SUMMARY
In mammalian cells, the SET1/MLL complexes are the main writer of the H3K4 methyl mark that is associated with active gene expression. The activities of these complexes are critically dependent on the association of the catalytic subunit with their shared core subunits, WDR5, RBBP5, ASH2L, and DPY30, collectively referred as WRAD. In addition, some of these core subunits can bind to proteins other than the SET1/MLL complex components. This review starts with discussion of the molecular activities of these core subunits, with an emphasis on DPY30 in organizing the assembly of the SET1/MLL complexes with other associated factors. This review then focuses on the roles of the core subunits in stem cells and development, as well as in diseased cell states, mainly cancer, and ends with discussion on dissecting the responsible activities of the core subunits and how we may target them for potential disease treatment.
1. Introduction
Histone H3 Lysine 4 (H3K4) methylation is one of the most prominent chemical modifications associated with potentiated or active transcription in the eukaryotic genomes [1]. Moreover, the three different levels (mono-, di-, tri-) of methylation (me1, me2, me3) at H3K4 have different biological functions. H3K4me3 definitely marks active and poised transcription start sites, H3K4me2 marks active gene body, and H3K4me1 marks active and poised enhancers [2, 3]. Although H3K4 methylation is not obligatory for all transcription reactions in cells and it is often hard to unambiguously demonstrate the impact of H3K4 methylation at a particular gene on its transcription in cells, this epigenetic mark nevertheless functionally influences the transcriptome and potentially other DNA-based processes in one way or another. While the word “epigenetic” has multiple definitions, it refers to histone post-translational modifications throughout this review.
The most notable writer of H3K4 methyl mark in mammals is the SET1/MLL complexes. All these complexes share the common composition of one catalytic subunit, four core subunits, and a few additional subunits that are relatively specific to each sub-group of complexes. All of the six catalytic subunits, KMT2A/MLL1, KMT2B/MLL2, KMT2C/MLL3, KMT2D/MLL4, KMT2F/SET1A, KMT2G/SET1B, rely on the SET domain located near the C-terminus for their intrinsic methyltransferase activity, which is weak in the absence of the core subunits. The core subunits, WDR5, RBBP5, ASH2L, and DPY30 (together as WRAD), have no or very weak intrinsic catalytic activity, but are all required for levels of H3K4 methylation that are significant and biologically meaningful in cells [4, 5]. The stimulatory effects of most cores are mostly direct at the biophysical level, as they can be consistently demonstrated in vitro on complexes reconstituted with all purified proteins, and structural studies have shown how the effects are realized through the structural coordination of the core subunits with the catalytic subunits.
While best known for their activity in H3K4 methylation, the SET1/MLL complex components, either as a complex or as isolated proteins, possess activities that are not directly related to H3K4 methylation. These “non-canonical” activities are usually mediated by interaction with molecules not typically found in the SET1/MLL complexes. This review will also touch upon some of the “non-canonical” activities of SET1/MLL complexes, with a focus on the core subunits.
While the catalytic subunits have greatly diverged in evolution, the core subunits have remained highly conserved from yeast to human, suggesting their fundamental importance in eukaryotes. In this review, the molecular activities of the core subunits will be discussed first, especially with regard to recent findings on the assembly of the SET1/MLL complexes for functional coordination. It is followed by review of roles of the core subunits in development and disease, especially in mouse models and human. The later part of the review discusses how we approach the key activities of the core subunits in regulating the diverse physiological and disease processes, and how we might target them for potential disease (mainly cancer) treatment.
2. Molecular activities of the SET1/MLL complex core subunits
The catalytic subunits of the SET1/MLL complexes are covered in other reviews in this issue ( ) and thus not a focus there, but it is important to keep the activities of the whole complexes in mind in reviewing the core subunits. One outstanding question on the biochemistry of these KMT2s is how regions outside the SET domain either by themselves or via interaction with other factors regulate the methylation activity, and/or contribute to non-catalytic activities of the proteins. The methylation activity of yeast Set1 is regulated by regions far from the SET domain, through intertwined inhibitory and anti-inhibitory effects [6] as well as regulation of the genomic localization [7]. Most of the mammalian KMT2s are large proteins with a few shared domains, and thus almost all of the in vitro reconstituted studies have relied on truncated and SET-containing regions of KMT2s recombinantly expressed from bacterial or insect cells. To dissect the function of other regions on KMT2s in vitro, we may express the tagged full-length or truncated KMT2s in complexes with other endogenously subunits in mammalian cells, and tag-purify the complexes that often exhibit robust H3K4 methylation activities in vitro [8, 9]. The drawback of this approach includes the difficulty to identify the contribution of a specific interacting factor, which is endogenously expressed and cannot be easily manipulated.
2.1. WDR5, RBBP5, and ASH2L
The biochemistry of these core subunits is covered in previous reviews [5, 10] and in this issue (). While all four cores are required for the full methylation activity of the SET1/MLL complexes in vivo, their contributions to the different catalytic subunits remain incompletely understood. A minimized RBBP5-ASH2L heterodimer serves as the essential structural unit that directly binds and activates all catalytic subunits [11], and an internal interaction in RBBP5 also contributes to the maintenance of the compact conformation of the MLL1 complex [12]. As a bridging molecule, WDR5 appears to be more specifically required for MLL1 than other catalytic subunits [11, 13] in vitro. However, different assay conditions bring out various effects on other catalytic subunits as well [14, 15], including even inhibitory effect on the activity of MLL3 [11, 16, 17]. Moreover, WDR5 depletion leads to marked reduction of global H3K4 methylation in cells [18] and in animals [19], which is not observed when MLL1 is lost from cells [20]. This suggests that WDR5 may indirectly affect intracellular H3K4 methylation as it also binds to a large number of other proteins and RNAs, especially long non-coding RNAs (lncRNAs) [21].
A recent study has resolved the structure of MLL1 and MLL3 catalytic modules in complex with WRAD and associated with nucleosome core particles that contain lysine 120-ubiquitinated or unmodified H2B [17]. Strikingly, H3K4 methylation is facilitated by the extensive contacts between MLL1 and histones beyond H3, and those between RBBP5 and the DNA backbone, histones including H4 tail, H3, and H2B-conjugated ubiquitin. All of these interactions probably stabilize the conformation of the MLL complexes on the nucleosome surface [17]. This study has thus brought unprecedented insights into how the different subunits contribute to the regulation of the complex activity on the physiologically relevant nucleosome structure.
2.2. DPY30
DPY30 is a small protein (99 amino acids in human and mouse) in the large SET1/MLL complexes, but deserves a special focus in this review because it is unique in a number of ways among the four core subunits. DPY30 is the only core subunit that does not directly contact the catalytic subunits [22–24]. Unlike the other three core subunits, DPY30 only modestly enhances methylation of the catalytic subunits in vitro, yet is profoundly important for genome-wide H3K4 methylation (though not uniformly as discussed below). Moreover, DPY30 is the only subunit that readily forms homodimer and weak oligomer (mainly tetramer) [25, 26] with potentially important biological roles as discussed below.
A relatively consistent understanding of how DPY30 interacts with the SET1/MLL complexes is emerging after studies over the past several years on both mammalian and yeast counterparts [22–29], taking advantage of the highly conserved nature of the key domains on the DPY30 (Sdc1 in yeast) and ASH2L (Bre2 in yeast) subunits. DPY30 is incorporated into SET1/MLL complexes by directly interacting with ASH2L, which is the only subunit in the entire complex that DPY30 contacts. The highly conserved DPY30 domain at the C-terminal region mediates DPY30 homodimerization and provides a hydrophobic groove at the inner surface of the dimer, which engages extensive interactions with the hydrophobic ridge of the amphipathic α helix at the C-terminal region of ASH2L [28]. Moreover, both ends of the ASH2L C-terminal helix make extra and critical interactions with the DPY30 dimer [22]. It is thought that the Sdc1 (DPY30) dimer stimulates the methylation activity of the SET1/MLL complexes by clamping the Bre2 (ASH2L) helical tail onto the RBBP5-interacting domain on Bre2 (ASH2L) and thus allosterically reinforcing the entire Bre2 (ASH2L) protein fold [23, 24]. With this basic structural knowledge of DPY30 in the SET1/MLL complexes, its activity is discussed in depth in the following few sections.
2.2.1. The biochemical role of DPY30 in regulating H3K4 methylation
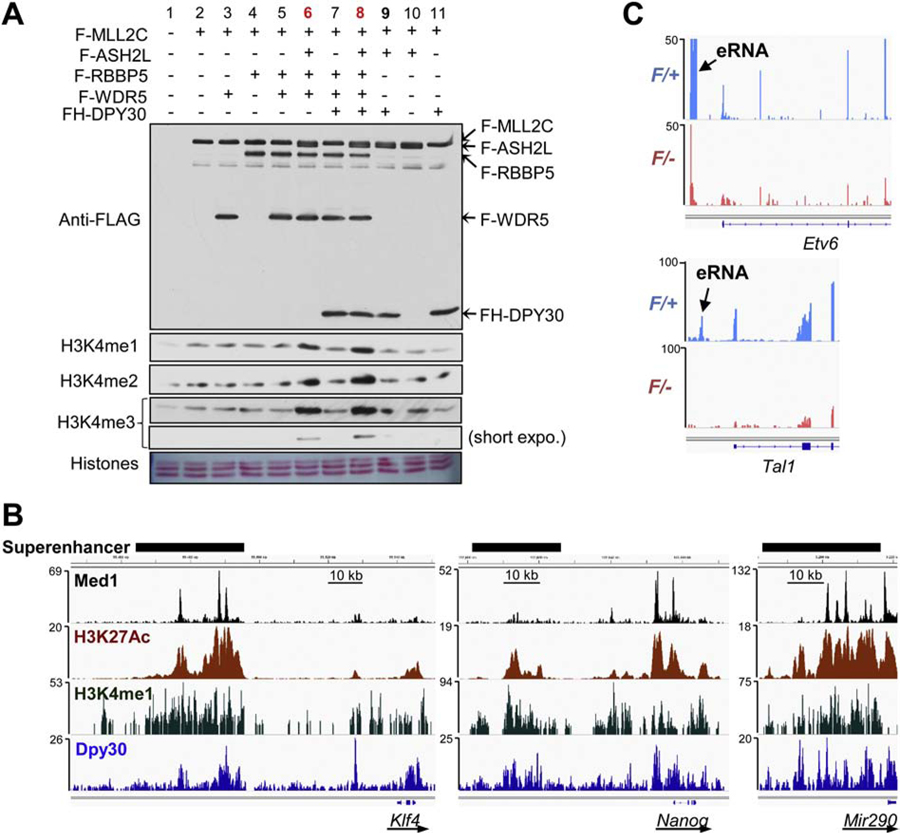
DPY30 facilitates all three levels of H3K4 methylation both in vitro (Figure 1A) [22] and in yeast [30–32], zebrafish, mouse, and human cells [33, 34]. While its best appreciated effect is on H3K4me3 at transcription start sites, its effect on H3K4me1 is also considerable in metazoans. This is relevant to the less studied activity of DPY30 in complex with MLL3 and MLL4, which mainly regulate enhancer H3K4me1 [35]. ChIP-seq data [36] indicate that DPY30 co-occupies enhancers including super-enhancers in mouse embryonic stem (ES) cells, and aligns well with established enhancer signatures both in signal locations and magnitudes (Figure 1B). DPY30 loss in hematopoietic cells markedly downregulates expression of certain transcription factors including Etv6 and Tal1 [36] and the associated enhancer RNAs (eRNAs) (Figure 1C), consistent with a direct or indirect effect of DPY30 on enhancer activity. As the direct interaction partner of DPY30, ASH2L also binds and regulates super-enhancers for pluripotency circuitry genes [37]. Further studies are needed to better understand the role of DPY30 in regulating the enhancer activity, which is undoubtedly pivotal for gene regulation in development and disease [2].
Figure 1. DPY30 may regulate enhancers including superenhancers.
(A) DPY30 enhances all three levels of H3K4 methylation in an in vitro histone methylation assay on purified bovine histones using reconstituted MLL2 complex, including FLAG- (F-) or FLAG-HA- (FH-) tagged recombinant subunits of human MLL2 (C-fragment) complex purified from Sf9 cells. Compare lanes 6 and 8.
(B) ChIP-seq profiles at indicated gene loci for Med1 (GSE44288), H3K27Ac (GSE62380), H3K4me1 (GSE34975), and Dpy30 (GSE26136) in mouse ES cells.
(C) Expression of eRNAs at Etv6 and Tal1 was greatly reduced in the Dpy30 knockout HSPCs, shown by RNA-seq from sorted Lin−Sca1+cKit+ cells derived from Mx1Cre; Dpy30F/+ (control) and Mx1Cre; Dpy30F/- (knockout) donors in the bone marrow transplant recipients after pIpC injection to induce Dpy30 deletion. Based on [34].
While each of WDR5, RBBP5, and ASH2L is required to tremendously (hundreds of fold) enhance the methylation activities of SET1/MLL complexes in reconstituted systems in the absence of DPY30, addition of DPY30 only modestly (~2 fold) stimulates the methylation activities in vitro (Figure 1A) [22]. Paradoxically, loss of DPY30 profoundly reduces global H3K4 methylation in cells [30–34], comparable to the effect of loss of the other cores. Loss or mutation of yeast Sdc1 that disrupts Bre2 binding profoundly reduces global H3K4me3 throughout the genome, but leaves a subset of the genome with largely unaltered H3K4me2. As this subset of the genome tends to be highly transcribed, it is proposed that the high turnover of RNA polymerase II maintains the loading and the di-methylation (not tri-methylation) activities of Set1 complexes in those regions [22]. These results are also reminiscent of the preferential impact of DPY30 loss on H3K4me3 of hematopoietic genes in mouse hematopoietic cells [34], and reveal the heterogeneous response of H3K4 methylation in the genome to DPY30 loss.
The apparent discrepancy between the in vitro and in vivo effects may be in part because association with DPY30 (Bre2) is important for ASH2L (Bre2) to be efficiently incorporated into the SET1/MLL complexes [6, 30, 31] and recruited to genomic targets in cells [22]. However, as many reports have reconstituted SET1/MLL complexes in the absence of DPY30 to demonstrate structure and robust (and ASH2L-dependent) histone methylation activities, sometimes on chromatin substrate, it seems that the requirement of DPY30-ASH2L interaction for stable incorporation of ASH2L into the complexes is not absolute and can be bypassed in vitro. Further studies on the genome-wide binding of ASH2L and other subunits of the SET1/MLL complexes in cells lacking DPY30 will be very helpful to test this hypothesis. Moreover, the biochemical activity of DPY30 will be better understood in in vitro studies using more physiologically relevant substrates (nucleosomes and chromatin) and larger parts if not full-length of the catalytic subunits.
