Abstract
Among several potential applications, sigma receptor ligands can be used as antipsychotics, antiamnesics, and against other neurodegenerative disorders as well as neuroprotective agents. We present herein a new series of diazepane-containing derivatives as σR ligands obtained by a conformational expansion approach of our previously synthesized piperidine-based compounds. The best results were reached by benzofurane 2c, 3c and quinoline 2d, 3d-substituted diazepane derivatives, which showed the highest σR affinity. The cytotoxic activities of synthesized compounds were evaluated against two cancer cell lines, and the results indicated that none of the compounds induced significant toxicity in these cells. We also evaluated the antioxidant activity by radical scavenging capacity of our best compounds on ABTS and H2O2. The results obtained reveal that our new derivatives possess an excellent antioxidant profile and could be protective for the cells. Overall, the benzofurane derivative 2c due to its strong interaction with the active site of the receptor, as confirmed by molecular dynamic simulations, emerged as the optimum compound with high σ1R affinity, low cytotoxicity, and a potent antioxidant activity.
Keywords: Sigma receptor, molecular dynamics, binding studies, cytotoxicity, antioxidant activity
The sigma receptors (σR) are a class of proteins initially classified, by Martin and co-workers,1 as a subtype of the opiate receptors. Further studies revealed them to be a different receptor class comprising two distinct subtypes: σ1 and σ2.2−5 The σ1R is a chaperone protein, cloned in 1996 from several tissues including human, consisting of 223 amino acids6,7 with a MW of 25.3 kDa.8 Crystallized 20 years later, it revealed a trimeric protein organization.9 The σ1R subtype is primarily localized to mitochondria-associated ER membranes (MAM) of neuronal and peripheral cells, such as cardiac myocytes and hepatocytes. This receptor can also translocate to the plasma membrane or ER-membrane and regulate the activity of other proteins by modulating different ionic channels via an IP3-indipendent mechanism.10,11 The σ1Rs have neuroprotective and antiamnesic activity12,13 and modulate opioid analgesia14 as well as drug addiction,15 and their antagonists seem to be effective against the negative manifestations of schizophrenia without producing extrapyramidal side effects.16,17 In addition, several studies suggest a role for σ1R in tumor biology, since its expression increased in some cancers.18
After 40 years from the discovery of σRs,1 in 2017, the σ2R subtype has been purified and identified as transmembrane protein-97 (TMEM97),19 an endoplasmic-reticulum-resident transmembrane molecule implicated in cholesterol homeostasis due to its association with the lysosomal transporter NPC1.20,21 The σ2R crystal structure is still elusive, but several pharmacophore models have been proposed.22−25 The σ2Rs are overexpressed in many cancer cell lines including lung cancer,26,27 breast cancer,28 ovarian cancer,29 glioma cancer,30 and gastric cancer.31 In this context, since σ2R agonists can induce tumor cell death, they have been proposed as potential antitumor drugs. On the other hand, the σ2Rs are widely expressed in cerebellum, red nucleus, and substantia nigra and are a potential target for the treatment of movement disorders and of neuroleptic-induced acute dystonia.32 In addition, σ1R antagonists as well as σ2R agonists can modulate neuropathic pain.33,34
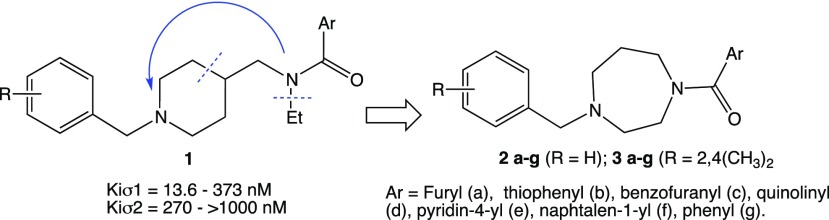
In the past decade, our group has synthesized and biologically evaluated an extensive series of compounds both of preferential affinity for σ1R and σ2R subtypes. Following up our studies in this field, we report herein the development of a new class of sigma ligands designed through the expansion of the conformational selection paradigm applied to our previously synthesized piperidine-based σ1R ligands 1 (Figure 1).35
Figure 1.
Conformational expansion approach starting from previously synthesized sigma ligands 1.
