Abstract
Pancreatic cancer is a dismal disorder that is histologically characterized by a dense fibrotic stroma around the tumor cells. As the extracellular matrix comprises the bulk of the stroma, matrix degrading proteases may play an important role in pancreatic cancer. It has been suggested that matrix metalloproteases are key drivers of both tumor growth and metastasis during pancreatic cancer progression. Based upon this notion, changes in matrix metalloprotease expression levels are often considered surrogate markers for pancreatic cancer progression and/or treatment response. Indeed, reduced matrix metalloprotease levels upon treatment (either pharmacological or due to genetic ablation) are considered as proof of the anti-tumorigenic potential of the mediator under study. In the current review, we aim to establish whether matrix metalloproteases indeed drive pancreatic cancer progression and whether decreased matrix metalloprotease levels in experimental settings are therefore indicative of treatment response. After a systematic review of the studies focusing on matrix metalloproteases in pancreatic cancer, we conclude that the available literature is not as convincing as expected and that, although individual matrix metalloproteases may contribute to pancreatic cancer growth and metastasis, this does not support the generalized notion that matrix metalloproteases drive pancreatic ductal adenocarcinoma progression.
Keywords: MMP, MMP2, MMP9, MMP7, MMP14, matrix metalloproteases, PDAC, pancreatic cancer
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a devastating disease with the worst survival outcome of any cancer [1]. Its incidence, which is around 10 per 100,000 individuals, is rising in developed countries [2,3], with 458 thousand new cases and 432 thousand deaths in 2018 worldwide [4]. The 5-year survival rate is around 9%, and the 10-year mortality is approaching 99% [5]. Progress towards improving survival has been slow, and current treatment options are inadequate. The only significant progress that has been made is in the form of lower mortality rates for patients eligible for resections, and a slight prolongation and improved quality of life in patients with inoperable disease with the use of chemotherapeutic agents. Single-agent gemcitabine treatment has been the standard of care for inoperable PDAC for many years, although the observed benefits are small in daily practice [6,7,8,9] and seem restricted to patients with a good performance status [10]. More recently, nanoparticle albumin-bound paclitaxel was shown to exert superior antitumor activity compared to gemcitabine monotherapy, thereby establishing nab-paclitaxel and gemcitabine combination therapy as first-line chemotherapy regimens in PDAC [11]. In patients with a good performance status, combination therapy with folinic acid, fluorouracil, irinotecan and oxaliplatin (FOLFIRINOX) is superior over other treatments [12] and FOLFIRINOX is consequently emerging as the new standard of care for relatively fit patients [13]. Importantly however, even in the specific group of patients eligible for FOLFIRINOX treatment, the survival benefit is limited [14].
1.1. Tumor Microenvironment of PDAC
PDAC is characterized by a strong desmoplastic reaction, which results in an archetypal tumor microenvironment, consisting of a dense stroma surrounding the tumor cells [15,16]. The stroma forms the bulk of the tumor, taking up to 90% of the total tumor mass and consists of many cellular and acellular components like (myo)fibroblasts, macrophages, blood vessels and extracellular matrix components such as, among others, collagen I, collagen IV, laminin and fibronectin. In the stroma, the extracellular matrix has traditionally been considered to be a stable structure that mainly plays a supportive role in maintaining tissue morphology. Nowadays, however, it is evident that the extracellular matrix forms a dynamic and versatile milieu that affects the fundamental processes of the surrounding cells [17,18]. Accordingly, the loss of extracellular matrix homeostasis and integrity is considered one of the hallmarks of cancer and typically defines transitional events, resulting in cancer progression and metastasis [19]. Moreover, the loss of extracellular matrix homeostasis due to stromal depletion aggravates pancreatic cancer progression in preclinical animal models [20,21,22].
1.2. Matrix Metalloproteases in the Tumor Microenvironment
The desmoplastic PDAC stroma contains many different proteases that play a key role in the crosstalk between tumor and stromal cells. An intriguing group of proteases in the tumor microenvironment consist of matrix metalloproteases (MMPs), which are primarily known for their ability to degrade extracellular matrix components. Altered expression and/or activity of MMPs in the tumor microenvironment is likely to lead to the loss of homeostasis of the extracellular matrix, thereby driving PDAC progression. Based upon this notion, MMPs are considered important contributors to PDAC progression and experimental PDAC studies frequently use MMPs as surrogate markers for treatment responses. Decreased MMP levels are, nowadays, considered as important signs of the anti-tumorigenic potential of the gene/compound/miRNA under study. In the current review, we address whether the literature supports the concept that MMPs drive PDAC progression and if decreased MMP levels under experimental settings are indicative of the treatment response. To this end, we performed a systematic review of patient and experimental animal studies, focusing on MMPs in PDAC.
1.3. Overview of Matrix Metalloproteases
MMPs are calcium-dependent zinc-containing endopeptidases of the metzincin protease superfamily. They typically contain an N-terminal propeptide of approximately 80–90 amino acids, with a conserved PRCGXPD motif that is responsible for maintaining latency via the binding of the cysteine residue to the zinc atom in the active site [23]. After the proteolytic removal of the propeptide, the active form of MMP contains a calcium-dependent catalytic domain of around 200 amino acids, which contains a hydrophobic S1′-pocket that determines substrate specificity, proceeded by a linker region of variable length, and the C-terminal hemopexin-like domain, which spans approximately 200 amino acids. The hemopexin-like domain, which is absent in some MMP family members, plays a functional role in substrate binding and/or in interactions with tissue inhibitors of metalloproteases (TIMPs), a family of specific MMP protein inhibitors [24].
Since the identification of a diffusible collagenolytic factor in living amphibian tissue that is capable of degrading undenatured calf skin collagen [25], a total of 24 MMPs have been identified in humans [26]. According to their substrate specificity, MMPs are classified into subfamilies: (1) collagenases, (2) gelatinases, (3) stromelysins, (4) matrilysins, (5) membrane-type MMPs and (6) others. Despite the general acceptance of the classification system based on extracellular matrix substrates, MMPs are rather promiscuous in substrate recognition and also proteolytically cleave substrates beyond extracellular matrix proteins.
2. Methods
To provide a comprehensive overview of the role of MMPs in PDAC, a systematic PubMed search without restrictions was performed. A combination of the search terms “pancreatic cancer” and every individual MMP (both using the official gene name and the common name; see Supplementary Materials Table S1) was used to retrieve papers published up to 1 March 2020. All papers were independently screened by their title and abstract, followed by full text assessment to include papers that contained MMP expression analysis in PDAC patients and papers that contained animal experiments that targeted (either genetically or pharmacologically) MMPs in pancreatic cancer models. The excluded papers were those that contained in vitro data only, papers that assayed MMP levels in experimental animal models without interventions or genetic modifications, or papers that did not focus on PDAC.
3. Results
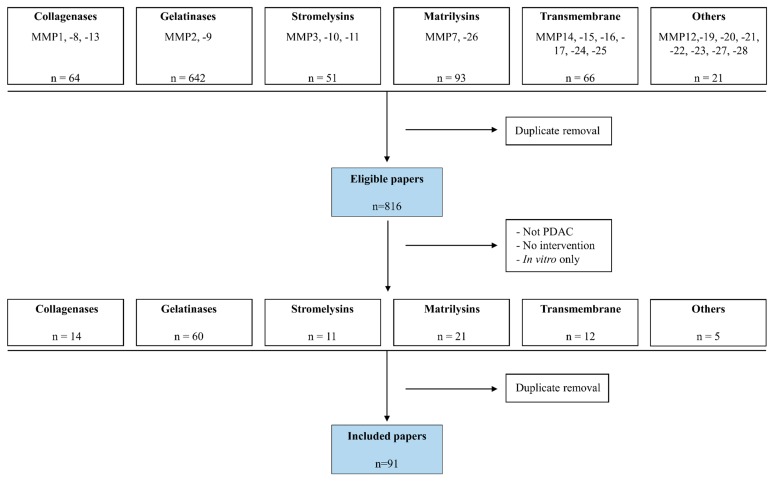
We retrieved 64 papers focusing on collagenases, 642 papers focusing on gelatinases, 51 papers focusing on stromelysins, 93 papers focusing on matrilysins, 66 papers focusing on transmembrane MMPs and 21 papers focusing on other MMPs (Figure 1). After the removal of duplicates, 816 eligible studies were identified and were vigorously screened to obtain those that contained patient data and/or animal experiments in which MMPs were targeted. This resulted in the inclusion of 14 papers focusing on collagenases, 60 on gelatinases, 11 on stromelysins, 21 on matrilysins, 12 on transmembrane-type MMPs and five on the so-called “other” MMPs. As several of the eligible papers contained data on multiple MMPs, the total number of papers including patient/experimental animal data selected for the review was 91.
Figure 1.
Flowchart of paper inclusion. Using the search criteria indicated in Supplementary Materials Table S1, we obtained 814 eligible papers that we screened for the presence of patient and/or matrix metalloprotease (MMP) intervention in animal models. After the exclusion of duplicate papers, we ended up with 91 papers that were included in the review.
3.1. Collagenases in PDAC
Despite the general notion that collagenases (MMP1, MMP8 and MMP13) are key players in cancer biology [27,28,29], relatively little is known about collagenases in PDAC. Although MMP-1 is consistently shown to be overexpressed in PDAC patients compared to healthy controls [30,31,32,33,34,35,36], its effects on cancer progression are inconsistent (Table 1). For example, MMP1 overexpression has been reported as being associated with both a poor prognosis [30] and prolonged survival [37], although no correlations with tumor size, differentiation status and lymph node involvement have been observed [30,36,38]. Despite an elegant recent study showing that MMP1-dependent protease activated receptor (PAR)-1 drives PDAC cell migration and perineural invasion [33], the important role of MMP1 in PDAC is not supported by the experimental data. Besides MMP1 overexpression, MMP8 [36,39] and MMP13 [34,40] are also overexpressed in PDAC patients compared to healthy controls. The relevance of increased MMP expression is not well documented and only a single study showed that MMP-13 expression is associated with lymph node metastasis and the tumor’s pathological stage [41]. Interestingly however, MMP13 overexpression significantly promoted the invasion of the PDAC cells in vitro, whereas MMP13 inhibition blocked leptin-mediated PDAC cell invasion [41], while CD40 agonist-dependent resolution of fibrosis and enhanced chemotherapy efficacy were diminished by MMP13 inhibition [42].
Table 1.