2.2.2. Other DPY30-associated proteins
Unbiased proteomic approaches have shown that DPY30 is mainly associated with SET1/MLL complexes, NURF complex (an ATP-dependent chromatin remodeling complex), AKAP95 (also known as AKAP8), and a guanine nucleotide exchange factor BIG1 [8, 38, 39]. A DPY30-binding motif was identified, which was used to predict a few more proteins that may bind DPY30 [28]. Some of these proteins were also experimentally validated as DPY30-associated proteins, including the BAP18 subunit of the NURF complex [28]. Sharing strong sequence [8] and structural [28] similarity with the regulatory subunit of the protein kinase A (PKA), the DPY30 dimerization domain can accommodate amphipathic α helices of ASH2L, AKAP95 (known to bind to PKA regulatory subunit), BIG1, BAP18, and potentially other proteins [28]. Moreover, the hydrophobic Leu69 of DPY30 is commonly required for binding to ASH2L, BAP18 [28] and BIG1 [40]. It is thus clear that all these factors bind to DPY30 on the same site.
AKAP95 physically associates with DPY30 [8, 40, 41] and the entire SET1/MLL complexes, as shown by co-immunoprecipitation with other subunits and co-elution with the complexes in gel filtration analysis [8]. Strikingly, AKAP95 directly stimulates MLL2 enzymatic activity on chromatin [8], making it the only protein other than the core subunits that are known to be able to directly regulate the enzymatic activity of the H3K4 methyltransferases. The mechanism of stimulation is unknown. AKAP95 is recently shown to undergo liquid-liquid phase separation that underlies its activity in promoting tumorigenesis [42]. This allows the speculation that AKAP95 phase separation helps get the methyltransferases into high local concentration in the dynamic liquid droplets and thus work more effectively on the substrate. It remains unclear if AKAP95 regulates intracellular H3K4 methylation and gene regulation through SET1/MLL complexes, and if the role of AKAP95 in regulating RNA splicing [42, 43] is linked to DPY30 or SET1/MLL complexes.
Associations of DPY30 with most other factors have not been demonstrated to impact the biochemical activities of either the SET1/MLL complexes or the associated factors. A structural question highly relevant to the functional studies also remains to be addressed: “are these factors in the same complex as the SET1/MLL complexes?” Because these different factors including ASH2L bind to the same groove formed by the DPY30 dimer [28], they are unlikely to be simultaneously associated with the same DPY30 dimer. A similar question also exists for WDR5, which binds to numerous protein and RNA factors at sites also bound by the SET1/MLL complex subunits [21]. These questions are discussed in the section below.
2.2.3. Role of the dimerization/oligomerization property of DPY30
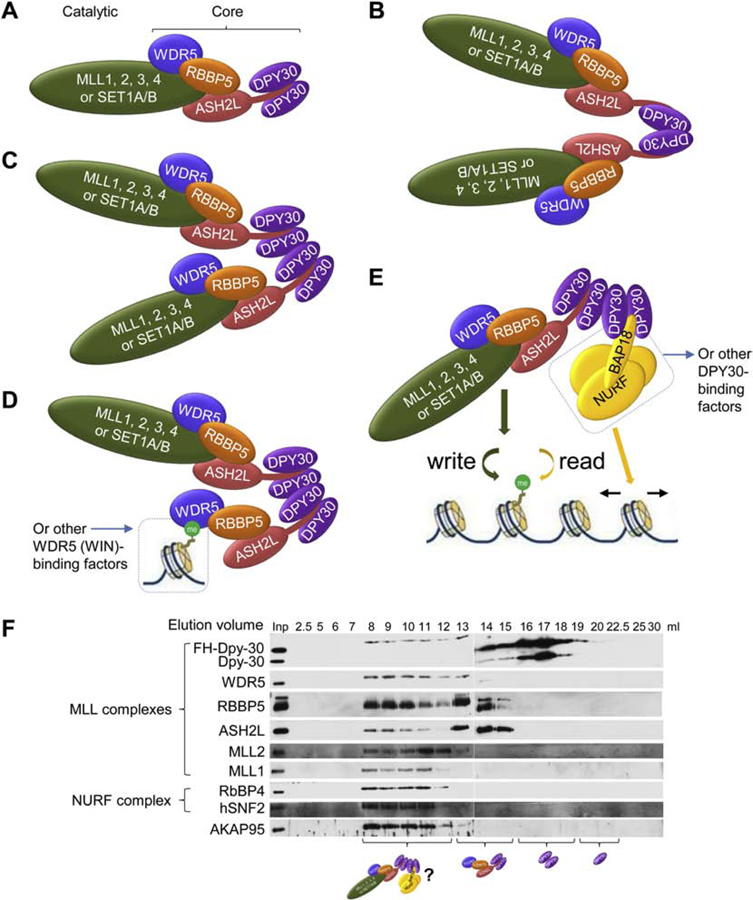
Structural and functional studies indicate that DPY30 homodimerization enables it to bind to the C-terminal tail of ASH2L and facilitates the methylation activity, and the catalytic subunit and WDR5, RBBP5, ASH2L, and DPY30 are assembled with a stoichiometry of 1:1:1:1:2 [22–24, 26–29] (Figure 2A). DPY30 is often found at higher than 2:1 stoichiometry relative to other subunits [38]. A recent study [31] shows that the yeast Set1 complex in cell extract adopts a dimeric state, which requires Bre2 and Sdc1 and is disrupted by mutation of the dimeric interface of Sdc1 (V130A, L134A, L135A, labeled as V129A, L133A, L134A likely not counting the first Methionine). The monomeric Set1 complex greatly reduces global levels of H3K4me3 and me2 and increases the levels of asymmetric H3K4-methylated nucleosomes. Overall, the Set1 complex is proposed to form dimer via Sdc1 homodimerization and conveys symmetrical H3K4 tri-methylation (Figure 2B). This new model suggests that the stoichiometry of Sdc1: Bre2 (and other subunits) is 2:2 or 1:1 instead of 2:1, and that Sdc1 dimerization is unnecessary for interaction with Bre2 and the Set1 complex (Figure 2B) [31]. This is puzzling given all other available structural data and that the key residues in ASH2L C-terminal region make extensive contacts with the hydrophobic pockets contributed from both DPY30 protomers [28].
Figure 2. Possible assembly states of the SET1/MLL complexes.
(A) Monomeric SET1/MLL complex with the C-terminal tail of ASH2L binding to the groove between the DPY30 dimer.
(B) Dimeric SET1/MLL complex mediated by the dimerization of DPY30, based on the yeast Set1 complex dimer model. Note that ASH2L C-terminal region only binds to monomeric DPY30.
(C) Dimeric SET1/MLL complex mediated by the tetramerization of DPY30, with ASH2L C-terminal tail binding to the groove between the DPY30 dimer.
(D) Alternative factors may directly bind to the WIN site on WDR5 and get incorporated into the physical complex with SET1/MLL united by the tetramerization of DPY30. Shown is WDR5 binding to a modified H3 in chromatin.
(E) Alternative factors may directly bind to the groove between the DPY30 dimer and incorporated into the physical complex with SET1/MLL united by the tetramerization of DPY30. Shown is the NURF complex that reads the H3K4me3 mark written by the methyltransferase in the same complex and mediates chromatin remodeling, thereby efficiently coordinates gene activation.
(F) Gel filtration chromatography results of complexes that were affinity purified by anti-FLAG from nuclear extract of cells stably expressing FLAG-HA-DPY30 (FH-DPY30). Details in [8].
Note that these different states of the complexes may dynamically convert into each other, possibly depending on the available local concentration of the factors.
Here I propose a reconciling model that is consistent with most biochemical and structural data and can be tested by the community. In this model (Figure 2C–2E), DPY30 can form dimer that interacts with one molecule of ASH2L or other protein, and may also form tetramer, sometimes in conjunction with the weakly dimeric and stabilized SPRY domain of RBBP5 [25, 26]. The different assembly states of the complexes may dynamically change to one another, likely depending on the available local concentration of the factors. DPY30 tetramer brings in two copies of the SET1/MLL complexes (Figure 2C) to carry out symmetrical methylation on nucleosomes, but, alternatively, may bring one copy of the whole SET1/MLL complex and other proteins into the same complex (Figure 2D and 2E). The latter possibility offers a great combinatorial diversity, and potentially provides a solution to the question: how can the cores allow association of different factors and the SET1/MLL complex in the same complex through the same binding sites.
WDR5 is thought to promote the recruitment of SET1/MLL complexes to genomic targets as it can bind to both the SET1/MLLs and histone H3 with methylated K4 [19] or symmetrically methylated R2 [44], but how is this achieved with the same site on WDR5 interacting with both the SET1/MLLs Win motif and the modified H3 [5, 44]? This would be possible if two copies of WDR5 exists in the same complex via DPY30 oligomerization, so that one copy of WDR5 brings in SET1/MLL complex and the other copy recognizes the modified H3 in the genome (Figure 2D). SET1/MLL complex regulates chromosome alignment and spindle assembly in mitosis by targeting the kinesin motor protein Kif2A to spindle poles, in a manner dependent on a Win motif of Kif2A that directly binds WDR5 [45]. As MLL also uses its Win motif to bind WDR5, one possible model of a joint complex containing all these molecules for functional coordination is presented as MLL-WRAD and Kif2A-WRAD associated by oligomeric DPY30.
ASH2L, BAP18, AKAP95, and BIG1 have all been shown to directly bind to DPY30 at the DPY30 dimer-based groove, and AKAP95 and BIG1 individually coexists in a same complex as the SET1/MLL complexes with functional implications. Co-elution of subunits of the NURF complex (containing BAP18) with the SET1/MLL complexes in gel-filtration chromatography (Figure 2F) is consistent with the existence of a co-complex formed by the NURF and SET1/MLL complex components. The physical association of an H3K4me3 mark reader, the PHD domain-containing BPTF subunit of the NURF complex [46], with the writer of the same mark may allow efficient recognition of the mark (compared to scanning the entire genome for the mark) to produce biological effects through its chromatin remodeling activity (Figure 2E). Cytoplasmic DPY30 is proposed to function together with WRAD and BIG1 in regulation of endosomal trafficking [39] that may underlie a novel epistatic effect of DPY30 haploinsufficiency on hereditary spastic paraplegia [47]. A coordinated activity of WRAD with either AKAP95 or BIG1 in the same complex would be made possible through the oligomerized DPY30 bringing in different interacting factors.
A few issues need to be addressed for a better understanding of DPY30 oligomerization. While an oligomeric (likely tetrameric) state of purified (and un-crosslinked) DPY30 is shown by gel-filtration analysis [25], other analyses do not provide strong evidence of oligomerization in solution unless stabilized by crosslinkers, suggesting that the oligomerization is weak [25, 26]. Moreover, the apparent N-terminal helix of DPY30 may occupy the DPY30 C-terminal groove that is also used for ASH2L [26], and may thus interfere with the DPY30 oligomer binding to ASH2L and the whole complex. However, this structural information was obtained in the absence of ASH2L and was likely artificially induced by molecular packing in the crystal [26]. The dimerization mutant used by Choudhury et al. [31], Sdc1 (V130A, L134A, L135A), still allows Bre2 association with Set1, and was thus considered to disrupt only Sdc1 dimerization but not Bre2 interaction. This is in contrast to the previous data showing that Sdc1 (L134A, L135A) disrupts binding to Bre2 and also the association of Bre2 with Set1 [30]. As discussed above, Bre2 may be (weakly) incorporated into a Sdc1-less Set1 complex shown by in vitro pulldown. Therefore, the monomeric Set1 complex may frequently experience dissociation from Sdc1 (V130A, L134A, L135A) and thus not get stimulated by Sdc1, consistent with the almost identical and profound genome-wide impact on H3K4 methylation by this mutation and the complete loss of Sdc1 [31]. More work will help further separate the impact of Sdc1 dissociation from that of Sdc1 monomerization.
Further structural and functional characterizations are needed to better understand whether and how DPY30 dimerization/oligomerization mediates the dimerization of the entire SET1/MLL complexes. The stoichiometry of Set1 complex can be determined by photon counting histogram analysis of yEGFP-Sdc1, in addition to Swd1 (RBBP5) fused to another fluorescence tag [31]. Moreover, it is important but probably more challenging to determine the dimerization state of these complexes in cells (as opposed to in extracts). Structural studies will help understand how a multimeric Set1 complex supports the symmetrical nucleosome recognition. We do not know if the mammalian SET1/MLL complexes are also dimeric via DPY30 di- or oligo-merization, and how the assembly status of SET1/MLL complexes affects the symmetry of the histone marks and gene regulation in development and disease. These studies will help elucidate how the conserved oligomerization property of the tiny DPY30 may play a central role in coordinating and potentially diversifying the biological functions of the WRAD or even the entire SET1/MLL complexes.
Finally, as a largely speculative note, is it possible that the multimerization of DPY30 and thus SET1/MLL complexes plays a role in spreading the chromosomal H3K4 methylation with far-reaching biological consequences? This tantalizing idea is consistent with the often stronger effect on breadth than intensity of the H3K4me3 domains following DPY30 loss in hematopoietic cells [34]. This may link the SET1/MLL complexes to animal development [48, 49] through control of transcriptional consistency associated with breadth of H3K4me3 domains [50]. This notion has gained support from single cell RNA-sequence assays that show increase in transcriptional variation in DPY30-deficient pancreatic cells associated with impaired differentiation capacity [51].
3. Physiological functions of the core subunits
Most of the SET1/MLL complex subunits were originally discovered and characterized based on their developmental roles before the demonstration of their presence in the H3K4 methyltransferase complex. Among the core subunits, Ash2 was originally identified as a developmental regulator of Drosophila [52, 53]. Dpy-30 was discovered as a gene important for dose compensation and development of C. elegans [54, 55]. WDR5 was earlier on characterized as an essential regulator of mammalian bone development by promoting osteoblast differentiation [56–58]. Consistent with the universal association of H3K4 methylation with transcription, all core subunits are expressed in most tissues and developmental stages, and play important roles in a very wide range of development and physiology. Reports on their physiological roles thus more likely reflect the interests of the investigators rather than the specificity of these subunits. Considering the fundamental role of stem cells in development, the role of the core subunits in regulating the fate determination of stem cells (and progenitors) are the focus of review below.
3.1. Role of the core subunits in development of embryos and embryonic stem (ES) cells
ES cells are a particularly relevant system to understanding the biological role of the H3K4 methylation, considering the poised and “primed” state of the bivalently marked developmental genes in ES cells [59]. It is somewhat surprising that DPY30 or RBBP5 depletion in mouse ES cells does not affect their self-renewal or expression of key pluripotency genes [36]. However, DPY30 and RBBP5 are critical for ES cell differentiation, possibly by potentiating the induction of the developmental genes [36]. WDR5 is required for maintaining self-renewal of mouse ES cells. WDR5 is under control of the core pluripotency transcription factors including OCT4 and NANOG, and interacts with OCT4, and cooperates with these factors in binding to and regulating the expression of key pluripotency genes [60]. The ability of WDR5 in maintaining pluripotency requires its interactions with a group of lncRNAs to facilitate its long residence on chromatin targets [61]. More extensive interactions exist between the core pluripotency factors and ASH2L as well [37, 62]. ASH2L is also important for maintaining pluripotency of mouse ES cells, probably by maintaining a relatively open chromatin state [63]. While ASH2L appears to bind to genomic sites via its DNA-binding domain [64, 65] unique among WRAD, the strongly overlapping binding pattern with the other core subunits and H3K4me3 [60, 63] suggest that these cores largely act together on chromatin. ASH2L also binds to the super-enhancers of the pluripotency circuitry genes and facilitates the recruitment of the core pluripotency transcription factors to the super-enhancers and activation of these genes [37]. The core subunits are thus all crucial regulators of pluripotency at the chromatin level.
