Abstract
Background and Purpose
Dravet syndrome is a severe, genetic form of paediatric epilepsy associated with premature mortality and co‐morbidities such as anxiety, depression, autism, motor dysfunction and memory deficits. Cannabidiol is an approved anticonvulsive drug in the United States and Europe for seizures associated with Dravet syndrome in patients 2 years of age and older. We investigated its potential to prevent premature mortality and improve associated co‐morbidities.
Experimental Approach
The efficacy of sub‐chronic cannabidiol administration in two mouse models of Dravet syndrome was investigated. The effect of cannabidiol on neonatal welfare and survival was studied using Scn1a −/− mice. We then used a hybrid, heterozygote Scn1a +/− mouse model to study the effect of cannabidiol on survival and behavioural co‐morbidities: motor deficits (rotarod and static‐beam test), gait abnormality (gait test), social anxiety (social interaction test), anxiety‐like (elevated plus maze) and depressive‐like behaviours (sucrose preference test) and cognitive impairment (radial arm maze test).
Key Results
In Scn1a −/− mice, cannabidiol increased survival and delayed worsening of neonatal welfare. In Scn1a +/− mice, chronic cannabidiol administration did not show any adverse effect on motor function and gait, reduced premature mortality, improved social behaviour and memory function, and reduced anxiety‐like and depressive‐like behaviours.
Conclusion and Implications
We are the first to demonstrate a potential disease‐modifying effect of cannabidiol in animal models of Dravet syndrome. Cannabidiol treatment reduced premature mortality and improved several behavioural co‐morbidities in Dravet syndrome mice. These crucial findings may be translated into human therapy to address behavioural co‐morbidities associated with Dravet syndrome.
Abbreviations
- AED
Anti‐epileptic Drug
- ASPA
Animals (Scientific Procedures)Act
- CBD
cannabidiol
- TNW
Total Neonatal Welfare
What is already known about this subject
Dravet syndrome is associated with premature mortality, seizures and associated co‐morbidities.
Cannabidiol's (CBD's) anticonvulsive action in Dravet syndrome has been demonstrated in preclinical and clinical studies.
What this study adds
Chronic CBD administration reduces premature mortality in two mouse models of Dravet syndrome.
This study demonstrates that CBD improves co‐morbidities in a mouse model of Dravet syndrome.
What is the clinical significance
CBD may improve survival in human Dravet syndrome patients.
CBD may be a suitable treatment to reduce the possibility of behavioural co‐morbidities in human Dravet syndrome patients.
1. INTRODUCTION
Dravet syndrome is a severe form of myoclonic epilepsy in children (Dravet, Bureau, Oguni, Fukuyama, & Cokar, 2005). It is typically triggered by a fever which initiates the first generalised or partial seizure at usually 6 to 9 months of age (Dravet et al., 2005). During the first year of life, seizures are relatively infrequent but over time, can lead to status epilepticus. In subsequent years, the symptoms become more severe with generalised tonic–clonic, myoclonic, absence and focal seizures being common, status epilepticus rarely occurs after 10 years of age (Akiyama, Kobayashi, Yoshinaga, & Ohtsuka, 2010). The https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=578 gene encodes https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=578, a voltage‐gated sodium channel, which plays a pivotal role in Dravet syndrome where 70–80% of patients exhibit a deletion or mutation (truncating, missense, or splice site mutations leading to loss of function) of this gene on chromosome 2q (Harkin et al., 2007; Marini et al., 2011).
In addition to seizures, premature mortality occurs in approximately 21% Dravet syndrome patients (Dravet et al., 2005; Genton, Velizarova, & Dravet, 2011). Patients also exhibit multiple co‐morbidities including psychomotor delay, gait abnormality, hyperactivity, attention deficits, autism, sleep disorders, anxiety, depression, language impairment and severe cognitive deficits, profoundly affecting quality of life (Genton et al., 2011). The current standard treatment includes the combination of two or more antiepileptic drugs, such as benzodiazepines, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7009, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6849, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6826 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5469 (Devinsky et al., 2017). However, these drugs often fail to adequately control seizures and are associated with severe drug‐induced motor and psychiatric adverse effects including anxiety, depression and memory impairments (Chen et al., 2017; Ristić, Vojvodić, Janković, Sindelić, & Sokić, 2006).
