Abstract
Background and Purpose
The enteric neurotransmitter nitric oxide (NO) regulates gastrointestinal motility by relaxing smooth muscle. Pharmacological cAMP induction also relaxes gastrointestinal smooth muscle, but it is uncertain whether cAMP augments or suppresses enteric NO signalling. In other organ systems, cAMP can increase neuronal NO production by stimulating protein kinase A (PKA) to phosphorylate neuronal NOS (nNOS) Serine‐1412 (S1412). We hypothesized that cAMP also increases nNOS S1412 phosphorylation by PKA in enteric neurons to augment nitrergic relaxation of mouse ileum.
Experimental Approach
We measured contractile force and nNOS S1412 phosphorylation in ileal rings suspended in an organ bath. We used forskolin to induce cAMP‐dependent relaxation of wild type, nNOSS1412A knock‐in and nNOSα‐null ileal rings in the presence or absence of PKA, protein kinase B (Akt) and NOS inhibitors.
Key Results
Forskolin stimulated phosphorylation of nNOS S1412 in mouse ileum. Forskolin relaxed nNOSα‐null and nNOSS1412A ileal rings less than wild‐type ileal rings. PKA inhibition blocked forskolin‐induced nNOS phosphorylation and attenuated relaxation of wild type but not nNOSS1412A ileum. Akt inhibition did not alter nNOS phosphorylation with forskolin but did attenuate relaxation of wild type and nNOSS1412A. NOS inhibition with l‐NAME eliminated the effects of PKA and Akt inhibitors on relaxation.
Conclusion and Implications
PKA phosphorylation of nNOS S1412 augments forskolin‐induced nitrergic ileal relaxation. The relationship between cAMP/PKA and NO is therefore synergistic in enteric nitrergic neurons. Because NO regulates gut motility, selective modulation of enteric neuronal cAMP synthesis may be useful for the treatment of gastrointestinal motility disorders.
Abbreviations
- 1400W
N‐([3‐(aminomethyl)‐phenyl]‐methyl)‐ethanimidamide HCl
- CaM
calmodulin
- CFKB
calcium‐free Krebs buffer
- cPTIO
2‐(4‐carboxyphenyl)‐4,4,5,5‐tetramethylimidazoline‐1‐oxyl‐3‐oxide
- eNOS KO nNOSS1412A
mice doubly homozygous for the nNOSS1412A mutation and eNOS knockout
- eNOS KO
mice lacking eNOS
- eNOS
endothelial NOS
- EPAC
exchange factor activated by cAMP
- FSK
forskolin
- GI
gastrointestinal
- H‐89
N‐[2‐(p‐Br‐cinnamylamino)‐ethyl]‐5‐isoquinolinesulfonamide HCl
- IJP
inhibitory junction potential
- KB
Krebs buffer
- l‐NAME
l‐Nitro‐l‐arginine methyl ester
- Myr‐PKI
myristoylated PKA inhibitor peptide, residues 14–22
- NANT
N‐[(4S)‐4‐amino‐5‐[(2‐aminoethyl)‐amino]‐pentyl]‐N′‐nitroguanidinetris trifluoroacetate
- nNOS
neuronal NOS
- nNOSS1412A
mice with knock‐in mutation of nNOS serine‐1412 to alanine
- nNOSα KO
mice lacking the first exon of nNOS
- ODQ
1H‐[1,2,4]‐oxadiazolo‐[4,3‐a]‐quinoxalin‐1‐one
- pS1412
nNOS phosphorylated at serine‐1412
- S1179
serine‐1179 of mouse eNOS
- S1412
serine‐1412 of mouse nNOS
- sGC
soluble GC
- TTX
tetrodotoxin
- WT
wild type
What is already known
nNOS and cAMP each regulate gastrointestinal motility, and PKA activates nNOS in other organ systems.
cAMP/PKA is reported to both facilitate and antagonize nitrergic gastrointestinal relaxation.
What does this study add
Forskolin stimulates PKA via cAMP to phosphorylate and activate nNOS in mouse ileum.
In enteric neurons, forskolin‐stimulated NO signalling is partly mediated by nNOS Serine‐1412 phosphorylation.
What is the clinical significance
Enteric neuronal PKA may influence gastrointestinal motility and transit rates.
Selective stimulation of cAMP pathways in enteric nitrergic neurons may improve gastrointestinal dysmotility symptoms.
1. INTRODUCTION
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2509 (NO) is a NANC neurotransmitter that relaxes smooth muscle via the soluble guanylate cyclase (sGC)‐https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2347‐https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=287 pathway in myocytes (D. D. Guerra & Hurt, 2019; Lefebvre, Smits, & Timmermans, 1995). The primary NO source in the gastrointestinal (GI) tract is https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1251 (nNOS), expressed in inhibitory myenteric neurons (Mang, Truempler, Erbelding, & Kilbinger, 2002). Neuronal depolarization increases NO synthesis by raising intracellular [Ca2+], which stimulates nNOS via Ca2+/https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2351 (CaM; Thatte, He, & Goyal, 2009). nNOS Serine‐1412 (S1412) phosphorylation by https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1694 promotes nitrergic relaxation of penile smooth muscle (Hurt et al., 2012). We recently showed that low frequency depolarization relaxes mouse ileum via https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=285 phosphorylation of nNOS S1412 (D.D. Guerra et al., 2019); this may explain why NO responses are tuned to depolarization frequency in the gut (Spencer et al., 2018) and why nitrergic inhibitory junction potentials (IJPs) have a longer duration than action potentials (Hurt et al., 2012; Keef et al., 2013).
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2352 is a second messenger for https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=4 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1152 receptors that relax GI smooth muscle by stimulating PKA phosphorylation of myocyte https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=133 (Koh, Sanders, & Carl, 1996; Smith et al., 1993). cAMP pathways are less clear in enteric neurons. The adenylate cyclase (AC) activator https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5190 (FSK) depolarizes afferent neurons in guinea pig small intestine myenteric plexus (Nemeth, Palmer, Wood, & Zafirov, 1986), and selective neuronal expression of a dominant negative https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1472 causes intestinal pseudo‐obstruction in mice (Howe et al., 2006). These studies suggest that neuronal cAMP/PKA may regulate GI motility.
Interactions between cAMP and NO pathways are not clearly defined in the gut. A cAMP analogue relaxes ileum from wild type (WT) and protein kinase G type 1 (PKG1)‐deficient mice with equal efficacy (Bonnevier, Fässler, Somlyo, Somlyo, & Arner, 2004), and forskolin does not depolarize guinea pig myenteric plexus efferent motor neurons (Nemeth et al., 1986). However, pharmacological cAMP elevation blocks nitrergic IJPs in mucosa‐denuded mouse colon (Hwang et al., 2008), suggesting cAMP‐ and NO‐relaxation pathways are antagonistic or independent. Other studies have drawn contrary conclusions. For example forskolin sensitizes rat ileum to NO donor relaxation (Ekblad & Sundler, 1997), and pharmacological inhibition of cAMP and cGMP additively impairs mouse ileal relaxation by https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1989 (Idrizaj, Garella, Francini, Squecco, & Baccari, 2018). These findings imply that cAMP and NO pathways can interact. Prior work has not demonstrated a mechanism by which physiological cAMP–NO synergy promotes GI smooth muscle relaxation, and the cell type (e.g. myocytes, neurons and endothelium) has not been identified.
Forskolin relaxation of human corpus cavernosum requires cGMP (Uckert et al., 2004). cAMP–NO synergy may occur in neurons because forskolin relaxes rat corpus cavernosum by activating PKA to phosphorylate cavernosal nerve nNOS S1412 (Hurt et al., 2012). We reasoned that cAMP–NO synergy in enteric neurons might also promote GI smooth muscle relaxation. Using kinase and NO pathway inhibitors and non‐phosphorylatable nNOSS1412A knock‐in mice, we assessed forskolin relaxation of ileal rings. We found that PKA phosphorylation of nNOS S1412 mediates a significant portion of forskolin relaxation of mouse ileum, suggesting that enteric neuronal cAMP promotes nitrergic GI relaxation. Our findings reconcile prior conflicting reports on the relationship between cAMP/PKA and NO‐cGMP‐PKG signalling in forskolin relaxation of GI smooth muscle.
2. METHODS
2.1. Animals
2.1.1. Ethics statement and husbandry
The University of Colorado Institutional Animal Care and Use Committee approved all animal procedures (IACUC protocol 90). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology. All animals were male C57Bl/6 mice (Mus musculus) and maintained by Office of Laboratory Animal Research (OLAR) veterinary technicians at 20°C in a 12‐hr day/night cycle vivarium with ad libitum standard rodent chow (Teklad 8640). Mice were housed two to five animals per cage in Allentown 75 JAG modular ventilated cages with hardwood bedding. To maintain hybrid vigour, we backcrossed mutant males to WT C57Bl/6 females and genotyped pups at postnatal day 9 via tail snip. All mutant mice were F5 generation or later. Mice were 10–40 weeks of age (median 16 weeks) and weighed 29–43 g (median 33 g) at euthanasia via CO2 asphyxiation and cervical dislocation.
2.1.2. Mouse strains
Non‐phosphorylatable nNOS knock‐in mutant (nNOS S1412A ): The nNOSS1412A knock‐in mouse is homozygous for the nNOSS1412A allele, in which the nNOS Serine‐1412 phosphorylation site is replaced with non‐phosphorylatable alanine. nNOS knockout (nNOSα KO): The nNOSα null strain (Jackson Laboratory 002986) is homozygous for the NOS1 tm1Plh allele, which lacks the membrane localizing PDZ domain (amino acids 1–159). These mice show ~90% reduced nNOS activity, but nNOSβ is still expressed throughout the cytoplasm. https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1249 knockout (eNOS KO): The eNOS null strain (Jackson Laboratory 002684) is homozygous for the NOS3 tm1Unc allele, which abolishes eNOS enzymatic activity. nNOS S1412A /eNOS KO double mutants: Mice doubly homozygous for the nNOSS1412A knock‐in and eNOS knockout mutations were obtained by nNOSS1412A × eNOS KO crosses. WT: WT mice were obtained from Charles River laboratories or by heterozygous nNOSS1412A crosses.
2.2. Study design
2.2.1. Groups
For all forskolin experiments, we defined control and treatment groups as follows:
Chemical treatments of one genotype: Controls received vehicle, 0.1% (v/v) DMSO or water. The treatment group received one drug in the same volume of DMSO or water. For l‐NAME experiments, controls received l‐NAME and vehicle, while treatment groups received l‐NAME and a drug in the same volume as vehicle.
Comparison by genotype: The control group was WT or eNOS KO. The treatment groups were nNOSS1412A, nNOSα KO or eNOS KO/nNOSS1412A.
Immunoblots of nNOS S1412 phosphorylation: The control group received 0.1% (v/v) DMSO vehicle. The treatment group received 30‐ or 300‐nM forskolin. If the experiment used kinase inhibitors, controls received Akt or PKA inhibitor and vehicle, while treatment groups received the same volume of Akt or PKA inhibitor and 300‐nM forskolin.