WDR5 is also required for transcription factor-mediated formation of induced pluripotent stem cells (iPSCs) [60], and this is dependent on interaction of WDR5 with MYC [66]. WDR5 also regulates cell reprogramming by functionally interacting with BRCA1 and BARD1 and regulating DNA repair gene expression and DNA damage response [67]. An isoform of ASH2L highly expressed in mouse ES cells is induced by OCT4 and required for efficient iPSC formation [68]. OCT4 and WDR5 mutually promote the binding of each other to subsets of chromatin targets [60], and DPY30 regulates genomic binding of OCT4 during iPSC formation in a manner that is not necessarily dependent on the associated H3K4me3 [62]. Together with the importance of DPY30 and RBBP5 in efficient iPSC formation [62], these findings indicate that the core subunits are important for the two-way transitions between the pluripotent and differentiated states.
In human ES cells, DPY30 cooperates with transcription factors NANOG and SMAD2/3 under the control of Activin/Nodal signaling pathway, and regulates H3K4 methylation and expression of key developmental genes. DPY30 depletion in human ES cells affects their capacity in self-renewal as well as differentiation into all derivatives of the three germ layers [69]. Deletion of Dpy30 in mice shows a critical role of DPY30 in maintaining pluripotency of the post-implantation epiblast and also the proper differentiation of the three germ layers in vivo. This study provides a nice example in which the SET1/MLL complexes control stem cell fate by integrating the signals from extracellular factors relayed through transcription factors. Compared to mouse ES cells, human ES cells are more related to the “primed” post-implantation epiblast. Therefore, this and the earlier study [36] together suggest that, in addition to a crucial role in ES cell differentiation, DPY30 is preferentially required in establishment or maintenance of the epiblast pluripotency but not the “naïve” pluripotency. Strikingly, MLL1 appears to be a crucial determinant for the chromatin landscape of the epiblast versus naive stem cells, as pharmacological inhibition of MLL1 activity promotes reversion of the epiblast pluripotency to naïve pluripotency via redistribution of H3K4me1 at enhancers and reprogramming of transcription circuitry [70].
Loss of ASH2L [71] or DPY30 [34, 69] in mouse leads to early embryonic lethality, and WDR5 depletion in Xenopus embryos exhibit multilineage defects [19], indicating an essential role of the cores and probably SET1/MLL complexes in embryonic survival and/or development. WDR5 joins a complex named WHHERE that also contains RERE, HDAC1, and HDAC2 in mouse embryos, and plays an important role in retinoic acid-dependent regulation of the bilateral symmetry of embryo [72]. Wdr5 also plays a critical role in left-right patterning in development of asymmetrical organs [73]. Mediated by a lncRNA linc1405, WDR5 forms complex with a transcription factor EOMES and the histone acetyltransferase GCN5 at enhancers of target genes including Mesp1, and regulates its expression during cardiac mesoderm specification [74]. ASH2L, but not WDR5 or RBBP5, is recruited to the inactive X chromosome by interacting with a specific region on Xist RNA in embryonic development. Surprisingly, deletion of this region leads to even higher level of expression of a subset of escape genes without affecting X-linked gene silencing, suggesting a possible role of SET1/MLL complexes-independent role of ASH2L in maintaining the proper expression level of escape genes during random X-chromosome inactivation [75].
3.2. Role of the core subunits in somatic tissues and stem cells
3.2.1. In hematopoietic development
DPY30 is required for hematopoiesis in zebrafish [33] and mouse [34, 76]. It plays a critical role in the long-term maintenance and multilineage differentiation of both fetal [76] and adult hematopoietic stem cells (HSCs) [34]. Loss of DPY30 in fetal or adult mouse hematopoietic system results in pancytopenia but striking accumulation of hematopoietic stem and progenitor cells (HSPCs) that are functionally defective [34, 76]. DPY30-deficient HSCs fail to differentiate or turn on lineage-regulatory genes, and fail to maintain HSC signature gene expression. DPY30 directly regulates expression of key glycolytic genes and energy metabolism that enables HSC activation, and ensures efficient DNA damage repair capacity in HSPCs. The metabolic and genotoxic stresses in DPY30-deficient HSPCs are likely partially funneled through increase in CDK inhibitor p21 that contributes to the attrition of HSC functionality [76].
Loss of ASH2L in the mouse hematopoietic system results in phenotypes largely similar to DPY30 loss, including pancytopenia yet strong accumulation of HSPCs. ASH2L-deficient HSPCs cannot proliferate in vitro and appear to be arrested in S and G2/M phases in the cell cycle, consistent with downregulation of genes associated with the late stage of cell cycle [77]. The accumulation of phenotypic HSPCs at the expense of downstream hematopoietic cells is shared by loss or depletion of other subunits of the SET1/MLL complexes in the hematopoietic system, including SET1A [78], CFP1 (an important subunit of SET1A/B complexes) [79], MLL3 [80], and MLL4 [81]. Altered differentiation was found upon loss of some of these subunits [33, 34, 80, 81], and their deficiency can erode the cell type signature gene expression and rigidifies the transcriptional program (which defines cell identity) during cell fate transitions. Therefore, the stability and plasticity of cell identity appears to be under tight control of the SET1/MLL complexes.
3.2.2. In neural development
Through conditional deletion in specific brain regions, MLL1 was previously found to be required for postnatal neurogenesis, but not gliogenesis [82]. However, MLL1 deficiency does not affect global or local H3K4 methylation, but rather increases H3K27me3 at a target gene promoter. This suggests a possible activity of Mll1 in recruiting an H3K27 demethylase [82], probably indirectly, since H3K27 demethylases associates with MLL3 and MLL4, but not with MLL1 or MLL2 [4].
ASH2L is a target induced by nicotinic stimulation and, via interaction with a transcription factor MEF2C, critically mediates developmental nicotine exposure to changes in neuronal structure and behavior [83]. Deletion of Ash2l in the mouse dorsal cortex at the early neocortical developmental stage results in malformation of neocortex and low number of neurons, which are also abnormal in composition and positioning of projection. ASH2L is required for maintaining neural progenitor cell pool and their proliferation in late neurogenesis, likely through regulation of the Wnt signaling pathway [84].
Deletion of Dpy30 in neural stem cells in mouse brain leads to reduction of H3K4 methylation and disrupts postnatal neurogenesis and gliogenesis. DPY30 is required for neural stem cell self-renewal in a cell-autonomous manner, and also important for differentiation of mouse and human neural progenitors to neuronal and glial lineages [85]. SET1/MLL complexes cooperate with ZNF335, a causative gene for severe microcephaly, to regulate human and mouse neurogenesis and neuronal differentiation. Most of the core and at least two catalytic subunits (MLL1 and SET1A) associate with ZNF335 and regulate histone methylation and expression of ZNF335 target genes, including REST [86]. These studies together indicate a crucial role of the SET1/MLL complexes in neurodevelopment, with important implications in human neurodevelopmental disorders associated with the SET1/MLL complex components.
3.2.3. In other tissue development
A very recent report shows important yet distinct roles of WDR5 and DPY30 in pancreas progenitor fate specification [51]. Depletion of WDR5 from pancreas progenitor spheroids prevents endocrine and acinar lineage differentiation and reduces sphere maintenance. Depletion of DPY30 in pancreas progenitors in mice, however, leads to premature acinar cell specification at the expense of endocrine cell lineage, but also impairs further differentiation of acinar precursor-like cells. DPY30 loss also results in reduced proliferation and increased apoptosis in pancreas progenitors. Interestingly, single cell RNA-seq analyses revealed increased cell-to-cell variation of gene expression upon loss of DPY30 and H3K4 methylation. The study concludes that the assembly and co-activator function of the SET1/MLL complexes is important for gene activation in pancreas progenitor differentiation, while their H3K4 methylation activity regulates transcriptional stability and proper specification into correct lineage [51]. Such general conclusion needs to be taken with caution as the effects of WDR5 or DPY30 loss could result from their activities unrelated to H3K4 methylation, as discussed above.
3.3. Role of the core subunits in lifespan regulation
Changes in chromatin state not only are associated with, but also regulate organism aging and longevity [87, 88]. Mutations or depletion of several core subunits in the C. elegans H3K4 methyltransferase complexes, including the orthologues of ASH2L, WDR5, and SET1, significantly extend the worm lifespan along with loss of global H3K4 methylation. Loss of an H3K4 demethylase increases H3K4me3 level and reduces the worm lifespan, and overexpression of the H3K4 demethylase extends lifespan [89]. These studies suggest that H3K4 methylation is important for lifespan regulation, with the low level being beneficial for worm longevity [90]. Strikingly, the extended longevity by the methyltransferase deficiencies only in the parents can be inherited through several descendants in C. elegans [91]. The H3K4 methyltransferase deficiency results in downregulation of germline gene targets, which signals alteration of key enzymes in fat metabolism and promotes accumulation of mono-unsaturated fatty acids to extend the lifespan [90]. As H3K4 methylation marks an open chromatin state, these data are congruent with other findings suggesting that tight packaging of chromatin (by more histones [92] or less H4K16 acetylation [93]) appears to promote longevity.
It is tempting to ask if perturbing H3K4 methylation in higher animals like mice and, ultimately human, will also affect lifespan. DPY30 is significantly upregulated in aged macaque brain, and H3K4me2 increases at regulatory elements globally (and especially stress-response related genes) during postnatal development and aging [94], suggesting that increase in H3K4 methylation may be a consequence of experiencing cellular stress. Dpy30+/− mice show a very modest and insignificant increase in lifespan compared to the wild type littermates based on a relatively small number of samples [95]. It remains an interesting possibility if partial inactivation of other core subunits affects (increases in particular) mammalian lifespan, especially considering that WDR5 [66] and DPY30 [95] control the chromatin binding activity of MYC, and Myc+/− mice have significantly increased lifespan [96]. Since H3K4 methylation is generally associated with active transcription, it would be interesting to determine if reduced activity of the SET1/MLL complexes fits the emerging theme of “less is better” in longevity studies across species [96].
3.4. Role of the core subunits in other processes
SET1/MLL complexes were found to play an important role in mediating genetic compensation response that contributes to genetic robustness in a zebrafish model [97]. UPF3A, a member of the nonsense-mediated mRNA decay pathway, recruits the H3K4 methylation activity via directly binding to WDR5 to promote the expression of the compensatory genes [97].
Invading microbes (usually viruses) can exploit cellular WDR5. WDR5 is upregulated upon human cytomegalovirus infection, and is important for efficient viral replication by promoting the nuclear egress of viral capsids without major effects on viral genome replication or gene expression [98]. Upon infection by measles virus, WDR5 is selectively recruited to virally induced inclusion bodies in cytoplasm and enhances virus replication, apparently in a manner independent of other subunits of SET1/MLL complexes [99]. These studies suggest that viral pathogens usurp WDR5 to promote their infection and that WDR5 can be a potential target for antiviral therapy.
On the other hand, SET1/MLL complex subunits (often via H3K4 methylation activities) also contribute to our immune response to infection. Mediated by a lncRNAs NeST, WDR5 promotes H3K4me3 at the IFN-γ locus and contributes to control of microbial susceptibility [100]. Another lncRNA, UMLILO, directs WDR5 and probably its associated MLL1 to immune-related promoters brought in close proximity through chromatin topology, and promotes their H3K4me3 for robust transcription in trained immune response [101]. Upon viral infection, WDR5 is translocated from the nucleus to mitochondria to associate with the mitochondrial outer membrane protein VISA, and is important for virus-triggered type I interferon induction through IRF3 and NF-κB activation [102]. In addition, ASH2L associates with MKL1 in response to TNF-α stimulation and promotes pro-inflammatory transcription in macrophages, likely through H3K4 methylation [103]. Therefore, either in the context of the SET1/MLL complexes or not, the core subunits play important roles in both sides of the constant battle between the infectious microbes and our immune system.
While the SET1/MLL complexes are best known to function on chromatin, their localization is not limited to nucleus. DPY30 can be found at the trans-Golgi network in the cytosol, and together with ASH2L and RBBP5, functions in endosomal trafficking [39]. During mitosis, WDR5, RBBP5, and MLL1 (and thus likely the whole MLL1 complex) localize to the spindle apparatus and serve a critical role in spindle assembly and chromosomal misalignment. Via interactions (including the direct binding of WDR5) with Kinesin motor protein Kif2A on its Win motif, MLL1 complex helps target KIF2A to spindle poles [45]. WDR5 also localizes to the midbody during cytokinesis and promotes the final step of cytokinesis in a manner dependent on its MLL-interaction region [104].
4. Roles of the core subunits in disease
4.1. Role in cancer
Consistent with the important role of the SET1/MLL complexes in regulating cell survival, proliferation, and death, subunits of these complexes are critically involved in cancer. Genetic mutations and deletions of the catalytic subunits are extensively associated with human cancers, but their functional roles in cancer are complex and can vary in different context [105]. Chromosomal translocations of MLL1 are drivers of mixed lineage leukemias, but wild type MLL1, MLL2, and SET1A play roles in sustaining certain cancers. MLL3 and MLL4 are well-established tumor suppressors, but MLL4 is also required for supporting MLL-rearranged leukemia [81]. Recurrent mutations in SET1B are found in primary hepatic neuroendocrine tumors, and one of the mutations promotes cell proliferation, migration and invasion [106]. In contrast, genetic mutations or deletions of the core subunits are rarely found in cancer. Instead, frequent amplification and upregulated expression of the cores are associated with human cancers [95], and all of the four core subunits have been shown to play important roles in unanimously promoting tumorigenesis as further reviewed in the following sections.
4.1.1. WDR5 in cancer
As WDR5 directly interacts with numerous diverse macromolecules including nuclear non-histone and histone proteins, cytosolic proteins, and an ever-increasing number of different lncRNAs, one might expect it to exhibit different roles in cancer including promoting and suppressing cancer. However, though genetic mutations and deletions do occur at a low frequency to WDR5 [95, 106], all existing literature indicate a tumor-promoting activity of WDR5.
WDR5 is overexpressed in bladder cancer and elevated WDR5 protein levels correlate with advanced tumor stage and poor survival. WDR5 promotes proliferation and chemo-resistance of bladder cancer, likely through promoting expression of multiple cyclins genes, cell survival genes, and Nanog [107]. WDR5 preferentially interacts with a C/EBPα isoform (p30) commonly associated with acute myeloid leukemia, and is critical for p30-dependent self-renewal of leukemia in vivo [108]. WDR5 associates with Cbx8 in non-canonical PRC1 complexes and regulates H3K4me3 on Notch-network genes, thereby promoting breast tumorigenesis [109]. WDR5 upregulation is also found in acute leukemia and hepatocellular carcinoma, and correlates with poor remission rate [110, 111]. WDR5 is also important for survival of leukemia cells, target expression and H3K4me3 in leukemia [110], as well as proliferation of hepatocellular carcinoma cells [111]. Elevated in colorectal cancer, WDR5 promotes colorectal cancer metastasis through promoting epithelial-mesenchymal transition process in response to the PI3K/AKT signaling pathway, likely via directly regulating ZNF407 expression [112].
