Abstract
Background and Purpose
Liver fibrosis is a serious cause of morbidity and mortality worldwide and has no adequate treatment. Accumulating evidence suggests that cannabinoid CB1 receptors regulate a variety of physiological and pathological processes in the liver, and blockage of CB1 receptor signalling shows promise as a new therapy for several liver diseases. The aim of this study was to investigate the potential therapeutic effects of CB1 receptors and a peripheral CB1 receptor antagonist JD5037 in liver fibrogenesis.
Experimental Approach
Liver samples from both humans and mouse models were investigated. The peripheral CB1 receptor antagonist JD5037, β‐arr1 wild type (β‐arr1‐WT) and β‐arr1 knockout (β‐arr1‐KO) littermate models, and primary hepatic stellate cells (HSCs) were also used. The mechanisms underlying CB1 receptor‐regulated HSCs activation in fibrosis and the therapeutic potential of JD5037 were further analysed.
Key Results
CB1 receptors were induced in samples from patients with liver fibrosis and from mouse models. These receptors promoted activation of HSCs in liver fibrosis via recruiting β‐arrestin1 and Akt signalling, while blockage of CB1 receptors with JD5037 attenuated CB1 receptor‐regulated HSCs activation and liver fibrosis by suppressing β‐arrestin1/Akt signalling.
Conclusions and Implications
CB1 receptors promote the activation of HSCs and liver fibrosis via the β‐arrestin1/Akt signalling pathway. The peripheral CB1 receptor antagonist JD5037 blocked this pathway, the activation of HSCs and liver fibrosis. This compound and the associated pathway may be a novel approach to the treatment of liver fibrosis.
Abbreviations
- COL‐I
collagen‐I
- COL‐IV
collagen‐IV
- ECM
extracellular matrix
- HSCs
hepatic stellate cells
- PI3K
phosphatidylinositol‐3 kinase
- α‐SMA
α‐smooth muscle actin
What is already known
CB1 receptors are up‐regulated in hepatic myofibroblasts in response to extracellular stimuli.
What does this study add
The peripheral CB1 receptor antagonist JD5037 blocked hepatic stellate cell activation and attenuated liver fibrosis.
What is the clinical significance
Analysis of the actions of JD5037 may provide a new therapeutic approach to liver fibrosis.
1. INTRODUCTION
Liver fibrosis is characterized by excessive production and deposition of extracellular matrix (ECM) substances by myofibroblasts and this pathological state lacks effective treatment (Lee, Wallace, & Friedman, 2015; Yin, Evason, Asahina, & Stainier, 2013). The activation of resident hepatic stellate cells (HSCs) is considered one of the major initiators and an attractive target for antifibrotic therapy (Coll et al., 2018; Inzaugarat et al., 2018; Suh et al., 2012), and as a result, several studies have explored the therapeutic potential of HSCs in liver fibrosis. However, our knowledge of their cellular biology is still far from complete.
The endocannabinoid system has emerged as a pivotal regulator of various aspects of liver pathophysiology, through the actions of the cannabinoid https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=57 s (Caraceni, Domenicali, Giannone, & Bernardi, 2009; Izzo & Camilleri, 2008). The CB1 receptors are expressed by a variety of cell types, including high expression in the mammalian brain and low levels in a large number of peripheral tissues, such as the haematopoietic system and various cell types of the liver, whereas the CB2 receptors are restricted primarily to immune cells and haematopoietic cells, with a low level of CB2 receptors also detected in the liver, in certain pathological states (Dai et al., 2017; Mallat, Teixeira‐Clerc, & Lotersztajn, 2013). The expression of cannabinoid receptors in normal liver is very low, and many studies have demonstrated the up‐regulation of CB1 receptors in hepatic myofibroblasts and vascular endothelial cells in alcohol‐induced liver disease, non‐alcoholic fatty liver disease, liver regeneration/injury, hepatic fibrogenesis/cirrhosis, and hepatocarcinogenesis (Mukhopadhyay et al., 2015; Parfieniuk & Flisiak, 2008; Tian et al., 2017).
Teixeira‐Clerc et al. (2006) demonstrated that genetic inactivation of CB1 receptors or pharmacological antagonism of these receptors repressed the matrix remodelling and fibrogenic response associated with acute or chronic liver injury, suggesting a novel crucial antifibrotic role of the CB1 receptor antagonist. Many studies have also suggested that the non‐selective CB1 receptor antagonist https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=743 is effective in reducing body weight and waist circumference and ameliorating both lipid and glucose control, and it can improve metabolic disorders such as Type 2 diabetes, obesity, insulin resistance, fatty liver, and dyslipidaemia. Based on these promising clinical trials, rimonabant was licensed and used for the treatment of obesity worldwide (Christensen, Kristensen, Bartels, Bliddal, & Astrup, 2007). However, due to the increased reported risk of adverse psychiatric events (including anxiety, depression, and suicidal ideation), this drug was subsequently withdrawn from the market (Gruden, Barutta, Kunos, & Pacher, 2016).
Thus, peripherally restricted CB1 receptor antagonists that do not readily cross the blood–brain barrier (brain‐impermeable) and are thus devoid of centrally mediated psychiatric adverse events have been developed to reassess the therapeutic potential of CB1 receptor blockage. A recent study showed a type of peripherally restricted and non‐brain‐penetrant hybrid compound, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=10521, which is a dual antagonist of CB1 receptors and inducible NOS (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1250), was able not only to slow the progression of liver fibrosis but also to attenuate established liver fibrosis (Cinar et al., 2016). Another compound, JD5037, is a potent and peripherally restricted antagonist of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56 s and has therapeutic potential in disorders of the peripheral tissues, such as the liver, ileum, and pancreas (Cota, 2017; Gruden et al., 2016; Tam et al., 2017). These data indicate that CB1 receptors and its peripheral antagonists have potential for the treatment of liver fibrosis.
The CB1 receptors, as members of the https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=694, have a high sequence identity and mediate a variety of hepatic physiological and pathological processes through G‐proteins of the Gi/o type (Caraceni et al., 2009; Huang et al., 2016). Activity of GPCRs is always regulated following the formation of a complex with β‐arrestins (β‐arrestin1 and β‐arrestin2). β‐Arrestins have the advantage of being generic in a G‐protein‐subtype‐independent manner and are suitable for screening G‐proteins of the Gi/o type. CB1 receptors recruit β‐arrestins upon activation by (partial) agonists, and such recruitment is always associated with the termination/activation by the phosphorylation of their C‐terminal tails to signal independently or synergistically to a wide variety of downstream effectors. Van der Lee et al. (2009) revealed the pharmacological properties of compounds on CB1 receptor intracellular status, and β‐arrestin recruitment assays in a G‐protein‐independent manner could be used for the identification, optimization, and development of new drugs or compounds for cannabinoid‐based therapies, such as the specific agonists, antagonists, and inverse agonists. Other studies have also established that β‐arrestins are involved in CB1 receptor‐biased signalling and intracellular effects by a CB1 receptor allosteric modulator (Ahn, Mahmoud, Shim, & Kendall, 2013). Moreover, we recently discovered that β‐arrestin1 promoted HSCs growth with activation in liver fibrogenesis (Tan et al., 2019). The above‐mentioned observations suggest a possible functional link between the CB1 receptor system and β‐arrestin1 in the activation of HSCs in liver fibrogenesis.
In the current study, we have demonstrated that CB1 receptors contribute to activation of HSCs in liver fibrosis via recruiting β‐arrestin1 and the https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=285 signalling pathway. Moreover, the peripheral CB1 receptor antagonist JD5037 blocked the activation of HSCs by the CB1 receptor/β‐arrestin1/Akt pathway and attenuated liver fibrosis.
2. METHODS
2.1. Human liver samples
This study protocol was approved by the Clinical Research Ethics Committee of The Third Affiliated Hospital of Sun Yat‐Sen University and written informed consent was obtained from each patient before inclusion in the study. Six samples of normal liver were obtained from parahemangioma sites of hepatic haemangioma patients, and the paired tissues of liver fibrosis were acquired from six hepatitis B virus‐related hepatic fibrosis patients. The characteristics of the donors of the non‐fibrotic tissue and the patients with liver fibrosis are shown in Table S1.
2.2. Mouse genotyping and modelling
All animal care and experimental studies were approved by the Institutional Animal Care and Use Committee of The Third Affiliated Hospital of Sun Yat‐Sen University. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology. Eight‐ to 10‐week‐old male mice (20–25 g) were housed in micro‐isolator cages in a room illuminated from 8:00 a.m. to 8:00 p.m. (12:12‐hr light/dark cycle) and were given water and chow ad libitum. The animal cages (U‐TEMP Polyetherimide, Techniplast, UK) were 820 cm2 by 15.5 cm depth, and bedding was acquired from the Institutional Animal Care and Use Committee at Sun Yat‐Sen University. β‐Arrestin1 (β‐arr1), β‐arr1 wild type (β‐arr1‐WT), and β‐arr1 knockout (β‐arr1‐KO) littermates on C57BL/6J (IMSR Cat# JAX:000664, RRID:IMSR_JAX:000664) background were obtained from Dr. R. J. Lefkowitz (Duke University Medical Center, Durham, NC, USA). All mice were randomly allocated to each group and to collect and process data, and the experimenters were blinded towards the treatments or genetic background in all the experiments.
The mouse model of hepatic fibrosis was induced by carbon tetrachloride (CCl4). A solution of CCl4 (20% in olive oil) was injected i.p. (5 ml·kg−1) twice per week for 8 weeks (Tan et al., 2015, 2019). The control group (Ctrl) was injected i.p. with the same volume of olive oil only. For bile duct ligation (BDL)‐induced hepatic fibrosis (Garcia‐Ruiz et al., 2019), the peritoneal cavity was opened by laparotomy through a 2‐cm midline incision during isoflurane anaesthesia. The bile duct was carefully isolated, and a 5‐0 suture was placed around the bile duct and tied in two surgical knots, and then another 5‐0 suture was also tied in the similar manner near the hepatic hilum. After that, the peritoneal cavity was rinsed with a 0.9% NaCl solution, and the abdominal wall was closed carefully. The sham operation (as Sham) was performed similarly without BDL. The mice that received BDL or the sham operation were killed 15 days later. For blockage of the CB1 receptors, different doses (1, 2, 3, 4, or 5 mg·kg−1) of the peripheral CB1 receptor antagonist JD5037 were given by oral gavage, daily, and the effects analysed in preliminary experiments. As a result, 3 mg·kg−1·day−1 JD5037 was selected for further experiments (daily, over 8 weeks for CCl4‐induced models; over 2 weeks for BDL‐induced models). The control group was given, by gavage, the same volume of vehicle (1% Tween80, 4% DMSO, and 95% saline). In some experiments, mice were injected i.p. with the Akt inhibitor, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5921, at a dose of 50 mg·kg−1 three times per week for 8 weeks. The control group was injected i.p. with equivalent volume of vehicle (10% DMSO, 5% Tween80, 40% PEG300, and 45% saline).
2.3. Isolation of primary HSCs
Primary HSCs were prepared from mice using non‐recirculating perfusion of collagenases, as previously described (Deng et al., 2008; Papeleu et al., 2006; Tan et al., 2019). The mice were anaesthetised with Ketamine & Xylazine mixed anaesthetic and the liver was perfused through the portal vein in situ, successively with Ca2+‐free HBSS for 15 min, with 100‐ml 0.2% pronase solution, and lastly with 0.2% collagenase type IV (Sigma‐Aldrich). This resulting cell suspension was filtered through 100‐μm pore size mesh nylon filter (Sinopharm Chemical Reagent) and centrifuged for 10 min at 200× g. The supernatant was collected for the isolation of primary HSCs. Commercially available 11.5% Percoll (Sigma‐Aldrich) was prepared, and the supernatant was added to the upper layer of Percoll carefully and then was centrifuged at 450× g for 10 min. After that, the pellet was re‐suspended with 0.5‐ml HBSS and centrifuged at 1,400× g for 25 min. Finally, the top of the Optiprep layer was collected for primary HSCs and cultured in RPMI medium 1640.