2.2.2. Sample sizes and randomization
For all experiments, “n” is the number of individual ileal rings obtained from two or more mice. In our prior study (D.D. Guerra et al., 2019), nNOSS1412A mutants exhibited impaired EFS relaxation, with relative effect sizes and SDs of ~1.5 and ~30% of the mean respectively. Assuming similar parameters for forskolin ileal relaxation and using power = 0.8 and α = 0.05, we calculated a sample size of at least n = 5. Considering previous pharmacological studies, we planned to use N = 6–15 (Mo, Michel, Lee, Kaumann, & Molenaar, 2017; Shuttleworth, Sanders, & Keef, 1993; Zavala‐Mendoza, Grasa, Zavala‐Sánchez, Pérez‐Gutiérrez, & Murillo, 2016). Deviation from equal allocation occurred when some rings were unresponsive or failed to respond to https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2098 and were therefore discarded prior to the experiment. The number of vehicle samples exceeds that of specific chemical treatments because controls accompanied each chemical treatment (Motulsky & Michel, 2018) and identically treated rings from different experiments and mice were combined for analysis. We divided each mouse ileum into rings that were randomly placed into separate organ bath chambers and assigned to control or treatment conditions. We were unable to perform blinding because ear tags identified strain and because many chemical treatments predictably altered ileal tension.
2.3. Ileal ring preparation and organ bath pharmacology
We removed mesentery from the distal 8 cm of small intestine 1 cm proximal to the ileocecal valve and placed the ileum in ice‐cold 95% O2/5% CO2 (95/5)‐perfused Ca2+‐free Krebs buffer (CFKB; 118‐mM NaCl +4.7‐mM KCl + 1.2‐mM MgSO4 heptahydrate + 25‐mM NaHCO3 + 1.2‐mM KH2PO4 + 11 mM https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4536 + 5‐mM HEPES + 50‐μM EDTA + 100‐μM EGTA). We flushed the lumen with CFKB using an 18‐gauge 1.8 × 40 mm blunt tip syringe, replaced buffer with fresh CFKB, and cut the ileum into 0.5‐cm circular segments with a clean razor blade. We performed all organ bath experiments at 37°C using tandem Radnoti 159920‐X1/10 systems and 95/5 oxygenated Krebs buffer (KB; CFKB + 3.3‐mM CaCl2, without EGTA). To maintain NANC conditions (Shuttleworth et al., 1993), we added https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=320, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=564, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=502 (all 1 μM) and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1909 (10 μM). Ileal rings were positioned in organ bath chambers between supports and stationary mounts (Radnoti 158817 and 160152‐14) and MLT0201/RAD force transducers (AD instruments). Powerlab 16/35 and LabChart7.0 (AD Instruments) enabled detection and recording of ileal tensile force.
Ileal rings were equilibrated for 15 min under NANC conditions before adding vehicle or inhibitors. To inhibit PKA, we used 1‐μM https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5983, 25‐μM https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5262, or 10‐μM Myr‐PKI. To inhibit Akt, we used 10‐μM https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7945. To inhibit sGC and PKG, we used 5‐μM https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5234 or 5‐μM Rp‐8‐Br‐cGMPS respectively. cPTIO (300 μM) was used to scavenge NO. To inhibit all NOS activity non‐selectively, we used 1‐mM l‐ https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=summary&ligandId=5213. To inhibit nNOS selectively over eNOS, we used 100‐μM NANT (N‐[(4S)‐4‐amino‐5‐[(2‐aminoethyl)‐amino]‐pentyl]‐N′‐nitroguanidinetris trifluoroacetate) or 50‐μM https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5102. l‐ https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=721 (2 mM) was used to reverse l‐NAME effects. To inhibit neuronal depolarization we used 10‐μM https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2616. Prior publications indicate that these concentrations of H‐89, Myr‐PKI, MK‐2206, ODQ, cPTIO, l‐NAME, 1400W and TTX are selective (Denisova et al., 2014; Kaya et al., 2012; Lefebvre et al., 1995; Mang et al., 2002; May et al., 2019; Paisley & Martin, 1996; Tajima et al., 2012).
After 15 min of vehicle or inhibitor treatment, we added 1‐μM https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2098 (Briejer, Akkermans, Meulemans, Lefebvre, & Schuurkes, 1993) to ensure tissue viability and promote consistent contractility during the entire concentration–response curve. We discarded rings that did not respond to substance P; 5 min after substance P and with stable regular baseline contractility, we cumulatively increased [forskolin] every 2 min to 0.1‐, 1.1‐, 11.1‐, 44.1‐, 144‐, 477‐ and 1480‐nM forskolin. To evaluate relaxation with the stable cAMP analogue Sp‐cAMPs, we added 25‐μM Sp‐cAMPs or water 8 min after substance P.
Control experiments with WT ileal rings (Figure S2) revealed no loss of tensile force at 0.1‐nM forskolin relative to vehicle (0.01% (v/v) DMSO). Therefore, we normalized mean tensile force at each [forskolin] to mean tensile force after 2‐min incubation with 0.1‐nM forskolin. Mean tensile force is the average phasic contraction force relative to baseline and was calculated by dividing the AUC by the time interval.
2.4. Estimating the proportion of nitrergic forskolin relaxation
To estimate the contribution of nitrergic/NOS sources to forskolin relaxation, we compared mean tensile force at multiple [forskolin] for control (WT or vehicle) and experimental groups (mutant strains or pharmacological treatments). The groups were WT treated with vehicle (Figure 4c), l‐NAME (Figure 1c), NANT (Figure 4c) or H‐89 (Figure 1b); nNOSS1412A treated with vehicle (Figure 3c), l‐NAME (Fig., 3D) or H‐89 (Figure 3c); and eNOS KO treated with vehicle (Figure 4a) or l‐NAME (Figure 4a). Our procedure precluded variance calculation and was therefore a non‐statistical exploratory analysis giving a quantitative estimate of the contribution of NO synthesis or nNOS S1412 phosphorylation to forskolin relaxation. We defined relaxation as the inverse of mean tensile force (i.e. 100—mean tensile force). To calculate total percent relaxation due to treatment (Figure S7), we subtracted experimental relaxation from control relaxation. To calculate relative relaxation (i.e. proportion of relaxation at a given forskolin concentration due to treatment; Figure S8), we divided the difference in control and experimental relaxation by control relaxation and expressed as a new percentage. Values less than or equal to zero were expressed as zero.
Figure 4.
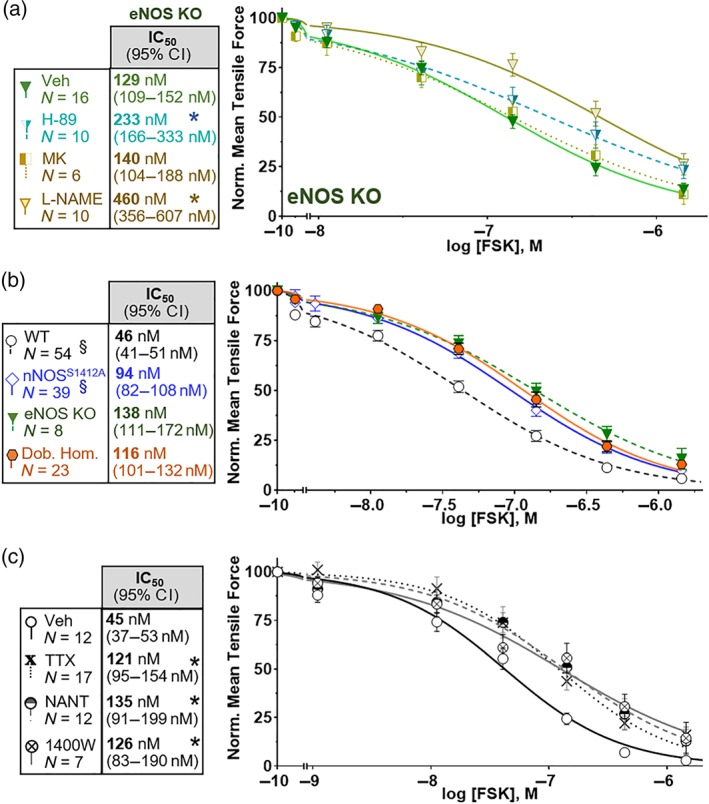
NO‐dependent forskolin (FSK) relaxation primarily requires nNOS. (a) FSK relaxation of endothelial NOS‐null (eNOS KO) ilea is sensitive to PKA (H‐89) and NOS (l‐NAME) inhibition, but not Akt inhibition (MK). (b) FSK relaxation of the eNOS KO nNOSS1412A double homozygote (Dob. Hom.) is indistinguishable from the eNOS KO and nNOSS1412A single mutants. (c) Inhibition of neuronal depolarization (TTX; 10 μM) or nNOS (100 μM NANT or 50 μM 1400W) blocks most NOS‐dependent FSK relaxation. * P < .05 versus Veh IC50 (a,c). §Datasets from Figure 3a repeated to illustrate non‐additivity of eNOS KO and nNOSS1412A mutations
Figure 1.
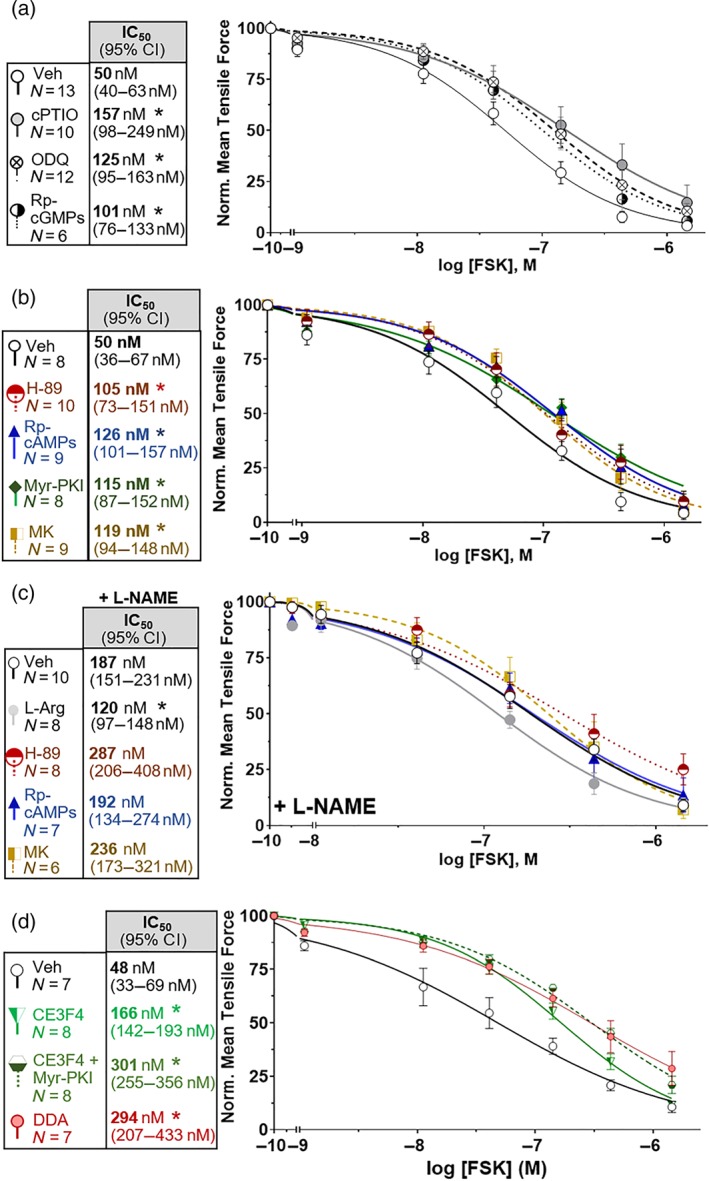
PKA and Akt inhibitors attenuate nitrergic forskolin (FSK) relaxation of ileum. (a) The NO signalling inhibitors cPTIO (300 μM), ODQ (5 μM), and Rp‐cGMPs (5 μM) attenuate FSK relaxation. (b) PKA inhibitors (H‐89, 1 μM; Myr‐PKI, 10 μM; and Rp‐cAMPs, 25 μM) and the Akt inhibitor MK‐2206 (MK; 10 μM) attenuate FSK relaxation. (c) Akt and PKA inhibitors do not further attenuate FSK relaxation under NOS blockade with l‐NAME (1 mM), but the NOS substrate l‐Arginine (l‐Arg; 2 mM) partially restores FSK relaxation. (d) The EPAC inhibitor CE3F4 (10 μM) attenuates FSK relaxation independently of PKA, and adenylate cyclase blockade with dideoxyadenosine (DDA; 20 μM) attenuates relaxation as much as combined PKA and EPAC inhibition. FSK IC50 values are bold, and 95% confidence intervals are in parentheses. * P < .05 versus Veh IC50. Veh: DMSO = control. Error bars: SEM. n, number of ileal rings
Figure 3.