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4150 (CBD) is one of the most abundant plant‐derived cannabinoids. It is non‐euphoric and has shown potential for use in treating seizures, epilepsy, epileptogenesis and related neuroprotection in a number of animal models including Dravet syndrome (Kaplan, Stella, Catterall, & Westenbroek, 2017; Patra et al., 2019; Rosenberg, Patra, & Whalley, 2017). CBD was demonstrated to be an effective anticonvulsant in Phase 3 clinical trials for the treatment of Dravet syndrome and Lennox Gastaut Syndrome and in 2018 received FDA approval in the United States for seizures associated with these disorders (Devinsky et al., 2017; FDA, 2018; Thiele et al., 2018). However, little is known about its potential effect on the premature mortality and co‐morbidities associated with Dravet syndrome.
Here, we first evaluated the effect of CBD on survival and neonatal welfare in Scn1a −/− knockout mice Scn1a −/− mice are obtained from mice with a 129S background. They differ from human Dravet syndrome by their complete loss of expression of NaV1.1 channels, but they reproduce the ataxia, seizures and premature mortality commonly observed in Dravet syndrome patients (Kalume, Yu, Westenbroek, Scheuer, & Catterall, 2007; Yu et al., 2006). They exhibit symptoms such as seizures, ataxia and a poor righting reflex from post‐natal day 9 (P9) onwards which become progressively worse over time, with animals typically dying before they reach P16 (Kalume et al., 2007; Miller, Hawkins, McCollom, & Kearney, 2014; Yu et al., 2006). Due to the extreme severity and early mortality, this model is not suitable for studying complex behavioural co‐morbidities but does allow rapid assessment of antiepileptic drug (AED) efficacy and was used here as a proof‐of‐concept for CBD efficacy.
We then conducted a study in the hybrid heterozygote Scn1a +/− mouse Dravet model to investigate the effect of chronic CBD treatment in prevention of premature mortality and a range of complex behavioural co‐morbidities such as motor impairment, anxiety, depression, social and memory deficits. This model of Dravet syndrome uses a hybrid heterozygote Scn1a +/− mouse model breeding male heterozygotic mice from a 129S background with female wild‐type (WT) mice from the C57/Black six (C57/B6) background. The resultant heterozygote offspring recapitulate several features of Dravet syndrome including seizures, premature mortality and co‐morbidities such as social deficit, anxiety and memory impairments (Han et al., 2012; Yu et al., 2006). They are considered a standard model to study drug effects on Dravet syndrome and complex behavioural co‐morbidities (Anderson, Hawkins, Thompson, Kearney, & George, 2017; Hawkins et al., 2017; Kaplan et al., 2017).
2. METHODS
Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology and UK Home Office regulations (Animals [Scientific Procedures] Act, 1986; ASPA) under licence 70/8397 “Mouse Model of Dravet Syndrome” and were approved by the Animal Welfare and Ethics Review Board at the University of Reading (McGrath & Lilley, 2015). The study used a total of 182 mice: 95 in Study I (19 for the development of the survival algorithm, 36 for breeding/colony maintenance and 40 for the study) and 87 in Study II (15 for breeding/colony maintenance, 52 in test groups, and a further 20 WT “novel” animals for social interaction test). All mice were maintained in 12 hr:12 hr light:dark cycle, a room temperature of 21°C and humidity of 50 ± 10%, with ad libitum access to food and water. Both male and female mice were used in all experimental groups.
2.1. Study I: Assessment of neonatal welfare and survival in Scn1a −/− mice
2.1.1. Animals
129S‐Scn1a tm1Kea/Mmjax (RRID:MMRRC_037107‐JAX) heterozygote mice (Jackson Laboratory, USA) were maintained in the BioResource Unit at the University of Reading (UK) and bred together to obtain Scn1a −/− and WT animals used for this study (n = 10 per group; Figure 1a). All experiments were conducted during light phase 8:00 a.m.–8:00 p.m. The maternal behaviour (see Table S1) of the dams was also assessed simultaneously to ensure that any of the parameters observed in the study animals (Scn1a −/−/WT mice) were not affected by the dam's behaviour. In this study, dam scores remained 0 throughout the study and so the responses of the pups were not considered to have been affected by variations in maternal behaviours. At the end of the study, animals were humanely killed by a Schedule 1 method (cervical dislocation).