Interactions of WDR5 with lncRNAs play important roles in a number of cancers. WDR5 cooperates with TWIST1 and a lncRNA, HOTTIP, to facilitate prostate cancer metastasis by promoting the expression of HOXA9, a well-established target of WDR5-MLL1 complex [113]. The HOTTIP-WDR5-HOXA9 pathway regulates pancreatic cancer stem cell properties [114]. Another lncRNA, BLACAT2, directly associates with WDR5 to promote H3K4 methylation and expression of VEGF-C, which contributes to the lymphatic metastasis of bladder cancer [115]. Association of WDR5 with another lncRNA, GCAWKR promotes gastric cancer development partly via regulation of the target gene PTP4A1 [116].
The property of WDR5 in binding multiple different histone modifications [19, 44, 117, 118] may contribute to its role in cancer. Overexpressed in prostate cancer, WDR5 is critical for androgen-dependent prostate cancer cell proliferation. Androgen stimulation leads to H3T11 phosphorylation, which facilitates the direct recruitment of WDR5 and MLL1 complexes to promote expression of the androgen receptor targets [117]. WDR5 also associates with ACK1-mediated H4Y88 phosphorylation, and, together with KMT2D, drives H3K4me3 and expression of androgen receptor. Such epigenetic circuit is important for maintenance of the malignant castration-resistant prostate cancer, and global H3K4me3 is indeed elevated in high grade prostate cancer [118]. Moreover, WDR5 is recruited to a H3R2me1/H3R2me2s chromatin state that is activated by β-catenin signaling following genotoxic stress, and thus contributes to reestablishment of redox homeostasis and genotoxic resistance in ovarian cancer cells [119]. These studies suggest that WDR5 can promote tumorigenesis by integrating reading of different histone modifications with writing of the H3K4 methyl mark.
WDR5 expression is promoted by N-MYC oncogene in neuroblastoma cells, and elevated WDR5 levels in neuroblastoma specimens are associated with poor survival. WDR5 interacts with N-MYC at key tumorigenic genes including MDM2, and drives their H3K4 methylation and activation. Depletion or pharmacological suppression of WDR5 inhibits N-MYC target gene expression and neuroblastoma cell growth [120]. WDR5 also directly binds to c-MYC oncoprotein via the WIN site, and mutations on MYC that disrupt this interaction attenuate the ability of MYC in binding to most of its chromosomal targets, promoting formation of iPSCs, and driving tumorigenesis [66]. These studies establish that WDR5 is a key cofactor for MYC oncoproteins in transcriptional activation for tumorigenesis and a novel therapeutic target.
One important mechanism by which WDR5 sustains cancer is likely to be mitigating genotoxic stress. WDR5 and other SET1/MLL complex subunits are critical for pancreatic cancer cell proliferation and protect cancer cells from replicative stress and DNA damage [121]. Similarly, WDR5 depletion or inhibition induces DNA damage response in colon cancer cells, which overexpress WDR5 [122]. Moreover, WDR5 confers resistance to genotoxic tress on ovarian cancer cells in vivo by promoting the expression of genes in the glutathione metabolic pathway [119]. WDR5 also regulates DNA repair gene expression and its loss leads to increase in DNA damage response in cells undergoing reprograming to iPSCs [67].
In addition to WDR5, other SET1/MLL complex components are also involved in handling genotoxic stress in normal and cancer cells. DPY30 deletion in HSPCs results in increased DNA damage and metabolic stress [76]. SET1A promotes DNA repair gene expression and genome integrity in HSCs [78], but uses a novel cyclin K-interacting domain to regulate DNA damage response in leukemias [123]. MLL4 regulates antioxidant transcriptional programs, and loss of MLL4 leads to increased DNA damage and oxidative stress on cells, leading to cell differentiation [81]. MLL4 also protects genome integrity by resolving transcription stress through implementing co-transcriptional H3K4 methylation [124]. Moreover, H3K4 methylation may be directly involved in guarding genome integrity at the damaged sites including stressed replication sites [125, 126]. Therefore, a general role of SET1/MLL complexes in protecting against the oxidative and genotoxic stress is emerging. This probably constitutes a major mechanism by which cancer cells are particularly dependent on the SET1/MLL complex components to initiate or sustain the cancerous state, which is usually associated with increased genotoxic and metabolic stress.
4.1.2. RBBP5 in cancer
RBBP5 expression is upregulated in glioma samples and correlates with the pathology grades [127]. High RBBP5 expression level was also found in hepatocellular carcinoma and significantly associated with poor prognosis [128]. Both of these studies also show that RBBP5 knockdown inhibits cancer cell proliferation and induces apoptosis. Another study shows an important role of RBBP5 in promoting the persistence of cancer stem cells in hostile microenvironments [129]. Glioblastoma cancer stem cells downregulate the expression of TLR4, a member of the Toll-like receptor family, to allow them to evade innate immune suppression. As TLR4 suppresses the expression of RBBP5 via activation of the TBK1 kinase signaling pathway, RBBP5 expression is elevated in glioblastoma cancer stem cells to promote H3K4me3 and expression of key pluripotency transcription factors [129]. RBBP5 depletion inhibits self-renewal and tumorigenicity of glioblastoma cancer stem cells [129], providing a potential therapeutic opportunity.
4.1.3. ASH2L in cancer
ASH2L is overexpressed at the protein level in a broad range of human cancers and its knockdown inhibits the proliferation of cancer cells [130]. Strikingly, ASH2L cooperates with HRAS to transform primary rat embryonic fibroblasts into cells capable of forming rapidly growing and poorly differentiated tumors, suggesting that ASH2L functions as a oncoprotein. In line with the direct binding of ASH2L and MYC [131], ASH2L is required for transformation of primary rat embryonic fibroblasts by MYC and HRAS oncogenes, but not for that by either the E1a-HRAS or mutant p53-HRAS combination [130]. Consistent with the oncogenic role of ASH2L, a lower level of ASH2L protein appears to be beneficial to patients with acute myeloid leukemia [132].
4.1.4. DPY30 in cancer
DPY30 is required for the growth and clonogenicity of MLL-rearranged leukemia cell [33]. DPY30 expression is upregulated in gastric cancer cell lines and patient tissues. DPY30 knockdown inhibits proliferation, migration, and invasion of gastric cancer cells, whereas its overexpression has the opposite effects [133]. DPY30 expression is high in epithelial ovarian cancer while undetectable in normal ovarian tissues and very low in benign ovarian epithelial tumor tissues, and associated with clinicopathological variables. High DPY30 expression is significantly associated with poor prognosis of patients with epithelial ovarian cancer. DPY30 knockdown inhibits growth, migration, and invasion of epithelial ovarian cancer cell lines [134]. DPY30 thus plays an important role of in promoting these two types of cancer.
What drives the frequent upregulation of the core subunits in a diverse range of cancers? N-MYC promotes WDR5 expression in neuroblastoma cells [120]. c-MYC binds to and drives the expression of all four core subunits, but not most of the catalytic subunits, in different cell lines, human Burkitt lymphoma samples with MYC/Ig translocations, and Eμ-Myc pretumor B cells [62, 95]. This suggests functional roles of the core subunits in MYC-driven cancers.
At the molecular level, DPY30 is important for the activity of the MYC oncoprotein in binding to its genomic targets. A 50% reduction in DPY30 expression significantly suppresses MYC-driven lymphomagenesis without affecting normal mouse physiology including blood homeostasis and lifespan. While the full-level of DPY30 activity is dispensable for normal cells, it is critically required for combatting oncogene-induced apoptotic stress and MYC and HRAS-mediated transformation of primary mouse embryonic fibroblasts. Moreover, very much like ASH2L [130], the DPY30 and HRAS combination is able to transform mouse embryonic fibroblasts, suggesting that DPY30 and ASH2L can both function as oncoproteins. Therefore, the important oncogene c-MYC has evolved to hijack a major epigenetic pathway to promote tumorigenesis in the blood system. While this might be a clever strategy for tumor cells, it also creates a basis for “epigenetic vulnerability’, which may be exploited for therapeutics.
4.2. Role in other diseases
The extensive roles of the core subunits in physiology suggests that their alterations will likely cause many diseases, but the essentiality of these proteins suggests that major genetic alterations would not be tolerated and thus not commonly found in patients. Though not as prevalently reported as in cancer, genetic alterations of the SET1/MLL complex components (including the cores) are associated with certain developmental disorders as reviewed below.
De novo mutations in the writers, readers, and erasers of H3K4 methylation, including frameshift mutations of KMT2D/MLL4 and missense mutations of WDR5, are associated with congenital heart disease, often with neurodevelopmental anomaly [135, 136]. This strongly suggests the importance of the tight regulation of the H3K4 methylation state in development. The WDR5 (K7Q) mutation was found in a patient with congenital heart disease, neurodevelopmental anomaly, and also a mild heterotaxy phenotype of a right aortic arch rather than a normal left one [135]. WDR5 (K7Q) was later shown to be a loss-of-function allele in a Drosophila-based functional system [137] and also in left-right patterning of developing Xenopus [73].
SET1/MLL complexes are strongly associated with neurodevelopmental disorders. Mutations in MLL1 are associated with Wiedemann-Steiner syndrome [138]. Rare loss of function variants found in SET1A are associated with schizophrenia and developmental disorders [139]. Mutations in MLL4 are the more frequent genetic cause of Kabuki syndrome [140]. Genetic alterations in ASH2L are possibly associated with congenital brain malformations in humans [141]. Mutations in SPAST are the most frequent causal genetic alterations for hereditary spastic paraplegias, and often affect DPY30, a neighboring gene of SPAST [142, 143]. Genomic deletions of SPAST that extend into DPY30 lead to a significantly younger onset age in patients, likely due to impaired activity of the cytoplasmic DPY30 in regulating endosomal tabulation and endosome-to-Golgi traffic of mannose 6-phosphate receptors [47].
5. Determining the key activities of core subunits in development and disease
For the vast majority of studies showing the role of the core subunits in development and disease, the underlying mechanisms are usually proposed to be through promotion of H3K4 methylation and thus the expression of relevant target genes. Several different modes of target regulation by the cores are summarized in Figure 3. These studies typically involve characterizations to show that the identified targets have functional impact or are known to have a role in the process of interest, and the key targets are bound by the subunit and alter expression and H3K4 methylation upon perturbation of the subunit expression. In addition, the relationship with H3K4 methylation can be strengthened by addressing two different layers of dependence: Is the effect dependent on association with the other cores as well as the whole SET1/MLL complexes? Is the activity dependent on the SET domain and H3K4 methylation activity of the complexes?
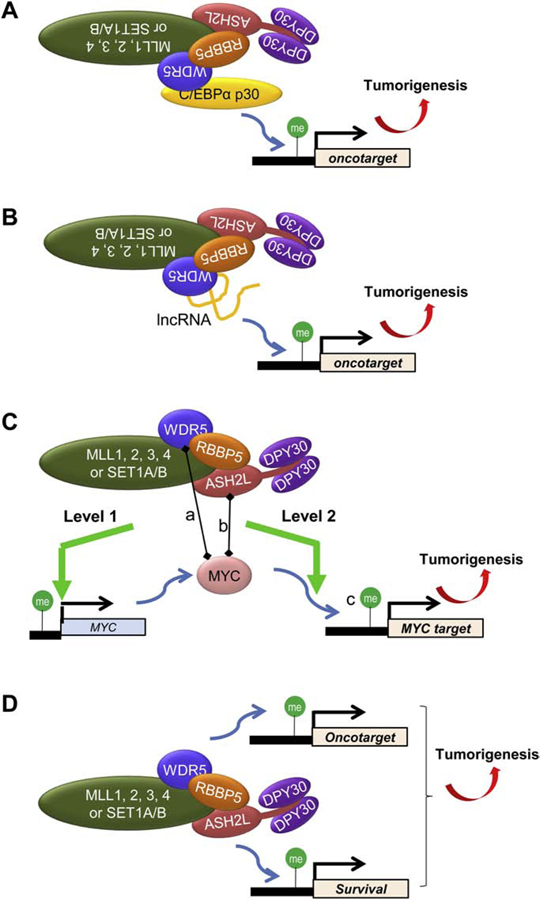
Figure 3. Various modes of target gene regulation by SET1/MLL complexes in tumorigenesis.
(A) Oncoprotein recruits SET1/MLL complexes to its oncogenic targets via physical interaction with WRAD.
(B) LncRNA recruits SET1/MLL complexes via WRAD to its oncogenic targets. An existing question is how WDR5 can bind to both lncRNAs and RBBP5 through the same site.
(C) WRAD and/or SET1/MLL complexes facilitate the binding of oncoprotein to its target genes. Shown here are two levels of regulation of MYC by the complexes. Level 1, the complexes directly promote the expression of MYC itself. Level 2, the core subunits are important for efficient recruitment of MYC to its genomic targets on chromatin. This level of regulation is likely contributed by three related but not identical molecular mechanisms: a. direct interaction of WDR5 and MYC, possibly independent of the SET1/MLL complexes, is important for MYC recruitment to targets; b. direct interaction of ASH2L and MYC; c. DPY30-associated activity lays out the accessible local chromatin environment for MYC binding.
(D) SET1/MLL complexes can support tumorigenesis without interaction with oncoproteins, but rather through regulating expression of key survival genes, including those regulating genotoxic stress, which is elevated in cancer.
However, it is extremely difficult to unambiguously demonstrate such a linear causality, due to multiple level of complexities: (1) Most catalytic and non-catalytic subunits of the SET1/MLL complexes have molecular activities unrelated to H3K4 methylation. (2) Changes of H3K4 methylation in cells are not necessarily associated with transcriptional changes or changes in the expected direction. (3) It is not known if the cores have activities that are unrelated to H3K4 methylation but still require them to be associated with other cores or the whole SET1/MLL complexes. In fact, it has been reported that WRAD in complex with MLL1 regulate cell cycle progression in a SET-domain-independent manner [144]. (4) Even the effect is through the H3K4 methylation activity of the associated SET1/MLL complexes, it is likely that the observed effects are indirect results of methylation and expression changes of other genes.
On the other hand, when the gene of interest does not show a change of H3K4 methylation, it is relatively safe to conclude that the effect is likely not through methylation activity. For example, DPY30 appears to be required for efficient genomic binding of Oct4 during cell reprogramming, but a role of H3K4 methylation in OCT4 binding is not strongly supported as the affected OCT4 binding was not accompanied by the expected changes of H3K4 methylation [62]. However, methods in determining H3K4 methylation can affect how thorough we gain the methylation information. Unaffected methylation determined by ChIP-qPCR on specific genomic sites may miss truly affected sites. ChIP-seq reveals effects on all sites, but there is a possibility that we may miss potentially important information such as peak width, which may be linked to transcription stability with important biological roles [48–50].
How do we identify new molecular activities of the core subunits responsible for the observed phenotypes? This often requires extensive studies of structure-function relationship by mapping the minimal regions on protein that are critical for the observed function. Further biochemical characterizations of the minimal regions may provide clues to the associated activities. This is well exemplified in the identification of a novel molecular activity of SET1A in supporting MLL-rearranged leukemogenesis [123]. If a key region on the cores is similarly involved in different processes due to its capacity in binding to different molecules, finer dissections of individual amino acid residues may be necessary. This scenario probably applies to the two major sites (Win and WBM sites) on WDR5 and the dimerization domain of DPY30. Though the WBM site on WDR5 is shared in binding to both RBBP5 and a group of lncRNAs, the relatively selective requirement of Phe266 for binding to lncRNAs compared to MLL complex made it possible to identify the role of lncRNA binding for WDR5 in pluripotency maintenance [61]. Moreover, novel localization can also provide clues to new activities. WDR5 was reported to play a critical role in left-right patterning in development through both chromatin-dependent and –independent mechanisms, the latter likely involving the localization of WDR5 at the bases of cilia instead on chromatin. In particular, WDR5 (K7Q) mutation is in a region not essential for regulating H3K4 methylation, and is thus likely to affect heart development through a H3K4-independent mechanism [73]. All these different approaches will together help elucidate the different activities of the cores.
