg J. (1991). Mythicomyces corneipes (Fr.) Redhead & Smith. Mystisk skivling identifierad. Jordstjärnan 12: 64–67. [Google Scholar]
- Stamatakis A. (2006). RAxML-VI-HPC: maximum likelihood based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Stridvall A, Stridvall L. (1996). Fynd av två för Sverige nya skivlingar Russula rutila Romagn. och Stagnicola perplexa (Orton) Redhead & Smith. Jordstjärnan 17: 11–16. [Google Scholar]
- Strittmatter E, Obenauer H. (2013). Ein Fund des Hornstieligen Scheinschwefelkopfes Mythicomyces corneipes (Fr.) Redhead & A. H. Sm. in Südwestdeutschland. Zeitschrift für Mykologie 79: 337–349. [Google Scholar]
- Tamura K, Stecher G, Peterson D, et al. (2013). MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudell SA, Rygiewicz PT, Edmonds RL. (2004). Patterns of nitrogen and carbon stable isotope ratios in macrofungi, plants and soils in two old-growth conifer forests. New Phytologist 164: 317–335. [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volders J. (2002). Het genus Tubaria in Vlaanderen en het Brussels Gewest. Sterbeeckia 21–22: 3–28. [Google Scholar]
- Watling R, Gregory NM. (1993). British Fungus Flora 7. Cortinariaceae pp. Royal Botanic Garden, Edinburgh, UK. [Google Scholar]
- Walther G, Garnica S, Weiss M. (2005). The systematic relevance of conidiogenesis modes in the gilled Agaricales. Mycological Research 109: 525–544. [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns TD, Lee S, et al. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky J, White TJ, eds). Academic Press, San Diego, USA: 315–322. [Google Scholar]
- Wood AE. (2001). Studies in the genus Galerina (Agaricales) in Australia. Australian Systematic Botany 14: 615–676. [Google Scholar]
- Zhao R-L, Li G-J, Sánchez-Ramírez S, et al. (2017). A six-gene phylogenetic overview of Basidiomycota and allied phyla with estimated divergence times of higher taxa and a phyloproteomics perspective. Fungal Diversity 84: 43–74. [Google Scholar]


