Abstract
Background and Purpose
Despite recent advances in understanding its pathophysiology, treatment of acute kidney injury (AKI) remains a major unmet medical need, and novel therapeutic strategies are needed. Cathelicidin‐related antimicrobial peptide (CRAMP) with immunomodulatory properties has an emerging role in various disease contexts. Here, we aimed to investigate the role of CRAMP and its underlying mechanisms in AKI.
Experimental Approach
The human homologue LL‐37 and CRAMP were measured in blood samples of AKI patients and in experimental AKI mice respectively. Experimental AKI was induced in wild‐type and CRAMP‐deficient (Cnlp −/−) mice by ischaemia/reperfusion (I/R). Therapeutic evaluation of CRAMP was performed with exogenous CRAMP (5 mg·kg−1, i.p.) treatment.
Key Results
Cathelicidin expression was inversely related to clinical signs in patients and down‐regulated in renal I/R‐induced injury in mice. Cnlp −/− mice exhibited exacerbated I/R‐induced renal dysfunction, aggravated inflammatory responses and apoptosis. Moreover, over‐activation of the NLRP3 inflammasome in Cnlp −/− mice was associated with I/R‐induced renal injury. Exogenous CRAMP treatment markedly attenuated I/R‐induced renal dysfunction, inflammatory response and apoptosis, correlated with modulation of immune cell infiltration and phenotype. Consistent with Cnlp −/− mouse data, CRAMP administration suppressed renal I/R‐induced NLRP3 inflammasome activation, and its renal protective effects were mimicked by a specific NLRP3 inhibitor CY‐09. The reno‐protective and NLRP3 inhibitory effects of CRAMP required the EGF receptor.
Conclusion and Implications
Our results suggest that CRAMP acts as a novel immunomodulatory mediator of AKI and modulation of CRAMP may represent a potential therapeutic strategy.
Abbreviations
- AG1478
inhibitor of EGFR
- AKI
acute kidney injury
- AMP
antimicrobial peptide
- Cnlp−/−
CRAMP deficient
- CRAMP
mouse cathelicidin‐related antimicrobial peptide
- E‐cadherin
epithelial cadherin
- I/R
ischaemia/reperfusion
- LL‐37
human cathelicidin
- MPO
myeloperoxidase
- NETs
neutrophil extracellular traps
- NLRP3
nucleotide‐binding domain leucine‐rich repeat containing family, pyrin domain containing 3
- PBS
phosphate buffer saline
- RT‐qPCR
real‐time quantitative PCR
- TUNEL
terminal deoxyribonucleotide transferase (TdT)‐mediated dUTP nick‐end labelling
- WT
wild type
What is already known
Effective treatments for acute kidney injury remain a major unmet medical need.
The cathelicidin‐related antimicrobial peptide CRAMP exhibits host‐defence activities in infections and inflammatory conditions.
What this study adds
In mice, treatment with exogenous CRAMP protects against experimental ischaemia/reperfusion‐induced acute kidney injury.
Such protection involves inhibition of NLRP3 inflammasomes, mediated by EGF receptors.
What is the clinical significance
Modulation of CRAMP represents a novel potential therapeutic strategy for acute kidney injury.
1. INTRODUCTION
Acute kidney injury (AKI) is a globally common clinical problem associated with high morbidity, mortality, and clinical costs (Zuk & Bonventre, 2016). Its pathophysiology is multifaceted and involves inflammation, tubular injury, and vascular damage (Togel & Westenfelder, 2014). Ischaemia/reperfusion (I/R) is a common cause of AKI, characterized by injury to tubular epithelial cells and vascular endothelium, and robust inflammatory responses with immune cell infiltration and cytokine release, as well as cellular apoptosis in kidneys (Raup‐Konsavage et al., 2018). However, no effective drug has been approved for treating AKI patients.
Cathelicidins are a family of antimicrobial peptides (AMPs) expressed at epithelial linings and by immune cells under steady‐state or inflamed conditions (Hilchie, Wuerth, & Hancock, 2013). A single cathelicidin AMP is found in the human and rodents, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5527 and cathelicidin‐related antimicrobial peptide (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6266) respectively. As effector host defence molecules that possess broad‐spectrum antimicrobial and multifaceted immunomodulatory activities, cathelicidins have important roles in not only infection but also inflammation and related conditions (Hancock, Haney, & Gill, 2016). We have earlier demonstrated that CRAMP exerts positive immuno‐regulatory effect on macrophages and conventional dendritic cells and thus plays a key role in maintenance of pancreatic immune homeostasis (Sun, Furio, et al., 2015). In addition, ours and other studies have shown that CRAMP directly attenuates stimuli‐triggered inflammatory responses in various cell types by directly interacting with bacterial endotoxin, activating https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=302&familyType=CATALYTICRECEPTOR, or affecting intracellular inflammatory signalling (Hilchie et al., 2013; Mookherjee et al., 2006; Sun et al., 2016). Further, we also demonstrated that CRAMP could prevent cellular apoptosis by modulating apoptotic pathways and associated proteins (Bei et al., 2019; Sun et al., 2016).
In kidney‐related diseases, earlier studies have focused on the role of CRAMP on infectious conditions and largely described its antimicrobial properties (Becknell, Schwaderer, Hains, & Spencer, 2015; Chromek, 2015; Chromek et al., 2006). For example, AMPs have been involved in urinary tract infections including pyelonephritis (Becknell et al., 2015). Cathelicidin from epithelial cells of the urinary tract is thought to afford initial protective effects against urinary tract infection by defending against uropathogens via antimicrobial mechanisms (Chromek, 2015; Chromek et al., 2006). However, the role of CRAMP on renal I/R injury remains unknown. Considering the role of CRAMP in modulating diverse cellular processes and inflammatory responses, we postulated that CRAMP might play an important role in renal I/R injury. Thus, here, we investigated the role and underlying mechanism of CRAMP in renal I/R injury with CRAMP‐deficient (Cnlp −/−) and wild‐type (WT) mice and assessed the protective effects of exogenous CRAMP against I/R‐induced AKI in mice.
2. METHODS
2.1. Animals
All animal care and experimental protocols complied with guidelines of, and were approved by, the Animal Ethics Committee of Jiangnan University (JN. No20161018‐20161026[72]). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology. Male C57BL/6J (Su Pu Si Biotechnology Co., Ltd., Jiangsu, China, RRID:IMSR_JAX:000664) and CRAMP‐deficient Cnlp −/− mice (C57BL/6J background; 8 weeks old; the Jackson Laboratory, CA, USA, RRID:IMSR_JAX:017799) were maintained at the Animal Housing Unit of Jiangnan University (Jiangsu, China) under a controlled temperature (23–25°C) and a 12‐hr light/12‐hr dark cycle. Animal studies were designed to generate groups of equal size, using randomization and blinded analysis.
2.2. Renal ischaemia–reperfusion injury
Mice were randomized into different experimental groups of n ≥ 6, where n is the number of mice in each group. Renal I/R injury was performed as described previously (Leemans et al., 2005). Briefly, mice were anaesthetized with sodium pentobarbital (50 mg·kg−1, i.p.) and renal arteries were bilaterally clamped for 45 min, using microaneurysm clamps. Reperfusion was confirmed visually upon release of the clamps. Groups of animals received CRAMP (5 mg·kg−1, i.p., >98% purity, GL Biochem, Shanghai, China), and/or https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1797 kinase inhibitor https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4862 (20 mg·kg−1, i.p., Selleck, Shanghai, China) or NOD‐like receptor family pyrin domain containing 3 (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1770) inhibitor https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=10057 (40 mg·kg−1, i.p., MedChemExpress, New Jersey, USA) or vehicle (saline, i.p.) 1 hr before renal pedicle clamping. All groups were subjected to the same surgical procedure except the sham groups which were not clamped. Surgical wounds were closed, and mice were given 1 ml of warm saline, i.p. The mice were kept in a warm incubator until they regained consciousness. Blood and kidney tissue were collected 24 hr after reperfusion.
2.3. Clinical samples
The human investigation protocol conformed to the principles outlined in the WMA Declaration of Helsinki and the Department of Health and Human Services Belmont Report and was approved by the Ethics Committee of Affiliated Hospital of Jiangnan University (JN. No2017[018]). Informed consent was obtained from all subjects. Blood samples were collected from both patients and volunteers. Inclusion criteria for patients with AKI were an abrupt (within 48 hr) reduction in kidney function currently defined as an absolute increase in serum creatinine of more than or equal to 3 mg·l−1 (≥26.4 μM), a percentage increase in serum creatinine of more than or equal to 50% (1.5‐fold from baseline), or a reduction in urine output (documented oliguria of less than 0.5 ml·kg−1 per hour for more than 6 hr).
2.4. Assessment of renal injury
Blood urea nitrogen and serum creatinine levels in serum collected at the end of experiments were measured by automatic biochemical analyzer (Cobas6000, Roche Diagnostics, IN, USA).
2.5. CRAMP and LL‐37 measurements by ELISA
The levels of CRAMP and LL‐37 in serum or plasma were analysed using the mouse CRAMP ELISA kit or human LL‐37 ELISA kit (MyBiosource, Inc., CA, USA).
2.6. Cytokine and chemokine measurements
The levels of cytokines (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4998 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4974) in serum were measured by ELISA kits (R&D systems, MN, USA) following the standard procedure of the manufacturer.
2.7. Detection of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2789 activity
Myeloperoxidase (MPO) activity measurements were determined by an MPO assay kit (Jian Cheng Bioengineering Institute, Jiangsu, China).