Figure 1.
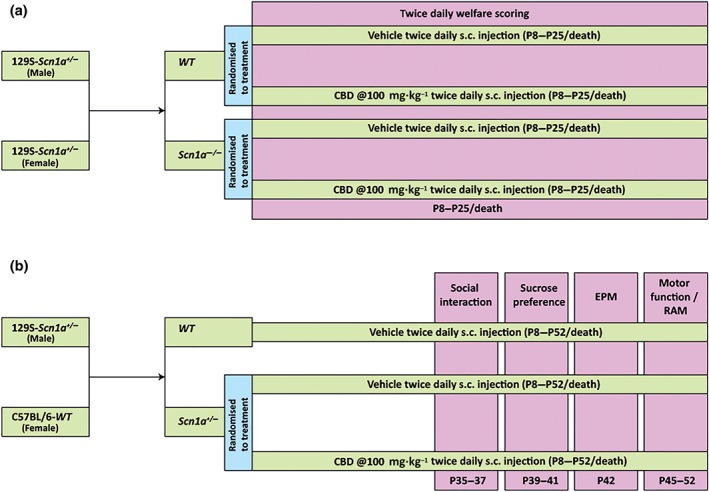
Schematic showing the timeline of the drug administration and behavioural tests. (a) Timeline of drug administration and welfare scoring in Study I. The wild‐type (WT) and Scn1a −/− mice were injected (s.c.) twice daily with either vehicle or CBD (100 mg·kg−1) from post‐natal day 8 (P8) to P25/death. Twice daily welfare scoring was conducted in all the groups throughout the experimental period. (b) Timeline of drug administration and co‐morbidity tests in Study II. The Scn1a −/− mice were injected (s.c.) twice daily with either vehicle or CBD (100 mg·kg−1) from P8 to P52/death. A WT vehicle‐treated control group was also taken. Social interaction (P35–P37), sucrose preference (P39–P41), elevated plus maze (EPM; P42) and motor function and radial arm maze (RAM; P45–P52) tests were conducted in all the groups
2.1.2. Experimental design
Following genotyping (see supplementary material 1), animals were randomly divided into four groups: WT vehicle treated, WT CBD treated, Scn1a −/− vehicle treated, and Scn1a −/− CBD treated (n = 10 per group). They were injected subcutaneously twice daily with either CBD (100 mg·kg−1) or its vehicle (ethanol: Kolliphor®: 0.9% saline = 2:1:17) from P8 until P25 or death (whichever was earlier). A twice daily welfare check was conducted throughout the entire duration of the study. Drug administration was conducted at 08:00 a.m. and followed by welfare checks. Conversely, afternoon welfare checks were conducted from 04:00 p.m. and followed by drug administration in order to provide the maximum possible time between doses. The experimental timeline is depicted in Figure 1a.
2.1.3. Assessment of welfare scores
Welfare scoring of neonates was conducted twice daily using a blinded spreadsheet that lacks the information on the genotype of the animals and the treatment (CBD/vehicle) given to them, to ensure the experimenter remained blind to both treatment and genotype. Neonatal welfare scoring (Table S2) was based upon a previously validated standardised approach used widely in murine models (Langford et al., 2010; Ullman‐Culleré & Foltz, 1999; Wolfensohn & Lloyd, 2007). Here, the parameters used for the welfare assessment were weight, natural activity (NA; 0–3), reflex/response to touch (RT; 0–3), orbital tightening (OT; 0–2), body condition (BC) score (1–3) and surface temperature (ST; 0–2; data not shown). Finally, a total neonatal welfare (TNW) score (range 0–8) was calculated by adding together scores from NA, RT and ST. CBD treatment significantly delayed the worsening of NA, RT, OT, BC and TNW scores in the Scn1a −/− mice compared to their vehicle‐treated counterparts. For clarity only, the TNW data have been reported in this manuscript; the rest of the welfare data have been supplied in Figure S2.
