Abstract
Since their discovery, the orphan nuclear receptors constitutive androstane receptor (CAR;NR1I3) and pregnane X receptor (PXR;NR1I2) have been regarded as master regulators of drug disposition and detoxification mechanisms. They regulate the metabolism and transport of endogenous mediators and xenobiotics in organs including the liver, intestine and brain. However, with proposals of new physiological functions for NR1I3 and NR1I2, there is increasing interest in the role of these receptors in influencing brain function. This review will summarise key findings regarding the expression and function of NR1I3 and NR1I2 in the brain, hereby highlighting the need for further research in this field.
Abbreviations
- 3α,5α‐THP
3α,5α‐tetrahydroprogesterone
- ABC
ATP‐binding cassette
- AhR
aryl hydrobon receptor
- BBB
blood–brain barrier
- BCRP
breast cancer resistance protein
- NR1I3/CAR
constitutive androstane receptor
- CITCO
6‐(4‐chlorophenyl)‐imidazo[2,1‐b]thiazole‐5‐carbaldehyde
- CPT1A
Carnitine palmitoyltransferase 1
- CYP450
cytochrome P450
- DBD
DNA‐binding domain
- G6P‐ase
glucose‐6‐phosphatase
- GADD45B
DNA‐damage inducible β protein
- GST
GSH‐S‐transferase
- HMGCS2
3‐hydroxymethylglutaryl CoA synthase 2
- LBD
ligand‐binding domain
- MCP
monocrotophos
- MRP
multidrug resistance‐associated protein
- NR
nuclear receptor
- PEPCK
phosphoenolpyruvate carboxykinase
- P‐GP
P‐glycoprotein
- NR1I2/PXR
pregnane X receptor
- RXR/NR2B
retinoid X receptor α, β, γ
- SCD‐1
stearoyl‐CoA desaturase 1
- TCPOBOP
(1,4‐bis‐[2‐(3,5‐dichloropyridyloxy)]benzene
- UGT
uridine glucuronosyl transferase
1. INTRODUCTION
The nuclear receptor (NR) superfamily is a group of proteins that function as cellular transcription factors of target genes responsible for the expression of proteins critical for cell development, activity and survival (Sever & Glass, 2013). In order to exert their effects, NRs must be activated via binding with ligands that are chemically diverse and include endobiotics, such as hormones, vitamins and their metabolites, dietary components including nutrients and xenobiotics including drugs and endocrine disruptors (Krasowski, Ni, Hagey, & Ekins, 2011).
To date, the NR superfamily is composed of 48 members in human, 49 in mice and 47 in the rat (Sever & Glass, 2013; Zhang et al., 2004). NRs share a common structure, composed of an N‐terminal containing the ligand‐independent transcription activation function‐1 (AF‐1) domain, a highly conserved DNA‐binding domain (DBD), which allows direct binding with response elements, and a C‐terminal ligand‐binding domain (LBD) (Huang, Chandra, & Rastinejad, 2010).
Understanding the functional properties of NRs that have a known nominal ligand, as in the case with steroid and vitamin‐activated receptors, has increased significantly due to the development of synthetic ligands with agonistic or antagonistic properties. However, with the identification of NRs with no known nominal ligands over recent decades, an increasing number of studies have attempted to elucidate their physiological functions (Banerjee, Robbins, & Chen, 2015; Mullican, Dispirito, & Lazar, 2013; Oladimeji & Chen, 2018). These so‐called orphan NRs tend to possess much broader ligand selectivity, such ligands including numerous xenobiotics (Ihunnah, Jiang, & Xie, 2011). In this regard, signalling via the constitutive androstane receptor (CAR;https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=607) and pregnane X receptor (PXR;https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=606) are major mechanisms for cellular response to exposure to xenobiotics and endogenous molecules (Chai, Zeng, & Xie, 2013; Tolson & Wang, 2010). Drugs used for treatment of disease conditions with numerous aetiologies (e.g. epilepsy, inflammatory diseases and bacterial infections) and endogenous compounds, including hormones and their metabolites, are ligands for both receptors and therefore influence the expression of metabolising enzymes and membrane transporters involved in drug disposition (Cerveny et al., 2007; Jigorel, Le Vee, Boursier‐Neyret, Parmentier, & Fardel, 2006; Martin, Riley, Back, & Owen, 2008). Although the evidence provided by in vitro and in vivo studies appears to support a potential role of both NR1I3 and NR1I2 in influencing drug disposition, recent studies have proposed additional functions (Figure 1). For example, NR1I3 and NR1I2 can exert anti‐inflammatory effects by blocking the actions of https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=996 in a cell‐ or tissue‐dependent ways (Dou et al., 2013; Malekshah, Lage, Bahrami, Afshari, & Behravan, 2012; Mencarelli et al., 2011) and influence the metabolism of endogenous compounds, including bile acids and hormones, as well as vitamins and nutrients. Interestingly, NR1I3 and NR1I2 can also regulate cell proliferation and the activity of pro‐apoptotic pathways (Oladimeji, Cui, Zhang, & Chen, 2016).
FIGURE 1.
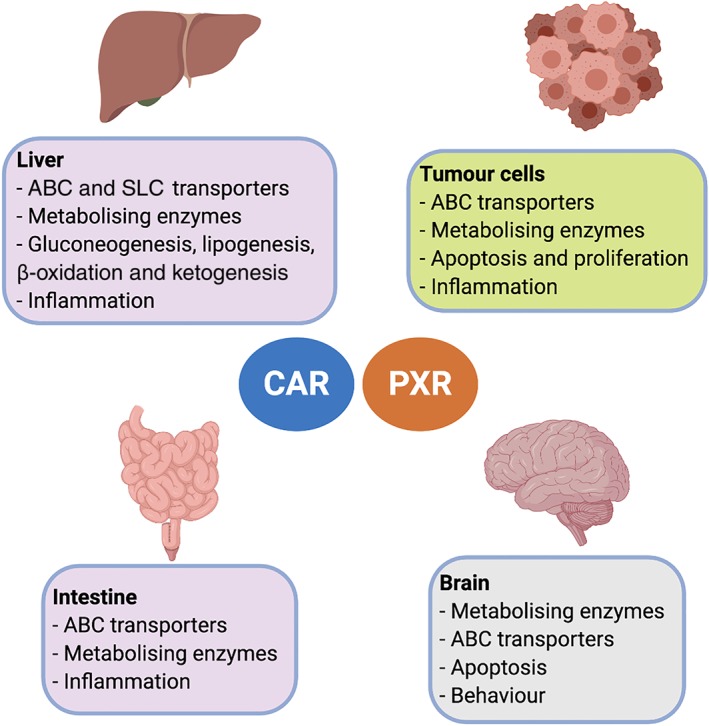
Known biological actions of NR1I3 (CAR) and NR1I2 (PXR). Since both nuclear receptors are ubiquitously expressed in organs and tissues of mammalians, they regulate the expression of proteins involved in metabolism and transport of nutrients, endogenous mediators and xenobiotics in a tissue‐dependent manner. Furthermore, NR1I3 and NR1I2 can also influence biological processes including inflammation and apoptosis through protein‐protein interactions. Recent research have demonstrated that in the brain, apart from their role in some of the above‐mentioned processes, NR1I3 and NR1I2 may participate in complex functions such as behaviour
The effects of NR1I2 and NR1I3 were initially studied in organs including the liver and small intestine (Figure 1). In subsequent years, expression of both receptors has been reported in endothelial cells of the blood–brain barrier (BBB), with several reports focusing on the effects of ligand‐based activation of NR1I2 and NR1I3 on the expression of transporters and enzymes (Dauchy et al., 2009; Miller, 2015; Qosa, Miller, Pasinelli, & Trotti, 2015). More recently, the study of NR1I2 and NR1I3 in the brain has been extended to other cell types, including neurons, with interesting findings that could define new biological roles.
The aim of this review is to summarise the key actions of NR1I3 and NR1I2 in the brain in light of what is known in other cells and tissues.
2. AN OVERVIEW OF NR1I2 (PXR) AND NR1I3 (CAR)
NR1I2 and NR1I3 possess many structural features present in classic NRs, including the DNA‐binding domain, an N‐terminal AF‐1 domain and a C‐terminal LB ligand‐binding domain D (Khorasanizadeh & Rastinejad, 2016; Mullican et al., 2013). NR1I2 and NR1I3 are 50‐kDa proteins highly expressed in liver and intestine (Giessmann et al., 2004; Nannelli et al., 2010). Further studies have demonstrated that their expression pattern is more ubiquitous than initially thought, as other organs including brain, kidneys and heart also express both of these receptors (Lamba et al., 2004; Marini et al., 2007; Miki, Suzuki, Tazawa, Blumberg, & Sasano, 2005; Nannelli et al., 2010; Nishimura, Naito, & Yokoi, 2004; Savkur et al., 2003).
Their cellular localisation is still a matter of controversy since, depending on the cell model employed, NR1I3 and NR1I2 can exhibit a nuclear or cytosolic localisation (Kanno, Suzuki, Nakahama, & Inouye, 2005; Kawamoto et al., 1999; Saradhi, Sengupta, Mukhopadhyay, & Tyagi, 2005; Squires, Sueyoshi, & Negishi, 2004; Yoshinari, Kobayashi, Moore, Kawamoto, & Negishi, 2003). For example, in primary cultures of rat hepatocytes, the ligand‐based activation of NR1I3 induces its nuclear translocation, while in several human‐derived immortalised cell lines, NR1I3 is primarily constitutively active and localised in the nucleus (Chan, Hoque, Cummins, & Bendayan, 2011; Kanno et al., 2005; Kobayashi, Sueyoshi, Inoue, Moore, & Negishi, 2003). Although ligand‐based activation of NR1I3 does not necessarily elicit nuclear translocation, it has been reported that use of 6‐(4‐chlorophenyl)‐imidazo[2,1‐b]thiazole‐5‐carbaldehyde (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2758), a selective human NR1I3 (hNR1I3) ligand, results in increased expression of the ATP‐binding cassette (https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=136&familyType=TRANSPORTER) transporter P‐glycoprotein (P‐GP; https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=768) in the immortalised brain endothelial cell line hCMEC/d3 (Chan et al., 2011). The authors attributed this finding to increased nuclear NR1I3 activity, but as a recent study demonstrated that CITCO is a dual hNR1I3/human NR1I2 (hNR1I2) ligand (Lin et al., 2020), the above‐mentioned outcome might also be the result of NR1I2 activation.
As is the case with NR1I3, NR1I2 exhibits nuclear localisation in immortalised cell lines (Saradhi et al., 2005) and cytosolic localisation in primary cultures (Ott, Fricker, & Bauer, 2009; Squires et al., 2004) but is not constitutively active when it accumulates in the nucleus.
In the cytosol, NR1I3 and NR1I2 are bound to a chaperone protein complex composed of the https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=784 90 and the NR1I3 retention protein (Mackowiak & Wang, 2016; Wang, Ong, Chai, & Chen, 2012). Upon activation, NR1I2 and NR1I3 are released from this protein complex and translocate to the nucleus to form a heterodimer with the α, β, γ isoforms of the https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=92 (RXR;NR2B) (Koutsounas et al., 2015). The NR1I2/RXR and NR1I3/RXR heterodimers are permissive partners within the heterodimer, such that the activity of this interaction can be enhanced through further binding with an RXR ligand (Chen, Wang, & Wan, 2010; Wang et al., 2006). The heterodimers can bind to co‐activators or co‐repressors, prior to interacting with the respective response elements, subsequently initiating or repressing the transcription of target genes (Oladimeji et al., 2016).
Although NR1I2 and NR1I3 are activated by direct ligand binding, evidence suggests that both receptors can be activated via an indirect mechanism. Indirect activation of NR1I3 involves the activation of the ERK (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1494/https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1495) and https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=621 signalling pathways (Mackowiak & Wang, 2016). The mechanism for indirect activation of NR1I2 remains elusive, but studies propose that the https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=570 signalling pathway may be involved (Dong, Lin, Wu, & Chen, 2010; Lin et al., 2008). Furthermore, the activities of NR1I3 and NR1I2 can be regulated by post‐translational modifications including phosphorylation, ubiquitination, acetylation and SUMOylation (Staudinger, Xu, Biswas, & Mani, 2011; Wang et al., 2012).
One important feature of NR1I2 and NR1I3 is that ligand‐mediated receptor activation is species dependent, due to differences in the amino acid sequence of the ligand‐binding domain (Gray, Peacock, & Squires, 2009; Gray, Pollock, Schook, & Squires, 2010). This issue makes comparisons of NR1I2 and NR1I3 activation between species difficult, although a limited number of selective ligands for rodent and hNR1I2 and hNR1I3 are available (Table 1) (Chan et al., 2011; Lemmen, Tozakidis, Bele, & Galla, 2013; Lemmen, Tozakidis, & Galla, 2013). To help address this issue, humanised rodent models have been developed (Bauer et al., 2006; Haines et al., 2018).