2.8. Western blot analysis
Western blotting procedures and analysis comply and adhere with BJP Guidelines (Alexander et al., 2019). Kidney tissues were homogenized in ice‐cold lysis buffer RIPA containing protease and phosphatase inhibitor cocktail (Sigma‐Aldrich, Shanghai, China). Samples were centrifuged at 4°C, 10,000× g for 10 min and equal amounts of protein (30 mg), as determined using standard bicinchoninic acid assay (BCA) method by BCA Protein Assay Kit (Beyotime Institute of Biotechnology, Shanghai, China). Samples were separated by electrophoresis in 10% sodium dodecyl sulfate polyacrylamide electrophoresis gel and transferred to polyvinylidene difluoride membranes. Membranes were blocked with blocking buffer for 1.5 hr at room temperature, washed with TBS‐Tween 20, and finally incubated overnight at 4°C with anti‐CRAMP pAb (Cat# PA‐CRPL‐100, 1:1,000, Innovagen AB, Lund, Sweden), anti‐GAPDH pAb (Cat# AP0063, RRID:AB_2651132), and anti‐β‐Actin mAb (Cat# AP0731, RRID:AB_2797410; 1:10,000, Bioworld Technology Inc., MN, USA), anti‐NLRP3 mAb (Cat# 15101, RRID:AB_2722591), anti‐cleaved‐caspase1 pAb (Cat# 67314, RRID:AB_2714037), anti‐cleaved IL‐1β mAb (Cat# 52718, RRID:AB_2799421), anti‐Bax pAb (Cat# 2772, RRID:AB_10695870), anti‐https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2844&familyId=910&familyType=OTHER mAb (Cat# 3498, RRID:AB_1903907), anti‐cleaved caspase‐3 pAb (Cat# 9661, RRID:AB_2341188; all in 1:1,000, Cell Signaling Technology, MA, USA), anti‐cleaved‐IL‐18 pAb (Cat# ab71495, RRID:AB_1209302, 1:1,000), and anti‐EGF receptor (EGFR) mAb (Cat# ab52894, RRID:AB_869579, 1:5,000; Abcam, Shanghai, China). Subsequently, incubation with anti‐rabbit HRP secondary pAb (Cat# PA1‐28767, RRID:AB_2540021, 1:5,000, ThermoFisher Scientific, MA, USA) was performed for 2 hr at room temperature, and immunoreactivity was analysed by Western Lightening Plus enhanced chemiluminescence (Millipore, MA, USA) according to the manufacturer's instructions.
2.9. Histology and immunostaining
Kidney tissues were fixed with 4% paraformaldehyde overnight and embedded in paraffin wax. The Skiving Machine Slicer PM2245 (Leica, Shanghai, China) diced 5‐μm sections were stained with haematoxylin and eosin. For kidney injury evaluation, a DM2000 light microscope (Leica, Shanghai, China) was used at ×40 magnification. Tubular injury was assessed in haematoxylin and eosin stained sections using a semi‐quantitative scale in which the percentage of cortical tubules showing epithelial cell necrosis, brush border loss, cast formation, and apoptotic bodies in the cortex was assigned a score: 0 = normal; 1 ≤ 10%; 2 = 10–25%; 3 = 26–75%; 4 ≥ 75%. Ten fields of 20 magnifications were examined and averaged. The individual scoring of the slides was blinded to the genotype of the animal. Immunofluorescent staining was performed according to the procedure described in our previous study (Sun, Furio, et al., 2015). For immunofluorescent staining, the following primary antibodies were applied: anti‐CRAMP pAb (Cat# sc‐66843, RRID:AB_2068699, 1:100; Santa Cruz Biotechnology, Shanghai, China), anti‐E‐cadherin pAb (Cat# 610181, RRID:AB_397580, 1:100; BD Biosciences, MD, USA), and anti‐Ly6G mAb (Cat# ab25377, RRID:AB_470492, 1:100; Abcam, Shanghai, China). Anti‐rabbit AlexaFluor 555 (CRAMP), anti‐mouse AlexaFluor 488 (E‐cadherin), and anti‐rat AlexaFluor 647 (Ly6G) polyclonal secondary antibodies (all in 1:500, Invitrogen, Shanghai, China) were applied. The DAPI staining was conducted according to the protocol provided by the manufacturer (Beyotime, Shanghai, China). The antibody‐based procedures used in this study comply with the recommendations made by the British Journal of Pharmacology.
2.10. Quantification of mRNA by real‐time quantitative PCR
Transcription of mRNA for IL‐6, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5074, IL‐1β, formyl peptide receptor (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=223), EGFR, and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=484 receptors was analysed by real‐time quantitative PCR (RT‐qPCR). Total RNA was isolated from kidney tissues using TRIzol (Life Technologies, MA, USA) and was subjected to reverse transcription using Prime‐Script RT reagent kit (TaKaRa Bio, Kyoto, Japan) following the manufacturer's instructions. SYBR® Green RT‐qPCR was performed using RT‐qPCR system (BIO RAD CFX Connect, CA, USA). The relative mRNA levels were normalized to mRNA levels of β‐actin (housekeeping control), and calculations for fold change of each mRNA were made on comparative cycle threshold method (2−ΔΔCt). The primers used in this study are provided in Table 1.
Table 1.
List of primers used for RT‐qPCR
| Gene | Forward (5′‐3′) | Reverse (5′‐3′) |
|---|---|---|
| β‐actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
| IL‐6 | ACCCCAATTTCCAATGCTCTC | AACGCACTAGGTTTGCCGAG |
| TNF‐α | GCATGATCCGCGACGTGGAA | AGATCCATGCCGTTGGCCAG |
| IL‐1β | ATCTTTTGGGGTCCGTCAACT | GCAACTGTTCCTGAACTCAACT |
| FPR2 | CCTGGCTAGGAAGGTGGTTG | GACATGGGCATGCTGAAACC |
| EGFR | GGGATTGGCCTATTCATGCG | AATGCTCCCGAACCCAGAAC |
| P2X7 | TAGGGGCCATCTGCGTCTAT | CTAACTTCGTCACCCCACCC |
Abbreviations: EGFR, EGF receptor; FPR2, formyl peptide receptor; P2X7, purinergic receptor P2X, ligand‐gated ion channel, 7.
2.11. Assessment of apoptosis
In situ detection of DNA fragments by terminal deoxyribonucleotide transferase (TdT)‐mediated dUTP nick‐end labelling (TUNEL) was performed using the one‐step TUNEL apoptosis assay kit (Beyotime Institute of Biotechnology, Shanghai, China).
2.12. Flow cytometry
2.12.1. Preparation of kidney cell suspension
Unilateral renal artery clipping was carried out for 45 min in adult mice, under pentobarbital sodium anaesthesia as described previously (Leemans et al., 2005). Kidneys were dissected, cut into 1–2 mm3 pieces, placed in DMEM containing 1.6 mg·ml−1 of collagenase I (Sigma‐Aldrich, Shanghai, China) and 200 mg·ml−1 of DNase I (Sigma‐Aldrich, Shanghai, China), dissociated using gentle MACS tissues dissociator (Miltenyi Biotec, Bergisch Gladbach, Germany) and digested for 40 min at 37°C with intermittent agitation, washed, resuspended in phosphate buffer saline. Following erythrocyte lysis, cells were resuspended in phosphate buffer saline. To ensure that large cellular clumps and non‐cellular material were not included, kidney cell suspensions were filtered through 75‐μm filters for use in these assays.
2.12.2. Reagents
Single‐cell suspensions were prepared from kidney tissues and were stained for 30 min at 4°C after FcγRII/III blocking (Miltenyi Biotec, Bergisch Gladbach, Germany). Surface staining was performed with the following monoclonal antibodies: PE‐Vio770 Anti‐Mouse CD45 (Miltenyi Biotec, Bergisch Gladbach, Germany), Brilliant Violet 421 Rat Anti‐Mouse https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=182, Brilliant Violet 605 anti‐mouse/human CD11b, Alexa Fluor 647 Anti‐Mouse Ly6G, and APC Anti‐Mouse CD206 (BioLegend, CA, USA). Gating method of FACS was programmed as CD45+ CD11b+ F4/80+ (for macrophages), CD45+ CD11b+ Ly6G+ (for neutrophils), and CD45+ CD11b+ F4/80+ CD206+ (for M2 macrophages). To detect M2 macrophages, cells were surface stained with CD45+ CD11b+ F4/80+, and then cells were fixed and permeabilized, stained for CD206 expression. Isotype‐matched controls were included in all experiments. Flow cytometry was performed on an Attune NxT (Thermo Fisher Scientific, MA, USA), and data were analysed using FlowJo software (RRID:SCR_008520).
2.13. Data and analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). The sample size declared in the different experimental groups was the number of independent mice in each group, and statistical analysis was done using these independent values. Statistical analysis was undertaken only for experiments where each group size was at least n = 5. Normal distribution was confirmed using the Kolmogorov–Smirnov test. Statistical analysis between two groups was performed by independent t test, or when multiple comparisons were made, by one‐way ANOVA followed by Tukey's post hoc test using GraphPad Prism (v5; GraphPad Software Inc., CA, USA, RRID:SCR_002798). For all one‐way ANOVAs, post hoc tests were run only if F achieved P < .05 and there was no significant variance inhomogeneity. For Western blot and RT‐qPCR analysis, the relative protein or mRNA expression values were expressed as “fold difference” by comparing to the corresponding control value, and the control value was normalized to 1.0. Potential outliers were tested using with Grubbs' test. P < .05 were considered as a statistically significant difference.
2.14. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos et al., 2019; Alexander, Fabbro et al., 2019a, 2019b; Alexander, Kelly et al., 2019; Alexander, Mathie et al., 2019).
3. RESULTS
3.1. Cathelicidins are down‐regulated in AKI patients and mouse kidneys after I/R
To establish the biological role for cathelicidins in AKI, we assessed relevance of human cathelicidin LL‐37 to clinical AKI and CRAMP to I/R‐induced AKI in mice. We first measured human plasma levels of LL‐37 in normal healthy subjects and in AKI patients. The clinical characteristics of these patients are presented in Table S1. As shown in Figure 1a, plasma LL‐37 was markedly lower in AKI patients than in healthy controls. In experimental renal I/R injury, systemic CRAMP levels were also decreased (Figure 1b). Concomitantly, expression of both the precursor form of CRAMP and the mature peptide in kidneys were markedly decreased after I/R (Figure 1c). CRAMP has been shown to be produced from different cellular sources in different tissues (Gallo & Hooper, 2012; Sun, Furio, et al., 2015). We therefore investigated the cellular localization and regulation of CRAMP in mouse kidneys by immunofluorescent staining. E‐cadherin, essential in maintaining structural integrity of renal epithelium, was stained to identify renal epithelium. Reduced E‐cadherin expression has been reported during renal I/R (Yang & Liu, 2001). As shown in Figure 1d, CRAMP staining was co‐localized with E‐cadherin‐expressing epithelial cells under normal conditions, which was largely lost after I/R, suggesting that renal epithelium constitutively expressed CRAMP in mouse kidneys and that CRAMP was down‐regulated in renal I/R.
Figure 1.
