Abstract
Background and Purpose
The transient receptor potential (TRP) ion channel TRPM3 functions as a noxious heat sensor, plays a key role in acute pain sensation and inflammatory hyperalgesia in rodents. Despite its potential as a novel analgesic drug target, little is known about the expression, function and modulation in the humans.
Experimental Approach
We studied TRPM3 in freshly isolated human dorsal root ganglion (hDRG) neurons and human stem cell‐derived sensory (hSCDS) neurons. Expression was analysed at the mRNA level using RT‐qPCR. Channel function was assessed using Fura‐2‐based calcium imaging and whole‐cell patch‐clamp recordings.
Key Results
TRPM3 was detected at the mRNA level in both hDRG and hSCDS neurons. The TRPM3 agonists pregnenolone sulphate (PS) and CIM0216 evoked robust intracellular Ca2+ responses in 52% of hDRG and 58% of hSCDS neurons. Whole‐cell patch‐clamp recordings in hSCDS neurons revealed pregnenolone sulphate (PS)‐ and CIM0216‐evoked currents exhibiting the characteristic current–voltage relation of TRPM3. PS‐induced calcium responses in hSCDS neurons were reversed in a dose‐dependent manner by the flavonoid isosakuranetin and by antiseizure drug primidone. Finally, the μ‐opioid receptor agonist DAMGO and the GABAB receptor agonist baclofen inhibited PS‐evoked TRPM3 responses in a subset of hSCDS neurons.
Conclusion and Implications
These results provide the first direct evidence of functional expression of the pain receptor TRPM3 in human sensory neurons, largely mirroring the channel's properties observed in mouse sensory neurons. hSCDS neurons represent a valuable and readily accessible in vitro model to study TRPM3 regulation and pharmacology in a relevant human cellular context.
Abbreviations
- Caps
Capsaincin
- hDRG
human dorsal root ganglion
- hESC
human embryonic stem cells
- hSCDS
human stem cell‐derived sensory
- ISOSA
isosakuranetin (ISOSA)
- MO
mustard oil
- PS
pregnenolone sulphate
- TRP
transient receptor potential (V, vanilloid; M, melastin; A, ankyrin)
What is already known
The cation channel TRPM3 plays a key role in acute and inflammatory pain in rodents.
What this study adds
TRPM3 is functional in a large subset of human DRG neurons and stem cell‐derived nociceptors.
Pharmacological properties and modulation by GPCR signalling are conserved in human sensory neurons.
What is the clinical significance
These data endorse TRPM3 as a relevant target for analgesic drug development in humans.
Stem cell‐derived nociceptors represent an accessible human cellular model for the development of TRPM3‐based therapies.
1. INTRODUCTION
Pain is a global problem, being the most common symptom why patients seek medical attention (Goldberg & McGee, 2011). Contradictory to this high medical need, the available therapies to treat pain conditions are often inadequate. Indeed, about half of the chronic pain sufferers report insufficient pain relief with the prescribed medication and opioid‐based pain therapies are associated with serious side effects, tolerance and addiction. In order to resolve this unmet need, preclinical pain research is exploring new analgesic strategies of the last few decades (Brennan, 2015) and there is a continuous quest for new pain targets (Yaksh, Woller, Ramachandran, & Sorkin, 2015; Yekkirala, Roberson, Bean, & Woolf, 2017).
Transient receptor potential (TRP) channels are a superfamily of cationic channels with diverse properties and a broad repertoire of physiological functions (Clapham, Runnels, & Strubing, 2001; Vangeel & Voets, 2019). A subset of these TRP channels are expressed in sensory neurons, where they function as molecular chemosensors and thermosensors in somatosensation and pain (Julius, 2013; Vriens, Nilius, & Voets, 2014). As such, they represent promising therapeutic targets to treat pain at the level of the periphery (Patapoutian, Tate, & Woolf, 2009). In particular, extensive preclinical and clinical research has been performed in the last two decades modulating the activity of the sensory neuron‐specific TRP channels https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=507, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=485 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=500 to treat different forms of pain (Moran & Szallasi, 2018). Whereas significant progress has been made in targeting these channels to treat pain in a variety of preclinical animal models, translation of these results to humans remains limited and challenging (Voets, Vriens, & Vennekens, 2019). In particular, the species‐specific expression profile and pharmacological modulation of several TRP channels turned out to be a significant hurdle in drug development (Klionsky et al., 2007; Papakosta et al., 2011; Rostock, Schrenk‐Siemens, Pohle, & Siemens, 2018). Therefore, it is of utmost importance to investigate the expression and pharmacological modulation of targeted TRP channels in the context of human sensory neurons.
Recently, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=495 has been identified as a novel key player in somatosensation and pain (Vandewauw et al., 2018). TRPM3 is a calcium‐permeable cation channel that is activated by heat and by chemical ligands such as the neurosteroid https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4290 (PS; Wagner et al., 2008) and the potent synthetic ligand https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=10297 (Held et al., 2015). In mouse, TRPM3 is expressed in approximately 60% of somatosensory neurons and plays a central role in the detection of noxious heat (Vriens et al., 2011). Strikingly, TRPM3−/− mice do not develop inflammatory hyperalgesia in response to injection of complete Freud's adjuvant (Vriens et al., 2011) and systemic TRPM3 antagonists were shown to alleviate mechanical and thermal hyperalgesia in mouse and rat models of inflammatory and neuropathic pain (Chen, Chen, Qian, Fang, & Zhu, 2014; Jia, Zhang, & Yu, 2017; Krugel, Straub, Beckmann, & Schaefer, 2017). Moreover, TRPM3 activity in sensory neurons is strongly suppressed by μ‐opioid receptor activation in sensory neurons, suggesting that TRPM3 may contribute to peripheral analgesic effects of opioids (Dembla et al., 2017; Quallo, Alkhatib, Gentry, Andersson, & Bevan, 2017; Yudin & Rohacs, 2019).
These data highlight the role of TRPM3 in nociception in acute and persistent pain models, emphasizing its significance as a novel target in the development of new analgesics (Voets et al., 2019). However, at this point, very little is known about TRPM3 expression, function or modulation in human sensory neurons. Here, we describe functional expression of TRPM3 in a large subset of human dorsal root ganglion (hDRG) neurons and show that human stem cell‐derived sensory (hSCDS) neurons can be used as a valuable alternative to donor material to study the pharmacological properties and modulation of TRPM3 in a relevant human cellular context.
2. METHODS
2.1. Human dorsal root ganglion (hDRG) neurons isolation and culture
All hDRGs used for this study were obtained from brain‐dead organ donors in the United States after obtaining informed consent in accordance to state and federal regulations and United Network for Organ Sharing policies. All donor tissue collections for the DRGs used in the present study were performed in accordance to Federal and State regulations using protocols approved by the Organ Procurement Organization. DRGs from the first thoracic vertebra (T1) through the first sacral vertebra (S1) from four donors were used in the present study. The DRGs used for RT‐PCR analysis were from two male donors aged 19 and 47 years, respectively. The DRGs used for calcium imaging were from one 49‐year‐old male and one 33‐year‐old female. These latter DRGs were stripped of connective tissue and enzymatically digested at 37°C for 2 hr using the methods described elsewhere (Davidson et al., 2014). Dissociated cells were seeded on 96‐well plastic bottom plates (Corning) that had been pre‐coated with poly‐d‐lysine. Cells were maintained in culture at 37°C with 5% CO2 in DMEM/F12 supplemented with 10% horse serum (Thermo Fisher Scientific), 2‐mM glutamine, 10 ng·ml−1 hNGF (Cell Signaling Technology), 10 ng·ml−1 GDNF (Peprotech) and penicillin/streptomycin (Thermo Fisher Scientific). Half of the culture medium was replaced with fresh medium every 3 days, and experiments were performed between 3 and 6 days after isolation (Davidson et al., 2014).
2.2. Embryonic stem cell culture and differentiation to sensory neurons
Human embryonic stem cells (hESCs; WA09, WiCell®, Madison, USA) were grown on six‐well plates coated with hESC‐qualified Matrigel® Matrix (corning®, Massachusetts, USA) and incubated at 37°C with 5% CO2. Medium (½ Essential 8™ Flex medium [Gibco Life Technologies, Grand Island, NY, USA] and ½ StemFlex [Gibco]) was changed every 2 days and fortified with RevitCell™ (Gibco). Cells were passaged every 5–7 days. Briefly, cells were washed with PBS and treated with Versene® EDTA 0.02% (Lonza, Verviers, Belgium) for 1 min at room temperature. Thereafter, cells were collected from the bottom of the well using a cell scraper and the mixed cell suspension was plated onto freshly coated plates.