MMP expression levels in Pancreatic ductal adenocarcinoma (PDAC) patients and controls. Red indicates increased MMP levels, blue indicates no difference and green indicates decreased MMP levels in PDAC patients.
| Member | Patient Number | Method | Difference | Reference |
|---|---|---|---|---|
| MMP1 | 45 PC, 10 CO | IHC | no difference | [36] |
| MMP1 | 8 PC, 8 CO | RNA | no difference | [59] |
| MMP1 | 248 PC, 216 CO | Serum | no difference | [69] |
| MMP1 | 46 PC, 5 CO | IHC | up vs healthy | [30] |
| MMP1 | 25 PC | RNA | up vs adjacent CO | [31] |
| MMP1 | 10 PC, 12 CP, 5 CO | IHC | up vs CO | [32] |
| MMP1 | 45 PC | RNA | up vs adjacent CO | [33] |
| MMP1 | 30 PC | IHC | up vs adjacent CO | [33] |
| MMP1 | 104 PC, 62 CO | IHC | up vs CO | [34] |
| MMP1 | 17 PC, 17 CO | RNA | up vs CO | [35] |
| MMP1 | 18 PC, 8 CO | RNA | up vs healthy | [36] |
| MMP2 | 75 PC, 10 CO | IHC | no difference | [36] |
| MMP2 | 18 PC, 8 CO | RNA | no difference | [36] |
| MMP2 | 70 PC and 10 CO | IHC | no difference | [38] |
| MMP2 | 92 PC, 43 CP, 91 CO | Serum | no difference | [68] |
| MMP2 | 35 PC | RNA/IHC | no difference | [70] |
| MMP2 | 46 PC, 13 CO | Serum | no difference | [71] |
| MMP2 | 104 PC, 62 CO | IHC | up vs CO | [34] |
| MMP2 | 17 PC, 17 CO | RNA | upvsCO | [35] |
| MMP2 | 122 PC | IHC | up vs adjacent CO | [43] |
| MMP2 | 18 PC, 9 CP, 9 CO | RNA | up vs both others | [44] |
| MMP2 | 12 PC, 11 CP, 7 CO | pancreatic juice | up vs both others | [45] |
| MMP2 | 127 PC | IHC | up vs CO | [46] |
| MMP2 | 20 PC | IHC | up vs CO | [47] |
| MMP2 | 32 PC, 31 CP | ELISA on tissue | up vs CP | [48] |
| MMP2 | 110 PC, 24 BT | Plasma | up vs BT | [49] |
| MMP2 | 37 PC, 7 CP | IHC | up vs CP and CO | [50] |
| MMP2 | 45 PC | IHC | up vs CO | [51] |
| MMP2 | 51 PC, 60 CO | Urine | up vs CO | [52] |
| MMP2 | 44 PC, 8 CO | IHC | up vs CO | [52] |
| MMP2 | 30 PC, 17 CO | IHC | up vs CO | [53] |
| MMP2 | 29 PC | IHC | up vs adjacent CO | [54] |
| MMP2 | 127 PC, 25 CP, 25 CO | Plasma | up vs CP and CO | [55] |
| MMP2 | 106 PC | RNA/WB | up vs adjacent CO | [56] |
| MMP2 | 40 PC, 10 CO | IHC | up vs CO | [57] |
| MMP2 | 67 PC, 20 CO | IHC | up vs adjacent CO | [58] |
| MMP2 | 8 PC, 8 CO | RNA | upvsCO | [59] |
| MMP2 | 10 PC, 3 CO | ZG | upvsCO | [60] |
| MMP2 | 33 PC, 14 CP, 13 CO | ZG/WB | upvsCO | [61] |
| MMP2 | 22 PC, 9 CP, 9 CO | RNA | up vs adjacent CO | [62] |
| MMP2 | 10 PC, 213 CO | Serum | down vs CO | [118] |
| MMP3 | 45 PC, 10 CO | IHC | no difference | [36] |
| MMP3 | 18 PC, 8 CO | RNA | no difference | [36] |
| MMP3 | 8 PC, 8 CO | RNA | no difference | [59] |
| MMP3 | 104 PC, 62 CO | IHC | up vs CO | [34] |
| MMP3 | 17 PC, 17 CO | RNA | up vs CO | [35] |
| MMP3 | 140 PC, 12 CO | IHC | up vs CO | [94] |
| MMP3 | 140 PC, 12 CO | IHC | up vs CO | [95] |
| MMP7 | 18 PC, 8 CO | RNA | no difference | [36] |
| MMP7 | 104 PC, 62 CO | IHC | up vs CO | [34] |
| MMP7 | 17 PC, 17 CO | RNA | up vs CO | [35] |
| MMP7 | 45 PC, 10 CO | IHC | up vs CO | [36] |
| MMP7 | 29 PC | IHC | up vs adjacent CO | [54] |
| MMP7 | 248 PC, 216 CO | Serum | up vs CO | [68] |
| MMP7 | 44 PC, 17 CP | RNA | up vs CP | [91] |
| MMP7 | 70 PC | RNA | up vs adjacent CO | [97] |
| MMP7 | 32 PC, ? CO | IHC | up vs CO | [98] |
| MMP7 | 47 PC, 10 CO | IHC | up vs CO | [99] |
| MMP7 | 63 PC, 31 CP | Plasma | up vs CP | [100] |
| MMP7 | 30 PC | RNA | up vs adjacent CO | [101] |
| MMP7 | 5 PC, 5 CP, 62 CO | IHC | up vs CP and CO | [102] |
| MMP7 | 131 PC, 30 CP, 131 CO | Plasma | up vs CO | [103] |
| MMP7 | 10 PC | RNA | up vs adjacent CO | [104] |
| MMP8 | 248 PC, 216 CO | Serum | no difference | [69] |
| MMP8 | 75 PC, 10 CO | IHC | up vs CO | [36] |
| MMP8 | 91 PC, 41 CP, 30 CO | RNA (PBMCs) | up vs CO | [39] |
| MMP9 | 18 PC, 8 CO | RNA | no difference | [36] |
| MMP9 | 70 PC, 10 CO | IHC | no difference | [38] |
| MMP9 | 51 PC, 60 CO | urine | no difference | [52] |
| MMP9 | 10 PC, 3 CO | ZG | no difference | [60] |
| MMP9 | 33 PC, 14 CP, 13 CO | ZG/WB | no difference | [61] |
| MMP9 | 248 PC, 216 CO | Serum | no difference | [69] |
| MMP9 | 35 PC | RNA/IHC | no difference | [70] |
| MMP9 | 104 PC, 62 CO | IHC | up vs CO | [34] |
| MMP9 | 45 PC, 10 CO | IHC | up vs CO | [36] |
| MMP9 | 91 PC, 41 CP, 30 CO | RNA (PBMCs) | up vs CP and CO | [39] |
| MMP9 | 32 PC, 31 CP | ELISA on tissue | up vs CP | [48] |
| MMP9 | 110 PC, 24 BT | Plasma | up vs BT | [49] |
| MMP9 | 30 PC, 17 CO | IHC | up vs CO | [53] |
| MMP9 | 29 PC | IHC | up vs adjacent CO | [54] |
| MMP9 | 8 PC, 8 CO | RNA | up vs CO | [59] |
| MMP9 | 22 PC, 9 CP, 9 CO | RNA | up vs adjacent CO | [62] |
| MMP9 | 36 PC | IHC | up vs CO | [63] |
| MMP9 | 9 PC, 9 CO | MS/MS | up vs CO | [64] |
| MMP9 | 78 PC, 45 CP, 70 CO | Serum | up vs both | [65] |
| MMP9 | 62 PC, 16 CO | IHC | up vs CO | [66] |
| MMP9 | 103 PC, 6 CO | IHC | up vs CO | [67] |
| MMP10 | 17 PC, 17 CO | RNA | no difference | [35] |
| MMP11 | 17 PC, 17 CO | RNA | up vs CO | [35] |
| MMP11 | 18 PC, 8 CO | RNA | up vs CO | [36] |
| MMP11 | 75 PC, 10 CO | IHC | up vs CO | [36] |
| MMP11 | 44 PC, 17 CP | RNA | up vs CP | [91] |
| MMP11 | 12 PC, 16 CO | Blood | up vs CO | [92] |
| MMP11 | 21 PC, 9 CO | IHC | up vs CO | [93] |
| MMP12 | 75 PC, 10 CO | IHC | no difference | [36] |
| MMP12 | 39 PC, 13 CO | RNA/WB/IHC | up vs CO | [111] |
| MMP13 | 104 PC, 62 CO | IHC | up vs CO | [34] |
| MMP13 | 45 PC | RNA | up vs adjacent CO | [40] |
| MMP14 | 75 PC, 10 CO | IHC | no difference | [36] |
| MMP14 | 35 PC | RNA/IHC | no difference | [111] |
| MMP14 | 18 PC, 9 CP, 9 CO | RNA | up vs both others | [44] |
| MMP14 | 64 PC, 9 CO | IHC | up vs CO | [110] |
| MMP15 | 18 PC, 9 CP, 9 CO | RNA | up vs both others | [44] |
| MMP15 | 18 PC, 8 CO | RNA | reduced vs CO | [36] |
| MMP16 | 18 PC, 9 CP, 9 CO | RNA | no difference | [44] |
| MMP16 | 12 PC | IHC | up vs adjacent CO | [119] |
| MMP19 | 102 PC | IHC | up vs adjacent CO | [112] |
| MMP20 | 102 PC | IHC | up vs adjacent CO | [112] |
| MMP21 | 25 PC, 18 CO | IHC | up vs CO | [106] |
| MMP26 | 25 PC, 18 CO | IHC | up vs CO | [106] |
Pancreatic cancer (PC); pancreatitis (CP); healthy control (CO); benign tumor (BT); immunohistochemistry (IHC); Western blot (WB); zymography (DG).
3.2. Gelatinases in PDAC
The most studied MMPs in PDAC are, without a doubt, the gelatinases (MMP2 and MMP9; see Figure 1). The vast majority of studies show that both MMP2 [34,35,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62] and MMP9 [34,36,39,48,49,53,54,59,62,63,64,65,66,67] are upregulated in PDAC patients (Table 1), while a minority of studies fail to show a difference in expression between PDAC and the controls [36,38,52,60,61,68,69,70,71]. The potential clinical relevance is less pronounced, as just half of the studies reported associations between increased MMP2 or MMP9 levels with clinical characteristics such as survival, metastasis or tumor stage [43,46,47,48,50,51,53,56,57,58,61,63,65,67,68,72,73], whereas in the other half of the studies no such correlations were observed (Table 2). Despite the rather diverse observations in patients, initial preclinical experimental animal experiments showed promising results (Table 3). Batimastat treatment of mice harboring orthotopic pancreatic cancers reduced cancer growth, metastasis and death compared to control-treated mice, while also potentiating gemcitabine sensitivity [74,75,76,77]. Batimastat was also shown to reduce metastasis and death when PDAC cells were directly injected into the spleen of recipient mice, in order to mimic liver metastasis in PDAC [78]. Although batimastat is not specific to MMP2 and MMP9 and also inhibits MMP1, MMP3, MMP7, MMP8 and several ADAM family members, based on the gelatin zymography of tumor samples before and after treatment, it was hypothesized that the tumor-inhibiting effect of batimastat was dependent on MMP2 and, to a lesser extent, MMP9. The potential importance of MMP2 and MMP9 in PDAC progression is further supported by studies using more specific inhibitors like MMI-166, RO28-2653 and OPB-3206. Indeed, the selective MMP2, MMP9 and MMP14 inhibitor MMI-166 inhibited PDAC growth in both mice and Syrian hamsters [79,80], whereas RO28-2653 and OPB-3206 (both also selective MMP2, MMP9 and MMP14 inhibitors) reduced chemically induced pancreatic carcinogenesis in Syrian hamsters [81,82]. Finally, treatment with the selective MMP2 and MMP9 inhibitor SB-3CT reduced the lung metastasis of subcutaneously implanted PDAC cells [83].