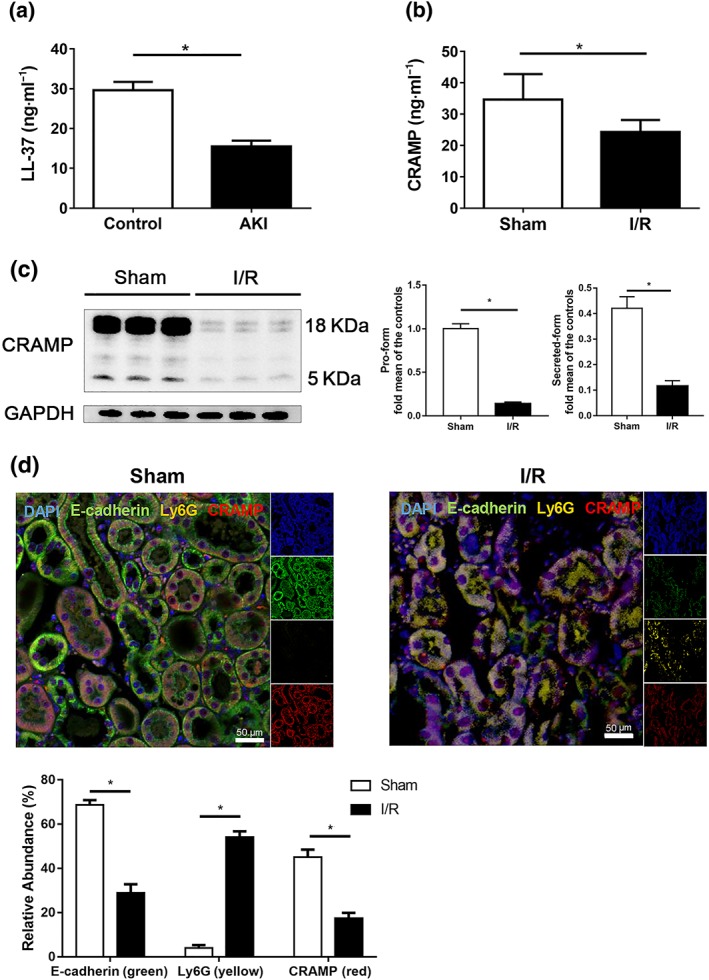
Decreased CRAMP production is found in patients with acute kidney injury (AKI) and in mice with experimental renal ischaemia/reperfusion (I/R). (a) Plasma LL‐37 levels from patients with AKI and healthy donors (n = 18 in healthy control group, n = 20 in AKI patient group) by ELISA. (b–d) Mice were subjected to renal I/R‐induced injury and compared with sham group mice. (b) Serum CRAMP determination by ELISA (n = 8 per group). (c) Western blot and densitometry analysis of CRAMP precursor and of the mature peptide in kidney tissues (n = 8 per group), GAPDH was used as loading control. (d) Localization and expression of CRAMP (red), E‐cadherin (green) and Ly6G (yellow) in kidney tissues by immunofluorescent staining (n = 8 minimum per group). Representative photomicrographs of individual and merged staining are shown. Nuclei are stained with DAPI (blue). Scale bar: 50 μm. Data are means ± SEM. *P < .05, significantly different as indicated; grouped t test. [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.2. CRAMP deficiency exacerbates I/R‐induced renal injury
Next, we examined the potential role of CRAMP in I/R‐induced renal injury by using Cnlp −/− mice. CRAMP deficiency did not have any effect on normal renal function, compared with WT control mice (Figure 2a–i). However, Cnlp −/− mice exhibited aggravated I/R‐induced kidney dysfunction as shown by increased serum creatinine (Figure 2a) and urea nitrogen production (Figure 2b), accompanied by increased MPO activities (Figure 2c), a marker for neutrophil infiltration, compared with values in WT mice. The results were also confirmed by our exploratory immunofluorescent staining that CRAMP deficiency aggravated loss of E‐cadherin‐expressing epithelial cells and infiltration of Ly6G‐positive cells (Figure S1). Histologically, renal I/R results in kidney tubular necrosis, cast formation, tubular dilation, and loss of brush border (Bai et al., 2018). Exacerbation of renal injury with CRAMP deficiency was further confirmed by worsened I/R‐mediated tubular injury and increased necrosis score in kidney tubular epithelial cells (Figure 2d). Additionally, I/R‐caused increases of renal TNFα, IL‐6, and IL‐1β mRNA expression (Figure 2e–g) as well as serum IL‐6 and IL‐1β production in WT mice. These measures were further enhanced in Cnlp −/− mice (Figure 2h, i).
Figure 2.
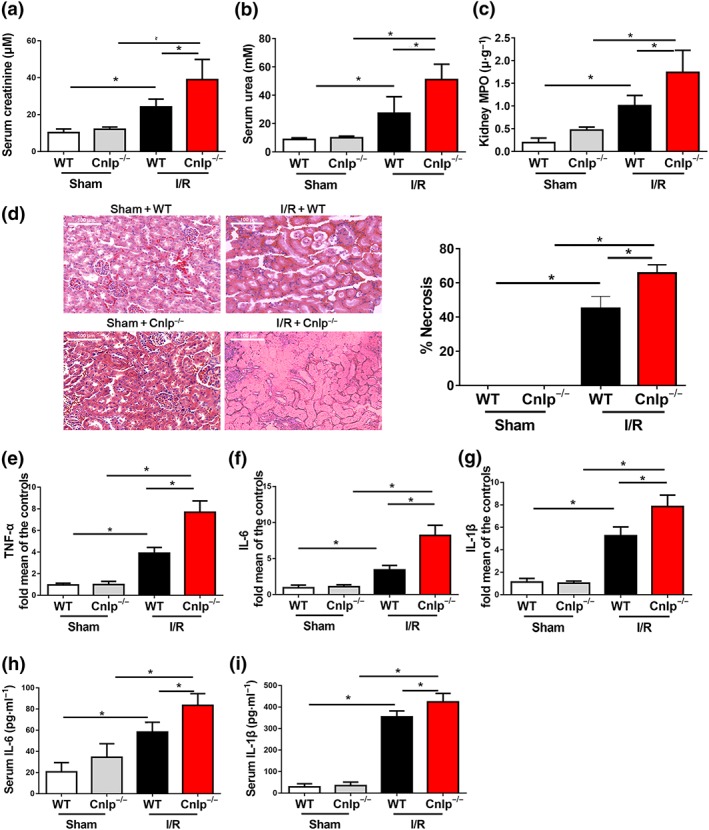
CRAMP deficiency aggravates I/R‐induced renal dysfunction. CRAMP deficient (Cnlp −/−) mice and WT mice were subjected to renal I/R. (a,b) Serum creatinine and urea levels were measured after I/R (n = 8 per group). (c) Myeloperoxidase (MPO) activities in kidney tissues were measured after I/R (n = 8 per group). (d) Representative histological sections and quantification of necrosis in tubular epithelial cells (n = 8 per group). Scale bar: 100 μm. (e–g) The mRNA levels of inflammatory cytokines in kidneys were measured by real‐time qPCR. (h,i) Serum levels of IL‐6 and IL‐1β were measured by ELISA (n = 8 per group). The results are shown as mean ± SEM. *P < .05, significantly different as indicated; one‐way ANOVA followed by Tukey's test [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.3. CRAMP deficiency worsens I/R‐induced apoptosis and NLRP3 inflammasome activation in kidneys
Tubular epithelial apoptosis is importantly involved in the pathophysiology of renal injury following I/R (Andrade‐Oliveira et al., 2015; Deng et al., 2017; Togel & Westenfelder, 2014). Thus, we also examined whether CRAMP modulated I/R‐mediated apoptosis in mouse kidneys. No detectable apoptotic nuclei were observed in sham‐operated kidneys. TUNEL staining revealed that Cnlp −/− mice displayed worsened renal apoptosis after I/R (Figure 3a). Western blot analyses of apoptosis‐related proteins confirmed that CRAMP deficiency caused more severe apoptotic responses after I/R with increased https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=910 ratio and cleaved https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1619 expression, compared with those in WT mice (Figure 3b).
Figure 3.
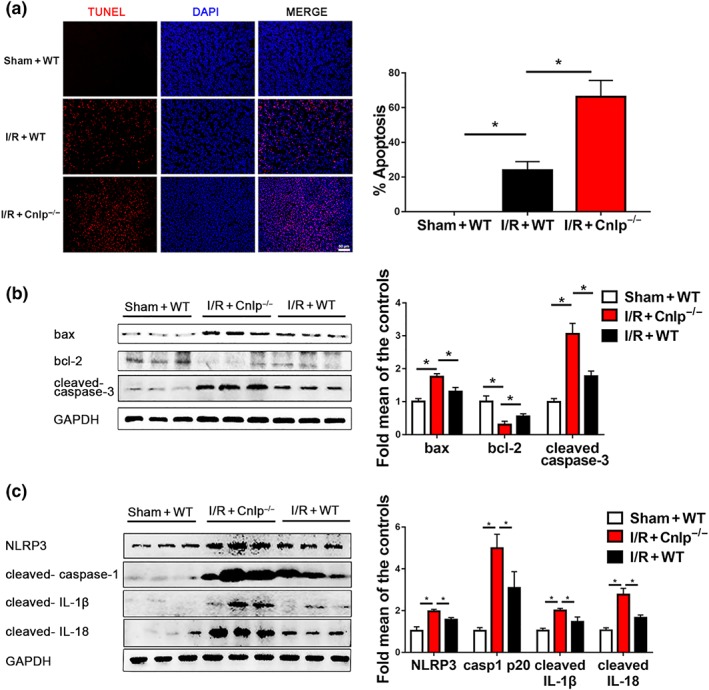
CRAMP deficiency causes NLRP3 inflammasome over‐activation and increased apoptosis in renal I/R‐induced injury. (a) Cell apoptosis was estimated by TUNEL staining. The percentage of apoptosis was measured relative to the photographic area, scale bar: 50 μm. (b) Western blot and quantitative analysis of Bax, Bcl‐2, and cleaved caspase‐3 in kidney tissues. GAPDH was used as loading control (n = 8 per group). (c) Western blot and quantitative analysis of NLRP3, cleaved caspase‐1 p20, cleaved IL‐1β, and cleaved IL‐18 expression in kidney tissues. GAPDH was used as loading control (n = 8 per group). TUNEL, terminal deoxynucleotidyl transferase‐mediated digoxigenin‐deoxyuridine nick‐end labelling. The results are shown as mean ± SEM. *P < .05, significantly different as indicated; one‐way ANOVA followed by Tukey's test [Colour figure can be viewed at http://wileyonlinelibrary.com]
The NLRP3 inflammasomes have been increasingly recognized to play a critical role in the pathogenesis of AKI (Andrade‐Oliveira et al., 2015). Next, we investigated whether CRAMP modulated NLRP3 inflammasome activation during I/R‐induced AKI. Renal I/R induced significant activation of NLRP3 inflammasomes, as shown by markedly increased expression of NLRP3, cleaved https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1617 p20, cleaved IL‐1β, and cleaved IL‐18. CRAMP ablation caused over‐activation of NLRP3 inflammasome with higher expression levels of NLRP3, cleaved caspase‐1 p20, cleaved IL‐1β, and cleaved IL‐18 after I/R, compared to WT mice (Figure 3c).