TABLE 1.
Ligands and biological targets of NR1I3 (CAR) and NR1I2 (PXR)
| Nuclear receptor | NR1I3 (CAR) | (NR1I2) PXR | Reference | ||
|---|---|---|---|---|---|
| Species | Rodent | Human a | Rodent | Human a | |
| Ligands | |||||
| Selective agonists | TCPOBOP, phenobarbital | CITCO, phenobarbital | PCN |
Rifampicin Hyperforin SR12813 |
(Jimenez, Quattrochi, Yockey, & Guzelian, 2000; Jones et al., 2000; Kliewer et al., 1998; Moore et al., 2000; Tzameli, Pissios, Schuetz, & Moore, 2000) |
| Antagonists | https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2760 |
CINPA‐1 https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2757 |
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2568 | Ketoconazole, l‐https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6569, coumestrol, SPA70 | (Cherian et al., 2018; Forman et al., 1998; Huang et al., 2007; Lemmen, Tozakidis, Bele, & Galla, 2013; Lin et al., 2017; Wang et al., 2008; Yeung, Sueyoshi, Negishi, & Chang, 2008) |
| Biological targets | |||||
| Enzymes | |||||
| CYP450 isoforms | Cyp1a1, Cyp1a2, Cyp2a4, Cyp2b10, Cyp2c29, Cyp2c37, Cyp2c55, Cyp3a11 | CYP1A1, CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP3A4, CYP3A5 | Cyp3a, Cyp3a11, Cyp2b9, Cyp2b10, Cyp2c55 | CYP3A4, CYP3A23, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP1A | (Aleksunes & Klaassen, 2012; Chen, Ferguson, Negishi, & Goldstein, 2003; Ferguson, Chen, LeCluyse, Negishi, & Goldstein, 2005; Ferguson, LeCluyse, Negishi, & Goldstein, 2002; Goodwin, Hodgson, D'Costa, Robertson, & Liddle, 2002; Maglich et al., 2004; Xu, Wang, & Staudinger, 2009; Yoshinari, Yoda, Toriyabe, & Yamazoe, 2010; Zhang, Huang, Chua, Wei, & Moore, 2002) |
| UGTs | Ugt1a1, Ugt1a9, Ugt2b34, Ugt2b35, Ugt2b36 | UGT1A1 | Ugt1a1, Ugt1a5, Ugt1a9 | UGT1A1, UGT1A6, UGT1A3, UGT1A4 | (Aleksunes & Klaassen, 2012; Maglich et al., 2004; Sugatani et al., 2005) |
| Sulfotransferases | Sult1a1, Sult1d1, Sult1e1, Sult2a1, Sult2a2, Sult3a1, Sult5a1 | SULT2A1 | Sult1a1, Sult1e1, Sult2a1, Sult2a2, Sult5a1 | SULT2A1 | (Aleksunes & Klaassen, 2012; Alnouti & Klaassen, 2008; Chen, Zhang, Baker, & Chen, 2007; Echchgadda et al., 2007; Maglich et al., 2004) |
| GSH‐S‐transferases | Gsta1, Gsta3, Gsta4, Gstm1, Gstm2, Gstm3, Gstm4, Gstp, Gstt1, Gstt3 | Gsta1, Gsta3, Gsta4, Gstm1, Gstm2, Gstm3, Gstm4, Gstm6, Gstp, Gstt1, MGst1 | GSTA1, GSTA2, GSTM1, GSTP1‐1 | (Aleksunes & Klaassen, 2012; Cui, Choudhuri, Knight, & Klaassen, 2010; Knight, Choudhuri, & Klaassen, 2008; Singh et al., 2012; Zhang et al., 2002) | |
| Gluconeogenic enzymes | PEPCK, G6P‐ase | PEPCK, G6P‐ase | PEPCK, G6P‐ase | (Kodama, Koike, Negishi, & Yamamoto, 2004; Kodama, Moore, Yamamoto, & Negishi, 2007; Mackowiak et al., 2019) | |
| Lipogenic enzymes | Scd‐1 | Scd‐1 | SCD‐1 | (Nakamura, Moore, Negishi, & Sueyoshi, 2007; Zhang et al., 2013) | |
| β‐oxidation‐related enzymes | Cpt1a | Cpt1a | CPT1A | (He et al., 2013; Moreau et al., 2009; Nakamura et al., 2007; Ueda et al., 2002) | |
| Ketogenic enzymes | Hmgcs2 | Hmgcs2 | (Gao, He, Zhai, Wada, & Xie, 2009; He et al., 2013) | ||
| Transporters | |||||
| ABC | P‐gp, Bcrp, Mrp2, Mrp4 | P‐GP, BCRP, MRP2 | P‐gp, Bcrp, Mrp2, Mrp4 |
P‐GP , BCRP, MRP2, ABCBA1, ABCG1 |
(Bauer, Hartz, Fricker, & Miller, 2004; Kast et al., 2002; Lemmen, Tozakidis, Bele, & Galla, 2013; Lemmen, Tozakidis, & Galla, 2013; Martin et al., 2008; Narang et al., 2008; Oswald et al., 2006; Ott et al., 2009; Petrick & Klaassen, 2007; Teng & Piquette‐Miller, 2005; Wang, Sykes, & Miller, 2010; Whyte‐Allman, Hoque, Jenabian, Routy, & Bendayan, 2017; Yamasaki, Kobayashi, & Chiba, 2018) |
| SLC | https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=165#876, https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=238#1219 | https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=238#1219, https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=238#1219 | (Aleksunes & Klaassen, 2012; Hassani‐Nezhad‐Gashti et al., 2018; Li et al., 2015; Meyer zu Schwabedissen, Tirona, Yip, Ho, & Kim, 2008) | ||
Studies performed in higher mammalian models including porcine are included since ligands for human NR1I3 (CAR) and NR1I2 (PXR) also activate these orthologues.
2.1. Ligands and biological effects
Both NR1I3 and NR1I2 possess broad ligand specificity that confers the ability to sense structurally diverse endogenous and exogenous compounds. Exposure to such ligands, including therapeutic drugs, environmental contaminants and endocrine disruptors, elicits the activation of overlapping and distinct sets of genes that encode metabolising enzymes, including members of the https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=242 (CYP450) family, sulfotransferases, uridine glucuronosyl transferases (https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=988) and GSH‐S‐transferases (https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=270#1381) (Table 1), resulting in xenobiotic inactivation (Aleksunes & Klaassen, 2012; Wang et al., 2012). Furthermore, NR1I2 and NR1I3 are involved in the regulation of expression of membrane transporters belonging to the ABC and solute carrier families (Table 1), responsible for the disposition of xenobiotics and their metabolites (Klaassen & Aleksunes, 2010). Due to this body of evidence, NR1I3 and NR1I2 were mainly regarded as modulators of detoxification mechanisms and drug disposition. However, more extensive research has unveiled further biological roles attributed to NR1I3 and NR1I2 activation (Table 1), including regulation of nutrient metabolism, inflammation and apoptosis (Hakkola, Rysa, & Hukkanen, 2016; Hukkanen, Hakkola, & Rysa, 2014; Robbins, Bakke, Cherian, Wu, & Chen, 2016).
The role of NR1I3 and NR1I2 in hepatic glucose and lipid metabolism has been studied in rodent and human‐based models. These studies demonstrated that ligand‐based activation of NR1I3 and NR1I2 plays a significant role in controlling energy status, including regulation of gluconeogenesis and lipogenesis, in a model‐dependent manner (Hakkola et al., 2016). For example, the ligand‐based activation of NR1I2 is a negative regulator of gluconeogenesis in human‐derived liver cell lines and rodent models, with NR1I2 activation leading to down‐regulation of the expression of key enzymes including phosphoenolpyruvate carboxykinase (PEPCK) and glucose‐6‐phosphatase (G6P‐ase) (Kodama et al., 2004; Kodama et al., 2007). However, studies carried out in human primary hepatocytes demonstrated ligand activation of NR1I2 results in induction of gluconeogenesis (Gotoh & Negishi, 2015). The findings of studies investigating the effects of NR1I3 activation on the regulation of gluconeogenesis are more consistent across models, with NR1I3 activation leading to down‐regulation of gluconeogenesis (Kodama et al., 2004; Mackowiak et al., 2019).
In regard to lipid metabolism, NR1I3 is thought to be a negative regulator of lipogenesis by down‐regulating the expression of lipogenic enzymes including stearoyl‐CoA desaturase 1 (SCD‐1) (Dong et al., 2009; Gao et al., 2009; Marmugi et al., 2016). Furthermore, the ligand‐based activation of NR1I3 decreases the expression of carnitine palmitoyltransferase 1 (CPT1A), an enzyme involved in mitochondrial β‐oxidation of fatty acids and the ketogenic enzyme 3‐hydroxymethylglutaryl CoA synthase 2 (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2432) (Gao et al., 2009). Conversely, NR1I2 exerts an inductive effect on lipogenesis through up‐regulation of SCD‐1 (Nakamura et al., 2007; Zhang et al., 2013), the fatty acid transporter https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=3104 (Zhou et al., 2008) and ttps://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=185#981 (Li et al., 2015), a transporter for citrate, which acts as a precursor for the synthesis of cholesterol and fatty acids. The ligand‐based activation of NR1I2 also decreases the expression of CPT1A and HMGCS2 (Bitter et al., 2015; He et al., 2013; Moreau et al., 2009; Nakamura et al., 2007).
In the regulation of inflammation, the ligand‐based activation of NR1I2 blocks https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=996‐mediated expression of pro‐inflammatory genes (Zhou et al., 2006) and this discovery has led to the development of new therapeutic approaches for management of intestinal‐associated inflammation (Calanni, Renzulli, Barbanti, & Viscomi, 2014). Furthermore, studies in human hepatocytes demonstrate the pro‐inflammatory cytokine https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4998 repressed the ligand‐based activation of both NR1I3 and NR1I2 (Pascussi et al., 2000).
Both NR1I3 and NR1I2 can also influence the survival of tumour cells by regulating additional cell processes including apoptosis and cell proliferation through other distinct mechanisms. In rodent, NR1I3 inhibits apoptosis by interacting with the DNA‐damage inducible β protein, GADD45B (Yamamoto, Moore, Flavell, Lu, & Negishi, 2010). The role of NR1I2 in cancer is more complex since the receptor can elicit, in a cell‐dependent manner, pro‐ or anti‐apoptotic effects by regulating the activity of the tumour suppressor protein p53 (Oladimeji et al., 2016). Furthermore, the interaction between NR1I2‐p53 and the biological outcome is dependent on the expression of the wild‐type (Robbins, Cherian, Wu, & Chen, 2016) or mutant (Ouyang et al., 2010) variant of p53.
3. NR1I3 (CAR) AND NR1I2 (PXR): EXPRESSION AND EFFECTS IN THE BRAIN
3.1. Expression profile in areas of the brain
Several early reports attempted to characterise the expression profile of NR1I3 and NR1I2 in different areas of the brain (Table 2). In human, levels of receptor expression are markedly lower (or even undetectable) when compared to liver and intestine (Lamba et al., 2004; Miki et al., 2005; Nishimura et al., 2004; Savkur et al., 2003). Further studies carried out in different regions of the brain from mammalians found expression of NR1I3 and/or NR1I2 in the cortex (Kajta et al., 2019; Marini et al., 2007; Nannelli et al., 2010), hippocampus (Litwa et al., 2016; Marini et al., 2007; Nannelli et al., 2010), midbrain (Frye, Koonce, & Walf, 2014d; Marini et al., 2007; Nannelli et al., 2010) and cerebellum (Marini et al., 2007; Nannelli et al., 2010; Xia et al., 2017) (Table 2). NR1I3 and NR1I2 expression have also consistently been reported in BBB endothelial cells isolated from cortex (Bauer et al., 2004; Chan et al., 2011; Lemmen, Tozakidis, Bele, & Galla, 2013; Ott et al., 2009).
TABLE 2.