2.4. Cell culture and transfection
The human stellate cells line (LX2 cells) was transfected with plasmid encoding pcDNA3.0‐CB1R‐GFP (CB1R‐GFP) for the overexpression of CB1 receptors, using the Lipofectamine 3000 according to the manufacturer's instructions, and the construct that contained only the pcDNA3.0‐GFP was also transfected as the control group. Small interfering RNA of CB1R (CB1R‐siRNA) was introduced into cells according to the manufacturer's instruction (GenePharma, Shanghai, China).
2.5. Histological staining and liver function assay
The experimental details of the immunohistochemical techniques used in this study comply with the BJP guidelines (Alexander et al., 2018). Sirius red was used for collagen determination, haematoxylin–eosin staining, immunohistochemical, immunofluorescence (IF), and double IF staining were performed as previously described (Tan et al., 2019; Yang et al., 2015). The semi‐quantitative analysis of the histological staining was analysed using Image‐Pro Plus 6.0 (Image‐Pro Plus, RRID:SCR_007369). The collagen deposition in the liver sections was quantified by measuring Sirius red‐stained areas using Image‐Pro Plus 6.0. The images were taken from at least six randomly selected fields from each liver section, and the value of Sirius red area was presented as the percentage of the total area of the section occupied by Sirius red staining (Tan et al., 2019). Immunohistochemical and IF staining were performed by using antibodies for β‐arrestin1 (Santa Cruz Biotechnology Cat# sc‐53780, RRID:AB_781486, Santa Cruz, CA, USA); collagen‐IV (COL‐IV, Abcam Cat# ab6586, RRID:AB_305584), collagen‐I (COL‐I, Abcam Cat# ab34710, RRID:AB_731684), α‐SMA (Abcam Cat# ab5694, RRID:AB_2223021), and CB1 receptors (Abcam Cat# ab23703, RRID:AB_447623) (all from Abcam, Cambridge, MA, USA); p‐Akt (Cell Signaling Technology Cat# 4060, RRID:AB_2315049) and PCNA (Cell Signaling Technology Cat# 13110, RRID:AB_2636979) were all from Cell Signaling Technology (Danvers, MA, USA).
TUNEL staining was performed using an in situ cell death detection kit. The apoptotic index was determined by dividing the number of apoptotic cells by the total number of cells in the section of at least 20 randomly selected fields, and the proliferation index was also performed as above‐mentioned methods of the apoptotic index (Tan et al., 2017). The concentrations of four serum markers of hepatic fibrosis, laminin, pro‐collagen III N‐terminal peptide, hyaluronic acid, and type IV collagen, were measured with ELISA kits (Sinopharm Chemical Reagent). Liver function tests, such as transaminase aspartate aminotransferase and alanine aminotransferase concentrations in the different groups, were determined utilizing ELISA kits (CUSABIO, Wuhan Huamei Biotech, China). The hydroxyproline content of the liver samples was determined by the hydroxyproline assay kit (Abcam) according to the manufacturer's instructions. The body weight and wet liver weight were determined and the liver wet weight index was calculated by dividing the wet weight of the liver by the body weight.
2.6. Western blotting
The antibody‐based procedures used in this study comply with the recommendations made by the British Journal of Pharmacology. Total liver proteins were analysed by western blotting using anti‐α‐SMA, ‐collagen‐IV, ‐collagen‐I, ‐CB1 receptor, ‐CB2 receptor (Abcam Cat# ab3561, RRID:AB_303908), ‐Akt (Cell Signaling Technology Cat# 4691, RRID:AB_915783), ‐p‐Akt, ‐PCNA, ‐cleaved caspase‐3 (Cell Signaling Technology Cat# 9661, RRID:AB_2341188), ‐TGF‐β1 (Abcam Cat# ab92486, RRID:AB_10562492), ‐Smad2 (Cell Signaling Technology Cat# 5339, RRID:AB_10626777), ‐Smad3 (Cell Signaling Technology Cat# 9523, RRID:AB_2193182), ‐p‐Smad2 (Cell Signaling Technology Cat# 18338, RRID:AB_2798798), ‐p‐Smad3 (Cell Signaling Technology Cat# 9520, RRID:AB_2193207), ‐β‐actin (Sigma‐Aldrich Cat# A5441, RRID:AB_476744) antibodies. Appropriate HRP conjugated secondary antibodies were adopted to detect the primary antibody/antigen complexes, and the signals were detected using western blotting detection reagents (Amersham Pharmacia Biotech, Piscataway, NJ, USA) and then was quantified for densitometry analysis as previously described (Tan et al., 2019; Yang et al., 2015). Lastly, for controlling unwanted sources of variation, the quantitative densitometry results were calculated and normalized to the loading control β‐actin.
2.7. Total RNA extraction and real‐time PCR
Total RNA was isolated using the RNAgents Total RNA Isolation System (Promega) and reversed transcribed with SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. The expressions of the indicated mRNA were analysed by real‐time PCR that performed with a Mini Opticon Real‐time PCR System (BioRad, Hercules, CA, USA) with SYBR Green (Invitrogen) as previously described (Tan et al., 2019). Relative quantitative algorithm (as relative mRNA ratio) was used to analyse the data. The expression of β‐actin in each tissue was quantified as the internal control, and for controlling unwanted sources of variation, the indicated mRNA in each sample was normalized by β‐actin gene from the same sample and expressed as fold changes relative to the matched control values. The formula was the relative expression of genes = 2−ΔΔCt, and ΔΔCt value = [(target gene Ct value in the treatment group − β‐actin Ct value in the treatment group) − (target gene Ct value in the control group − β‐actin Ct value in the control group)]. These data were presented as means ± SD.
2.8. Co‐immunoprecipitation
Co‐immunoprecipitation was carried out by using the Thermo Scientific Pierce Co‐immunoprecipitation Kit (Thermo Scientific, Rockford, AL, USA) as previously described (Yang et al., 2015). In brief, the primary HSCs after isolation were lysed in the RIPA (Gibco BRL, Rockville, MD, USA), and the indicated immunoprecipitating antibody or normal IgG (negative control, Santa Cruz Biotechnology Cat# sc‐2762, RRID:AB_737179) were added; after that, the lysates was incubated on a rotator at 4°C overnight. The 25‐μl G/A agarose beads were added into the 200‐μl above‐mentioned lysates and then was incubated for 3 hr with gentle agitation at room temperature according to the manufacturer's instructions. Lastly, the beads were removed by centrifugation, and the final lysates were analysed by western blotting.
2.9. Microarray analysis
Microarray analysis was performed as previously described (Tan et al., 2019). Briefly, total RNA was isolated, amplified, labelled, and purified by Affymetrix WT PLUS Reagent Kit (Affymetrix, Santa Clara, CA, US) and FL‐Ovation cDNA Biotin Module V2 (NuGEN, San Carlos, CA, USA) according to the manufacturer's protocol to gain the biotin‐labelled cDNA. Array hybridization and washing were performed utilizing GeneChip Hybridization, Wash and Stain Kit (Affymetrix) in a Hybridization Oven 645 and a Fluidics Station 450, and then the arrays were analysed by Affymetrix GeneChip Scanner 3000, and Command Console Software was adopted to control the scanner and summarize probe cell intensity data with default settings. The array data were analysed by using the GeneSpring software V12 (Agilent, GENESPRING GT, RRID:SCR_009196) and then further analysed with hierarchical clustering with average linkage as previously described. Finally, tree visualization was performed using Java Treeview (Stanford University School of Medicine, Stanford, CA, USA).
2.10. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). Statistical analysis was performed only for studies where each group size was at least n = 6, unless otherwise stated. The group size was the number of independent values and that statistical analysis was done using these independent values. These data were normally distributed and are presented as means ± SD. Comparisons of multiple groups were analysed using Student's two‐tailed t test or a one‐way ANOVA or two‐way ANOVA, followed by Bonferroni's comparison post hoc test; post hoc tests were run only if F achieved P < .05 and there was no significant variance in homogeneity. Differences between group means were considered statistically significant at the level of P < .05.
2.11. Materials
CCl4 and olive oil were supplied by Sinopharm Chemical Reagents (Shanghai, China) and JD5037 was supplied by TargetMol (Wellesley Hills, MA, USA). The Akt inhibitor VIII was from Sigma‐Aldrich (St Louis, MO, USA).
2.12. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos et al., 2017; Alexander, Fabbro et al., 2017; Alexander, Kelly et al., 2017).
3. RESULTS
3.1. CB1 receptors were involved in liver fibrosis in both humans and mice
To evaluate the status of CB1 and CB2 receptors in liver fibrosis, liver specimens from healthy volunteers and liver fibrosis patients were analysed. A gene expression microanalysis was performed to screen for differences in the gene expression between normal liver tissues and fibrotic tissues. We found that CB1R, rather than CB2R, presented distinctly higher expression in fibrotic tissues than in normal liver tissues (Figure 1a), and real‐time PCR also confirmed a similar finding (Figure 1b). Histological analysis revealed a loss of preserved architecture and excess deposition of ECM, accompanied by up‐regulated α‐SMA and CB1 receptors, in the fibrotic liver samples, compared with those of the normal liver samples (Figure 1c). Western blotting showed the same results (Figure 1d). Moreover, in the model of CCl4‐induced fibrotic liver in mice, we found that CCl4 injection induced marked liver fibrosis in mice, as shown by Sirius red staining (Figure 1e). The expression of α‐SMA and CB1 receptors was up‐regulated in CCl4‐treated fibrotic mice, (Figure 1e). Furthermore, western blotting confirmed the increase in α‐SMA and CB1 receptors in samples of fibrotic liver from mice (Figure 1f). These results indicate that CB1 receptors are involved in liver fibrosis in both humans and mice.
Figure 1.