3.4. CRAMP administration protects against I/R‐induced renal injury
The protective effect of CRAMP in renal I/R was further confirmed by treatment of mice with exogenous CRAMP. We first confirmed that exogenous CRAMP treatment resulted in increased serum and renal CRAMP levels at the point of determination after I/R‐induced injury (Figure S2). Exogenous CRAMP alone did not cause any effects in sham‐operated mice (Figure S3). In contrast, administration of exogenous CRAMP significantly attenuated I/R‐induced increases of serum creatinine (Figure 4a) and urea nitrogen production (Figure 4b) and renal MPO activity (Figure 4c). By histological examination, CRAMP administration significantly improved I/R‐mediated tubular injury and acute tubular necrosis scores, 24 hr after reperfusion (Figure 4d). Additionally, CRAMP attenuated I/R‐induced increases of renal TNFα, IL‐6, and IL‐1β mRNA expression (Figure 4e–g) and serum IL‐6 and IL‐1β production (Figure 4h,i). To confirm the immunomodulatory effects of exogenous CRAMP, we analysed, by flow cytometry, the renal‐infiltrating immune cells, including neutrophils and macrophages, which play an important role in the initiation and propagation of the kidney injury. CRAMP treatment reduced the numbers of infiltrating neutrophils in kidneys after renal I/R‐induced injury (Figure S4a). In contrast, CRAMP did not affect the total number of macrophages infiltrating the kidneys (Figure S4b) but led to increased percentage of M2 macrophages (Figure S4c), suggesting that CRAMP promoted polarization of renal macrophages towards the M2 phenotype.
Figure 4.
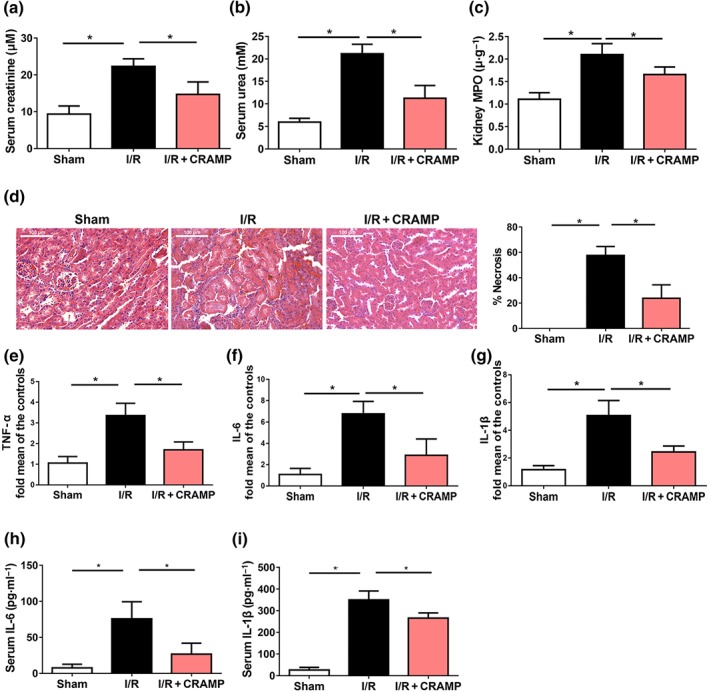
CRAMP ameliorates I/R‐induced renal dysfunction. Mice were treated with CRAMP (5 mg·kg−1) at 1 hr before I/R. (a–b) Serum creatinine and urea levels were measured after I/R (n = 8 per group). (c) MPO activities in kidney tissues were measured after I/R (n = 8 per group). (d) Representative histological sections and quantification of necrosis in tubular epithelial cells (n = 8 per group). Scale bar: 100 μm. (e–g) The mRNA levels of inflammatory cytokines in kidneys were measured by real‐time qPCR. (h,i) Serum levels of IL‐6 and IL‐1β were measured by ELISA (n = 8 per group). The results are shown as mean ± SEM. *P < .05, significantly different as indicated; one‐way ANOVA followed by Tukey's test [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.5. CRAMP suppresses I/R‐induced apoptosis and NLRP3 inflammasome activation in kidneys
We also investigated whether exogenous CRAMP affected renal apoptosis and NLRP3 inflammasome activation during renal I/R injury. By TUNEL staining, we observed that exogenous CRAMP treatment markedly reduced I/R‐mediated apoptosis in kidneys of WT mice (Figure 5a). Western blot analyses demonstrated that exogenous CRAMP treatment reversed the I/R‐induced increase of Bax/Bcl‐2 ratio and cleaved caspase‐3 expression in kidneys (Figure 5b). Consistent with the data in Cnlp −/− mice, administration of exogenous CRAMP markedly suppressed I/R‐mediated NLRP3 inflammasome activation and up‐regulation of cleaved caspase‐1 p20, cleaved IL‐1β, and cleaved IL‐18 (Figure 5c).
Figure 5.
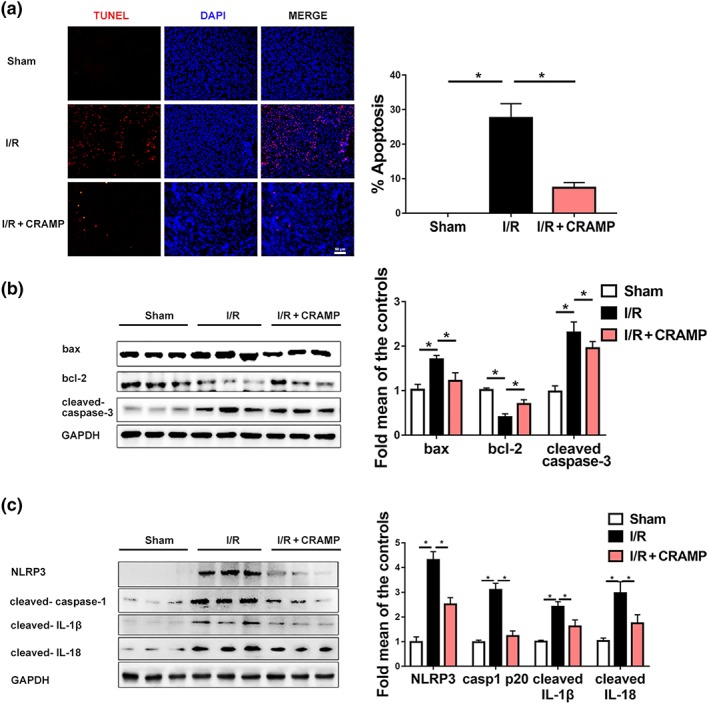
CRAMP suppresses renal I/R‐induced NLRP3 inflammasome activation and apoptosis in kidney tissues. (a) Cell apoptosis was estimated by TUNEL staining. The percentage of apoptosis was measured relative to the photographic area, scale bar: 50 μm. (b) Western blot and quantitative analysis of bax, bcl‐2, and cleaved caspase‐3 in kidney tissues. GAPDH was used as loading control (n = 8 per group). (c) Western blot and quantitative analysis of NLRP3, cleaved caspase‐1 p20, cleaved IL‐1β, and cleaved IL‐18 expression in kidney tissues. GAPDH was used as loading control (n = 8 per group). TUNEL, terminal deoxynucleotidyl transferase‐mediated digoxigenin‐deoxyuridine nick‐end labelling. The results are shown as mean ± SEM. *P < .05, significantly different as indicated; one‐way ANOVA followed by Tukey's test [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.6. CRAMP protects against I/R‐caused renal dysfunction partly by inhibition of NLRP3 inflammation activation
Given the evidence for a role of NLRP3 inflammasome activation in mediating ischaemic AKI (Kim et al., 2013), we next investigated whether NLRP3 was a potential mechanistic target of CRAMP. A specific NLRP3 inhibitor CY‐09 was administered prior to I/R‐induced renal injury, and its effects were compared to CRAMP treatment. Specific effects of CY‐09 on NLRP3 inflammasome activation and signalling were confirmed by Western blot (Figure 6a). Moreover, we found that NLRP3 inhibition by CY‐09 mimicked the protective effects of CRAMP in mouse kidneys during I/R renal injury, as shown by decreased serum creatinine (Figure 6b), urea nitrogen levels (Figure 6c), and renal MPO activity (Figure 6d). Histological examination confirmed that pretreatment with CY‐09 protected against kidney injury and tubular necrosis (Figure 6e). Attenuation of I/R‐induced renal injury by CY‐09 was accompanied by reduced mRNA expression of inflammatory cytokines IL‐6, TNF‐α, and IL‐1β (Figure 6f) in kidneys as well as circulating IL‐6 and IL‐1β levels (Figure 6g). Similar to CRAMP treatment, CY‐09 reduced I/R‐induced apoptosis as determined by TUNEL staining (Figure 6h). These data suggest that, in our model, CRAMP protected against AKI via inhibition of the activation of NLRP3 inflammasomes.
Figure 6.