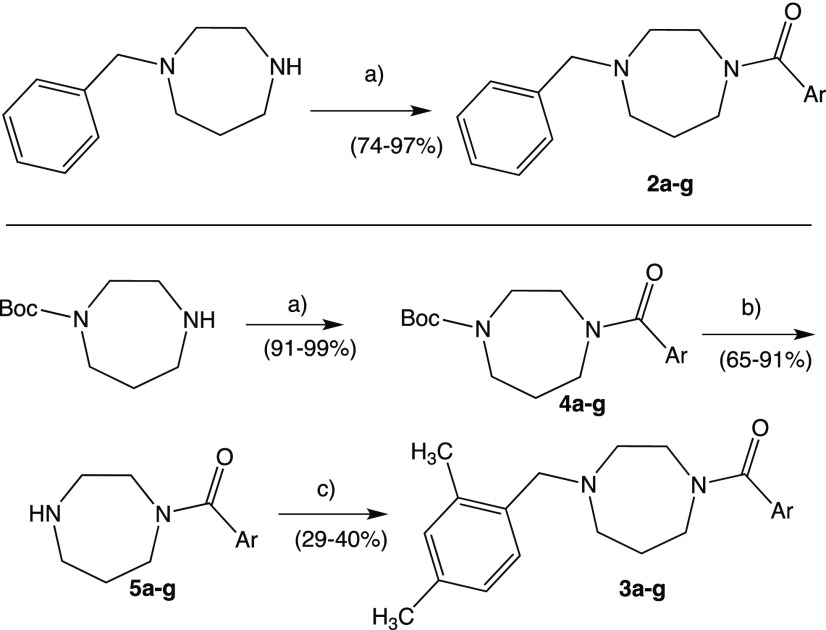
In addition to the spacer replacement, we opted to expand the new series of compounds by using various aromatic fragments, including heterocycles, both monocycles and bicycles, linked to the amide carbonyl group. Moreover, in order to verify the influence on the sigma affinity of the substitution on the benzyl moiety, we decided to retain the unsubstituted phenyl ring, present in many σ1R ligands and the 2,4-dimethyl substituted phenyl ring, typical of several σ2R ligands.36 The synthesis of our new diazepane-based derivatives 2a–g and 3a–g is depicted in Scheme 1.
Scheme 1. Synthesis of Compounds 2a–g and 3a–g.
(a) DCM, Ar-COCl, Et3N, 0 °C; (b) DCM, TFA, rt; (c) DCM, 2,4-dimethylbenzaldheyde, NaCNBH3, rt.
The synthetic route of our new series of compounds (Supporting Information) was carried out by treating the appropriate, commercially available, 1-benzyl-1,4-diazepane, which was made to react with the appropriate aroyl chloride to give the corresponding first subseries 2a–g. These compounds did not need further purification after the classical workup. The 2,4-dimethyl derivatives 3a–g were obtained in three reaction steps, starting from 1-Boc-1,4-diazepane and the corresponding aroyl chloride to provide the acylated intermediates 4a–g. The cleavage of protecting N-Boc group with TFA led to the intermediates 5a–g which were subsequently N-alkylated, with a direct reductive amination using 2,4-dimetylbenzaldheyde and NaCNBH3, to give the final subseries 3a–g. These compounds were purified by DCVC technique.
The σ1R and σ2R affinities of the test compounds were determined in competition experiments by radiometric assays, using [3H]-(+)-Pentazocine as radioligand for the σ1R assay and [3H]-DTG (di-o-tolylguanidine) as radioligand in the σ2R assay. Compounds 2a–g and 3a–g were tested against σ1R and σ2R of animal origin, prepared from guinea pig brain and rat liver membranes by homogenization, centrifugation, and washing of the respective tissues. We also performed a competition experiment toward GluN2b subunit containing NMDA receptors in a radioligand binding assay. This receptor subtype plays important roles in synaptic transmission and plasticity, learning, memory, and other physiological and pathological processes.37,38 Hence, antagonists of the GluN2b subunit are of interest as neuroprotective drugs for various CNS disorders. The radioligand used in the competition assay was [3H]-labeled Ifenprodil, a prototypical allosteric inhibitor of the GluN2b subunit (Supporting Information).