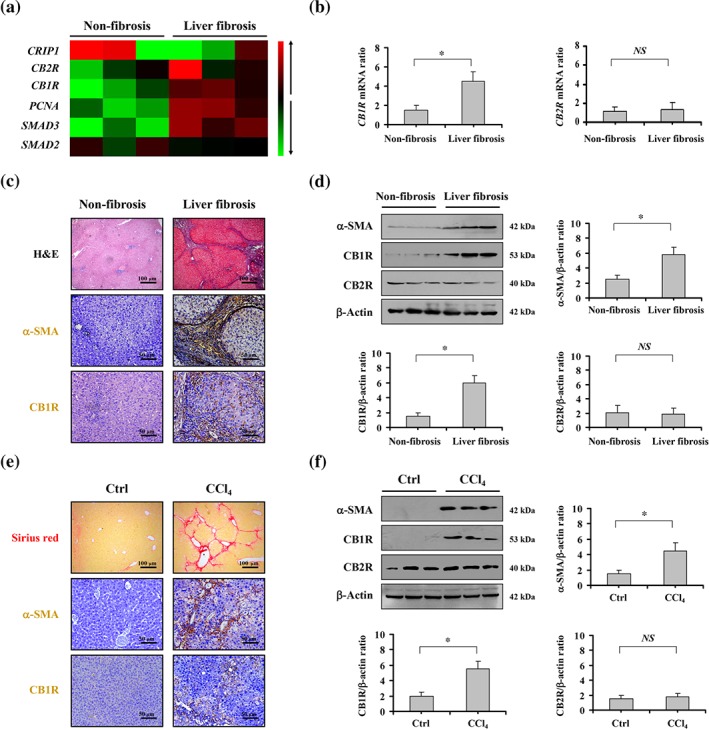
CB1 receptors were involved in liver fibrosis in both humans and mice. (a) Two‐dimensional hierarchical clustering results for the indicated mRNA between the human samples from patients with liver fibrosis and those from healthy volunteers are shown. The mRNA levels in fibrotic tissues are shown as decreased (green) or increased (red) relative to non‐fibrotic tissues. (b) The mRNA levels of CB1R and CB2R in the fibrosis patients and non‐fibrosis volunteers were analysed by PCR. The expression of β‐actin was quantified as the internal control, and the indicated mRNA in each sample were normalized by β‐actin gene from the same sample. (c) Histological staining (haematoxylin–eosin, 100×), α‐SMA, and CB1 receptor (CB1R) immunohistochemical staining (brown, 200×, n = 6 per group) of liver tissues of the fibrosis patients and the non‐fibrosis volunteers. (d) The levels of α‐SMA, CB1 receptors and CB2 receptors (CB2R) in the representative tissues were determined by western blotting, and β‐actin was used as the loading control. The ratio of densitometry units of the normalized α‐SMA/β‐actin, CB1 receptor/β‐actin and CB2 receptor/β‐actin was also presented. (e) Sirius red staining (red, 200×), α‐SMA, and CB1 receptor staining (brown, 200×) were shown in the mouse liver tissues treated by olive oil (as Ctrl) or 20% CCl4. (f) Levels of α‐SMA, CB1 receptor and CB2 receptor protein in the indicated groups of mice were analysed by western blotting, and the ratio of densitometry units of the normalized α‐SMA/β‐actin, CB1 receptor/β‐actin, and CB2 receptor/β‐actin was also shown. All summary data are shown as means ± SEM from n = 6 samples per group. *P<.05, significantly different; NS, not significant, as indicated
3.2. The peripheral CB1 receptor antagonist JD5037 attenuated liver fibrosis in mice
Based on the finding that CB1 receptors were up‐regulated in liver fibrosis, the peripheral CB1 receptor antagonist JD5037 was used to investigate the role of these receptors in liver fibrogenesis (Knani et al., 2016; Mukhopadhyay et al., 2015). Based on the analysis of the effect of different doses of the CB1 receptor antagonist JD5037 on liver fibrosis in mice, we chose 3 mg·kg−1·day−1 JD5037 for further experiments (Figure S1). At this dose JD5037 ameliorated collagen deposition and the expression of α‐SMA and CB1 receptors in CCl4‐treated mice, without affecting those of the control group (Figure 2a–c). Next, we analysed the liver function and found that JD5037 attenuated liver dysfunction in CCl4‐induced fibrotic mice (Figure 2c). Consistent with the histological analysis, western blotting showed that JD5037 blocked the increase of collagen‐IV (COL‐IV), collagen‐I (COL‐I), and α‐SMA protein in CCl4‐treated mice (Figure 2d). By examining the concentrations of the four serum markers of hepatic fibrosis, hyaluronic acid, type IV collagen, pro‐collagen III N‐terminal peptide and laminin, we found that these markers were up‐regulated in CCl4‐treated mice, while the peripheral CB1 receptor antagonist JD5037 decreased their concentrations (Figure 2e). Moreover, using the bile duct ligation (BDL)‐induced model, we also found that the peripheral CB1 receptor antagonist JD5037 attenuated BDL‐induced liver fibrosis in mice (Figure S2A–C). These results suggest that inhibition of CB1 receptors by JD5037 attenuates liver fibrosis in mice.
Figure 2.
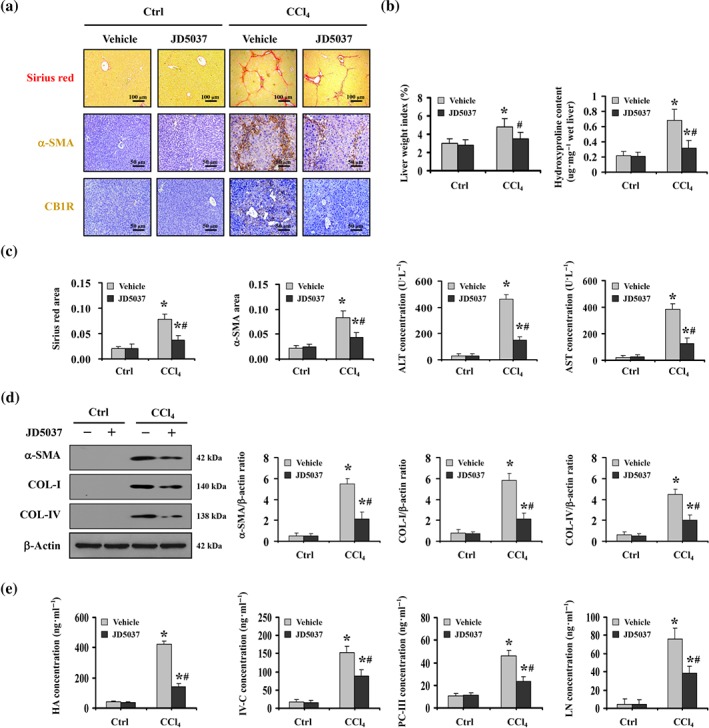
The peripheral CB1 receptor antagonist JD5037 attenuated liver fibrosis in mice. (a) Sirius red staining (red), α‐SMA, and CB1 receptor (CB1R) staining (brown) are presented in the indicated sections from olive oil‐ (as Ctrl) or CCl4‐treated mice, with or without JD5037 administration (n = 6 per group). (b) The liver wet weight ratio and the hydroxyproline content of the indicated sections were determined. Data shown are means ± SD from n = 6 mice per group. *P < .05, significantly different from Ctrl mice, # P < .05, significantly different from CCl4‐treated mice without JD5037 treatment, n = 6 per group. (c) Sirius red area and α‐SMA area from histological staining were shown (left panel). The liver function of the related mice was also determined (right panel). Data shown are means ± SD from n = 6 mice per group. *P < .05, significantly different from Ctrl mice, # P < .05, significantly different from CCl4‐treated mice without JD5037 treatment, (d) Western blotting assays were presented for the indicated treatments. The ratio of densitometry units of the normalized α‐SMA/β‐actin, COL‐I/β‐actin, and COL‐IV/β‐actin was also determined. Data shown are means ± SD from n = 6 mice per group. *P < .05, significantly different from Ctrl mice, # P < .05, significantly different from CCl4‐treated mice without JD5037 treatment. (e) The concentrations of hyaluronic acid (HA), Type IV collagen (IV‐C), pro‐collagen III N‐terminal peptide (PC‐III), and laminin (LN) from the indicated mice were measured by an ELISA kit. Data shown are means ± SD from n = 6 mice per group. *P < .05, significantly different from Ctrl mice, # P < .05, significantly different from CCl4‐treated mice without JD5037 treatment
3.3. β‐Arrestin1 was associated with the inhibition of the activation of HSCs and liver fibrosis, by JD5037
Previously, we reported that β‐arrestin1 was involved in liver fibrosis (Tan et al., 2019). Both histopathological assay and western blotting suggested that treatment with the peripheral CB1 receptor antagonist JD5037 alleviated the up‐regulation of β‐arrestin1 and α‐SMA in fibrotic liver sections from CCl4‐treated mice (Figure 3a,b). Furthermore, double immunofluorescence staining revealed a potential interaction between CB1 receptors and β‐arrestin1 contributing to liver fibrosis in both humans and mice, although there was also some cellular colocalization in the normal sections (Figure 3c,d). By using primary HSCs isolated from the mouse models, we found enhanced cellular colocalization of CB1 receptors and β‐arrestin1 in primary HSCs dissociated from CCl4‐treated mice (Figure 3e). Labelling primary HSCs with α‐SMA staining demonstrated that CB1 receptors and β‐arrestin1 contribute to the activation of HSCs in liver fibrosis (Figure 3f). Consistent with the above findings, immunoblotting showed decreased CB1 receptor, β‐arrestin1 and α‐SMA expression in primary HSCs from CCl4‐treated mice with JD5037, compared with expression in HSCs from CCl4‐treated mice without JD5037 (Figure 3g).
Figure 3.
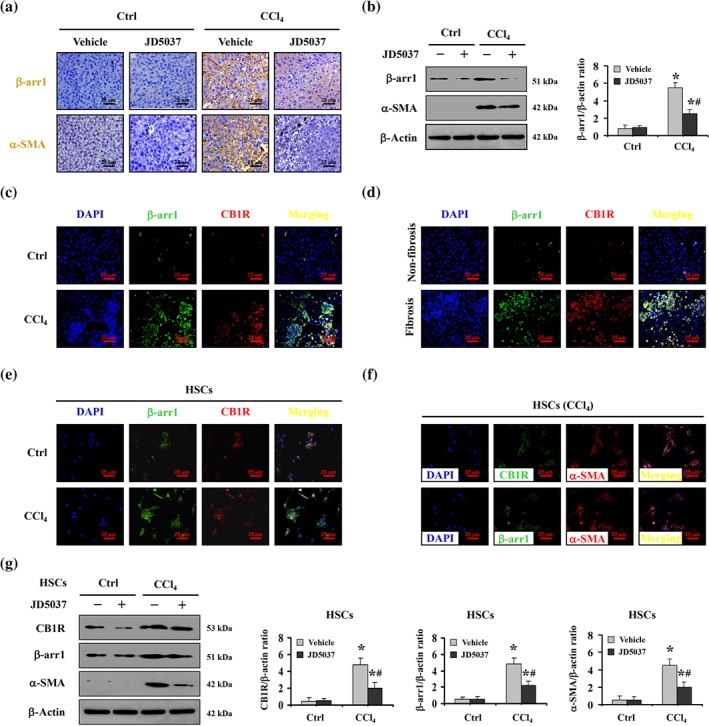
JD5037 suppressed the activation of HSCs and liver fibrosis by CB1 receptors with the involvement of β‐arrestin1. (a) Representative image of β‐arrestin1 (β‐arr1) and α‐SMA immunohistochemical staining (brown) in the indicated groups. (b) β‐Arrestin1 and α‐SMA levels were determined by western blotting, and the ratio of densitometry units of β‐arrestin1/β‐actin was shown. Data shown are means ± SD from n = 6 mice per group. *P < .05, significantly different from Ctrl mice, # P < .05, significantly different from CCl4‐treated mice without JD5037 treatment. (c) Double staining for β‐arrestin1 (green) and CB1 receptors (CB1R; red) with DAPI (blue) counterstaining for DNA in mouse models. (d) Co‐staining of β‐arrestin1 (green) and CB1 receptors (red) with DAPI (blue) counterstaining for DNA in human samples. (e) Double staining of β‐arrestin1 and CB1 receptors indicated that up‐regulation of β‐arrestin1 followed induction of CB1 receptors in primary hepatic stellate cells (HSCs) dissociated from CCl4‐treated mice. (f) Co‐staining of CB1 receptors (green) or β‐arrestin1 (green) and α‐SMA (red) in primary HSCs dissociated from CCl4‐treated mice. Cell nuclei (blue) were counterstained by DAPI. (g) Western blotting showed that JD5037 inhibited CB1 receptors, β‐arrestin1 and α‐SMA in primary HSCs dissociated from CCl4‐treated mice. The ratio of densitometry units of the normalized CB1 receptor/β‐actin, β‐arrestin1/β‐actin, and α‐SMA/β‐actin was shown. Data shown are means ± SD from n = 6 mice per group. *P < .05, significantly different from primary HSCs from the Ctrl group, # P < .05, significantly different from primary HSCs from CCl4‐treated mice without JD5037 treatment
Furthermore, CCl4‐induced β‐arr1‐WT and β‐arr1‐KO mouse models were used. As expected, targeted deletion of β‐arr1 ameliorated collagen deposition and α‐SMA level in the treated mice, although there was no significant distinction between β‐arr1‐WT and β‐arr1‐KO mice from the control group. Furthermore, JD5037 also repressed the collagen deposition and α‐SMA level in CCl4‐treated β‐arr1‐WT mice compared to that of β‐arr1‐KO mice (Figure 4a–c), suggesting that β‐arrestin1 played a prominent role in mediating CB1 receptor signalling in liver fibrosis. Both JD5037 and β‐arr1 deficiency alleviated the activation of primary HSCs dissociated from CCl4‐treated mice, as detected by α‐SMA expression (Figure 4d). Using α‐SMA as a marker of the activation of HSCs demonstrated that such activation was enhanced following CB1 receptor signalling in CCl4‐treated β‐arr1‐WT mice, compared with control mice, while JD5037 administration inhibited that process (Figure 4e). JD5037 suppressed the increase in α‐SMA and β‐arrestin1 in CCl4‐treated β‐arr1‐WT mice compared to that of β‐arr1‐KO mice, although deletion of β‐arr1 inhibited the expression of α‐SMA in CCl4‐treated mice, without affecting the state of CB1 receptors (Figure 4f). Altogether, these observations reveal that β‐arrestin1, as a downstream modulator of CB1 receptor signalling, was involved in the blockage by JD5037, of HSCs activation and liver fibrosis.