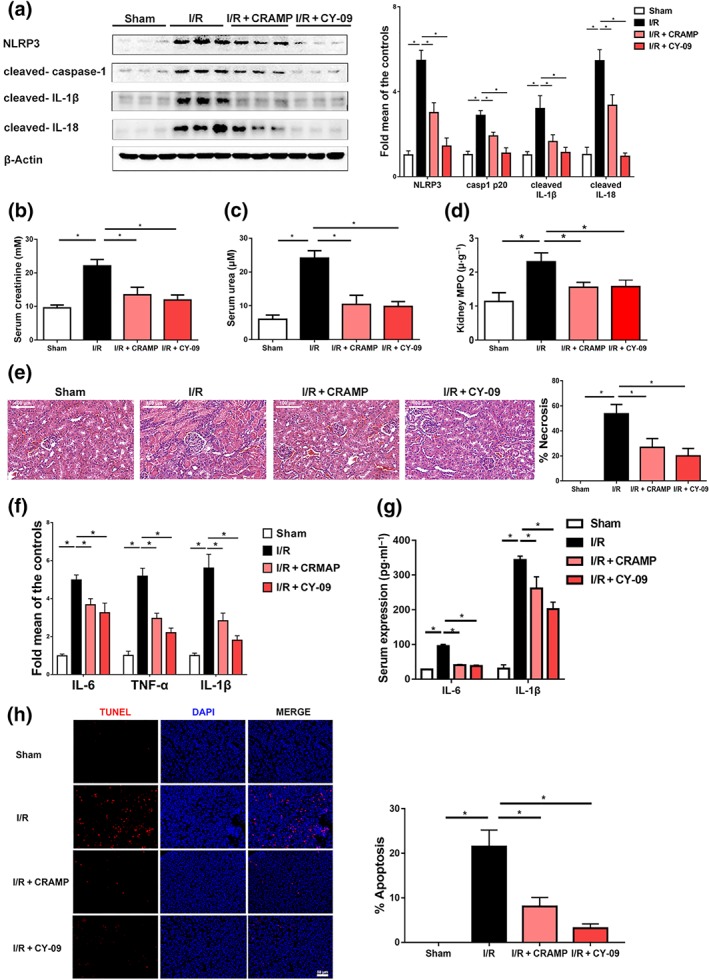
CRAMP alleviates I/R‐induced renal injury and apoptosis partly by inhibition of NLRP3 inflammasome activation. Mice were treated with CRAMP (5 mg·kg−1) or CY‐09 (40 mg·kg−1) 1 hr before I/R. (a) Western blot and densitometry analyses of NLRP3 inflammasome activation, β‐actin was used as loading control. (b) Serum creatinine (n = 8 per group). (c) Serum urea (n = 8 per group). (d) Myeloperoxidase (MPO) activities in kidney tissues (n = 8 per group). (e) Representative histological sections and quantification of necrosis in tubular epithelial cells (n = 8 per group). Scale bar: 100 μm. (f) The mRNA levels of proinflammatory cytokines (IL‐6, TNF‐α, and IL‐1β) in the kidneys (n = 5 minimum per group). (g) Serum levels of IL‐6 and IL‐1β (n = 8 per group). (h) Cell apoptosis was estimated by TUNEL staining. The percentage of apoptosis was measured relative to the photographic area, scale bar: 50 μm. TUNEL, terminal deoxynucleotidyl transferase‐mediated digoxigenin‐deoxyuridine nick‐end labelling. The results are shown as mean ± SEM. *P < .05, significantly different as indicated; one‐way ANOVA followed by Tukey's test [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.7. Renal protective and NLRP3 inhibitory effects of CRAMP require EGFR
Lastly, to further elucidate the mechanism and receptor involvement in renal protective and NLRP3 inhibitory effects of CRAMP on renal I/R renal injury, potential receptors of CRAMP including FPR2, P2X7 receptors and EGFR were analysed. EGFR but not FPR2 or P2X7 receptor mRNA was down‐regulated in kidneys after I/R‐mediated injury (Figure 7a). EGFR expression by Western blot was even lower in CRAMP‐deficient mice and partially restored with CRAMP administration (Figure 7b). Next, a specific EGFR inhibitor AG1478 was administered prior to I/R‐induced renal injury and CRAMP treatment. We found that inhibition of EGFR by AG1478 abolished CRAMP‐associated protective effects in the mouse kidneys during I/R renal injury, as shown by increased serum creatinine (Figure 7c), urea nitrogen levels (Figure 7d). Histological examination confirmed that pretreatment with AG1478 also blocked CRAMP‐mediated protective effects on kidney injury and tubular necrosis (Figure 7e), which was accompanied by increased mRNA expression of inflammatory cytokines IL‐6, TNFα, and IL‐1β (Figure 7e) as well as serum levels of IL‐6 and IL‐1β (Figure 7f). Furthermore, the suppressive effects of CRAMP on I/R‐induced NLRP3 inflammasome activation was abolished by the EGFR inhibitor AG1478 (Figure 7h). Protective effects of CRAMP on I/R‐induced apoptosis as demonstrated by Western blot analyses of Bax, Bcl‐2, and cleaved caspase‐3 expression were blocked by AG1478 (Figure 7i). Taken together, these results suggest that renal protective effects of CRAMP are mediated by EGFR.
Figure 7.
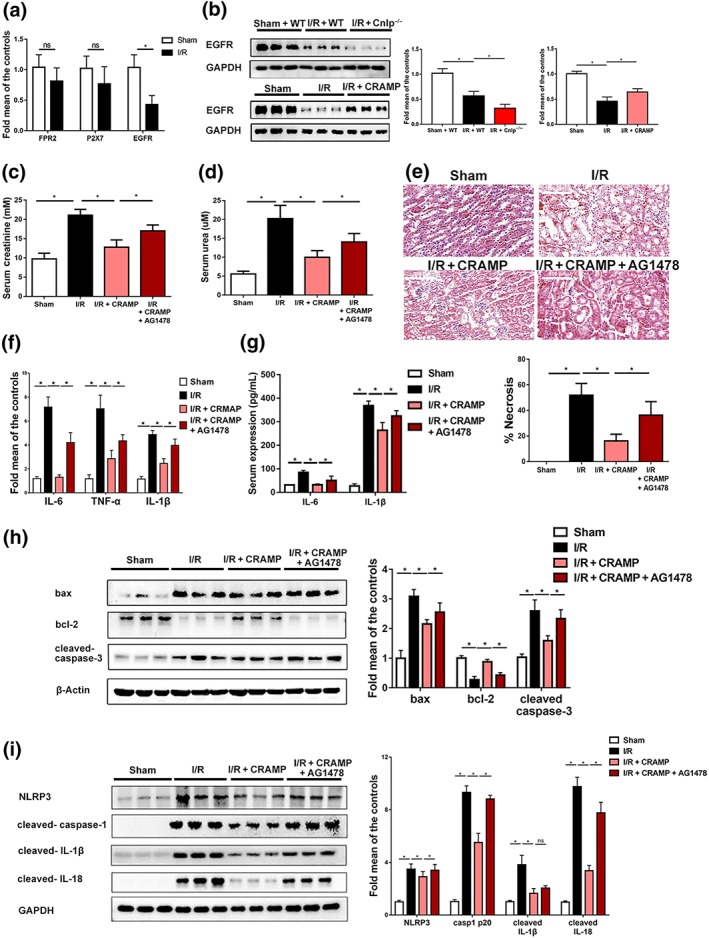
CRAMP protects renal I/R through EGFR. (a) The mRNA levels of CRAMP receptors (including FPR2, P2X7, and EGFR) after renal I/R measured by real‐time PCR in kidneys (n = 8 per group). Grouped t test was used. (b–i) Mice were treated with CRAMP (5 mg·kg−1) at 1 hr before I/R. AG1478 was administered 30 min before CRAMP treatment. (b) EGFR expression determined by Western blot and densitometry analyses. (c) Serum creatinine (n = 8 per group). (d) Serum urea (n = 8 per group). (e) Representative histological sections and quantification of necrosis in tubular epithelial cells (n = 8 per group). Scale bar: 100 μm. (f) The mRNA levels of proinflammatory cytokines (IL‐6, TNF‐α, and IL‐1β) in the kidneys (n = 5 minimum per group). (g) Serum levels of IL‐6 and IL‐1β (n = 8 per group). (h) Western blot and densitometry analyses of NLRP3 inflammasome activation in kidney tissues. (i) Western blot and quantitative analysis of Bax, Bcl‐2, and cleaved caspase‐3 in kidney tissues, β‐actin or GAPDH was used as loading control (n = 8 per group). The results are shown as mean ± SEM. *P < .05, significantly different as indicated; one‐way ANOVA followed by Tukey's test [Colour figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
Our recent work has demonstrated that peripheral non‐immune cells are a source of CRAMP with cytoprotective and immunomodulatory actions (Bei et al., 2019; Sun, Furio, et al., 2015; Sun et al., 2016). In this study, we extended our investigations to analyse the effects of CRAMP on renal I/R injury and the underlying mechanisms. Renal epithelial CRAMP expression was defective in clinical AKI and experimental I/R‐induced renal injury. CRAMP‐deficient mice exhibited aggravated I/R‐induced renal dysfunction while exogenous CRAMP protected against the effects of I/R. Mechanistic investigations revealed that the renal protective effects of CRAMP were mediated via EGFR to suppress NLRP3 inflammasome activation, consequently alleviating renal I/R injury. Overall, our findings indicate that CRAMP is a novel key modulator in the pathogenesis of AKI (Figure 8).
Figure 8.
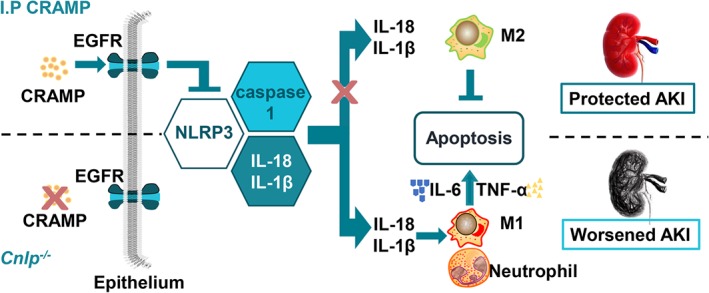
Cathelicidin‐related antimicrobial peptide (CRAMP) is a critical modulator of ischaemia/reperfusion (I/R)‐induced renal injury in mice. CRAMP protects against I/R‐mediated renal injury and associated inflammation by inhibition of NOD‐like receptor family pyrin domain containing‐3 (NLRP3) inflammasome via EGF receptors. [Colour figure can be viewed at http://wileyonlinelibrary.com]
As a traditional AMP with increasing recognized host defence functions, roles of cathelicidin in inflammatory, immunological, and cardiovascular disorders have been earlier documented (Bei et al., 2019; Diana et al., 2013; Gilliet & Lande, 2008; Soehnlein et al., 2011; Sun, Furio, et al., 2015). In renal tissues, CRAMP is mainly expressed in tubular epithelium and by infiltrating leukocytes, including neutrophils and monocytes (Ali, Townes, Hall, & Pickard, 2009; Zasloff, 2007). CRAMP appears to be a key factor in maintaining homeostasis of epithelial and mucosal immunity in kidney (Ali et al., 2009; Chromek, 2015; Chromek et al., 2006; Zasloff, 2007). Recent studies including ours demonstrate that CRAMP expression can be induced by a variety of nutrients and vitamins in various epithelial cells. For example, cathelicidin can be induced by vitamin D and gut microbiota metabolites such as short‐chain fatty acids (Sun, Furio, et al., 2015; Zasloff, 2007). Previous studies involving CRAMP in renal diseases have mostly described its antimicrobial properties and/or been confined to infectious conditions in the urinary tract (Ali et al., 2009; Chromek et al., 2006; Peng, Purkerson, Schwaderer, & Schwartz, 2017). Thus the possible roles of CRAMP on AKI had not been assessed. Here, the observation that circulating LL‐37 level significantly decreased in patients with AKI suggested its relevance to this clinical condition. Furthermore, defective CRAMP production by renal E‐cadherin‐positive tubular epithelial cells was demonstrated in a murine model of renal I/R injury. While a previous study has shown that intercalated cells of the collecting duct express cathelicidin (Peng et al., 2017), we found localization of CRAMP to E‐cadherin‐positive cells in kidneys, and the loss of CRAMP, corresponding to E‐cadherin‐expressing epithelial cells, during renal I/R confirmed its involvement in this condition (Abed et al., 2014). Although immune cells infiltrated into the kidneys could contribute to CRAMP production (Figure S1), the overall fall in CRAMP expression is largely attributable to the apoptotic loss of the epithelial cells which comprise a large proportion of total renal cells. The results were consistent with earlier indications that epithelium‐derived, but not immune cell‐derived, cathelicidin mostly contributes to its protective functions in kidney (Zasloff, 2007). Apart from its cellular localization and regulation being inversely correlated with the condition, an overall protective role of CRAMP in AKI was confirmed by evidence collected from two approaches: CRAMP deficiency exacerbated renal I/R‐induced injury, whereas exogenous CRAMP treatment markedly alleviated it. Thus, we have provided direct evidence that CRAMP is protective against renal I/R.