Table 2.
Association between MMP expression and clinical characteristics of PDAC. Red indicates that MMP levels are associated with poor outcome, blue indicates no association and green indicates that MMP levels are associated with improved survival.
| Member | Patient Number | Method | Correlation | Reference | |
|---|---|---|---|---|---|
| MMP1 | 45 PC, 10 CO | IHC | no | [36] | |
| MMP1 | 70 PC | IHC | no | [38] | |
| MMP1 | 46 PC, 5 CO | IHC | OS, | LM, Size, Stage | [30] |
| MMP1 | 30 PC | IHC | PNI | [33] | |
| MMP1 | 51 PC | IHC/serum | OS | [37] | |
| MMP2 | 75 PC, 10 CO | IHC | no | [36] | |
| MMP2 | 70 PC, 10 CO | IHC | no | [38] | |
| MMP2 | 51 PC | IHC/serum | no | [37] | |
| MMP2 | 32 PC, 31 CP | ELISA on tissue | no | [48] | |
| MMP2 | 37 PC, 7 CP | IHC | no | [50] | |
| MMP2 | 29 PC | IHC | no | [54] | |
| MMP2 | 127 PC, 25 CP, 25 CO | plasma | no | [55] | |
| MMP2 | 35 PC | RNA/IHC | no | [70] | |
| MMP2 | 32 PC | IHC | no | [120] | |
| MMP2 | 67 PC | IHC | LM, | PNI, OS, DF | [121] |
| MMP2 | 122 PC | IHC | OS, DF | [43] | |
| MMP2 | 127 PC | IHC | OS, Stage | [46] | |
| MMP2 | 20 PC | IHC | LM | [47] | |
| MMP2 | 37 PC, 7 CP | IHC | LM, DM | [50] | |
| MMP2 | 45 PC | IHC | OS, LM, Stage | [51] | |
| MMP2 | 30 PC, 17 CO | IHC | LM, Stage, Size | [53] | |
| MMP2 | 106 PC | RNA/WB | DM, Stage | [56] | |
| MMP2 | 40 PC, 10 CO | IHC | LM | [57] | |
| MMP2 | 67 PC, 20 CO | IHC | LM, Stage, PNI | [58] | |
| MMP2 | 33 PC, 14 CP, 13 CO | ZG/WB | Stage | [61] | |
| MMP2 | 92 PC, 43 CP, 91 CO | serum | LM, DM | [68] | |
| MMP2 | 32 PC | IHC | VI | [72] | |
| MMP2 | 88 PC | IHC | OS | [73] | |
| MMP3 | 45 PC, 10 CO | IHC | no | [36] | |
| MMP3 | 18 PC, 8 CO | RNA | no | [36] | |
| MMP3 | 70 PC | IHC | no | [38] | |
| MMP3 | 140 PC, 12 CO | IHC | OS | [95] | |
| MMP7 | 51 PC | IHC/serum | no | [37] | |
| MMP7 | 29 PC | IHC | no | [54] | |
| MMP7 | 88 PC | IHC | no | [73] | |
| MMP7 | 45 PC, 10 CO | IHC | OS, | LM, DIF, Stage | [36] |
| MMP7 | 70 PC | IHC | OS, | Size, DIF | [38] |
| MMP7 | 134 PC | IHC | Stage, PNI, | OS | [122] |
| MMP7 | 70 PC | RNA | LM, Size | [97] | |
| MMP7 | 47 PC, 10 CO | IHC | OS, DM | [99] | |
| MMP7 | 10 PC | RNA | OS | [104] | |
| MMP7 | 101 PC | serum | OS | [105] | |
| MMP7 | 39 PC | IHC | LM, OS | [123] | |
| MMP8 | 75 PC, 10 CO | IHC | no | [36] | |
| MMP8 | 91 PC, 41 CP, 30 CO | RNA (PBMCs) | no | [39] | |
| MMP9 | 45 PC, 10 CO | IHC | no | [36] | |
| MMP9 | 70 PC, 10 CO | IHC | no | [38] | |
| MMP9 | 51 PC | IHC/serum | no | [37] | |
| MMP9 | 91 PC, 41 CP, 30 CO | RNA (PBMCs) | no | [39] | |
| MMP9 | 29 PC | IHC | no | [54] | |
| MMP9 | 33 PC, 14 CP, 13 CO | ZG/WB | no | [61] | |
| MMP9 | 9 PC, 9 CO | MS/MS | no | [64] | |
| MMP9 | 35 PC | RNA/IHC | no | [70] | |
| MMP9 | 32 PC | IHC | no | [123] | |
| MMP9 | 27 PC | IHC | no | [124] | |
| MMP9 | 62 PC, 16 CO | IHC | PNI, | LM, Stage, Size | [66] |
| MMP9 | 63 PC | IHC | VI, | OS, LM, DM | [125] |
| MMP9 | 62 PC | IHC | LM, | OS | [126] |
| MMP9 | 32 PC, 31 CP | ELISA on tissue | LM | [48] | |
| MMP9 | 30 PC, 17 CO | IHC | LM, Stage, Size | [53] | |
| MMP9 | 36 PC | IHC | LM, DM | [63] | |
| MMP9 | 78 PC, 45 CP, 70 CO | serum | OS | [65] | |
| MMP9 | 103 PC, 6 CO | IHC | OS, LM, DM, VI, Stage | [67] | |
| MMP9 | 32 PC | IHC | VI | [72] | |
| MMP9 | 88 PC | IHC | OS, DF, | DM | [73] |
| MMP10 | 51 PC | IHC/serum | no | [37] | |
| MMP11 | 75 PC, 10 CO | IHC | OS, LM, | DIF, Size | [36] |
| MMP11 | not indicated | RNA | OS | [92] | |
| MMP12 | 75 PC, 10 CO | IHC | no | [36] | |
| MMP12 | 39 PC, 13 CO | RNA/WB/IHC | OS, | LM, Stage | [111] |
| MMP13 | 60 PC | IHC | LM | [41] | |
| MMP14 | 70 PC | IHC | no | [38] | |
| MMP14 | 75 PC, 10 CO | IHC | no | [36] | |
| MMP14 | 37 PC | RNA/IHC | no | [70] | |
| MMP15 | 78 PC | IHC | OS, DF, | PNI, LM, DM, Stage | [127] |
| MMP19 | 102 PC | IHC | OS, DF, PNI, Stage | [112] | |
| MMP20 | 102 PC | IHC | OS, DF, Stage, PNI | [112] | |
| MMP21 | 25 PC, 18 CO | IHC | no | [106] | |
| MMP26 | 25 PC, 18 CO | IHC | LM | [106] | |
| MMP28 | not indicated | RNA | OS | [113] | |
Pancreatic cancer (PC); pancreatitis (CP); healthy control (CO); benign tumor (BT); immunohistochemistry (IHC); Western blot (WB); zymography (DG); overall survival (OS); disease-free survival (DF); lymph node metastasis (LM); perineural invasion (PNI); venous invasion (VI); distant metastasis (DM); differentiation (DIF).
Table 3.
Experimental animal models that target MMPs.
| Target | Model | “Treatment” | Result | Reference |
|---|---|---|---|---|
| MMP1 | Sciatic nerve invasion | shMMP1 PANC1 cells | Reduced perineural invasion | [33] |
| MMP2/9? | Orthotopic injection HPAC cells | Batimastat (day −7 till death/sacrifice) | Increased gemcitabine sensitivity, No effect single treatment | [74] |
| Orthotopic injection HPAC cells | Batimastat (day −4 till death/sacrifice) | Reduced tumor growth, metastasis and death | [75] | |
| Orthotopic injection HPAC cells | Batimastat (day 7 till death/sacrifice) | Reduced local invasion and death | [76] | |
| Orthotopic injection HPAC cells | Batimastat (day 7 till death/sacrifice) | Reduced tumor weight | [77] | |
| Injection AsPC1 or Capan-1 cells in spleen | Batimastat (day −7 till day 14) | Reduced metastasis and death | [78] | |
| Subcutaneous injection SW1990 cells | MMI-166 from day 7 till sacrifice at day 28 | Reduced tumor growth | [79] | |
| Orthotopic injection PGHAM cells (Syrian hamster) | MMI-166 (day 1 till sacrifice) | Reduced tumor growth, liver metastasis and MVD | [80] | |
| BOP injections (Syrian hamster) | RO28-2653 (week 6 till week 14) | Reduced liver metastasis, No effect death | [81] | |
| BOP injections (Syrian hamster) | OPB-3206 in diet from day 48 till sacrifice | Reduced invasive carcinoma | [82] | |
| Subcutaneous injection Panc02 or MIAPaca2 cells | SB-3CT (day 1 till sacrifice) | Reduced lung metastasis | [83] | |
| MMP2 | Subcutaneous injection organoid and PSC | shMMP2 PSC | Reduced tumor growth | [84] |
| Subcutaneous injection PANC-1 or CFPAC-1 cells | MMP2 blocking peptides after tumor take | Reduced growth and MVD | [85] | |
| MMP3 | Kras(G12D) mice | MMP3 overexpression | Increased neoplastic alterations | [94] |
| MMP7 | Ductal ligation | MMP7 deficient mice | Reduced ductal metaplasia | [98] |
| Pfta1-Cre/KrasG12D mice | MMP7 deficiency | No effect acinar to ductal metaplasia | [105] | |
| Pdx1-Crelate/KrasG12D mice | MMP7 deficiency | Reduced tumor development | [105] | |
| Pdx1-CreLate/KrasG12D/p53f/+ mice | MMP7 deficiency | Reduced tumor growth and metastasis | [105] | |
| Tail vein injection PANC1 cells | shMMP7 | Reduced liver and lung metastasis | [101] | |
| SCTPSPA (day −2 till day 25) | Reduced lung metastasis | [101] | ||
| MMP9 | Subcutaneous injection Panc02 cells | MMP9 deficient mice | Reduced lung metastasis | [83] |
| Orthotopic injection Panc02 cells | MMP9 overexpression | Enhanced tumor growth, No effect metastasis | [86] | |
| Subcutaneous injection AsPC-1 cells | aMMP9 antibody AB0046 (day 1 till day 14) | No effect on tumor weight | [87] | |
| Injection AsPC-1 cells in peritoneal cavity | aMMP9 antibody AB0046 (day 14 till day 56) | Increased gemcitabine/nab-paclitaxel sensitivity, No effect metastasis | [87] | |
| Subcutaneous injection Capan-1 cells | Doxycycline (day 1 till day 14) | Reduced growth and MVD | [88] | |
| Orthotopic injection L3.6pl cells | MMP9 deficient mice | Reduced tumor take, growth and MVD | [89] | |
| Pdx-1+/Cre;KrasG12D;Trp53 mice | MMP9 deficiency | Increased progression and invasive growth | [90] | |
| Intravenous injection 9801 or Panc02 cells | MMP9 deficient mice | Increased metastasis | [90] | |
| MMP14 | Subcutaneous injection organoid and PSC | shMMP14 PSC | Reduced tumor growth | [84] |
| KrasG12D mice | MMP14 overexpression | Increased number of PanIN lesions | [107] | |
| Subcutaneous injection PANC1 or HPAF-II cells | MMP14 overexpression | Reduced gemcitabine sensitivity, No effect single treatment | [108] | |
| Orthotopic injection DanG or BxPc3 cells | MTCBP-1 overexpression | Reduced metastasis, No effect tumor growth | [109] |
Note: All experiments were performed using mice unless indicated otherwise.