To differentiate hESCs to sensory neurons, we used a previously described protocol (Chambers et al., 2012; Desiderio et al., 2019; Young et al., 2014). Briefly, neuronal differentiation protocol is executed in different phases, starting with the formation of neurectoderm (Chambers et al., 2009). Confluent hESCs were dissociated using 5‐min incubation with Accutase™ (Sigma‐Aldrich, Missouri, USA) and replated at a high density on Matrigel® matrix covered six‐well plates. Two days later, fully confluent cells were ready for neuronal induction (Day 0). Neural induction medium was prepared as a 1:1 mixture of N2 medium (DMEM/F12, 1X N2 supplement [Gibco], 5 μg·ml−1 insulin, 1‐mM l‐glutamine, 1X MEM Non‐essential Amino Acid Solution, 90‐μM β‐mercaptoethanol and 50 U·ml−1 Pen + 50 mg·ml−1 Strep) and B27 medium (neurobasal medium with 1X B27 supplement [Gibco], 1‐mM l‐glutamine and 50 U·ml−1 Pen + 50 mg·ml−1 Strep) and spiked with small molecule inhibitors LDN193189 (1 μM, STEMCELL Technologies, USA) and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=8216 (10 μM, Tocris Bioscience, Bristol, UK). Medium was changed every other day until Day 5. After, neuronal precursors were driven into a sensory neuron phenotype using a tailored differentiation and maturation protocol (Chambers et al., 2012; Young et al., 2014). From Day 5 until Day 10, specification was performed using N2B27 neuronal induction medium supplemented with CHIR99021 (5 μM), DAPT (5 μM; both from STEMCELL Technologies), SU5402 (5 μM; Tocris Bioscience), LDN193189 (1 μM; STEMCELL Technologies) and SB‐431542 (10 μM; Tocris Bioscience) with medium changes every other day. On Day 6 of the protocol, cells were transferred into 24‐well plates using a 5‐min incubation with Accutase™ (Sigma‐Aldrich) at 37°C and replated into Matrigel® matrix‐coated 24‐well plates with or without glass coverslips (see below) for continuation of the protocol. Sensory neuron maturation into mature nociceptors (Day 15–Day 30) consisted of medium changes with DMEM/F12 (10% FBS; Gibco), supplemented with neurotropins https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4872 (10 ng·ml−1, STEMCELL Technologies), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4940 (10 ng·ml−1, STEMCELL Technologies), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5033 (10 ng·ml−1, STEMCELL Technologies), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5026 (10 ng·ml−1, PeproTech, London, UK) and ascorbic acid (200 μM, Sigma‐Aldrich) three times a week. An in‐depth characterization of the derived neurons, including an expression profile of several neuronal and nociceptive markers, was performed in Young et al. (2014).
2.3. RNA extraction and RT‐qPCR
RNA isolation from hDRGs and hSCDS neurons was done using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Quantity and quality of the obtained RNA were checked using DropSense 16 (Trinean, Gent, Belgium). cDNA was synthesized with Ready‐To‐Go YouPrime First‐Strand Beads (GE Healthcare Life Sciences, Buckinghamshire, UK) and used for quantitative RT‐PCR using specific TaqMan™ gene expression assays (see Table 1) together with the accompanying TaqMan universal mastermix (Applied Biosystems®, Foster City, USA). Experiments were carried out using a 7500 Real‐Time PCR system and software (Applied Biosystems, Lennik, Belgium), using a protocol of 40 replication cycles. Sample threshold cycles were analysed relative to housekeeping gene HPRT as endogenous control (ΔCt), and data are shown as 2−ΔCt (mean ± SEM).
Table 1.
TaqMan™ gene expression assays used in this study
| Gene | Assay |
|---|---|
| TRPA1 | Hs00175798_m1 |
| TRPM3 | Hs00257553_m1 |
| TRPM8 | Hs01066596_m1 |
| TRPV1 | Hs00218912_m1 |
| SCN9A (Nav1.7) | Hs01076699_m1 |
| SCN10A (Nav1.8) | Hs01045137_m1 |
| GAPDH | Hs99999905_m1 |
| OPRM1 (μ‐opioid receptor) | Hs01053957_m1 |
| HPRT1 | Hs01003267_m1 |
| Isl‐1 | Hs00158126_m1 |
| NTRK1 (TrkA) | Hs01021011_m1 |
| NTRK2 (TrkB) | Hs00178811_m1 |
| PRDM12 | Hs00964106_m1 |
| NANOG | Hs02387400_g1 |
| CALCA (CGRP) | Hs01100741_m1 |
| POU5F1B | Hs04995079_g1 |
| RUNX1 | Hs01021970_m1 |
| TAC1 | Hs00243225_m1 |
| TRPV2 | Hs00901640_m1 |
| TRPV3 | Hs00376854_m1 |
| TRPV4 | Hs01099348_m1 |
| TRPV5 | Hs00219765_m1 |
| TRPV6 | Hs00367960_m1 |
| TRPM1 | Hs00170127_m1 |
| TRPM2 | Hs01066071_m1 |
| TRPM4 | Hs00214167_m1 |
| TRPM5 | Hs00175822_m1 |
| TRPM6 | Hs00214306_m1 |
| TRPM7 | Hs00292383_m1 |
| TRPC1 | Hs00608195_m1 |
| TRPC2 | Hs03453918_m1 |
| TRPC3 | Hs00162985_m1 |
| TRPC4 | Hs01077392_m1 |
| TRPC5 | Hs00202960_m1 |
| TRPC6 | Hs00175753_m1 |
| TRPC7 | Hs00220638_m1 |
| TRPML1 | Hs01100653_m1 |
| TRPML2 | Hs00401920_m1 |
| TRPML3 | Hs00217663_m1 |
| TRPP1 | Hs00165517_m1 |
| TRPP2 | Hs00175850_m1 |
| TRPP3 | Hs00950467_m1 |
2.4. Functional assays
For intracellular calcium imaging, hDRG neurons were loaded with 3‐μM Fluo‐8‐AM (AAT Bioquest) containing 0.1% Pluronic F‐127 (Sigma‐Aldrich) for 20 min at room temperature. Extracellular solution contained (in mM) 145 NaCl, 3 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose adjusted to pH 7.4 with NaOH. Fluo‐8‐loaded cells were excited at 480 nm, and emission was collected at 520 nm with a pcoEDGE sCMOS camera (PCO) mounted on an inverted microscope (Olympus IX71). Images were acquired at 0.2 Hz for 10 min in constant perfusion of the extracellular solution while adding the agonist and/or antagonist in the perfusion. Image acquisition and data analysis were performed using MetaMorph software (Molecular Devices). At the end of the experiment, a high K+ solution was applied, which contained (in mM) 100 NaCl, 50 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose adjusted to pH 7.4 with NaOH. Cells that did not respond to this depolarizing solution were considered to be either unhealthy or non‐neuronal and omitted from analysis.
Intracellular calcium measurements in hSCDS neurons were performed after incubation with 2‐μM ratiometric calcium indicator Fura‐2 acetoxymethyl (AM) for 30 min. Fluorescence was measured during alternating illumination at 340 and 380 nm using an Eclipse Ti (Nikon) fluorescence microscopy system and absolute calcium concentration was calculated from the ratio of the fluorescence signals at these two wavelengths (R = F 340/F 380) as [Ca2+] = K m × (R − R min) / (R max − R), where K m, R min, and R max were estimated from in vitro calibration experiments with known calcium concentrations (Grynkiewicz, Poenie, & Tsien, 1985). The standard bath solution contained (in mM) 150 NaCl, 6 KCl, 10 HEPES, 2 CaCl2, 1 MgCl2, pH 7.4 with NaOH. https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2420 (MO), pregnenolone sulphate (PS), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2486, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2430, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5338, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1647 ([d‐Ala2,N‐MePhe4,Gly‐ol5]enkephalin) and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1084 were obtained from Sigma‐Aldrich, CIM0216 from Tocris Bioscience, and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=10299 from Carl Roth. At the end of each experiment, cells were subjected to a depolarizing high K+ solution containing (in mM) 100 NaCl, 50 KCl, 10 HEPES, 2 CaCl2, 1 MgCl2, pH 7.4 with NaOH, and non‐responding cells were considered to be either unhealthy or non‐neuronal and hence omitted from analysis.
Whole‐cell patch‐clamp experiments were performed using an EPC‐10 amplifier and associated PatchMasterPro software version V2x73.5 (HEKA Elektronik). In current‐clamp recordings, the pipette solution contained (in mM) 140 aspartic acid, 10 NaCl, 10 EGTA, 1 MgCl2, 10 HEPES, pH 7.2 with KOH. In voltage‐clamp recordings, the pipette solution contained (in mM) 100 aspartic acid, 45 CsCl, 10 EGTA, 1 MgCl2, 10 HEPES, pH 7.2 with CsOH. The extracellular solution contained (in mM) 150 NaCl, 6 KCl, 1 MgCl2, 10 HEPES, 10 glucose, pH 7.4 with NaOH. Note that Ca2+ was omitted from the external solution to attenuate rundown of TRPM3 currents (Vriens, Held, et al., 2014). Pipettes had resistances between 2.5 and 6 MΩ when filled with pipette solutions. Approximately 50–70% of the series resistance was compensated before starting the measurements.
In current‐clamp experiments, steps of current injections of increasing amplitude ranging between −200 and +200 pA were applied, until an action potential was evoked. The threshold voltage was then defined as the voltage at the inflection point, that is, where the second derivative of the voltage trace changes from negative to positive.
The voltage protocol in voltage‐clamp experiments consisted of 200‐ms inverted voltage ramps from +100 to −150 mV, applied at 0.5 Hz from a holding potential of −70 mV. The use of the inverted ramp resulted in an effective suppression of voltage‐gated Na+ currents through voltage‐dependent inactivation, whereas voltage‐gated K+ currents were minimized by the use of a Cs+‐based intracellular solution and voltage‐gated Ca2+ currents by the omission of extracellular Ca2+.