Expression profile of NR1I3 (CAR) and NR1I2 (PXR) in areas of the brain
| NR1I3 | NR1I2 | Reference | |
|---|---|---|---|
| Rodent | Cortex (mRNA, protein) | Cortex (mRNA, protein) | (Bauer et al., 2004; Chan et al., 2013; Frye, Koonce, Walf, & Rusconi, 2013; Kajta et al., 2019; Litwa et al., 2016; Narang et al., 2008; Wang et al., 2010) |
| Hippocampus (mRNA, protein) | Hippocampus (mRNA, protein) | ||
| Cortical capillaries (mRNA, protein) | Midbrain (mRNA, protein) | ||
| Cortical capillaries (mRNA, protein) | |||
| Quail | Cerebrum (mRNA) | Cerebrum (mRNA) | (Du et al., 2017; Lin et al., 2018; Xia et al., 2017) |
| Cerebellum (mRNA) | Cerebellum (mRNA) | ||
| Rabbit | Cortex (mRNA) | Cortex (mRNA) | (Marini et al., 2007) |
| Hippocampus (mRNA) | Midbrain (mRNA) | ||
| Hypothalamus (mRNA) | Cerebellum (mRNA) | ||
| Midbrain (mRNA) | |||
| Striatum (mRNA) | |||
| Cerebellum (mRNA) | |||
| Porcine | Cortex (mRNA) | Cortex (mRNA) | (Lemmen, Tozakidis, Bele, & Galla, 2013; Lemmen, Tozakidis, & Galla, 2013; Nannelli et al., 2010; Ott et al., 2009) |
| Cerebellum (mRNA) | Cerebellum (mRNA) | ||
| Midbrain (mRNA) | Midbrain (mRNA) | ||
| Hippocampus (mRNA) | Hippocampus (mRNA) | ||
| Meninges (mRNA) | Meninges (mRNA) | ||
| Cortical capillaries (mRNA, protein) | Cortical capillaries (mRNA, protein) | ||
| Human | Brain tissue a (mRNA) | Brain tissue a (mRNA) | (Chan et al., 2011; Lamba et al., 2004; Miki et al., 2005; Nishimura et al., 2004; Savkur et al., 2003) |
| Caudate nucleus (mRNA) | Cerebrum (mRNA) | ||
| Cortical capillaries (mRNA, protein) | Cortex (mRNA) | ||
| Cerebellum (mRNA) | |||
| Cortical capillaries (mRNA, protein) |
Refers to no particular area of the brain.
As the brain is an organ whose divisions are both functional and anatomical, it is plausible to hypothesise that the regional expression of NR1I3 and NR1I2 may exert distinctive biological actions. Furthermore, as each cell subtype (i.e. neurons, glial cells and BBB endothelial cells) is phenotypically different, the set of genes activated by both NRs may vary. These premises have been demonstrated by studies conducted in animal models. For instance, ligand‐based activation of NR1I2 up‐regulated the mRNA levels of CYP enzymes in specific regions of the rodent (Yadav, Dhawan, Seth, Singh, & Parmar, 2006), porcine (Nannelli et al., 2010) and brain, while the total (Boussadia et al., 2016) or localised (Frye, Koonce, & Walf, 2014b) inhibition of NR1I2 expression in rodents influence brain functions including behaviour (Table 3).
TABLE 3.
Biological actions of NR1I3 (CAR) and NR1I2 (PXR) in neural cells
| Species | Model | Ligand | Receptor | Level of expression | Biological effect | Reference |
|---|---|---|---|---|---|---|
| Mouse | Primary hippocampal neurons from Swiss CD‐1 mice embryos | Nonylphenol | NR1I3, NR1I2 | mRNA, protein |
Up‐regulation of NR1I3, Pxr and Rxrα Induction of apoptosis |
(Litwa et al., 2016) |
| Primary neocortical neurons from Swiss CD‐1 mice embryos | Triclocarban | NR1I3 | mRNA, protein | Induction of apoptosis | (Kajta et al., 2019) | |
| NR1I3 (−/−) and Pxr (−/−) C57BL/6J mice | NR1I3, NR1I2 | Impaired recognition memory and anxiety | (Boussadia et al., 2016; Boussadia et al., 2018) | |||
| Rat | Male albino Wistar rats | PCN, dexamethasone | NR1I2 | mRNA | Up‐regulation of CYP3A in hypothalamus and hippocampus | (Yadav et al., 2006) |
| Knocked down expression of Pxr in midbrain of Long‐Evans rats | NR1I2 | mRNA |
Reduced metabolism of progesterone to 3α,5α‐THP Impaired mating behaviour Reduced hippocampal BDNF levels |
(Frye et al., 2013; Frye et al., 2014d; Frye, Koonce, & Walf, 2014a; Frye, Koonce, & Walf, 2014c) | ||
| Quail |
Atrazine, di‐(2‐ethylhexyl)‐phthalate |
NR1I3, NR1I2 | mRNA |
Up‐regulation of NR1I3, NR1I2 and AhR Induction of apoptosis Oxidative and mitochondrial damage Cerebral and cerebellar toxicity |
(Du et al., 2017; Lin et al., 2018; Xia et al., 2017) | |
| Porcine | 4‐day treatment with rifampicin in White large × Landrace hybrid pigs | Rifampicin | NR1I2 | mRNA |
Up‐regulation of CYP3A22, CYP3A29 in cortex and hippocampus Up‐regulation of CYP2B22 in meninges |
(Nannelli et al., 2010) |
| Human | Cord blood CD34+ stem cell‐derived differentiating neuronal cells | Rifampicin | NR1I2 | Protein | Up‐regulation of CYP1A1, 2B6, 2E1, 3A4, AhR, NR1I3, NR1I2 and GSTP1‐1 | (Singh et al., 2012) |
| SHSY‐5Y cells | Monocrotophos | NR1I3, NR1I2 | mRNA |
Up‐regulation of NR1I3, NR1I2, CYP2C8 and 3A4 Induction of apoptosis Oxidative damage |
(Tripathi et al., 2017) |
3.2. NR1I3 (CAR) and NR1I2 (PXR): Comparison of activities between brain and liver
Since it has been reported that the brain expresses metabolising enzymes and transporters present in the liver (an organ commonly used as reference), it was thought that their expression could also be regulated by NRs such as NR1I3 and NR1I2. Although a number of studies have supported the above premise, they also demonstrate that the roles of NR1I3 and NR1I2 in the brain cannot always be widely extrapolated.
Several in vivo comparative studies conducted in animal models showed that the response to treatments with NR1I3 and/or NR1I2 ligands may vary between organs expressing both NRs (Marini et al., 2007; Messina, Nannelli, Fiorio, Longo, & Gervasi, 2009; Nannelli et al., 2009; Nannelli et al., 2010; Yamasaki et al., 2018). Furthermore, as discussed above, the effects of ligand‐based activation of NR1I3/NR1I2 differ between areas of the brain.
In rodent, administration of the NR1I3 ligand https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2804 to rats selectively up‐regulated the expression of CYP2B (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1324 in cortex and midbrain (Schilter & Omiecinski, 1993; Upadhya, Chinta, Pai, Boyd, & Ravindranath, 2002). A later study showed that rats and mice treated with phenobarbital and the selective rodent NR1I3 ligand (1,4‐bis‐[2‐[3,5‐dichloropyridyloxy)]benzene (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2756), respectively, exhibited increased protein expression of P‐gp https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=768) the multidrug resistance‐associated protein‐2 (MRP2;https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=780) and the breast cancer resistance protein (BCRP;https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=792) in brain capillaries (Wang et al., 2010). One common feature of the above studies is that the effects elicited by ligand‐based activation of NR1I3 were comparable to those observed in liver, which was used as a positive control. A similar approach was employed for the study of ligand‐based activation of NR1I2 in the rodent brain, where reports demonstrated that rats exposed to the selective rodent NR1I2 ligands pregnelolone‐16α‐carbonitrile (PCN) and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2768 showed increased expression of Cyp450 enzymes and ABC transporters in liver and specific areas of the brain. For example, PCN and dexamethasone up‐regulated the mRNA expression and activity Cyp3a1 (in midbrain, hypothalamus and hippocampus (Yadav et al., 2006), while P‐gp and Mrp2 were up‐regulated at the protein level in isolated brain capillaries, but not brain homogenates (Bauer et al., 2004; Bauer et al., 2008). A recent study showed that administration of PCN to mice up‐regulated P‐gp mRNA expression in liver, intestine, whole brain and brain cortex (Yamasaki et al., 2018). However, these changes were not observed at the protein level in both liver and whole brain. Interestingly, transgenic mice expressing hNR1I2 exhibited up‐regulated P‐gp protein levels in both liver and isolated brain capillaries upon treatment with rifampicin (Bauer et al., 2006).
In terms of higher animal models, treatment of rabbits with phenobarbital increased the hepatic mRNA expression of several CYP450 isoforms (CYP2A10, CYP2B4, CYP2B5 and CYP3A6), but these changes were not observed in areas of the brain including cortex, midbrain, striatum, hippocampus, hypothalamus and cerebellum (Marini et al., 2007). In pigs, the intraperitoneal administration of the hNR1I2 ligand https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2765 up‐regulated the mRNA expression of CYP450 isoforms (CYP3A22 CYP3A29, CYP3A46 and CYP2B22) and ABC transporters (P‐GP) in liver (Nannelli et al., 2010). However, at brain level, the mRNA expression of CYP3A22 and CYP3A29 was up‐regulated only in the cortex and hippocampus, while CYP2B22 was up‐regulated in meninges, with no significant increases in both microsomal and mitochondrial activities. Although transcripts of ABC transporters including P‐GP, MRP1 and MRP2 were found at different levels in all the brain regions studied, rifampicin failed to up‐regulate their expression. This outcome contrasts with those reported by studies conducted in rodent models, described above (Bauer et al., 2004; Bauer et al., 2006).
The study of relationships between NR1I3/NR1I2 activation and the expression of target genes in both liver and brain has also been extended to disease states. A recent report demonstrated that the induction of status epilepticus and spontaneous recurrent seizures in rats, through intra‐hippocampal injection of kainic acid, up‐regulated the expression of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1330 and Cyp3a13 mRNA in liver and hippocampus of rats under spontaneous recurrent seizures, with no changes in the mRNA expression of NR1I3 and NR1I2 (Runtz et al., 2018). Although the authors did not demonstrate if NR1I3 and/or NR1I2 were involved in the up‐regulation of the Cyp450 isoforms, the fact that both brain and liver responded similarly to a common stimulus not related to direct ligand‐based activation of NR1I3 and NR1I2 with pharmacological agents is an interesting outcome that should be studied in greater depth.
Taken together, the above studies suggest that while certain areas of the brain respond in a similar manner to the liver upon ligand‐based activation of NR1I3 and NR1I2, this is not true of all brain regions. With a better understanding of the precise signalling pathways active in organs and tissues of the studied species, it would therefore be easier to predict the effects elicited by NR1I3 and NR1I2. In cells of the brain, there is evidence that both NR1I3 and NR1I2 translocate to the nucleus upon their ligand‐based activation (Kajta et al., 2019; Lemmen, Tozakidis, & Galla, 2013), but knowledge of any similarities or differences with liver in terms of post‐translational modifications, or how they latter interact with RXR and co‐activators/co‐repressors, is still scarce (Figure 2).
FIGURE 2.
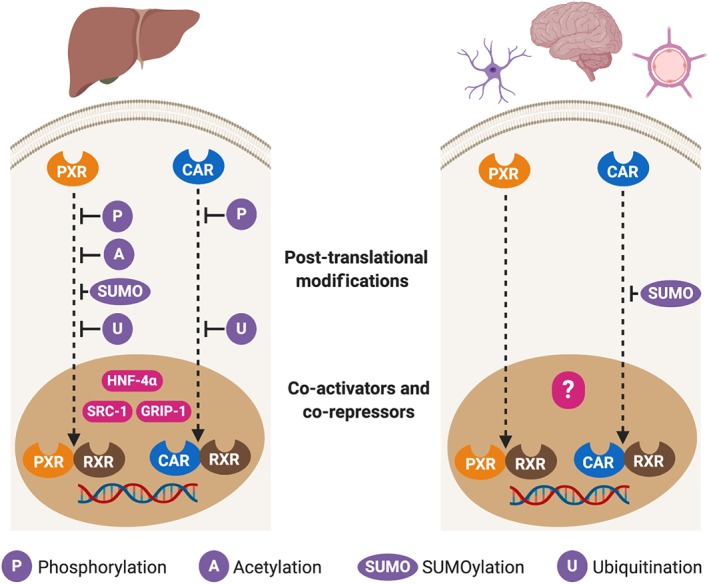
Comparison of the downstream events of the NR1I3 (CAR) and NR1I2 (PXR) signalling pathways in liver and brain. Most of the research aiming to characterise the signalling pathways of NR1I3 and NR1I2 has been conducted in liver, whilst organs such as the brain are much less studied. This issue is particularly important as brain endothelial cells and neurons express both receptors and the response to the ligand‐based activation of NR1I3 and NR1I2 appears vary amongst areas of the brain. This outcome is consistent with the tissue‐dependent effects of
3.3. NR1I3 (CAR) and NR1I2 (PXR) in the BBB
The study of NR1I3 and NR1I2 function in BBB endothelial cells has been primarily focused on their effects on the expression of ABC transporters and metabolising enzymes involved in drug disposition. The effects of ligand‐based activation of NR1I3 and NR1I2 were first studied in rodent models (Bauer et al., 2004; Chan et al., 2013; Wang et al., 2010), with subsequent studies carried out in higher models (Lemmen, Tozakidis, Bele, & Galla, 2013; Ott et al., 2009) including human (Chan et al., 2011) (Table 4).