The most conclusive evidence of the role of MMP2 in PDAC progression comes from subcutaneous models, in which the injection of shMMP2-silenced PANC1 cells resulted in smaller tumors compared to the injection of control shRNA transduced cells [84], whereas treatment with MMP2-blocking peptides limited tumor growth and angiogenesis [85].
In a similar way to the inconclusive association studies in patients (see above and Table 2), experimental animal experiments specifically targeting MMP9 show inconsistent results (Table 3). Orthotopic injections of MMP9-overexpressing Panc02 cells led to bigger tumors than injections of their control counterparts, but the absence/presence of MMP9 did not affect metastasis [86]. Treatment with a MMP9-blocking antibody did not affect the tumor growth of subcutaneously implanted PDAC cells, but did enhance gemcitabine and nab-paclitaxel sensitivity when PDAC cells were injected into the peritoneal cavity [87]. Doxycycline treatment, suggested to specifically target MMP9, reduced the growth of subcutaneously injected Capan-1 cells [88]. Finally, subcutaneous or orthotopic implantation of PDAC cells in MMP9-deficient mice diminished tumor take, tumor growth, angiogenesis and metastasis [83,89] but tumor progression and metastasis increased in MMP9-deficient mice on the Kras(G12D)/Tp53 background [90].
3.3. Stromelysins in PDAC
Clinical studies do not support the general role of stromelysins (MMP3, MMP10 and MMP11) in PDAC (Table 1). Although MMP11 is consistently upregulated and associated with clinical characteristics in PDAC patients [35,36,91,92,93], the data for MMP3 is more controversial. Only half of the studies focusing on MMP3 suggest its expression is increased in PDAC patients compared to control tissue [34,35,94,95], and only a single study suggests that MMP3 is associated with patient survival [95]. Besides clinical studies, preclinical animal models also do not support an important role for stromelysins in PDAC progression. Apart from a study which suggests, but does not prove, that MMP10 drives the invasion and metastasis of PDAC [96], it has only been shown that MMP3 overexpression on the Kras(G12D) background increases neoplastic alterations in pancreatic acinar cells [94]. These premalignant morphological changes were accompanied by the recruitment of infiltrating immune cells and the expression of smooth muscle actin and collagen, indicating that MMP3 is not only a coconspirator of Kras in inducing tumorigenic changes in epithelial cells, but also that it promotes the establishment of a tumorigenic microenvironment. Though it has been suggested that MMP3 may play a role in PDAC initiation, the actual importance of endogenous MMP3 (as opposed to overexpressed MMP3) in PDAC progression and its potential clinical relevance remains elusive.
3.4. Matrilysins in PDAC
MMP7 and MMP26 are the only two members of the matrilysin subfamily. A large number of studies have compared MMP7 expression in PDAC patients with pancreatitis patients and/or healthy controls and have consistently shown that MMP7 levels are elevated in PDAC patients (Table 1) [34,35,36,54,69,91,97,98,99,100,101,102,103,104]. More importantly, MMP7 levels correlate with metastasis and/or survival in most, but not all, studies. Based upon these reports, it is suggested that MMP7 is an important regulator of tumor formation. In line with this notion, preclinical experimental animal models show that MMP7 expression is intimately linked with acinar-to-ductal metaplasia and that pancreatic duct ligation-dependent acinar cell loss, caspase-3 activation, and subsequent metaplasia is significantly reduced in MMP7-deficient mice (Table 3) [98]. The effect of MMP7 on acinar-to-ductal metaplasia seems model-specific, however, as MMP7 deficiency did not affect pancreatitis driven-PanIN development in Pfta1-Cre Kras(G12D) mice [105]. In addition to PDAC initiation, MMP7 also seems to drive PDAC progression. Using several genetic Kras-driven PDAC models, it was shown that both tumor size and metastasis were significantly reduced by MMP7 deficiency. The percentage of mice with lymph node metastasis reduced from around 60 in MMP7-proficient mice to 0 in MMP7-deficient mice, whereas the percentage of mice with liver metastasis dropped from 67% to 13% due to MMP7 deficiency [105]. In line with these findings, the metastasis of MMP7-silenced PANC1 cells was largely reduced compared to control PANC1 cells, whereas pharmacological MMP7 inhibition with sulfur-2-(4-chlorine-3-trifluoromethyl phenyl)-sulfonamido-4-phenylbutyric acid (SCTPSPA) also significantly reduced the metastasis of PANC1 cells [101]. MMP26 expression was also induced in PDAC patients compared to the controls and, intriguingly, MMP26 was expressed significantly more often in tumors with lymph node involvement. Although this is suggestive of the general role of matrilysins in PDAC progression, experimental data confirming the pro-tumorigenic role of MMP26 in PDAC is lacking and it remains to be established whether MMP26 is indeed a driver of disease progression or merely acts as a marker of PDAC metastasis [106].
3.5. Membrane-Type MMPs in PDAC
Seven membrane-bound MMPs have been described so far: the transmembrane members MMP14, MMP15, MMP16, MMP23 and MMP24, and the GPI-anchored members MMP17 and MMP25. Of the membrane-bound MMPs, MMP14 seems most relevant in the setting of PDAC (Table 1, Table 2 and Table 3). Indeed, the overexpression of MMP14 in mice expressing an activating Kras(G12D) mutation led to more large, dysplastic mucin-containing papillary lesions compared to the control Kras(G12D) mice (Table 3) [107]. Using subcutaneous models, MMP14 overexpression in cancer cells seems to reduce the cytotoxic effect of gemcitabine [108], whereas MMP14 inhibition in pancreatic stellate cells limits tumor growth [84]. Moreover, the cancer cell-specific overexpression of membrane-type 1 matrix metalloproteinase cytoplasmic tail binding protein-1 (MTCBP-1; MMP14 binding protein inhibiting its activity) restricts metastasis in orthotopic PDAC models, further suggesting that MMP14 may enhance tumor progression [109]. However, clinical data do not support the important role of MMP14 in PDAC progression (Table 1 and Table 2). Although MMP14 may be overexpressed in PDAC [44,110], MMP14 does not correlate with clinical characteristics such as tumor differentiation, tumor size, lymph node status, or patient survival [31,37,111].
3.6. Other MMPs in PDAC
The so-called other MMPs (i.e., MMP12, MMP19, MMP20, MMP21, MMP27 and MMP28) are not very well characterized in PDAC. Although some members seem to be overexpressed in PDAC [106,111,112] and may be associated with tumor stage and patients survival (Table 1) [111,112,113], no preclinical studies have addressed the role of these MMPs in PDAC (Table 2). Therefore, their actual importance remains to be established.
3.7. Clinical Trials with MMP Inhibitors in PDAC
Only two phase 3 trials focusing on MMP inhibition in PDAC have been published [114,115]. One trial showed that the addition of marimastat (a broad-spectrum MMP inhibitor targeting MMP1, MMP2, MMP7, MMP9 and MMP14) to gemcitabine in a double-blind placebo-controlled, randomized study was well-tolerated but did not show clinical benefits in PDAC patients [114]. The overall response rates (11% and 16% with and without the addition of marimastat, respectively), progression-free survival and time to treatment failure were similar in both treatment arms. Another phase 3 trial showed that BAY 12-9566 (tanomastat; MMP2, MMP3 and MMP9 inhibitor) treatment was also well tolerated by PDAC patients but was inferior to gemcitabine, with median survival times of 3.74 and 6.59 months for the BAY 12-9566 and gemcitabine arm, respectively [115]. Median progression-free survival and quality-of-life analyses also favored gemcitabine, arguing against MMP inhibition in the setting of PDAC.
The fact that there are no clinical benefits obtained through MMP inhibition does not imply that MMPs do not contribute to PDAC progression. As elegantly discussed [116,117], the disappointing clinical trial results may be due to several reasons, of which the inclusion of advanced stage disease seems most relevant. Broad spectrum MMP inhibitors may also lack efficacy as they could block the potential tumor inhibitory activities of specific MMPs. As indicated above, MMP9 deficiency on the Kras(G12D) background enhanced tumor progression and invasive growth [90], supporting this notion and providing an alternative explanation for the negative marimastat and BAY 12-9566 results in PDAC patients. Finally, the poor clinical efficacy of MMP inhibitors could also be explained by the overestimation of the role of MMPs in PDAC progression based on preclinical models that do not fully capture the complexity of human disease.
4. Conclusions
The potential clinical relevance of MMPs in PDAC has largely been addressed using patient-derived tumor material. These studies show a rather consistent picture with respect to MMP overexpression in tumors compared to control sections, although almost 25% of the studies do not show significant differences between patients and controls. However, the association of MMP overexpression with clinical characteristics is not as convincing as suggested in the literature. Half of the studies show that high MMP levels are associated with (lymph node) metastasis and reduced survival, whereas the other half of the studies do not show any correlation with clinical characteristics. Patient-derived data do not, therefore, seem to allow firm conclusions that MMP expression levels (in general) are associated with PDAC progression and poor prognosis to be drawn, especially when considering that publication bias may have resulted in negative studies not being published.
Initial preclinical experimental animal models using broad spectrum MMP inhibitors are more in line with the general role of MMPs in PDAC progression, as different inhibitors limit tumor growth and metastasis in subcutaneous, orthotopic and spontaneous PDAC models. The contribution of individual MMPs in PDAC progression is, however, not very well established. Only MMP2, MMP7 and MMP14 are shown to potentiate tumor growth and/or metastasis in multiple independent papers. For others, the literature is conflicting or missing and no clear conclusions can be drawn. Importantly, however, conflicting results do not indicate that the individual MMPs have no effect in PDAC. The biology of PDAC and MMP is complex and MMPs may act in a context-dependent manner, with both tumor-promoting and tumor-inhibiting effects. The conflicting role of MMP9 serves as an excellent example for this notion. The data rather convincingly show that tumor MMP9 expression drives PDAC progression, but systemic MMP9 ablation triggers invasive growth and metastasis by blocking MMP9-dependent tumor-inhibiting effects in the bone marrow.
Despite the presence of a large range of MMP-deficient animals and the relative ease of generating MMP deficient cells with CRISPR technology, the majority of MMPs have not been studied in preclinical PDAC animal models. To fully appreciate the importance of individual MMPs in PDAC progression and to assess their potential clinical relevance, we have to await studies that combine (pharmacological inhibition in) genetic Kras-driven spontaneous models with subcutaneous and/or orthotopic models, in which MMPs are specifically depleted in stromal or tumor cells. In particular, experiments that address pharmacological treatment with specific MMP inhibitors after tumors could turn out to be invaluable for establishing the context-dependent role of individual MMPs in PDAC. Before such studies have been performed, we should be careful not to generalize the available literature.