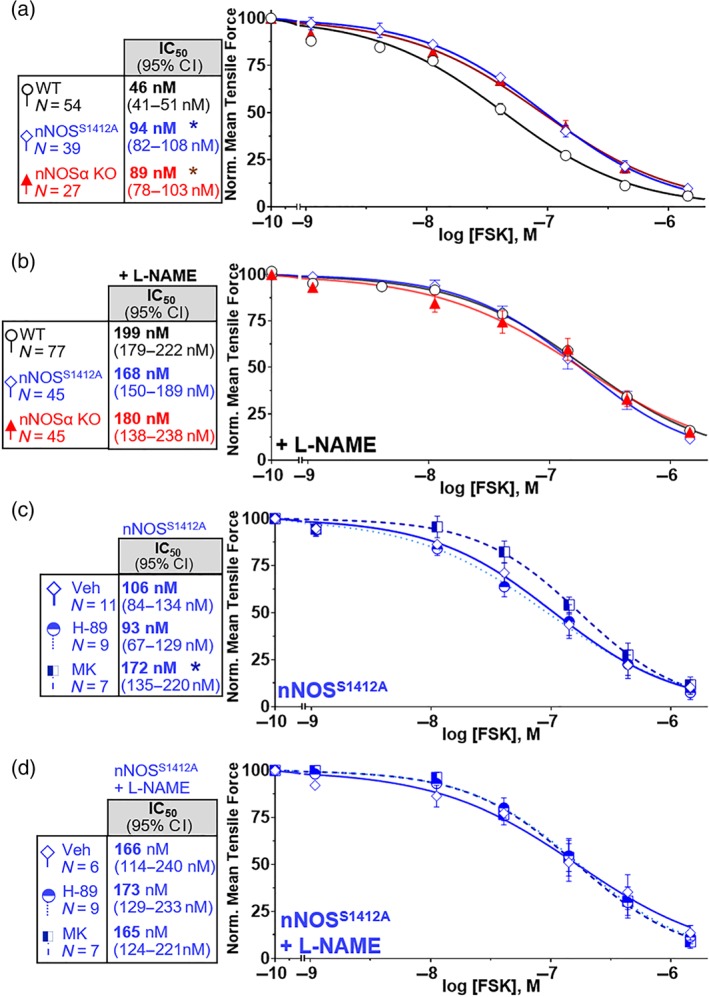
nNOS Serine‐1412 potentiates forskolin (FSK) ileal relaxation. (a) FSK relaxation is reduced in both nNOSS1412A and nNOSα‐null (nNOSα KO) compared to wild‐type (WT) mice. (b) WT, nNOSS1412A and nNOSα KO ilea exhibit the same sensitivity to FSK under NOS blockade with l‐NAME. (c) Akt (MK), but not PKA (H‐89), inhibition further attenuates nNOSS1412A ileal relaxation by FSK. D. Under NOS blockade with l‐NAME, neither PKA (H‐89) nor Akt (MK) inhibitors further attenuate FSK relaxation. * P < .05 versus WT IC50 (a) or Veh IC50 (c)
2.5. Tissue collection, protein extraction, and immunoblotting
The immuno‐related procedures used comply with the recommendations made by the British Journal of Pharmacology (Alexander et al., 2018). To evaluate nNOS S1412 phosphorylation, we added vehicle (0.1% DMSO), 30‐ or 300‐nM forskolin to ileal rings in organ baths 5 min after substance P. To assess PKA and Akt inhibition, we added H‐89, Rp‐cAMPs or MK‐2206 to organ baths 15 min prior to substance P. Ileal rings were vitrified in liquid nitrogen 2 min after FORSKOLIN or vehicle treatment and homogenized in 25‐mM Tris–HCl (pH 7.5) + 1‐mM EGTA + 1‐mM DTT + 0.4% (v/v) Triton X‐100 + 1× protease and phosphatase inhibitors. Homogenates were centrifuged at 16,000× g, and supernatant protein concentration was determined by the ThermoFisher Pierce 660‐nm assay. Supernatants were resolved by SDS‐PAGE, transferred to PVDF membranes, and immunoblotted with antibodies to nNOS (ImmunoStar, RRID AB_572255; 1:500) or phospho‐nNOS S1412 (pS1412; Abcam, RRID 304964; 1:250) as described previously (Hurt et al., 2012). We quantified protein band fluorescence with Li‐Cor Odyssey Image Studio 5.2. We expressed nNOS pS1412 relative to total nNOS in the same sample and normalized to the mean pS1412/total nNOS ratio for vehicle treatments without inhibitors.
2.6. Statistical tests
We calculated statistics using GraphPad Prism 7.04 with groups of N ≥ 6 and significance set at P < .05 (denoted by *). “N” refers to independent biological samples. We plotted normalized tensile force versus log [forskolin] and fit data with log [Inhibitor]—normalized response least squares sigmoidal regression models with variable Hill slopes to determine relative IC50 values for forskolin relaxation. We used normalized data to compare IC50 values, an accepted procedure to correct for initial variation (i.e., variable contractility due to differences in ileal ring size and positioning; Sebaugh, 2011). Sigmoidal curves were compared via extra sum of squares F tests to determine if one curve adequately explained all data. All regressions exhibited P > .05 for the replicates test (Table S1), suggesting goodness of fit. To determine if forskolin promoted nNOS S1412 phosphorylation and if Sp‐cAMPs relaxed WT and nNOSS1412A rings, we used Kruskal–Wallis non‐parametric ANOVA. We conducted Dunn's post hoc tests only if ANOVA 'F' values coincided with p<0.05 and variance inhomogeneity was not significant. All samples subjected to statistical analysis contained a minimum of n = 5 ileal rings, where n = biologically independent values. To determine if Akt or PKA inhibitors affected forskolin‐induced nNOS S1412 phosphorylation, we performed unpaired, two‐tailed non‐parametric Mann–Whitney tests for each drug treatment. To estimate fold‐relaxation of a treatment relative to a control group, we divided the IC50 of the treatment group by the IC50 of the control group. The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018).
2.7. Materials
Forskolin, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4100, dithiothreitol (DTT), protease and phosphatase inhibitors, NANC inhibitors, substance P, l‐Nitro‐l‐arginine methyl ester (l‐NAME), 1H‐[1,2,4]‐oxadiazolo‐[4,3‐a]‐quinoxalin‐1‐one (ODQ), tetrodotoxin (TTX) and all bulk reagents were from Millipore‐Sigma (Burlington, USA). The 100× protease inhibitor cocktail comprised 104‐mM AEBSF + 80‐μM aprotinin + 4‐mM bestatin + 1.4‐mM E‐64 + 2‐mM leupeptin + 1.5‐mM pepstatin A. The 100× phosphatase inhibitor cocktail was a proprietary mixture of Na3VO4, Na2MoO4, tartaric acid and imidazole. Myristoylated PKA inhibitor residues 14–22 (Myr‐PKI), and PVDF membranes were from ThermoFisher (Waltham, USA). N‐[2‐(p‐Br‐cinnamylamino)‐ethyl]‐5‐isoquinolinesulfonamide HCl (H‐89), N‐([3‐(aminomethyl)‐phenyl]‐methyl)‐ethanimidamide HCl (1400W) and 2‐(4‐carboxyphenyl)‐4,4,5,5‐tetramethylimidazoline‐1‐oxyl‐3‐oxide (cPTIO) were from Cayman Chemical (Ann Arbor, USA). MK‐2206 was from Apex Bio (Boston, USA). Rp‐ and Sp‐cAMPs, Rp‐8‐Br‐cGMPs and N‐[(4S)‐4‐amino‐5‐[(2‐aminoethyl)‐amino]‐pentyl]‐N′‐nitroguanidine tris (trifluoroacetate; NANT) were from Santa Cruz Biotech (Dallas, USA).
2.8. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org/, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos, et al., 2019; Alexander, Fabbro, et al., 2019; Alexander, Kelly, et al., 2019).
3. RESULTS
3.1. PKA, Akt and NOS facilitate forskolin relaxation of mouse ileum
NO and cAMP can each promote GI relaxation in rodents, but reports vary on whether cAMP facilitates or antagonizes nitrergic relaxation in the gut (Bonnevier et al., 2004; Hwang et al., 2008). To assess if cAMP acts via NO, we treated ileal rings from WT mice with forskolin in the presence of NO signalling pathway inhibitors and measured circular smooth muscle relaxation. Forskolin selectively activates AC over sGC (Allerston, von Delft, & Gileadi, 2013). An NO scavenger (cPTIO), a sGC inhibitor (ODQ) and a PKG inhibitor (Rp‐8‐Br‐cGMPs) increased the IC50 of forskolin 2‐ to 3‐fold over vehicle (Figures 1a and S1), indicating that the NO‐cGMP‐PKG pathway facilitates a portion of forskolin relaxation. While forskolin promoted ileal relaxation, vehicle DMSO and the inactive analogue 1,9‐dideoxyforskolin did not (Figure S2).
Next, we examined which forskolin‐induced relaxation mechanisms require NO. Downstream targets of cAMP include the protein kinases PKA and Akt (Garcia‐Morales, Luaces‐Regueira, & Campos‐Toimil, 2017), both of which activate nNOS via S1412 phosphorylation in other organ systems (Hurt et al., 2012; Rameau et al., 2007). When we inhibited PKA with competitive (H‐89 and Myr‐PKI) and allosteric (Rp‐cAMPs) antagonists, we observed a 2‐ to 2.5‐fold increase in the IC50 of forskolin (Figure 1b). The NOS inhibitor l‐NAME increased the IC50 of forskolin 3.7‐fold over vehicle treatment, and PKA inhibitors failed to shift the IC50 further than l‐NAME alone. However, the NOS substrate l‐arginine partly reversed the l‐NAME attenuation of forskolin relaxation, confirming that l‐NAME inhibition was related to NO (Figure 1c). Although we cannot rule out incomplete PKA inhibition, similar IC50 values with multiple PKA inhibitors imply that NO facilitates most of the PKA‐dependent relaxation. We also tested whether forskolin relaxation requires Akt. The Akt inhibitor MK‐2206 attenuated forskolin relaxation to the same extent as PKA inhibitors, and MK‐2206 did not shift the IC50 further than l‐NAME alone (Figure 1b–c), indicating that cAMP targets other than PKA may be involved. Because exchange factor activated by cAMP https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=259) mediates cAMP‐dependent Akt activation (Garcia‐Morales et al., 2017), we tested the EPAC inhibitor CE3F4. We found that CE3F4 alone or in combination with Myr‐PKI increased the forskolin IC50 3.4‐fold and 6.3‐fold respectively (Figure 1d). The AC inhibitor https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5108 attenuated relaxation as much as combined PKA and EPAC inhibition, but complete relaxation still occurred (Figure 1d), indicating that some forskolin relaxation is cAMP‐independent. Together, these data suggest that forskolin stimulates relaxation via distinct PKA and Akt pathways, both of which are cAMP‐dependent. Forskolin induction of NO synthesis could involve one or both of these kinases.