6. Therapeutic strategies based on core subunits
Among the reported strategies targeting the SET1/MLL complexes for potential cancer treatment, one targets the interaction of MLL1 with MENIN [145–147], a subunit of MLL1/2 complexes, and the rest focus on the interactions between a core with a catalytic subunit or another core. Some of the earlier works on targeting the SET1/MLL complexes have been previously reviewed [148].
6.1. Targeting WDR5
The extensive studies of WDR5 in disease have attracted several major series of efforts to develop its inhibitors, all targeting its WIN site and thus blocking the interaction with MLL1. MM-101, 102, and 401, a series of peptidomimetics [13, 149, 150] have been developed to achieve high affinity (~1 nM for MM-401) with WDR5. MM-401 selectively inhibits the methylation activity of MLL1 in vitro, and preferentially suppresses MLL-rearranged leukemia growth over normal hematopoietic cells [13]. It is noteworthy that this inhibitor is also vital in discovery of the striking role of MLL1 as a crucial regulator of the epiblast versus naive pluripotency [70], demonstrating a unique advantage of the pharmacologic approach in basic discoveries due to the ease in dose and temporal control. Another series of work generated WDR5–0101, −0102, −0103 [151], and finally OICR-9429 [108], which has been used in a number of studies [108, 119, 122, 152]. OICR-9429 inhibits growth of human acute myeloid leukemia driven by an C/EBPα isoform that binds to WDR5 [108], and cancer driven by the gain-of-function p53 mutants [152]. More recently, a group of compounds that bind to the WDR5 WIN site with very high affinity (picomolar) have been developed by fragment-based screening and structure-based design. They potently inhibit the histone methylation activity of MLL1 in vitro, and preferentially suppress growth of MLL-rearranged leukemia cells. Rather than inhibiting H3K4 methylation, they bind to the WDR5 WIN site and evict WDR5 off chromatin targets, most prominently at ribosomal protein genes. This results in imbalanced production of ribosome subunits and nucleolar stress, which in turn activates p53-dependent inhibitor of cell growth [153, 154].
6.2. Targeting DPY30
A recent proof-of-principle study exploits the epigenetic vulnerability of MYC-driven cancer [95] and targets DPY30 for pharmacological intervention of cancer cell growth [155]. The engineered cell-permeable peptides derived from the ASH2L C-terminal α helix bind to DPY30, interfere with the activity of DPY30 in binding to ASH2L and enhancing H3K4 methylation. They specifically inhibit growth of MLL-rearranged leukemia cells and MYC-hyperactivated Burkitt lymphoma cells without affecting growth of normal human HSPCs, and confer hypersensitivity to other epigenetic inhibitors [155]. These results provide foundation for further development of agents with better pharmacological properties to target the DPY30-ASH2L interface for cancer treatment.
The studies reviewed above suggest the potential of targeting a well-structured modulator (WDR5 or DPY30) that conveys non-oncogene addiction in combating cancers driven by an oncoprotein (e.g. MYC) not amenable to drug targeting. Moreover, as DPY30 and WDR5 are important for genomic binding of MYC oncoprotein (Figure 3C), targeting them (and potentially other cores) may offer a novel option for treating cancers that have evolved to evade repression of MYC gene expression following other approaches, such as inhibition of BET family proteins [156–160].
6.3. Considerations for targeting the core subunits
Targeting the core subunits for disease treatment is supported by the considerations below. (1) In contrast to the different roles of the catalytic subunits in cancer, all cores show a striking unanimity in promoting cancer. (2) While targeting each catalytic subunit offers more specificity, their partial redundancy may cause insufficient efficacy when only one is targeted. (3) Though the SET domain is well-structured and thus “druggable”, its similarity among all KMT2s [11] makes it challenging for specific targeting. (4) The key activities of some catalytic subunits in disease are independent of H3K4 methylation and not well defined. (5) The interactions of the core subunits involve well-defined structure and are thus likely to be amenable to small molecule perturbation.
On the other side, targeting the cores also has a few concerns. (1) Since loss-of-function mutations of the catalytic subunits, especially MLL3 and MLL4, are extensively associated with cancer, it needs to be taken into consideration if targeting the cores will affect the activity of MLL3/4 and promote cancer. However, as the activity of the wild type KMT2 allele (including MLL1 and MLL4) that is retained in KMT2-mutated cancers is often important for maintaining the viability of cancer cells [105], it is possible that targeting the cores will overall inhibit cancer. (2) The association of the cores with all six catalytic subunits and to many other factors (for WDR5 and DPY30) makes it difficult to identify the specific mechanisms. Although, the same could be argued for the KMT2s due to their complex activities as well. It is not clear how the available inhibitors of WDR5 affect WDR5 interacting with other proteins, many of which bind the same WIN site that the inhibitors occupy. The DPY30-inhibitory peptides are most likely inhibitory for interaction of DPY30 with not just ASH2L, but also NURF complex, AKAP95, and BIG1. This may partially explain their specific and significant effects on cancer cell growth, but only modest effect on cellular H3K4me3 and murky effect on gene expression [155].
Another important question regarding targeting the cores is the potential therapeutic windows, given the essentiality of these proteins. WDR5 inhibitors have shown selectivity on MLL-fusion-transformed cells [13]. A therapeutic window for targeting DPY30 is suggested by the following: (1) Dpy30+/− cells show normal growth, but remarkably resistant to oncogenic transformation [95]. (2) It is perhaps helpful to compare DPY30 with BRD4, a major target of the BET family inhibitors considered highly promising in cancer therapy. Constitutive and whole body inactivation of one allele of ASH2L [71] or DPY30 [95] in mouse has no obviate phenotype. Dpy30+/− mice have normal blood profiles and normal (if not improved) lifespan [95], whereas Brd4+/− mice suffer from prenatal and postnatal growth defects, anatomical defects in multiple organs, and postnatal lethality [161]. (3) H3K4me3 at genes associated with fundamental pathways is not profoundly affected upon Dpy30 KO [34]. (4) The DPY30-inhibitory peptides suppress growth of certain blood cancer cells but not normal human hematopoietic progenitor cells [155].
7. Conclusions
The core subunits of the SET1/MLL complexes critically regulate H3K4 methylation at biochemical and genomic levels, and also serve as a crucial link of other macromolecule activities to regulation of DNA processes, especially transcription. The dynamic assembly of the SET1/MLL complexes and other molecules associated with the different subunits may offer a very diverse regulation of coordinated activities, and warrants further studies in the future.
It is clear that all core subunits of the SET1/MLL complexes play important roles in diverse aspects of physiology and development, but it remains difficult to pinpoint their specific activities that underlie these roles in the different processes. Further studies will need to tease apart their activities in the context of different binding partners. Regardless of the uncertainty of the underlying molecular activities, the large amount of literature point to a consistent requirement of the core subunits in cancer development. Increasing evidence suggests the excessive reservoir of at least some of the core subunits for normal animal physiology, implying a potential therapeutic window. Equipped with the increasing structural and functional knowledge of these core subunits associated with the SET1/MLL complexes and other proteins/RNAs, we see a promising future in targeting these subunits for treatment of disease, especially cancer.
Highlights.
The core subunits of the SET1/MLL complexes are critical for H3K4 methylation activity
The core subunits may have high order assembly for functional coordination
The core subunits play important roles in development and disease
The core subunits unanimously promote cancer and can be targeted
Acknowledgements
The author thanks Dr. Kushani Shah for critical reading of the manuscript. Work in author’s laboratory was supported by the NIH grant R01DK105531, American Society of Hematology Scholar Award, American Cancer Society Research Scholar Award (128609-RSG-15-166-01-DMC), and the Leukemia and Lymphoma Society Scholar Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The author declare that no potential conflicts of interest were disclosed.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Shilatifard A, Molecular implementation and physiological roles for histone H3 lysine 4 (H3K4) methylation, Curr Opin Cell Biol, 20 (2008) 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rickels R, Shilatifard A, Enhancer Logic and Mechanics in Development and Disease, Trends in cell biology, 28 (2018) 608–630. [DOI] [PubMed] [Google Scholar]
- [3].Ruthenburg AJ, Allis CD, Wysocka J, Methylation of lysine 4 on histone H3: intricacy of writing and reading a single epigenetic mark, Mol Cell, 25 (2007) 15–30. [DOI] [PubMed] [Google Scholar]
- [4].Shilatifard A, The COMPASS family of histone H3K4 methylases: mechanisms of regulation in development and disease pathogenesis, Annu Rev Biochem, 81 (2012) 65–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ernst P, Vakoc CR, WRAD: enabler of the SET1-family of H3K4 methyltransferases, Brief Funct Genomics, 11 (2012) 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kim J, Kim JA, McGinty RK, Nguyen UT, Muir TW, Allis CD, Roeder RG, The n-SET domain of Set1 regulates H2B ubiquitylation-dependent H3K4 methylation, Mol Cell, 49 (2013) 1121–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Thornton JL, Westfield GH, Takahashi YH, Cook M, Gao X, Woodfin AR, Lee JS, Morgan MA, Jackson J, Smith ER, Couture JF, Skiniotis G, Shilatifard A, Context dependency of Set1/COMPASS-mediated histone H3 Lys4 trimethylation, Genes Dev, 28 (2014) 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jiang H, Lu X, Shimada M, Dou Y, Tang Z, Roeder RG, Regulation of transcription by the MLL2 complex and MLL complex-associated AKAP95, Nat Struct Mol Biol, 20 (2013) 1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dou Y, Milne TA, Tackett AJ, Smith ER, Fukuda A, Wysocka J, Allis CD, Chait BT, Hess JL, Roeder RG, Physical association and coordinate function of the H3 K4 methyltransferase MLL1 and the H4 K16 acetyltransferase MOF, Cell, 121 (2005) 873–885. [DOI] [PubMed] [Google Scholar]
- [10].Bochynska A, Luscher-Firzlaff J, Luscher B, Modes of Interaction of KMT2 Histone H3 Lysine 4 Methyltransferase/COMPASS Complexes with Chromatin, Cells, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li Y, Han J, Zhang Y, Cao F, Liu Z, Li S, Wu J, Hu C, Wang Y, Shuai J, Chen J, Cao L, Li D, Shi P, Tian C, Zhang J, Dou Y, Li G, Chen Y, Lei M, Structural basis for activity regulation of MLL family methyltransferases, Nature, 530 (2016) 447–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Han J, Li T, Li Y, Li M, Wang X, Peng C, Su C, Li N, Li Y, Xu Y, Chen Y, The internal interaction in RBBP5 regulates assembly and activity of MLL1 methyltransferase complex, Nucleic acids research, 47 (2019) 10426–10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cao F, Townsend EC, Karatas H, Xu J, Li L, Lee S, Liu L, Chen Y, Ouillette P, Zhu J, Hess JL, Atadja P, Lei M, Qin ZS, Malek S, Wang S, Dou Y, Targeting MLL1 H3K4 methyltransferase activity in mixed-lineage leukemia, Molecular cell, 53 (2014) 247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang P, Lee H, Brunzelle JS, Couture JF, The plasticity of WDR5 peptide-binding cleft enables the binding of the SET1 family of histone methyltransferases, Nucleic acids research, 40 (2012) 4237–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Alicea-Velazquez NL, Shinsky SA, Loh DM, Lee JH, Skalnik DG, Cosgrove MS, Targeted Disruption of the Interaction between WD-40 Repeat Protein 5 (WDR5) and Mixed Lineage Leukemia (MLL)/SET1 Family Proteins Specifically Inhibits MLL1 and SETd1A Methyltransferase Complexes, The Journal of biological chemistry, 291 (2016) 22357–22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shinsky SA, Cosgrove MS, Unique Role of the WD-40 Repeat Protein 5 (WDR5) Subunit within the Mixed Lineage Leukemia 3 (MLL3) Histone Methyltransferase Complex, The Journal of biological chemistry, 290 (2015) 25819–25833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xue H, Yao T, Cao M, Zhu G, Li Y, Yuan G, Chen Y, Lei M, Huang J, Structural basis of nucleosome recognition and modification by MLL methyltransferases, Nature, 573 (2019) 445–449. [DOI] [PubMed] [Google Scholar]
- [18].Dou Y, Milne TA, Ruthenburg AJ, Lee S, Lee JW, Verdine GL, Allis CD, Roeder RG, Regulation of MLL1 H3K4 methyltransferase activity by its core components, Nat Struct Mol Biol, 13 (2006) 713–719. [DOI] [PubMed] [Google Scholar]
- [19].Wysocka J, Swigut T, Milne TA, Dou Y, Zhang X, Burlingame AL, Roeder RG, Brivanlou AH, Allis CD, WDR5 associates with histone H3 methylated at K4 and is essential for H3 K4 methylation and vertebrate development, Cell, 121 (2005) 859–872. [DOI] [PubMed] [Google Scholar]