2.5. Immunohistochemical staining
hSCDS neurons on glass coverslip were fixated for 1 min in ethanol/formol (4%) and subsequently washed with TBS (0.01 M) and TBS‐T (0.1% Tween 80). Cells were permeabilized using 0.25% Triton X‐100. After blocking, TRPM3 antibody (Alomone Labs, Cat# ACC‐050, RRID:AB_10918820; 0.2 μg·ml−1) was incubated overnight. Later, coverslips were washed with TBS‐T and blocked in 1% milk before using secondary antibody (PO conjugated AffiniPure goat anti‐rabbit IgG, Jackson ImmunoResearch). Brown reaction product was obtained by ImmPACT DAB (Vector Laboratories), and nuclei were stained with Mayer's haematoxylin. Finally, coverslips were mounted using fluorescence mounting medium (Depex mounting medium, BDH Prolabo). The Immuno‐related procedures used comply with the recommendations made by the British Journal of Pharmacology (Alexander et al., 2018). We note that the specificity of the anti‐TRPM3 antibody for TRPM3 has not been fully established in human cells.
2.6. Data analyses
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). Group data are shown as mean ± SEM. In this study, n refers to the number of analysed hDRG neurons or hSCDS neurons. Statistical analyses were only performed when n was equal or larger than 5. Since most experiments were aimed at describing the functional properties of TRPM3 in human sensory neurons and do not include direct statistical comparisons between experimental groups, no blinding or randomizing was performed. The only exception is Figure 1e, where the difference between vehicle and isosakuranetin treatment was assessed using a two‐way repeated measures ANOVA, folllowed by a Tukey's post hoc test if F in ANOVA achieved P < .05 and there was no significant variance inhomogeneity. Offline analysis of all cellular experiments was performed in an automated manner using home‐written routines in Igor Pro 8 (http://www.wavemetrics.com), thereby limiting operator bias.
Figure 1.
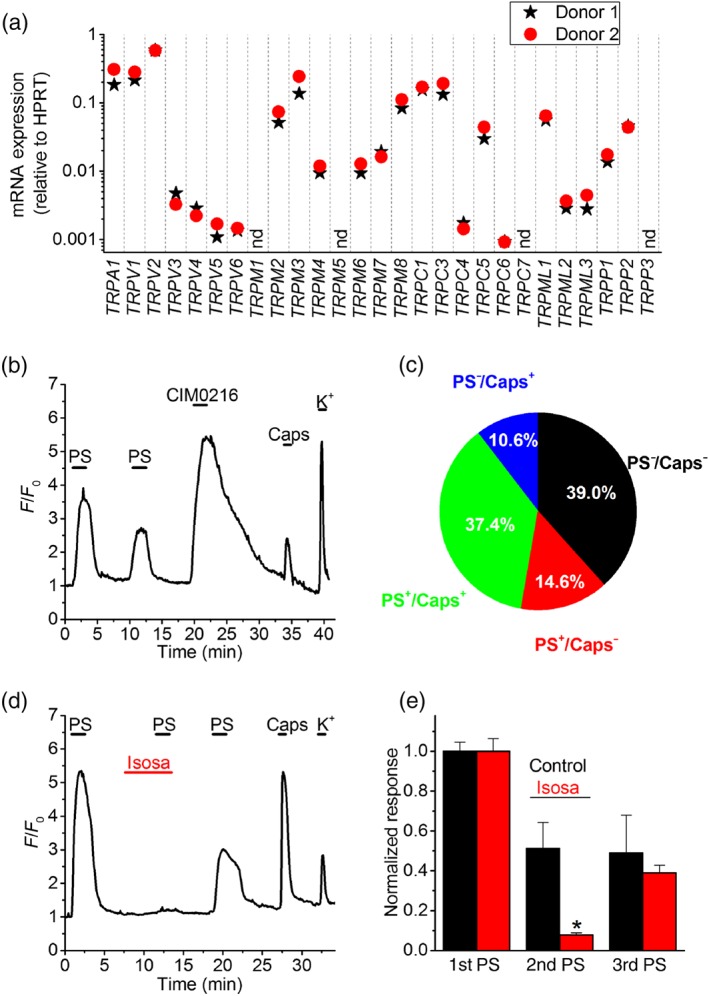
Functional TRPM3 expression in human dorsal root ganglion (DRG) neurons. (a) Expression levels of TRP channel mRNA relative to HPRT in DRG neurons from two donors, determined using RT‐qPCR. Access to fresh human DRG tissue is scarce, hence the limited number of samples in this exploratory experiment. nd, not detected. (b) Representative example of changes in Fluo‐8‐fluorescence in human DRG neurons in response to the TRPM3 agonists pregnenolone sulphate (PS; 50 μM) and CIM0216 (10 μM) and to the TRPV1 agonist capsaicin (Caps; 200 nM). A high K+ solution was applied at the end of the experiment to confirm neuronal excitability. A trace showing the mean ± SEM of the changes in Fluo‐8‐fluorescence for all cells in this particular experiment is provided in Figure S1A. (c) Pie chart showing the distribution of neurons responding to PS, capsaicin or both (n = 206 hDRG neurons). (d) Representative example showing the reversible inhibition of PS‐induced responses in hDRG neurons by the selective TRPM3 antagonist isosakuranetin (Isosa; 20 μM). A trace showing the mean ± SEM of the changes in Fluo‐8‐fluorescence for all cells in this particular experiment is provided in Figure S1B. (e) Normalized responses to repeated PS applications. Neurons were stimulated three times with PS, and the second application occurred in the presence of either isosakuranetin (10 μM; n = 50) or vehicle control (n = 39). *P < .05 (two‐way repeated measures ANOVA with Tukey's post hoc test) [Colour figure can be viewed at http://wileyonlinelibrary.com]
The concentration dependence of ligand‐induced calcium responses from individual cells were fitted using a Hill function of the form:
where Δ[Ca2+]i,normalized represents the ligand‐induced increase in intracellular calcium normalized to the maximal increase in the same cell, EC50 the concentration for half‐maximal activation, [A] the concentration of ligand, and n H the Hill coefficient. The concentration‐dependent inhibition curves were fitted using a Hill function of the form:
where [B] is the concentration of channel blocker and IC50 the concentration for half‐maximal inhibition. Here, the increase in intracellular calcium in the presence of blocker was normalized to that in vehicle‐treated cells. The normalizations reduced variation due to cell–cell differences in intracellular calcium buffering and extrusion, affecting maximal calcium increases. OriginPro 9.0 was used for statistical analyses and data display.
2.7. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org/, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
3. RESULTS
3.1. Functional TRPM3 expression in human dorsal root ganglion cells (hDRGs)
We used RT‐qPCR to evaluate mRNA expression of TRPM3 and all other members of the TRP superfamily in DRG tissue from two human donors. These exploratory experiments indicate expression of TRPM3 at levels comparable to those of other TRP channels known to be involved in somatosensation and pain, including TRPA1, TRPV1 and TRPM8 (Figure 1a). Next, we used Fluo‐8‐based intracellular Ca2+ imaging to probe functional TRPM3 expression in hDRG neurons from two additional donors. We found that a large subset of hDRG neurons (107 out of 206; 52%) displayed a robust Ca2+ response to two TRPM3 agonists, the neurosteroid pregnenolone sulphate (PS; 50 μM) and the synthetic TRPM3 agonist CIM0216 (1 μM; Figure 1b,c; Figure S1A). In these experiments, we also used capsaicin to probe for functional expression of TRPV1, an established marker of nociceptor neurons (Caterina et al., 1997) and found that the majority of PS‐sensitive neurons (77 out of 107; 72%) also responded to capsaicin (Figure 1b,c). Next, we tested the effect of the flavonoid isosakuranetin, a selective TRPM3 antagonist (Straub et al., 2013), on PS responses in hDRG neurons. Neurons were stimulated three times with PS and the second application occurred in the presence of either isosakuranetin (10 μM) or vehicle control (Figure 1c). Responses to the second PS application were almost completely suppressed in the presence of isosakuranetin and partially recovered upon washout (Figure 1d,e; Figure S1B). The range of response amplitudes to the first PS application was similar in hDRGs from the two different donors (Figure S1C). Taken together, these results demonstrate molecular and functional expression of TRPM3 in hDRG neurons.
3.2. Human embryonic stem cells (hESC)‐derived nociceptors represent a somatosensory phenotype
Access to fresh hDRG tissue for functional studies is restricted in most research laboratories, thus limiting their use as a model to study the pharmacology and modulation of TRPM3 in the context of human sensory neuron. As an alternative, we examined TRPM3 expression and function in sensory neurons derived from hESCs. We used a previously described three‐phased protocol, which results in the differentiation towards neurons that exhibit several hallmarks of sensory neurons, in particular small‐diameter nociceptors (Chambers et al., 2012; Young et al., 2014). The resulting cultures of human stem cell‐derived sensory (hSCDS) neurons had the expected cellular morphology, consisting of clusters of small‐diameter cell bodies connecting through neurites (Figure 2a). In whole‐cell current‐clamp experiments, differentiated hSCDS neurons had a resting potential of −52 ± 5 mV and fired action potentials upon current injection with a threshold at −32 ± 8 mV (n = 5; Figure 2b).
Figure 2.