3.2. Forskolin stimulates PKA phosphorylation of nNOS Serine‐1412
Because PKA and Akt phosphorylate nNOS S1412 in rat pelvic ganglia and hippocampal neurons (Hurt et al., 2012; Rameau et al., 2007), we examined whether forskolin induces PKA and/or Akt to phosphorylate nNOS S1412 in the ileum. Compared with vehicle treatment, 300‐nM forskolin increased nNOS‐S1412 phosphorylation twofold (Figure 2a). While the PKA inhibitors H‐89 and Rp‐cAMPs blocked forskolin‐stimulated phosphorylation of nNOS S1412, the Akt inhibitor MK‐2206 did not (Figure 2b). Thus, PKA and Akt both facilitate relaxation, but forskolin only induces PKA to phosphorylate nNOS S1412 in ileal neurons.
Figure 2.
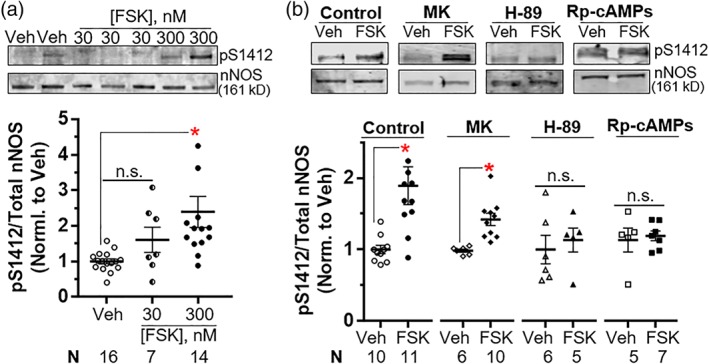
Forskolin (FSK) promotes nNOS S1412 phosphorylation by PKA in the ileum. (a) FSK enhances phosphorylation of nNOS at S1412 (pS1412). (b) PKA (H‐89 and Rp‐cAMPs), but not Akt (MK), inhibition blocks FSK‐enhanced phosphorylation of nNOS S1412. Representative immunoblots and quantifications are shown on the top and bottom panels respectively. Quantification of pS1412/total nNOS is normalized to Veh (a) or Veh for each treatment (b). Symbols represent individual ileal samples, and bars are means ± SEM. FSK: 300 nM. * P < .05 versus Veh by Kruskal–Wallis and Dunn's post hoc tests (a) or versus Veh for each treatment by Mann–Whitney tests (b). n.s., not significant
3.3. A component of forskolin relaxation requires nNOS Serine‐1412
Forskolin relaxation coincides with nNOS S1412 phosphorylation, so we wondered if nNOS S1412 phosphorylation facilitates forskolin relaxation. We previously developed a nNOSS1412A knock‐in mouse and determined that mutation of serine‐1412 to alanine attenuates electrical field stimulation‐induced ileal relaxation under NANC conditions. The nNOSS1412A mutation increased the IC50 of forskolin by 2.1‐fold compared to WT (Figures 3a and S3). The IC50 increased by 1.8‐fold more under NOS blockade with l‐NAME (Figure 3b). Because l‐NAME blocks all NOS activity (Toque et al., 2013), this indicates that nNOS S1412 phosphorylation facilitates about half of NOS‐dependent forskolin relaxation. The nNOSS1412A mutation reduced ileal forskolin sensitivity to a similar extent as loss of nNOSα (nNOSα KO; Figure 3a). nNOSα KO mice lack the nNOS membrane‐anchoring domain and exhibit only 5–10% of WT nNOS activity under physiological conditions (Huang, Dawson, Bredt, Snyder, & Fishman, 1993; Hurt et al., 2006). Interestingly, ileal rings from WT, nNOSS1412A and nNOSα KO mice exhibited indistinguishable forskolin IC50 values under NOS blockade with l‐NAME (Figure 3b). Thus, both membrane localization and S1412 phosphorylation likely facilitate nNOS‐dependent forskolin relaxation.
To confirm that forskolin promotes PKA phosphorylation of nNOS S1412, we treated WT and nNOSS1412A ileal rings with the direct PKA activator Sp‐cAMPs. Compared with WT, nNOSS1412A relaxed significantly less with Sp‐cAMPs, and l‐NAME blocked WT relaxation (Figure S4). We observed that l‐NAME increased the basal tone of WT rings, but not of nNOSS1412A rings, although subsequent substance P treatment abolished the l‐NAME effect (Figure S4). Next, we measured forskolin relaxation of nNOSS1412A ileal rings in the presence of H‐89 or MK‐2206. While MK‐2206 increased the forskolin IC50 1.6‐fold, H‐89 had no effect (Figure 3c). Thus, the nNOSS1412A mutation blocks PKA‐dependent forskolin relaxation but not the Akt‐dependent effect. H‐89 and MK‐2206 also failed to increase the forskolin IC50 in nNOSS1412A more than l‐NAME alone (Figure 3d). Together, these data imply that forskolin stimulates PKA to promote ileal relaxation via nNOS S1412 phosphorylation.
3.4. Nitrergic forskolin relaxation is primarily nNOS‐dependent
In the presence of l‐NAME, the Akt inhibitor MK‐2206 did not increase the forskolin IC50 for WT rings (Figure 1c), suggesting that Akt‐dependent relaxation requires NO synthesis. However, MK‐2206 induced a similar approximately twofold increase in forskolin IC50 for both WT and nNOSS1412A ileal rings, so Akt could mediate relaxation independently of nNOS S1412. https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1250 (iNOS) is not typically expressed in small intestine in the absence of inflammation (Cui et al., 1997), and histology showed no inflammatory infiltrate in WT or nNOSS1412A ilea (D.D. Guerra et al., 2019). The GI microvasculature expresses eNOS (Palatka et al., 2005), and Akt can phosphorylate serine 1179 in eNOS to increase Ca2+‐independent NO synthesis (Dimmeler et al., 1999; Fulton et al., 1999; Hurt et al., 2002). We found that eNOS‐deficient (eNOS KO) rings were sensitive to the PKA inhibitor H‐89 (1.8‐fold forskolin IC50 increase), but MK‐2206 had no effect (Figures 4a and S5). This is consistent with eNOS mediating Akt‐dependent forskolin relaxation. Therefore, we assessed the eNOS contribution to overall nitrergic forskolin relaxation by treating eNOS KO rings with l‐NAME. We observed an increase in the forskolin IC50 of 3.5‐fold (Figure 4a). This mirrors attenuation of WT relaxation by l‐NAME (Figure 3a–b) and shows that NO synthesis contributes similarly to forskolin relaxation in WT and eNOS KO ilea. Additionally, forskolin relaxation was similar for nNOSS1412A single mutants and eNOS KO/nNOSS1412A double mutants. Compared with WT, nNOSS1412A and eNOS KO/nNOSS1412A rings exhibited 2.1‐ and 2.5‐fold higher forskolin IC50 values (Figure 4b). l‐NAME increased the IC50 1.6‐fold for eNOS KO/nNOSS1412A rings (Figure S6), comparable to the 1.8‐fold increase for nNOSS1412A rings, indicating that nitrergic forskolin relaxation is similar in both mutants. These data are consistent with a small role for Akt‐mediated eNOS activation in NOS‐dependent forskolin relaxation and affirms that eNOS is not the major source of nitrergic relaxation in the gut (Mang et al., 2002).
Forskolin can directly and indirectly promote K+ channel closure and depolarization of enteric neurons (Hoshi, Garber, & Aldrich, 1988; Nemeth et al., 1986). Therefore, to estimate the contributions of neuronal depolarization and nNOS activity to forskolin‐dependent relaxation, we measured forskolin relaxation of WT ilea in the presence of the neuronal Na+ channel blocker TTX or the nNOS selective inhibitors NANT and 1400W (Boer et al., 2000; Hah, Roman, Martasek, & Silverman, 2001). TTX increased the forskolin IC50 2.7‐fold for WT ileal rings (Figure 4c), indicating that neuronal depolarization partially mediates forskolin‐stimulated relaxation. Inhibition of nNOS with NANT and 1400W increased the IC50 2.8‐ to 3‐fold (Figure 4c). These values were slightly less than the 3.7‐fold increase in IC50 obtained via blockade of all NOS activity with l‐NAME (Figure 3b). To estimate the nitrergic component of forskolin relaxation, we calculated differences in ileal relaxation upon drug treatment or genetic knock‐in mutation (nNOSS1412A) compared with vehicle or WT controls. We estimated that nNOS mediates about 20% total relaxation at moderate forskolin concentrations (44–477 nM), of which two‐thirds is due to nNOS S1412 (Figure S7). Thus, nNOS facilitates most nitrergic forskolin relaxation (via nNOS S1412 phosphorylation) with minimal additional contribution from eNOS. While low forskolin concentrations (1–44 nM) stimulate very slight absolute relaxation, the relative contribution of nNOS is 50–80% (Figure S8). At the highest forskolin concentration (1,440 nM), forskolin relaxation is large and predominantly NOS‐ and PKA‐independent (Figures S7 and S8).
4. DISCUSSION AND CONCLUSIONS
Our primary finding in this study is that cAMP increases nitrergic relaxation of the ileum by activating PKA in enteric neurons to phosphorylate nNOS S1412 (Figure 5). This complements our prior report that low frequency electrical field stimulation promotes nitrergic ileal relaxation via nNOS S1412 phosphorylation (D.D. Guerra et al., 2019) and shows that two different kinases can augment nNOS inhibitory signals in the gut. Our findings further suggest that exchange factor activated by cAMP (EPAC) may facilitate PKA‐independent Akt activation of eNOS, but knockout animal experiments indicate that eNOS contributes only a minor fraction of forskolin relaxation. Other non‐PKA targets of cAMP may also be involved. Nonetheless, this and our previous study demonstrate that distinct stimuli can activate Akt or PKA to phosphorylate nNOS S1412 and alter ileal tone. This broadens potential therapeutic targets for nitrergic GI dysmotility. nNOS mutant mouse strains allowed us to isolate the neuronal component of the cAMP–NO interaction because only neurons express nNOS (Huber, Saur, Kurjak, Schusdziarra, & Allescher, 1998; Nasser, Ho, & Sharkey, 2006). However, bath application of forskolin elevates cAMP in all cells expressing AC, and we found that most forskolin relaxation was both non‐nitrergic and PKA‐independent at concentrations over 400‐nM forskolin (Figures S7 and S8).
Figure 5.