Figure 4.
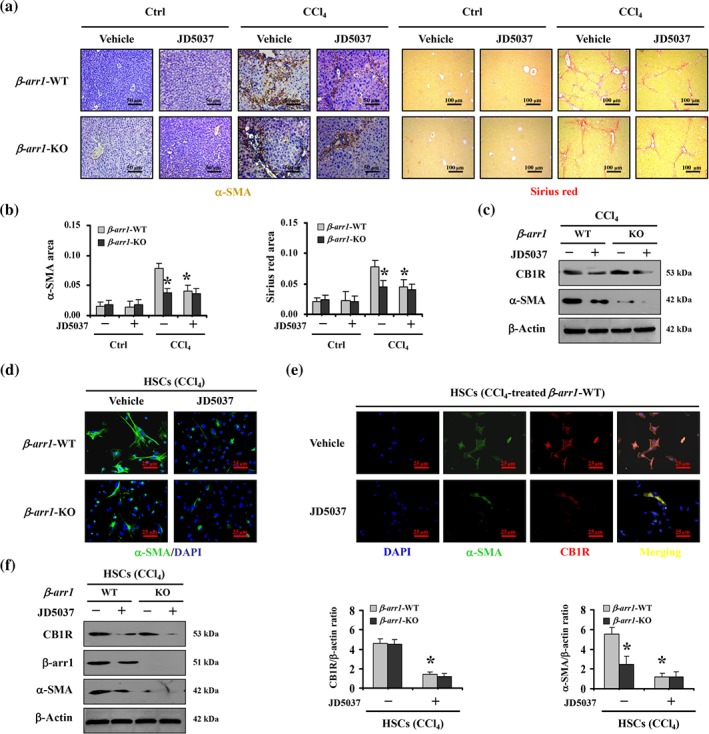
β‐Arrestin1 was involved in the blockage of HSCs activation and liver fibrosis, by JD5037. (a) α‐SMA staining (brown) and Sirius red staining (red) were represented both in β‐arr1‐WT mice and in β‐arr1‐KO mice from CCl4‐ or olive oil‐treated (as Ctrl) mice, with or without JD5037 treatment (n = 6 per group). (b) α‐SMA area and Sirius red area from histological staining were determined. Data shown are means ± SD from n = 6 mice per group. *P < .05, significantly different from CCl4‐treated β‐arr1‐WT mice without JD5037. (c) Western blotting presented JD5037 ameliorated the levels of α‐SMA in CCl4‐treated β‐arr1‐WT mice (n = 6 per group). (d) JD5037 or β‐arr1 deficiency inhibited α‐SMA expression of hepatic stellate cells (HSCs) in CCl4‐treated mice. Nuclei (blue) were counterstained with DAPI (400×, n = 6 per group). (e) Co‐staining of α‐SMA (green) and CB1 receptors (CB1R; red) in primary HSCs dissociated from olive oil‐ and CCl4‐treated β‐arr1‐WT mice, respectively. Cell nuclei (blue) were counterstained by DAPI. (f) The indicated proteins from primary HSCs in the mouse models were detected by western blotting. The ratio of densitometry units of the normalized CB1 receptor/β‐actin and α‐SMA/β‐actin was also determined. Data shown are means ± SD from n = 6 mice per group. *P < .05, significantly different from primary HSCs from CCl4‐treated β‐arr1‐WT mice without JD5037
3.4. JD5037 ameliorated the CB1 receptor/β‐arrestin1‐mediated activation of HSCs and liver fibrosis by suppressing Akt signalling
The Akt signalling pathway is related to cellular proliferation, and previous reports have shown that β‐arrestin1 mediates Akt phosphorylation to activate Akt signalling in response to a variety of stimuli (New, Wu, Kwok, & Wong, 2007; Smith & Rajagopal, 2016). Immunostaining and immunoblotting showed that the levels of p‐Akt (phosphorylated Akt), the active form of Akt, were enhanced in the sections from patients with liver fibrosis (Figure 5a,b). Subsequently, by assessing the liver tissues in mouse models, we found that CCl4‐treated β‐arr1‐WT mice showed enhanced expression of p‐Akt and α‐SMA compared with that in CCl4‐treated β‐arr1‐KO mice, and JD5037 administration repressed the elevations of p‐Akt and α‐SMA in CCl4‐treated β‐arr1‐WT mice compared to that of CCl4‐treated KO mice, although JD5037 treatment or β‐arr1 deficiency did not affect the status of p‐Akt and α‐SMA in the normal sections (Figure 5c–f). We also examined the key elements of the TGF‐β1‐induced canonical Smad‐dependent pathway and found that β‐arr1 deficiency did not affect the level of the TGF‐β1/Smad2/3 networks in CCl4‐treated mice, while JD5037 treatment suppressed the expression of TGF‐β1/Smad2/3 signalling in CCl4‐treated mice, regardless of β‐arr1 knockout (Figure S3). Furthermore, we investigated whether β‐arrestin1 and Akt interacted with CB1 receptors in the primary HSCs isolated from olive oil‐ or CCl4‐treated mice. As expected, interaction between β‐arrestin1, Akt, and CB1 receptors was detected in the primary HSCs isolated from CCl4‐treated WT mice but not in those from the control group in WT mice. In KO mice following CCl4 treatment, no definite interaction was observed (Figure 6a). Primary HSCs lysates from β‐arr1‐WT and KO mice were immunoprecipitated with anti‐CB1 receptor antibody and then detected with anti‐Akt and anti‐β‐arrestin1 antibodies, showing a definite mutual effect among CB1 receptors, β‐arrestin1 and Akt (Figure 6b). Using an anti‐Akt antibody to immunoprecipitate with primary HSCs lysates, we also detected a similar effect (Figure 6b).
Figure 5.
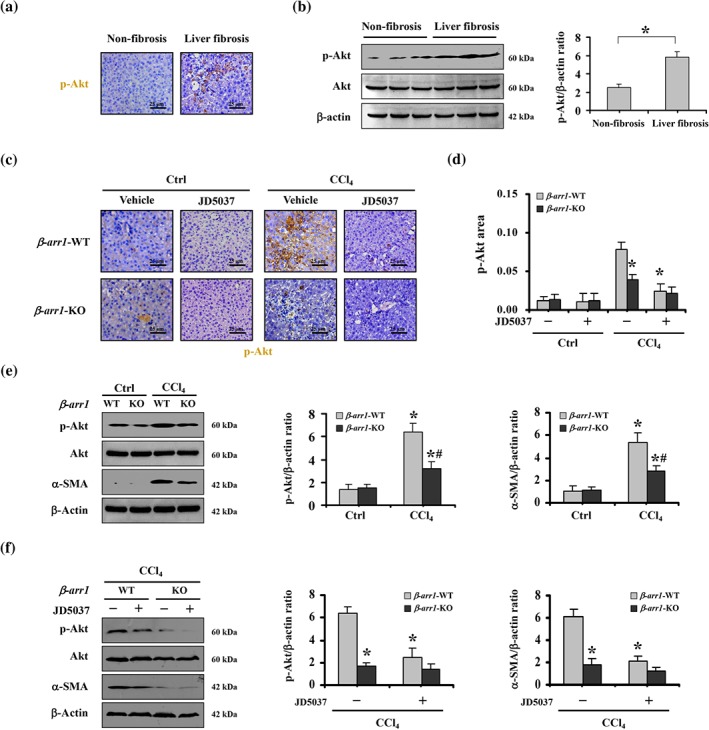
JD5037 ameliorated the activation of HSCs and liver fibrosis mediated by the CB1 receptor/β‐arrestin1 pathway by suppressing Akt signalling. (a) p‐Akt expression in non‐fibrotic and fibrotic liver tissues was evaluated by immunohistochemical staining (brown, 400×). (b) p‐Akt expression in non‐fibrotic and fibrotic liver tissues was analysed by western blotting andthe ratio of densitometry units of the normalized p‐Akt/β‐actin was also determined. Data shown are means ± SD from n = 6 mice per group. *P < .05, significantly different as indicated. (c) p‐Akt expression from the indicated group was examined by immunohistochemical staining (brown, 400×). (d) p‐Akt area from immunohistochemical staining was evaluated. Data shown are means ± SD from n = 6 mice per group. *P < .05, significantly different from CCl4‐treated β‐arr1‐WT mice without JD5037 treatment. (e) Targeted deletion of β‐arr1 blocked the up‐regulation of p‐Akt and α‐SMA in CCl4‐treated mice. The ratio of densitometry units of the normalized p‐Akt/β‐actin and α‐SMA/β‐actin was shown. Data shown are means ± SD from n = 6 mice per group. *P < .05, significantly different from Ctrl mice, # P < .05, significantly different from β‐arr1‐WT mice in the CCl4‐treated mice. (f) JD5037 inhibited the levels of p‐Akt and α‐SMA in CCl4‐treated β‐arr1‐WT mice, without affecting that in β‐arr1‐KO mice. The ratio of densitometry units of the normalized p‐Akt/β‐actin and α‐SMA/β‐actin was shown. Data shown are means ± SD from n = 6 mice per group. *P < .05, significantly different from CCl4‐treated β‐arr1‐WT mice without JD5037 treatment
Figure 6.