TABLE 4.
Effect of ligand‐based activation of NR1I3 (CAR) and NR1I2 (PXR) on expression of ABC transporters and metabolising enzymes in blood–brain barrier endothelial cells
| Receptor | Species | Model | Ligand | Effect on expression and/or activity | Reference |
|---|---|---|---|---|---|
| NR1I3 | Mouse | Isolated capillaries from wild‐type C3H mice | TCPOBOP | ↑ P‐gp, Mrp2, Bcrp | (Wang et al., 2010) |
| Isolated capillaries from NR1I3 (−/−) C3H mice | N/E | ||||
| Rat | Isolated capillaries from Sprague–Dawley rats | Phenobarbital | ↑ P‐gp, Mrp2, Bcrp | (Wang et al., 2010) | |
| Porcine | Primary culture | CITCO | ↑ P‐GP, BCRP | (Lemmen, Tozakidis, Bele, & Galla, 2013) | |
| Human | hCMEC/D3 cells | CITCO | ↑ P‐GP | (Chan et al., 2011) | |
| NR1I32 | Mouse | Isolated capillaries from wild‐type CB6F1 mice | PCN | ↑ P‐gp | (Bauer et al., 2006) |
| Isolated capillaries from hNR1I2 humanised CB6F1 mice |
Rifampicin Hyperforin |
↑ P‐gp | |||
| CD‐1 mice | Dexamethasone | ↑ P‐gp (cortical capillaries), N/E Cyp3a11 | (Chan et al., 2013) | ||
| Rat | Isolated capillaries from Sprague–Dawley rats |
PCN Dexamethasone |
↑ P‐gp, Gstp | (Bauer et al., 2004; Bauer et al., 2008) | |
| Primary culture from Sprague–Dawley rats | Dexamethasone | ↑ P‐GP, Mrp2, Bcrp | (Narang et al., 2008) | ||
| Porcine | Primary culture |
Rifampicin Hyperforin |
↑ P‐GP, BCRP | (Ott et al., 2009); (Lemmen, Tozakidis, & Galla, 2013) | |
| White large × Landrace hybrid pigs | Rifampicin |
N/E CYP3A22 CYP3A29, CYP3A46 and CYP2B22, P‐GP, MRP1, MRP2 |
(Nannelli et al., 2010) | ||
| Human | hCMEC/D3 cells |
Rifampicin SR12813 |
↑ P‐GP | (Chan et al., 2011) | |
| Primary culture | ↑ CYP3A4, CYP2C9, CYP2E1 | (Ghosh et al., 2017) |
Note: ↑ denotes increased expression or activity, and ↓ decreased expression or activity.
Abbreviation: N/E, no effect.
Studies investigating NR1I3 and NR1I2 expression in rodent (mouse and rat) brain endothelial cells have provided consistent results in both in vitro and in vivo models. In mice, exposure of brain capillaries to PCN and dexamethasone up‐regulated the expression of PGP1(Bauer et al., 2006), while humanised mice for hNR1I2 were responsive to the ligands rifampicin and hyperforin by increasing both P‐gp expression and activity, findings that were reproduced in an in vivo model. The rodent NR1I2 ligand dexamethasone up‐regulated the expression of P‐gp in cortical brain capillaries but not in whole brain homogenates of mice (Chan et al., 2013). A later study that employed PCN in mice demonstrated a similar outcome, but using brain cortex homogenates instead of isolated brain capillaries (Yamasaki et al., 2018). The authors suggested that the PCN‐mediated up‐regulation of cortical P‐gp is a result of its effect on BBB endothelial cells, but the study did not provide findings supporting this statement.
Activation of NR1I2 has been reported to increase both P‐gp (Bauer et al., 2004), Mrp2 and the Gst isoform Gstp (Bauer et al., 2008) expression and activity in brain capillaries of rats exposed to PCN or dexamethasone, with similar findings observed in isolated brain capillaries. In primary cultures of rat brain endothelial cells, dexamethasone up‐regulated the expression of P‐gp, Mrp2 and the BCRP (Narang et al., 2008). Evidence of the expression of functional NR1I3 in rodent brain endothelial cells is provided by a study (Wang et al., 2010), which reported that in vivo and in vitro activation of NR1I3 with TCPOBOP and phenobarbital increased expression and activity of PGP1, MRP2 and the BCRP in mouse and rat brain capillaries, respectively.
Recent studies have proposed that alterations in NR1I2 and NR1I3 function could impair the expression of tight junction proteins at the BBB since deletion of NR1I2 and NR1I3 in rats leads to reduced expression of zonula occuldens‐1 protein in isolated brain microvessels, with no changes in claudin‐5 expression reported (Boussadia et al., 2016; Boussadia et al., 2018). Although this is the only evidence available to date at brain level, it is supported by a previous study that demonstrated the relationship between NR1I2 and expression of TJ proteins in the intestine, in which NR1I2 (−/−) mice exhibited higher intestinal permeability (Venkatesh et al., 2014).
There is convincing evidence of NR1I2 and NR1I3 expression in porcine brain endothelial cells. Although one study has reported that porcine NR1I2 (pNR1I2) is not expressed at the mRNA level in whole brain (Pollock, Rogatcheva, & Schook, 2007), a later report (Nannelli et al., 2010) found porcine NR1I3 and NR1I2 mRNA transcripts in porcine brain capillaries, albeit at lower levels compared to liver. As described in a previous section, the hNR1I2 ligand rifampicin in pigs did not affect capillary mRNA expression of CYP450 isoforms and ABC transporters such as P‐GP, MRP1 and MRP2 (Nannelli et al., 2010). However, in primary cultures of porcine brain endothelial cells, activation of pNR1I2 with the hNR1I2 ligands https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2764 and rifampicin increased expression of P‐GP at the mRNA and protein levels and the activity of P‐GP, although no such effect was achieved with the rodent NR1I2 ligand PCN (Ott et al., 2009). More recently, studies have confirmed the findings of Ott et al. (2009) and shown that activation of pNR1I2 up‐regulated the expression and activity of P‐GP and BCRP in porcine brain endothelial cells (Lemmen, Tozakidis, & Galla, 2013). Furthermore, studies have demonstrated that porcine brain endothelial cells exposed to the hNR1I3 ligand CITCO up‐regulated expression and activity of P‐GP and BCRP, but this up‐regulation was not achieved using TCPOBOP (Lemmen, Tozakidis, Bele, & Galla, 2013).
In human, the evidence is somewhat conflicting but tends to support the expression of functional NR1I3 and NR1I2 in the BBB. A quantitative proteomic analysis performed in freshly isolated human brain capillaries found that NR1I3 and NR1I2 are not expressed at the protein level (Shawahna et al., 2011). However, a later study demonstrated that NR1I2 is expressed in human brain endothelial cells, with a marked overexpression at both mRNA and protein levels in cultured endothelial cells isolated from epileptic brains (Ghosh et al., 2017). The authors suggested that overexpression of NR1I2 might be responsible for the increased expression of CYP enzymes, including https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1337, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1326 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1330.
The hCMEC/D3 human brain endothelial cell line is widely used to study human BBB characteristics. Evidence of key orphan NR expression in these cells is mixed, with one study reporting NR1I3 and NR1I2 mRNA transcripts were not detectable (Dauchy et al., 2009). However, a later study confirmed expression of both receptors at the protein level, demonstrating that exposure of this cell line to CITCO (hNR1I3 ligand), rifampicin and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2763 (hNR1I2 ligands), increased P‐GP mRNA and protein expression (Chan et al., 2011). Furthermore, the same study showed that the NR1I3 and NR1I2‐mediated up‐regulation of P‐GP expression can be counteracted by employing antagonists of both NRs or down‐regulating their expression through use of siRNA.
3.4. NR1I3 (CAR) and NR1I2 (PXR): Roles in neurotoxicity
The mechanistic roles of NR1I2 and NR1I3 in the brain in neurotoxicity induced by xenobiotics, including chemotherapeutic drugs, pesticides and environmental contaminants, are of increasing interest (Table 3). One of the first pieces of evidence of a potential relationship between the ligand‐based activation of orphan NRs and neurotoxicity is the case of the NR1I3 ligand phenobarbital. For example, chronic exposure of rats to phenobarbital up‐regulated the expression of Cyp2b1 and enhanced the neurotoxic actions of the anticancer drug 9‐methoxy‐N2‐methtyellipticinium in reticular neurons (Upadhya et al., 2002). Although the authors did not experimentally demonstrate that NR1I3 is responsible for the outcome, the use of a well‐known NR1I3 ligand strongly suggests involvement of this NR.
Later studies focused their efforts in characterising the molecular mechanisms involved in the NR1I3 and NR1I2‐mediated neurotoxicity. A report showed that in isolated mouse embryonic hippocampal neurons maintained in culture, exposure to the environmental contaminant nonylphenol up‐regulated expression of NR1I3, NR1I2 and Rxrα (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=610) at both the mRNA and protein levels (Litwa et al., 2016). These effects were accompanied by induction of apoptosis, due to increased https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1619 and lactate dehydrogenase activities. A later study showed that treatment of neocortical primary mouse neurons with the environmental contaminant triclocarban resulted in apoptosis mediated by NR1I3 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2951 (AhR) activation (Kajta et al., 2019). Furthermore, triclocarban inhibited SUMOylation, a post‐translational modification that could serve as a protective mechanism by reducing the activity of NR1I3 and AhR.
In cultures of human cord blood CD34+ stem cell‐derived differentiating neuronal cells, treatment with rifampicin up‐regulated the expression of NR1I3, NR1I2, CYP450 enzymes, including the 1A1, 2B6, 3A4 and 2E1 isoforms, and the GSTP1 isoform (Singh et al., 2012). The same study demonstrated that the pesticide and developmental toxicant monocrotophos (MCP) exerted similar effects on the above enzymes, which were enhanced by co‐treatment with MCP and rifampicin. This evidence showed that differentiating neural cells express inducible enzymes that respond to the ligand‐based activation of NR1I2, probably as a mechanism for inactivation of potentially neurotoxic xenobiotics.
Further studies with the human neuroblastoma SH‐SY5Y cell line confirmed the inductive effects elicited by MCP on the expression of NR1I3, NR1I2 and CYP3A4 and also reported enhanced induction of NR1I3, NR1I2 and CYP3A4 mRNA following co‐treatment with MCP and the anticancer drug https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7154 (Tripathi et al., 2017). Furthermore, exposure of SH‐SY5Y cells to MCP increased the production of ROS, decreased the levels of reduced GSH and up‐regulated expression of pro‐apoptotic markers. Docking studies have demonstrated that MCP binds to both NR1I3 and NR1I2, suggesting that the up‐regulation of mitochondrial CYP450 enzymes, mediated by ligand‐based activation of both receptors, may be responsible for changes in levels of ROS and reduced GSH. However, no association between the pro‐apoptotic effects of MCP and the activation of NR1I3/NR1I2 is described.
Similar findings have been reported in a study carried out in quail that evaluated the actions of atrazine, a NR1I2 ligand (Abass et al., 2012) and widely employed neurotoxic herbicide (Lin et al., 2018). Chronic exposure to increasing doses of atrazine resulted in mitochondrial dysfunction, elevated ROS production, induction of apoptosis in the cerebrum and cerebellar toxicity (Lin et al., 2018; Xia et al., 2017). Furthermore, atrazine increased the cerebral and cerebellar expression of NR1I2, NR1I3 and AhR, suggesting that activation of these NRs is responsible for the up‐regulation of expression of CYP450 isoforms (Lin et al., 2018; Xia et al., 2017). The authors of the above studies proposed that the NR1I3, NR1I2 and AhR‐mediated changes in the expression of mitochondrial CYP450 enzymes are responsible for the mitochondrial dysfunction that leads to oxidative stress in the brain. This experimental approach was replicated for the assessment of the effects of the plasticizer di‐(2‐ethylhexyl)‐phthalate on cerebellar toxicity in quail, and the outcome was comparable to that observed for atrazine (Du et al., 2017).
The above findings are interesting since the ligand‐based activation of NR1I3 and NR1I2 partly mediates changes in expression and activities of key proteins involved in neurotoxicity. However, as most of the research attempting to characterise the cellular mechanisms has been conducted in cell‐based models with non‐selective ligands of NR1I3 and NR1I2, more studies are required to establish if the activity of both receptors is directly related to the neurotoxic effect of chemicals in an in vivo setting. This issue is important since the neurotoxic effects observed in animal models exposed to environmental contaminants and other chemicals might be the result of the activation of NRs other than NR1I3 and NR1I2.
3.5. NR1I3 (CAR) and NR1I2 (PXR): Roles in behaviour and other brain functions
The influence of NR1I3 and NR1I2 in the regulation of behaviour and other brain function, including memory, is receiving increased attention. Studies have proposed that the ligand‐based activation of NR1I2 may influence the metabolism of sex hormones, which themselves are ligands of this receptor, thereby altering levels of hormone metabolites involved in the regulation of behaviour.