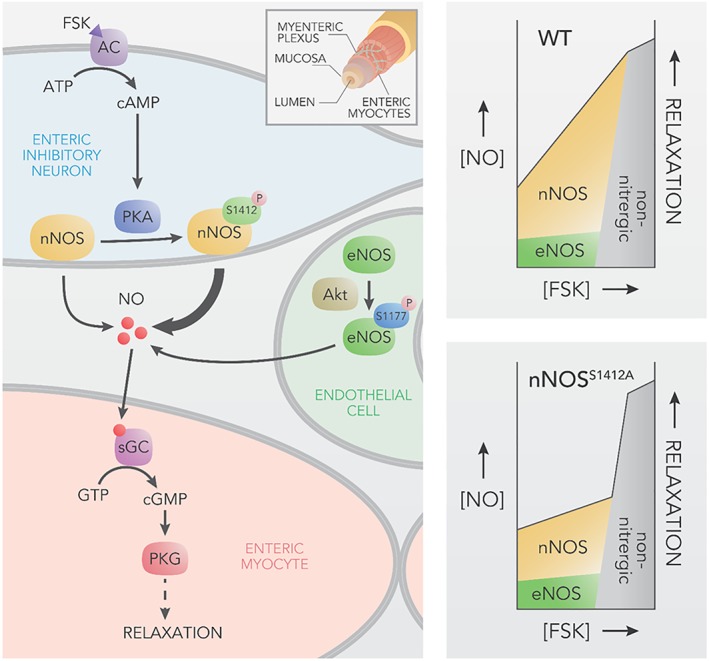
nNOS S1412 phosphorylation augments cAMP‐dependent enteric relaxation. Forskolin (FSK) relaxes GI smooth muscle via nitrergic (NO‐dependent) and non‐nitrergic mechanisms. Left. A model for nitrergic FSK relaxation. FSK stimulates AC to synthesize cAMP, which activates PKA phosphorylation of nNOS S1412 in myenteric inhibitory neurons. Phosphorylation enhances nNOS activity, thereby increasing NO‐cGMP‐dependent relaxation in myocytes. Akt phosphorylation of eNOS facilitates basal NO release, but eNOS stimulation plays a small role in nitrergic FSK relaxation. Inset shows gut layers. Right. Contribution of nNOS, eNOS and other factors in FSK relaxation. Top. At low to moderate [FSK], nNOS activation is the primary relaxation mechanism in WT animals with a small contribution from eNOS. At high [FSK], relaxation is primarily non‐nitrergic and may involve AC‐independent effects of FSK. Bottom. nNOSS1412A mutation abolishes most nitrergic FSK relaxation, but non‐phosphorylation dependent nNOS activation and possibly eNOS can still mediate nitrergic relaxation at low to moderate [FSK]
Prior disagreements concerning cAMP and nitrergic relaxation may derive from pharmacological effects in multiple GI cellular compartments. Forskolin stimulates myocyte AC in addition to neuronal AC. NO promotes GI myocyte relaxation via PKG. PKG activation of K+ channels decreases Ca2+ entry, and PKG activation of myosin light chain phosphatase decreases Ca2+ sensitivity of the myocyte contractile apparatus (Khromov et al., 2006; Koh et al., 2001). While cAMP can activate transient https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=81 current in myocytes, prolonged PKA stimulation blocks K+ current, opens l‐type Ca2+ https://www.guidetopharmacology.org/GRAC/FamilyIntroductionForward?familyId=80, and thereby attenuates nitrergic IJPs (Hwang et al., 2008). The strengths of our current study include using multiple NO signalling, NO synthesis and PKA inhibitors to test forskolin relaxation with several mutant NOS mouse strains. Our approach allowed us to estimate that a substantial portion of forskolin relaxation is via nNOS S1412 phosphorylation. The twofold increase in forskolin IC50 due to nNOSS1412A mutation may reflect simultaneous cAMP elevation in ileal neurons (IJP inducing) and myocytes (IJP inhibitory at low to moderate cAMP levels). Additionally, because AC can activate CNS excitatory neurons (Zhang et al., 2008), perhaps it can also facilitate enteric excitatory pathways. AC can be activated in nitrergic neurons by https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=29s and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=9 that co‐localize with nNOS in myenteric nerve cells (M. T. Liu, Kuan, Wang, Hen, & Gershon, 2009; Nasser et al., 2006), suggesting a means of endogenous nNOS S1412 phospho‐regulation in the gut. In our studies, we used substance P to maintain reliable contractions during the concentration–response curve. Substance P stimulates guinea pig ileum NO synthesis and up‐regulates cAMP in astrocytoma cells (Fowler & Brannstrom, 1994; Garcia‐Villar, Dupuis, Martinolle, Fioramonti, & Bueno, 1996). Thus, substance P may influence cAMP‐dependent nNOS S1412 phosphorylation. However, basal nNOS S1412 phosphorylation in vehicle‐treated ileal rings is minimal despite substance P (D.D. Guerra et al., 2019), and we employed substance P in every treatment. Studies using inducible neuron‐ and smooth muscle‐specific PKA mutant mice, selective AC agonists and additional contractile stimulants could confirm compartment specific cAMP signalling. The interactions between cyclic nucleotides and their degrading https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=260 (PDEs) may also be cell‐specific. NO elevates cGMP that can inhibit cAMP‐specific https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1298 (D.D. Guerra, Bok, & Hurt, 2020; Murthy, Zhou, & Makhlouf, 2002). nNOS S1412 phosphorylation could therefore enhance cGMP attenuation of PDE3 activity in myocytes.
The functional phenotype of nNOSα KO ileum affirms a role for nNOS S1412 phosphorylation in facilitating forskolin relaxation. Classically, NO synthesis increases after neuronal depolarization elevates cytosolic Ca2+, which binds calmodulin (CaM) and stimulates nNOS (Thatte et al., 2009). The nNOSα KO mutation abolishes 90% of physiological nNOS activity by eliminating membrane‐associated nNOSα (Huang et al., 1993). Although nNOSα KO ileum was less sensitive than WT to forskolin, l‐NAME further attenuated nNOSα KO relaxation, indicating that forskolin still induces NOS activity in nNOSα KO ileum, probably via alternatively spliced nNOSβ (Huber et al., 1998; Hurt et al., 2006). Our data are consistent with S1412 phosphorylation of nNOSβ mediating some NOS‐dependent forskolin relaxation in nNOSα KO ileum. GI motility does not require eNOS in mice (Mang et al., 2002), but we found that eNOS ablation influences forskolin relaxation. While cAMP activates eNOS by phosphorylating S1179 in endothelial cells (Boo et al., 2002), nNOS is the primary locus of cAMP–NO synergy in GI relaxation. l‐NAME induces similar fold changes in forskolin IC50 for WT and eNOS KO ilea, and eNOS KO and nNOSS1412A mutations are non‐additive. Endogenous cAMP may promote an EPAC‐Akt‐eNOS pathway that contributes to ileal relaxation, but forskolin does not significantly enhance eNOS activity (Figure 5). Alternatively, the eNOS contribution to forskolin relaxation could be a procedural artefact because our ex vivo model lacks the potent NO scavenging of circulating red blood cells (Wennmalm, Benthin, & Petersson, 1992). Recent work suggests that red blood cells do not scavenge all eNOS‐derived NO, particularly in microvasculature (Cortese‐Krott & Kelm, 2014). Like eNOS, iNOS is probably not involved in forskolin ileal relaxation. 1400W has greater affinity for iNOS than nNOS (Boer et al., 2000), but NANT and 1400W shifted the forskolin IC50 to the same extent, and rat small intestine does not express iNOS in the absence of inflammation (Cui et al., 1997).
Our findings are relevant to the pathophysiology of GI motility disorders. Slow transit constipation, gastroparesis and oesophageal achalasia are associated with diminished or excess nitrergic neurotransmission (Grover et al., 2011; X. Liu, Liu, Xu, Liu, & Sun, 2015; Shteyer et al., 2015). Although non‐selective AC activation (e.g. forskolin) may not be clinically useful, selective modulation of enteric neuronal AC could be a therapeutic strategy to enhance nitrergic relaxation. Human myenteric neurons express AC‐coupled A2 https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=3 (Christofi, 2008). Purinergic IJPs precede nitrergic IJPs during GI smooth muscle relaxation (Christofi, 2008; Keef et al., 2013), and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=19‐dependent colon relaxation requires NO synthesis (Blandizzi et al., 2006). Selective A2 agonists may modulate nNOS S1412 phosphorylation. Another potential AC‐activator is https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2267 (PACAP), which co‐localizes with nNOS in myenteric neurons and regulates nNOS membrane association (Hannibal, Ekblad, Mulder, Sundler, & Fahrenkrug, 1998; Ohnishi et al., 2008).
In summary, we present pharmacological and genetic evidence that enteric neuronal cAMP and nNOS synergistically regulate GI muscle tone. PKA mediates a component of forskolin relaxation via phosphorylation of nNOS S1412, which does not preclude a role for Akt in nNOS‐mediated GI relaxation by other stimuli such as neuronal depolarization (D.D. Guerra et al., 2019). GI motility disorders often feature altered nNOS activity (Grover et al., 2011; X. Liu et al., 2015; Shteyer et al., 2015), and rodent models of GI dysmotility feature PKA hyperactivity and hypoactivity (Howe et al., 2006; Yu et al., 2017). Thus, selective modulation of AC‐coupled receptors on enteric nitrergic neurons may be clinically useful. Along with the GI tract and the cavernosal nerve (Hurt et al., 2012), PKA phosphorylation of nNOS S1412 could be important in other areas of neuronal communication, such as regulation of synaptic strength (Bhattacharyya, Biou, Xu, Schluter, & Malenka, 2009; Colledge et al., 2000). The role of PKA in central and peripheral nitrergic neurotransmission deserves renewed attention.
AUTHOR CONTRIBUTIONS
D.D.G., R.A.L., and K.J.H. designed the research. D.D.G. and R.B. conducted the experiments. All authors analysed the data. D.D.G. and K.J.H. wrote the manuscript.
CONFLICT OF INTEREST
The authors have no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14208, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Table S1: Goodness‐of‐fit tests for sigmoidal regressions. A replicates test with Prism indicates that individual data points for FSK dose‐dependent relaxation do not significantly deviate from regression curves.
Figure S1: PKA and Akt facilitate nitrergic FSK relaxation of ileum. Left: Representative force‐time plots. Right: Summary regression statistics. Hill coefficients are bold, and 95% confidence intervals are in parentheses. A. cPTIO, ODQ and Rp‐cGMPs (NO signaling inhibitors) decrease sensitivity to FSK‐dependent relaxation. B. H‐89, Myr‐PKI, and Rp‐cAMPs (PKA inhibitors) and MK‐2206 (Akt inhibitor) decrease sensitivity to FSK‐dependent relaxation. C. Under NOS blockade with L‐NAME, Akt and PKA inhibitors do not affect FSK‐dependent ileal relaxation. L‐Arg (NOS substrate) partially rescues L‐NAME attenuation of FSK relaxation. D. EPAC and PKA inhibition additively attenuate FSK relaxation, and adenylate cyclase inhibition attenuates relaxation as much as combined PKA and EPAC inhibition. Scale bars: 0.08 g (A‐C) and 0.12 g (D) × 120 sec.
Figure S2: FSK relaxes WT ileal rings. A. WT ileal rings relax when treated with 1 μM FSK, but not with 1 μM 1,9‐dideoxyforskolin (dFSK). B. Cumulative FSK relaxes WT ileal rings, but cumulative DMSO vehicle does not. N: ileal rings. *: p < 0.05 vs dFSK (A) or vs. DMSO vehicle at each [FSK] or [DMSO] (B) by Mann–Whitney tests.