2.1.4. Assessment of survival
Animal suffering was minimised by employing a validated, welfare scoring system (Table S2) alongside a mathematical model to predict death; see Data S2 and Data S3 for the original welfare data used to construct the model (Animals 1–19 only) and the welfare data for animals used in the study. In this way, any animal for which the model predicted death could be killed 0.5 day before enduring the maximal severity of the disease and enabling us to comply with welfare requirements of ASPA. The model used an algorithm to predict death based on prior data obtained from untreated Scn1a −/− mice (n = 19) that exhibited the maximum severity of the disease and died a natural death (Data S3). In this algorithm, the thresholds for each parameter (TNW, NA, RT, OT and BC scores) to predict death were obtained using the following procedure: (i) Each parameter, measured every half day from birth for each animal, is averaged with a moving mean with a 1.5‐day window; (ii) the least severe score for each parameter observed across the 19 animals over 0.5 day before their death was found; (iii) each of the five parameters exhibited by the animals in the study was compared to scores obtained in (ii) twice a day; (iv) if each of the five parameters reached their respective threshold defined in (ii) at least once since P8, the animal would undergo a Schedule 1 procedure (cervical dislocation) within 0.5 day. Additionally, ST threshold was employed such that if the sum of the ST scores over the last 1.5 days was equal to or greater than 3, the animal would be killed by Schedule 1 procedure (cervical dislocation) within 0.5 day.
2.2. Study II: Assessment of survival and co‐morbidities in Scn1a +/− mice
2.2.1. Animals
The animals were group housed throughout the study except for 3 days during sucrose preference test when each animal was individually housed. This study was conducted in a reversed dark:light cycle with all experiments performed in the dark cycle (dim red light, 8:00 a.m.–8:00 p.m.). Male 129S‐Scn1a tm1Kea/Mmjax heterozygote mice (Jackson Laboratory, USA) maintained in the BioResource Unit, University of Reading (UK), were bred with female WT C57BL/6 mice (Charles River, UK; RRID:MGI:5656552) to obtain Scn1a +/− and WT littermate mice used in this study. At the end of the study, animals were humanely killed by a Schedule 1 method (cervical dislocation).
2.2.2. Experimental design
Here, hybrid Scn1a +/− of both sexes was randomly divided into two groups and subcutaneously injected with either CBD (100 mg·kg−1 twice daily; n = 12) or its vehicle (ethanol:Kolliphor®:0.9% saline = 2:1:17; n = 29) from P8 onwards until P52 or death (whichever was earlier). Similarly, WT littermate mice (n = 11) were injected with vehicle for the entire period of the study. Given that a significant number of deaths (~60%) were predicted to occur between P20–P27 in vehicle‐treated Scn1a +/−, a larger initial group size was utilised to obtain a minimum n = 10 animals per group for behavioural assessment from P35 onwards. Of note, we accounted the possibility that CBD‐treated animals might also die; therefore, in the first run, equal group sizes were taken. However, the mortality was higher in the vehicle‐treated Scn1a +/− group than in the CBD‐treated Scn1a +/− group, so in subsequent runs, a greater number of animals were included in the vehicle‐treated Scn1a +/− group. The experimental timeline has been depicted in Figure 1b.
2.2.3. Assessment of survival
As seizure‐related deaths in this model were unpredictable, animals were video monitored continuously (24 hr × 7 days) throughout the study, and any mortality observed was cross‐checked with the available video footage (1 hr prior to death) to confirm the reason of death.
2.2.4. Assessment of motor function
Fine motor control in animals were assessed by the accelerated rotarod and static beam tests. Animals were habituated to the stationary rotarod for 2 min a day for 2 days. In the accelerated rotarod test, each mouse was placed individually on a linearly accelerating rod (4–40 rpm over 5 min; LE8500, Letica Scientific Instruments, UK), and average latency to fall from the rod (maximum 300 s) was calculated from three consecutive trials (2 min interval between trials).