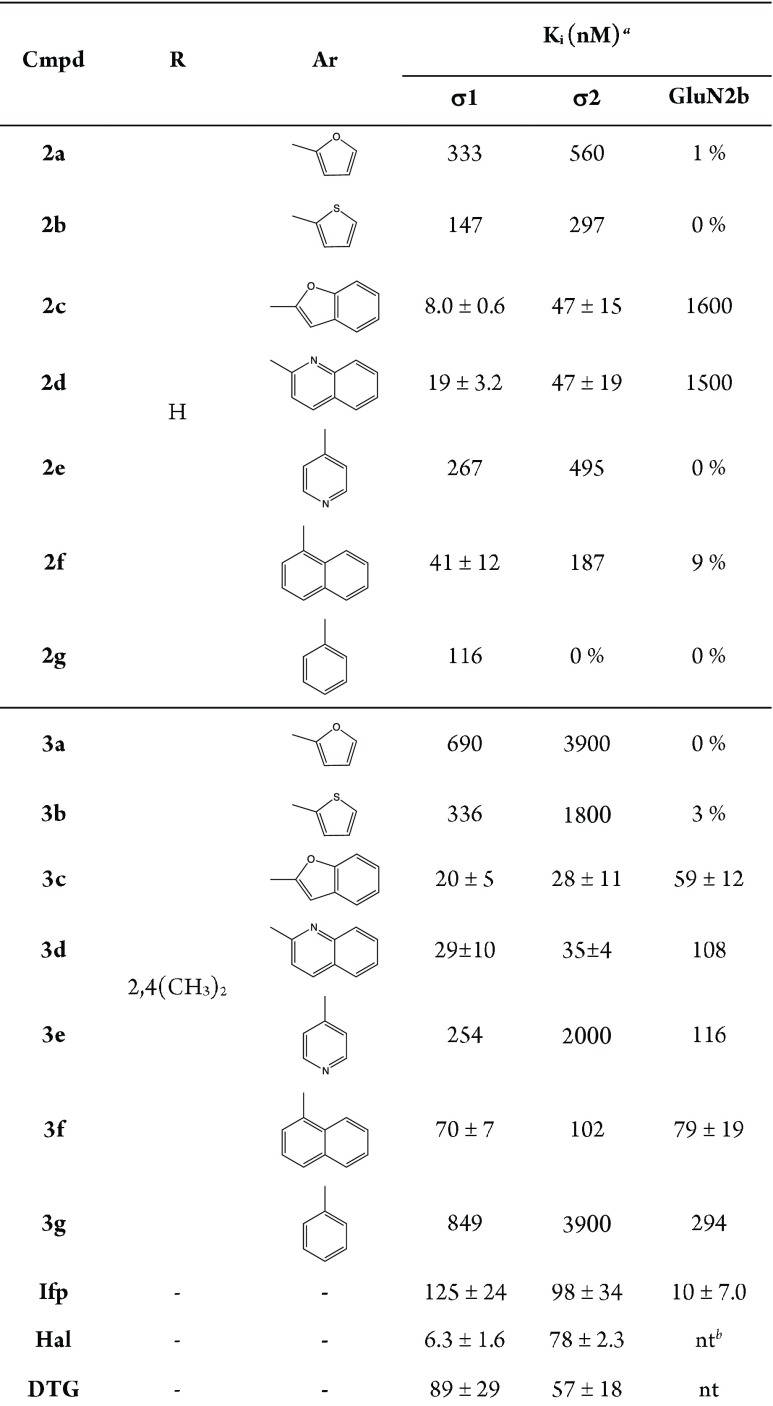
For compounds with affinity value higher than 100 nM, only one measure was performed. The σ1R, σ2R, and GluN2b affinities of compounds 2a–g and 3a–g are presented in Table 1.
Table 1. Affinities of Compounds 2a–g and 3a–g toward σ1, σ2, and GluN2b Receptors.
![]()
Only compounds with highest affinities (<100 nM) were tested in triplicate. For low-affinity compounds, the competition curves were recorded only once (single value), whereas the inhibition of the radioligand binding (shown as %) was assayed at a test compound concentration of 1 μM.
Not tested.
From the obtained data we can summarize the following: (i) the bulky diazepane spacer retained, or even improved, the σR affinity to both σ1 and σ2, with respect to the piperidine ring; (ii) only bicycle derivatives displayed moderate to high affinity toward both σR subtypes, while the corresponding monocycle analogues were weak inhibitors or avoiding of σR affinity; (iii) the best results against σ1R were reached by benzofurane derivative 2c, while its 2,4-dimethyl substituted analogue 3c gave the best pan-affinity with Ki values of 8.0 and 28 nM toward both σR subtypes and also the best GluN2b inhibition value of 59 nM; (iv) the 2,4-dimethyl substitution on benzyl moiety derivatives improves the σ2 over σ1 affinity of the bicycle derivatives, as well as the affinity toward the GluN2b subunit receptor.
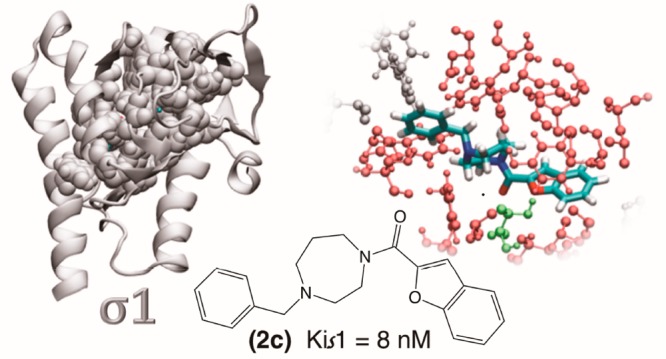
To get insight into the interaction of our compounds into the σ1R active site, we performed a computational assessment of the best σ1R ligand of the series, 2c, in comparison with its monocycle analogue 2a.
We prepared the σ1R following our previous procedure36 (Supporting Information) and we docked compounds 2a and 2c to the target by following the same protocol.
The comparison between the optimum pose obtained for each compound (Figure 2) suggests that compound 2c slides further into the pocket than 2a pushing its benzene ring to interact with Trp164 and Phe133, closed at the bottom by Tyr206, and forming an H-bond with Thr181. Moreover, compound 2c’s optimum pose is predicted to be in touch through 17 hydrophobic interactions with target residues and a hydrogen bond with Thr181 (inset in Figure 2a). Also compound 2a interacts with the target with 17 hydrophobic interactions including Thr181 and shares 14 of those interactions with compound 2c (inset in Figure 2b).
Figure 2.