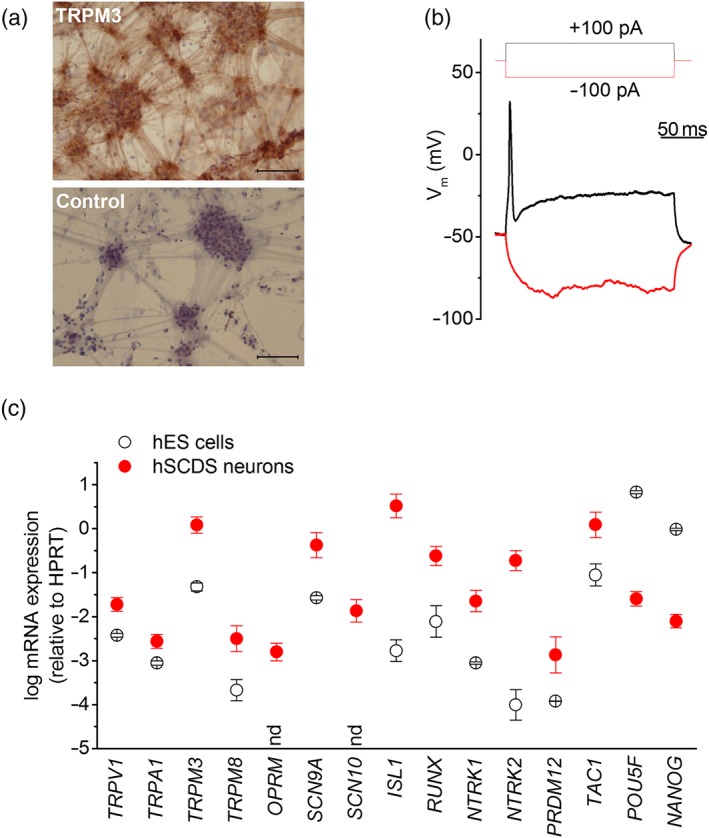
Properties of human stem cell‐derived sensory neurons (hSCDS) neurons. (a) Brightfield image of immunohistochemically stained hSCDS neurons with anti‐TRPM3 antibody and negative control (only secondary antibody) showing a neuronal morphology with cell body clusters interconnected by neurites. Brown peroxidase‐based TRPM3 staining is visible in cell bodies as well as neurites. Scale bar: 500 μm. (b) Whole‐cell current‐clamp recordings on hSCDS neurons demonstrate the ability to fire action potentials upon current injection. (c) RT‐qPCR comparing relative expression of sensory TRP channels, nociceptor marker genes, and pluripotency genes between human embryonic stem (hES) cells (black) and hSCDS neurons (red). Results represent mean ± SEM from five independent experiments with three technical replicates each. nd, not detected in hES cells human embryonic stem cells [Colour figure can be viewed at http://wileyonlinelibrary.com]
We used RT‐qPCR to compare mRNA expression of relevant genes between hESCs and differentiated hSCDS neurons (Figure 2c), based on markers used elsewhere (Chambers et al., 2012; Young et al., 2014). Differentiation resulted in a down‐regulation of pluripotency genes POU5F1 and NANOG, whereas several sensory neuronal markers were significantly up‐regulated. These included the genes encoding the transcription factors RUNX1 and ISL‐1, the neurotrophin receptors https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1817 (NTRK1) and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1818 (NTRK2), the voltage‐gated Na+ channels NaV https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=584 (SNC9A) and NaV https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=585&familyId=82&familyType=IC (SNC10A), the https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=319 (OPRM1) and the somatosensory TRP channels TRPA1, TRPM8, TRPV1 and TRPM3 (Figure 2c). Using an anti‐TRPM3 antibody (Papanikolaou, Lewis, & Butt, 2017), we detected extensive immunostaining of cell bodies and neurites of hSCDS neurons (Figure 2a).
Using Fura‐2‐based intracellular Ca2+ imaging, we tested the functional expression of somatosensory TRP channels by examining responses to the chemical ligands menthol (a TRPM8 agonist and weak TRPA1 agonist), mustard oil (MO; a potent TRPA1 agonist), capsaicin (a potent TRPV1 agonist) and PS (Figure 3a). We observed responses to all four agonists in different, partly overlapping subsets of hSCDS neurons (Figure 3a,b). Most prominently, 58% of all hSCDS neurons responded to PS, indicating widespread TRPM3 expression (Figure 3b). Overall, the neuronal responsiveness to TRP channel agonists corresponded well with the relative TRP channel expression at the mRNA levels determined by RT‐qPCR (TRPM3 > TRPV1 > TRPM8 > TRPA1). PS‐induced calcium responses were fully and reversible inhibited by isosakuranetin and all PS‐responsive neurons also responded to CIM0216 (Figure 3c,d). Taken together, these results indicate that a large fraction of hSCDS neurons express functional TRPM3.
Figure 3.
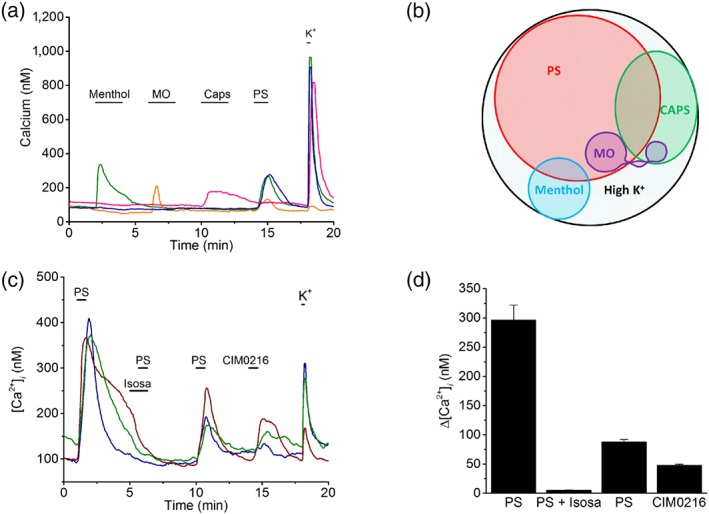
Functional expression profile of somatosensory TRP channels in human stem cell‐derived sensory neurons (hSCDS) neurons. (a) Changes in intracellular calcium in single hSCDS neurons stimulated with the TRP channel agonists menthol (100 μM), MO (100 μM), capsaicin (Caps;1 μM), and pregnenolone sulphate (PS;40 μM) and with a high K+ solution to probe excitability. (b) Venn diagram showing the pattern of responses to TRP channel agonists in hSCDS neurons (n = 1,180 cells in five independent differentiations). (c) Intracellular calcium measurements showing reversible inhibition of PS‐evoked responses by isosakuranetin (5 μM). PS‐responsive hSCDS neurons also responded to the synthetic TRPM3 agonist CIM0216 (1 μM). (d) Quantification of calcium responses for experiments as in panel (c) (n = 72). Isosa, isosakuranetin; MO, mustard oil [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.3. Electrophysiological properties of TRPM3 in human stem cell‐derived sensory (hSCDS) neurons
Next, we performed whole‐cell patch‐clamp recordings to characterize the electrophysiological properties of TRPM3‐mediated currents in hSCDS neurons. In these experiments, extracellular Ca2+ was omitted to reduce channel desensitization (Vriens, Held, et al., 2014). The agonists PS and CIM0216 reversibly activated whole‐cell currents in hSCDS neurons (Figure 4a,b). PS‐activated currents were relatively small and outwardly rectifying and therefore primarily detectable at positive potentials (Figure 4a–d). Currents activated by CIM0216 were much more robust and showed characteristic double rectification (Figure 4a–d). Moreover, these currents were reversibly inhibited by isosakuranetin (5 μM; Figure 4e,f). The shape of the current–voltage relations (outwardly rectifying for PS vs. doubly rectifying in the presence of CIM0216; Figure 4c,d,f) is in accordance with the properties of heterologously expressed mouse TRPM3 and of endogenous TRPM3‐mediated currents in mouse sensory neurons and beta cells (Held et al., 2015; Vriens, Held, et al., 2014; Wagner et al., 2008).
Figure 4.