Although broad spectrum MMP inhibitors limit PDAC progression in preclinical animal models [73,74,75,76,77,78,79,80,81,82], they seem to lack efficacy in a clinical setting [115,116]. This disparity between preclinical data and clinical trials can be attributed to several factors—for instance, differences in pharmacokinetics, pharmacodynamics and metabolism and the failure to accurately model the tumor microenvironment [128]. In particular, xenograft models, which lack a functional immune system, show a reduced complexity and cellular diversity compared to human disease models. Moreover, the degree of aneuploidy in human tumors results in great variety within inter-tumoral gene modifications, in a different manner compared to how it occurs in mice [129,130]. All of these species-related differences limit the capacity of preclinical mouse models to accurately predict the response of MMP inhibitors in PDAC patients.
In conclusion, based on our systematic review on the role of matrix metalloproteases in PDAC, we conclude that the available literature is not as consistent as envisioned and that, although individual matrix metalloproteases seem to contribute to PDAC growth and metastasis, our review does not support the generalized notion that matrix metalloproteases drive PDAC progression.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-7737/9/4/80/s1, Table S1: Search terms used and number of papers retrieved.
Funding
This research was funded by grants from the Dutch Cancer Foundation (UVA 2017-11174 and UVA 2014-6782) and the Netherlands Organization for Scientific Research (VENI grant 016.186.046).
Conflicts of Interest
The authors declare no conflict of interest. M.F.B. has acted as a consultant to Servier, and received research funding from Celgene.
References
- 1.Neoptolemos J.P., Urrutia R., Abbruzzese J.L., Büchler M.W., editors. Pancreatic Cancer. Volume XXXIII. Springer; New York, NY, USA: 2018. pp. 3–18. [Google Scholar]
- 2.Rawla P., Sunkara T., Gaduputi V. Epidemiology of pancreatic cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019;10:10–27. doi: 10.14740/wjon1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simard E.P., Ward E.M., Siegel R., Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J. Clin. 2012;62:118–128. doi: 10.3322/caac.20141. [DOI] [PubMed] [Google Scholar]
- 4.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 5.Siegel R.L., Miller K.D., Jemal A. Cancer statistics 2019. CA Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 6.Burris H.A., III, Moore M.J., Andersen J., Green M.R., Rothenberg M.L., Modiano M.R., Cripps M.C., Portenoy R.K., Storniolo A.M., Tarassoff P., et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham D., Chau I., Stocken D.D., Valle J.W., Smith D., Steward W., Harper P.G., Dunn J., Tudur-Smith C. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J. Clin. Oncol. 2009;27:5513–5518. doi: 10.1200/JCO.2009.24.2446. [DOI] [PubMed] [Google Scholar]
- 8.Heinemann V., Quietzsch D., Gieseler F., Gonnermann M., Schönekäs H., Rost A., Neuhaus H., Haag C., Clemens M., Heinrich B., et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J. Clin. Oncol. 2006;24:3946–3952. doi: 10.1200/JCO.2005.05.1490. [DOI] [PubMed] [Google Scholar]
- 9.Louvet C., Labianca R., Hammel P., Lledo G., Zampino M.G., André T., Zaniboni A., Ducreux M., Aitini E., Taïeb J., et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: Results of a GERCOR and GISCAD phase III trial. J. Clin. Oncol. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Reni M., Cordio S., Milandri C., Passoni P., Bonetto E., Oliani C., Luppi G., Nicoletti R., Galli L., Bordonaro R., et al. Gemcitabine versus cisplatin, epirubicin, fluorouracil, and gemcitabine in advanced pancreatic cancer: A randomised controlled multicentre phase III trial. Lancet Oncol. 2005;6:369–376. doi: 10.1016/S1470-2045(05)70175-3. [DOI] [PubMed] [Google Scholar]
- 11.Awasthi N., Zhang C., Schwarz A.M., Hinz S., Wang C., Williams N.S., Schwarz M.A., Schwarz R.E. Comparative benefits of Nab-paclitaxel over gemcitabine or polysorbate-based docetaxel in experimental pancreatic cancer. Carcinogenesis. 2013;34:2361–2369. doi: 10.1093/carcin/bgt227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinemann V., Haas M., Boeck S. Systemic treatment of advanced pancreatic cancer. Cancer Treat. Rev. 2012;38:843–853. doi: 10.1016/j.ctrv.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Rombouts S.J., Mungroop T.H., Heilmann M.N., van Laarhoven H.W., Busch O.R., Molenaar I.Q., Besselink M.G., Wilmink J.W. FOLFIRINOX in Locally Advanced and Metastatic Pancreatic Cancer: A Single Centre Cohort Study. J. Cancer. 2016;7:1861–1866. doi: 10.7150/jca.16279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suker M., Beumer B.R., Sadot E., Marthey L., Faris J.E., Mellon E.A., El-Rayes B.F., Wang-Gillam A., Lacy J., Hosein P.J., et al. FOLFIRINOX for locally advanced pancreatic cancer: A systematic review and patient level meta-analysis. Lancet Oncol. 2016;17:801–810. doi: 10.1016/S1470-2045(16)00172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hidalgo M. Pancreatic cancer. N. Engl. J. Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 16.Bijlsma M., van Laarhoven H. The conflicting roles of tumor stroma in pancreatic cancer and their contribution to the failure of clinical trials: A systematic review and critical appraisal. Cancer Metastasis Rev. 2015;34:97–114. doi: 10.1007/s10555-014-9541-1. [DOI] [PubMed] [Google Scholar]
- 17.Hynes R.O. The extracellular matrix: Not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu P., Weaver V.M., Werb Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filipe E.C., Chitty J.L., Cox T.R. Charting the unexplored extracellular matrix in cancer. Int. J. Exp. Pathol. 2018;99:58–76. doi: 10.1111/iep.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Özdemir B.C., Pentcheva-Hoang T., Carstens J.L., Zheng X., Wu C., Simpson T.R., Laklai H., Sugimoto H., Kahlert C., Novitskiy S.V., et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhim A.D., Oberstein P.E., Thomas D.H., Mirek E.T., Palermo C.F., Sastra S.A., Dekleva E.N., Saunders T., Becerra C.P., Tattersall I.W., et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Catenacci D.V., Junttila M.R., Karrison T., Bahary N., Horiba M.N., Nattam S.R., Marsh R., Wallace J., Kozloff M., Rajdevet L., et al. Randomized Phase Ib/II Study of Gemcitabine Plus Placebo or Vismodegib, a Hedgehog Pathway Inhibitor, in Patients With Metastatic pancreatic cancer. J. Clin. Oncol. 2015;33:4284–4292. doi: 10.1200/JCO.2015.62.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massova I., Kotra L.P., Fridman R., Mobashery S. Matrix metalloproteinases: Structures, evolution, and diversification. FASEB J. 1998;12:1075–1095. doi: 10.1096/fasebj.12.12.1075. [DOI] [PubMed] [Google Scholar]
- 24.Brew K., Dinakarpandian D., Nagase H. Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim. Biophys. Acta. 2000;1477:267–283. doi: 10.1016/S0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 25.Gross J., Lapiere C. Collagenolytic activity in amphibian tissues: A tissue culture assay. Proc. Natl. Acad. Sci. USA. 1962;48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mittal R., Patel A.P., Debs L.H., Nguyen D., Patel K., Grati M., Mittal J., Yan D., Chapagain P., Liu X.Z. Intricate Functions of Matrix Metalloproteinases in Physiological and Pathological Conditions. J. Cell Physiol. 2016;231:2599–2621. doi: 10.1002/jcp.25430. [DOI] [PubMed] [Google Scholar]
- 27.Juurikka K., Butler G.S., Salo T., Nyberg P., Åström P. The Role of MMP8 in Cancer: A Systematic Review. Int. J. Mol. Sci. 2019;20:4506. doi: 10.3390/ijms20184506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foley C.J., Kuliopulos A. Mouse Matrix metalloprotease-1a (Mmp1a) Gives New Insight into MMP Function. J. Cell Physiol. 2014;229:1875–1880. doi: 10.1002/jcp.24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang S.H., Law C.H., Kuo P.H., Hu R.Y., Yang C.C., Chung T.W., Li J.M., Lin L.H., Liu Y.C., Liao E.C., et al. MMP13 is involved in oral cancer cell metastasis. Oncotarget. 2016;7:17144–171161. doi: 10.18632/oncotarget.7942. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Ito T., Ito M., Shiozawa J., Naito S., Kanematsu T., Sekine I. Expression of the MMP-1 in Human Pancreatic Carcinoma: Relationship with Prognostic Factor. Mod. Pathol. 1999;12:669–674. [PubMed] [Google Scholar]
- 31.Rogers A., Smith M.J., Doolan P., Clarke C., Clynes M., Murphy J.F., McDermott A., Swan N., Crotty P., Ridgway P.F., et al. Invasive Markers Identified by Gene Expression Profiling in pancreatic cancer. Pancreatology. 2012;12:130–140. doi: 10.1016/j.pan.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Tahara H., Sato K., Yamazaki Y., Ohyama T., Horiguchi N., Hashizume H., Kakizaki S., Takagi H., Ozaki I., Arai H., et al. Transforming Growth Factor-α Activates Pancreatic Stellate Cells and May Be Involved in Matrix metalloproteinase-1 Upregulation. Lab. Investig. 2013;93:720–732. doi: 10.1038/labinvest.2013.59. [DOI] [PubMed] [Google Scholar]
- 33.Huang C., Li Y., Guo Y., Guo Y., Zhang Z., Lian G., Chen Y., Li J., Su Y., Li J., et al. MMP1/PAR1/SP/NK1R paracrine loop modulates early perineural invasion of pancreatic cancer cells. Theranostics. 2018;8:3074–3086. doi: 10.7150/thno.24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohlund D., Ardnor B., Oman M., Naredi P., Sund M. Expression pattern and circulating levels of endostatin in patients with pancreas cancer. Int. J. Cancer. 2008;122:2805–2810. doi: 10.1002/ijc.23468. [DOI] [PubMed] [Google Scholar]