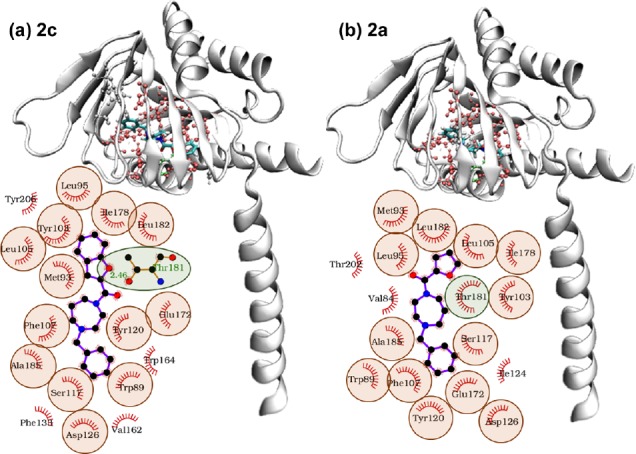
3D putative of (a) compound 2c and (b) compound 2a in the optimum AutoDock pose. Protein residues interacting with both compounds by van der Waals interactions are highlighted in red, Thr 181 in green, other interacting residues (Trp 164, Phe 133, Tyr 206 in panel (a) and Thr202, Val84, Ile124 in panel (b)) are white. In the insets: schematic diagrams of the interaction between the receptor and the respective compounds in the same optimum AutoDock pose. Protein residues interacting with the compounds by van der Waals interactions are highlighted in red, while hydrogen bonds are indicated by green dotted lines. The hydrogen bond distances are also indicated. Residues interacting with both compounds through van der Waals interactions are circled in red. Thr 181 (circled in green) forms a hydrophobic interaction with compounds 2a, while a hydrogen bond with compound 2c.
To confirm the docking result and understand the different behaviors of the two compounds, we ran 250 ns of molecular dynamics (MD) simulation of the complexes in water solvent. The ligand topologies were built with ATB.39 The topologies were validated as the molecular mechanics minimized structure of compound 2a had root-mean-square deviation (RMSD) of 0.01007 nm with respect to the semiempirical minimized structure, while for compound 2c the same RMSD was 0.0082 nm.
The trajectories, scored with the Autodock Vina scoring function,40 showed a constant binding score for both compounds (Figure 3a,b). For both systems the protein backbone RMSD diverged along the dynamics up to 0.6 nm (Figure 3c,d), as expected by simulating only a monomer of the extracellular domain. The ligands RMSD with respect to the fixed protein backbone, below 0.4 nm for compound 2c (Figure 3c), revealed a major conformational change for compound 2a (RMSD > 1.0 nm, Figure 3d). These variations were not reflected in the protein backbone radius of gyration constant at 1.6 nm for both systems (Figure 3e,f). Larger protein rearrangements peak at residues 190–200 for both systems, as observed in the protein backbone root-mean-square fluctuation (RMSF > 0.5 nm, Figure 3g,h). These are part of the helical structure delimitating the pocket but not directly interacting with the ligands (Figure 3i,j and insets in Figure 2). Along the whole simulated time ligands were not observed to leave the pocket with compound 2c maintaining its position but rotating its benzene ring (Figure 3i) and compound 2a changing position by both flipping its orientation and further sliding inside the pocket (Figure 3j), a movement not associated with a gain in binding energy as calculated by Autodock Vina score (Figure 3b).
Figure 3.
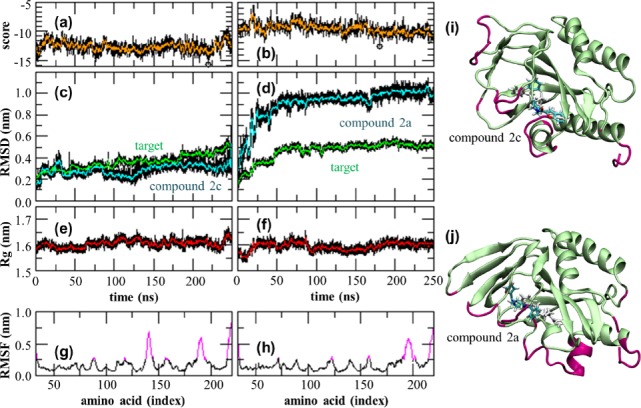
Molecular dynamics simulations analysis for compound 2c (left column) and 2a (right column): (a,b) Autodock Vina score, (c,d) protein backbone RMSD (green) and ligands RMSD in the protein backbone frame (blue), (e,f) protein backbone radius of gyration, (g,h) protein backbone RMSF with values above 0.25 nm highlighted in magenta. All values measured with respect to the starting configuration correspond to the minimized optimum pose identified by docking. Simulation snapshots taken at the lowest Autodock Vina score (circled in panels a,b) for (i) compound 2c and (j) compound 2a. Protein residues with RMSF above 0.25 nm are highlighted (magenta). The starting ligand configuration is also indicated (white).
