Abstract
Background
Lobular capillary hemangiomas (LCH) are acquired benign vascular lesions of the skin and mucous membranes mostly affecting the head and neck region. Involvement of the nasal cavity is extremely rare and can manifest as epistaxis and nasal obstruction.
Case series
In this case series, we present five cases of intranasal LCH. Three cases are of pregnant women that presented with epistaxis and nasal obstruction. The first was surgically treated during her pregnancy with preoperative embolization of the tumor for vascular control, while the other two patients were treated after delivery. The two other cases are of a post trauma pediatric patient, and an elderly lady with multiple co-morbidities, both presenting with recurrent nose bleeds and nasal obstruction. Surgical excision was performed with no complications observed post-operatively.
Discussion
The etiology of LCH is unknown, but certain predisposing factors have been associated with the development of LCH and include pregnancy and trauma. The anterior portion of the nasal septal mucosa and the tip of the inferior turbinate are commonly involved sites. Computed tomography scans and histopathology are used to diagnose LCH. Treatment is surgical excision with or without pre-operative embolization.
Conclusion
LCH are rare tumors of the nasal cavity. Treatment of these lesions is surgical with or without preoperative vascular control.
Keywords: Lobular capillary hemangioma, Pyogenic granuloma, Endoscopic surgery, Epistaxis
1. Introduction
Lobular Capillary Hemangioma (LCH) is a benign acquired fast-growing vascular lesion of the skin and mucous membranes most commonly affecting the head and neck region (60% of LCH cases) [1,2]. The most common sites affected are the skin and lips, followed by the nose, oral mucosa and tongue. Within the nasal cavity, the most commonly affected area is the anterior nasal septum (Little's area), the inferior turbinate and the vestibule [[1], [2], [3], [4]]. The most common presenting symptoms of LCH in the nasal cavity are epistaxis and nasal obstruction [5]. Examination of LCH under microscopy reveals extensive endothelial proliferation with a distinctive histopathological appearance where the capillaries appear dilated and arranged in lobules [2,6]. Its etiology is not clearly understood but certain predisposing factors to the development of LCH have been reported in the literature and include a history of trauma and pregnancy [2,7,8]. Despite multiple forms of treatment, surgical endoscopic excision with complete electrodessication of the tumor base is the treatment of choice [3,4,9]. In this case series, we present five cases of intranasal LCH.
2. Description of case series
2.1. Case 1
A 29-year-old medically free pregnant lady, gravida 5 para 3 + 1, in her second trimester, presented to the emergency department with a one-month history of right sided nasal obstruction and epistaxis associated with severe right sided headache for the last 2 weeks. She describes her headache as throbbing in character and is associated with photophobia and nausea. There was no history of trauma, bleeding from any other orifice and the patient was not on any anticoagulants or antiplatelet medications. The patient was referred to our service and endoscopic examination showed a large right nasal fleshy mass obstructing the right nasal cavity. A brain (magnetic resonance imaging) MRI was performed and showed a heterogenous polypoidal mass lesion in the right nasal cavity extending to the nasopharynx containing internal flow void. A biopsy was taken from the right nasal mass in the clinic to further identify the lesion and the results were suggestive of lobular capillary hemangioma. With an agreement made with the patient, and due to her pregnant state, the decision to postpone surgical excision until after delivery was made with a possibility of pre-op angiography.
The patient presented to the emergency department before delivery with an aggravation in symptoms associated with decreased oral intake, disturbed sleep, dysphagia, dry mouth, obstructive sleep apnea symptoms and halitosis. The patient could no longer tolerate the symptoms. Due to the large size of the vascular mass, the patient was booked for external carotid angiography prior to surgical excision to reduce the incidence of bleeding in the operating room. A successful embolization of the branches of the distal right internal maxillary artery feeding the hyper-vascular nasal tumor was achieved. The patient was then booked for surgery, during which, complete resection of the tumor was achieved endoscopically (Fig. 1). Post-operatively, the patient was stable with no complications and was consequently discharged home the next day.