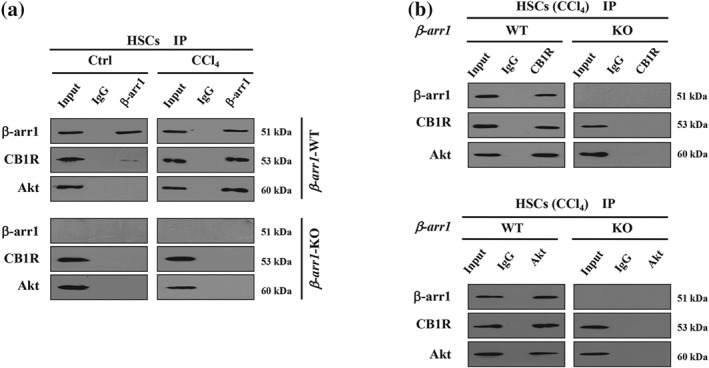
The interactions between β‐arrestin1, Akt, and CB1 receptors involved in the activation of HSCs during liver fibrosis in mice. (a) Lysates of primary HSCs from β‐arr1‐WT and β‐arr1‐KO mice were immunoprecipitated with anti‐β‐arrestin1 (β‐arr1) antibody and then analysed by anti‐CB1 receptor (CB1R) and anti‐Akt antibodies, respectively. (b) Primary HSCs lysates from the relative β‐arr1‐WT and β‐arr1‐KO mice were immunoprecipitated with anti‐CB1 receptor (upper panel) or anti‐Akt antibody (lower panel) and then analysed by the indicated antibodies
Further analysis using the Akt inhibitor VIII revealed that, as seen with JD5037, the Akt inhibitor blocked α‐SMA up‐regulation and collagen deposition following CCl4 treatment (Figure 7a,b). Furthermore, in primary HSCs, we found that α‐SMA levels in cells isolated from CCl4‐treated β‐arr1‐WT mice were visibly up‐regulated, while both JD5037 and Akt inhibitor VIII decreased the activation of HSCs, as assesed by α‐SMA levels (Figure 7c). Consistent with the histological analysis, western blotting confirmed that inhibition of Akt suppressed α‐SMA up‐regulation but had no effect on the induction of CB1 receptor, β‐arrestin1 and TGF‐β1/Smad2/3 signalling in primary HSCs isolated from CCl4‐treated WT mice. JD5037 suppressed all of them in these cells (Figure 7d,e). Moreover, primary HSCs isolated from the BDL‐induced mouse model showed similar conditions as those from CCl4‐treated model (Figure S2D). To sum up, these data showed that CB1 receptors recruit β‐arrestin1 to up‐regulate Akt signalling during the development of liver fibrosis and that JD5037 ameliorates the activation of HSCs and liver fibrosis, through the CB1 receptor/β‐arrestin1/Akt signalling pathway.
Figure 7.
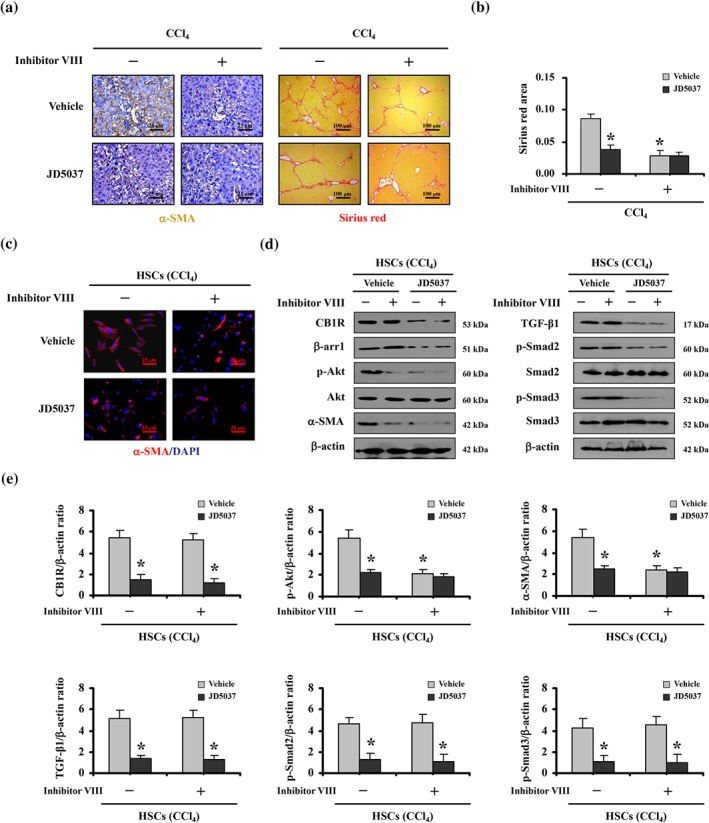
Inhibition of Akt ameliorated CB1 receptor/β‐arrestin1‐regulated activation of HSCs and liver fibrosis. (a) Treatment with the Akt inhibitor VIII down‐regulated α‐SMA expression (brown) and Sirius red area (red) in mice. (b) Sirius red area was analysed. Data shown are means ± SD from n = 6 mice per group. *P < .05, significantly different from vehicle‐treated mice without Akt inhibitor VIII treatment. (c) Primary HSCs dissociated from CCl4‐treated mice with the indicated treatment were stained by α‐SMA (red). Nuclei (blue) were counterstained with DAPI (400×). (d) The indicated proteins from primary HSCs analysed by western blotting revealed that Akt inhibitor VIII suppressed α‐SMA up‐regulation, while having no effect on the regulation of CB1 receptors (CB1R), β‐arrestin1 (β‐arr1) or TGF‐β1/Smad2/3 signalling in primary HSCs isolated from CCl4‐treated mice. (e) The ratio of densitometry units of the normalized CB1 receptor/β‐actin, p‐Akt/β‐actin, α‐SMA/β‐actin, TGF‐β1/β‐actin, p‐Smad2/β‐actin, and p‐Smad3/β‐actin from western blotting assay was shown. Data shown are means ± SD from n = 6 mice per group. *P < .05, significantly different from vehicle‐treated mice without Akt inhibitor VIII treatment
3.5. JD5037 promoted apoptosis and inhibited proliferation of HSCs in liver fibrosis through the CB1 receptor/β‐arrestin1/Akt signalling pathway
It has been known that the activation leading to growth of HSCs is the major source of ECM and the hallmark of liver fibrosis, and the loss or death of HSCs is commonly regarded as a target for antifibrotic therapy. To evaluate the role of JD5037 in the status of HSCs during liver fibrosis, the apoptotic and proliferative states of liver sections were analysed. We found that the level of the proliferative protein PCNA was enhanced in the livers of fibrotic mice, while JD5037 administration suppressed proliferation (PCNA staining) and enhanced hepatic apoptosis (TUNEL staining), which was associated with attenuated liver fibrosis (Figure 8a,b). By using primary HSCs isolated from the mouse models, we found that enhanced HSCs proliferation was present in primary HSCs dissociated from CCl4‐treated mice, and JD5037 treatment suppressed HSC proliferation and promoted HSC apoptosis during liver fibrogenesis (Figure 8c). Furthermore, immunoblotting analysis showed that JD5037 inhibited the elevation of CB1 receptors, β‐arrestin1, p‐Akt, α‐SMA, and PCNA, while enhancing the level of cleaved caspase‐3 in primary HSCs isolated from CCl4‐treated mice model, but it did not affect the status of HSCs isolated from the normal mouse models (Figure 8d). JD5037 also suppressed the elevation of TGF‐β1 and the phosphorylation of Smad2/3 in the primary HSCs isolated from CCl4‐treated mice (Figure 8e). In addition, we transfected the CB1R plasmid into the human HSC line LX2 and found that the levels of β‐arrestin1, p‐Akt, α‐SMA, and PCNA were significantly increased, accompanied by down‐regulation of the pro‐apoptotic element cleaved caspase‐3 (Figure S4A). Then, we down‐regulated CB1R by siRNA in the LX2 cell line and observed that the expressions of β‐arrestin1, p‐Akt, α‐SMA, and PCNA were inhibited, and the expression of cleaved caspase‐3 was clearly enhanced by CB1R knockdown (Figure S4B). These results reveal that the status of CB1 receptors influences the activation of HSCs via the β‐arrestin1/Akt signalling pathway. In summary, these findings indicate that the peripheral CB1 receptor antagonist JD5037 promotes apoptosis and inhibits proliferation of HSCs in liver fibrosis, mainly by modulating the CB1 receptor/β‐arrestin1/Akt signalling pathway (Figure 9).
Figure 8.
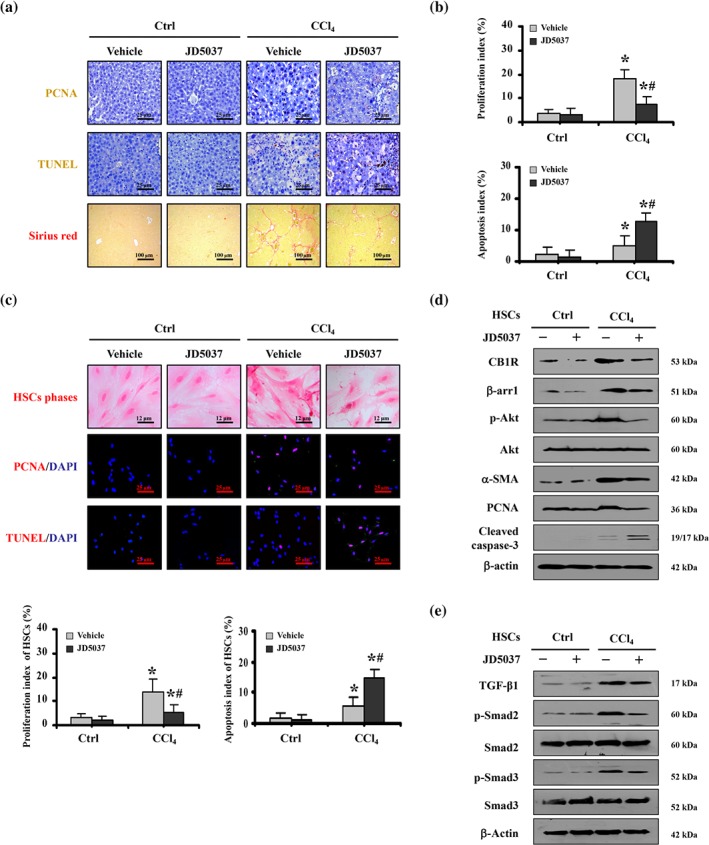
JD5037 promoted apoptosis and inhibited proliferation of HSCs in liver fibrosis mediated by CB1 receptor/β‐arrestin1/Akt signalling. (a) Treatment with JD5037 suppressed hepatic proliferation (PCNA staining, 400×, brown) and fibrosis (Sirius red, 100×, red), and enhanced the apoptosis (TUNEL staining, 400×, brown) in CCl4‐treated mice. n = 6 per group. (b) Summary data on the proliferation index and the apoptosis index. Data shown are means ± SD from n = 6 mice per group. *P < .05, significantly different from Ctrl mice, # P < .05, significantly different from CCl4‐treated mice without JD5037 treatment. (c) HSCs phases (Sirius red, 800×, red) and staining for PCNA (400×, red) and TUNEL (400×, red) in primary HSCs dissociated from mice with the indicated treatments. Cell nuclei (blue) were counterstained by DAPI. The proliferation and apoptosis indices were shown (lower panel), Data shown are means ± SD from n = 6 mice per group. *P < .05, significantly different from Ctrl mice, # P < .05, significantly different from CCl4‐treated mice without JD5037 treatment. (d) The indicated proteins from primary HSCs in the related mouse models were detected by western blotting. (e) TGF‐β1/Smad2/3 signalling in primary HSCs isolated from the indicated mice was also analysed by western blotting
Figure 9.