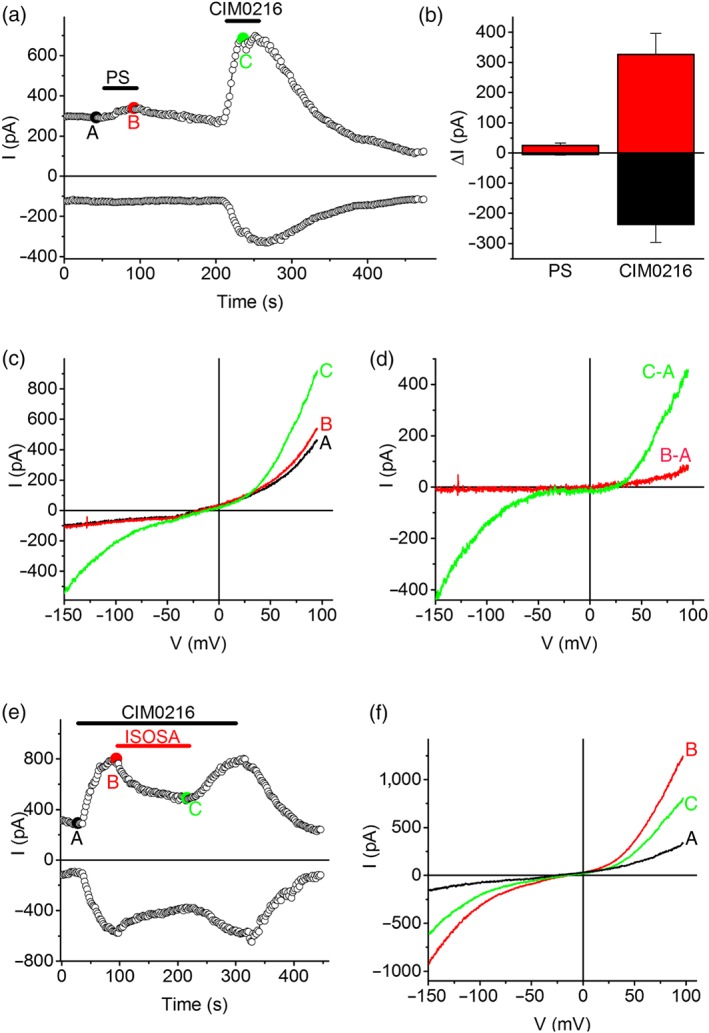
Electrophysiological properties of TRPM3 in human stem cell‐derived sensory neurons (hSCDS) neurons. (a) Time course of inward (at −120 mV) and outward (at +80 mV) whole‐cell currents in hSCDS neurons showing the effects of the TRPM3 agonists pregnenolone sulphate (PS; 40 μM) and CIM0216 (1 μM). (b) Quantification of the amplitude of inward and outward currents activated by PS and CIM0216 (n = 6). (c) IV relationships corresponding to the indicated time points in panel (a). (d) Difference currents illustrate the typical outward rectification of PS‐activated currents and double rectification of CIM0216‐activated currents. (e,f) CIM0216‐activated whole‐cell currents were partially and reversibly inhibited by isosakuranetin (ISOSA; 5 μM) [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.4. Pharmacological modulation of TRPM3 in human stem cell‐derived sensory (hSCDS) neurons
The high levels of functional TRPM3 expression in the hSCDS neurons render them an excellent in vitro model to study human TRPM3 in a relevant cellular environment and to compare its pharmacological properties with those of heterologously expressed and/or rodent TRPM3. To evaluate the concentration dependence of the agonistic effect of PS, we used Fura‐2‐based intracellular Ca2+ imaging and measured responses to PS concentrations increasing from 1 to 300 μM. This assay yielded a concentration‐dependent increase in intracellular Ca2+ with an EC50 of 48 ± 3 μM and a Hill coefficient (n H) of 2.1 ± 0.1 (Figure 5a,b). In comparison, PS activates heterologously expressed mouse TRPM3 tested using calcium imaging or voltage clamp with EC50 values of 1 and 23 μM, respectively (Majeed et al., 2010; Wagner et al., 2008).
Figure 5.
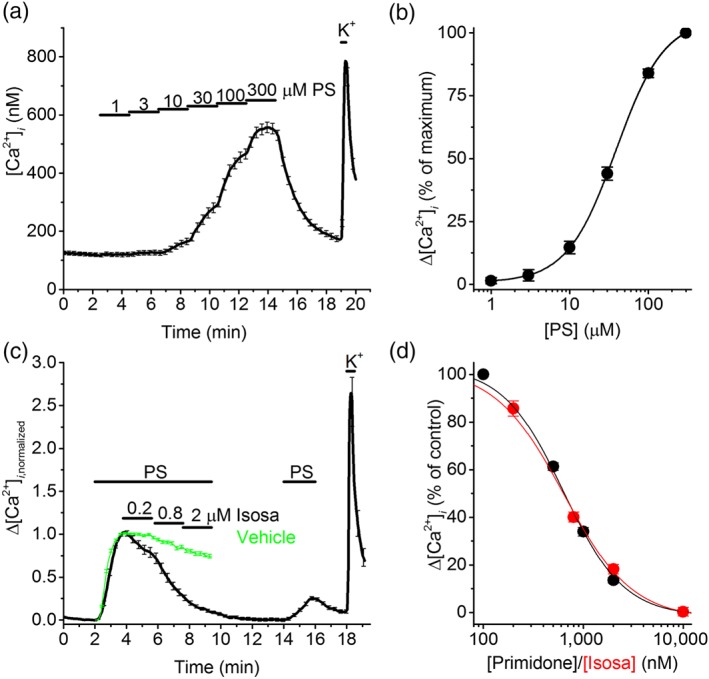
Pharmacological characterization of TRPM3 in human stem cell‐derived sensory neurons (hSCDS) neurons. (a) Time course of the intracellular Ca2+ concentration in PS responding hSCDS neurons upon increasing the concentration of pregnenolone sulphate (PS) from 1 to 300 μM. (b) Concentration dependence of the PS‐induced calcium response in hSCDS neurons. Responses were normalized to the maximal response (n = 117). Solid line represents a fit using a Hill function. (c) Normalized calcium traces showing the effect of increasing concentrations of the TRPM3 antagonist isosakuranetin (Isoas) on the response to PS (40 μM). Green trace represents the vehicle control, showing mild rundown of the signal in the absence of antagonist. (d) Concentration–response curve for the inhibition of PS‐evoked calcium responses by isosakuranetin (n = 93; red squares) and primidone (n = 61; black dots). The mean normalized calcium response of vehicle‐treated hSCDS neurons (green trace in panel (c)) was used as control. Solid line represents a fit using a Hill function [Colour figure can be viewed at http://wileyonlinelibrary.com]
To determine the concentration dependence of the TRPM3 antagonists isosakuranetin and primidone (Krugel et al., 2017; Straub et al., 2013), we stimulated hSCDS neurons with PS and then applied increasing concentrations of the inhibitory compound (Figure 5c,d). The percentage of antagonist‐induced inhibition was calculated after correction for rundown of the response to a sustained PS application in the absence of antagonists (Figure 5c). This approach yielded IC50 values of 659 ± 18 nM for isosakuranetin (n H = 1.9 ± 0.1) and 705 ± 28 nM for primidone (n H = 1.7 ± 0.1; Figure 5d).
In rodents, it has been previously shown that TRPM3 activity is suppressed following activation of a variety of GPCRs, including the μ‐opioid receptor, via a mechanism involving the Gβγ subunit of trimeric G proteins (Badheka et al., 2017; Dembla et al., 2017; Quallo et al., 2017; Yudin & Rohacs, 2019). Considering the role of TRPM3 in nociception, it has been proposed that this pathway could contribute to the analgesic effects of peripheral opioids. To investigate whether μ‐opioid‐dependent regulation is conserved in hSCDS neurons, we performed Fura‐2‐based intracellular Ca2+ imaging and tested the effect of DAMGO, a selective μ‐opioid agonist (Seki et al., 1998), on the response to PS (Figure 6a). DAMGO caused a rapid and reversible inhibition of the PS response in hSCDS neurons, with a mean inhibition of 24%. Notably, the extent of the DAMGO‐induced inhibition varied substantially between neurons (Figure 6a,b). Indeed, in some neurons, the PS response was suppressed by more than 80% by DAMGO, whereas in other neurons, the inhibition was more moderate or absent (Figure 6a,b). Overall, DAMGO inhibited the PS response by at least 20% in 50% (115 out of 230) of hSCDS neurons. In contrast, subsequent addition of isosakuranetin inhibited the PS response in all tested neurons (Figure 6a). In a similar approach, we also tested the effect of baclofen, an agonist of the https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=26 B receptor (Badheka et al., 2017; Quallo et al., 2017). Like DAMGO, baclofen evoked a rapid and reversible inhibition of the PS response (Figure 6c). Notably, the inhibitory effect of baclofen was more pronounced than that of DAMGO neurons, with a mean inhibition of 61% (Figure 6d). Overall, baclofen inhibited the PS response by at least 20% in 98% of the hSCDS neurons (Figure 6d).
Figure 6.