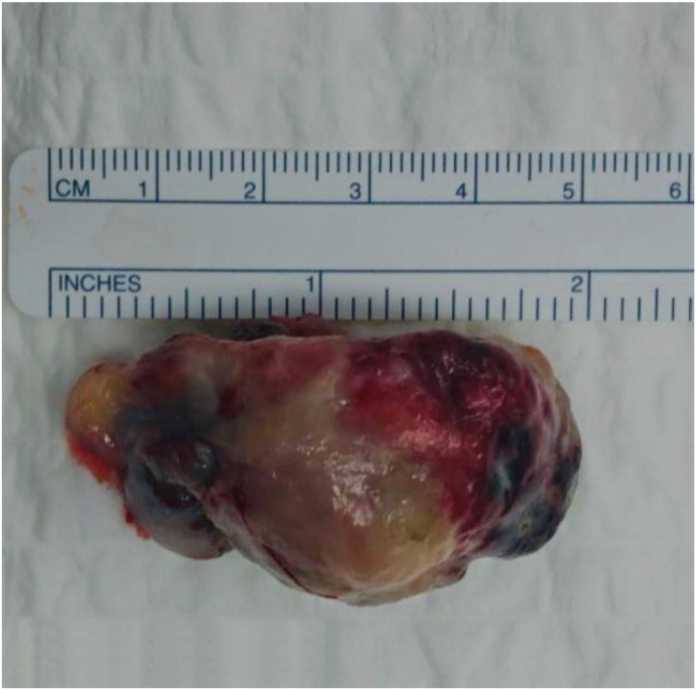
Fig. 1.
Gross appearance of the highly vascular excised mass.
2.2. Case 2
A 13-year-old boy was referred from the emergency medicine to our department with a history of recurrent epistaxis and progressive nasal obstruction following nasal trauma four days ago. The patient has a documented history of recurrent epistaxis, snoring, mouth breathing and progressively worsening nasal obstruction for 3 months prior to the trauma. Left nostril examination revealed a soft tissue mass in the anterior nasal septum with no septal hematoma. Right nostril examination showed a severely deviated nasal septum almost touching the lateral nasal wall. Sinus CT with contrast was performed and showed an enhancing left nostril mass lesion inseparable from the septum. The patient was booked for surgery to excise the mass and to assess the size of the adenoid tissue. In the operating room, findings were a left nasal mass measuring 1 × 1 cm originating from the nasal septum (Fig. 2), grade 2 adenoid hypertrophy, and confirmed severe right sided nasal septal deviation. The mass was excised (Fig. 3), and adenoidectomy was performed. The excised mass was sent for histopathology and a histopathological diagnosis of lobular capillary hemangioma was made.
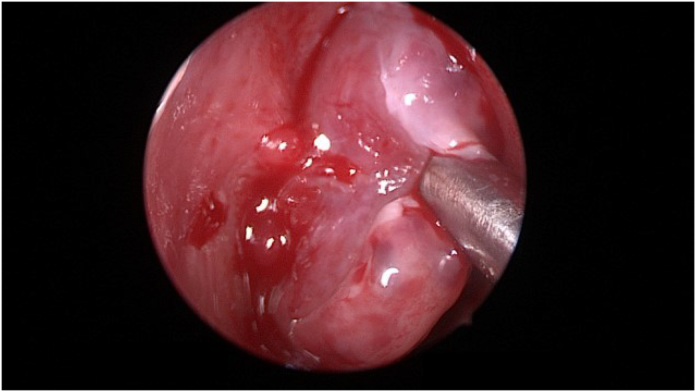
Fig. 2.
Intraoperative endoscopic examination revealed a left sided anterior nasal mass originating from the septum with a narrow pedicle.
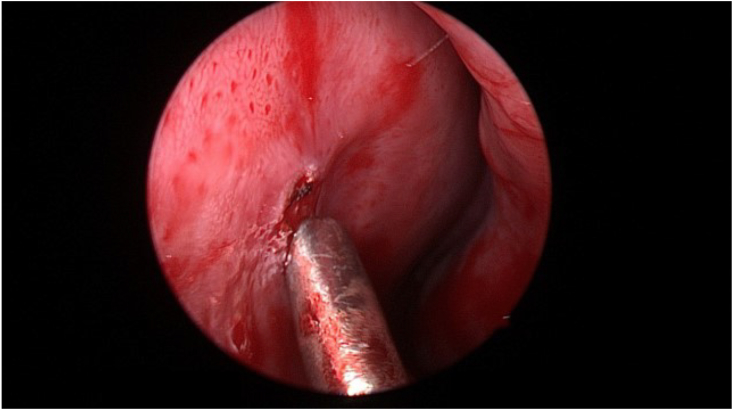
Fig. 3.