The MM/PBSA analysis confirmed that the van der Waals interactions were the major liable for binding the compounds, a contribution that is more relevant for compound 2c which also benefits of a less unfavorable polar solvation energy than that calculated for compound 2a (Figure 4a,b). This result is reflected by the single amino acid contribution to the binding energy. Indeed, for compound 2a several amino acids opposed to the binding with Arg119 and Glu172 contribution larger than 1 kcal/mol, followed by Gln135, Asp126, and His154. Instead, compound 2c is synergistically kept bound to its interacting site by several residues (Figure 4d). More in detail (Figure 4e,f) there are seven residues (namely Leu105, Ile124, Phe107, Trp89, Val84, Leu182, and Tyr103) contributing to the binding energy with more than 0.9 kcal/mol with the major contributing force to be ascribed to van der Waals forces and hydrophobic interactions.
Figure 4.
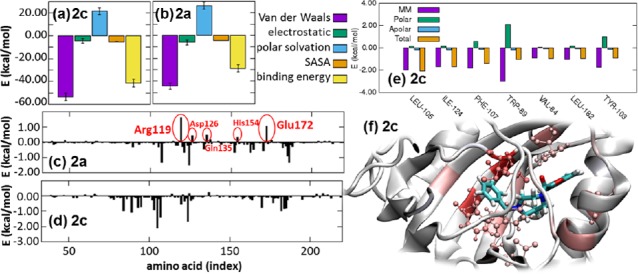
MMPBSA analysis: energetic contributions to the receptor binding of (a) compound 2c and (b) compound 2a, and amino acids contribution to the total binding energy of (c) compound 2a and (d) compound 2c with highlighted the amino acids opposed to the binding with predicted energy larger than 0.3 kcal/mol; (e) details of the contributions of each amino acid with binding energy larger than 0.9 kcal/mol for compound 2c; (f) snapshot of the complex σ1/2c with highlighted amino acids (shades of red) contributing to the binding energy with more than ±0.9 kcal/mol. All data averaged over the last 150 ns of the molecular dynamics trajectory.
Overall the larger ring keeps the ligand fixed in its position, which is more accessible to Thr181 for H-bonding. A larger number of contacts deep into the pocket further inhibited the molecular rearrangement inside the protein pocket.
The effects of this new set of σ1R ligands on cell health were evaluated by testing the cytotoxic response of the human pancreatic carcinoma (PANC1) and human neuroblastoma (SH-SY5Y) cell lines, selected because they express high levels of σ1R.18 To this aim, we selected the most interesting compounds (2c, 2d, 3c, and 3d) and tested their potential toxicity by MTT assay (Supporting Information, Table S1 and Table S2). The experiments revealed that none of our diazepane-containing derivatives showed significant cytotoxicity at different concentrations, with the exception of compound 3d, which exhibited a moderate toxicity toward PANC1 cells, but only at 100 μM concentration (viability of 51%). Interestingly, compounds 2c and 2d, which exhibited the best σ1R affinities (Ki = 8 and 19 nM, respectively), resulted to be the less toxic (viability: 127 and 196% at 50 μM; 98 and 127% at 100 μM, in SH-SY5Y and PANC1, respectively). Therefore, considering the general consensus that σ1R agonists promote cell survival,41,42 these results support the hypothesis that compounds 2c, 2d, 3c, and 3d can be included in this category.
Motivated by these results, we evaluated the in vitro antioxidant activity of the same compounds tested in the aforementioned cytotoxic assay. We tested the ability to scavenge ABTS derived radicals and H2O2 oxidant. Ascorbic acid and Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) were used as standard antioxidants in a comparison test. The assayed compounds potently inhibited ABTS radicals and H2O2, compared to the standards (Table 2).
Table 2. In Vitro Antioxidant Activity of Compounds 2c, 2d, 3c, and 3d.
| IC50 (μg/mL)a |
||
|---|---|---|
| Cmpd | ABTS | H2O2 |
| 2c | 12.71 ± 0.25 | 15.89 ± 0.18 |
| 2d | 14.26 ± 0.15 | 20.35 ± 0.27 |
| 3c | 10.05 ± 0.09 | 18.56 ± 0.31 |
| 3d | 9.43 ± 0.11 | 17.44 ± 0.18 |
| Ascorbic Acid | 12.75 ± 0.12 | 19.27 ± 0.54 |
| Trolox | 18.73 ± 0.26 | 20.38 ± 0.19 |
All measurements were performed in triplicate.
Among the series, the dimethyl substituted compounds 3c and 3d exhibited a significant radical scavenging capacity on both ABTS and H2O2 with values of 10.05 and 18.56 μg/mL for 3c and 9.43 and 17.44 μg/mL for 3d, lower than compared standards, ascorbic acid and Trolox (12.75, 19.27 μg/mL and 18.73, 20.38 μg/mL, respectively).
Furthermore, in order to evaluate their drug likeness and the potential ability to cross the BBB, the compounds 2a–g and 3a–g were also in-silico scored for their physiochemical and pharmacokinetic parameters (ADME) by using the extended version of Lipinski’s rule of five. All the compounds were found to be BBB permeant, and none of them violate any Lipinski’s RO5 (Supporting Information).