The static beam task was further employed to analyse balance and coordination (Sedy, Urdzikova, Jendelova, & Sykova, 2008), where the animals were required to walk along a cylindrical elevated beam (100 cm long, 0.9 cm diameter, and 50 cm height from floor) and enter a dark enclosure at the beam end. The mice were habituated to the task for three consecutive days before the test day. Each day of the habituation period, the animals were placed 30, 60, and 100 cm away from the enclosure and allowed to traverse along the beam. On the test day, each mouse performed two consecutive trials (2‐min interval between trials) with a maximum given time of 2 min to complete the task (the nose entering the box was taken as task completion). The test was video monitored (Sony DCR‐SX21E), and blinded offline analysis was conducted (Observer XT 12, Noldus, the Netherlands) to evaluate the average number of foot slips made from two consecutive trials.
2.2.5. Assessment of gait
Gait test was conducted to assess the cerebellar function of the animals (Patel & Hillard, 2001). In this test, the hind paws of each mouse were marked with a non‐toxic ink, and the mouse was allowed to walk on a white paper (50 × 10 cm) placed on the floor of a custom‐made plexiglass tunnel (50 × 10 × 10 cm). To obtain the left and right stride length, the distance between two ipsilateral paw prints was measured, whereas stride width was calculated from the distance between a foot print and its contralateral stride length at right angle (Wecker et al., 2013). The initial and last footprints were not considered in measurements. All the animals were habituated to the test procedures and the apparatus for 2 days prior to the test. On the day of test, two trials were conducted for each animal to obtain mean stride length (left or right) and width for that animal.
2.2.6. Assessment of social interaction
The social interaction test was conducted in the home cage of test mouse to assess the social behaviour of the animals (Sato, Mizuguchi, & Ikeda, 2013). On test day, cage mate(s) were removed from the home cage of the test mouse, and the mouse remained in isolation for 15 min. A novel WT mouse of same strain, same sex, and similar weight to the test mouse was then introduced to the home cage of the test mouse. Activity was video recorded (Sony DCR‐SX21E) for 10 min, and the obtained video files were blinded at the end of all experiments. Time spent in active interactions (e.g. close following, sniffing, allogrooming/social grooming and mounting) and number of rearing (lifting the front paws on the air) occasions were coded offline using Observer XT 12 (Noldus, the Netherlands). Aggressive behaviours were not considered as social interactions and were not coded. In this test, a reduced social interaction is considered as autistic‐like behaviour (Sato et al., 2013), while increased rearing occasions is sign of defensive escapes (Kaplan et al., 2017).
2.2.7. Assessment of anxiety‐like behaviours
The elevated plus maze (EPM) test was performed to assess the level of anxiety in animals (Chen et al., 2017). The wooden test apparatus consists of two closed arms (50 × 10 × 40 cm) and two open arms (50 × 10 cm) connected via a central platform (10 × 10 cm) and raised at a height of 50 cm above the floor. Each animal was placed on the central platform facing towards an open arm. Activity was video recorded (Swann SRDVR‐16440H, UK) for 5 min. The video files were blinded and coded offline at the end of all experiments using Observer XT 12 (Noldus, the Netherlands). Time spent on open arms was inversely related to the level of anxiety.
2.2.8. Assessment of depression‐like behaviour
The sucrose preference test was carried out to assess the depression‐like behaviour (Serova, Mulhall, & Sabban, 2017). The animals were separately housed during this test. Here, 24 hr before the test, animals were trained to drink from two bottles each containing 2% sucrose. On the first day of test, the animals were provided with a pre‐weighed bottle of 2% sucrose and another containing a pre‐weighed volume of tap water. The positions of the bottles were swapped after 24 hr to avoid any side preferences. After 48 hr, both bottles were weighed and sucrose preference was calculated by using the following formula.
2.2.9. Assessment of cognition
A radial arm maze (RAM) consisting of eight arms (each arm 60 × 10 cm; raised at 50 cm above the floor) was used to assess the reference memory and working memory (WM) of the animals. On four consecutive days, animals were given two 10‐min sessions of habituation to the test apparatus and rules of the test, separated by a 90‐min interval. During the first 2 days of habituation, food rewards (1/4 Cheerios®, Nestle) were randomly scattered on the floor of the apparatus covering all arms and food troughs at the end of each arm. On the third and fourth habituation day, food rewards were placed only in food troughs of four randomly selected arms (fixed for each animal during the habituation and test day). Food was withdrawn 4–6 hr before the trial (both during habituation and test days) to motivate the animals to locate the rewards and thus perform the task. On the test day, two trials of 10 min were conducted at 90‐min interval and the activity of the animals were video recorded for offline blinded coding after the end of experiment. Entry to a non‐baited arm was considered as a reference memory error (RME), whereas re‐entry to a previously baited arm from which the food was already taken is considered as a working memory error (WME). The mean working memory error or reference memory error was calculated from the two test trials.