- [20].Mishra BP, Zaffuto KM, Artinger EL, Org T, Mikkola HK, Cheng C, Djabali M, Ernst P, The histone methyltransferase activity of MLL1 is dispensable for hematopoiesis and leukemogenesis, Cell reports, 7 (2014) 1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Guarnaccia AD, Tansey WP, Moonlighting with WDR5: A Cellular Multitasker, Journal of clinical medicine, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Haddad JF, Yang Y, Takahashi YH, Joshi M, Chaudhary N, Woodfin AR, Benyoucef A, Yeung S, Brunzelle JS, Skiniotis G, Brand M, Shilatifard A, Couture JF, Structural Analysis of the Ash2L/Dpy-30 Complex Reveals a Heterogeneity in H3K4 Methylation, Structure (London, England : 1993), 26 (2018) 1594–1603.e1594. [DOI] [PubMed] [Google Scholar]
- [23].Hsu PL, Li H, Lau HT, Leonen C, Dhall A, Ong SE, Chatterjee C, Zheng N, Crystal Structure of the COMPASS H3K4 Methyltransferase Catalytic Module, Cell, 174 (2018) 1106–1116.e1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Qu Q, Takahashi YH, Yang Y, Hu H, Zhang Y, Brunzelle JS, Couture JF, Shilatifard A, Skiniotis G, Structure and Conformational Dynamics of a COMPASS Histone H3K4 Methyltransferase Complex, Cell, 174 (2018) 1117–1126.e1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dong X, Peng Y, Xu F, He X, Wang F, Peng X, Qiang B, Yuan J, Rao Z, Characterization and crystallization of human DPY-30-like protein, an essential component of dosage compensation complex, Biochim Biophys Acta, 1753 (2005) 257–262. [DOI] [PubMed] [Google Scholar]
- [26].Zhang H, Li M, Gao Y, Jia C, Pan X, Cao P, Zhao X, Zhang J, Chang W, Structural implications of Dpy30 oligomerization for MLL/SET1 COMPASS H3K4 trimethylation, Protein & cell, 6 (2015) 147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang X, Lou Z, Dong X, Yang W, Peng Y, Yin B, Gong Y, Yuan J, Zhou W, Bartlam M, Peng X, Rao Z, Crystal structure of the C-terminal domain of human DPY-30-like protein: A component of the histone methyltransferase complex, J Mol Biol, 390 (2009) 530–537. [DOI] [PubMed] [Google Scholar]
- [28].Tremblay V, Zhang P, Chaturvedi CP, Thornton J, Brunzelle JS, Skiniotis G, Shilatifard A, Brand M, Couture JF, Molecular Basis for DPY-30 Association to COMPASS-like and NURF Complexes, Structure (London, England : 1993), 22 (2014) 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen Y, Cao F, Wan B, Dou Y, Lei M, Structure of the SPRY domain of human Ash2L and its interactions with RbBP5 and DPY30, Cell research, 22 (2012) 598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].South PF, Fingerman IM, Mersman DP, Du HN, Briggs SD, A conserved interaction between the SDI domain of Bre2 and the Dpy-30 domain of Sdc1 is required for histone methylation and gene expression, The Journal of biological chemistry, 285 (2010) 595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Choudhury R, Singh S, Arumugam S, Roguev A, Stewart AF, The Set1 complex is dimeric and acts with Jhd2 demethylation to convey symmetrical H3K4 trimethylation, Genes & development, 33 (2019) 550–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dehe PM, Dichtl B, Schaft D, Roguev A, Pamblanco M, Lebrun R, Rodriguez-Gil A, Mkandawire M, Landsberg K, Shevchenko A, Rosaleny LE, Tordera V, Chavez S, Stewart AF, Geli V, Protein interactions within the Set1 complex and their roles in the regulation of histone 3 lysine 4 methylation, J Biol Chem, 281 (2006) 35404–35412. [DOI] [PubMed] [Google Scholar]
- [33].Yang Z, Augustin J, Chang C, Hu J, Shah K, Chang CW, Townes T, Jiang H, The DPY30 subunit in SET1/MLL complexes regulates the proliferation and differentiation of hematopoietic progenitor cells, Blood, 124 (2014) 2025–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yang Z, Shah K, Khodadadi-Jamayran A, Jiang H, Dpy30 is critical for maintaining the identity and function of adult hematopoietic stem cells, The Journal of experimental medicine, 213 (2016) 2349–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sze CC, Shilatifard A, MLL3/MLL4/COMPASS Family on Epigenetic Regulation of Enhancer Function and Cancer, Cold Spring Harbor perspectives in medicine, 6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Jiang H, Shukla A, Wang X, Chen WY, Bernstein BE, Roeder RG, Role for Dpy-30 in ES Cell-Fate Specification by Regulation of H3K4 Methylation within Bivalent Domains, Cell, 144 (2011) 513–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tsai PH, Chien Y, Wang ML, Hsu CH, Laurent B, Chou SJ, Chang WC, Chien CS, Li HY, Lee HC, Huo TI, Hung JH, Chen CH, Chiou SH, Ash2l interacts with Oct4-stemness circuitry to promote super-enhancer-driven pluripotency network, Nucleic acids research, (2019). [DOI] [PMC free article] [PubMed]
- [38].van Nuland R, Smits AH, Pallaki P, Jansen PW, Vermeulen M, Timmers HT, Quantitative dissection and stoichiometry determination of the human SET1/MLL histone methyltransferase complexes, Molecular and cellular biology, 33 (2013) 2067–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Xu Z, Gong Q, Xia B, Groves B, Zimmermann M, Mugler C, Mu D, Matsumoto B, Seaman M, Ma D, A role of histone H3 lysine 4 methyltransferase components in endosomal trafficking, J Cell Biol, 186 (2009) 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bieluszewska A, Weglewska M, Bieluszewski T, Lesniewicz K, Poreba E, PKA-binding domain of AKAP8 is essential for direct interaction with DPY30 protein, The FEBS journal, 285 (2018) 947–964. [DOI] [PubMed] [Google Scholar]
- [41].Lopez-Soop G, Ronningen T, Rogala A, Richartz N, Blomhoff HK, Thiede B, Collas P, Kuntziger T, AKAP95 interacts with nucleoporin TPR in mitosis and is important for the spindle assembly checkpoint, Cell Cycle, 16 (2017) 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Li W, Hu J, Shi B, Jiang H, Protein condensates with appropriate material properties regulate tumorigenesis, BioRxiv, 536839 (2019) [Preprint]. January 31, 2019. Available from:
- [43].Hu J, Khodadadi-Jamayran A, Mao M, Shah K, Yang Z, Nasim MT, Wang Z, Jiang H, AKAP95 regulates splicing through scaffolding RNAs and RNA processing factors, Nature communications, 7 (2016) 13347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Migliori V, Muller J, Phalke S, Low D, Bezzi M, Mok WC, Sahu SK, Gunaratne J, Capasso P, Bassi C, Cecatiello V, De Marco A, Blackstock W, Kuznetsov V, Amati B, Mapelli M, Guccione E, Symmetric dimethylation of H3R2 is a newly identified histone mark that supports euchromatin maintenance, Nature structural & molecular biology, 19 (2012) 136–144. [DOI] [PubMed] [Google Scholar]
- [45].Ali A, Veeranki SN, Chinchole A, Tyagi S, MLL/WDR5 Complex Regulates Kif2A Localization to Ensure Chromosome Congression and Proper Spindle Assembly during Mitosis, Developmental cell, 41 (2017) 605–622.e607. [DOI] [PubMed] [Google Scholar]
- [46].Wysocka J, Swigut T, Xiao H, Milne TA, Kwon SY, Landry J, Kauer M, Tackett AJ, Chait BT, Badenhorst P, Wu C, Allis CD, A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling, Nature, 442 (2006) 86–90. [DOI] [PubMed] [Google Scholar]
- [47].Newton T, Allison R, Edgar JR, Lumb JH, Rodger CE, Manna PT, Rizo T, Kohl Z, Nygren AOH, Arning L, Schule R, Depienne C, Goldberg L, Frahm C, Stevanin G, Durr A, Schols L, Winner B, Beetz C, Reid E, Mechanistic basis of an epistatic interaction reducing age at onset in hereditary spastic paraplegia, Brain : a journal of neurology, 141 (2018) 1286–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Balazsi G, van Oudenaarden A, Collins JJ, Cellular decision making and biological noise: from microbes to mammals, Cell, 144 (2011) 910–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Raj A, Rifkin SA, Andersen E, van Oudenaarden A, Variability in gene expression underlies incomplete penetrance, Nature, 463 (2010) 913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Benayoun BA, Pollina EA, Ucar D, Mahmoudi S, Karra K, Wong ED, Devarajan K, Daugherty AC, Kundaje AB, Mancini E, Hitz BC, Gupta R, Rando TA, Baker JC, Snyder MP, Cherry JM, Brunet A, H3K4me3 breadth is linked to cell identity and transcriptional consistency, Cell, 158 (2014) 673–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Campbell SA, McDonald CL, Krentz NAJ, Lynn FC, Hoffman BG, TrxG Complex Catalytic and Non-catalytic Activity Play Distinct Roles in Pancreas Progenitor Specification and Differentiation, Cell reports, 28 (2019) 1830–1844.e1836. [DOI] [PubMed] [Google Scholar]
- [52].Adamson AL, Shearn A, Molecular genetic analysis of Drosophila ash2, a member of the trithorax group required for imaginal disc pattern formation, Genetics, 144 (1996) 621–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].LaJeunesse D, Shearn A, Trans-regulation of thoracic homeotic selector genes of the Antennapedia and bithorax complexes by the trithorax group genes: absent, small, and homeotic discs 1 and 2, Mech Dev, 53 (1995) 123–139. [DOI] [PubMed] [Google Scholar]
- [54].Hsu DR, Meyer BJ, The dpy-30 gene encodes an essential component of the Caenorhabditis elegans dosage compensation machinery, Genetics, 137 (1994) 999–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hsu DR, Chuang PT, Meyer BJ, DPY-30, a nuclear protein essential early in embryogenesis for Caenorhabditis elegans dosage compensation, Development, 121 (1995) 3323–3334. [DOI] [PubMed] [Google Scholar]
- [56].Gori F, Divieti P, Demay MB, Cloning and characterization of a novel WD-40 repeat protein that dramatically accelerates osteoblastic differentiation, The Journal of biological chemistry, 276 (2001) 46515–46522. [DOI] [PubMed] [Google Scholar]
- [57].Gori F, Friedman LG, Demay MB, Wdr5, a WD-40 protein, regulates osteoblast differentiation during embryonic bone development, Developmental biology, 295 (2006) 498–506. [DOI] [PubMed] [Google Scholar]
- [58].Zhu ED, Demay MB, Gori F, Wdr5 is essential for osteoblast differentiation, The Journal of biological chemistry, 283 (2008) 7361–7367. [DOI] [PubMed] [Google Scholar]
- [59].Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES, A bivalent chromatin structure marks key developmental genes in embryonic stem cells, Cell, 125 (2006) 315–326. [DOI] [PubMed] [Google Scholar]
- [60].Ang YS, Tsai SY, Lee DF, Monk J, Su J, Ratnakumar K, Ding J, Ge Y, Darr H, Chang B, Wang J, Rendl M, Bernstein E, Schaniel C, Lemischka IR, Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network, Cell, 145 (2011) 183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yang YW, Flynn RA, Chen Y, Qu K, Wan B, Wang KC, Lei M, Chang HY, Essential role of lncRNA binding for WDR5 maintenance of active chromatin and embryonic stem cell pluripotency, eLife, 3 (2014) e02046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Yang Z, Augustin J, Hu J, Jiang H, Physical Interactions and Functional Coordination between the Core Subunits of Set1/Mll Complexes and the Reprogramming Factors, PloS one, 10 (2015) e0145336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wan M, Liang J, Xiong Y, Shi F, Zhang Y, Lu W, He Q, Yang D, Chen R, Liu D, Barton M, Songyang Z, The trithorax group protein Ash2l is essential for pluripotency and maintaining open chromatin in embryonic stem cells, The Journal of biological chemistry, 288 (2013) 5039–5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chen Y, Wan B, Wang KC, Cao F, Yang Y, Protacio A, Dou Y, Chang HY, Lei M, Crystal structure of the N-terminal region of human Ash2L shows a winged-helix motif involved in DNA binding, EMBO Rep, 12 (2011) 797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Sarvan S, Avdic V, Tremblay V, Chaturvedi CP, Zhang P, Lanouette S, Blais A, Brunzelle JS, Brand M, Couture JF, Crystal structure of the trithorax group protein ASH2L reveals a forkhead-like DNA binding domain, Nature structural & molecular biology, 18 (2011) 857–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Thomas LR, Wang Q, Grieb BC, Phan J, Foshage AM, Sun Q, Olejniczak ET, Clark T, Dey S, Lorey S, Alicie B, Howard GC, Cawthon B, Ess KC, Eischen CM, Zhao Z, Fesik SW, Tansey WP, Interaction with WDR5 promotes target gene recognition and tumorigenesis by MYC, Molecular cell, 58 (2015) 440–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Penalosa-Ruiz G, Bousgouni V, Gerlach JP, Waarlo S, van de Ven JV, Veenstra TE, Silva JCR, van Heeringen SJ, Bakal C, Mulder KW, Veenstra GJC, WDR5, BRCA1, and BARD1 Co-regulate the DNA Damage Response and Modulate the Mesenchymal-to-Epithelial Transition during Early Reprogramming, Stem cell reports, 12 (2019) 743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Li S, Xiao F, Zhang J, Sun X, Wang H, Zeng Y, Hu J, Tang F, Gu J, Zhao Y, Jin Y, Liao B, Disruption of OCT4 Ubiquitination Increases OCT4 Protein Stability and ASH2L-B-Mediated H3K4 Methylation Promoting Pluripotency Acquisition, Stem cell reports, 11 (2018) 973–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Bertero A, Madrigal P, Galli A, Hubner NC, Moreno I, Burks D, Brown S, Pedersen RA, Gaffney D, Mendjan S, Pauklin S, Vallier L, Activin/Nodal signaling and NANOG orchestrate human embryonic stem cell fate decisions by controlling the H3K4me3 chromatin mark, Genes & development, 29 (2015) 702–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhang H, Gayen S, Xiong J, Zhou B, Shanmugam AK, Sun Y, Karatas H, Liu L, Rao RC, Wang S, Nesvizhskii AI, Kalantry S, Dou Y, MLL1 Inhibition Reprograms Epiblast Stem Cells to Naive Pluripotency, Cell Stem Cell, 18 (2016) 481–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Stoller JZ, Huang L, Tan CC, Huang F, Zhou DD, Yang J, Gelb BD, Epstein JA, Ash2l interacts with Tbx1 and is required during early embryogenesis, Exp Biol Med (Maywood), 235 (2010) 569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Vilhais-Neto GC, Fournier M, Plassat JL, Sardiu ME, Saraf A, Garnier JM, Maruhashi M, Florens L, Washburn MP, Pourquie O, The WHHERE coactivator complex is required for retinoic acid-dependent regulation of embryonic symmetry, Nature communications, 8 (2017) 728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kulkarni SS, Khokha MK, WDR5 regulates left-right patterning via chromatin-dependent and -independent functions, Development (Cambridge, England), 145 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Guo X, Xu Y, Wang Z, Wu Y, Chen J, Wang G, Lu C, Jia W, Xi J, Zhu S, Jiapaer Z, Wan X, Liu Z, Gao S, Kang J, A Linc1405/Eomes Complex Promotes Cardiac Mesoderm Specification and Cardiogenesis, Cell stem cell, 22 (2018) 893–908.e896. [DOI] [PubMed] [Google Scholar]