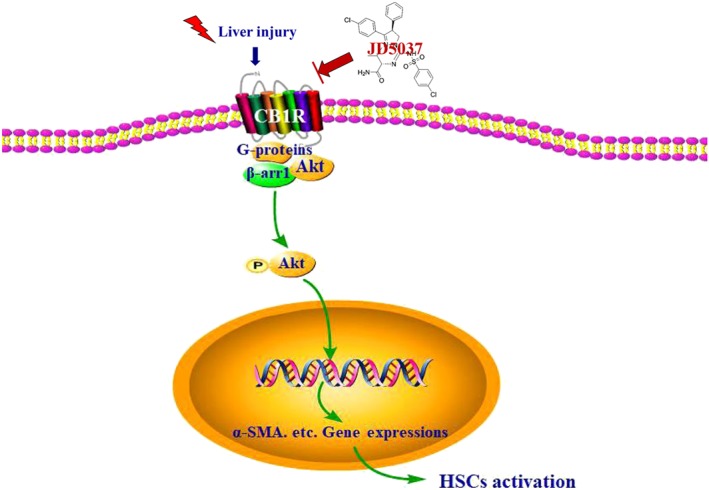
Diagram of the role of the peripheral CB1 receptor antagonist JD5037 in the blockage of the activation of HSCs, driven by the CB1 receptor/β‐arrestin1/Akt signalling pathway, in liver fibrosis. CB1R, CB1 receptor; β‐arr1, β‐arrestin1
4. DISCUSSION
Liver fibrosis is a complex reversible fibrogenic process that results from a variety of liver injuries and is characterized by excess accumulation of ECM in the liver (Lee et al., 2015; Sato‐Matsubara et al., 2017). Studies of the behaviour of HSCs during development, regeneration, and fibrotic formation have provided substantial insight that a reduction in the number of HSCs is critical to the reversibility of fibrosis (Matsuda et al., 2018). In the current study, we found that the up‐regulation of CB1 receptors promoted activation of HSCs and enhanced the progression of liver fibrosis via regulating the β‐arrestin1/Akt pathway. As a GPCR, the CB1 receptor is one of the most abundant receptors in the mammalian CNS, albeit it also presents and functions in a variety of cells and tissues (Mallat et al., 2013; Wang, Meng, Chang, & Tang, 2016). Studies have revealed that CB1 receptors are enhanced in hepatic myofibroblasts/HSCs of cirrhotic patients and fibrotic mouse models following bile duct ligation, chronic exposure to CCl4 or thioacetamide, and prolonged high fat feeding. Furthermore, treatment with the relevant antagonists (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=743 or MRI‐1867) or genetic ablation of CB1R could ameliorate hepatic fibrogenesis (Cinar et al., 2016; Liu et al., 2016; Mallat et al., 2013; Teixeira‐Clerc et al., 2006). Given the critical roles that the CB1 receptor plays in liver diseases, by analysing the liver sections from both humans and mice, we found that CB1 receptors, which were accompanied by up‐regulated α‐SMA and excess ECM, presented distinctly higher expression in the fibrotic tissues than in normal liver sections. Furthermore, the peripheral CB1 receptor antagonist JD5037 ameliorated ECM deposition, α‐SMA and CB1 receptor expressions, and liver dysfunction in CCl4‐treated mice, suggesting the potential effect of JD5037 in blocking liver fibrogenesis.
β‐Arrestin1 was originally identified as a signal terminator for GPCR signalling, but accumulating evidence over the last decade demonstrates that β‐arrestin1 also serves as a scaffold protein and functions as a signal transducer by facilitating the interaction of signalling molecules and scaffolding upon activation of GPCRs, including the Hedgehog, https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=964, https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=914, and TGF‐β pathways, and downstream kinases such as the MAPKs and https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=781/Akt pathways (Ahn et al., 2013; Gu, Sun, Zhang, Wu, & Wei, 2015; Whalen, Rajagopal, & Lefkowitz, 2011). β‐Arrestin1 has been established as an important modulator of GPCR signalling and plays a role in the pathogenesis of bleomycin‐induced pulmonary fibrosis, renal fibrosis, multiple sclerosis, and primary biliary cirrhosis (Gu et al., 2015; Lovgren et al., 2011; Nakaya et al., 2012; Tan et al., 2019). Consistent with these findings, a distinctly higher expression of β‐arrestin1 was present in fibrotic tissues than in normal liver tissues, and enhanced cellular colocalization of CB1 receptors and β‐arrestin1 was detected in the primary HSCs isolated from CCl4‐treated mice. Simultaneously, peripheral CB1 receptor blockage by JD5037 alleviated the up‐regulation of β‐arrestin1, α‐SMA, and ECM deposition in fibrotic liver sections, revealing that β‐arrestin1 contributes to the CB1 receptor‐regulated activation of HSCs and liver fibrosis, and JD5037 attenuates activation of HSCs and liver fibrosis through regulating CB1 receptor/β‐arrestin1 signalling.
A recent study revealed that loss of function of either β‐arrestin1 or β‐arrestin2 protected mice from excessive ECM deposition and architectural distortion by limiting the ability of myofibroblasts in pulmonary fibrosis (Lovgren et al., 2011). We demonstrated that deletion of β‐arr1 alleviated collagen deposition, α‐SMA level, and HSCs activation in CCl4‐treated mice without influencing the state of CB1 receptors. Moreover, inhibition of CB1 receptors by JD5037 suppressed collagen deposition and HSCs activation in CCl4‐treated β‐arr1‐WT mice rather than that in β‐arr1‐KO mice, revealing β‐arrestin1 as a downstream modulator in the CB1 receptor pathway in liver fibrogenesis. These results strongly suggest a potential role for CB1 receptor/β‐arrestin1 signalling in the modulation of HSC activation in liver fibrosis and clearly indicate that JD5037 potentially blocks HSCs activation during this process. Furthermore, several pharmacological studies showed that JD5037 alleviated obesity by reversing https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5015 resistance in diet‐induced obesity mice, reversed hepatic insulin resistance, and improved metabolic parameters (Cinar et al., 2014; Cota, 2017; Tam et al., 2017). Thus, the peripherally restricted CB1 receptor antagonist JD5037 could represent a novel and rational therapeutic approach to treat complicated liver disorders.
Recent years have produced a growing body of evidence that clearly established GPCRs and their scaffolding proteins, such as β‐arrestin1, are the key initiators of the modulation of Akt‐dependent signalling events in a variety of cells and tissues (Niu et al., 2018; Smith & Rajagopal, 2016). Our previous data revealed that β‐arrestin1 promoted hepatocellular proliferation through Akt signalling, and β‐arrestin1 regulated mucosal proliferation by promoting https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2206/https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1797/Akt/PCNA signalling activation in portal hypertensive gastropathy (Tan et al., 2017; Yang et al., 2015). Furthermore, Akt signalling played a crucial role in the regulation of HSCs function and HSCs‐modulated ECM in hepatic fibrosis (Krycer, Sharpe, Luu, & Brown, 2010; Li, Ou‐Yang, & Han, 2014). JD5037 was similarly effective in reversing insulin resistance in diet‐induced obesity mouse models, and hepatic insulin resistance has been linked to the repression of insulin‐induced Akt phosphorylation (the active form of Akt) in the case of both ceramide and anandamide, revealing that JD5037 could modulate Akt signalling in several conditions (Cinar et al., 2014; Liu et al., 2012; Schmitz‐Peiffer, 2010). In the current study, consistent with these studies, we found that Akt phosphorylation was enhanced in the sections from fibrotic livers. In addition to decreased α‐SMA levels, targeted deletion of β‐arr1 ameliorated Akt phosphorylation in fibrotic mice. JD5037 administration repressed Akt phosphorylation and α‐SMA elevation in CCl4‐treated β‐arr1‐WT mice rather than that in CCl4‐treated KO mice. As expected, further analysis using the Akt inhibitor revealed that Akt was the downstream executor of the CB1 receptor/β‐arrestin1‐mediated signalling in HSC activation, and CB1 receptors could recruit β‐arrestin1 to enhance Akt phosphorylation in the activation of HSCs during liver fibrogenesis.
TGF‐β1 signalling has been widely demonstrated to phosphorylate Smad2, and Smad3 associates with Smad4 to translocate into the nucleus to regulate the development of liver fibrosis. Some studies have revealed that the antagonism or genetic ablation of CB1 receptors attenuates TGF‐β signalling to mitigate liver fibrosis (Cinar et al., 2016; Liu et al., 2016; Mallat et al., 2013; Teixeira‐Clerc et al., 2006). In our current study, we also examined the key elements of the TGF‐β1‐induced canonical Smad‐dependent pathway and found that β‐arr1 deficiency did not affect the level of the TGF‐β1/Smad2/3 networks in CCl4‐treated mice, while JD5037 treatment suppressed the expression of TGF‐β1/Smad2/3 signalling in CCl4‐treated mice, regardless of β‐arr1 knockout. Moreover, JD5037 administration also inhibited TGF‐β1/Smad2/3 signalling in the primary HSCs isolated from CCl4‐treated mice. These data suggest that JD5037 regulates TGF‐β1/Smad2/3 signalling to modulate liver fibrosis development via a β‐arrestin1/Akt‐independent pathway, and further study is needed to delineate whether combined approaches with the β‐arrestin1/Akt pathway and TGF‐β1/Smad2/3 signalling may pave the way for synergistic antifibrotic treatments. Finally, we demonstrated that JD5037 promoted HSCs apoptosis and inhibited HSCs proliferation by blocking the CB1 receptor/β‐arrestin1/Akt signalling pathway to attenuate liver fibrosis, showing that CB1 receptor blockage by JD5037 may be a viable option to combat liver fibrosis and may move to clinical testing in the near future. The induction of CB1 receptors is a common feature of liver injuries of diverse origins, and these receptors are expressed by multiple cell types in liver, including HSCs, hepatocytes, macrophages (Kupffer cells), and endothelial cells, which contribute to the pathogenesis of liver fibrosis (Cinar et al., 2016). In our current study, we solely revealed that JD5037 promoted apoptosis and inhibited activation or proliferation of HSCs by regulating the CB1 receptor/β‐arrestin1/Akt signalling pathway to mitigate liver fibrosis. Further study is needed to explore the relative contributions of the different types of liver cells as potential targets of the antifibrotic effect of CB1 receptor antagonism.
Many data reveal that the pharmacological modulation of CB1 receptor signalling should be able to not only prevent the progression of fibrosis by starting drug administration immediately after fibrogenic initiation but also mitigate established fibrosis even in advanced cirrhosis. Our current study mainly assessed the prevention of fibrosis by starting treatments at the beginning of fibrogenesis, and further study on the therapeutic potential of JD5037 in established fibrosis/cirrhosis is needed. Moreover, due to the multiple molecular mechanisms and signalling pathways involved in liver fibrosis, the efficacy of a single‐target antagonist would not be enough in the treatment of liver fibrosis, and the engagement of antagonists targeting more than one molecular or signalling would have promise of an exciting higher antifibrotic efficacy. Based on this, Cinar et al. introduced a dual‐target peripheral CB1 receptor/iNOS antagonist, MRI‐1867, which significantly enhanced the antifibrotic efficacy of the traditional antagonists (Cinar et al., 2016). Thus, a peripherally restricted CB1 receptor antagonist, which could accumulate in the liver and release some metabolites with the antifibrogenic activity to affect more than one molecular or signalling mechanism would contribute to the robust antifibrotic efficacy and higher drug safety in future clinical applications.