2.3. Statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018).
In Study I, the welfare parameters were analysed using SPSS 24 (IBM SPSS Statistics®, UK; RRID:SCR_002865), while survival data were analysed using GraphPad Prism 6 software (GraphPad Software, Inc., USA; RRID:SCR_002798). Data obtained from welfare parameters were compared using a three‐way ANOVA to observe the main effects of treatment, genotype and time, and their two‐way and three‐way interactions. If significant two‐way interactions were found, Bonferroni post hoc tests were conducted on any Treatment × Genotype interactions to assess the effect of CBD treatment on different genotypes (WT/Scn1a −/−). Bonferroni post hoc tests were also conducted for any significant three‐way Treatment × Genotype × Time interactions to compare the effect of CBD treatment with vehicle treatment at every time point of welfare assessment in both the WT and Scn1a −/− groups. In all cases, post hoc analyses were corrected for multiple comparisons. Data from 2.2% welfare scores were outliers and were excluded from further analysis (±2.5*SD; Miller, 1991). For the survival data, survival curves from Scn1a −/− vehicle‐treated and CBD‐treated groups were compared using a Mantel–Cox test. No WT animals died during the study, so survival curves were not compared. All the data are expressed as mean ± SEM of n animals. In all cases, P < .05 is considered as the level of significance.
In Study II, the data were analysed in GraphPad Prism 6 software (RRID:SCR_002798). Survival curves of Scn1a +/− vehicle‐treated and Scn1a +/− CBD‐treated group were compared using a Mantel–Cox test. The percentage of animals from the Scn1a +/− vehicle‐treated and Scn1a +/− CBD‐treated groups that survived until the end of the study (P52) was compared by Fisher's exact test. Further, data obtained from the co‐morbidity assessment were checked for normality by D'Agostino and Pearson omnibus normality test. Data obtained from rotarod, gait, social interaction (active interaction), elevated plus maze, sucrose preference, and radial arm maze (reference memory error) tests were normally distributed and the differences between the three groups were analysed by one‐way ANOVA. If a significant difference was found, then Holm–Sidak post hoc test was conducted among the groups. On the other hand, data obtained from static beam, social interaction (rearing occasions) and radial arm maze (working memory errors) were found to be non‐parametric, thus were analysed by Kruskal–Wallis test. Upon observing a significant difference, the Dunn's post hoc test was employed to compare the groups. Multiple comparisons were corrected in all cases. Parametric data are presented in scattered dot plot in the figures and are expressed as mean ± SEM. Non‐parametric data are presented in box plot in the figures and are expressed as median, min to max, and interquartile range. In all cases, n = the number of mice in an experimental group, and P < .05 is considered as the level of significance.
2.4. Materials
CBD with batch no. 6046727R (Study I) and 070214 (Study II) were supplied by the GW Pharmaceuticals (Cambridge, UK). Ethanol and Kolliphor® were purchased from Sigma‐Aldrich (Poole, UK).
2.5. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2017), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
3. RESULTS
3.1. Study I
3.1.1. CBD improved neonatal welfare in Scn1a −/− mice
In this study, animals (n = 10 per group) were treated with either vehicle or CBD from P8 until P25 or death (whichever was earlier) and welfare was monitored twice daily. The mean TNW scores in WT vehicle‐treated, WT CBD‐treated, Scn1a −/− vehicle‐treated and Scn1a −/− CBD‐treated groups were respectively 0.39 ± 0.04, 0.24 ± 0.04, 3.66 ± 0.04 and 2.85 ± 0.04. Main significant effects of treatment where genotype and time, on TNW scores were found. A significant three‐way interaction among Treatment × Genotype × Time was observed, while significant two‐way interactions were observed for Treatment × Genotype, Treatment × Time and Genotype × Time. The post hoc comparison for Treatment × Genotype × Time interactions revealed that CBD delayed significantly the worsening of welfare scores in Scn1a −/− mice from P12 to P16 compared to the vehicle‐treated Scn1a −/− mice on respective days (Figure 2a). This post hoc test further showed that CBD significantly improved TNW score in WT animals from P8 to P8.5, that is, in first day of treatment compared to the WT vehicle‐treated animals on respective occasions (Figure 2a).