- 35.Bramhall S.R., Neoptolemos J.P., Stamp G.W., Lemoine N.R. Imbalance of expression of matrix metalloproteinases (MMPs) and tissue inhibitors of the matrix metalloproteinases (TIMPs) in human pancreatic carcinoma. J. Pathol. 1997;182:347–355. doi: 10.1002/(SICI)1096-9896(199707)182:3<347::AID-PATH848>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 36.Jones L.E., Humphreys M.J., Campbell F., Neoptolemos J.P., Boyd M.T. Comprehensive analysis of matrix metalloproteinase and tissue inhibitor expression in pancreatic cancer: Increased expression of matrix metalloproteinase-7 predicts poor survival. Clin. Cancer Res. 2004;10:2832–2845. doi: 10.1158/1078-0432.CCR-1157-03. [DOI] [PubMed] [Google Scholar]
- 37.Kahlert C., Fiala M., Musso G., Halama N., Keim S., Mazzone M., Lasitschka F., Pecqueux M., Klupp F., Schmidt T., et al. Prognostic Impact of a Compartment-Specific Angiogenic Marker Profile in Patients With pancreatic cancer. Oncotarget. 2014;5:12978–12989. doi: 10.18632/oncotarget.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto H., Itoh F., Iku S., Adachi Y., Fukushima H., Sasaki S., Mukaiya M., Hirata K., Imai K. Expression of Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in Human Pancreatic Adenocarcinomas: Clinicopathologic and Prognostic Significance of Matrilysin Expression. J. Clin. Oncol. 2001;19:1118–1127. doi: 10.1200/JCO.2001.19.4.1118. [DOI] [PubMed] [Google Scholar]
- 39.Moz S., Basso D., Padoan A., Padoan A., Bozzato D., Fogar P., Zambon C.F., Pelloso M., Sperti C., Vigili de Kreutzenberg S., et al. Blood expression of matrix metalloproteinases 8 and 9 and of their inducers S100A8 and S100A9 supports diagnosis and prognosis of pancreatic cancer -associated diabetes mellitus. Clin. Chim. Acta. 2016;456:24–30. doi: 10.1016/j.cca.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 40.Tréhoux S., Duchêne B., Jonckheere N., Van Seuningen I. The MUC1 oncomucin regulates pancreatic cancer cell biological properties and chemoresistance. Implication of p42–44 MAPK, Akt, Bcl-2 and MMP13 pathways. Biochem. Biophys. Res. Commun. 2015;456:757–762. doi: 10.1016/j.bbrc.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 41.Fa Y., Gan Y., Shen Y., Cai X., Song Y., Zhao F., Yao M., Gu J., Tu H. Leptin signaling enhances cell invasion and promotes the metastasis of human pancreatic cancer via increasing MMP-13 production. Oncotarget. 2015;6:16120–16134. doi: 10.18632/oncotarget.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Long K.B., Gladney W.L., Tooker G.M., Graham K., Fraietta J.A., Beatty G.L. IFNγ and CCL2 Cooperate to Redirect Tumor-Infiltrating Monocytes to Degrade Fibrosis and Enhance Chemotherapy Efficacy in Pancreatic Carcinoma. Cancer Discov. 2016;6:400–413. doi: 10.1158/2159-8290.CD-15-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhai L.L., Cai C.Y., Wu Y., Tang Z.G. Correlation and prognostic significance of MMP-2 and TFPI-2 differential expression in pancreatic carcinoma. Int. J. Clin. Exp. Pathol. 2015;8:682–691. [PMC free article] [PubMed] [Google Scholar]
- 44.Ellenrieder V., Alber B., Lacher U., Hendler S.F., Menke A., Boeck W., Wagner M., Wilda M., Friess H., Büchler M., et al. Role of MT-MMPs and MMP-2 in pancreatic cancer progression. Int. J. Cancer. 2000;85:14–20. doi: 10.1002/(SICI)1097-0215(20000101)85:1<14::AID-IJC3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 45.Yokoyama M., Ochi K., Ichimura M., Mizushima T., Shinji T., Koide N., Tsurumi T., Hasuoka H., Harada M. Matrix metalloproteinase-2 in pancreatic juice for diagnosis of pancreatic cancer. Pancreas. 2002;24:344–347. doi: 10.1097/00006676-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Juuti A., Lundin J., Nordling S., Louhimo J., Haglund C. Epithelial MMP-2 expression correlates with worse prognosis in pancreatic cancer. Oncology. 2006;71:61–68. doi: 10.1159/000100988. [DOI] [PubMed] [Google Scholar]
- 47.He Y., Liu X.D., Chen Z.Y., Zhu J., Xiong Y., Li K., Dong J.H., Li X. Interaction between cancer cells and stromal fibroblasts is required for activation of the uPAR-uPA-MMP-2 cascade in pancreatic cancer metastasis. Clin. Cancer Res. 2007;13:3115–3124. doi: 10.1158/1078-0432.CCR-06-2088. [DOI] [PubMed] [Google Scholar]
- 48.Lekstan A., Lampe P., Lewin-Kowalik J., Olakowski M., Jablonska B., Labuzek K., Jedrzejowska-Szypulka H., Olakowska E., Gorka D., Filip I., et al. Concentrations and activities of metalloproteinases 2 and 9 and their inhibitors (TIMPS) in chronic pancreatitis and pancreatic adenocarcinoma. J. Physiol. Pharmacol. 2012;63:589–599. [PubMed] [Google Scholar]
- 49.Śmigielski J., Piskorz Ł., Talar-Wojnarowska R., Malecka-Panas E., Jabłoński S., Brocki M. The estimation of metaloproteinases and their inhibitors blood levels in patients with pancreatic tumors. World J. Surg. Oncol. 2013;11:137. doi: 10.1186/1477-7819-11-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh N., Das P., Datta Gupta S., Sahni P., Pandey R.M., Gupta S., Chauhan S.S., Saraya A. Prognostic significance of extracellular matrix degrading enzymes-cathepsin L and matrix metalloproteases-2 [MMP-2] in human pancreatic cancer. Cancer Investig. 2013;31:461–471. doi: 10.3109/07357907.2013.820318. [DOI] [PubMed] [Google Scholar]
- 51.Xu L., Ding X., Tan H., Qian J. Correlation between B7-H3 expression and matrix metalloproteinases 2 expression in pancreatic cancer. Cancer Cell Int. 2013;13:81. doi: 10.1186/1475-2867-13-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roy R., Zurakowski D., Wischhusen J., Frauenhoffer C., Hooshmand S., Kulke M., Moses M.A. Urinary TIMP-1 and MMP-2 levels detect the presence of pancreatic malignancies. Br. J. Cancer. 2014;111:1772–1779. doi: 10.1038/bjc.2014.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiang T., Xia X., Yan W. Expression of Matrix Metalloproteinases-2/-9 is Associated with Microvessel Density in Pancreatic Cancer. Am. J. Ther. 2017;24:e431–e434. doi: 10.1097/MJT.0000000000000424. [DOI] [PubMed] [Google Scholar]
- 54.Jakubowska K., Pryczynicz A., Januszewska J., Sidorkiewicz I., Kemona A., Niewiński A., Lewczuk L., Kędra B., Guzińska-Ustymowicz K. Expressions of Matrix Metalloproteinases 2, 7, and 9 in Carcinogenesis of Pancreatic Ductal Adenocarcinoma. Dis. Markers. 2016;2016:9895721. doi: 10.1155/2016/9895721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh N., Gupta S., Pandey R.M., Sahni P., Chauhan S.S., Saraya A. Prognostic significance of plasma matrix metalloprotease-2 in pancreatic cancer patients. Indian J. Med. Res. 2017;146:334–340. doi: 10.4103/ijmr.IJMR_1348_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mao L., Le S., Jin X., Liu G., Chen J., Hu J. CSN5 promotes the invasion and metastasis of pancreatic cancer by stabilization of FOXM1. Exp. Cell Res. 2019;374:274–281. doi: 10.1016/j.yexcr.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 57.Li Y., Song T., Chen Z., Wang Y., Zhang J., Wang X. Pancreatic Stellate Cells Activation and Matrix Metallopeptidase 2 Expression Correlate With Lymph Node Metastasis in Pancreatic Carcinoma. Am. J. Med. Sci. 2019;357:16–22. doi: 10.1016/j.amjms.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 58.Zhu C.L., Huang Q. Overexpression of the SMYD3 Promotes Proliferation, Migration, and Invasion of Pancreatic Cancer. Dig. Dis. Sci. 2020;65:489–499. doi: 10.1007/s10620-019-05797-y. [DOI] [PubMed] [Google Scholar]
- 59.Gress T.M., Müller-Pillasch F., Lerch M.M., Friess H., Büchler M., Adler G. Expression and in-situ localization of genes coding for extracellular matrix proteins and extracellular matrix degrading proteases in pancreatic cancer. Int. J. Cancer. 1995;62:407–413. doi: 10.1002/ijc.2910620409. [DOI] [PubMed] [Google Scholar]
- 60.Koshiba T., Hosotani R., Wada M., Fujimoto K., Lee J.U., Doi R., Arii S., Imamura M. Detection of matrix metalloproteinase activity in human pancreatic cancer. Surg. Today. 1997;27:302–304. doi: 10.1007/BF00941802. [DOI] [PubMed] [Google Scholar]
- 61.Koshiba T., Hosotani R., Wada M., Miyamoto Y., Fujimoto K., Lee J.U., Doi R., Arii S., Imamura M. Involvement of matrix metalloproteinase-2 activity in invasion and metastasis of pancreatic carcinoma. Cancer. 1998;82:642–650. doi: 10.1002/(SICI)1097-0142(19980215)82:4<642::AID-CNCR5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 62.Kuniyasu H., Ellis L.M., Evans D.B., Abbruzzese J.L., Fenoglio C.J., Bucana C.D., Cleary K.R., Tahara E., Fidler I.J. Relative expression of E-cadherin and type IV collagenase genes predicts disease outcome in patients with resectable pancreatic carcinoma. Clin. Cancer Res. 1999;5:25–33. [PubMed] [Google Scholar]
- 63.Pryczynicz A., Guzińska-Ustymowicz K., Dymicka-Piekarska V., Czyzewska J., Kemona A. Expression of matrix metalloproteinase 9 in pancreatic ductal carcinoma is associated with tumor metastasis formation. Folia Histochem. Cytobiol. 2007;45:37–40. [PubMed] [Google Scholar]
- 64.Tian M., Cui Y.Z., Song G.H., Zong M.J., Zhou X.Y., Chen Y., Han J.X. Proteomic analysis identifies MMP-9, DJ-1 and A1BG as overexpressed proteins in pancreatic juice from pancreatic ductal adenocarcinoma patients. BMC Cancer. 2008;8:241. doi: 10.1186/1471-2407-8-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mroczko B., Lukaszewicz-Zajac M., Wereszczynska-Siemiatkowska U., Groblewska M., Gryko M., Kedra B., Jurkowska G., Szmitkowski M. Clinical significance of the measurements of serum matrix metalloproteinase-9 and its inhibitor (tissue inhibitor of metalloproteinase-1) in patients with pancreatic cancer: Metalloproteinase-9 as an independent prognostic factor. Pancreas. 2009;38:613–618. doi: 10.1097/MPA.0b013e3181a488a0. [DOI] [PubMed] [Google Scholar]