In summary, the present investigation provides strong evidence for the effect of CB1 receptors and its peripheral antagonist JD5037 on the activation of HSCs in liver fibrosis and that blocking the CB1 receptor/β‐arrestin1/Akt signalling pathway with JD5037 might provide a novel, potentially therapeutic strategy for liver fibrosis (Figure 9).
AUTHOR CONTRIBUTIONS
S.W.T. performed the experiments and analysed the data. H.L.L. collected the clinical samples and conducted clinical study. B.L.K. contributed the essential reagents and conducted the animals study. J.J. planned and conducted primary cells isolation and cells culture study. B.W. designed the whole project, supervised the research, and wrote the paper.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14208, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1. Analysis of the effect of different doses of the CB1R antagonist JD5037 on liver fibrosis in mice. (A) The influence of different doses of JD5037 on liver fibrosis was examined by Sirius red staining (red, ×100). (B) Sirius red area from histological staining was shown. *P<0.05 versus the other groups from CCl4‐treated mice with different JD5037 doses, n = 6 per group. (C) The influence of different doses of JD5037 on liver fibrosis was determined by western blotting analysis of α‐SMA levels, and the ratio of densitometry units of α‐SMA/β‐actin was also revealed. *P<0.05 versus the other groups from CCl4‐treated mice with different JD5037 doses, n = 6 per group.
Figure S2. CB1R antagonist JD5037 attenuated bile duct ligation (BDL)‐induced liver fibrosis in mice. (A) Sirius red staining (red), α‐SMA and CB1R staining (brown) were presented in the indicated sections from Sham‐operated control (as Sham) or BDL‐treated mice, with or without JD5037 administration. (B) Sirius red area and α‐SMA area from histological staining were shown (upper panel). The liver wet weight index and the hydroxyproline content of the indicated sections were also determined (lower panel). *P<0.05 versus Sham mice, #P<0.05 versus BDL‐treated mice without JD5037 treatment, n = 6 per group. (C) Western blotting assay were presented in the indicated treatment, revealing that JD5037 blocked the proteins elevations of collagen‐IV (COL‐IV), collagen‐I (COL‐I) and α‐SMA in BDL‐treated mice. n = 6 per group. (D) The indicated proteins from primary HSCs isolated from the related mouse models were detected by western blotting. n = 6 per group.
Figure S3. JD5037 repressed TGF‐β1/Smad2/3 signaling in CCl4‐treated mice via a β‐arr1‐independent pathway. (A) Targeted deletion of β ‐arr1 did not affect the levels of TGF‐β1/Smad2/3 signaling networks in CCl4‐treated mice. The ratio of densitometry units of p‐Smad2/β‐actin and p‐Smad3/β‐actin was revealed. *P<0.05 versus Ctrl mice, n = 6 per group. (B) JD5037 inhibited the signal activation of TGF‐β1/Smad2/3 in CCl4‐treated mice, regardless of β ‐arr1 knockout. The ratio of densitometry units of p‐Smad2/β‐actin and p‐Smad3/β‐actin was shown. *P<0.05 versus CCl4‐treated mice without JD5037 treatment, n = 6 per group.
Figure S4. The status of CB1R influenced the activation of HSCs via β‐arr1/Akt networks. (A) CB1R transfection increased the levels of β‐arr1, p‐Akt, α‐SMA and PCNA, while repressed the level of cleaved caspase‐3 in human stellate cells line (LX2 cells). (B) CB1R expression was silenced by CB1R‐siRNA. CB1R knockdown inhibited the levels of β‐arr1, p‐Akt, α‐SMA and PCNA, while increased the level of cleaved caspase‐3 in LX2 cells
Table S1. Characteristics of the non‐fibrosis individuals and liver fibrosis patients
ACKNOWLEDGEMENTS
This work was partly funded by the grants from the National Natural Science Foundation of China (81700536 and U1501224), the Natural Science Foundation Team Project of Guangdong Province (2018B03031200), the Science and Technology Developmental Special Foundation of Guangdong Province (2017B020226003), the Basic Research Program of Young Teachers' Training Project of Sun Yat‐Sen University (17ykpy51), and the Science and Technology Planning Projects of Guangzhou City (201804010026 and 201604020002).
Tan S, Liu H, Ke B, Jiang J, Wu B. The peripheral CB1 receptor antagonist JD5037 attenuates liver fibrosis via a CB1 receptor/β‐arrestin1/Akt pathway. Br J Pharmacol. 2020;177:2830–2847. 10.1111/bph.15010
Siwei Tan and Huiling Liu contributed equally.
REFERENCES
- Ahn, K. H. , Mahmoud, M. M. , Shim, J. Y. , & Kendall, D. A. (2013). Distinct roles of β‐arrestin 1 and β‐arrestin 2 in ORG27569‐induced biased signaling and internalization of the cannabinoid receptor 1 (CB1). The Journal of Biological Chemistry, 288, 9790–9800. 10.1074/jbc.M112.438804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … Davies, J. A. (2017). The concise guide to pharmacologY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174, S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … Davies, J. A. (2017). The concise guide to pharmacology 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. , Peters, J. A. , Kelly, E. , Marrion, N. V. , Faccenda, E. , Harding, S. D. , … Davies, J. A. (2017). The concise guide to pharmacology 2017/18: Ligand‐gated ion channels. British Journal of Pharmacology, 174, S130–S159. 10.1111/bph.13879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. , Roberts, R. E. , Broughton, B. , Sobey, C. G. , George, C. H. , … Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology . British Journal of Pharmacology, 175(3), 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraceni, P. , Domenicali, M. , Giannone, F. , & Bernardi, M. (2009). The role of the endocannabinoid system in liver diseases. Best Practice & Research Clinical Endocrinology & Metabolism, 23, 65–77. 10.1016/j.beem.2008.10.009 [DOI] [PubMed] [Google Scholar]
- Christensen, R. , Kristensen, P. K. , Bartels, E. M. , Bliddal, H. , & Astrup, A. (2007). Efficacy and safety of the weight‐loss drug rimonabant: A meta‐analysis of randomised trials. Lancet, 370, 1706–1713. 10.1016/S0140-6736(07)61721-8 [DOI] [PubMed] [Google Scholar]
- Cinar, R. , Godlewski, G. , Liu, J. , Tam, J. , Jourdan, T. , Mukhopadhyay, B. , … Kunos, G. (2014). Hepatic cannabinoid‐1 receptors mediate diet‐induced insulin resistance by increasing de novo synthesis of long‐chain ceramides. Hepatology, 59, 143–153. 10.1002/hep.26606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinar, R. , Iyer, M. R. , Liu, Z. , Cao, Z. , Jourdan, T. , Erdelyi, K. , … Kunos, G. (2016). Hybrid inhibitor of peripheral cannabinoid‐1 receptors and inducible nitric oxide synthase mitigates liver fibrosis. JCI Insight, 1(11), e87336 10.1172/jci.insight.87336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll, M. , Perea, L. , Boon, R. , Leite, S. B. , Vallverdu, J. , Mannaerts, I. , … Sancho‐Bru, P. (2018). Generation of hepatic stellate cells from human pluripotent stem cells enables in vitro modeling of liver fibrosis. Cell Stem Cell, 23, 101–113. 10.1016/j.stem.2018.05.027 [DOI] [PubMed] [Google Scholar]
- Cota, D. (2017). The brain strikes back: Hypothalamic targets for peripheral CB1 receptor inverse agonism. Molecular Metabolism, 6, 1077–1078. 10.1016/j.molmet.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175(7), 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, E. , Zhang, L. , Ye, L. , Wan, S. , Feng, L. , Qi, Q. , … Li, Z. (2017). Hepatic expression of cannabinoid receptors CB1 and CB2 correlate with fibrogenesis in patients with chronic hepatitis B. International Journal of Infectious Diseases, 59, 124–130. 10.1016/j.ijid.2017.03.008 [DOI] [PubMed] [Google Scholar]
- Deng, X. , Chen, Y. X. , Zhang, X. , Zhang, J. P. , Yin, C. , Yue, H. Y. , … Xie, W. F. (2008). Hepatic stellate cells modulate the differentiation of bone marrow mesenchymal stem cells into hepatocyte‐like cells. Journal of Cellular Physiology, 217, 138–144. 10.1002/jcp.21481 [DOI] [PubMed] [Google Scholar]
- Garcia‐Ruiz, I. , Blanes, R. N. , Rada, P. , Pardo, V. , Ruiz, L. , Blas‐Garcia, A. , … Valverde, A. M. (2019). Protein tyrosine phosphatase 1b deficiency protects against hepatic fibrosis by modulating nadph oxidases. Redox Biology, 26, 101263 10.1016/j.redox.2019.101263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruden, G. , Barutta, F. , Kunos, G. , & Pacher, P. (2016). Role of the endocannabinoid system in diabetes and diabetic complications. British Journal of Pharmacology, 173, 1116–1127. 10.1111/bph.13226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, Y. J. , Sun, W. Y. , Zhang, S. , Wu, J. J. , & Wei, W. (2015). The emerging roles of β‐arrestins in fibrotic diseases. Acta Pharmacologica Sinica, 36, 1277–1287. 10.1038/aps.2015.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, S. S. , Chen, D. Z. , Wu, H. , Chen, R. C. , Du, S. J. , Dong, J. J. , … Chen, Y. P. (2016). Cannabinoid receptors are involved in the protective effect of a novel curcumin derivative C66 against CCl4‐induced liver fibrosis. European Journal of Pharmacology, 779, 22–30. 10.1016/j.ejphar.2016.02.067 [DOI] [PubMed] [Google Scholar]
- Inzaugarat, M. E. , Johnson, C. D. , Holtmann, T. M. , McGeough, M. D. , Trautwein, C. , Papouchado, B. G. , … Feldstein, A. E. (2018). NLRP3 inflammasome activation in hepatic stellate cells induces murine liver fibrosis. Hepatology, 69, 845–859. 10.1002/hep.30252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo, A. A. , & Camilleri, M. (2008). Emerging role of cannabinoids in gastrointestinal and liver diseases: Basic and clinical aspects. Gut, 57, 1140–1155. 10.1136/gut.2008.148791 [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. 10.1111/j.1476-5381.2010.00872.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knani, I. , Earley, B. J. , Udi, S. , Nemirovski, A. , Hadar, R. , Gammal, A. , … Tam, J. (2016). Targeting the endocannabinoid/CB1 receptor system for treating obesity in Prader–Willi syndrome. Molecular Metabolism, 5, 1187–1199. 10.1016/j.molmet.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krycer, J. R. , Sharpe, L. J. , Luu, W. , & Brown, A. J. (2010). The Akt‐SREBP nexus: Cell signaling meets lipid metabolism. Trends in Endocrinology and Metabolism, 21, 268–276. 10.1016/j.tem.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Lee, Y. A. , Wallace, M. C. , & Friedman, S. L. (2015). Pathobiology of liver fibrosis: A translational success story. Gut, 64, 830–841. 10.1136/gutjnl-2014-306842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. J. , Ou‐Yang, P. H. , & Han, X. P. (2014). Profibrotic effect of miR‐33a with Akt activation in hepatic stellate cells. Cellular Signaling, 26, 141–148. 10.1016/j.cellsig.2013.09.018 [DOI] [PubMed] [Google Scholar]