Intraoperative excision was performed using Colorado needle. Hemostasis was maintained and packing was applied.
2.3. Case 3
An 87-year-old female with a past medical history of hypertension, dyslipidemia and asthma, was referred to our out-patient clinic from the emergency department as case of recurrent left nasal epistaxis for three months associated with left nasal obstruction and facial tenderness. The patient gave no history of any traumatic events, dizziness, vomiting or bleeding from any other orifice. The patient was not on any anticoagulants or antiplatelet medications.
In the clinic, nasal endoscopy revealed a hemorrhagic mass in the left nasal cavity. A computed tomography (CT) scan of the sinuses with contrast was performed and showed a significant strong enhancing soft tissue mass originating from the left anterior septum, suggestive of a highly vascular lesion. The mass is confined to the left nasal cavity with no signs of bony erosion. Biopsy was taken in the clinic and was inconclusive. The patient was started on steroids for 2 weeks prior to surgery. A face MRI was performed and revealed an anteriorly located soft tissue mass which is of high signal intensity on T2 weighted imaging, measuring 1.8 cm in anteroposterior dimension and 1 cm in transverse dimension. There were also associated findings of chronic polypoidal mucosal thickening of the maxillary sinuses. Endoscopic excision of the mass was performed, and histopathology confirmed LCH.
2.4. Case 4
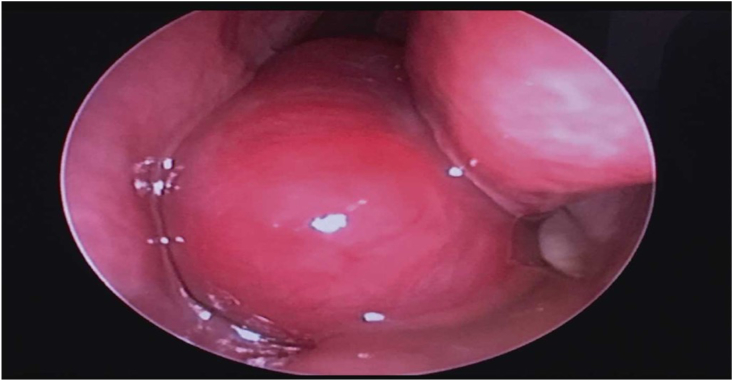
A 33-year-old medically free pregnant lady, gravida 3 para 2, in her third trimester, presented to the emergency department with left sided epistaxis and nasal obstruction. The patient denied any history of trauma or nose picking. Her initial hemoglobin level was 9.9 mg/dL. Packing of the anterior nose was done, and after 2 h, bleeding had stopped. Irrigation was done with normal saline and the patient was given instructions on first aid measures in case it recurs. Packs were removed and the patient was instructed on when to come back to the ED. Within the next two weeks of initial presentation, the patient presented to the ED three times with the same complaint with progressive decline in hemoglobin level, reaching 8.7 mg/dL. Otolaryngology – Head and Neck Surgery were involved, and endoscopic examination of the nose revealed an erythematous, large, rounded, smooth mass in the left nasal cavity that actively bled on touch. The left anterior nose was packed again. Due to multiple episodes of bleeding and decline in hemoglobin levels, a decision on how to control the bleeding had to be made. Since the gestational age of the fetus was 38 weeks, Obstetrics and Gynecology and Otorhinolaryngology – Head and Neck Surgery made a decision to admit the patient for delivery and to retain the nasal pack for 48 more hours. Two days later, the patient delivered the baby. The day following delivery, the nasal pack was removed, and the bleeding recurred again. Endoscopic examination showed no alteration in the size of the mass. Her hemoglobin level further dropped to 7.8 mg/dL. Blood transfusion was recommended, but the patient refused. A contrast enhanced CT scan of the paranasal sinuses was done and showed a heterogeneous strongly enhancing soft tissue mass involving the middle and lower meatus of the left nasal cavity measuring 3.2 × 2.2 × 1.5 cm with preserved architecture of surrounding bony structures (Fig. 4). The patient desired quick symptomatic relief, and a written consent was obtained for complete surgical excision. During intraoperative endoscopic examination, a large, erythematous, well-defined, smooth mass was found occupying the left nasal cavity having its origin from the medial surface of the inferior turbinate and the inferior surface of the posterior part of the middle turbinate with no attachment to the nasal septum (Fig. 5). Using bipolar diathermy, the mass was excised from its origin with no significant bleeding. The patient's symptoms resolved and had four follow up appointments in the six months following the excision. The patient remained asymptomatic with healthy nasal mucosa and no evidence of lesion recurrence noted on nasal endoscopic examination.