Studies conducted in rat demonstrate that NR1I2 in the midbrain is required for formation of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4108 (3α,5α‐THP), a metabolite of progesterone and neurosteroid that regulates motivated behaviours (e.g. mating) (Frye et al., 2013). The injection of a Nr1i2 antisense oligonucleotide into the ventral tegmental area of pro‐oestrous rats abrogated expression of NR1I2 and reduced 3α,5α‐THP levels, resulting in diminished sexual receptivity. A later study confirmed the above findings, also reporting that NR1I2 deletion reduced hippocampal levels of brain‐derived neurotropic factor, influencing behaviour and neural plasticity (Frye et al., 2014a).
More recently, studies report that deletion of NR1I3 and NR1I2 in rodents severely impairs recognition memory and increases development of anxiety‐like behaviours (Boussadia et al., 2016; Boussadia et al., 2018). Furthermore, both studies associated the absence of these NRs with alterations in the electroencephalographic signatures during sleep/awake periods. The authors suggested that these findings could be the result of alterations in the metabolism of endogenous mediators that regulate certain behaviours, thereby supporting the findings of previous studies.
4. CONCLUDING REMARKS
The current knowledge of the biological functions elicited by NR1I3 (CAR) and NR1I2 (PXR) in selected organs, such as the liver and intestine, has vastly improved over recent years; however, significantly less is known of the roles of these receptors in the brain. Constant exposure to endogenous and exogenous NR1I3 and NR1I2 ligands therefore necessitates an increased fundamental understanding of how the ligand‐based activation of these receptors, in healthy or pathological states, regulate brain biochemical functions, including proliferation and apoptosis, and psychological functions, such as behaviour. Despite an understanding of NR1I3 and NR1I2 functions in peripheral tissues, responses to ligand‐based activation of NR1I3 and NR1I2 are cell and tissue dependent, with differences attributed to downstream events (e.g. post‐translational modifications, co‐activation and co‐repression) within the NR1I3 and NR1I2 signalling pathways. The evidence from studies in cell lines and animal models have demonstrated that the effects of ligand‐based activation of NR1I3 and NR1I2 are cell dependent, so the approach to analyse their function in brain cells should be exploratory and circumscribed to the cell type used rather than based on assumptions generated from findings obtained on other models. In this regard, the development of selective ligands, comparative studies to assess responses in different tissues, characterisation of downstream events, studies of signalling pathways and the constitutive activity of NR1I3 in cells of the brain, and improved analyses of regional expression of NR1I3 and NR1I2 in the brain would prove useful in defining the roles of these receptors within the CNS.
4.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Cidilowski et al., 2019; Alexander, Fabbro et al., 2019; Alexander, Kelly et al., 2019).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the support of the Comision Nacional de Investigacion Cientifica y Tecnologica (CONICYT), through Grants REDI170373 and CMA BIO‐BIO ECM‐12 PIA‐CONICYT.
Torres‐Vergara P, Ho YS, Espinoza F, Nualart F, Escudero C, Penny J. The constitutive androstane receptor and pregnane X receptor in the brain. Br J Pharmacol. 2020;177:2666–2682. 10.1111/bph.15055
Contributor Information
Pablo Torres‐Vergara, Email: pabltorr@udec.cl.
Jeffrey Penny, Email: jeff.penny@manchester.ac.uk.
REFERENCES
- Abass, K. , Lamsa, V. , Reponen, P. , Kublbeck, J. , Honkakoski, P. , Mattila, S. , … Hakkola, J. (2012). Characterization of human cytochrome P450 induction by pesticides. Toxicology, 294, 17–26. 10.1016/j.tox.2012.01.010 [DOI] [PubMed] [Google Scholar]
- Aleksunes, L. M. , & Klaassen, C. D. (2012). Coordinated regulation of hepatic phase I and II drug‐metabolizing genes and transporters using AhR‐, CAR‐, PXR‐, PPARα‐, and Nrf2‐null mice. Drug Metabolism and Disposition, 40, 1366–1379. 10.1124/dmd.112.045112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Mathie, A. , Peters, J. A. , … GTP colaborators . (2019). The concise guide to pharmacology 2019/2020: Nuclear hormones receptors. British Journal of Pharmacology, 176, S229–S246. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. … CGTP collaborators (2019). The concise guide to pharmacology 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , … GTP collaborators . (2019). The concise guide to pharmacology 2019/20: Transporters. British Journal of Pharmacology, 176, S397–S493. 10.1111/bph.14753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnouti, Y. , & Klaassen, C. D. (2008). Regulation of sulfotransferase enzymes by prototypical microsomal enzyme inducers in mice. The Journal of Pharmacology and Experimental Therapeutics, 324, 612–621. 10.1124/jpet.107.129650 [DOI] [PubMed] [Google Scholar]
- Banerjee, M. , Robbins, D. , & Chen, T. (2015). Targeting xenobiotic receptors PXR and CAR in human diseases. Drug Discovery Today, 20, 618–628. 10.1016/j.drudis.2014.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, B. , Hartz, A. M. , Fricker, G. , & Miller, D. S. (2004). Pregnane X receptor up‐regulation of P‐glycoprotein expression and transport function at the blood‐brain barrier. Molecular Pharmacology, 66, 413–419. 10.1124/mol.66.3 [DOI] [PubMed] [Google Scholar]
- Bauer, B. , Hartz, A. M. , Lucking, J. R. , Yang, X. , Pollack, G. M. , & Miller, D. S. (2008). Coordinated nuclear receptor regulation of the efflux transporter, Mrp2, and the phase‐II metabolizing enzyme, GSTpi, at the blood‐brain barrier. Journal of Cerebral Blood Flow and Metabolism, 28, 1222–1234. 10.1038/jcbfm.2008.16 [DOI] [PubMed] [Google Scholar]
- Bauer, B. , Yang, X. , Hartz, A. M. S. , Olson, E. R. , Zhao, R. , Kalvass, J. C. , … Miller, D. S. (2006). In vivo activation of human pregnane X receptor tightens the blood‐brain barrier to methadone through P‐glycoprotein up‐regulation. Molecular Pharmacology, 70, 1212–1219. 10.1124/mol.106.023796 [DOI] [PubMed] [Google Scholar]
- Bitter, A. , Rümmele, P. , Klein, K. , Kandel, B. A. , Rieger, J. K. , Nüssler, A. K. , … Burk, O. (2015). Pregnane X receptor activation and silencing promote steatosis of human hepatic cells by distinct lipogenic mechanisms. Archives of Toxicology, 89, 2089–2103. 10.1007/s00204-014-1348-x [DOI] [PubMed] [Google Scholar]
- Boussadia, B. , Gangarossa, G. , Mselli‐Lakhal, L. , Rousset, M. C. , de Bock, F. , Lassere, F. , … Marchi, N. (2016). Lack of CAR impacts neuronal function and cerebrovascular integrity in vivo. Experimental Neurology, 283, 39–48. 10.1016/j.expneurol.2016.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussadia, B. , Lakhal, L. , Payrastre, L. , Ghosh, C. , Pascussi, J. M. , Gangarossa, G. , & Marchi, N. (2018). Pregnane X receptor deletion modifies recognition memory and electroencephalographic activity. Neuroscience, 370, 130–138. 10.1016/j.neuroscience.2017.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calanni, F. , Renzulli, C. , Barbanti, M. , & Viscomi, G. C. (2014). Rifaximin: Beyond the traditional antibiotic activity. Journal of Antibiotics (Tokyo), 67, 667–670. [DOI] [PubMed] [Google Scholar]
- Cerveny, L. , Svecova, L. , Anzenbacherova, E. , Vrzal, R. , Staud, F. , Dvorak, Z. , … Pavek, P. (2007). Valproic acid induces CYP3A4 and MDR1 gene expression by activation of constitutive androstane receptor and pregnane X receptor pathways. Drug Metabolism and Disposition, 35, 1032–1041. 10.1124/dmd.106.014456 [DOI] [PubMed] [Google Scholar]
- Chai, X. , Zeng, S. , & Xie, W. (2013). Nuclear receptors PXR and CAR: Implications for drug metabolism regulation, pharmacogenomics and beyond. Expert Opinion on Drug Metabolism & Toxicology, 9, 253–266. 10.1517/17425255.2013.754010 [DOI] [PubMed] [Google Scholar]
- Chan, G. N. , Hoque, M. T. , Cummins, C. L. , & Bendayan, R. (2011). Regulation of P‐glycoprotein by orphan nuclear receptors in human brain microvessel endothelial cells. Journal of Neurochemistry, 118, 163–175. 10.1111/j.1471-4159.2011.07288.x [DOI] [PubMed] [Google Scholar]
- Chan, G. N. , Saldivia, V. , Yang, Y. , Pang, H. , de Lannoy, I. , & Bendayan, R. (2013). In vivo induction of P‐glycoprotein expression at the mouse blood‐brain barrier: An intracerebral microdialysis study. Journal of Neurochemistry, 127, 342–352. 10.1111/jnc.12344 [DOI] [PubMed] [Google Scholar]
- Chen, S. , Wang, K. , & Wan, Y. J. (2010). Retinoids activate RXR/CAR‐mediated pathway and induce CYP3A. Biochemical Pharmacology, 79, 270–276. 10.1016/j.bcp.2009.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. , Zhang, J. , Baker, S. M. , & Chen, G. (2007). Human constitutive androstane receptor mediated methotrexate induction of human dehydroepiandrosterone sulfotransferase (hSULT2A1). Toxicology, 231, 224–233. 10.1016/j.tox.2006.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Ferguson, S. S. , Negishi, M. , & Goldstein, J. A. (2003). Identification of constitutive androstane receptor and glucocorticoid receptor binding sites in the CYP2C19 promoter. Molecular Pharmacology, 64, 316–324. 10.1124/mol.64.2.316 [DOI] [PubMed] [Google Scholar]
- Cherian, M. T. , Chai, S. C. , Wright, W. C. , Singh, A. , Alexandra Casal, M. , Zheng, J. , … Chen, T. (2018). CINPA1 binds directly to constitutive androstane receptor and inhibits its activity. Biochemical Pharmacology, 152, 211–223. 10.1016/j.bcp.2018.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, J. Y. , Choudhuri, S. , Knight, T. R. , & Klaassen, C. D. (2010). Genetic and epigenetic regulation and expression signatures of glutathione S‐transferases in developing mouse liver. Toxicological Sciences, 116, 32–43. 10.1093/toxsci/kfq115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauchy, S. , Miller, F. , Couraud, P. O. , Weaver, R. J. , Weksler, B. , Romero, I. A. , … Declèves, X. (2009). Expression and transcriptional regulation of ABC transporters and cytochromes P450 in hCMEC/D3 human cerebral microvascular endothelial cells. Biochemical Pharmacology, 77, 897–909. 10.1016/j.bcp.2008.11.001 [DOI] [PubMed] [Google Scholar]
- Dong, B. , Saha, P. K. , Huang, W. , Chen, W. , Abu‐Elheiga, L. A. , Wakil, S. J. , … Moore, D. D. (2009). Activation of nuclear receptor CAR ameliorates diabetes and fatty liver disease. Proceedings of the National Academy of Sciences of the United States of America, 106, 18831–18836. 10.1073/pnas.0909731106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, H. , Lin, W. , Wu, J. , & Chen, T. (2010). Flavonoids activate pregnane × receptor‐mediated CYP3A4 gene expression by inhibiting cyclin‐dependent kinases in HepG2 liver carcinoma cells. BMC Biochemistry, 11, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou, W. , Zhang, J. , Zhang, E. , Sun, A. , Ding, L. , Chou, G. , … Mani, S. (2013). Chrysin ameliorates chemically induced colitis in the mouse through modulation of a PXR/NF‐κB signaling pathway. The Journal of Pharmacology and Experimental Therapeutics, 345, 473–482. 10.1124/jpet.112.201863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, Z. H. , Xia, J. , Sun, X. C. , Li, X. N. , Zhang, C. , Zhao, H. S. , … Li, J. L. (2017). A novel nuclear xenobiotic receptors (AhR/PXR/CAR)‐mediated mechanism of DEHP‐induced cerebellar toxicity in quails (Coturnix japonica) via disrupting CYP enzyme system homeostasis. Environmental Pollution, 226, 435–443. 10.1016/j.envpol.2017.04.015 [DOI] [PubMed] [Google Scholar]