- Liu, J. , Zhou, L. , Xiong, K. , Godlewski, G. , Mukhopadhyay, B. , Tam, J. , … Kunos, G. (2012). Hepatic cannabinoid receptor‐1 mediates diet‐induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology, 142, 1218–1228. 10.1053/j.gastro.2012.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. Y. , Alexa, K. , Cortes, M. , Schatzman‐Bone, S. , Kim, A. J. , Mukhopadhyay, B. , … Goessling, W. (2016). Cannabinoid receptor signaling regulates liver development and metabolism. Development, 143, 609–622. 10.1242/dev.121731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovgren, A. K. , Kovacs, J. J. , Xie, T. , Potts, E. N. , Li, Y. , Foster, W. M. , … Noble, P. W. (2011). β‐Arrestin deficiency protects against pulmonary fibrosis in mice and prevents fibroblast invasion of extracellular matrix. Science Translational Medicine, 3, 23–74. 10.1126/scitranslmed.3001564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat, A. , Teixeira‐Clerc, F. , & Lotersztajn, S. (2013). Cannabinoid signaling and liver therapeutics. Journal of Hepatology, 59, 891–896. 10.1016/j.jhep.2013.03.032 [DOI] [PubMed] [Google Scholar]
- Matsuda, M. , Tsurusaki, S. , Miyata, N. , Saijou, E. , Okochi, H. , Miyajima, A. , & Tanaka, M. (2018). Oncostatin M causes liver fibrosis by regulating cooperation between hepatic stellate cells and macrophages in mice. Hepatology, 67, 296–312. 10.1002/hep.29421 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay, B. , Schuebel, K. , Mukhopadhyay, P. , Cinar, R. , Godlewski, G. , Xiong, K. , … Kunos, G. (2015). Cannabinoid receptor 1 promotes hepatocellular carcinoma initiation and progression through multiple mechanisms. Hepatology, 61, 1615–1626. 10.1002/hep.27686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya, M. , Chikura, S. , Watari, K. , Mizuno, N. , Mochinaga, K. , Mangmool, S. , … Kurose, H. (2012). Induction of cardiac fibrosis by β‐blocker in G protein‐independent and G protein‐coupled receptor kinase 5/β‐arrestin2‐dependent Signaling pathways. The Journal of Biological Chemistry, 287, 35669–35677. 10.1074/jbc.M112.357871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- New, D. C. , Wu, K. , Kwok, A. W. , & Wong, Y. H. (2007). G protein‐coupled receptor‐induced Akt activity in cellular proliferation and apoptosis. The FEBS Journal, 274, 6025–6036. 10.1111/j.1742-4658.2007.06116.x [DOI] [PubMed] [Google Scholar]
- Niu, S. , Li, H. , Chen, W. , Zhao, J. , Gao, L. , & Bo, T. (2018). β‐Arrestin 1 mediates liver thyrotropin regulation of cholesterol conversion metabolism via the Akt‐dependent pathway. International Journal of Endocrinology, 2018, 4371396 10.1155/2018/4371396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papeleu, P. , Vanhaecke, T. , Henkens, T. , Elaut, G. , Vinken, M. , Snykers, S. , & Rogiers, V. (2006). Isolation of rat hepatocytes. Methods in Molecular Biology, 320, 229–237. 10.1385/1-59259-998-2:229 [DOI] [PubMed] [Google Scholar]
- Parfieniuk, A. , & Flisiak, R. (2008). Role of cannabinoids in chronic liver diseases. World Journal of Gastroenterology, 14, 6109–6114. 10.3748/wjg.14.6109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato‐Matsubara, M. , Matsubara, T. , Daikoku, A. , Okina, Y. , Longato, L. , Rombouts, K. , … Kawada, N. (2017). Fibroblast growth factor 2 (FGF2) regulates cytoglobin expression and activation of human hepatic stellate cells via JNK signaling. The Journal of Biological Chemistry, 292, 18961–18972. 10.1074/jbc.M117.793794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz‐Peiffer, C. (2010). Targeting ceramide synthesis to reverse insulin resistance. Diabetes, 59, 2351–2353. 10.2337/db10-0912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, J. S. , & Rajagopal, S. (2016). The β‐arrestins: Multifunctional regulators of G protein‐coupled receptors. The Journal of Biological Chemistry, 291, 8969–8977. 10.1074/jbc.R115.713313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan, C. , Sharman, J. L. , Benson, H. E. , Faccenda, E. , Pawson, A. J. , Alexander, S. P. , … Davies, J. A. (2016). The IUPHAR/BPS guide to pharmacology in 2016: Towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Research, 44, D1054–D1068. 10.1093/nar/gkv1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh, Y. G. , Kim, J. K. , Byun, J. S. , Yi, H. S. , Lee, Y. S. , Eun, H. S. , … Jeong, W. I. (2012). CD11b+ Gr1+ bone marrow cells ameliorate liver fibrosis by producing interleukin‐10 in mice. Hepatology, 56, 1902–1912. 10.1002/hep.25817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam, J. , Szanda, G. , Drori, A. , Liu, Z. , Cinar, R. , Kashiwaya, Y. , … Kunos, G. (2017). Peripheral cannabinoid‐1 receptor blockade restores hypothalamic leptin signaling. Molecular Metabolism, 6, 1113–1125. 10.1016/j.molmet.2017.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, S. W. , Chen, X. L. , Xu, M. Y. , Huang, X. L. , Liu, H. L. , Jiang, J. , … Wu, B. (2017). PGE2/EP4 receptor attenuated mucosal injury via β‐arrestin1/Src/EGFR‐mediated proliferation in portal hypertensive gastropathy. British Journal of Pharmacology, 174, 848–866. 10.1111/bph.13752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, S. W. , Li, L. J. , Chen, T. T. , Chen, X. L. , Tao, L. , Lin, X. Y. , … Wu, B. (2015). β‐Arrestin‐1 protects against endoplasmic reticulum stress/p53‐upregulated modulator of apoptosis‐mediated apoptosis via repressing p‐p65/inducible nitric oxide synthase in portal hypertensive gastropathy. Free Radical Biology and Medicine, 87, 69–83. 10.1016/j.freeradbiomed.2015.06.004 [DOI] [PubMed] [Google Scholar]
- Tan, S. W. , Lu, Y. , Xu, M. Y. , Huang, X. L. , Liu, H. L. , Jiang, J. , … Wu, B. (2019). β‐Arrestin1 enhances liver fibrosis through autophagy‐mediated Snail signaling. FASEB Journal, 33, 2000–2016. 10.1096/fj.201800828RR [DOI] [PubMed] [Google Scholar]
- Teixeira‐Clerc, F. , Julien, B. , Grenard, P. , Tran, V. N. J. , Deveaux, V. , Li, L. , … Lotersztajn, S. (2006). CB1 cannabinoid receptor antagonism: A new strategy for the treatment of liver fibrosis. Nature Medicine, 12(6), 671–676. 10.1038/nm1421 [DOI] [PubMed] [Google Scholar]
- Tian, L. , Li, W. , Yang, L. , Chang, N. , Fan, X. , Ji, X. , … Li, L. (2017). Cannabinoid receptor 1 participates in liver inflammation by promoting M1 macrophage polarization via RhoA/NF‐κB p65 and ERK1/2 pathways, respectively, in mouse liver fibrogenesis. Frontiers in Immunology, 8, 1214 10.3389/fimmu.2017.01214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Lee, M. M. , Blomenrohr, M. , van der Doelen, A. A. , Wat, J. W. , Smits, N. , Hanson, B. J. , … Zaman, G. J. (2009). Pharmacological characterization of receptor redistribution and β‐arrestin recruitment assays for the cannabinoid receptor 1. Journal of Biomolecular Screening, 14(7), 811–823. 10.1177/1087057109337937 [DOI] [PubMed] [Google Scholar]
- Wang, M. , Meng, N. , Chang, Y. , & Tang, W. (2016). Endocannabinoids signaling: Molecular mechanisms of liver regulation and diseases. Frontiers in Bioscience (Landmark Edition), 21, 1488–1501. 10.2741/4468 [DOI] [PubMed] [Google Scholar]
- Whalen, E. J. , Rajagopal, S. , & Lefkowitz, R. J. (2011). Therapeutic potential of β‐arrestin‐ and G protein‐biased agonists. Trends in Molecular Medicine, 17(3), 126–139. 10.1016/j.molmed.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Guo, Y. , Tan, S. , Ke, B. , Tao, J. , Liu, H. , … Wu, B. (2015). β‐Arrestin1 enhances hepatocellular carcinogenesis through inflammation‐mediated Akt signaling. Nature Communications, 6, 7369 10.1038/ncomms8369 [DOI] [PubMed] [Google Scholar]
- Yin, C. , Evason, K. J. , Asahina, K. , & Stainier, D. Y. (2013). Hepatic stellate cells in liver development, regeneration, and cancer. The Journal of Clinical Investigation, 123, 1902–1910. 10.1172/JCI66369 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Analysis of the effect of different doses of the CB1R antagonist JD5037 on liver fibrosis in mice. (A) The influence of different doses of JD5037 on liver fibrosis was examined by Sirius red staining (red, ×100). (B) Sirius red area from histological staining was shown. *P<0.05 versus the other groups from CCl4‐treated mice with different JD5037 doses, n = 6 per group. (C) The influence of different doses of JD5037 on liver fibrosis was determined by western blotting analysis of α‐SMA levels, and the ratio of densitometry units of α‐SMA/β‐actin was also revealed. *P<0.05 versus the other groups from CCl4‐treated mice with different JD5037 doses, n = 6 per group.
Figure S2. CB1R antagonist JD5037 attenuated bile duct ligation (BDL)‐induced liver fibrosis in mice. (A) Sirius red staining (red), α‐SMA and CB1R staining (brown) were presented in the indicated sections from Sham‐operated control (as Sham) or BDL‐treated mice, with or without JD5037 administration. (B) Sirius red area and α‐SMA area from histological staining were shown (upper panel). The liver wet weight index and the hydroxyproline content of the indicated sections were also determined (lower panel). *P<0.05 versus Sham mice, #P<0.05 versus BDL‐treated mice without JD5037 treatment, n = 6 per group. (C) Western blotting assay were presented in the indicated treatment, revealing that JD5037 blocked the proteins elevations of collagen‐IV (COL‐IV), collagen‐I (COL‐I) and α‐SMA in BDL‐treated mice. n = 6 per group. (D) The indicated proteins from primary HSCs isolated from the related mouse models were detected by western blotting. n = 6 per group.
Figure S3. JD5037 repressed TGF‐β1/Smad2/3 signaling in CCl4‐treated mice via a β‐arr1‐independent pathway. (A) Targeted deletion of β ‐arr1 did not affect the levels of TGF‐β1/Smad2/3 signaling networks in CCl4‐treated mice. The ratio of densitometry units of p‐Smad2/β‐actin and p‐Smad3/β‐actin was revealed. *P<0.05 versus Ctrl mice, n = 6 per group. (B) JD5037 inhibited the signal activation of TGF‐β1/Smad2/3 in CCl4‐treated mice, regardless of β ‐arr1 knockout. The ratio of densitometry units of p‐Smad2/β‐actin and p‐Smad3/β‐actin was shown. *P<0.05 versus CCl4‐treated mice without JD5037 treatment, n = 6 per group.
Figure S4. The status of CB1R influenced the activation of HSCs via β‐arr1/Akt networks. (A) CB1R transfection increased the levels of β‐arr1, p‐Akt, α‐SMA and PCNA, while repressed the level of cleaved caspase‐3 in human stellate cells line (LX2 cells). (B) CB1R expression was silenced by CB1R‐siRNA. CB1R knockdown inhibited the levels of β‐arr1, p‐Akt, α‐SMA and PCNA, while increased the level of cleaved caspase‐3 in LX2 cells
Table S1. Characteristics of the non‐fibrosis individuals and liver fibrosis patients