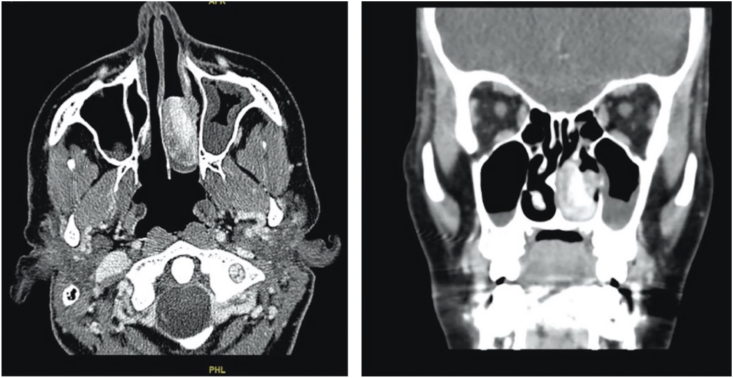
Fig. 4.
Axial and coronal sinus contrast enhanced CT scans showing a heterogeneous strongly enhancing mass involving the middle and lower meatus of the left nasal cavity with preserved architecture of adjacent bony structures. The mass measured 3.2 × 2.2 × 1.5 cm in its maximum dimensions.
Fig. 5.
Intraoperative endoscopic examination showing a large mass occupying the left nasal cavity.
2.5. Case 5
A 25-year-old medically free primigravida presented to the primary care clinic in her third trimester with a history of recurrent epistaxis, nasal obstruction and nasal pain for 3 months. Examination in the primary care setting showed a left nasal mass. Two weeks after presenting to the primary care clinic the patient was admitted to the hospital for labor and delivery. After delivery she was referred to the otolaryngology – head and neck surgery clinic for further evaluation of the mass. The mass had regressed in size after delivery, but still the patient had episodes of recurrent epistaxis. Endoscopic examination in the clinic showed a soft tissue mass originating from the floor of the left nasal cavity at the level of the internal nasal valve. Sinus CT scan was done, and the patient was booked for excisional biopsy of the mass under General Anesthesia (GA). Histopathology confirmed the diagnosis of LCH. A summary of the five cases is presented in Table 1.
Table 1.
A summary of the cases presented.
| Case | Gender | Age | Medical History | Symptoms | Site | Treatment |
|---|---|---|---|---|---|---|
| 1 | F | 29 | Second trimester pregnancy | Epistaxis and headache | Attached to the posterior end of the right inferior turbinate protruding posteriorly to the nasopharynx obstructing bilateral nasal choana | Excision during pregnancy under GA |
| 2 | M | 13 | Post trauma | Epistaxis, nasal obstruction and snoring | Left anterior nasal septum | Excision under GA |
| 3 | F | 87 | Hypertension, asthma and dyslipidemia | Epistaxis, nasal obstruction and facial tenderness | Left anterior nasal septum | Excision under GA |
| 4 | F | 33 | Third trimester pregnancy | Epistaxis and nasal obstruction | The medial surface of the left inferior turbinate and the inferior surface of the posterior part of the left middle turbinate | Postpartum excision under GA |
| 5 | F | 25 | Third trimester pregnancy | Epistaxis and nasal obstruction | Originating from the left nasal floor at the level of the internal nasal valve | Postpartum excision under GA |
3. Discussion
LCH was initially described in the late 19th century by Poncet and Dor as “human botryomycosis” based on the speculation that the lesion arose secondary to a fungal infection [10]. In the early 20th century, the term “pyogenic granuloma” was introduced to describe the lesion by Hartzell given the assumption that it was a mass of granulation tissue that arose in response to a bacterial infection [11]. It was only until 1980 when Mills et al. proved that both these terms were misnomers and the lesion was termed LCH based on its unique histopathological characteristics [12].