Figure 2.
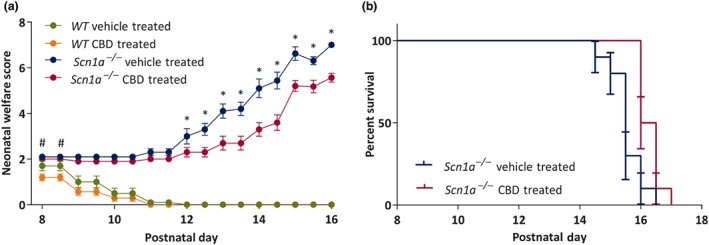
Plots showing chronic administration of CBD to wild‐type (WT) and Scn1a −/− mice on neonatal welfare (TNW) score and survival. (A) Neonatal welfare score. CBD significantly improved the TNW score in WT mice from P8 to P8.5 compared to the vehicle‐treated WT mice. CBD significantly improved the TNW score in Scn1a −/− mice from P12 onwards compared to the vehicle‐treated Scn1a −/− mice. Data are expressed as mean ± SEM; data were analysed by a three‐way ANOVA with Bonferroni's post hoc test; # P < .05; *P < .05; #represents WT vehicle‐treated versus WT CBD‐treated; *represents Scn1a −/− vehicle‐treated versus Scn1a −/− CBD‐treated. (b) Survival. CBD treatment significantly (P < .05) increased survival compared to the vehicle‐treated Scn1a −/− group. Error bars are SEM; data were analysed by Mantel–Cox test
3.1.2. CBD improved survival in Scn1a −/− mice
As expected, none of the WT animals died during the study. In the two Scn1a −/− groups, the median survival in the CBD‐treated Scn1a −/− mice was significantly higher (16.25 days) compared to the vehicle‐treated Scn1a −/− mice (15.5 days; Figure 2b).
3.2. Study II
3.2.1. CBD prevented premature mortality in Scn1a +/− mice
The mortality rate was highest between P20 and P27 in Scn1a +/− mice except for a single animal from the Scn1a +/− vehicle‐treated group which died at P47. We reviewed the recorded video footage and confirmed that tonic–clonic seizures were the cause of death in all cases. Survival was significantly less in Scn1a +/− vehicle‐treated group compared to the Scn1a +/− CBD‐treated group (Figure 3a). Approximately 66% (19 of 29) Scn1a +/− vehicle‐treated animals died before the completion of the study in contrast to only 17% (two of 12) Scn1a +/− CBD‐treated animals (Figure 3b). In total, 15 mice died during the dark (active) cycle, and six died during the light (inactive) cycle.
Figure 3.
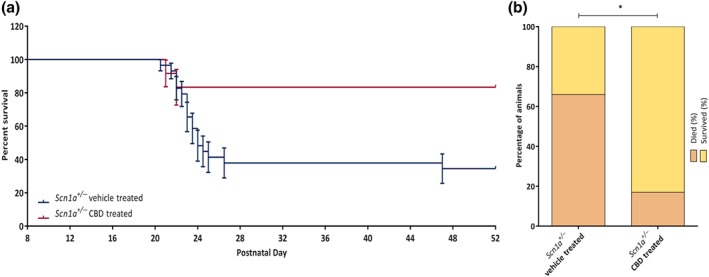
Plots showing chronic administration of CBD to Scn1a +/− mice on survival. (a) Survival of Scn1a +/− vehicle‐treated and Scn1a +/− CBD‐treated group. CBD treatment significantly increased survival compared to the vehicle‐treated Scn1a +/− group. Error bars are SEM; data were analysed by Mantel–Cox test. (b) Percentage of Scn1a +/− vehicle‐treated and Scn1a +/− CBD‐treated animals that survived until the completion of experiment (P52). A significantly greater number of CBD treated animals survived until the end of experiment compared to the Scn1a +/− vehicle‐treated animals. Data were analysed by Fisher's exact test. *P < .05
3.2.2. CBD did not exert any adverse effect on motor function
Motor function was assessed by both the accelerating rotarod and static beam test. In the accelerating rotarod test, no significant difference in time spent on rod was observed between the groups (Figure 4a).