Cathelicidin may be produced by various cell types and tissues (Diana et al., 2013; Gallo & Hooper, 2012; Sun, Furio, et al., 2015; Sun et al., 2016). Several studies including ours suggested that cathelicidin from non‐immune cells has beneficial effects (Bei et al., 2019; Sun, Furio, et al., 2015; Sun et al., 2016). Cathelicidin from pancreatic islet cells under the control of gut microbiota and its metabolite butyrate, affects the pancreatic immune environment and dampens the development of Type 1 diabetes (Sun, Furio, et al., 2015). Recently, we have shown that c‐Jun was induced in the heart upon I/R injury. Aberrantly induced c‐Jun as a negative regulator of CRAMP may down‐regulate its expression (Bei et al., 2019). Exogenous CRAMP therapy protected against cardiomyocyte apoptosis via activation of https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=285 and https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=514 as well as phosphorylation and nuclear export of FoxO3a (Bei et al., 2019). In contrast, neutrophil‐derived cathelicidins somehow demonstrate contrasting effects. Soehnlein et al. (2011) showed that neutrophil‐derived cathelicidin may protect from neointimal hyperplasia. However, in autoimmune diabetes or skin inflammation, cathelicidin released from neutrophils could form complexes with self‐DNA to trigger autoimmune responses (Diana et al., 2013; Gilliet & Lande, 2008). The distinct roles of cathelicidins seem to be dependent on its origin, targeted cells and their activation state, tissue micro‐environment, and the disease context.
The pathogenesis of AKI is multifactorial, and a robust inflammatory process engaging both innate and adaptive immune responses is believed to cause the initial renal injury and long‐term structural remodelling (Gluba et al., 2010). Additionally, I/R injury involves the migration and activation of innate and adaptive immune cells into the kidneys (Andrade‐Oliveira et al., 2015). Inflammation and leukocyte recruitment are known to contribute to the development of AKI, and TNF‐α and IL‐6 are widely recognized mediators during AKI pathogenesis (Nishikawa et al., 2018). Consistent with its overall protective effects, anti‐inflammatory and immune modulatory effects of CRAMP by suppressing inflammatory cytokine production, inhibiting infiltration of neutrophils and modulating macrophage phenotypic conversion to M2 were observed in renal I/R‐induced injury. Accordingly, both indirect association study and direct evidence have previously indicated positive effects of CRAMP associated with its anti‐inflammatory and immune‐regulatory properties in I/R and related kidney diseases (Hilchie et al., 2013; Hu et al., 2014). For example, PR‐39, a porcine CRAMP, has been reported to reduce infarct size and preserve cardiac function after myocardial I/R by inhibiting leukocyte recruitment (Hoffmeyer, Scalia, Ross, Jones, & Lefer, 2000), reducing MPO activity, and down‐regulating the expression of leukocyte adhesion molecules (Bao et al., 2001). In addition, upregulation of LL‐37 by vitamin D supplementation could reduce complications in peritoneal dialysis patients on peritoneal dialysis increased expression of LL‐37 and reduced complications in chronic kidney diseases (Bacchetta et al., 2014). Our findings that CRAMP modulated macrophage phenotype was in line with our previous study and others that CRAMP stimulated a regulatory phenotype and anti‐inflammatory activity of macrophages in peripheral tissues (Brown et al., 2011; Sun, Furio, et al., 2015). Inhibition of neutrophil infiltration together with skewed phenotype conversion to M2 subtype by CRAMP are desirable because it provides a new strategy to control the initial inflammation that causes tissue damage and to facilitate repair and inhibits inflammation (Lee et al., 2011; Zhang et al., 2017). Thus, our results are clearly compatible with the proposition that CRAMP attenuated I/R‐mediated acute renal injury through the modulation of immune‐inflammatory processes.
Renal I/R is a result of a complex sequence of events that, besides inflammatory responses, involves apoptosis of tubular epithelial cells (Havasi & Borkan, 2011; Stokman et al., 2017). Our observations that CRAMP deletion enhanced cell apoptosis during I/R while CRAMP administration markedly reduced I/R‐induced apoptosis in kidneys were in line with previous studies that CRAMP possesses anti‐apoptotic properties (Sun et al., 2016). Clinical and experimental studies have demonstrated that activation of inflammation was crucial for tissue damage and cell apoptosis during renal I/R, particularly during the process of reperfusion (Rabadi, Kim, D'Agati, & Lee, 2016). Therefore, modulation of immune‐inflammation by CRAMP might contribute to its anti‐apoptotic effects on tubular epithelial cells during I/R.
The NLRP3 inflammasomes, as a cytoplasmic receptor complex, responds to an extensive array of molecular ligands associated with cellular stress, including those induced by ischaemic tissue injury (Anders, 2016). Emerging evidence has suggested a pivotal role for NLRP3 in triggering renal inflammation and apoptosis in the pathogenesis of AKI (Kim et al., 2013; Shen et al., 2016; Shigeoka et al., 2010). NLRP3 deficiency confers anti‐inflammatory and anti‐apoptotic effects in acute renal injury in a model‐dependent manner (Anders & Muruve, 2011; Kim et al., 2013; Shen et al., 2016; Shigeoka et al., 2010). Consistent with these studies, renal protective and anti‐apoptotic effect of CRAMP on I/R induced injury was mimicked by a specific NLRP3 inhibitor CY‐09 and is thus at least partly dependent on suppression of NLRP3 inflammasome activation. It has been suggested that epithelial‐expressed NLRP3 may exert a direct effect on tubular epithelia, independent of inflammasome‐induced proinflammatory cytokines, contributing to renal I/R injury (Shigeoka et al., 2010). It remains possible that CRAMP regulates NLRP3 and downstream cytokines via different mechanisms. In contrast, human LL‐37 externalized in the neutrophil extracellular traps directly activated NLRP3 inflammasomes in lupus macrophages to promote perpetuation of inflammatory responses, a process dependent on P2X7 receptor‐mediated potassium efflux (Kahlenberg, Carmona‐Rivera, Smith, & Kaplan, 2013). Nevertheless, this NLRP3‐activating mechanism was found in macrophages and proposed with aberrant neutrophil extracellular trap formation during autoimmune diseases. Indeed, cathelicidins from different sources have demonstrated either pro‐inflammatory or immunomodulatory effects, as discussed above (Hancock et al., 2016).
The bioactive functions of CRAMP important for immune‐inflammatory responses may be mediated by several different receptors, including FPR2, P2X7, and EGFR (Rekha et al., 2015; Sun, Dahlen, Agerberth, & Haeggstrom, 2013; Sun, Furio, et al., 2015). EGFR is widely expressed in the mammalian renal tissue at sites that include epithelial cells (Tang, Liu, & Zhuang, 2013). The present results demonstrated that EGFR mediated the renal protective actions of CRAMP, which is in agreement with our previous studies that CRAMP produced by non‐immune cells modulated immune‐inflammatory processes in vivo via EGFR (Sun, Furio, et al., 2015). EGFR is associated with tubular epithelial cell proliferation, reparative response, and fibrogenesis after AKI (He, Liu, Bayliss, & Zhuang, 2013; Tang, Liu, Tolbert, et al., 2013; Tang, Liu, & Zhuang, 2013). EGFR and its natural ligand EGF have been shown to be important in the repair of renal tubules following kidney injury (Zhou et al., 2017). Activation of EGFR can enhance recovery of renal function and structure following AKI, through activation of PI3K‐Akt and ERK1/2 signalling pathways (Chen, Chen, & Harris, 2012; He et al., 2013). Furthermore, EGFR‐PI3K‐Akt‐dependent YAP activation plays an essential role in mediating epithelial cell regeneration during kidney recovery from AKI (Chen et al., 2018). The results were consistent with our previous study that CRAMP inhibited I/R‐mediated cardiomyocyte apoptosis by activation of Akt and ERK1/2 and phosphorylation and nuclear export of FoxO3a (Bei et al., 2019). In converse, renal function recovery was significantly delayed following I/R injury in mice with a specific EGFR deletion in the renal proximal tubule (Chen et al., 2012). Meanwhile, waved‐2 mice showing reduced EGFR activity developed more severe acute renal tubular damage with less reparative responses after renal ischaemia as indicated by enhanced tubular cell apoptosis and reduced dedifferentiation and proliferation as compared to WT mice (Tang, Liu, Tolbert, et al., 2013). In accordance with these earlier studies, we have observed a decrease in total EGFR expression 24 hr after I/R, likely to be correlated with loss of tubular epithelial cells. An overall protective effect of CRAMP with reduced renal apoptosis and injury was associated with restored EGFR expression. Together, our data indicate a protective role of CRAMP engaging EGFR during the acute inflammatory stage in AKI.
In conclusion, the present study demonstrates that CRAMP modulates I/R‐mediated immune‐inflammatory responses, contributing to the loss of epithelial cells and renal dysfunction. The attenuating effect of CRAMP on I/R‐mediated renal dysfunction is at least in part dependent on NLRP3 inflammasome inhibition and mediated via EGFR. Our findings indicate that CRAMP is a novel modulator of AKI pathogenesis and raise the possibility that modulation of CRAMP might represent a novel therapeutic strategy for AKI.
AUTHOR CONTRIBUTIONS
L.‐L.P., W.L., and Z.R. performed the experiments and analysed the data. C.L. and Y.C. helped with obtaining the clinical samples. W.N., X.F., H.L., and M.Z. assisted the experiments. J.D. and B.A. contributed to the data acquisition and critically reviewed the manuscript. J.S. and L.‐L.P. designed and interpreted the experiments. L.‐L.P. and J.S. wrote the paper.
CONFLICT OF INTEREST
The authors have declared no competing interests.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14208, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Table S1. Clinical features of patients with acute kidney injury (no. or mean ± SEM)
Figure S1. Localization and expression of CRAMP (red) in kidney tissues by immunofluorescent staining (n = 5 minimum per group). Representative photomicrographs of individual and merged stainings are shown. Blue: nuclei staining with DAPI; green: kidney epithelial marker E‐cadherin; yellow: Ly6G; red: CRAMP. Scale bar: 50 μm.