LCH is the most common vascular tumor of the nasal cavity [12]. It is most commonly seen in patients in their third and fifth decades [3]. It is more prevalent in females and in males under 18 years of age [1]. It has an unusual predilection for women of reproductive age, and an association with pregnancy has been reported in 2–5% of cases, mostly observed in the last two trimesters [1,2,6]. After the age of 40 years, there is no gender predilection, and the male to female ratio is 1:1 [2].
The most common location for LCH development in the head and neck region is the lip (38%) followed by the nasal cavity (29%), oral mucosa (18%) and tongue (15%) [[1], [2], [3], [4]].
LCH can manifest in a mucosal or cutaneous variant. The former is more frequently seen in women, whereas the latter is mostly seen in men [8].
Certain predisposing factors to the development of LCH have been described in the literature, and include a history of oral contraceptive pill use, pregnancy and a history of pre-existing trauma [2,7,8].
Also, it was observed that these lesions spontaneously regress after delivery, implicating a hormonal role in the development of the LCH [2,6].
The development of the mucosal variant of LCH most commonly occurs in the anterior nasal septum (Little's area), the inferior turbinate and the vestibule [[1], [2], [3], [4]]. These sensitive sites nearest the nares are more prone to local trauma and support the theory of trauma as a factor to the development of LCH [13].
The differential diagnosis for LCH includes a list of benign and malignant lesions. Examples of benign lesions are Wegener's granulomatosis, meningoencephalocele, sarcoidosis, nasal polyps, glioma, hemangioma, lipoma, osteoma, fibroma, nasopharyngeal cyst, and histiocytoma. Examples of malignant lesions include angiosarcoma, aesthesioneuroblastoma, squamous cell carcinoma, achromic melanoma, Kaposi sarcoma, and adenocarcinoma lymphoma [1,6,8,9]. Due to the wide list of differential diagnoses for LCH, establishing a diagnosis of LCH can only be made after thorough investigations. Radiological studies are frequently used to assess intranasal masses (degree of bony destruction and intracranial extension). In cases of LCH, contrast enhanced computed tomography of the sinuses typically shows a well-defined strongly enhancing soft tissue mass. Magnetic Resonance Imaging, on the other hand, demonstrates hypo intensity and hyperintensity in T1 and T2 weighted images, respectively. It has been reported in the literature that LCH usually respects bony architecture, however, bony destruction in LCH can occur and is primarily secondary to the compressive devascularization effect of the mass [[1], [2], [3],14,15].
Microscopic histopathologic examination of LCH will show extensive endothelial proliferation with prominent vascular spaces, capillaries in lobular arrangement, epithelial ulceration and a fibrovascular tissue base [2,6,8].
For treatment of intranasal LCH, endoscopic surgical excision with complete electrodesiccation of the tumor base is the treatment of choice [3]. Pre-operative vascular control in the form of embolization has been utilized in select cases and found to be useful [2,16]. On the other hand, others believe that surgery alone is the treatment of choice for LCH and that preoperative vascular control is unnecessary [17].
Unfortunately, the recurrence rate of LCH can be as high 16% [4]. This is mostly due to incomplete resection of the mass [2,4]. Complete excision of the mass with healthy mucosal margins as shown on frozen section at the time of resection minimizes the rate of recurrence [8].
4. Conclusion
LCH is the most common vascular lesion arising within the nasal cavity. Due to a large number of differential diagnoses, tissue diagnosis is critical to establishing the diagnosis of LCH. Treatment of LCH is surgical excision with pre-operative embolization & vascular ligation being useful in certain select cases.
5. Registration of research studies
Non Applicable.
Guarantor
Bassam Alghamdi.
Mohammed J Al Mahdi.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Research has been approved by the Research Ethical Committee of King Abdullah International Medical Research Centers.
Consent
Consent was taken directly from patients & from the guardian of the pediatric patient for publication purposes.
Author contribution
Bassam Alghamdi: Data collection, data analysis & interpretation and writing the paper.
Mohammad Al-Kadi: Performing surgery, study concept & design, data collection, data analysis & interpretation and writing the paper.
Norah Alkhayal: Data collection, data analysis & interpretation and writing the paper.
Riyadh Alhedaithy: Performing surgery, data collection, data analysis & interpretation and writing the paper.
Mohammed J Al Mahdi: Performing surgery, study concept & design, data collection, data analysis & interpretation and writing the paper.
Declaration of competing interest
The authors declare that there are no conflicts of interest to disclose with regards to this article.