Figure S3: The nNOSS1412A and nNOSα KO mutations partially block nitrergic FSK relaxation of ileum. Left: Representative force‐time plots. Right: Summary regression statistics. Hill coefficients are bold, and 95% confidence intervals are in parentheses. A. FSK relaxation is reduced for nNOSS1412A and nNOSα KO ileal rings compared with WT ileal rings. B. In the presence of the NOS inhibitor L‐NAME, WT, nNOSS1412A, and nNOSα KO ilea are equally sensitive to FSK relaxation. C. FSK relaxation of nNOSS1412A ilea is sensitive to MK (Akt inhibitor), but not to H‐89 (PKA inhibitor). C. Under NOS blockade with L‐NAME, Akt and PKA inhibitors do not affect FSK‐dependent relaxation of nNOSS1412A ilea. Scale bars: 0.15 g × 120 sec.
Figure S4: NO synthesis and nNOS Serine‐1412 facilitate ileal relaxation. A. L‐ NAME (1 mM) and the nNOSS1412A mutation curtail ileal relaxation induced by Sp‐cAMPs (25 μM). B. The nNOSS1412A mutation attenuates L‐NAME enhancement of basal ileal tone. Subsequent substance P (SP) treatment abolishes differences in tensile force caused by L‐NAME. C, D. Quantification of A, B. *: p < 0.05 by Dunn's post tests after Kruskal‐Wallis. n.s.: not significant. Veh: water. Scale bars: 0.13 g × 120 sec (A) and 0.15 g × 60 sec (B).
Figure S5: nNOS facilitates most nitrergic FSK relaxation. Left: Representative force‐ time plots. Right: Summary regression statistics. Hill coefficients are bold, and 95% confidence intervals are in parentheses. A. L‐NAME (NOS inhibitor) and H‐89 (PKA inhibitor) reduce eNOS KO ileal sensitivity to FSK relaxation, but MK (Akt inhibitor) has no effect on eNOS KO relaxation. B. Ilea from eNOS KO nNOSS1412A double homozygotes (Dob. Hom.) are as sensitive to FSK relaxation as eNOS KO and nNOSS1412A single mutants. C. TTX (neuronal depolarization inhibitor), NANT (specific nNOS inhibitor), and 1400 W (selective iNOS/nNOS inhibitor) attenuate FSK relaxation of WT ileal rings. Scale bars: 0.08 g (A‐B) and 0.18 g (C) × 120 sec.
Figure S6: WT and eNOS KO nNOSS1412A ilea exhibit similar non‐nitrergic FSK relaxation. A. FSK IC50 values are the same for WT and eNOS KO and nNOSS1412A double mutants treated with the NOS inhibitor L‐NAME. B. Representative force‐time plots and summary regression statistics. §: Dataset from Figure S1D for comparison. Scale bars: 0.12 g × 120 sec.
Figure S7: Contributions of NO synthesis and PKA to absolute (total) ileal relaxation. A. Graphical representation of percent relaxation due to NO synthesis: the absolute difference in percent relaxation with vehicle and L‐NAME at each [FSK]. B. Percent relaxation for WT, eNOS KO, and nNOSS1412A ileal rings treated with vehicle, L‐NAME (NO synthesis inhibitor), NANT (nNOS inhibitor), or H‐89 (PKA inhibitor). The first two columns (WT treated with vehicle or L‐NAME) are depicted in A. C. Total percent relaxation of WT, eNOS KO, or nNOSS1412A ileal rings due to NO synthesis, nNOS, or PKA. The first column (WT relaxation due to NO synthesis) is depicted in A. The first row of each column includes the formula to calculate percent relaxation.
Figure S8: Relative ileal relaxation by FSK due to NO synthesis or PKA. This method calculates the proportion of relaxation at a particular [FSK] due to NO synthesis or PKA, relative to control relaxation at the same [FSK]. Thus, nitrergic relaxation is the difference in percent relaxation with vehicle and L‐NAME, divided by percent relaxation with vehicle, at each [FSK]. A. Graphical depiction. B. Percent relaxation for WT, eNOS KO, and nNOSS1412A ileal rings treated with vehicle, L‐NAME, NANT, or H‐89 during FSK relaxation. C. Relative percent relaxation of WT, eNOS KO, or nNOSS1412A ileal rings due to NO synthesis, nNOS, or PKA. The first row of each column includes the formula used to calculate percent relaxation values for that column.
ACKNOWLEDGEMENTS
The authors thank John Riggs for manuscript input. This work was supported by an NICHD T32 training grant (5T32HD007186‐37, DDG) and a Society for Maternal Fetal Medicine/American Association of Obstetricians and Gynecologists Foundation Scholar Award (K.J.H.).
Guerra DD, Bok R, Lorca RA, Hurt KJ. Protein kinase A facilitates relaxation of mouse ileum via phosphorylation of neuronal nitric oxide synthase. Br J Pharmacol. 2020;177:2765–2778. 10.1111/bph.15001
REFERENCES
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … Davies, J. A. (2019). The concise guide to pharmacology 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176(Suppl 1), S21–s141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … Davies, J. A. (2019). The concise guide to pharmacology 2019/20: Enzymes. British Journal of Pharmacology, 176(Suppl 1), S297–s396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , … Davies, J. A. (2019). The concise guide to pharmacology 2019/20: Introduction and other protein targets. British Journal of Pharmacology, 176(Suppl 1), S1–s20. 10.1111/bph.14747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , … Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology. British Journal of Pharmacology, 175(3), 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allerston, C. K. , von Delft, F. , & Gileadi, O. (2013). Crystal structures of the catalytic domain of human soluble guanylate cyclase. PLoS ONE, 8(3), 1–9. e57644 10.1371/journal.pone.0057644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya, S. , Biou, V. , Xu, W. , Schluter, O. , & Malenka, R. C. (2009). A critical role for PSD‐95/AKAP interactions in endocytosis of synaptic AMPA receptors. Nature Neuroscience, 12(2), 172–181. 10.1038/nn.2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandizzi, C. , Del Tacca, M. , Antonioli, L. , Ghisu, N. , Fornai, M. , & Colucci, R. (2006). A2a receptors mediate inhibitory effects of adenosine on colonic motility in the presence of experimental colitis. Inflammatory Bowel Diseases, 12(2), 117–122. 10.1097/01.MIB.0000198535.13822.a9 [DOI] [PubMed] [Google Scholar]
- Boer, R. , Ulrich, W.‐R. , Klein, T. , Mirau, B. , Haas, S. , & Baur, I. (2000). The inhibitory potency and selectivity of arginine substrate site nitric‐oxide synthase inhibitors is solely determined by their affinity toward the different isoenzymes. Molecular Pharmacology, 58(5), 1026–1034. 10.1124/mol.58.5.1026 [DOI] [PubMed] [Google Scholar]
- Bonnevier, J. , Fässler, R. , Somlyo, A. P. , Somlyo, A. V. , & Arner, A. (2004). Modulation of Ca2+ sensitivity by cyclic nucleotides in smooth muscle from protein kinase G‐deficient mice. The Journal of Biological Chemistry, 279(7), 5146–5151. 10.1074/jbc.M306532200 [DOI] [PubMed] [Google Scholar]
- Boo, Y. C. , Sorescu, G. , Boyd, N. , Shiojima, I. , Walsh, K. , Du, J. , & Jo, H. (2002). Shear stress stimulates phosphorylation of endothelial nitric‐oxide synthase at Ser1179 by Akt‐independent mechanisms: role of protein kinase A. The Journal of Biological Chemistry, 277(5), 3388–3396. 10.1074/jbc.M108789200 [DOI] [PubMed] [Google Scholar]
- Briejer, M. R. , Akkermans, L. M. , Meulemans, A. L. , Lefebvre, R. A. , & Schuurkes, J. A. (1993). Substance P‐induced contractions of the guinea‐pig proximal colon through stimulation of post‐junctional tachykinin NK1 receptors. European Journal of Pharmacology, 250(1), 181–183. 10.1016/0014-2999(93)90640-4 [DOI] [PubMed] [Google Scholar]
- Christofi, F. L. (2008). Purinergic receptors and gastrointestinal secretomotor function. Purinergic Signalling, 4(3), 213–236. 10.1007/s11302-008-9104-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge, M. , Dean, R. A. , Scott, G. K. , Langeberg, L. K. , Huganir, R. L. , & Scott, J. D. (2000). Targeting of PKA to glutamate receptors through a MAGUK‐AKAP complex. Neuron, 27(1), 107–119. 10.1016/s0896-6273(00)00013-1 [DOI] [PubMed] [Google Scholar]
- Cortese‐Krott, M. M. , & Kelm, M. (2014). Endothelial nitric oxide synthase in red blood cells: key to a new erythrocrine function? Redox Biology, 2, 251–258. 10.1016/j.redox.2013.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, L. , Takagi, Y. , Wasa, M. , Iiboshi, Y. , Khan, J. , Nezu, R. , & Okada, A. (1997). Induction of nitric oxide synthase in rat intestine by interleukin‐1α may explain diarrhea associated with zinc deficiency. The Journal of Nutrition, 127(9), 1729–1736. 10.1093/jn/127.9.1729 [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175(7), 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denisova, O. V. , Söderholm, S. , Virtanen, S. , Von Schantz, C. , Bychkov, D. , Vashchinkina, E. , … Kainov, D. E. (2014). Akt inhibitor MK2206 prevents influenza pH1N1 virus infection in vitro. Antimicrobial Agents and Chemotherapy, 58(7), 3689–3696. 10.1128/aac.02798-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler, S. , Fleming, I. , Fisslthaler, B. , Hermann, C. , Busse, R. , & Zeiher, A. M. (1999). Activation of nitric oxide synthase in endothelial cells by Akt‐dependent phosphorylation. Nature, 399(6736), 601–605. 10.1038/21224 [DOI] [PubMed] [Google Scholar]
- Ekblad, E. , & Sundler, F. (1997). Motor responses in rat ileum evoked by nitric oxide donors vs. field stimulation: Modulation by pituitary adenylate cyclase‐activating peptide, forskolin and guanylate cyclase inhibitors. J Pharmacol Exper Therap, 283(1), 23–28. [PubMed] [Google Scholar]
- Fowler, C. J. , & Brannstrom, G. (1994). Substance P enhances forskolin‐stimulated cyclic AMP production in human UC11MG astrocytoma cells. Methods and Findings in Experimental and Clinical Pharmacology, 16(1), 21–28. [PubMed] [Google Scholar]
- Fulton, D. , Gratton, J. P. , McCabe, T. J. , Fontana, J. , Fujio, Y. , Walsh, K. , … Sessa, W. C. (1999). Regulation of endothelium‐derived nitric oxide production by the protein kinase Akt. Nature, 399(6736), 597–601. 10.1038/21218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Morales, V. , Luaces‐Regueira, M. , & Campos‐Toimil, M. (2017). The cAMP effectors PKA and Epac activate endothelial NO synthase through PI3K/Akt pathway in human endothelial cells. Biochemical Pharmacology, 145, 94–101. 10.1016/j.bcp.2017.09.004 [DOI] [PubMed] [Google Scholar]