- [75].Yue M, Ogawa A, Yamada N, Charles Richard JL, Barski A, Ogawa Y, Xist RNA repeat E is essential for ASH2L recruitment to the inactive X and regulates histone modifications and escape gene expression, PLoS genetics, 13 (2017) e1006890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Yang Z, Shah K, Khodadadi-Jamayran A, Jiang H, Control of Hematopoietic Stem and Progenitor Cell Function through Epigenetic Regulation of Energy Metabolism and Genome Integrity, Stem Cell Reports, 13 (2019) 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Luscher-Firzlaff J, Chatain N, Kuo CC, Braunschweig T, Bochynska A, Ullius A, Denecke B, Costa IG, Koschmieder S, Luscher B, Hematopoietic stem and progenitor cell proliferation and differentiation requires the trithorax protein Ash2l, Scientific reports, 9 (2019) 8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Arndt K, Kranz A, Fohgrub J, Jolly A, Bledau AS, Di Virgilio M, Lesche M, Dahl A, Hofer T, Stewart AF, Waskow C, SETD1A protects HSCs from activation-induced functional decline in vivo, Blood, 131 (2018) 1311–1324. [DOI] [PubMed] [Google Scholar]
- [79].Chun KT, Li B, Dobrota E, Tate C, Lee JH, Khan S, Haneline L, HogenEsch H, Skalnik DG, The epigenetic regulator CXXC finger protein 1 is essential for murine hematopoiesis, PloS one, 9 (2014) e113745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chen C, Liu Y, Rappaport AR, Kitzing T, Schultz N, Zhao Z, Shroff AS, Dickins RA, Vakoc CR, Bradner JE, Stock W, LeBeau MM, Shannon KM, Kogan S, Zuber J, Lowe SW, MLL3 Is a Haploinsufficient 7q Tumor Suppressor in Acute Myeloid Leukemia, Cancer cell, 25 (2014) 652–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Santos MA, Faryabi RB, Ergen AV, Day AM, Malhowski A, Canela A, Onozawa M, Lee JE, Callen E, Gutierrez-Martinez P, Chen HT, Wong N, Finkel N, Deshpande A, Sharrow S, Rossi DJ, Ito K, Ge K, Aplan PD, Armstrong SA, Nussenzweig A, DNA-damage-induced differentiation of leukaemic cells as an anti-cancer barrier, Nature, 514 (2014) 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lim DA, Huang YC, Swigut T, Mirick AL, Garcia-Verdugo JM, Wysocka J, Ernst P, Alvarez-Buylla A, Chromatin remodelling factor Mll1 is essential for neurogenesis from postnatal neural stem cells, Nature, 458 (2009) 529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Jung Y, Hsieh LS, Lee AM, Zhou Z, Coman D, Heath CJ, Hyder F, Mineur YS, Yuan Q, Goldman D, Bordey A, Picciotto MR, An epigenetic mechanism mediates developmental nicotine effects on neuronal structure and behavior, Nature neuroscience, 19 (2016) 905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Li L, Ruan X, Wen C, Chen P, Liu W, Zhu L, Xiang P, Zhang X, Wei Q, Hou L, Yin B, Yuan J, Qiang B, Shu P, Peng X, The COMPASS Family Protein ASH2L Mediates Corticogenesis via Transcriptional Regulation of Wnt Signaling, Cell reports, 28 (2019) 698–711.e695. [DOI] [PubMed] [Google Scholar]
- [85].Shah K, King GD, Jiang H, A chromatin modulator sustains self-renewal and enables differentiation of postnatal neural stem and progenitor cells, Journal of molecular cell biology, (2019). [DOI] [PMC free article] [PubMed]
- [86].Yang YJ, Baltus AE, Mathew RS, Murphy EA, Evrony GD, Gonzalez DM, Wang EP, Marshall-Walker CA, Barry BJ, Murn J, Tatarakis A, Mahajan MA, Samuels HH, Shi Y, Golden JA, Mahajnah M, Shenhav R, Walsh CA, Microcephaly gene links trithorax and REST/NRSF to control neural stem cell proliferation and differentiation, Cell, 151 (2012) 1097–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sen P, Shah PP, Nativio R, Berger SL, Epigenetic Mechanisms of Longevity and Aging, Cell, 166 (2016) 822–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Booth LN, Brunet A, The Aging Epigenome, Molecular cell, 62 (2016) 728–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Greer EL, Maures TJ, Hauswirth AG, Green EM, Leeman DS, Maro GS, Han S, Banko MR, Gozani O, Brunet A, Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans, Nature, 466 (2010) 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Han S, Schroeder EA, Silva-Garcia CG, Hebestreit K, Mair WB, Brunet A, Mono-unsaturated fatty acids link H3K4me3 modifiers to C. elegans lifespan, Nature, 544 (2017) 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Greer EL, Maures TJ, Ucar D, Hauswirth AG, Mancini E, Lim JP, Benayoun BA, Shi Y, Brunet A, Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans, Nature, 479 (2011) 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Feser J, Truong D, Das C, Carson JJ, Kieft J, Harkness T, Tyler JK, Elevated histone expression promotes life span extension, Molecular cell, 39 (2010) 724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Dang W, Steffen KK, Perry R, Dorsey JA, Johnson FB, Shilatifard A, Kaeberlein M, Kennedy BK, Berger SL, Histone H4 lysine 16 acetylation regulates cellular lifespan, Nature, 459 (2009) 802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Han Y, Han D, Yan Z, Boyd-Kirkup JD, Green CD, Khaitovich P, Han JD, Stress-associated H3K4 methylation accumulates during postnatal development and aging of rhesus macaque brain, Aging cell, 11 (2012) 1055–1064. [DOI] [PubMed] [Google Scholar]
- [95].Yang Z, Shah K, Busby T, Giles K, Khodadadi-Jamayran A, Li W, Jiang H, Hijacking a key chromatin modulator creates epigenetic vulnerability for MYC-driven cancer, The Journal of clinical investigation, 128 (2018) 3605–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Hofmann JW, Zhao X, De Cecco M, Peterson AL, Pagliaroli L, Manivannan J, Hubbard GB, Ikeno Y, Zhang Y, Feng B, Li X, Serre T, Qi W, Van Remmen H, Miller RA, Bath KG, de Cabo R, Xu H, Neretti N, Sedivy JM, Reduced expression of MYC increases longevity and enhances healthspan, Cell, 160 (2015) 477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ma Z, Zhu P, Shi H, Guo L, Zhang Q, Chen Y, Chen S, Zhang Z, Peng J, Chen J, PTC-bearing mRNA elicits a genetic compensation response via Upf3a and COMPASS components, Nature, 568 (2019) 259–263. [DOI] [PubMed] [Google Scholar]
- [98].Yang B, Liu XJ, Yao Y, Jiang X, Wang XZ, Yang H, Sun JY, Miao Y, Wang W, Huang ZL, Wang Y, Tang Q, Rayner S, Britt WJ, McVoy MA, Luo MH, Zhao F, WDR5 Facilitates Human Cytomegalovirus Replication by Promoting Capsid Nuclear Egress, Journal of virology, 92 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Ma D, George CX, Nomburg JL, Pfaller CK, Cattaneo R, Samuel CE, Upon Infection, Cellular WD Repeat-Containing Protein 5 (WDR5) Localizes to Cytoplasmic Inclusion Bodies and Enhances Measles Virus Replication, Journal of virology, 92 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Gomez JA, Wapinski OL, Yang YW, Bureau JF, Gopinath S, Monack DM, Chang HY, Brahic M, Kirkegaard K, The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-gamma locus, Cell, 152 (2013) 743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Fanucchi S, Fok ET, Dalla E, Shibayama Y, Borner K, Chang EY, Stoychev S, Imakaev M, Grimm D, Wang KC, Li G, Sung WK, Mhlanga MM, Immune genes are primed for robust transcription by proximal long noncoding RNAs located in nuclear compartments, Nature genetics, 51 (2019) 138–150. [DOI] [PubMed] [Google Scholar]
- [102].Wang YY, Liu LJ, Zhong B, Liu TT, Li Y, Yang Y, Ran Y, Li S, Tien P, Shu HB, WDR5 is essential for assembly of the VISA-associated signaling complex and virus-triggered IRF3 and NF-kappaB activation, Proceedings of the National Academy of Sciences of the United States of America, 107 (2010) 815–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Song M, Fang F, Dai X, Yu L, Fang M, Xu Y, MKL1 is an epigenetic mediator of TNF-alpha-induced proinflammatory transcription in macrophages by interacting with ASH2, FEBS letters, 591 (2017) 934–945. [DOI] [PubMed] [Google Scholar]
- [104].Bailey JK, Fields AT, Cheng K, Lee A, Wagenaar E, Lagrois R, Schmidt B, Xia B, Ma D, WD repeat-containing protein 5 (WDR5) localizes to the midbody and regulates abscission, The Journal of biological chemistry, 290 (2015) 8987–9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Rao RC, Dou Y, Hijacked in cancer: the KMT2 (MLL) family of methyltransferases, Nature reviews. Cancer, 15 (2015) 334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Yang P, Huang X, Lai C, Li L, Li T, Huang P, Ouyang S, Yan J, Cheng S, Lei G, Wang Z, Yu L, Hong Z, Li R, Dong H, Wang C, Yu Y, Wang X, Li X, Wang L, Lv F, Yin Y, Yang H, Song J, Gao Q, Wang X, Zhang S, SET domain containing 1B gene is mutated in primary hepatic neuroendocrine tumors, International journal of cancer, (2019). [DOI] [PMC free article] [PubMed]
- [107].Chen X, Xie W, Gu P, Cai Q, Wang B, Xie Y, Dong W, He W, Zhong G, Lin T, Huang J, Upregulated WDR5 promotes proliferation, self-renewal and chemoresistance in bladder cancer via mediating H3K4 trimethylation, Scientific reports, 5 (2015) 8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Grebien F, Vedadi M, Getlik M, Giambruno R, Grover A, Avellino R, Skucha A, Vittori S, Kuznetsova E, Smil D, Barsyte-Lovejoy D, Li F, Poda G, Schapira M, Wu H, Dong A, Senisterra G, Stukalov A, Huber KVM, Schonegger A, Marcellus R, Bilban M, Bock C, Brown PJ, Zuber J, Bennett KL, Al-Awar R, Delwel R, Nerlov C, Arrowsmith CH, Superti-Furga G, Pharmacological targeting of the Wdr5-MLL interaction in C/EBPalpha N-terminal leukemia, Nature chemical biology, 11 (2015) 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Chung CY, Sun Z, Mullokandov G, Bosch A, Qadeer ZA, Cihan E, Rapp Z, Parsons R, Aguirre-Ghiso JA, Farias EF, Brown BD, Gaspar-Maia A, Bernstein E, Cbx8 Acts Non-canonically with Wdr5 to Promote Mammary Tumorigenesis, Cell reports, 16 (2016) 472–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Ge Z, Song EJ, Kawasawa YI, Li J, Dovat S, Song C, WDR5 high expression and its effect on tumorigenesis in leukemia, Oncotarget, 7 (2016) 37740–37754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Cui Z, Li H, Liang F, Mu C, Mu Y, Zhang X, Liu J, Effect of high WDR5 expression on the hepatocellular carcinoma prognosis, Oncology letters, 15 (2018) 7864–7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Tan X, Chen S, Wu J, Lin J, Pan C, Ying X, Pan Z, Qiu L, Liu R, Geng R, Huang W, PI3K/AKT-mediated upregulation of WDR5 promotes colorectal cancer metastasis by directly targeting ZNF407, Cell death & disease, 8 (2017) e2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Malek R, Gajula RP, Williams RD, Nghiem B, Simons BW, Nugent K, Wang H, Taparra K, Lemtiri-Chlieh G, Yoon AR, True L, An SS, DeWeese TL, Ross AE, Schaeffer EM, Pienta KJ, Hurley PJ, Morrissey C, Tran PT, TWIST1-WDR5-Hottip Regulates Hoxa9 Chromatin to Facilitate Prostate Cancer Metastasis, Cancer research, 77 (2017) 3181–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Fu Z, Chen C, Zhou Q, Wang Y, Zhao Y, Zhao X, Li W, Zheng S, Ye H, Wang L, He Z, Lin Q, Li Z, Chen R, LncRNA HOTTIP modulates cancer stem cell properties in human pancreatic cancer by regulating HOXA9, Cancer letters, 410 (2017) 68–81. [DOI] [PubMed] [Google Scholar]
- [115].He W, Zhong G, Jiang N, Wang B, Fan X, Chen C, Chen X, Huang J, Lin T, Long noncoding RNA BLACAT2 promotes bladder cancer-associated lymphangiogenesis and lymphatic metastasis, The Journal of clinical investigation, 128 (2018) 861–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Ma M, Zhang Y, Weng M, Hu Y, Xuan Y, Hu Y, Lv K, lncRNA GCAWKR Promotes Gastric Cancer Development by Scaffolding the Chromatin Modification Factors WDR5 and KAT2A, Molecular therapy : the journal of the American Society of Gene Therapy, 26 (2018) 2658–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Kim JY, Banerjee T, Vinckevicius A, Luo Q, Parker JB, Baker MR, Radhakrishnan I, Wei JJ, Barish GD, Chakravarti D, A role for WDR5 in integrating threonine 11 phosphorylation to lysine 4 methylation on histone H3 during androgen signaling and in prostate cancer, Molecular cell, 54 (2014) 613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Mahajan K, Malla P, Lawrence HR, Chen Z, Kumar-Sinha C, Malik R, Shukla S, Kim J, Coppola D, Lawrence NJ, Mahajan NP, ACK1/TNK2 Regulates Histone H4 Tyr88-phosphorylation and AR Gene Expression in Castration-Resistant Prostate Cancer, Cancer cell, 31 (2017) 790–803.e798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Cao L, Wu G, Zhu J, Tan Z, Shi D, Wu X, Tang M, Li Z, Hu Y, Zhang S, Yu R, Mo S, Wu J, Song E, Li M, Song L, Li J, Genotoxic stress-triggered beta-catenin/JDP2/PRMT5 complex facilitates reestablishing glutathione homeostasis, Nature communications, 10 (2019) 3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Sun Y, Bell JL, Carter D, Gherardi S, Poulos RC, Milazzo G, Wong JW, Al-Awar R, Tee AE, Liu PY, Liu B, Atmadibrata B, Wong M, Trahair T, Zhao Q, Shohet JM, Haupt Y, Schulte JH, Brown PJ, Arrowsmith CH, Vedadi M, MacKenzie KL, Huttelmaier S, Perini G, Marshall GM, Braithwaite A, Liu T, WDR5 Supports an N-Myc Transcriptional Complex That Drives a Protumorigenic Gene Expression Signature in Neuroblastoma, Cancer research, 75 (2015) 5143–5154. [DOI] [PubMed] [Google Scholar]
- [121].Carugo A, Genovese G, Seth S, Nezi L, Rose JL, Bossi D, Cicalese A, Shah PK, Viale A, Pettazzoni PF, Akdemir KC, Bristow CA, Robinson FS, Tepper J, Sanchez N, Gupta S, Estecio MR, Giuliani V, Dellino GI, Riva L, Yao W, Di Francesco ME, Green T, D’Alesio C, Corti D, Kang Y, Jones P, Wang H, Fleming JB, Maitra A, Pelicci PG, Chin L, DePinho RA, Lanfrancone L, Heffernan TP, Draetta GF, In Vivo Functional Platform Targeting Patient-Derived Xenografts Identifies WDR5-Myc Association as a Critical Determinant of Pancreatic Cancer, Cell reports, 16 (2016) 133–147. [DOI] [PubMed] [Google Scholar]