Figure S2. The effects of exogenous CRAMP treatment on renal and serum CRAMP levels. Mice were treated with CRAMP (5 mg·kg−1) 1 h hr before I/R, blood and kidney tissue were collected 24 h after reperfusion. (a) quantitative analysis of serum CRAMP levels by ELISA. (b) quantitative analysis of renal CRAMP levels by ELISA. The results are shown as mean ± SEM, n = 8 per group. *P < 0.05 by one‐way ANOVA followed by Tukey's test.
Figure S3. The effects of CRAMP treatment alone on renal function. Mice were treated with CRAMP (5 mg·kg−1) alone 1 h hour before sham surgical procedure, blood and kidney tissue were collected 24 h after reperfusion. (a) Serum urea (n = 8 per group). (b) Serum creatinine (n = 8 per group). (c) Myeloperoxidase (MPO) activities in kidney tissues (n = 8 per group). (d) Representative histological sections and quantification of necrosis in tubular epithelial cells (n = 8 per group). Scale bar: 100 μm. The results are shown as mean ± SEM. Grouped t test was used.
Figure S4. CRAMP modulates immune cell responses in renal I/R‐induced AKI. Mice (C57BL/6) were treated with CRAMP (5 mg·kg−1) at 1 hour before I/R. Flow cytometry was performed on kidney‐derived single‐cell suspensions to analyze intrarenal CD45+ leukocytes. Gr‐1+CD11b+ cells were considered neutrophils; F4/80+CD11b+ cells were considered macrophages. (a) Percentage of Gr‐1+CD11b+ neutrophils in CD45+ cells (n = 8 minimum per group). (b) Percentage of F4/80+ CD11b+ macrophages in CD45+ cells (n = 8 minimum per group). (c) Percentage of CD206+ M2 macrophages in F4/80+ CD11b+ macrophages (n = 8 minimum per group). Data are expressed as median ± interquartile range by one‐way ANOVA followed by Tukey's test. *P < 0.05. FSC, forward scatter; SSC, side scatter.
ACKNOWLEDGEMENTS
The work was supported by funds from the National Natural Science Foundation of China (Grants 81973322, 81870439 91642114, 31570915, 81573420, and National Youth 1000 Talents Plan), Jiangsu Province Recruitment Plan for High‐level, Innovative and Entrepreneurial Talents (Innovative Research Team), Collaborative innovation center of food safety and quality control in Jiangsu Province, the Fundamental Research Funds for the Central Universities (Grant JUSRP11866), National First‐Class Discipline Program of Food Science and Technology (Grant JUFSTR20180103), Wuxi Social Development Funds for International Science & Technology Cooperation (Grant WX0303B010518180007PB), Jiangsu Province Qing Lan Project, Jiangsu Province “Six Summit Talents” Program (Grant 2019‐YY‐038), and Postgraduate Research & Practice Innovation Program of Jiangnan University (Grant JNKY19_005).
Pan L‐L, Liang W, Ren Z, et al. Cathelicidin‐related antimicrobial peptide protects against ischaemia reperfusion‐induced acute kidney injury in mice. Br J Pharmacol. 2020;177:2726–2742. 10.1111/bph.14998
Li‐Long Pan Wenjie Liang and Zhengnan Ren contributed equally to this work.
REFERENCES
- Abed, A. , Toubas, J. , Kavvadas, P. , Authier, F. , Cathelin, D. , Alfieri, C. , … Chadjichristos, C. E. (2014). Targeting connexin 43 protects against the progression of experimental chronic kidney disease in mice. Kidney International, 86, 768–779. 10.1038/ki.2014.108 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … CGTP Collaborators (2019). The Concise Guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176, S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators (2019a). The Concise Guide to PHARMACOLOGY 2019/20: Catalytic receptors. British Journal of Pharmacology, 176, S247–S296. 10.1111/bph.14751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators (2019b). The Concise Guide to PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Faccenda, E. , … CGTP Collaborators (2019). The Concise Guide to PHARMACOLOGY 2019/20: Introduction and Other Protein Targets. British Journal of Pharmacology, 176, S1–S20. 10.1111/bph.14747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , … CGTP Collaborators (2019). The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology, 176, S142–S228. 10.1111/bph.14749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali, A. S. , Townes, C. L. , Hall, J. , & Pickard, R. S. (2009). Maintaining a sterile urinary tract: The role of antimicrobial peptides. The Journal of Urology, 182, 21–28. 10.1016/j.juro.2009.02.124 [DOI] [PubMed] [Google Scholar]
- Anders, H. J. (2016). Of inflammasomes and alarmins: IL‐1β and IL‐1α in kidney disease. J Am Soc Nephrol, 27, 2564–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders, H. J. , & Muruve, D. A. (2011). The inflammasomes in kidney disease. Journal of the American Society of Nephrology: JASN, 22, 1007–1018. 10.1681/ASN.2010080798 [DOI] [PubMed] [Google Scholar]
- Andrade‐Oliveira, V. , Amano, M. T. , Correa‐Costa, M. , Castoldi, A. , Felizardo, R. J. , de Almeida, D. C. , … Câmara, N. O. (2015). Gut bacteria products prevent AKI induced by ischemia‐reperfusion. J Am Soc Nephrol, 26, 1877–1888. 10.1681/ASN.2014030288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetta, J. , Chun, R. F. , Gales, B. , Zaritsky, J. J. , Leroy, S. , Wesseling‐Perry, K. , … Hewison, M. (2014). Antibacterial responses by peritoneal macrophages are enhanced following vitamin D supplementation. PLoS ONE, 9, e116530 10.1371/journal.pone.0116530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, M. , Zhang, L. , Fu, B. , Bai, J. , Zhang, Y. , Cai, G. , … Chen, X. (2018). IL‐17A improves the efficacy of mesenchymal stem cells in ischemic‐reperfusion renal injury by increasing Treg percentages by the COX‐2/PGE2 pathway. Kidney International, 93, 814‐825. [DOI] [PubMed] [Google Scholar]
- Bao, J. , Sato, K. , Li, M. , Gao, Y. , Abid, R. , Aird, W. , … Post, M. J. (2001). PR‐39 and PR‐11 peptides inhibit ischemia‐reperfusion injury by blocking proteasome‐mediated IκBα degradation. American Journal of Physiology. Heart and Circulatory Physiology, 281, H2612–H2618. 10.1152/ajpheart.2001.281.6.H2612 [DOI] [PubMed] [Google Scholar]
- Becknell, B. , Schwaderer, A. , Hains, D. S. , & Spencer, J. D. (2015). Amplifying renal immunity: The role of antimicrobial peptides in pyelonephritis. Nature Reviews Nephrology, 11, 642–655. 10.1038/nrneph.2015.105 [DOI] [PubMed] [Google Scholar]
- Bei, Y. , Pan, L. L. , Zhou, Q. , Zhao, C. , Xie, Y. , Wu, C. , … Xiao, J. (2019). Cathelicidin‐related antimicrobial peptide protects against myocardial ischemia/reperfusion injury. BMC Medicine, 17, 42 10.1186/s12916-019-1268-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, K. L. , Poon, G. F. T. , Birkenhead, D. , Pena, O. M. , Falsafi, R. , Dahlgren, C. , … Johnson, P. (2011). Host defense peptide LL‐37 selectively reduces proinflammatory macrophage responses. The Journal of Immunology, 186, 5497–5505. 10.4049/jimmunol.1002508 [DOI] [PubMed] [Google Scholar]
- Chen, J. , Chen, J. K. , & Harris, R. C. (2012). Deletion of the epidermal growth factor receptor in renal proximal tubule epithelial cells delays recovery from acute kidney injury. Kidney International, 82, 45–52. 10.1038/ki.2012.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , You, H. , Li, Y. , Xu, Y. , He, Q. , & Harris, R. C. (2018). EGF receptor‐dependent YAP activation is important for renal recovery from AKI. Journal of the American Society of Nephrology: JASN, 29, 2372–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chromek, M. (2015). The role of the antimicrobial peptide cathelicidin in renal diseases. Pediatric Nephrology, 30, 1225–1232. 10.1007/s00467-014-2895-3 [DOI] [PubMed] [Google Scholar]
- Chromek, M. , Slamova, Z. , Bergman, P. , Kovacs, L. , Podracka, L. , Ehren, I. , … Brauner, A. (2006). The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nature Medicine, 12, 636–641. 10.1038/nm1407 [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, B. Q. , Luo, Y. , Kang, X. , Li, C. B. , Morisseau, C. , Yang, J. , … Liu, J. Y. (2017). Epoxide metabolites of arachidonate and docosahexaenoate function conversely in acute kidney injury involved in GSK3β signaling. Proceedings of the National Academy of Sciences of the United States of America, 114, 12608–12613. 10.1073/pnas.1705615114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana, J. , Simoni, Y. , Furio, L. , Beaudoin, L. , Agerberth, B. , Barrat, F. , & Lehuen, A. (2013). Crosstalk between neutrophils, B‐1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nature Medicine, 19, 65–73. 10.1038/nm.3042 [DOI] [PubMed] [Google Scholar]
- Gallo, R. L. , & Hooper, L. V. (2012). Epithelial antimicrobial defence of the skin and intestine. Nature Reviews. Immunology, 12, 503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliet, M. , & Lande, R. (2008). Antimicrobial peptides and self‐DNA in autoimmune skin inflammation. Current Opinion in Immunology, 20, 401–407. 10.1016/j.coi.2008.06.008 [DOI] [PubMed] [Google Scholar]
- Gluba, A. , Banach, M. , Hannam, S. , Mikhailidis, D. P. , Sakowicz, A. , & Rysz, J. (2010). The role of Toll‐like receptors in renal diseases. Nature Reviews Nephrology, 6, 224–235. 10.1038/nrneph.2010.16 [DOI] [PubMed] [Google Scholar]
- Hancock, R. E. , Haney, E. F. , & Gill, E. E. (2016). The immunology of host defence peptides: Beyond antimicrobial activity. Nature Reviews. Immunology, 16, 321–334. [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res., 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havasi, A. , & Borkan, S. C. (2011). Apoptosis and acute kidney injury. Kidney International, 80, 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, S. , Liu, N. , Bayliss, G. , & Zhuang, S. (2013). EGFR activity is required for renal tubular cell dedifferentiation and proliferation in a murine model of folic acid‐induced acute kidney injury. American Journal of Physiology. Renal Physiology, 304, F356–F366. 10.1152/ajprenal.00553.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilchie, A. L. , Wuerth, K. , & Hancock, R. E. W. (2013). Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nature Chemical Biology, 9, 761‐768. [DOI] [PubMed] [Google Scholar]