- Echchgadda, I. , Song, C. S. , Oh, T. , Ahmed, M. , De La Cruz, I. J. , & Chatterjee, B. (2007). The xenobiotic‐sensing nuclear receptors pregnane X receptor, constitutive androstane receptor, and orphan nuclear receptor hepatocyte nuclear factor 4α in the regulation of human steroid‐/bile acid‐sulfotransferase. Molecular Endocrinology, 21, 2099–2111. 10.1210/me.2007-0002 [DOI] [PubMed] [Google Scholar]
- Ferguson, S. S. , Chen, Y. , LeCluyse, E. L. , Negishi, M. , & Goldstein, J. A. (2005). Human CYP2C8 is transcriptionally regulated by the nuclear receptors constitutive androstane receptor, pregnane X receptor, glucocorticoid receptor, and hepatic nuclear factor 4α. Molecular Pharmacology, 68, 747–757. 10.1124/mol.105.013169 [DOI] [PubMed] [Google Scholar]
- Ferguson, S. S. , LeCluyse, E. L. , Negishi, M. , & Goldstein, J. A. (2002). Regulation of human CYP2C9 by the constitutive androstane receptor: Discovery of a new distal binding site. Molecular Pharmacology, 62, 737–746. 10.1124/mol.62.3.737 [DOI] [PubMed] [Google Scholar]
- Forman, B. M. , Tzameli, I. , Choi, H. S. , Chen, J. , Simha, D. , Seol, W. , … Moore, D. D. (1998). Androstane metabolites bind to and deactivate the nuclear receptor CAR‐β. Nature, 395, 612–615. 10.1038/26996 [DOI] [PubMed] [Google Scholar]
- Frye, C. A. , Koonce, C. J. , & Walf, A. A. (2014a). Involvement of pregnane xenobiotic receptor in mating‐induced allopregnanolone formation in the midbrain and hippocampus and brain‐derived neurotrophic factor in the hippocampus among female rats. Psychopharmacology, 231, 3375–3390. 10.1007/s00213-014-3569-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye, C. A. , Koonce, C. J. , & Walf, A. A. (2014b). Novel receptor targets for production and action of allopregnanolone in the central nervous system: A focus on pregnane xenobiotic receptor. Frontiers in Cellular Neuroscience, 8, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye, C. A. , Koonce, C. J. , & Walf, A. A. (2014c). The pregnane xenobiotic receptor, a prominent liver factor, has actions in the midbrain for neurosteroid synthesis and behavioral/neural plasticity of female rats. Frontiers in Systems Neuroscience, 8, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye, C. A. , Koonce, C. J. , & Walf, A. A. (2014d). Role of pregnane xenobiotic receptor in the midbrain ventral tegmental area for estradiol‐ and 3α,5α‐THP‐facilitated lordosis of female rats. Psychopharmacology, 231, 3365–3374. 10.1007/s00213-013-3406-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye, C. A. , Koonce, C. J. , Walf, A. A. , & Rusconi, J. C. (2013). Motivated behaviors and levels of 3α,5α‐THP in the midbrain are attenuated by knocking down expression of pregnane xenobiotic receptor in the midbrain ventral tegmental area of proestrous rats. The Journal of Sexual Medicine, 10, 1692–1706. 10.1111/jsm.12173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, J. , He, J. , Zhai, Y. , Wada, T. , & Xie, W. (2009). The constitutive androstane receptor is an anti‐obesity nuclear receptor that improves insulin sensitivity. The Journal of Biological Chemistry, 284, 25984–25992. 10.1074/jbc.M109.016808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, C. , Hossain, M. , Solanki, J. , Najm, I. M. , Marchi, N. , & Janigro, D. (2017). Overexpression of pregnane X and glucocorticoid receptors and the regulation of cytochrome P450 in human epileptic brain endothelial cells. Epilepsia, 58, 576–585. 10.1111/epi.13703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giessmann, T. , May, K. , Modess, C. , Wegner, D. , Hecker, U. , Zschiesche, M. , … Siegmund, W. (2004). Carbamazepine regulates intestinal P‐glycoprotein and multidrug resistance protein MRP2 and influences disposition of talinolol in humans. Clinical Pharmacology and Therapeutics, 76, 192–200. 10.1016/j.clpt.2004.04.011 [DOI] [PubMed] [Google Scholar]
- Goodwin, B. , Hodgson, E. , D'Costa, D. J. , Robertson, G. R. , & Liddle, C. (2002). Transcriptional regulation of the human CYP3A4 gene by the constitutive androstane receptor. Molecular Pharmacology, 62, 359–365. 10.1124/mol.62.2.359 [DOI] [PubMed] [Google Scholar]
- Gotoh, S. , & Negishi, M. (2015). Statin‐activated nuclear receptor PXR promotes SGK2 dephosphorylation by scaffolding PP2C to induce hepatic gluconeogenesis. Scientific Reports, 5, 14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, M. A. , Peacock, J. N. , & Squires, E. J. (2009). Characterization of the porcine constitutive androstane receptor (CAR) and its splice variants. Xenobiotica, 39, 915–930. 10.3109/00498250903330348 [DOI] [PubMed] [Google Scholar]
- Gray, M. A. , Pollock, C. B. , Schook, L. B. , & Squires, E. J. (2010). Characterization of porcine pregnane X receptor, farnesoid X receptor and their splice variants. Experimental Biology and Medicine (Maywood, N.J.), 235, 718–736. [DOI] [PubMed] [Google Scholar]
- Haines, C. , Elcombe, B. M. , Chatham, L. R. , Vardy, A. , Higgins, L. G. , Elcombe, C. R. , et al. (2018). Comparison of the effects of sodium phenobarbital in wild type and humanized constitutive androstane receptor (CAR)/pregnane X receptor (PXR) mice and in cultured mouse, rat and human hepatocytes. Toxicology, 396(397), 23–32. [DOI] [PubMed] [Google Scholar]
- Hakkola, J. , Rysa, J. , & Hukkanen, J. (2016). Regulation of hepatic energy metabolism by the nuclear receptor PXR. Biochimica et Biophysica Acta, 1859, 1072–1082. 10.1016/j.bbagrm.2016.03.012 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani‐Nezhad‐Gashti, F. , Rysä, J. , Kummu, O. , Näpänkangas, J. , Buler, M. , Karpale, M. , … Hakkola, J. (2018). Activation of nuclear receptor PXR impairs glucose tolerance and dysregulates GLUT2 expression and subcellular localization in liver. Biochemical Pharmacology, 148, 253–264. 10.1016/j.bcp.2018.01.001 [DOI] [PubMed] [Google Scholar]
- He, J. , Gao, J. , Xu, M. , Ren, S. , Stefanovic‐Racic, M. , O'Doherty, R. M. , & Xie, W. (2013). PXR ablation alleviates diet‐induced and genetic obesity and insulin resistance in mice. Diabetes, 62, 1876–1887. 10.2337/db12-1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. , Wang, H. , Sinz, M. , Zoeckler, M. , Staudinger, J. , Redinbo, M. R. , … Mani, S. (2007). Inhibition of drug metabolism by blocking the activation of nuclear receptors by ketoconazole. Oncogene, 26, 258–268. 10.1038/sj.onc.1209788 [DOI] [PubMed] [Google Scholar]
- Huang, P. , Chandra, V. , & Rastinejad, F. (2010). Structural overview of the nuclear receptor superfamily: Insights into physiology and therapeutics. Annual Review of Physiology, 72, 247–272. 10.1146/annurev-physiol-021909-135917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hukkanen, J. , Hakkola, J. , & Rysa, J. (2014). Pregnane X receptor (PXR)—A contributor to the diabetes epidemic? Drug Metabolism and Drug Interactions, 29, 3–15. [DOI] [PubMed] [Google Scholar]
- Ihunnah, C. A. , Jiang, M. , & Xie, W. (2011). Nuclear receptor PXR, transcriptional circuits and metabolic relevance. Biochimica et Biophysica Acta, 1812, 956–963. 10.1016/j.bbadis.2011.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jigorel, E. , Le Vee, M. , Boursier‐Neyret, C. , Parmentier, Y. , & Fardel, O. (2006). Differential regulation of sinusoidal and canalicular hepatic drug transporter expression by xenobiotics activating drug‐sensing receptors in primary human hepatocytes. Drug Metabolism and Disposition, 34, 1756–1763. 10.1124/dmd.106.010033 [DOI] [PubMed] [Google Scholar]
- Jimenez, B. D. , Quattrochi, L. C. , Yockey, C. B. , & Guzelian, P. S. (2000). Identification by differential display of the IF1 inhibitor peptide of ATP synthase/ATPase as a gene inducible in rat liver by pregnenolone 16α‐carbonitrile. Life Sciences, 67, 1825–1832. 10.1016/s0024-3205(00)00769-4 [DOI] [PubMed] [Google Scholar]
- Jones, S. A. , Moore, L. B. , Shenk, J. L. , Wisely, G. B. , Hamilton, G. A. , McKee, D. D. , … Moore, J. T. (2000). The pregnane X receptor: A promiscuous xenobiotic receptor that has diverged during evolution. Molecular Endocrinology, 14, 27–39. 10.1210/mend.14.1.0409 [DOI] [PubMed] [Google Scholar]
- Kajta, M. , Wnuk, A. , Rzemieniec, J. , Lason, W. , Mackowiak, M. , Chwastek, E. , … Wojtowicz, A. K. (2019). Triclocarban disrupts the epigenetic status of neuronal cells and induces AHR/CAR‐mediated apoptosis. Molecular Neurobiology, 56, 3113–3131. 10.1007/s12035-018-1285-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno, Y. , Suzuki, M. , Nakahama, T. , & Inouye, Y. (2005). Characterization of nuclear localization signals and cytoplasmic retention region in the nuclear receptor CAR. Biochimica et Biophysica Acta, 1745, 215–222. 10.1016/j.bbamcr.2005.06.012 [DOI] [PubMed] [Google Scholar]
- Kast, H. R. , Goodwin, B. , Tarr, P. T. , Jones, S. A. , Anisfeld, A. M. , Stoltz, C. M. , … Edwards, P. A. (2002). Regulation of multidrug resistance‐associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X‐activated receptor, and constitutive androstane receptor. The Journal of Biological Chemistry, 277, 2908–2915. 10.1074/jbc.M109326200 [DOI] [PubMed] [Google Scholar]
- Kawamoto, T. , Sueyoshi, T. , Zelko, I. , Moore, R. , Washburn, K. , & Negishi, M. (1999). Phenobarbital‐responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Molecular and Cellular Biology, 19, 6318–6322. 10.1128/mcb.19.9.6318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorasanizadeh, S. , & Rastinejad, F. (2016). Visualizing the architectures and interactions of nuclear receptors. Endocrinology, 157, 4212–4221. 10.1210/en.2016-1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen, C. D. , & Aleksunes, L. M. (2010). Xenobiotic, bile acid, and cholesterol transporters: Function and regulation. Pharmacological Reviews, 62, 1–96. 10.1124/pr.109.002014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer, S. A. , Moore, J. T. , Wade, L. , Staudinger, J. L. , Watson, M. A. , Jones, S. A. , … Lehmann, J. M. (1998). An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell, 92, 73–82. 10.1016/s0092-8674(00)80900-9 [DOI] [PubMed] [Google Scholar]
- Knight, T. R. , Choudhuri, S. , & Klaassen, C. D. (2008). Induction of hepatic glutathione S‐transferases in male mice by prototypes of various classes of microsomal enzyme inducers. Toxicological Sciences, 106, 329–338. 10.1093/toxsci/kfn179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, K. , Sueyoshi, T. , Inoue, K. , Moore, R. , & Negishi, M. (2003). Cytoplasmic accumulation of the nuclear receptor CAR by a tetratricopeptide repeat protein in HepG2 cells. Molecular Pharmacology, 64, 1069–1075. 10.1124/mol.64.5.1069 [DOI] [PubMed] [Google Scholar]
- Kodama, S. , Koike, C. , Negishi, M. , & Yamamoto, Y. (2004). Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug‐metabolizing and gluconeogenic enzymes. Molecular and Cellular Biology, 24, 7931–7940. 10.1128/MCB.24.18.7931-7940.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama, S. , Moore, R. , Yamamoto, Y. , & Negishi, M. (2007). Human nuclear pregnane X receptor cross‐talk with CREB to repress cAMP activation of the glucose‐6‐phosphatase gene. The Biochemical Journal, 407, 373–381. 10.1042/BJ20070481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsounas, I. , Giaginis, C. , Alexandrou, P. , Zizi‐Serbetzoglou, A. , Patsouris, E. , Kouraklis, G. , & Theocharis, S. (2015). Pregnane X Receptor Expression in Human Pancreatic Adenocarcinoma: Associations With Clinicopathologic Parameters, Tumor Proliferative Capacity, Patients' Survival, and Retinoid X Receptor Expression. Pancreas, 44(7), 1134–1140. [DOI] [PubMed] [Google Scholar]