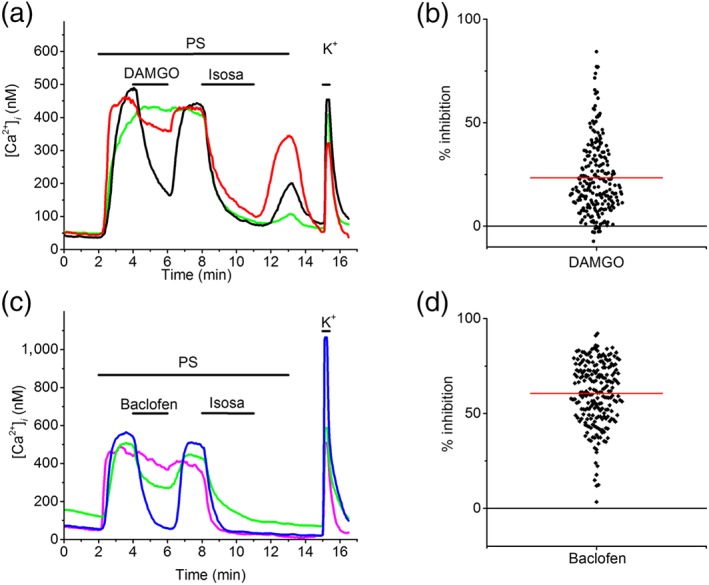
Modulation of TRPM3 in human stem cell‐derived sensory neurons (hSCDS) neurons by GPCRs. (a) Example intracellular calcium measurements showing variable degrees of inhibition of PS‐induced responses upon activation of endogenously expressed μ‐opioid receptors using DAMGO (300 nM). (b) Scatter plot showing the range of DAMGO‐induced inhibition of PS responses in hSCDS neurons. Red line represents the mean percentage of TRPM3 inhibition caused by DAMGO (data from six experiments, with hSCDS neurons from three different differentiations). (c) Example intracellular calcium traces showing inhibition of PS‐induced responses by the GABAB receptor agonist baclofen (25 μM). (d) Scatter plot showing the range of baclofen‐induced inhibition of PS. Red line represents the mean percentage of inhibition caused by baclofen (data from six experiments, with hSCDS neurons from two different differentiations) [Colour figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
In recent years, research in rodents has revealed that the cation channel TRPM3 plays a central role in the somatosensory pathway, where it is involved in acute noxious heat sensing and in the development of inflammatory hyperalgesia (Behrendt, 2019; Vriens et al., 2011). Moreover, pharmacological inhibition of TRPM3 alleviates pain in a variety of preclinical models in mice and rats (Jia et al., 2017; Krugel et al., 2017; Straub et al., 2013), highlighting the potential of TRPM3 as a target for novel analgesic drugs (Held, Voets, & Vriens, 2017). However, whether TRPM3 is implicated in somatosensation and pain in humans remains unknown. In this study, we have evaluated for the first time the functional expression and pharmacological properties of TRPM3 in the context of human sensory neurons. In the first part, we demonstrated that TRPM3 is functionally expressed in a large subset of hDRG neurons. The majority of these TRPM3‐expressing hDRG neurons also responded to capsaicin, indicating that they represent TRPV1‐expressing nociceptors. In the second part, to overcome the limited availability of sensory neurons from human donors (Meyer & Kaspar, 2014), we examined TRPM3 in nociceptor‐like neurons derived from human embryonic stem cells (hESCs). We found that human stem cell‐derived sensory (hSCDS) neurons obtained following a three‐phased small molecule inhibitor protocol (Young et al., 2014) can be used to study the properties and pharmacological modulation of human TRPM3. Our results demonstrate that the majority of hSCDS neurons express functional TRPM3, which can be readily assayed using whole‐cell patch‐clamp recordings and intracellular calcium measurements. Therefore, hSCDS neurons represent as an accessible and unlimited model to study the pharmacological properties and modulation of TRPM3 in the context of human sensory neurons.
It is now well established that at least four TRP channels (TRPV1, TRPM8, TRPA1 and TRPM3) function as thermoreceptors and chemoreceptors in the somatosensory system of rodents (Bautista et al., 2006, 2007; Caterina et al., 2000; Davis et al., 2000; Julius, 2013; Karashima et al., 2009; Kwan et al., 2006; Vandewauw et al., 2018; Vriens, Nilius, et al., 2014; Vriens et al., 2011). We found that these four channels are also expressed at similar levels in bulk mRNA isolated from DRG neurons from human donors. Importantly, we here provide the first direct evidence for functional TRPM3 expression in human somatosensory neurons, by showing responses to the agonists PS and CIM0216 in 52% of hDRG neurons, which were reversibly inhibited by the flavonoid TRPM3 antagonist isosakuranetin. In line with earlier findings in mice (Vandewauw et al., 2018; Vriens et al., 2011), our results also indicate significant co‐expression of TRPM3 with TRPV1, as 75% of PS‐responsive neurons also responded to capsaicin. These results indicate that TRPM3 is abundantly expressed in human nociceptors, supporting a role for the channel in human somatosensation and pain.
We also investigated the molecular and functional expression of TRPM3 in hSCDS neurons. These in vitro derived neurons exhibit the defining properties of primary nociceptive neurons, including the mRNA expression of the nociceptor‐specific neurotrophin receptor TrkA, the voltage‐gated sodium channels NaV1.7 and NaV1.8, and the capsaicin receptor TRPV1 (Young et al., 2014). Moreover, in line with earlier work using hSCDS neurons (Blanchard et al., 2015; Wainger et al., 2015; Young et al., 2014), agonists of TRPV1, TRPA1 and TRPM8 evoked calcium responses in different subsets of neurons. It should be noted, however, that the percentage of TRPV1‐ and TRPA1‐positive neurons (21% and 4%, respectively) is substantially lower than what has been described in mouse and hDRGs, where typically 30–50% of the neurons respond to capsaicin or MO (Figure S2). In contrast, we found that 58% of hSCDS neurons exhibited functional expression of TRPM3, which is comparable to the fraction of TRPM3‐positive DRG neurons found in human or mouse DRG (Vriens et al., 2011). Overall, these results indicated that hSCDS neurons functionally express four sensory TRP channels implicated in thermosensation and pain but also indicated quantitative differences in the expression patterns of these channels compared to other in vitro models.
Given the abundant functional expression of TRPM3, we used hSCDS neurons to further study the properties of human TRPM3 in the context of nociceptor‐like neurons. In whole‐cell patch‐clamp recordings, PS activated small, outwardly rectifying currents, in line with earlier findings in heterologous expression systems showing that TRPM3 is a voltage‐dependent channel whose open probability increases upon depolarization (Oberwinkler, Lis, Giehl, Flockerzi, & Philipp, 2005; Vriens, Held, et al., 2014; Vriens et al., 2011). Notably, TRPM3 currents were strongly potentiated in the presence of CIM0216 and exhibited a characteristic double rectification pattern. Earlier work using heterologously expressed mouse TRPM3 has shown that the CIM0216‐induced inwardly rectifying current component is mediated by an alternative permeation pathway in TRPM3, distinct from the central pore and located in the voltage sensing domain (Held et al., 2015; Vriens, Held, et al., 2014). Taken together, endogenously expressed TRPM3 in hSCDS neurons largely recapitulates the electrophysiological characteristics of heterologously expressed mouse TRPM3 (Vriens, Held, et al., 2014).
Currently, there are only few pharmacological tools that can be used to inhibit TRPM3 function in vitro and in vivo. The most widely used experimental TRPM3 antagonists are on the one hand isosakuranetin and related plant‐derived flavonoids (Straub et al., 2013) and on the other hand the anticonvulsant primidone (Krugel et al., 2017). These compounds inhibit rodent TRPM3 at submicromolar concentrations and suppress TRPM3‐mediated pain behaviour in mice (Krugel et al., 2017; Straub et al., 2013). Using Fura‐2‐based calcium imaging, we measured the concentration dependence of the inhibition of TRPM3 by isosakuranetin and primidone in hSCDS neurons. Both compounds were able to reverse PS‐induced calcium responses in a concentration‐dependent manner and the IC50 value for primidone (705 nM) closely matches the published IC50 value obtained for mouse TRPM3 expressed in HEK293 cells (Krugel et al., 2017). For isosakuranetin, we obtained an approximately 10‐fold higher IC50 value in comparison to the value obtained for heterologously expressed mouse TRPM3 (Straub et al., 2013). This difference in IC50 value may reflect variation in isosakuranetin affinity between species or may be related to the expression of different TRPM3 isoforms. Alternatively, the differences may reflect the distinct experimental approaches used to determine the concentration dependence. Indeed, Straub et al. (2013) pretreated their cells with isosakuranetin for 2.5 min before applying PS, whereas isosakuranetin was applied after full development of the PS response in our experiments.
Interestingly, it was recently shown that activation of the μ‐opioid receptor inhibits TRPM3‐mediated responses in mouse sensory neurons, via a mechanism that involved direct interaction of Gβγ with the channel. Accordingly, peripherally applied opioids such as morphine or DAMGO were shown to inhibit TRPM3‐dependent pain responses in mice (Dembla et al., 2017; Quallo et al., 2017; Yudin & Rohacs, 2019) and it has been suggested that this mechanism may contribute to the peripheral analgesic effect of opioids (Dembla et al., 2017; Quallo et al., 2017). We observed that PS responses were rapidly and reversibly inhibited by DAMGO in about 50% of the hSCDS neurons. Such a variable inhibition of TRPM3 by μ‐opioid receptor activation was also reported in isolated mouse sensory neurons, where PS responses were insensitive to DAMGO or morphine in between 5% and 30% of the neurons (Dembla et al., 2017; Quallo et al., 2017; Yudin & Rohacs, 2019). The variable inhibition of the TRPM3 responses may reflect the unequal expression of the μ‐opioid receptor or its downstream signalling pathway in sensory neurons. In this respect, we observed a more profound less variable inhibition of TRPM3‐dependent responses in hSCDS neurons upon activation of the GABAB receptor using baclofen (Badheka et al., 2017; Quallo et al., 2017). Overall, our data indicate that modulation of TRPM3 via the GPCR–Gβγ pathway is conserved in human sensory neurons.
There is a high unmet need for effective and safe analgesic treatments, fuelling continuous research into novel molecular players in the pain pathway (Brennan, 2015; Yaksh et al., 2015; Yekkirala et al., 2017). In this respect, sensory TRP channels have been intensively pursued as pain targets ever since the identification of the capsaicin receptor TRPV1 more than two decades ago (Voets et al., 2019; Yekkirala et al., 2017). However, despite promising preclinical results with antagonists of TRPV1, TRPA1 and TRPM8 in various animal models of acute and chronic pain, effective treatments in humans are still outstanding (Voets et al., 2019). In particular, translating findings in rodent models to humans has been challenging, in part due to species‐specific differences in TRP channel expression, function and pharmacology (Klionsky et al., 2007; Papakosta et al., 2011; Rostock et al., 2018). Analysing channel regulation and pharmacology in a relevant human cellular context therefore represents an important step in the development of novel TRP channel‐based pharmacotherapies. Our present results provide the first characterization of TRPM3, a more recently identified pain target, in hDRG neurons and reveal hSCDS neurons as an unlimited resource to study its pharmacological modulation. Thus, hSCDS neurons represent an instrumental human nociceptor‐like cellular model that can be used in the development of TRPM3‐specific antagonists for the treatment of various forms of pain. Recently, a de novo mutation in TRPM3's S4–S5 linker region was identified in seven probands with developmental and epileptic encephalopathy, which in some patients was associated with markedly altered pain and heat thresholds (Dyment et al., 2019). As such, hSCDS neurons represent an attractive cellular model to investigate the consequences of such mutations on TRPM3 function.