In conclusion, we have synthesized a new series of ring-expanded diazepane-based compounds. The new series showed enhanced affinity than its original counterpart35 toward both σR subtypes, and among the series, the benzofurane derivative 2c showed the best σ1R affinity and molecular dynamic simulations confirmed a strong interaction with the active site of the receptor. The benzofurane and quinoline derivatives 2c, 3c and 2d, 3d displayed the best Kiσ values and a safe profile toward two human cell lines. Altogether these data, along with the documented radical scavenging and cell survival promoting activities, support the interest for further studies aiming at evaluating the potential neuroprotective activity of this novel series of σ1R ligands.
Acknowledgments
We thank Dr. Fabio Hollan (University of Trieste, DSCF) for the MS data. We acknowledge the CINECA Awards N. HP10CKYM0P, 2019, for the availability of high performance computing resources and support.
Glossary
Abbreviations
- σ1R and σ2R
sigma-1 and sigma-2 receptor
- ABTS
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- ADME
absorption distribution metabolism excretion
- ATB
automated topology builder
- BBB
blood brain barrier
- Boc
tert-butyloxycarbonyl
- CNS
central nervous system
- DCM
dichloromethane
- DCVC
direct column vacuum chromatography
- DTG
di-o-tolylguanidine
- Et3N
triethylamine
- ER
endoplasmatic reticulum
- DMEM
Dulbecco’s Modified Eagle’s Medium
- MAM
mitochondrion-associated membrane
- MD
molecular dynamics
- MMPBSA
molecular mechanics Poisson–Boltzmann surface area
- MW
molecular weight
- NMDA
N-methyl-d-aspartic acid
- NPC1
Niemann–Pick cholesterol transporter type1
- RMSD
root-mean-square deviation
- RMSF
root mean squared fluctuation
- RO5
rule of five
- TLC
thin-layer chromatography
- TFA
trifluoroacetic acid
- TMEM97
transmembrane protein-97
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.9b00524.
Detailed experimental, synthetic procedures and characterization of compounds, additional computational details, pharmacology and cytotoxicity assays, antioxidant activity, and drug likeness prediction (PDF)
Author Contributions
DZ synthesized and characterized the compounds and wrote the manuscript; SF generated the computational results and analysis; AC performed the antioxidant assay; MR and RM tested the cytotoxicity of the compounds and wrote the manuscript; DS and BW performed the binding assay; DZ and MGM conceived the project. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Martin W. R.; Eades C. E.; Thompson J. A.; Huppler R. E.; Gilbert P. E. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976, 197, 517–532. [PubMed] [Google Scholar]
- Su T.-P. Evidence for sigma opioid receptor: binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. J. Pharmacol. Exp. Ther. 1982, 223, 284–290. [PubMed] [Google Scholar]
- Quirion R.; Bowen W. D.; Itzhak Y.; Junien J. L.; Musacchio J. M.; Rothman R. B.; Su T.-P.; Tam S. W.; Taylor D. P. A proposal for the classification of sigma binding sites. Trends Pharmacol. Sci. 1992, 13, 85–86. 10.1016/0165-6147(92)90030-A. [DOI] [PubMed] [Google Scholar]
- Walker J. M.; Bowen W. D.; Goldstein S. R.; Roberts A. H.; Patrick S. L.; Hohmann A. G.; DeCosta B. Autoradiographic distribution of [3H](+)-pentazocine and [3H]1,3-di-o-tolylguanidine (DTG) binding sites in guinea pig brain: a comparative study. Brain Res. 1992, 581, 33–38. 10.1016/0006-8993(92)90340-F. [DOI] [PubMed] [Google Scholar]
- Bouchard P.; Quirion R. [3H]1,3-di(2-tolyl)guanidine and [3H](+)pentazocine binding sites in the rat brain: autoradiographic visualization of the putative sigma1 sigma2 receptor subtypes. Neuroscience 1997, 76, 467–477. 10.1016/S0306-4522(96)00221-7. [DOI] [PubMed] [Google Scholar]
- Hanner M.; Moebius F. F.; Flandorfer A.; Knaus H. G.; Striessnig J.; Kempner E.; Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc. Natl. Acad. Sci. U. S. A. 1996, 93, 8072–8077. 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekuda R.; Prasad P. D.; Fei Y. J.; Leibach F. H.; Ganapathy V. Cloning and functional expression of the human type 1 sigma receptor (hSigmaR1). Biochem. Biophys. Res. Commun. 1996, 229, 553–558. 10.1006/bbrc.1996.1842. [DOI] [PubMed] [Google Scholar]