- 66.Duan L., Hu X.Q., Feng D.Y., Lei S.Y., Hu G.H. GPC-1 may serve as a predictor of perineural invasion and a prognosticator of survival in pancreatic cancer. Asian J. Surg. 2013;36:7–12. doi: 10.1016/j.asjsur.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 67.Xu Y., Li Z., Jiang P., Wu G., Chen K., Zhang X., Li X. The co-expression of MMP-9 and Tenascin-C is significantly associated with the progression and prognosis of pancreatic cancer. Diagn. Pathol. 2015;10:211. doi: 10.1186/s13000-015-0445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Łukaszewicz-Zając M., Gryko M., Pączek S., Szmitkowski M., Kędra B., Mroczko B. Matrix metalloproteinase 2 (MMP-2) and its tissue inhibitor 2 (TIMP-2) in pancreatic cancer (PC) Oncotarget. 2019;10:395–403. doi: 10.18632/oncotarget.26571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park H.D., Kang E.S., Kim J.W., Lee K.T., Lee K.H., Park Y.S., Park J.O., Lee J., Heo J.S., Choi S.H. Serum CA19-9, cathepsin D, and matrix metalloproteinase-7 as a diagnostic panel for pancreatic ductal adenocarcinoma. Proteomics. 2012;12:3590–3597. doi: 10.1002/pmic.201200101. [DOI] [PubMed] [Google Scholar]
- 70.Määttä M., Soini Y., Liakka A., Autio-Harmainen H. Differential expression of matrix metalloproteinase (MMP)-2, MMP-9, and membrane type 1-MMP in hepatocellular and pancreatic adenocarcinoma: Implications for tumor progression and clinical prognosis. Clin. Cancer Res. 2000;6:2726–2734. [PubMed] [Google Scholar]
- 71.Pezzilli R., Corsi M.M., Barassi A., Morselli-Labate A.M., Dogliotti G., Casadei R., Corinaldesi R., D’Eril G.M. The role of inflammation in patients with intraductal mucinous neoplasm of the pancreas and in those with pancreatic adenocarcinoma. Anticancer Res. 2010;30:3801–3805. [PubMed] [Google Scholar]
- 72.Nagakawa Y., Aoki T., Kasuya K., Tsuchida A., Koyanagi Y. Histologic features of venous invasion, expression of vascular endothelial growth factor and matrix metalloproteinase-2 and matrix metalloproteinase-9, and the relation with liver metastasis in pancreatic cancer. Pancreas. 2002;24:169–178. doi: 10.1097/00006676-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 73.Park J.K., Kim M.A., Ryu J.K., Yoon Y.B., Kim S.W., Han H.S., Kang G.H., Kim H., Hwang J.H., Kim Y.T. Postoperative prognostic predictors of pancreatic ductal adenocarcinoma: Clinical analysis and immunoprofile on tissue microarrays. Ann. Surg. Oncol. 2012;19:2664–2672. doi: 10.1245/s10434-012-2277-7. [DOI] [PubMed] [Google Scholar]
- 74.Haq M., Shafii A., Zervos E.E., Rosemurgy A.S. Addition of matrix metalloproteinase inhibition to conventional cytotoxic therapy reduces tumor implantation and prolongs survival in a murine model of human pancreatic cancer. Cancer Res. 2000;60:3207–3211. [PubMed] [Google Scholar]
- 75.Zervos E.E., Norman J.G., Gower W.R., Franz M.G., Rosemurgy A.S. Matrix metalloproteinase inhibition attenuates human pancreatic cancer growth in vitro and decreases mortality and tumorigenesis in vivo. J. Surg. Res. 1997;69:367–371. doi: 10.1006/jsre.1997.5086. [DOI] [PubMed] [Google Scholar]
- 76.Zervox E.E., Franz M.G., Salhab K.F., Shafii A.E., Menendez J., Gower W.R., Rosemurgy A.S. Matrix metalloproteinase inhibition improves survival in an orthotopic model of human pancreatic cancer. J. Gastrointest. Surg. 2000;4:614–619. doi: 10.1016/S1091-255X(00)80111-0. [DOI] [PubMed] [Google Scholar]
- 77.Zervos E.E., Shafii A.E., Rosemurgy A.S. Matrix metalloproteinase (MMP) inhibition selectively decreases type II MMP activity in a murine model of pancreatic cancer. J. Surg. Res. 1999;81:65–68. doi: 10.1006/jsre.1998.5447. [DOI] [PubMed] [Google Scholar]
- 78.Jimenez R.E., Hartwig W., Antoniu B.A., Compton C.C., Warshaw A.L., Fernández-Del Castillo C. Effect of matrix metalloproteinase inhibition on pancreatic cancer invasion and metastasis: An additive strategy for cancer control. Ann. Surg. 2000;231:644–654. doi: 10.1097/00000658-200005000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao C.C., Gong B.G., Wu J.B., Cheng P.G., Xu H.Y., Song D.K., Li F. MMI-166, a selective matrix metalloproteinase inhibitor, promotes apoptosis in human pancreatic cancer. Med. Oncol. 2015;32:418. doi: 10.1007/s12032-014-0418-5. [DOI] [PubMed] [Google Scholar]
- 80.Matsushita A., Onda M., Uchida E., Maekawa R., Yoshioka T. Antitumor effect of a new selective matrix metalloproteinase inhibitor, MMI-166, on experimental pancreatic cancer. Int. J. Cancer. 2001;92:434–440. doi: 10.1002/ijc.1199. [DOI] [PubMed] [Google Scholar]
- 81.Kilian M., Gregor J.I., Heukamp I., Hanel M., Ahlgrimm M., Schimke I., Kristiansen G., Ommer A., Walz M.L., Jacobi C.A., et al. Matrix metalloproteinase inhibitor RO 28-2653 decreases liver metastasis by reduction of MMP-2 and MMP-9 concentration in BOP-induced ductal pancreatic cancer in Syrian Hamsters: Inhibition of matrix metalloproteinases in pancreatic cancer. Prostaglandins Leukot. Essent. Fatty Acids. 2006;75:429–434. doi: 10.1016/j.plefa.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 82.Iki K., Tsutsumi M., Kido A., Sakitani H., Takahama M., Yoshimoto M., Motoyama M., Tatsumi K., Tsunoda T., Konishi Y. Expression of matrix metalloproteinase 2 (MMP-2), membrane-type 1 MMP and tissue inhibitor of metalloproteinase 2 and activation of proMMP-2 in pancreatic duct adenocarcinomas in hamsters treated with N-nitrosobis(2-oxopropyl)amine. Carcinogenesis. 1999;20:1323–1329. doi: 10.1093/carcin/20.7.1323. [DOI] [PubMed] [Google Scholar]
- 83.Andersson P., Yang Y., Hosaka K., Zhang Y., Fischer C., Braun H., Liu S., Yu G., Liu S., Beyaert R., et al. Molecular mechanisms of IL-33-mediated stromal interactions in cancer metastasis. JCI Insight. 2018;3:e122375. doi: 10.1172/jci.insight.122375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koikawa K., Ohuchida K., Ando Y., Kibe S., Nakayama H., Takesue S., Endo S., Abe T., Okumura T., Iwamoto C., et al. Basement membrane destruction by pancreatic stellate cells leads to local invasion in pancreatic ductal adenocarcinoma. Cancer Lett. 2018;425:65–77. doi: 10.1016/j.canlet.2018.03.031. [DOI] [PubMed] [Google Scholar]
- 85.Lu G., Zheng M., Zhu Y., Sha M., Wu Y., Han X. Selection of peptide inhibitor to matrix metalloproteinase-2 using phage display and its effects on pancreatic cancer cell lines PANC-1 and CFPAC-1. Int. J. Biol. Sci. 2012;8:650–662. doi: 10.7150/ijbs.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arnold S., Mira E., Muneer S., Korpanty G., Beck A.W., Holloway S.E., Mañes S., Brekken R.A. Forced expression of MMP9 rescues the loss of angiogenesis and abrogates metastasis of pancreatic tumors triggered by the absence of host SPARC. Exp. Biol. Med. (Maywood) 2008;233:860–873. doi: 10.3181/0801-RM-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Awasthi N., Mikels-Vigdal A.J., Stefanutti E., Schwarz M.A., Monahan S., Smith V., Schwarz R.E. Therapeutic efficacy of anti-MMP9 antibody in combination with nab-paclitaxel-based chemotherapy in pre-clinical models of pancreatic cancer. J. Cell Mol. Med. 2019;23:3878–3887. doi: 10.1111/jcmm.14242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bausch D., Pausch T., Krauss T., Hopt U.T., Fernandez-del-Castillo C., Warshaw A.L., Thayer S.P., Keck T. Neutrophil granulocyte derived MMP-9 is a VEGF independent functional component of the angiogenic switch in pancreatic ductal adenocarcinoma. Angiogenesis. 2011;14:235–243. doi: 10.1007/s10456-011-9207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nakamura T., Kuwai T., Kim J.S., Fan D., Kim S.J., Fidler I.J. Stromal metalloproteinase-9 is essential to angiogenesis and progressive growth of orthotopic human pancreatic cancer in parabiont nude mice. Neoplasia. 2007;9:979–986. doi: 10.1593/neo.07742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grünwald B., Vandooren J., Gerg M., Ahomaa K., Hunger A., Berchtold S., Akbareian S., Schaten S., Knolle P., Edwards D.R., et al. Systemic Ablation of MMP-9 Triggers Invasive Growth and Metastasis of Pancreatic Cancer via Deregulation of IL6 Expression in the Bone Marrow. Mol. Cancer Res. 2016;14:1147–1158. doi: 10.1158/1541-7786.MCR-16-0180. [DOI] [PubMed] [Google Scholar]
- 91.Bournet B., Pointreau A., Souque A., Muscari F., Lepage B., Senesse P., Barthet M., Lesavre N., Hammel P., Levy P., et al. Gene expression signature of advanced pancreatic ductal adenocarcinoma using low density array on endoscopic ultrasound-guided fine needle aspiration samples. Pancreatology. 2012;12:27–34. doi: 10.1016/j.pan.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 92.Lee J., Lee J., Kim J.H. Identification of Matrix Metalloproteinase 11 as a Prognostic Biomarker in Pancreatic Cancer. Anticancer Res. 2019;39:5963–5971. doi: 10.21873/anticanres.13801. [DOI] [PubMed] [Google Scholar]
- 93.Von Marschall Z., Riecken E.O., Rosewicz S. Stromelysin 3 is overexpressed in human pancreatic carcinoma and regulated by retinoic acid in pancreatic carcinoma cell lines. Gut. 1998;43:692–698. doi: 10.1136/gut.43.5.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mehner C., Miller E., Khauv D., Nassar A., Oberg A.L., Bamlet W.R., Zhang L., Waldmann J., Radisky E.S., Crawford H.C., et al. Tumor cell-derived MMP3 orchestrates Rac1b and tissue alterations that promote pancreatic adenocarcinoma. Mol. Cancer Res. 2014;12:1430–1439. doi: 10.1158/1541-7786.MCR-13-0557-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mehner C., Miller E., Nassar A., Bamlet W.R., Radisky E.S., Radisky D.C. Tumor cell expression of MMP3 as a prognostic factor for poor survival in pancreatic, pulmonary, and mammary carcinoma. Genes Cancer. 2015;6:480–489. doi: 10.18632/genesandcancer.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang J.J., Zhu Y., Xie K.L., Peng Y.P., Tao J.Q., Tang J., Li Z., Xu Z.K., Dai C.C., Qian Z.Y., et al. Yin Yang-1 suppresses invasion and metastasis of pancreatic ductal adenocarcinoma by downregulating MMP10 in a MUC4/ErbB2/p38/MEF2C-dependent mechanism. Mol. Cancer. 2014;13:130. doi: 10.1186/1476-4598-13-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fukushima H., Yamamoto H., Itoh F., Nakamura H., Min Y., Horiuchi S., Iku S., Sasaki S., Imai K. Association of matrilysin mRNA expression with K-ras mutations and progression in pancreatic ductal adenocarcinomas. Carcinogenesis. 2001;22:1049–1052. doi: 10.1093/carcin/22.7.1049. [DOI] [PubMed] [Google Scholar]