To conclude, we have demonstrated functional expression of TRPM3 in a large subset of hDRG neurons and in human nociceptor‐like neurons derived from embryonic stem cells. In this cellular context, human TRPM3 exhibits similar electrophysiological and pharmacological properties as have been described earlier in rodents, including reversible inhibition upon activation of μ‐opioid and GABAB receptors. These results support the hypothesis that TRPM3 is an important molecular player in the human pain pathway. Our findings demonstrate that human stem cell‐derived sensory (hSCDS) neurons represent an accessible human cellular model to study TRPM3 pharmacology and regulation, which may be highly instrumental for the development of TRPM3‐targeting therapies to treat various forms of pain.
AUTHOR CONTRIBUTIONS
L.V., M.B., Y.M., and K.D.C. conducted the experiments. L.V., P.E.M., P.C., C.V., J.V., and T.V. participated in the research design, data analysis, and interpretation. T.V. supervised the project. L.V. and T.V. wrote the manuscript. All authors reviewed and revised the final version of this manuscript and approved its submission.
CONFLICT OF INTEREST
J.V. and T.V. are co‐inventors on patents entitled “Treatment of pain” derived from WO2012149614, and their labs have received research funding for pain‐related research from industrial parties.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14208, and as recommended by funding agencies, publishers, and other organizations engaged with supporting research.
Supporting information
Figure S1: Human data (A,B) Mean ± SEM of all traces in the experiment shown in Figure 1B (n = 23) and Figure 1D (n = 27), respectively. (C) Amplitude of the first pregnenolone sulphate (PS) response of all neurons, separated by donor. Solid line indicates the mean.
Figure S2: Distribution of TRPV1 and TRPM3 responders in different DRG models. (A‐C) Piecharts showing the percentage of TRPV1 and/or TRPM3 responders in (A) mouse DRG (Vandewauw et al., 2018), (B) human DRG and (C) hSCDS neurons.
ACKNOWLEDGEMENTS
We thank all members of the Laboratory of Ion Channel Research for comments and discussion. This research was supported by grants from the VIB, KU Leuven Research Council (C1‐TRPLe to T.V.), the Research Foundation‐Flanders (FWO G.084515N to J.V. and T.V.), the Queen Elisabeth Medical Foundation for Neurosciences (to T.V.), the Belgian Foundation Against Cancer (to J.V. and T.V.), and the Planckaert‐De Waele fund (to J.V.).
Vangeel L, Benoit M, Miron Y, et al. Functional expression and pharmacological modulation of TRPM3 in human sensory neurons. Br J Pharmacol. 2020;177:2683–2695. 10.1111/bph.14994
REFERENCES
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. M. , Striessnig, J. , Kelly, E. , … CGTP collaboratories (2019). The coincise guide to pharmacology 2019/20: Ion channels. British Journal of Pharmacology, 176, S142–S228. 10.1111/bph.14749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , … Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology. British Journal of Pharmacology, 175(3), 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badheka, D. , Yudin, Y. , Borbiro, I. , Hartle, C. M. , Yazici, A. , Mirshahi, T. , & Rohacs, T. (2017). Inhibition of transient receptor potential melastatin 3 ion channels by G‐protein βγ subunits. eLife, 6 10.7554/eLife.26147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista, D. M. , Jordt, S. E. , Nikai, T. , Tsuruda, P. R. , Read, A. J. , Poblete, J. , … Julius, D. (2006). TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell, 124(6), 1269–1282. 10.1016/j.cell.2006.02.023 [DOI] [PubMed] [Google Scholar]
- Bautista, D. M. , Siemens, J. , Glazer, J. M. , Tsuruda, P. R. , Basbaum, A. I. , Stucky, C. L. , … Julius, D. (2007). The menthol receptor TRPM8 is the principal detector of environmental cold. Nature, 448(7150), 204–208. 10.1038/nature05910 [DOI] [PubMed] [Google Scholar]
- Behrendt, M. (2019). Transient receptor potential channels in the context of nociception and pain—Recent insights into TRPM3 properties and function. Biological Chemistry, 400(7), 917–926. 10.1515/hsz-2018-0455 [DOI] [PubMed] [Google Scholar]
- Blanchard, J. W. , Eade, K. T. , Szucs, A. , Lo Sardo, V. , Tsunemoto, R. K. , Williams, D. , … Baldwin, K. K. (2015). Selective conversion of fibroblasts into peripheral sensory neurons. Nature Neuroscience, 18(1), 25–35. 10.1038/nn.3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan, F. (2015). The US congressional “decade on pain control and research” 2001–2011: A review. Journal of Pain & Palliative Care Pharmacotherapy, 29(3), 212–227. 10.3109/15360288.2015.1047553 [DOI] [PubMed] [Google Scholar]
- Caterina, M. J. , Leffler, A. , Malmberg, A. B. , Martin, W. J. , Trafton, J. , Petersen‐Zeitz, K. R. , … Julius, D. (2000). Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science, 288(5464), 306–313. 10.1126/science.288.5464.306 [DOI] [PubMed] [Google Scholar]
- Caterina, M. J. , Schumacher, M. A. , Tominaga, M. , Rosen, T. A. , Levine, J. D. , & Julius, D. (1997). The capsaicin receptor: A heat‐activated ion channel in the pain pathway. Nature, 389(6653), 816–824. 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- Chambers, S. M. , Fasano, C. A. , Papapetrou, E. P. , Tomishima, M. , Sadelain, M. , & Studer, L. (2009). Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nature Biotechnology, 27(3), 275–280. 10.1038/nbt.1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers, S. M. , Qi, Y. , Mica, Y. , Lee, G. , Zhang, X. J. , Niu, L. , … Studer, L. (2012). Combined small‐molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nature Biotechnology, 30(7), 715–720. 10.1038/nbt.2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. , Chen, W. , Qian, X. , Fang, Y. , & Zhu, N. (2014). Liquiritigenin alleviates mechanical and cold hyperalgesia in a rat neuropathic pain model. Scientific Reports, 4, 5676–5674. 10.1038/srep05676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham, D. E. , Runnels, L. W. , & Strubing, C. (2001). The TRP ion channel family. Nature Reviews. Neuroscience, 2(6), 387–396. 10.1038/35077544 [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175(7), 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, S. , Copits, B. A. , Zhang, J. , Page, G. , Ghetti, A. , & Gereau, R. W. (2014). Human sensory neurons: Membrane properties and sensitization by inflammatory mediators. Pain, 155(9), 1861–1870. 10.1016/j.pain.2014.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, J. B. , Gray, J. , Gunthorpe, M. J. , Hatcher, J. P. , Davey, P. T. , Overend, P. , … Sheardown, S. A. (2000). Vanilloid receptor‐1 is essential for inflammatory thermal hyperalgesia. Nature, 405(6783), 183–187. 10.1038/35012076 [DOI] [PubMed] [Google Scholar]
- Dembla, S. , Behrendt, M. , Mohr, F. , Goecke, C. , Sondermann, J. , Schneider, F. M. , … Oberwinkler, J. (2017). Anti‐nociceptive action of peripheral mu‐opioid receptors by G‐beta‐gamma protein‐mediated inhibition of TRPM3 channels. eLife, 6 10.7554/eLife.26280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desiderio, S. , Vermeiren, S. , Van Campenhout, C. , Kricha, S. , Malki, E. , Richts, S. , … Bellefroid, E. J. (2019). Prdm12 directs nociceptive sensory neuron development by regulating the expression of the NGF receptor TrkA. Cell Reports, 26(13), 3522–3536e3525. 10.1016/j.celrep.2019.02.097 [DOI] [PubMed] [Google Scholar]
- Dyment, D. A. , Terhal, P. A. , Rustad, C. F. , Tveten, K. , Griffith, C. , Jayakar, P. , … Lines, M. A. (2019). De novo substitutions of TRPM3 cause intellectual disability and epilepsy. European Journal of Human Genetics, 27, 1611–1618. 10.1038/s41431-019-0462-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg, D. S. , & McGee, S. J. (2011). Pain as a global public health priority. BMC Public Health, 11, 770 10.1186/1471-2458-11-770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz, G. , Poenie, M. , & Tsien, R. Y. (1985). A new generation of Ca2+ indicators with greatly improved fluorescence properties. The Journal of Biological Chemistry, 260(6), 3440–3450. [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … Nc, I (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46(D1), D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held, K. , Kichko, T. , De Clercq, K. , Klaassen, H. , Van Bree, R. , Vanherck, J. C. , … Vriens, J. (2015). Activation of TRPM3 by a potent synthetic ligand reveals a role in peptide release. Proceedings of the National Academy of Sciences of the United States of America, 112(11), E1363–E1372. 10.1073/pnas.1419845112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held, K. , Voets, T. , & Vriens, J. (2017). The nociceptive TRPM3 channel as potential therapeutic target for chronic pain. Internal Medicine Review, 3(8), 1–16. 10.18103/imr.v3i8.535 [DOI] [Google Scholar]