- Krasowski, M. D. , Ni, A. , Hagey, L. R. , & Ekins, S. (2011). Evolution of promiscuous nuclear hormone receptors: LXR, FXR, VDR, PXR, and CAR. Molecular and Cellular Endocrinology, 334, 39–48. 10.1016/j.mce.2010.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba, J. K. , Lamba, V. , Yasuda, K. , Lin, Y. S. , Assem, M. , Thompson, E. , … Schuetz, E. (2004). Expression of constitutive androstane receptor splice variants in human tissues and their functional consequences. The Journal of Pharmacology and Experimental Therapeutics, 311, 811–821. 10.1124/jpet.104.069310 [DOI] [PubMed] [Google Scholar]
- Lemmen, J. , Tozakidis, I. E. , Bele, P. , & Galla, H. J. (2013). Constitutive androstane receptor upregulates Abcb1 and Abcg2 at the blood‐brain barrier after CITCO activation. Brain Research, 1501, 68–80. 10.1016/j.brainres.2013.01.025 [DOI] [PubMed] [Google Scholar]
- Lemmen, J. , Tozakidis, I. E. , & Galla, H. J. (2013). Pregnane X receptor upregulates ABC‐transporter Abcg2 and Abcb1 at the blood‐brain barrier. Brain Research, 1491, 1–13. 10.1016/j.brainres.2012.10.060 [DOI] [PubMed] [Google Scholar]
- Li, L. , Li, H. , Garzel, B. , Yang, H. , Sueyoshi, T. , Li, Q. , … Wang, H. (2015). SLC13A5 is a novel transcriptional target of the pregnane X receptor and sensitizes drug‐induced steatosis in human liver. Molecular Pharmacology, 87, 674–682. 10.1124/mol.114.097287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, J. , Zhao, H. S. , Qin, L. , Li, X. N. , Zhang, C. , Xia, J. , & Li, J. L. (2018). Atrazine triggers mitochondrial dysfunction and oxidative stress in quail (Coturnix C. coturnix) cerebrum via activating xenobiotic‐sensing nuclear receptors and modulating cytochrome P450 systems. Journal of Agricultural and Food Chemistry, 66, 6402–6413. 10.1021/acs.jafc.8b01413 [DOI] [PubMed] [Google Scholar]
- Lin, W. , Bwayi, M. , Wu, J. , Li, Y. , Chai, S. C. , Huber, A. D. , & Chen, T. (2020). CITCO directly binds to and activates human pregnane X receptor. Molecular Pharmacology, 97, 180–190. 10.1124/mol.119.118513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, W. , Wang, Y. M. , Chai, S. C. , Lv, L. , Zheng, J. , Wu, J. , … Chen, T. (2017). SPA70 is a potent antagonist of human pregnane X receptor. Nature Communications, 8, 741 10.1038/s41467-017-00780-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, W. , Wu, J. , Dong, H. , Bouck, D. , Zeng, F. Y. , & Chen, T. (2008). Cyclin‐dependent kinase 2 negatively regulates human pregnane X receptor‐mediated CYP3A4 gene expression in HepG2 liver carcinoma cells. The Journal of Biological Chemistry, 283, 30650–30657. 10.1074/jbc.M806132200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwa, E. , Rzemieniec, J. , Wnuk, A. , Lason, W. , Krzeptowski, W. , & Kajta, M. (2016). RXRα, PXR and CAR xenobiotic receptors mediate the apoptotic and neurotoxic actions of nonylphenol in mouse hippocampal cells. The Journal of Steroid Biochemistry and Molecular Biology, 156, 43–52. 10.1016/j.jsbmb.2015.11.018 [DOI] [PubMed] [Google Scholar]
- Mackowiak, B. , Li, L. , Lynch, C. , Ziman, A. , Heyward, S. , Xia, M. , & Wang, H. (2019). High‐content analysis of constitutive androstane receptor (CAR) translocation identifies mosapride citrate as a CAR agonist that represses gluconeogenesis. Biochemical Pharmacology, 168, 224–236. 10.1016/j.bcp.2019.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackowiak, B. , & Wang, H. (2016). Mechanisms of xenobiotic receptor activation: Direct vs. indirect. Biochimica et Biophysica Acta, 1859, 1130–1140. 10.1016/j.bbagrm.2016.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglich, J. M. , Watson, J. , McMillen, P. J. , Goodwin, B. , Willson, T. M. , & Moore, J. T. (2004). The nuclear receptor CAR is a regulator of thyroid hormone metabolism during caloric restriction. The Journal of Biological Chemistry, 279, 19832–19838. 10.1074/jbc.M313601200 [DOI] [PubMed] [Google Scholar]
- Malekshah, O. M. , Lage, H. , Bahrami, A. R. , Afshari, J. T. , & Behravan, J. (2012). PXR and NF‐κB correlate with the inducing effects of IL‐1β and TNF‐α on ABCG2 expression in breast cancer cell lines. European Journal of Pharmaceutical Sciences, 47, 474–480. [DOI] [PubMed] [Google Scholar]
- Marini, S. , Nannelli, A. , Sodini, D. , Dragoni, S. , Valoti, M. , Longo, V. , & Gervasi, P. G. (2007). Expression, microsomal and mitochondrial activities of cytochrome P450 enzymes in brain regions from control and phenobarbital‐treated rabbits. Life Sciences, 80, 910–917. 10.1016/j.lfs.2006.11.022 [DOI] [PubMed] [Google Scholar]
- Marmugi, A. , Lukowicz, C. , Lasserre, F. , Montagner, A. , Polizzi, A. , Ducheix, S. , … Mselli‐Lakhal, L. (2016). Activation of the Constitutive Androstane Receptor induces hepatic lipogenesis and regulates Pnpla3 gene expression in a LXR‐independent way. Toxicology and Applied Pharmacology, 303, 90–100. 10.1016/j.taap.2016.05.006 [DOI] [PubMed] [Google Scholar]
- Martin, P. , Riley, R. , Back, D. J. , & Owen, A. (2008). Comparison of the induction profile for drug disposition proteins by typical nuclear receptor activators in human hepatic and intestinal cells. British Journal of Pharmacology, 153, 805–819. 10.1038/sj.bjp.0707601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencarelli, A. , Renga, B. , Palladino, G. , Claudio, D.'. A. , Ricci, P. , Distrutti, E. , … Fiorucci, S. (2011). Inhibition of NF‐κB by a PXR‐dependent pathway mediates counter‐regulatory activities of rifaximin on innate immunity in intestinal epithelial cells. European Journal of Pharmacology, 668, 317–324. 10.1016/j.ejphar.2011.06.058 [DOI] [PubMed] [Google Scholar]
- Messina, A. , Nannelli, A. , Fiorio, R. , Longo, V. , & Gervasi, P. G. (2009). Expression and inducibility of CYP1A1, 1A2, 1B1 by β‐naphthoflavone and CYP2B22, 3A22, 3A29, 3A46 by rifampicin in the respiratory and olfactory mucosa of pig. Toxicology, 260, 47–52. 10.1016/j.tox.2009.03.003 [DOI] [PubMed] [Google Scholar]
- Meyer zu Schwabedissen, H. E. , Tirona, R. G. , Yip, C. S. , Ho, R. H. , & Kim, R. B. (2008). Interplay between the nuclear receptor pregnane X receptor and the uptake transporter organic anion transporter polypeptide 1A2 selectively enhances estrogen effects in breast cancer. Cancer Research, 68, 9338–9347. 10.1158/0008-5472.CAN-08-0265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki, Y. , Suzuki, T. , Tazawa, C. , Blumberg, B. , & Sasano, H. (2005). Steroid and xenobiotic receptor (SXR), cytochrome P450 3A4 and multidrug resistance gene 1 in human adult and fetal tissues. Molecular and Cellular Endocrinology, 231, 75–85. 10.1016/j.mce.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Miller, D. S. (2015). Regulation of ABC transporters blood‐brain barrier: The good, the bad, and the ugly. Advances in Cancer Research, 125, 43–70. 10.1016/bs.acr.2014.10.002 [DOI] [PubMed] [Google Scholar]
- Moore, L. B. , Goodwin, B. , Jones, S. A. , Wisely, G. B. , Serabjit‐Singh, C. J. , Willson, T. M. , … Kliewer, S. A. (2000). St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proceedings of the National Academy of Sciences of the United States of America, 97, 7500–7502. 10.1073/pnas.130155097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau, A. , Teruel, C. , Beylot, M. , Albalea, V. , Tamasi, V. , Umbdenstock, T. , … Navarro, F. (2009). A novel pregnane X receptor and S14‐mediated lipogenic pathway in human hepatocyte. Hepatology, 49, 2068–2079. 10.1002/hep.22907 [DOI] [PubMed] [Google Scholar]
- Mullican, S. E. , Dispirito, J. R. , & Lazar, M. A. (2013). The orphan nuclear receptors at their 25‐year reunion. Journal of Molecular Endocrinology, 51, T115–T140. 10.1530/JME-13-0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, K. , Moore, R. , Negishi, M. , & Sueyoshi, T. (2007). Nuclear pregnane X receptor cross‐talk with FoxA2 to mediate drug‐induced regulation of lipid metabolism in fasting mouse liver. The Journal of Biological Chemistry, 282, 9768–9776. 10.1074/jbc.M610072200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nannelli, A. , Rossignolo, F. , Tolando, R. , Rossato, P. , Longo, V. , & Gervasi, P. G. (2009). Effect of β‐naphthoflavone on AhR‐regulated genes (CYP1A1, 1A2, 1B1, 2S1, Nrf2, and GST) and antioxidant enzymes in various brain regions of pig. Toxicology, 265, 69–79. 10.1016/j.tox.2009.09.010 [DOI] [PubMed] [Google Scholar]
- Nannelli, A. , Rossignolo, F. , Tolando, R. , Rossato, P. , Pellegatti, M. , Longo, V. , & Gervasi, P. G. (2010). Expression and distribution of CYP3A genes, CYP2B22, and MDR1, MRP1, MRP2, LRP efflux transporters in brain of control and rifampicin‐treated pigs. Molecular and Cellular Biochemistry, 337, 133–143. 10.1007/s11010-009-0292-1 [DOI] [PubMed] [Google Scholar]
- Narang, V. S. , Fraga, C. , Kumar, N. , Shen, J. , Throm, S. , Stewart, C. F. , & Waters, C. M. (2008). Dexamethasone increases expression and activity of multidrug resistance transporters at the rat blood‐brain barrier. American Journal of Physiology. Cell Physiology, 295, C440–C450. 10.1152/ajpcell.00491.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, M. , Naito, S. , & Yokoi, T. (2004). Tissue‐specific mRNA expression profiles of human nuclear receptor subfamilies. Drug Metabolism and Pharmacokinetics, 19, 135–149. 10.2133/dmpk.19.135 [DOI] [PubMed] [Google Scholar]
- Oladimeji, P. , Cui, H. , Zhang, C. , & Chen, T. (2016). Regulation of PXR and CAR by protein‐protein interaction and signaling crosstalk. Expert Opinion on Drug Metabolism & Toxicology, 12, 997–1010. 10.1080/17425255.2016.1201069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oladimeji, P. O. , & Chen, T. (2018). PXR: More than just a master xenobiotic receptor. Molecular Pharmacology, 93, 119–127. 10.1124/mol.117.110155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald, S. , Haenisch, S. , Fricke, C. , Sudhop, T. , Remmler, C. , Giessmann, T. , … Siegmund, W. (2006). Intestinal expression of P‐glycoprotein (ABCB1), multidrug resistance associated protein 2 (ABCC2), and uridine diphosphate‐glucuronosyltransferase 1A1 predicts the disposition and modulates the effects of the cholesterol absorption inhibitor ezetimibe in humans. Clinical Pharmacology and Therapeutics, 79, 206–217. 10.1016/j.clpt.2005.11.004 [DOI] [PubMed] [Google Scholar]
- Ott, M. , Fricker, G. , & Bauer, B. (2009). Pregnane X receptor (PXR) regulates P‐glycoprotein at the blood‐brain barrier: Functional similarities between pig and human PXR. The Journal of Pharmacology and Experimental Therapeutics, 329, 141–149. 10.1124/jpet.108.149690 [DOI] [PubMed] [Google Scholar]
- Ouyang, N. , Ke, S. , Eagleton, N. , Xie, Y. , Chen, G. , Laffins, B. , … Tian, Y. (2010). Pregnane X receptor suppresses proliferation and tumourigenicity of colon cancer cells. British Journal of Cancer, 102, 1753–1761. 10.1038/sj.bjc.6605677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascussi, J. M. , Gerbal‐Chaloin, S. , Pichard‐Garcia, L. , Daujat, M. , Fabre, J. M. , Maurel, P. , & Vilarem, M. J. (2000). Interleukin‐6 negatively regulates the expression of pregnane X receptor and constitutively activated receptor in primary human hepatocytes. Biochemical and Biophysical Research Communications, 274, 707–713. 10.1006/bbrc.2000.3219 [DOI] [PubMed] [Google Scholar]