- Hellewell S. B.; Bruce A.; Feinstein G.; Orringer J.; Williams W.; Bowen W. D. Rat liver and kidney contain high densities of 1 and 2 receptors: characterization by ligand binding and photoaffinity labeling. Eur. J. Pharmacol., Mol. Pharmacol. Sect. 1994, 268, 9–18. 10.1016/0922-4106(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Schmidt H. R.; Zheng S.; Gurpinar E.; Koehl A.; Manglik A.; Kruse A. C. Crystal structure of the human, 1 receptor. Nature 2016, 532, 527–530. 10.1038/nature17391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T.; Su T.-P. Regulating ankyrin dynamics: Roles of sigma-1 receptors. Proc. Natl. Acad. Sci. U. S. A. 2001, 98, 491–496. 10.1073/pnas.98.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z.; Bowen W. D. Role of sigma-1 receptor C-terminal segment in inositol 1,4,5-trisphosphate receptor activation: constitutive enhancement of calcium signaling in MCF-7 tumor cells. J. Biol. Chem. 2008, 283, 28198–28215. 10.1074/jbc.M802099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos E. J.; Entrena J. M.; Nieto F. R.; Cendan C. M.; Pozo E. D. Pharmacology and therapeutic potential of sigma-1 ligands. Curr. Neuropharmocol. 2008, 6, 344–366. 10.2174/157015908787386113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T.; Lockhart B. P. Neuroprotective and anti-amnesic potentials of sigma (sigma) receptor ligands. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1997, 21, 69–102. 10.1016/S0278-5846(96)00160-1. [DOI] [PubMed] [Google Scholar]
- King M.; Pan Y. X.; Mei J.; Chang A.; Xu J.; Pasternak G. W. Enhanced kappa-opioid receptor-mediated analgesia by antisense targeting the sigma1 receptor. Eur. J. Pharmacol. 1997, 331 (1997), R5–6. 10.1016/S0014-2999(97)01064-9. [DOI] [PubMed] [Google Scholar]
- McCracken K. A.; Bowen W. D.; Walker F. O.; De Costa B.; Matsumoto R. R. Two novel sigma receptor ligands, BD1047 and LR172, attenuate cocaine-induced toxicity and locomotor activity. Eur. J. Pharmacol. 1999, 370, 225–232. 10.1016/S0014-2999(99)00113-2. [DOI] [PubMed] [Google Scholar]
- Modell S.; Naber D.; Holzbach R. Efficacy and safety of an opiate sigma-receptor antagonist (SL 82.0715) in schizophrenic patients with negative symptoms: an open dose-range study. Pharmacopsychiatry 1996, 29, 63–66. 10.1055/s-2007-979546. [DOI] [PubMed] [Google Scholar]
- Huber M. T.; Gotthardt U.; Schreiber W.; Krieg J. C. Efficacy and safety of the sigma receptor ligand EMD 57445 (panamesine) in patients with schizophrenia: an open clinical trial. Pharmacopsychiatry 1999, 32, 68–72. 10.1055/s-2007-979194. [DOI] [PubMed] [Google Scholar]
- Kim F. J.; Maher C. M.. Sigma1 Pharmacology in the Context of Cancer. Handbook of Experimental Pharmacology; Springer, 2017; pp 237–308. [DOI] [PubMed] [Google Scholar]
- Alon A.; Schmidt H. R.; Wood M. D.; Sahn J. J.; Martin S. F.; Kruse A. C. Identification of the gene that codes for the (2 receptor. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 7160–7165. 10.1073/pnas.1705154114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz F.; Kern L.; Erz D.; Zhu M.; Gilbert D.; Meinhof T.; Wirkner U.; Erfle H.; Muckenthaler M.; Pepperkok R.; Runz H. Identification of cholesterol-regulating genes by targeted RNAi screening. Cell Metab. 2009, 10, 63–75. 10.1016/j.cmet.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Riad A.; Zeng C.; Weng C. C.; Winters H.; Xu K.; Makvandi M.; Metz T.; Carlin S.; Mach R. H. Sigma-2 Receptor/TMEM97 and PGRMC-1 Increase the Rate of Internalization of LDL by LDL Receptor through the formation of a Ternary Complex. Sci. Rep. 2018, 8, 16845. 10.1038/s41598-018-35430-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cratteri P.; Romanelli M. N.; Cruciani G.; Bonaccini C.; Melani F. Grind-derived pharmacopore model for a series of alfa-trophanyl derivative ligands of the sigma-2 receptor. J. Comput.-Aided Mol. Des. 2004, 18, 361–374. 10.1023/B:JCAM.0000047815.22931.3b. [DOI] [PubMed] [Google Scholar]
- Laurini E.; Zampieri D.; Mamolo M. G.; Vio L.; Zanette C.; Florio C.; Posocco P.; Fermeglia M.; Pricl S. A 3D-Pharmacophore model for (2 receptors based on a series of substituted benzo[d]oxazol-2(3H)-one derivatives. Bioorg. Med. Chem. Lett. 2010, 20, 2954–2957. 10.1016/j.bmcl.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Rhoades D. J.; Kinder D. H.; Mahfouz T. M. A Comprehensive Ligand Based Mapping of the 2 Receptor Binding Pocket. Med. Chem. 2013, 10, 98–121. 10.2174/1573406409999131119103621. [DOI] [PubMed] [Google Scholar]