- [122].Neilsen BK, Chakraborty B, McCall JL, Frodyma DE, Sleightholm RL, Fisher KW, Lewis RE, WDR5 supports colon cancer cells by promoting methylation of H3K4 and suppressing DNA damage, BMC cancer, 18 (2018) 673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Hoshii T, Cifani P, Feng Z, Huang CH, Koche R, Chen CW, Delaney CD, Lowe SW, Kentsis A, Armstrong SA, A Non-catalytic Function of SETD1A Regulates Cyclin K and the DNA Damage Response, Cell, 172 (2018) 1007–1021.e1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Kantidakis T, Saponaro M, Mitter R, Horswell S, Kranz A, Boeing S, Aygun O, Kelly GP, Matthews N, Stewart A, Stewart AF, Svejstrup JQ, Mutation of cancer driver MLL2 results in transcription stress and genome instability, Genes & development, 30 (2016) 408–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Ray Chaudhuri A, Callen E, Ding X, Gogola E, Duarte AA, Lee JE, Wong N, Lafarga V, Calvo JA, Panzarino NJ, John S, Day A, Crespo AV, Shen B, Starnes LM, de Ruiter JR, Daniel JA, Konstantinopoulos PA, Cortez D, Cantor SB, Fernandez-Capetillo O, Ge K, Jonkers J, Rottenberg S, Sharan SK, Nussenzweig A, Replication fork stability confers chemoresistance in BRCA-deficient cells, Nature, 535 (2016) 382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Higgs MR, Sato K, Reynolds JJ, Begum S, Bayley R, Goula A, Vernet A, Paquin KL, Skalnik DG, Kobayashi W, Takata M, Howlett NG, Kurumizaka H, Kimura H, Stewart GS, Histone Methylation by SETD1A Protects Nascent DNA through the Nucleosome Chaperone Activity of FANCD2, Molecular cell, 71 (2018) 25–41.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Zhao C, Chen W, Dai S, Zhang X, Ban N, Fan S, Bao Z, Sun J, Shen C, Xia X, Zhang L, Ren J, Expression and clinical role of RBQ3 in gliomas, Journal of the neurological sciences, 359 (2015) 177–184. [DOI] [PubMed] [Google Scholar]
- [128].Zhou H, Bao J, Zhu X, Dai G, Jiang X, Jiao X, Sheng H, Huang J, Yu H, Retinoblastoma Binding Protein 5 Correlates with the Progression in Hepatocellular Carcinoma, BioMed research international, 2018 (2018) 1073432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Alvarado AG, Thiagarajan PS, Mulkearns-Hubert EE, Silver DJ, Hale JS, Alban TJ, Turaga SM, Jarrar A, Reizes O, Longworth MS, Vogelbaum MA, Lathia JD, Glioblastoma Cancer Stem Cells Evade Innate Immune Suppression of Self-Renewal through Reduced TLR4 Expression, Cell stem cell, 20 (2017) 450–461.e454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Luscher-Firzlaff J, Gawlista I, Vervoorts J, Kapelle K, Braunschweig T, Walsemann G, Rodgarkia-Schamberger C, Schuchlautz H, Dreschers S, Kremmer E, Lilischkis R, Cerni C, Wellmann A, Luscher B, The human trithorax protein hASH2 functions as an oncoprotein, Cancer research, 68 (2008) 749–758. [DOI] [PubMed] [Google Scholar]
- [131].Ullius A, Luscher-Firzlaff J, Costa IG, Walsemann G, Forst AH, Gusmao EG, Kapelle K, Kleine H, Kremmer E, Vervoorts J, Luscher B, The interaction of MYC with the trithorax protein ASH2L promotes gene transcription by regulating H3K27 modification, Nucleic acids research, 42 (2014) 6901–6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Butler JS, Qiu YH, Zhang N, Yoo SY, Coombes KR, Dent SY, Kornblau SM, Low expression of ASH2L protein correlates with a favorable outcome in acute myeloid leukemia, Leukemia & lymphoma, 58 (2017) 1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Lee YJ, Han ME, Baek SJ, Kim SY, Oh SO, Roles of DPY30 in the Proliferation and Motility of Gastric Cancer Cells, PloS one, 10 (2015) e0131863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Zhang L, Zhang S, Li A, Zhang A, Zhang S, Chen L, DPY30 is required for the enhanced proliferation, motility and epithelial-mesenchymal transition of epithelial ovarian cancer cells, International journal of molecular medicine, 42 (2018) 3065–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Zaidi S, Choi M, Wakimoto H, Ma L, Jiang J, Overton JD, Romano-Adesman A, Bjornson RD, Breitbart RE, Brown KK, Carriero NJ, Cheung YH, Deanfield J, DePalma S, Fakhro KA, Glessner J, Hakonarson H, Italia MJ, Kaltman JR, Kaski J, Kim R, Kline JK, Lee T, Leipzig J, Lopez A, Mane SM, Mitchell LE, Newburger JW, Parfenov M, Pe’er I, Porter G, Roberts AE, Sachidanandam R, Sanders SJ, Seiden HS, State MW, Subramanian S, Tikhonova IR, Wang W, Warburton D, White PS, Williams IA, Zhao H, Seidman JG, Brueckner M, Chung WK, Gelb BD, Goldmuntz E, Seidman CE, Lifton RP, De novo mutations in histone-modifying genes in congenital heart disease, Nature, 498 (2013) 220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Homsy J, Zaidi S, Shen Y, Ware JS, Samocha KE, Karczewski KJ, DePalma SR, McKean D, Wakimoto H, Gorham J, Jin SC, Deanfield J, Giardini A, Porter GA Jr., Kim R, Bilguvar K, Lopez-Giraldez F, Tikhonova I, Mane S, Romano-Adesman A, Qi H, Vardarajan B, Ma L, Daly M, Roberts AE, Russell MW, Mital S, Newburger JW, Gaynor JW, Breitbart RE, Iossifov I, Ronemus M, Sanders SJ, Kaltman JR, Seidman JG, Brueckner M, Gelb BD, Goldmuntz E, Lifton RP, Seidman CE, Chung WK, De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies, Science, 350 (2015) 1262–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Zhu JY, Fu Y, Nettleton M, Richman A, Han Z, High throughput in vivo functional validation of candidate congenital heart disease genes in Drosophila, eLife, 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Jones WD, Dafou D, McEntagart M, Woollard WJ, Elmslie FV, Holder-Espinasse M, Irving M, Saggar AK, Smithson S, Trembath RC, Deshpande C, Simpson MA, De novo mutations in MLL cause Wiedemann-Steiner syndrome, American journal of human genetics, 91 (2012) 358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Singh T, Kurki MI, Curtis D, Purcell SM, Crooks L, McRae J, Suvisaari J, Chheda H, Blackwood D, Breen G, Pietilainen O, Gerety SS, Ayub M, Blyth M, Cole T, Collier D, Coomber EL, Craddock N, Daly MJ, Danesh J, DiForti M, Foster A, Freimer NB, Geschwind D, Johnstone M, Joss S, Kirov G, Korkko J, Kuismin O, Holmans P, Hultman CM, Iyegbe C, Lonnqvist J, Mannikko M, McCarroll SA, McGuffin P, McIntosh AM, McQuillin A, Moilanen JS, Moore C, Murray RM, Newbury-Ecob R, Ouwehand W, Paunio T, Prigmore E, Rees E, Roberts D, Sambrook J, Sklar P, St Clair D, Veijola J, Walters JT, Williams H, Sullivan PF, Hurles ME, O’Donovan MC, Palotie A, Owen MJ, Barrett JC, Rare loss-of-function variants in SETD1A are associated with schizophrenia and developmental disorders, Nature neuroscience, 19 (2016) 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Bogershausen N, Wollnik B, Unmasking Kabuki syndrome, Clinical genetics, 83 (2013) 201–211. [DOI] [PubMed] [Google Scholar]
- [141].Karaca E, Harel T, Pehlivan D, Jhangiani SN, Gambin T, Coban Akdemir Z, Gonzaga-Jauregui C, Erdin S, Bayram Y, Campbell IM, Hunter JV, Atik MM, Van Esch H, Yuan B, Wiszniewski W, Isikay S, Yesil G, Yuregir OO, Tug Bozdogan S, Aslan H, Aydin H, Tos T, Aksoy A, De Vivo DC, Jain P, Geckinli BB, Sezer O, Gul D, Durmaz B, Cogulu O, Ozkinay F, Topcu V, Candan S, Cebi AH, Ikbal M, Yilmaz Gulec E, Gezdirici A, Koparir E, Ekici F, Coskun S, Cicek S, Karaer K, Koparir A, Duz MB, Kirat E, Fenercioglu E, Ulucan H, Seven M, Guran T, Elcioglu N, Yildirim MS, Aktas D, Alikasifoglu M, Ture M, Yakut T, Overton JD, Yuksel A, Ozen M, Muzny DM, Adams DR, Boerwinkle E, Chung WK, Gibbs RA, Lupski JR, Genes that Affect Brain Structure and Function Identified by Rare Variant Analyses of Mendelian Neurologic Disease, Neuron, 88 (2015) 499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Miura S, Shibata H, Kida H, Noda K, Toyama T, Iwasaki N, Iwaki A, Ayabe M, Aizawa H, Taniwaki T, Fukumaki Y, Partial SPAST and DPY30 deletions in a Japanese spastic paraplegia type 4 family, Neurogenetics, 12 (2011) 25–31. [DOI] [PubMed] [Google Scholar]
- [143].Racis L, Storti E, Pugliatti M, Agnetti V, Tessa A, Santorelli FM, Novel SPAST deletion and reduced DPY30 expression in a Spastic Paraplegia type 4 kindred, BMC medical genetics, 15 (2014) 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Ali A, Veeranki SN, Tyagi S, A SET-domain-independent role of WRAD complex in cell-cycle regulatory function of mixed lineage leukemia, Nucleic acids research, 42 (2014) 7611–7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Grembecka J, He S, Shi A, Purohit T, Muntean AG, Sorenson RJ, Showalter HD, Murai MJ, Belcher AM, Hartley T, Hess JL, Cierpicki T, Menin-MLL inhibitors reverse oncogenic activity of MLL fusion proteins in leukemia, Nature chemical biology, 8 (2012) 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Borkin D, He S, Miao H, Kempinska K, Pollock J, Chase J, Purohit T, Malik B, Zhao T, Wang J, Wen B, Zong H, Jones M, Danet-Desnoyers G, Guzman ML, Talpaz M, Bixby DL, Sun D, Hess JL, Muntean AG, Maillard I, Cierpicki T, Grembecka J, Pharmacologic inhibition of the Menin-MLL interaction blocks progression of MLL leukemia in vivo, Cancer cell, 27 (2015) 589–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Malik R, Khan AP, Asangani IA, Cieslik M, Prensner JR, Wang X, Iyer MK, Jiang X, Borkin D, Escara-Wilke J, Stender R, Wu YM, Niknafs YS, Jing X, Qiao Y, Palanisamy N, Kunju LP, Krishnamurthy PM, Yocum AK, Mellacheruvu D, Nesvizhskii AI, Cao X, Dhanasekaran SM, Feng FY, Grembecka J, Cierpicki T, Chinnaiyan AM, Targeting the MLL complex in castration-resistant prostate cancer, Nature medicine, 21 (2015) 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Vedadi M, Blazer L, Eram MS, Barsyte-Lovejoy D, Arrowsmith CH, Hajian T, Targeting human SET1/MLL family of proteins, Protein science : a publication of the Protein Society, 26 (2017) 662–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Karatas H, Li Y, Liu L, Ji J, Lee S, Chen Y, Yang J, Huang L, Bernard D, Xu J, Townsend EC, Cao F, Ran X, Li X, Wen B, Sun D, Stuckey JA, Lei M, Dou Y, Wang S, Discovery of a Highly Potent, Cell-Permeable Macrocyclic Peptidomimetic (MM-589) Targeting the WD Repeat Domain 5 Protein (WDR5)-Mixed Lineage Leukemia (MLL) Protein-Protein Interaction, Journal of medicinal chemistry, 60 (2017) 4818–4839. [DOI] [PubMed] [Google Scholar]
- [150].Karatas H, Townsend EC, Cao F, Chen Y, Bernard D, Liu L, Lei M, Dou Y, Wang S, High-affinity, small-molecule peptidomimetic inhibitors of MLL1/WDR5 protein-protein interaction, Journal of the American Chemical Society, 135 (2013) 669–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Senisterra G, Wu H, Allali-Hassani A, Wasney GA, Barsyte-Lovejoy D, Dombrovski L, Dong A, Nguyen KT, Smil D, Bolshan Y, Hajian T, He H, Seitova A, Chau I, Li F, Poda G, Couture JF, Brown PJ, Al-Awar R, Schapira M, Arrowsmith CH, Vedadi M, Small-molecule inhibition of MLL activity by disruption of its interaction with WDR5, The Biochemical journal, 449 (2013) 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Zhu J, Sammons MA, Donahue G, Dou Z, Vedadi M, Getlik M, Barsyte-Lovejoy D, Al-awar R, Katona BW, Shilatifard A, Huang J, Hua X, Arrowsmith CH, Berger SL, Gain-of-function p53 mutants co-opt chromatin pathways to drive cancer growth, Nature, 525 (2015) 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Aho ER, Wang J, Gogliotti RD, Howard GC, Phan J, Acharya P, Macdonald JD, Cheng K, Lorey SL, Lu B, Wenzel S, Foshage AM, Alvarado J, Wang F, Shaw JG, Zhao B, Weissmiller AM, Thomas LR, Vakoc CR, Hall MD, Hiebert SW, Liu Q, Stauffer SR, Fesik SW, Tansey WP, Displacement of WDR5 from Chromatin by a WIN Site Inhibitor with Picomolar Affinity, Cell reports, 26 (2019) 2916–2928.e2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Aho ER, Weissmiller AM, Fesik SW, Tansey WP, Targeting WDR5: A WINning Anti-Cancer Strategy?, Epigenetics insights, 12 (2019) 2516865719865282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Shah KK, Whitaker RH, Busby T, Hu J, Shi B, Wang Z, Zang C, Placzek WJ, Jiang H, Specific inhibition of DPY30 activity by ASH2L-derived peptides suppresses blood cancer cell growth, Experimental cell research, (2019). [DOI] [PMC free article] [PubMed]
- [156].Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, Magoon D, Qi J, Blatt K, Wunderlich M, Taylor MJ, Johns C, Chicas A, Mulloy JC, Kogan SC, Brown P, Valent P, Bradner JE, Lowe SW, Vakoc CR, RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia, Nature, 478 (2011) 524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, Philpott M, Munro S, McKeown MR, Wang Y, Christie AL, West N, Cameron MJ, Schwartz B, Heightman TD, La Thangue N, French CA, Wiest O, Kung AL, Knapp S, Bradner JE, Selective inhibition of BET bromodomains, Nature, 468 (2010) 1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI, Robson SC, Chung CW, Hopf C, Savitski MM, Huthmacher C, Gudgin E, Lugo D, Beinke S, Chapman TD, Roberts EJ, Soden PE, Auger KR, Mirguet O, Doehner K, Delwel R, Burnett AK, Jeffrey P, Drewes G, Lee K, Huntly BJ, Kouzarides T, Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia, Nature, 478 (2011) 529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, Kastritis E, Gilpatrick T, Paranal RM, Qi J, Chesi M, Schinzel AC, McKeown MR, Heffernan TP, Vakoc CR, Bergsagel PL, Ghobrial IM, Richardson PG, Young RA, Hahn WC, Anderson KC, Kung AL, Bradner JE, Mitsiades CS, BET bromodomain inhibition as a therapeutic strategy to target c-Myc, Cell, 146 (2011) 904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Mertz JA, Conery AR, Bryant BM, Sandy P, Balasubramanian S, Mele DA, Bergeron L, Sims RJ 3rd, Targeting MYC dependence in cancer by inhibiting BET bromodomains, Proceedings of the National Academy of Sciences of the United States of America, 108 (2011) 16669–16674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Houzelstein D, Bullock SL, Lynch DE, Grigorieva EF, Wilson VA, Beddington RS, Growth and early postimplantation defects in mice deficient for the bromodomain-containing protein Brd4, Molecular and cellular biology, 22 (2002) 3794–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]