References
- 1.Karagama Y.G., Howarth K., Steel P.R., Spencer M.G. Lobular capillary haemangioma of the nasal vestibule: a rare entity. Int. J. Pediatr. Otorhinolaryngol. 2002;66:71–75. doi: 10.1016/s0165-5876(02)00207-0. [DOI] [PubMed] [Google Scholar]
- 2.Delbrouck C., Chamiec M., Hassid S., Ghanooni R. Lobular capillary haemangioma of the nasal cavity during pregnancy. J. Laryngol. Otol. 2011;125:973–977. doi: 10.1017/S0022215111001654. [DOI] [PubMed] [Google Scholar]
- 3.Ifeacho S.N., Caulfield H.M. A rare cause of paediatric epistaxis: lobular capillary haemangioma of the nasal cavity. BMJ Case Rep. 2011;7:700–703. doi: 10.1136/bcr.07.2010.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jafarzadeh H., Sanatkhani M., Mohtasham N. Oral pyogenic granuloma: a review. J. Oral Sci. 2006;48:167–175. doi: 10.2334/josnusd.48.167. [DOI] [PubMed] [Google Scholar]
- 5.Nayak D.R., M Bhandarkar A., Shivamurthy A., Joy J. Intranasal lobular capillary haemangioma. BMJ Case Rep. 2014 doi: 10.1136/bcr-2014-207196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller F.R., D'Agostino M.A., Schlack K. Lobular capillary hemangioma of the nasal cavity. Otolaryngol. Head Neck Surg. 1999;120:783–784. doi: 10.1053/hn.1999.v120.a85324. [DOI] [PubMed] [Google Scholar]
- 7.Gregorio L.L., Wu C.L., Busaba N.Y. Lobular capillary hemangioma formation: an unusual complication of submucous resection with power instrumentation of the inferior turbinate. Laryngoscope. 2015;125:2654–2655. doi: 10.1002/lary.25355. [DOI] [PubMed] [Google Scholar]
- 8.Derkenne R., Coulet O., Varoquaux A., de Biasi C., Tomasi M. Nasal cavity lobular capillary hemangioma due to insect sting. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2012;129:278–280. doi: 10.1016/j.anorl.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Zarrinneshan A.A.Z., Zapanta P.E., Wall S.J. Nasal pyogenic granuloma. Otolaryngol. Head Neck Surg. 2007;136:130–131. doi: 10.1016/j.otohns.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 10.Poncet A., Dor L. Botyromycose humaine. Rev. Chirurgie (Paris) 1897;18:996. [Google Scholar]
- 11.Hartzell M.B. Granuloma pyogenicum. J. Cutan Dis. 1904;22:520–525. [Google Scholar]
- 12.Mills S.E., Cooper P.H., Fechner R.E. Lobular capillary hemangioma: the underlying lesion of pyogenic granuloma. A study of 73 cases from the oral and nasal mucous membranes. Am. J. Surg. Pathol. 1980;4:470–479. [PubMed] [Google Scholar]
- 13.Puxeddu R., Berlucchi M., Ledda G.P., Parodo G., Farina D., Nicolai P. Lobular capillary hemangioma of the nasal cavity: a retrospective study on 40 patients. Am. J. Rhinol. 2006;20:480–484. doi: 10.2500/ajr.2006.20.2878. [DOI] [PubMed] [Google Scholar]
- 14.Lee D.G., Lee S.K., Chang H.W. CT features of lobular capillary hemangioma of nasal cavity. AJNR Am. J. Neuroradiol. 2010;31:749–754. doi: 10.3174/ajnr.A1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee G.K., Suh K.J., Lee YH Y.H., Kang I.W. CT findings in two cases of lobular capillary haemangioma of the nasal cavity: focusing on the enhancement pattern. Dentomaxillofacial Radiol. 2012;41:165–168. doi: 10.1259/dmfr/85015314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamaki A., Babajanian E., D'Anza B., Rodriguez K. Lobular capillary hemangiomas: case report and review of literature of vascular lesions of the nasal cavity. Am. J. Otolaryngol. 2017;38:363–366. doi: 10.1016/j.amjoto.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Lin G., Bleier B. Surgical management of severe epistaxis. Otolaryngol. Clin. 2016;49:627–637. doi: 10.1016/j.otc.2016.01.003. [DOI] [PubMed] [Google Scholar]