- 98.Crawford H.C., Scoggins C.R., Washington M.K., Matrisian L.M., Leach S.D. Matrix metalloproteinase-7 is expressed by pancreatic cancer precursors and regulates acinar-to-ductal metaplasia in exocrine pancreas. J. Clin. Investig. 2002;109:1437–1444. doi: 10.1172/JCI0215051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li Y.J., Wei Z.M., Meng Y.X., Ji X.R. Beta-catenin up-regulates the expression of cyclinD1, c-myc and MMP-7 in human pancreatic cancer: Relationships with carcinogenesis and metastasis. World J. Gastroenterol. 2005;11:2117–2123. doi: 10.3748/wjg.v11.i14.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kuhlmann K.F., van Till J.W., Boermeester M.A., de Reuver P.R., Tzvetanova I.D., Offerhaus G.J., ten Kate F.J., Busch O.R., van Gulik T.M., Gouma D.J., et al. Evaluation of matrix metalloproteinase 7 in plasma and pancreatic juice as a biomarker for pancreatic cancer. Cancer Epidemiol. Biomark. Prev. 2007;16:886–891. doi: 10.1158/1055-9965.EPI-06-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yuan P., He X.H., Rong Y.F., Cao J., Li Y., Hu Y.P., Liu Y., Li D., Lou W., Liu M.F. KRAS/NF-κB/YY1/miR-489 Signaling Axis Controls Pancreatic Cancer Metastasis. Cancer Res. 2017;77:100–111. doi: 10.1158/0008-5472.CAN-16-1898. [DOI] [PubMed] [Google Scholar]
- 102.Banaei N., Foley A., Houghton J.M., Sun Y., Kim B. Multiplex detection of pancreatic cancer biomarkers using a SERS-based immunoassay. Nanotechnology. 2017;28:455101. doi: 10.1088/1361-6528/aa8e8c. [DOI] [PubMed] [Google Scholar]
- 103.Resovi A., Bani M.R., Porcu L., Anastasia A., Minoli L., Allavena P., Cappello P., Novelli F., Scarpa A., Morandi E., et al. Soluble stroma-related biomarkers of pancreatic cancer. EMBO Mol. Med. 2018;10:e8741. doi: 10.15252/emmm.201708741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang J., Cong X., Ren M., Sun H., Liu T., Chen G., Wang Q., Li Z., Yu S., Yang Q. Circular RNA hsa_circRNA_0007334 is Predicted to Promote MMP7 and COL1A1 Expression by Functioning as a miRNA Sponge in Pancreatic Ductal Adenocarcinoma. J. Oncol. 2019;2019:7630894. doi: 10.1155/2019/7630894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fukuda A., Wang S.C., Morris J.P., IV, Folias A.E., Liou A., Kim G.E., Akira S., Boucher K.M., Firpo M.A., Mulvihill S.J., et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19:441–455. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bister V., Skoog T., Virolainen S., Kiviluoto T., Puolakkainen P., Saarialho-Kere U. Increased expression of matrix metalloproteinases-21 and -26 and TIMP-4 in pancreatic adenocarcinoma. Mod. Pathol. 2007;20:1128–1140. doi: 10.1038/modpathol.3800956. [DOI] [PubMed] [Google Scholar]
- 107.Krantz S.B., Shields M.A., Dangi-Garimella S., Cheon E.C., Barron M.R., Hwang R.F., Rao M.R., Grippo P.J., Bentrem D.J., Munshi H.G. MT1-MMP cooperates with Kras(G12D) to promote pancreatic fibrosis through increased TGF-β signaling. Mol. Cancer Res. 2011;9:1294–1304. doi: 10.1158/1541-7786.MCR-11-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dangi-Garimella S., Krantz S.B., Barron M.R., Shields M.A., Heiferman M.J., Grippo P.J., Bentrem D.J., Munshi H.G. Three-dimensional collagen I promotes gemcitabine resistance in pancreatic cancer through MT1-MMP-mediated expression of HMGA2. Cancer Res. 2011;71:1019–1028. doi: 10.1158/0008-5472.CAN-10-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Qiang L., Cao H., Chen J., Weller S.G., Krueger E.W., Zhang L., Razidlo G.L., McNiven M.A. Pancreatic tumor cell metastasis is restricted by MT1-MMP binding protein MTCBP-1. J. Cell Biol. 2019;218:317–332. doi: 10.1083/jcb.201802032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ottaviano A.J., Sun L., Ananthanarayanan V., Munshi H.G. Extracellular matrix-mediated membrane-type 1 matrix metalloproteinase expression in pancreatic ductal cells is regulated by transforming growth factor-beta1. Cancer Res. 2006;66:7032–7040. doi: 10.1158/0008-5472.CAN-05-4421. [DOI] [PubMed] [Google Scholar]
- 111.Balaz P., Friess H., Kondo Y., Zhu Z., Zimmermann A., Büchler M.W. Human macrophage metalloelastase worsens the prognosis of pancreatic cancer. Ann. Surg. 2002;235:519–527. doi: 10.1097/00000658-200204000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhai L.L., Wu Y., Cai C.Y., Huang Q., Tang Z.G. High-Level Expression and Prognostic Significance of Matrix Metalloprotease-19 and Matrix Metalloprotease-20 in Human Pancreatic Ductal Adenocarcinoma. Pancreas. 2016;45:1067–1072. doi: 10.1097/MPA.0000000000000569. [DOI] [PubMed] [Google Scholar]
- 113.Khalid M., Idichi T., Seki N., Wada M., Yamada Y., Fukuhisa H., Toda H., Kita Y., Kawasaki Y., Tanoue K., et al. Gene Regulation by Antitumor miR-204-5p in Pancreatic Ductal Adenocarcinoma: The Clinical Significance of Direct RACGAP1 Regulation. Cancers. 2019;11:327. doi: 10.3390/cancers11030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bramhall S.R., Schulz J., Nemunaitis J., Brown P.D., Baillet M., Buckels J.A. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br. J. Cancer. 2002;87:161–167. doi: 10.1038/sj.bjc.6600446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moore M.J., Hamm J., Dancey J., Dagenais M., Fields A., Hagan K., Greenberg B., Colwell B., Zee B., Tuet D., et al. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 2003;21:3296–3302. doi: 10.1200/JCO.2003.02.098. [DOI] [PubMed] [Google Scholar]
- 116.Fields G.B. The Rebirth of Matrix Metalloproteinase Inhibitors: Moving Beyond the Dogma. Cells. 2019;8:984. doi: 10.3390/cells8090984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Winer A., Adams S., Mignatti P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures into Future Successes. Mol. Cancer Ther. 2018;17:1147–1155. doi: 10.1158/1535-7163.MCT-17-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fujimoto N., Mouri N., Iwata K., Ohuchi E., Okada Y., Hayakawa T. A one-step sandwich enzyme immunoassay for human matrix metalloproteinase 2 (72-kDa gelatinase/type IV collagenase) using monoclonal antibodies. Clin. Chim. Acta. 1993;221:91–103. doi: 10.1016/0009-8981(93)90024-X. [DOI] [PubMed] [Google Scholar]
- 119.Lin F., Wang X., Jie Z., Hong X., Li X., Wang M., Yu Y. Inhibitory effects of miR-146b-5p on cell migration and invasion of pancreatic cancer by targeting MMP16. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2011;31:509. doi: 10.1007/s11596-011-0481-5. [DOI] [PubMed] [Google Scholar]
- 120.Giannopoulos G., Pavlakis K., Parasi A., Kavatzas N., Tiniakos D., Karakosta A., Tzanakis N., Peros G. The expression of matrix metalloproteinases-2 and -9 and their tissue inhibitor 2 in pancreatic ductal and ampullary carcinoma and their relation to angiogenesis and clinicopathological parameters. Anticancer Res. 2008;28:1875–1881. [PubMed] [Google Scholar]
- 121.Lee S.H., Kim H., Hwang J.H., Shin E., Lee H.S., Hwang D.W., Cho J.Y., Yoon Y.S., Han H.S., Cha B.H. CD24 and S100A4 expression in resectable pancreatic cancers with earlier disease recurrence and poor survival. Pancreas. 2014;43:380–388. doi: 10.1097/MPA.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 122.Wang S.C., Parekh J.R., Porembka M.R., Nathan H., D’Angelica M.I., DeMatteo R.P., Fong Y., Kingham T.P., Jarnagin W.R., Allen P.J. A Pilot Study Evaluating Serum MMP7 as a Preoperative Prognostic Marker for Pancreatic Ductal Adenocarcinoma Patients. J. Gastrointest. Surg. 2016;20:899–904. doi: 10.1007/s11605-015-3057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nakamura H., Horita S., Senmaru N., Miyasaka Y., Gohda T., Inoue Y., Fujita M., Meguro T., Morita T., Nagashima K. Association of matrilysin expression with progression and poor prognosis in human pancreatic adenocarcinoma. Oncol. Rep. 2002;9:751–755. doi: 10.3892/or.9.4.751. [DOI] [PubMed] [Google Scholar]
- 124.Harvey S.R., Hurd T.C., Markus G., Martinick M.I., Penetrante R.M., Tan D., Venkataraman P., DeSouza N., Sait S.N., Driscoll D.L., et al. Evaluation of urinary plasminogen activator, its receptor, matrix metalloproteinase-9, and von Willebrand factor in pancreatic cancer. Clin. Cancer Res. 2003;9:4935–4943. [PubMed] [Google Scholar]
- 125.Cho K., Matsuda Y., Ueda J., Uchida E., Naito Z., Ishiwata T. Keratinocyte growth factor induces matrix metalloproteinase-9 expression and correlates with venous invasion in pancreatic cancer. Int. J. Oncol. 2012;40:1040–1048. doi: 10.3892/ijo.2011.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu Q., Sheng W., Dong M., Dong X., Dong Q., Li F. Gli1 promotes transforming growth factor-beta1- and epidermal growth factor-induced epithelial to mesenchymal transition in pancreatic cancer cells. Surgery. 2015;158:211–224. doi: 10.1016/j.surg.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 127.Zhu S.K., Zhou Y., Cheng C., Zhong S., Wu H.Q., Wang B., Fan P., Xiong J.X., Yang H.J., Wu H.S. Overexpression of membrane-type 2 matrix metalloproteinase induced by hypoxia-inducible factor-1α in pancreatic cancer: Implications for tumor progression and prognosis. Mol. Clin. Oncol. 2014;2:973–981. doi: 10.3892/mco.2014.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gopinathan A., Morton J.P., Jodrell D., Sansom O.J. GEMMs as preclinical models for testing pancreatic cancer therapies. Dis. Model. Mech. 2015;8:1185–1200. doi: 10.1242/dmm.021055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Swayden M., Soubeyran P., Iovanna J. Upcoming Revolutionary Paths in Preclinical Modeling of Pancreatic Adenocarcinoma. Front. Oncol. 2020;9:1443. doi: 10.3389/fonc.2019.01443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Biankin A.V., Waddell N., Kassahn K.S., Gingras M.C., Muthuswamy L.B., Johns A.L., Miller D.K., Wilson P.J., Patch A.M., Wu J., et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.