- Garcia‐Villar, R. , Dupuis, C. , Martinolle, J. P. , Fioramonti, J. , & Bueno, L. (1996). Functional evidence for NO‐synthase activation by substance P through a mechanism not involving classical tachykinin receptors in guinea‐pig ileum in vitro. British Journal of Pharmacology, 118(5), 1253–1261. 10.1111/j.1476-5381.1996.tb15531.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover, M. , Farrugia, G. , Lurken, M. S. , Bernard, C. E. , Faussone‐Pellegrini, M. S. , Smyrk, T. C. , … Consortium, N. G. C. R. (2011). Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology, 140(5), 1575–1585e1578. 10.1053/j.gastro.2011.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra, D. D. , Bok, R. , & Hurt, K. J. (2020). Cyclic nucleotides and myometrial contractility. Current Opinion in Physiology, 13, 102–107. 10.1016/j.cophys.2019.10.014 [DOI] [Google Scholar]
- Guerra, D. D. , Bok, R. , Vyas, V. , Orlicky, D. , Lorca, R. , & Hurt, K. (2019). Akt phosphorylation of neuronal nitric oxide synthase regulates gastrointestinal motility. Proceedings of the National Academy of Sciences of the United States of America, 116(35), 17541–17546. 10.1073/pnas.1905902116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra, D. D. , & Hurt, K. J. (2019). Gasotransmitters in pregnancy: from conception to uterine involution. Biology of Reproduction, 101(1), 4–25. 10.1093/biolre/ioz038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hah, J. M. , Roman, L. J. , Martasek, P. , & Silverman, R. B. (2001). Reduced amide bond peptidomimetics. (4S)‐N‐(4‐amino‐5‐[aminoakyl]aminopentyl)‐N'‐nitroguanidines, potent and highly selective inhibitors of neuronal nitric oxide synthase. Journal of Medicinal Chemistry, 44(16), 2667–2670. 10.1021/jm0101491 [DOI] [PubMed] [Google Scholar]
- Hannibal, J. , Ekblad, E. , Mulder, H. , Sundler, F. , & Fahrenkrug, J. (1998). Pituitary adenylate cyclase activating polypeptide (PACAP) in the gastrointestinal tract of the rat: Distribution and effects of capsaicin or denervation. Cell and Tissue Research, 291(1), 65–79. 10.1007/s004410050980 [DOI] [PubMed] [Google Scholar]
- Hoshi, T. , Garber, S. S. , & Aldrich, R. W. (1988). Effect of forskolin on voltage‐gated K+ channels is independent of adenylate cyclase activation. Science, 240(4859), 1652–1655. 10.1126/science.2454506 [DOI] [PubMed] [Google Scholar]
- Howe, D. G. , Clarke, C. M. , Yan, H. , Willis, B. S. , Schneider, D. A. , McKnight, G. S. , & Kapur, R. P. (2006). Inhibition of protein kinase A in murine enteric neurons causes lethal intestinal pseudo‐obstruction. Journal of Neurobiology, 66(3), 256–272. 10.1002/neu.20217 [DOI] [PubMed] [Google Scholar]
- Huang, P. L. , Dawson, T. M. , Bredt, D. S. , Snyder, S. H. , & Fishman, M. C. (1993). Targeted disruption of the neuronal nitric oxide synthase gene. Cell, 75(7), 1273–1286. 10.1016/0092-8674(93)90615-W [DOI] [PubMed] [Google Scholar]
- Huber, A. , Saur, D. , Kurjak, M. , Schusdziarra, V. , & Allescher, H. D. (1998). Characterization and splice variants of neuronal nitric oxide synthase in rat small intestine. The American Journal of Physiology, 275(5), G1146–G1156. 10.1152/ajpgi.1998.275.5.G1146 [DOI] [PubMed] [Google Scholar]
- Hurt, K. J. , Musicki, B. , Palese, M. A. , Crone, J. K. , Becker, R. E. , Moriarity, J. L. , … Burnett, A. L. (2002). Akt‐dependent phosphorylation of endothelial nitric‐oxide synthase mediates penile erection. Proceedings of the National Academy of Sciences of the United States of America, 99(6), 4061–4066. 10.1073/pnas.052712499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt, K. J. , Sezen, S. F. , Champion, H. C. , Crone, J. K. , Palese, M. A. , Huang, P. L. , … Burnett, A. L. (2006). Alternatively spliced neuronal nitric oxide synthase mediates penile erection. Proceedings of the National Academy of Sciences of the United States of America, 103(9), 3440–3443. 10.1073/pnas.0511326103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt, K. J. , Sezen, S. F. , Lagoda, G. F. , Musicki, B. , Rameau, G. A. , Snyder, S. H. , & Burnett, A. L. (2012). Cyclic AMP‐dependent phosphorylation of neuronal nitric oxide synthase mediates penile erection. Proceedings of the National Academy of Sciences of the United States of America, 109(41), 16624–16629. 10.1073/pnas.1213790109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, S. J. , O'Kane, N. , Singer, C. , Ward, S. M. , Sanders, K. M. , & Koh, S. D. (2008). Block of inhibitory junction potentials and TREK‐1 channels in murine colon by Ca2+ store‐active drugs. The Journal of Physiology, 586(4), 1169–1184. 10.1113/jphysiol.2007.148718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idrizaj, E. , Garella, R. , Francini, F. , Squecco, R. , & Baccari, M. C. (2018). Relaxin influences ileal muscular activity through a dual signaling pathway in mice. World Journal of Gastroenterology, 24(8), 882–893. 10.3748/wjg.v24.i8.882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya, A. I. , Onaran, H. O. , Özcan, G. , Ambrosio, C. , Costa, T. , Balli, S. , & Uğur, Ö. (2012). Cell contact‐dependent functional selectivity of β2‐adrenergic receptor ligands in stimulating cAMP accumulation and extracellular signal‐regulated kinase phosphorylation. The Journal of Biological Chemistry, 287(9), 6362–6374. 10.1074/jbc.M111.301820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keef, K. D. , Saxton, S. N. , McDowall, R. A. , Kaminski, R. E. , Duffy, A. M. , & Cobine, C. A. (2013). Functional role of vasoactive intestinal polypeptide in inhibitory motor innervation in the mouse internal anal sphincter. The Journal of Physiology, 591(6), 1489–1506. 10.1113/jphysiol.2012.247684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khromov, A. S. , Wang, H. , Choudhury, N. , McDuffie, M. , Herring, B. P. , Nakamoto, R. , … Somlyo, A. V. (2006). Smooth muscle of telokin‐deficient mice exhibits increased sensitivity to Ca2+ and decreased cGMP‐induced relaxation. Proceedings of the National Academy of Sciences of the United States of America, 103(7), 2440–2445. 10.1073/pnas.0508566103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh, S. D. , Monaghan, K. , Sergeant, G. P. , Ro, S. , Walker, R. L. , Sanders, K. M. , & Horowitz, B. (2001). TREK‐1 regulation by nitric oxide and cGMP‐dependent protein kinase. An essential role in smooth muscle inhibitory neurotransmission. The Journal of Biological Chemistry, 276(47), 44338–44346. 10.1074/jbc.M108125200 [DOI] [PubMed] [Google Scholar]
- Koh, S. D. , Sanders, K. M. , & Carl, A. (1996). Regulation of smooth muscle delayed rectifier K+ channels by protein kinase A. Pflügers Archiv, 432(3), 401–412. 10.1007/s004240050151 [DOI] [PubMed] [Google Scholar]
- Lefebvre, R. A. , Smits, G. J. , & Timmermans, J. P. (1995). Study of NO and VIP as non‐adrenergic non‐cholinergic neurotransmitters in the pig gastric fundus. British Journal of Pharmacology, 116(3), 2017–2026. 10.1111/j.1476-5381.1995.tb16406.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, M. T. , Kuan, Y. H. , Wang, J. , Hen, R. , & Gershon, M. D. (2009). 5‐HT4 receptor‐mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. The Journal of Neuroscience, 29(31), 9683–9699. 10.1523/jneurosci.1145-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Liu, S. , Xu, Y. , Liu, X. , & Sun, D. (2015). Bone morphogenetic protein 2 regulates the differentiation of nitrergic enteric neurons by modulating Smad1 signaling in slow transit constipation. Molecular Medicine Reports, 12(5), 6547–6554. 10.3892/mmr.2015.4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mang, C. F. , Truempler, S. , Erbelding, D. , & Kilbinger, H. (2002). Modulation by NO of acetylcholine release in the ileum of wild‐type and NOS gene knockout mice. American Journal of Physiology. Gastrointestinal and Liver Physiology, 283(5), G1132–G1138. 10.1152/ajpgi.00192.2002 [DOI] [PubMed] [Google Scholar]
- May, A. T. , Crowe, M. S. , Blakeney, B. A. , Mahavadi, S. , Wang, H. , Grider, J. R. , & Murthy, K. S. (2019). Identification of expression and function of the glucagon‐like peptide‐1 receptor in colonic smooth muscle. Peptides, 112, 48–55. 10.1016/j.peptides.2018.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo, W. , Michel, M. C. , Lee, X. W. , Kaumann, A. J. , & Molenaar, P. (2017). The β3‐adrenoceptor agonist mirabegron increases human atrial force through β1‐adrenoceptors: an indirect mechanism? British Journal of Pharmacology, 174(16), 2706–2715. 10.1111/bph.13897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky, H. J. , & Michel, M. C. (2018). Commentary on the BJP's new statistical reporting guidelines. British Journal of Pharmacology, 175(18), 3636–3637. 10.1111/bph.14441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy, K. S. , Zhou, H. , & Makhlouf, G. M. (2002). PKA‐dependent activation of PDE3A and PDE4 and inhibition of adenylyl cyclase V/VI in smooth muscle. American Journal of Physiology. Cell Physiology, 282(3), C508–C517. 10.1152/ajpcell.00373.2001 [DOI] [PubMed] [Google Scholar]
- Nasser, Y. , Ho, W. , & Sharkey, K. A. (2006). Distribution of adrenergic receptors in the enteric nervous system of the guinea pig, mouse, and rat. The Journal of Comparative Neurology, 495(5), 529–553. 10.1002/cne.20898 [DOI] [PubMed] [Google Scholar]
- Nemeth, P. R. , Palmer, J. M. , Wood, J. D. , & Zafirov, D. H. (1986). Effects of forskolin on electrical behaviour of myenteric neurones in guinea‐pig small intestine. The Journal of Physiology, 376, 439–450. 10.1113/jphysiol.1986.sp016162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi, T. , Okuda‐Ashitaka, E. , Matsumura, S. , Katano, T. , Nishizawa, M. , & Ito, S. (2008). Characterization of signaling pathway for the translocation of neuronal nitric oxide synthase to the plasma membrane by PACAP. Journal of Neurochemistry, 105(6), 2271–2285. 10.1111/j.1471-4159.2008.05325.x [DOI] [PubMed] [Google Scholar]
- Paisley, K. , & Martin, W. (1996). Blockade of nitrergic transmission by hydroquinone, hydroxocobalamin and carboxy‐PTIO in bovine retractor penis: Role of superoxide anion. British Journal of Pharmacology, 117(8), 1633–1638. 10.1111/j.1476-5381.1996.tb15333.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatka, K. , Serfozo, Z. , Vereb, Z. , Hargitay, Z. , Lontay, B. , Erdodi, F. , … Altorjay, I. (2005). Changes in the expression and distribution of the inducible and endothelial nitric oxide synthase in mucosal biopsy specimens of inflammatory bowel disease. Scandinavian Journal of Gastroenterology, 40(6), 670–680. 10.1080/00365520510015539 [DOI] [PubMed] [Google Scholar]