- Jia, S. , Zhang, Y. , & Yu, J. (2017). Antinociceptive effects of isosakuranetin in a rat model of peripheral neuropathy. Pharmacology, 100(3–4), 201–207. 10.1159/000478986 [DOI] [PubMed] [Google Scholar]
- Julius, D. (2013). TRP channels and pain. Annual Review of Cell and Developmental Biology, 29, 355–384. 10.1146/annurev-cellbio-101011-155833 [DOI] [PubMed] [Google Scholar]
- Karashima, Y. , Talavera, K. , Everaerts, W. , Janssens, A. , Kwan, K. Y. , Vennekens, R. , … Voets, T. (2009). TRPA1 acts as a cold sensor in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America, 106(4), 1273–1278. 10.1073/pnas.0808487106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky, L. , Tamir, R. , Gao, B. , Wang, W. , Immke, D. C. , Nishimura, N. , & Gavva, N. R. (2007). Species‐specific pharmacology of trichloro(sulfanyl)ethyl benzamides as transient receptor potential ankyrin 1 (TRPA1) antagonists. Molecular Pain, 3, 39 10.1186/1744-8069-3-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugel, U. , Straub, I. , Beckmann, H. , & Schaefer, M. (2017). Primidone inhibits TRPM3 and attenuates thermal nociception in vivo. Pain, 158(5), 856–867. 10.1097/j.pain.0000000000000846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan, K. Y. , Allchorne, A. J. , Vollrath, M. A. , Christensen, A. P. , Zhang, D. S. , Woolf, C. J. , & Corey, D. P. (2006). TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair‐cell transduction. Neuron, 50(2), 277–289. 10.1016/j.neuron.2006.03.042 [DOI] [PubMed] [Google Scholar]
- Majeed, Y. , Agarwal, A. K. , Naylor, J. , Seymour, V. A. , Jiang, S. , Muraki, K. , … Beech, D. J. (2010). Cis‐isomerism and other chemical requirements of steroidal agonists and partial agonists acting at TRPM3 channels. British Journal of Pharmacology, 161(2), 430–441. 10.1111/j.1476-5381.2010.00892.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, K. , & Kaspar, B. K. (2014). Making sense of pain: Are pluripotent stem cell‐derived sensory neurons a new tool for studying pain mechanisms? Molecular Therapy, 22(8), 1403–1405. 10.1038/mt.2014.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran, M. M. , & Szallasi, A. (2018). Targeting nociceptive transient receptor potential channels to treat chronic pain: Current state of the field. British Journal of Pharmacology, 175(12), 2185–2203. 10.1111/bph.14044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberwinkler, J. , Lis, A. , Giehl, K. M. , Flockerzi, V. , & Philipp, S. E. (2005). Alternative splicing switches the divalent cation selectivity of TRPM3 channels. The Journal of Biological Chemistry, 280(23), 22540–22548. 10.1074/jbc.M503092200 [DOI] [PubMed] [Google Scholar]
- Papakosta, M. , Dalle, C. , Haythornthwaite, A. , Cao, L. , Stevens, E. B. , Burgess, G. , … Grimm, C. (2011). The chimeric approach reveals that differences in the TRPV1 pore domain determine species‐specific sensitivity to block of heat activation. The Journal of Biological Chemistry, 286(45), 39663–39672. 10.1074/jbc.M111.273581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanikolaou, M. , Lewis, A. , & Butt, A. M. (2017). Store‐operated calcium entry is essential for glial calcium signalling in CNS white matter. Brain Structure & Function, 222(7), 2993–3005. 10.1007/s00429-017-1380-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian, A. , Tate, S. , & Woolf, C. J. (2009). Transient receptor potential channels: Targeting pain at the source. Nature Reviews. Drug Discovery, 8(1), 55–68. 10.1038/nrd2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quallo, T. , Alkhatib, O. , Gentry, C. , Andersson, D. A. , & Bevan, S. (2017). G protein βγ subunits inhibit TRPM3 ion channels in sensory neurons. eLife, 6 10.7554/eLife.26138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostock, C. , Schrenk‐Siemens, K. , Pohle, J. , & Siemens, J. (2018). Human vs. mouse nociceptors—Similarities and differences. Neuroscience, 387, 13–27. 10.1016/j.neuroscience.2017.11.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki, T. , Minami, M. , Nakagawa, T. , Ienaga, Y. , Morisada, A. , & Satoh, M. (1998). DAMGO recognizes four residues in the third extracellular loop to discriminate between μ‐ and κ‐opioid receptors. European Journal of Pharmacology, 350(2–3), 301–310. 10.1016/s0014-2999(98)00240-4 [DOI] [PubMed] [Google Scholar]
- Straub, I. , Krugel, U. , Mohr, F. , Teichert, J. , Rizun, O. , Konrad, M. , … Schaefer, M. (2013). Flavanones that selectively inhibit TRPM3 attenuate thermal nociception in vivo. Molecular Pharmacology, 84(5), 736–750. 10.1124/mol.113.086843 [DOI] [PubMed] [Google Scholar]
- Vandewauw, I. , De Clercq, K. , Mulier, M. , Held, K. , Pinto, S. , Van Ranst, N. , … Voets, T. (2018). A TRP channel trio mediates acute noxious heat sensing. Nature, 555(7698), 662–666. 10.1038/nature26137 [DOI] [PubMed] [Google Scholar]
- Vangeel, L. , & Voets, T. (2019). Transient receptor potential channels and calcium signaling. Cold Spring Harbor Perspectives in Biology, 11(6): a035048 10.1101/cshperspect.a035048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets, T. , Vriens, J. , & Vennekens, R. (2019). Targeting TRP channels—Valuable alternatives to combat pain, lower urinary tract disorders, and type 2 diabetes? Trends in Pharmacological Sciences, 40(9), 669–683. 10.1016/j.tips.2019.07.004 [DOI] [PubMed] [Google Scholar]
- Vriens, J. , Held, K. , Janssens, A. , Toth, B. I. , Kerselaers, S. , Nilius, B. , … Voets, T. (2014). Opening of an alternative ion permeation pathway in a nociceptor TRP channel. Nature Chemical Biology, 10(3), 188–195. 10.1038/nchembio.1428 [DOI] [PubMed] [Google Scholar]
- Vriens, J. , Nilius, B. , & Voets, T. (2014). Peripheral thermosensation in mammals. Nature Reviews. Neuroscience, 15(9), 573–589. 10.1038/nrn3784 [DOI] [PubMed] [Google Scholar]
- Vriens, J. , Owsianik, G. , Hofmann, T. , Philipp, S. E. , Stab, J. , Chen, X. , … Voets, T. (2011). TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron, 70(3), 482–494. 10.1016/j.neuron.2011.02.051 [DOI] [PubMed] [Google Scholar]
- Wagner, T. F. , Loch, S. , Lambert, S. , Straub, I. , Mannebach, S. , Mathar, I. , … Oberwinkler, J. (2008). Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells. Nature Cell Biology, 10(12), 1421–1430. 10.1038/ncb1801 [DOI] [PubMed] [Google Scholar]
- Wainger, B. J. , Buttermore, E. D. , Oliveira, J. T. , Mellin, C. , Lee, S. , Saber, W. A. , … Woolf, C. J. (2015). Modeling pain in vitro using nociceptor neurons reprogrammed from fibroblasts. Nature Neuroscience, 18(1), 17–24. 10.1038/nn.3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh, T. L. , Woller, S. A. , Ramachandran, R. , & Sorkin, L. S. (2015). The search for novel analgesics: Targets and mechanisms. F1000Prime Rep, 7, 56 10.12703/P7-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekkirala, A. S. , Roberson, D. P. , Bean, B. P. , & Woolf, C. J. (2017). Breaking barriers to novel analgesic drug development. Nature Reviews. Drug Discovery, 16, 545–564. 10.1038/nrd.2017.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, G. T. , Gutteridge, A. , Fox, H. , Wilbrey, A. L. , Cao, L. , Cho, L. T. , … Stevens, E. B. (2014). Characterizing human stem cell‐derived sensory neurons at the single‐cell level reveals their ion channel expression and utility in pain research. Molecular Therapy, 22(8), 1530–1543. 10.1038/mt.2014.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudin, Y. , & Rohacs, T. (2019). The G‐protein‐biased agents PZM21 and TRV130 are partial agonists of mu‐opioid receptor‐mediated signalling to ion channels. British Journal of Pharmacology, 176(17), 3110–3125. 10.1111/bph.14702 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Human data (A,B) Mean ± SEM of all traces in the experiment shown in Figure 1B (n = 23) and Figure 1D (n = 27), respectively. (C) Amplitude of the first pregnenolone sulphate (PS) response of all neurons, separated by donor. Solid line indicates the mean.
Figure S2: Distribution of TRPV1 and TRPM3 responders in different DRG models. (A‐C) Piecharts showing the percentage of TRPV1 and/or TRPM3 responders in (A) mouse DRG (Vandewauw et al., 2018), (B) human DRG and (C) hSCDS neurons.