- Petrick, J. S. , & Klaassen, C. D. (2007). Importance of hepatic induction of constitutive androstane receptor and other transcription factors that regulate xenobiotic metabolism and transport. Drug Metabolism and Disposition, 35, 1806–1815. 10.1124/dmd.107.015974 [DOI] [PubMed] [Google Scholar]
- Pollock, C. B. , Rogatcheva, M. B. , & Schook, L. B. (2007). Comparative genomics of xenobiotic metabolism: A porcine‐human PXR gene comparison. Mammalian Genome, 18, 210–219. 10.1007/s00335-007-9007-7 [DOI] [PubMed] [Google Scholar]
- Qosa, H. , Miller, D. S. , Pasinelli, P. , & Trotti, D. (2015). Regulation of ABC efflux transporters at blood‐brain barrier in health and neurological disorders. Brain Research, 1628, 298–316. 10.1016/j.brainres.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins, D. , Bakke, J. , Cherian, M. T. , Wu, J. , & Chen, T. (2016). PXR interaction with p53: A meeting of two masters. Cell Death & Disease, 7, e2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins, D. , Cherian, M. , Wu, J. , & Chen, T. (2016). Human pregnane X receptor compromises the function of p53 and promotes malignant transformation. Cell Death Discovery, 2, 16023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runtz, L. , Girard, B. , Toussenot, M. , Espallergues, J. , Fayd'Herbe De Maudave, A. , Milman, A. , … Bertaso, F. (2018). Hepatic and hippocampal cytochrome P450 enzyme overexpression during spontaneous recurrent seizures. Epilepsia, 59, 123–134. 10.1111/epi.13942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saradhi, M. , Sengupta, A. , Mukhopadhyay, G. , & Tyagi, R. K. (2005). Pregnane and Xenobiotic Receptor (PXR/SXR) resides predominantly in the nuclear compartment of the interphase cell and associates with the condensed chromosomes during mitosis. Biochimica et Biophysica Acta, 1746, 85–94. 10.1016/j.bbamcr.2005.10.004 [DOI] [PubMed] [Google Scholar]
- Savkur, R. S. , Wu, Y. , Bramlett, K. S. , Wang, M. , Yao, S. , Perkins, D. , … Burris, T. P. (2003). Alternative splicing within the ligand binding domain of the human constitutive androstane receptor. Molecular Genetics and Metabolism, 80, 216–226. 10.1016/j.ymgme.2003.08.013 [DOI] [PubMed] [Google Scholar]
- Schilter, B. , & Omiecinski, C. J. (1993). Regional distribution and expression modulation of cytochrome P‐450 and epoxide hydrolase mRNAs in the rat brain. Molecular Pharmacology, 44, 990–996. [PubMed] [Google Scholar]
- Sever, R. , & Glass, C. K. (2013). Signaling by nuclear receptors. Cold Spring Harbor Perspectives in Biology, 5, a016709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawahna, R. , Uchida, Y. , Decleves, X. , Ohtsuki, S. , Yousif, S. , Dauchy, S. , … Terasaki, T. (2011). Transcriptomic and quantitative proteomic analysis of transporters and drug metabolizing enzymes in freshly isolated human brain microvessels. Molecular Pharmaceutics, 8, 1332–1341. [DOI] [PubMed] [Google Scholar]
- Singh, A. K. , Kashyap, M. P. , Jahan, S. , Kumar, V. , Tripathi, V. K. , Siddiqui, M. A. , … Pant, A. B. (2012). Expression and inducibility of cytochrome P450s (CYP1A1, 2B6, 2E1, 3A4) in human cord blood CD34+ stem cell‐derived differentiating neuronal cells. Toxicological Sciences, 129, 392–410. 10.1093/toxsci/kfs213 [DOI] [PubMed] [Google Scholar]
- Squires, E. J. , Sueyoshi, T. , & Negishi, M. (2004). Cytoplasmic localization of pregnane X receptor and ligand‐dependent nuclear translocation in mouse liver. The Journal of Biological Chemistry, 279, 49307–49314. 10.1074/jbc.M407281200 [DOI] [PubMed] [Google Scholar]
- Staudinger, J. L. , Xu, C. , Biswas, A. , & Mani, S. (2011). Post‐translational modification of pregnane x receptor. Pharmacological Research, 64, 4–10. 10.1016/j.phrs.2011.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugatani, J. , Nishitani, S. , Yamakawa, K. , Yoshinari, K. , Sueyoshi, T. , Negishi, M. , & Miwa, M. (2005). Transcriptional regulation of human UGT1A1 gene expression: Activated glucocorticoid receptor enhances constitutive androstane receptor/pregnane X receptor‐mediated UDP‐glucuronosyltransferase 1A1 regulation with glucocorticoid receptor‐interacting protein 1. Molecular Pharmacology, 67, 845–855. 10.1124/mol.104.007161 [DOI] [PubMed] [Google Scholar]
- Teng, S. , & Piquette‐Miller, M. (2005). The involvement of the pregnane X receptor in hepatic gene regulation during inflammation in mice. The Journal of Pharmacology and Experimental Therapeutics, 312, 841–848. 10.1124/jpet.104.076141 [DOI] [PubMed] [Google Scholar]
- Tolson, A. H. , & Wang, H. (2010). Regulation of drug‐metabolizing enzymes by xenobiotic receptors: PXR and CAR. Advanced Drug Delivery Reviews, 62, 1238–1249. 10.1016/j.addr.2010.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi, V. K. , Kumar, V. , Pandey, A. , Vatsa, P. , Dhasmana, A. , Singh, R. P. , … Lohani, M. (2017). Monocrotophos induces the expression of xenobiotic metabolizing cytochrome P450s (CYP2C8 and CYP3A4) and neurotoxicity in human brain cells. Molecular Neurobiology, 54, 3633–3651. 10.1007/s12035-016-9938-7 [DOI] [PubMed] [Google Scholar]
- Tzameli, I. , Pissios, P. , Schuetz, E. G. , & Moore, D. D. (2000). The xenobiotic compound 1,4‐bis[2‐(3,5‐dichloropyridyloxy)]benzene is an agonist ligand for the nuclear receptor CAR. Molecular and Cellular Biology, 20, 2951–2958. 10.1128/mcb.20.9.2951-2958.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda, A. , Hamadeh, H. K. , Webb, H. K. , Yamamoto, Y. , Sueyoshi, T. , Afshari, C. A. , … Negishi, M. (2002). Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Molecular Pharmacology, 61, 1–6. 10.1124/mol.61.1.1 [DOI] [PubMed] [Google Scholar]
- Upadhya, S. C. , Chinta, S. J. , Pai, H. V. , Boyd, M. R. , & Ravindranath, V. (2002). Toxicological consequences of differential regulation of cytochrome p450 isoforms in rat brain regions by phenobarbital. Archives of Biochemistry and Biophysics, 399, 56–65. 10.1006/abbi.2001.2727 [DOI] [PubMed] [Google Scholar]
- Venkatesh, M. , Mukherjee, S. , Wang, H. , Li, H. , Sun, K. , Benechet, A. P. , … Mani, S. (2014). Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll‐like receptor 4. Immunity, 41, 296–310. 10.1016/j.immuni.2014.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Li, H. , Moore, L. B. , Johnson, M. D. , Maglich, J. M. , Goodwin, B. , … Mani, S. (2008). The phytoestrogen coumestrol is a naturally occurring antagonist of the human pregnane X receptor. Molecular Endocrinology, 22, 838–857. 10.1210/me.2007-0218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, K. , Mendy, A. J. , Dai, G. , Luo, H. R. , He, L. , & Wan, Y. J. (2006). Retinoids activate the RXR/SXR‐mediated pathway and induce the endogenous CYP3A4 activity in Huh7 human hepatoma cells. Toxicological Sciences, 92, 51–60. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Sykes, D. B. , & Miller, D. S. (2010). Constitutive androstane receptor‐mediated up‐regulation of ATP‐driven xenobiotic efflux transporters at the blood‐brain barrier. Molecular Pharmacology, 78, 376–383. 10.1124/mol.110.063685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. M. , Ong, S. S. , Chai, S. C. , & Chen, T. (2012). Role of CAR and PXR in xenobiotic sensing and metabolism. Expert Opinion on Drug Metabolism & Toxicology, 8, 803–817. 10.1517/17425255.2012.685237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte‐Allman, S. K. , Hoque, M. T. , Jenabian, M. A. , Routy, J. P. , & Bendayan, R. (2017). Xenobiotic nuclear receptors pregnane X receptor and constitutive androstane receptor regulate antiretroviral drug efflux transporters at the blood‐testis barrier. The Journal of Pharmacology and Experimental Therapeutics, 363, 324–335. 10.1124/jpet.117.243584 [DOI] [PubMed] [Google Scholar]
- Xia, J. , Qin, L. , Du, Z. H. , Lin, J. , Li, X. N. , & Li, J. L. (2017). Performance of a novel atrazine‐induced cerebellar toxicity in quail (Coturnix C. coturnix): Activating PXRCAR pathway responses and disrupting cytochrome P450 homeostasis. Chemosphere, 171, 259–264. 10.1016/j.chemosphere.2016.12.075 [DOI] [PubMed] [Google Scholar]
- Xu, C. , Wang, X. , & Staudinger, J. L. (2009). Regulation of tissue‐specific carboxylesterase expression by pregnane x receptor and constitutive androstane receptor. Drug Metabolism and Disposition, 37, 1539–1547. 10.1124/dmd.109.026989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav, S. , Dhawan, A. , Seth, P. K. , Singh, R. L. , & Parmar, D. (2006). Cytochrome P4503A: Evidence for mRNA expression and catalytic activity in rat brain. Molecular and Cellular Biochemistry, 287, 91–99. 10.1007/s11010-005-9080-8 [DOI] [PubMed] [Google Scholar]
- Yamamoto, Y. , Moore, R. , Flavell, R. A. , Lu, B. , & Negishi, M. (2010). Nuclear receptor CAR represses TNFα‐induced cell death by interacting with the anti‐apoptotic GADD45B. PLoS ONE, 5, e10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki, Y. , Kobayashi, K. , & Chiba, K. (2018). Effect of pregnenolone 16α‐carbonitrile on the expression of P‐glycoprotein in the intestine, brain and liver of mice. Biological & Pharmaceutical Bulletin, 41, 972–977. 10.1248/bpb.b18-00053 [DOI] [PubMed] [Google Scholar]
- Yeung, E. Y. , Sueyoshi, T. , Negishi, M. , & Chang, T. K. (2008). Identification of Ginkgo biloba as a novel activator of pregnane X receptor. Drug Metabolism and Disposition, 36, 2270–2276. 10.1124/dmd.108.023499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinari, K. , Kobayashi, K. , Moore, R. , Kawamoto, T. , & Negishi, M. (2003). Identification of the nuclear receptor CAR:HSP90 complex in mouse liver and recruitment of protein phosphatase 2A in response to phenobarbital. FEBS Letters, 548, 17–20. [DOI] [PubMed] [Google Scholar]
- Yoshinari, K. , Yoda, N. , Toriyabe, T. , & Yamazoe, Y. (2010). Constitutive androstane receptor transcriptionally activates human CYP1A1 and CYP1A2 genes through a common regulatory element in the 5′‐flanking region. Biochemical Pharmacology, 79, 261–269. 10.1016/j.bcp.2009.08.008 [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Huang, W. , Chua, S. S. , Wei, P. , & Moore, D. D. (2002). Modulation of acetaminophen‐induced hepatotoxicity by the xenobiotic receptor NR1I3. Science, 298, 422–424. 10.1126/science.1073502 [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Wei, Y. , Hu, B. , Huang, M. , Xie, W. , & Zhai, Y. (2013). Activation of human stearoyl‐coenzyme A desaturase 1 contributes to the lipogenic effect of PXR in HepG2 cells. PLoS ONE, 8, e67959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. , Burch, P. E. , Cooney, A. J. , Lanz, R. B. , Pereira, F. A. , Wu, J. , … Wheeler, D. A. (2004). Genomic analysis of the nuclear receptor family: New insights into structure, regulation, and evolution from the rat genome. Genome Research, 14, 580–590. 10.1101/gr.2160004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, C. , Tabb, M. M. , Nelson, E. L. , Grun, F. , Verma, S. , Sadatrafiei, A. , … Blumberg, B. (2006). Mutual repression between steroid and xenobiotic receptor and NF‐κB signaling pathways links xenobiotic metabolism and inflammation. The Journal of Clinical Investigation, 116, 2280–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J. , Febbraio, M. , Wada, T. , Zhai, Y. , Kuruba, R. , He, J. , … Xie, W. (2008). Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARγ in promoting steatosis. Gastroenterology, 134, 556–567. 10.1053/j.gastro.2007.11.037 [DOI] [PubMed] [Google Scholar]