- Floresta G.; Rescifina A.; Marrazzo A.; Dichiara M.; Pistarà V.; Pittalà V.; Prezzavento O.; Amata E. Hyphenated 3D-QSAR statistical model-scaffold hopping analysis for the identification of potentially potent and selective sigma-2 receptor ligands. Eur. J. Med. Chem. 2017, 139, 884–891. 10.1016/j.ejmech.2017.08.053. [DOI] [PubMed] [Google Scholar]
- Han K. Y.; Gu X.; Wang H. R.; Liu D.; Lv F. Z.; Li J. N. Overexpression of MAC30 is associated with poor clinical outcome in human non-small cell lung cancer. Tumor Biol. 2013, 34, 821–825. 10.1007/s13277-012-0612-z. [DOI] [PubMed] [Google Scholar]
- Ding H.; Gui X. H.; Lin X. B.; Chen R. H.; Cai H. R.; Fen Y.; Sheng Y. L. Prognostic value of MAC30 expression in human pure squamous cell carcinomas of the lung. Asian Pac. J. Cancer Prev. 2016, 17, 2705–2710. [PubMed] [Google Scholar]
- Xiao M.; Li H.; Yang S.; Huang Y.; Jia S.; Wang H.; Wang J.; Li Z. Expression of MAC30 protein is related to survival and clinicopathological variables in breast cancer. J. Surg. Oncol. 2013, 107, 456–462. 10.1002/jso.23269. [DOI] [PubMed] [Google Scholar]
- Yang S.; Li H.; Liu Y.; Ning X.; Meng F.; Xiao M.; Wang D.; Lou G.; Zhang Y. Elevated expression of MAC30 predicts lymph node metastasis and unfavourable prognosis in patients with epithelial ovarian cancer. Med. Oncol. 2013, 30, 324. 10.1007/s12032-012-0324-7. [DOI] [PubMed] [Google Scholar]
- Qiu G.; Sun W.; Zou Y.; Cai Z.; Wang P.; Lin X.; Huang J.; Jiang L.; Ding X.; Hu G. RNA interference against TMEM97 inhibits cell proliferation, migration, and invasion in glioma cells. Tumor Biol. 2015, 36, 8231–8238. 10.1007/s13277-015-3552-6. [DOI] [PubMed] [Google Scholar]
- Xu X. Y.; Zhang L. J.; Yu Y. Q.; Zhang X. T.; Huang W. J.; Nie X. C.; Song G. Q. Downregulated MAC30 expression inhibits proliferation and mobility of human gastric cancer cells. Cell. Physiol. Biochem. 2014, 33, 1359–1368. 10.1159/000358703. [DOI] [PubMed] [Google Scholar]
- Rousseaux C. G.; Greene S. F. Sigma receptors [σRs]: biology in normal and diseased states. J. Recept. Signal Transduction Res. 2016, 36, 327–388. 10.3109/10799893.2015.1015737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanillo D.; Romero L.; Merlos M.; Vela J. M. Sigma 1 receptor: a new therapeutic target for pain. Eur. J. Pharmacol. 2013, 716, 78–93. 10.1016/j.ejphar.2013.01.068. [DOI] [PubMed] [Google Scholar]
- Sahn J. J.; Mejia G. L.; Ray P. R.; Martin S. F.; Price T. J. Sigma 2 Receptor/Tmem97 Agonists Produce Long Lasting Antineuropathic Pain Effects in Mice. ACS Chem. Neurosci. 2017, 8, 1801–1811. 10.1021/acschemneuro.7b00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampieri D.; Laurini E.; Vio L.; Golob S.; Fermeglia M.; Pricl S.; Mamolo M. G. Synthesis and receptor binding studies of some new arylcarboxamide derivative as sigma-1 ligands. Bioorg. Med. Chem. Lett. 2014, 24, 1021–1025. 10.1016/j.bmcl.2014.01.032. [DOI] [PubMed] [Google Scholar]
- Zampieri D.; Fortuna S.; Calabretti A.; Romano M.; Menegazzi R.; Schepmann D.; Wǔnsch B.; Collina S.; Zanon D.; Mamolo M. G. Discovery of new potent dual sigma receptor/GluN2b ligands with antioxidant property as neuroprotective agents. Eur. J. Med. Chem. 2019, 180, 268–282. 10.1016/j.ejmech.2019.07.012. [DOI] [PubMed] [Google Scholar]
- Riedel G.; Platt B.; Micheau J. Glutamate receptor function in learning and memory. Behav. Brain Res. 2003, 140, 1–47. 10.1016/S0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Paoletti P.; Bellone C.; Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- Malde A. K.; Zuo L.; Breeze M.; Stroet M.; Poger D.; Nair P. C.; Oostenbrink C.; Mark A. E. An Automated Force Field Topology Builder (ATB) and Repository: Version 1.0. J. Chem. Theory Comput. 2011, 7, 4026–4037. 10.1021/ct200196m. [DOI] [PubMed] [Google Scholar]
- Trott O.; Olson A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T.; Su T. P. The pharmacology of sigma-1 receptors. Pharmacol. Ther. 2009, 124, 195–206. 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T.; Su T. P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 2007, 131, 596–610. 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.