- Rameau, G. A. , Tukey, D. S. , Garcin‐Hosfield, E. D. , Titcombe, R. F. , Misra, C. , Khatri, L. , … Ziff, E. B. (2007). Biphasic coupling of neuronal nitric oxide synthase phosphorylation to the NMDA receptor regulates AMPA receptor trafficking and neuronal cell death. The Journal of Neuroscience, 27(13), 3445–3455. 10.1523/jneurosci.4799-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebaugh, J. L. (2011). Guidelines for accurate EC50/IC50 estimation. Pharmaceutical Statistics, 10(2), 128–134. 10.1002/pst.426 [DOI] [PubMed] [Google Scholar]
- Shteyer, E. , Edvardson, S. , Wynia‐Smith, S. L. , Pierri, C. L. , Zangen, T. , Hashavya, S. , … Smith, B. C. (2015). Truncating mutation in the nitric oxide synthase 1 gene is associated with infantile achalasia. Gastroenterology, 148(3), 533–536e534. 10.1053/j.gastro.2014.11.044 [DOI] [PubMed] [Google Scholar]
- Shuttleworth, C. W. , Sanders, K. M. , & Keef, K. D. (1993). Inhibition of nitric oxide synthesis reveals non‐cholinergic excitatory neurotransmission in the canine proximal colon. British Journal of Pharmacology, 109(3), 739–747. 10.1111/j.1476-5381.1993.tb13636.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, T. K. , Ward, S. M. , Zhang, L. , Buxton, I. L. , Gerthoffer, W. T. , Sanders, K. M. , & Keef, K. D. (1993). Beta‐adrenergic inhibition of electrical and mechanical activity in canine colon: Role of cAMP. The American Journal of Physiology, 264(4 Pt 1), G708–G717. 10.1152/ajpgi.1993.264.4.G708 [DOI] [PubMed] [Google Scholar]
- Southan, C. , Sharman, J. L. , Benson, H. E. , Faccenda, E. , Pawson, A. J. , Alexander, S. P. , … Davies, J. A. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: Towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Research, 44(D1), D1054–D1068. 10.1093/nar/gkv1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer, N. J. , Hibberd, T. J. , Travis, L. , Wiklendt, L. , Costa, M. , Hu, H. , … Sorensen, J. (2018). Identification of a rhythmic firing pattern in the enteric nervous system that generates rhythmic electrical activity in smooth muscle. The Journal of Neuroscience, 38(24), 5507–5522. 10.1523/jneurosci.3489-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima, T. , Murata, T. , Aritake, K. , Urade, Y. , Michishita, M. , Matsuoka, T. , … Hori, M. (2012). EP2 and EP4 receptors on muscularis resident macrophages mediate LPS‐induced intestinal dysmotility via iNOS upregulation through cAMP/ERK signals. American Journal of Physiology. Gastrointestinal and Liver Physiology, 302(5), G524–G534. 10.1152/ajpgi.00264.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatte, H. S. , He, X. D. , & Goyal, R. K. (2009). Imaging of nitric oxide in nitrergic neuromuscular neurotransmission in the gut. PLoS ONE, 4(4), 1–7. e4990 10.1371/journal.pone.0004990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toque, H. A. , Nunes, K. P. , Yao, L. , Xu, Z. , Kondrikov, D. , Su, Y. , … Caldwell, R. W. (2013). Akita spontaneously type 1 diabetic mice exhibit elevated vascular arginase and impaired vascular endothelial and nitrergic function. PLoS ONE, 8(8), 1–14. e72277 10.1371/journal.pone.0072277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckert, S. , Hedlund, P. , Waldkirch, E. , Sohn, M. , Jonas, U. , Andersson, K. E. , & Stief, C. G. (2004). Interactions between cGMP‐ and cAMP‐pathways are involved in the regulation of penile smooth muscle tone. World Journal of Urology, 22(4), 261–266. 10.1007/s00345-003-0394-4 [DOI] [PubMed] [Google Scholar]
- Wennmalm, A. , Benthin, G. , & Petersson, A. S. (1992). Dependence of the metabolism of nitric oxide (NO) in healthy human whole blood on the oxygenation of its red cell haemoglobin. British Journal of Pharmacology, 106(3), 507–508. 10.1111/j.1476-5381.1992.tb14365.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, T. , Wang, Y. , Qian, D. , Sun, X. , Tang, Y. , Shen, X. , & Lin, L. (2017). Advanced glycation end products impair Ca2+ mobilization and sensitization in colonic smooth muscle cells via the CAMP/PKA pathway. Cellular Physiology and Biochemistry, 43(4), 1571–1587. 10.1159/000482005 [DOI] [PubMed] [Google Scholar]
- Zavala‐Mendoza, D. , Grasa, L. , Zavala‐Sánchez, M. , Pérez‐Gutiérrez, S. , & Murillo, M. (2016). Antispasmodic effects and action mechanism of essential oil of chrysactinia mexicana A. Gray on rabbit ileum. Molecules, 21(6), 1–12. 10.3390/molecules21060783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M. , Moon, C. , Chan, G. C. , Yang, L. , Zheng, F. , Conti, A. C. , … Wang, H. (2008). Ca‐stimulated type 8 adenylyl cyclase is required for rapid acquisition of novel spatial information and for working/episodic‐like memory. The Journal of Neuroscience, 28(18), 4736–4744. 10.1523/jneurosci.1177-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Goodness‐of‐fit tests for sigmoidal regressions. A replicates test with Prism indicates that individual data points for FSK dose‐dependent relaxation do not significantly deviate from regression curves.
Figure S1: PKA and Akt facilitate nitrergic FSK relaxation of ileum. Left: Representative force‐time plots. Right: Summary regression statistics. Hill coefficients are bold, and 95% confidence intervals are in parentheses. A. cPTIO, ODQ and Rp‐cGMPs (NO signaling inhibitors) decrease sensitivity to FSK‐dependent relaxation. B. H‐89, Myr‐PKI, and Rp‐cAMPs (PKA inhibitors) and MK‐2206 (Akt inhibitor) decrease sensitivity to FSK‐dependent relaxation. C. Under NOS blockade with L‐NAME, Akt and PKA inhibitors do not affect FSK‐dependent ileal relaxation. L‐Arg (NOS substrate) partially rescues L‐NAME attenuation of FSK relaxation. D. EPAC and PKA inhibition additively attenuate FSK relaxation, and adenylate cyclase inhibition attenuates relaxation as much as combined PKA and EPAC inhibition. Scale bars: 0.08 g (A‐C) and 0.12 g (D) × 120 sec.
Figure S2: FSK relaxes WT ileal rings. A. WT ileal rings relax when treated with 1 μM FSK, but not with 1 μM 1,9‐dideoxyforskolin (dFSK). B. Cumulative FSK relaxes WT ileal rings, but cumulative DMSO vehicle does not. N: ileal rings. *: p < 0.05 vs dFSK (A) or vs. DMSO vehicle at each [FSK] or [DMSO] (B) by Mann–Whitney tests.
Figure S3: The nNOSS1412A and nNOSα KO mutations partially block nitrergic FSK relaxation of ileum. Left: Representative force‐time plots. Right: Summary regression statistics. Hill coefficients are bold, and 95% confidence intervals are in parentheses. A. FSK relaxation is reduced for nNOSS1412A and nNOSα KO ileal rings compared with WT ileal rings. B. In the presence of the NOS inhibitor L‐NAME, WT, nNOSS1412A, and nNOSα KO ilea are equally sensitive to FSK relaxation. C. FSK relaxation of nNOSS1412A ilea is sensitive to MK (Akt inhibitor), but not to H‐89 (PKA inhibitor). C. Under NOS blockade with L‐NAME, Akt and PKA inhibitors do not affect FSK‐dependent relaxation of nNOSS1412A ilea. Scale bars: 0.15 g × 120 sec.
Figure S4: NO synthesis and nNOS Serine‐1412 facilitate ileal relaxation. A. L‐ NAME (1 mM) and the nNOSS1412A mutation curtail ileal relaxation induced by Sp‐cAMPs (25 μM). B. The nNOSS1412A mutation attenuates L‐NAME enhancement of basal ileal tone. Subsequent substance P (SP) treatment abolishes differences in tensile force caused by L‐NAME. C, D. Quantification of A, B. *: p < 0.05 by Dunn's post tests after Kruskal‐Wallis. n.s.: not significant. Veh: water. Scale bars: 0.13 g × 120 sec (A) and 0.15 g × 60 sec (B).
Figure S5: nNOS facilitates most nitrergic FSK relaxation. Left: Representative force‐ time plots. Right: Summary regression statistics. Hill coefficients are bold, and 95% confidence intervals are in parentheses. A. L‐NAME (NOS inhibitor) and H‐89 (PKA inhibitor) reduce eNOS KO ileal sensitivity to FSK relaxation, but MK (Akt inhibitor) has no effect on eNOS KO relaxation. B. Ilea from eNOS KO nNOSS1412A double homozygotes (Dob. Hom.) are as sensitive to FSK relaxation as eNOS KO and nNOSS1412A single mutants. C. TTX (neuronal depolarization inhibitor), NANT (specific nNOS inhibitor), and 1400 W (selective iNOS/nNOS inhibitor) attenuate FSK relaxation of WT ileal rings. Scale bars: 0.08 g (A‐B) and 0.18 g (C) × 120 sec.
Figure S6: WT and eNOS KO nNOSS1412A ilea exhibit similar non‐nitrergic FSK relaxation. A. FSK IC50 values are the same for WT and eNOS KO and nNOSS1412A double mutants treated with the NOS inhibitor L‐NAME. B. Representative force‐time plots and summary regression statistics. §: Dataset from Figure S1D for comparison. Scale bars: 0.12 g × 120 sec.
Figure S7: Contributions of NO synthesis and PKA to absolute (total) ileal relaxation. A. Graphical representation of percent relaxation due to NO synthesis: the absolute difference in percent relaxation with vehicle and L‐NAME at each [FSK]. B. Percent relaxation for WT, eNOS KO, and nNOSS1412A ileal rings treated with vehicle, L‐NAME (NO synthesis inhibitor), NANT (nNOS inhibitor), or H‐89 (PKA inhibitor). The first two columns (WT treated with vehicle or L‐NAME) are depicted in A. C. Total percent relaxation of WT, eNOS KO, or nNOSS1412A ileal rings due to NO synthesis, nNOS, or PKA. The first column (WT relaxation due to NO synthesis) is depicted in A. The first row of each column includes the formula to calculate percent relaxation.
Figure S8: Relative ileal relaxation by FSK due to NO synthesis or PKA. This method calculates the proportion of relaxation at a particular [FSK] due to NO synthesis or PKA, relative to control relaxation at the same [FSK]. Thus, nitrergic relaxation is the difference in percent relaxation with vehicle and L‐NAME, divided by percent relaxation with vehicle, at each [FSK]. A. Graphical depiction. B. Percent relaxation for WT, eNOS KO, and nNOSS1412A ileal rings treated with vehicle, L‐NAME, NANT, or H‐89 during FSK relaxation. C. Relative percent relaxation of WT, eNOS KO, or nNOSS1412A ileal rings due to NO synthesis, nNOS, or PKA. The first row of each column includes the formula used to calculate percent relaxation values for that column.