- Hoffmeyer, M. R. , Scalia, R. , Ross, C. R. , Jones, S. P. , & Lefer, D. J. (2000). PR‐39, a potent neutrophil inhibitor, attenuates myocardial ischemia‐reperfusion injury in mice. American Journal of Physiology. Heart and Circulatory Physiology, 279, H2824–H2828. [DOI] [PubMed] [Google Scholar]
- Hu, Z. , Murakami, T. , Suzuki, K. , Tamura, H. , Kuwahara‐Arai, K. , Iba, T. , & Nagaoka, I. (2014). Antimicrobial cathelicidin peptide LL‐37 inhibits the LPS/ATP‐induced pyroptosis of macrophages by dual mechanism. PLoS ONE, 9, e85765 10.1371/journal.pone.0085765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlenberg, J. M. , Carmona‐Rivera, C. , Smith, C. K. , & Kaplan, M. J. (2013). Neutrophil extracellular trap‐associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. Journal of Immunology, 190, 1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. J. , Lee, D. W. , Ravichandran, K. , Keys, D. O. , Akcay, A. , Nguyen, Q. , … Edelstein, C. L. (2013). NLRP3 inflammasome knockout mice are protected against ischemic but not cisplatin‐induced acute kidney injury. The Journal of Pharmacology and Experimental Therapeutics, 346, 465–472. 10.1124/jpet.113.205732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. , Huen, S. , Nishio, H. , Nishio, S. , Lee, H. K. , Choi, B. S. , … Cantley, L. G. (2011). Distinct macrophage phenotypes contribute to kidney injury and repair. Journal of the American Society of Nephrology: JASN, 22, 317–326. 10.1681/ASN.2009060615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans, J. C. , Stokman, G. , Claessen, N. , Rouschop, K. M. , Teske, G. J. , Kirschning, C. J. , … Florquin, S. (2005). Renal‐associated TLR2 mediates ischemia/reperfusion injury in the kidney. The Journal of Clinical Investigation, 115, 2894–2903. 10.1172/JCI22832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookherjee, N. , Brown, K. L. , Bowdish, D. M. , Doria, S. , Falsafi, R. , Hokamp, K. , … Powers, J. P. (2006). Modulation of the TLR‐mediated inflammatory response by the endogenous human host defense peptide LL‐37. Journal of Immunology, 176, 2455–2464. 10.4049/jimmunol.176.4.2455 [DOI] [PubMed] [Google Scholar]
- Nishikawa, H. , Taniguchi, Y. , Matsumoto, T. , Arima, N. , Masaki, M. , Shimamura, Y. , … Komatsu, T. (2018). Knockout of the interleukin‐36 receptor protects against renal ischemia‐reperfusion injury by reduction of proinflammatory cytokines. Kidney International, 93, 599‐614. [DOI] [PubMed] [Google Scholar]
- Peng, H. , Purkerson, J. M. , Schwaderer, A. L. , & Schwartz, G. J. (2017). Metabolic acidosis stimulates the production of the antimicrobial peptide cathelicidin in rabbit urine. American Journal of Physiology Renal Physiology, 313, F1061–F1067. 10.1152/ajprenal.00701.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabadi, M. , Kim, M. , D'Agati, V. , & Lee, H. T. (2016). Peptidyl arginine deiminase‐4‐deficient mice are protected against kidney and liver injury after renal ischemia and reperfusion. American Journal of Physiology Renal Physiology, 311, F437–F449. 10.1152/ajprenal.00254.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raup‐Konsavage, W. M. , Wang, Y. , Wang, W. W. , Feliers, D. , Ruan, H. , & Reeves, W. B. (2018). Neutrophil peptidyl arginine deiminase‐4 has a pivotal role in ischemia/reperfusion‐induced acute kidney injury. Kidney International, 93, 365‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekha, R. S. , Rao Muvva, S. S. , Wan, M. , Raqib, R. , Bergman, P. , Brighenti, S. , … Agerberth, B. (2015). Phenylbutyrate induces LL‐37‐dependent autophagy and intracellular killing of Mycobacterium tuberculosis in human macrophages. Autophagy, 11, 1688–1699. 10.1080/15548627.2015.1075110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, J. , Wang, L. , Jiang, N. , Mou, S. , Zhang, M. , Gu, L. , … Ni, Z. (2016). NLRP3 inflammasome mediates contrast media‐induced acute kidney injury by regulating cell apoptosis. Scientific Reports, 6, 34682 10.1038/srep34682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeoka, A. A. , Mueller, J. L. , Kambo, A. , Mathison, J. C. , King, A. J. , Hall, W. F. , … McKay, D. B. (2010). An inflammasome‐independent role for epithelial‐expressed Nlrp3 in renal ischemia‐reperfusion injury. Journal of Immunology, 185, 6277–6285. 10.4049/jimmunol.1002330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soehnlein, O. , Wantha, S. , Simsekyilmaz, S. , Doring, Y. , Megens, R. T. , Mause, S. F. , … Gries, T. (2011). Neutrophil‐derived cathelicidin protects from neointimal hyperplasia. Science Translational Medicine, 3, 103ra198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokman, G. , Kors, L. , Bakker, P. J. , Rampanelli, E. , Claessen, N. , Teske, G. J. D. , … Leemans, J. C. (2017). NLRX1 dampens oxidative stress and apoptosis in tissue injury via control of mitochondrial activity. The Journal of Experimental Medicine, 214, 2405–2420. 10.1084/jem.20161031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, J. , Dahlen, B. , Agerberth, B. , & Haeggstrom, J. Z. (2013). The antimicrobial peptide LL‐37 induces synthesis and release of cysteinyl leukotrienes from human eosinophils–implications for asthma. Allergy, 68, 304–311. [DOI] [PubMed] [Google Scholar]
- Sun, J. , Furio, L. , Mecheri, R. , van der Does, A. M. , Lundeberg, E. , Saveanu, L. , … Diana, J. (2015). Pancreatic β‐cells limit autoimmune diabetes via an immunoregulatory antimicrobial peptide expressed under the influence of the gut microbiota. Immunity, 43, 304–317. 10.1016/j.immuni.2015.07.013 [DOI] [PubMed] [Google Scholar]
- Sun, J. , Xu, M. , Ortsater, H. , Lundeberg, E. , Juntti‐Berggren, L. , Chen, Y. Q. , … Agerberth, B. (2016). Cathelicidins positively regulate pancreatic β‐cell functions. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 30, 884–894. 10.1096/fj.15-275826 [DOI] [PubMed] [Google Scholar]
- Tang, J. , Liu, N. , Tolbert, E. , Ponnusamy, M. , Ma, L. , Gong, R. , … Zhuang, S. (2013). Sustained activation of EGFR triggers renal fibrogenesis after acute kidney injury. The American Journal of Pathology, 183, 160–172. 10.1016/j.ajpath.2013.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, J. , Liu, N. , & Zhuang, S. (2013). Role of epidermal growth factor receptor in acute and chronic kidney injury. Kidney International, 83, 804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togel, F. , & Westenfelder, C. (2014). Recent advances in the understanding of acute kidney injury. F1000Prime Rep, 6, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. , & Liu, Y. (2001). Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. The American Journal of Pathology, 159, 1465–1475. 10.1016/S0002-9440(10)62533-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff, M. (2007). Antimicrobial peptides, innate immunity, and the normally sterile urinary tract. Journal of the American Society of Nephrology: JASN, 18, 2810–2816. 10.1681/ASN.2007050611 [DOI] [PubMed] [Google Scholar]
- Zhang, M. Z. , Wang, X. , Wang, Y. , Niu, A. , Wang, S. , Zou, C. , & Harris, R. C. (2017). IL‐4/IL‐13‐mediated polarization of renal macrophages/dendritic cells to an M2a phenotype is essential for recovery from acute kidney injury. Kidney International, 91, 375–386. 10.1016/j.kint.2016.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, X. , Zhang, W. , Yao, Q. , Zhang, H. , Dong, G. , Zhang, M. , … Dong, Z. (2017). Exosome production and its regulation of EGFR during wound healing in renal tubular cells. American Journal of Physiology Renal Physiology, 312, F963–F970. 10.1152/ajprenal.00078.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk, A. , & Bonventre, J. V. (2016). Acute Kidney Injury. Annual Review of Medicine, 67, 293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical features of patients with acute kidney injury (no. or mean ± SEM)
Figure S1. Localization and expression of CRAMP (red) in kidney tissues by immunofluorescent staining (n = 5 minimum per group). Representative photomicrographs of individual and merged stainings are shown. Blue: nuclei staining with DAPI; green: kidney epithelial marker E‐cadherin; yellow: Ly6G; red: CRAMP. Scale bar: 50 μm.
Figure S2. The effects of exogenous CRAMP treatment on renal and serum CRAMP levels. Mice were treated with CRAMP (5 mg·kg−1) 1 h hr before I/R, blood and kidney tissue were collected 24 h after reperfusion. (a) quantitative analysis of serum CRAMP levels by ELISA. (b) quantitative analysis of renal CRAMP levels by ELISA. The results are shown as mean ± SEM, n = 8 per group. *P < 0.05 by one‐way ANOVA followed by Tukey's test.
Figure S3. The effects of CRAMP treatment alone on renal function. Mice were treated with CRAMP (5 mg·kg−1) alone 1 h hour before sham surgical procedure, blood and kidney tissue were collected 24 h after reperfusion. (a) Serum urea (n = 8 per group). (b) Serum creatinine (n = 8 per group). (c) Myeloperoxidase (MPO) activities in kidney tissues (n = 8 per group). (d) Representative histological sections and quantification of necrosis in tubular epithelial cells (n = 8 per group). Scale bar: 100 μm. The results are shown as mean ± SEM. Grouped t test was used.
Figure S4. CRAMP modulates immune cell responses in renal I/R‐induced AKI. Mice (C57BL/6) were treated with CRAMP (5 mg·kg−1) at 1 hour before I/R. Flow cytometry was performed on kidney‐derived single‐cell suspensions to analyze intrarenal CD45+ leukocytes. Gr‐1+CD11b+ cells were considered neutrophils; F4/80+CD11b+ cells were considered macrophages. (a) Percentage of Gr‐1+CD11b+ neutrophils in CD45+ cells (n = 8 minimum per group). (b) Percentage of F4/80+ CD11b+ macrophages in CD45+ cells (n = 8 minimum per group). (c) Percentage of CD206+ M2 macrophages in F4/80+ CD11b+ macrophages (n = 8 minimum per group). Data are expressed as median ± interquartile range by one‐way ANOVA followed by Tukey's test. *P < 0.05. FSC, forward scatter; SSC, side scatter.