Figure 4.
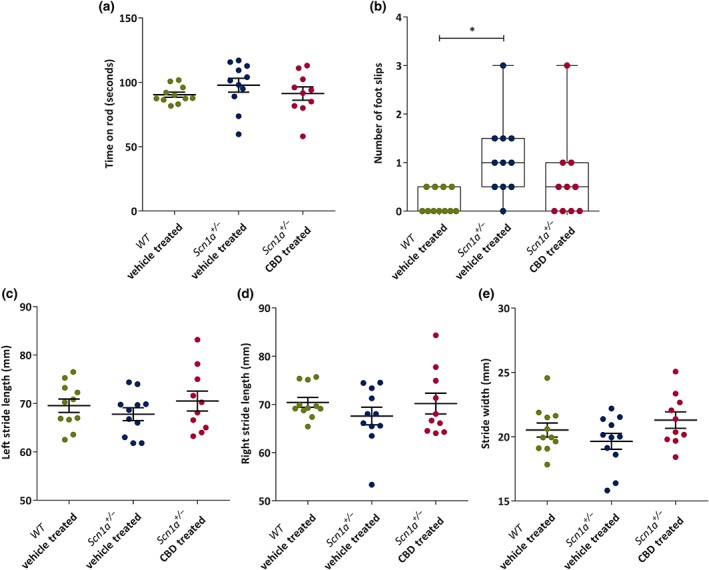
Dot plots or box and whisker plots showing chronic administration of CBD to Scn1a +/− mice on motor function and gait. (a) Mean time (seconds) spent on accelerated rotarod by the wild‐type (WT) vehicle‐treated (n = 11), Scn1a +/− vehicle‐treated (n = 11) and Scn1a +/− CBD‐treated (n = 10) groups. No difference was observed among the groups. (b) Median number of foot slips made in static beam test. CBD treatment had no adverse effect on foot slips. (c) There were no significant differences in mean left stride length (millimetres) (d) mean right stride length and (e) mean stride width among the groups. All the data are expressed as mean ± SEM except foot slips which are expressed as median, min to max and interquartile range (IQR). All data except foot slips were analysed by one‐way ANOVA with Holm–Sidak's post hoc test; foot slips data obtained from static beam test were analysed by Kruskal–Wallis test followed by Dunn's multiple comparison test; *P < .05; NS, not significant
On the other hand, in the static beam test, a significant difference in number of foot slips was found among the groups. Scn1a +/− vehicle‐treated group made significantly more foot slips compared to the WT vehicle‐treated group, however no significant difference was observed in between Scn1a +/− vehicle‐treated and Scn1a +/− CBD‐treated groups (Figure 4b). A further comparison between the WT vehicle‐treated and the Scn1a +/− CBD‐treated groups revealed no significant difference in number of foot slips between these two groups (P = .48).
3.2.3. CBD did not produce any gait abnormalities
In the gait test, no significant change was observed for left stride length (Figure 4c), right stride length (Figure 4d) and stride width (Figure 4e), between the three groups.
3.2.4. CBD improved social behaviour of Scn1a +/− mice
The social interaction test was conducted to assess the active social interaction and rearing behaviour exhibited in the home cage of the test animals.
The time spent on active interaction was significantly differed among the groups. The Scn1a +/− vehicle‐treated animals (n = 11) spent significantly less time in performing active interaction with the stranger mouse compared to both WT vehicle‐treated (n = 11) and Scn1a +/− CBD‐treated (n = 10) animals. The active interaction by the Scn1a +/− CBD‐treated group was similar to the WT vehicle‐treated group (P = .86).
On the other hand, a significant difference in number of rearing events was observed among the groups, with a significantly higher number of rearing occasions for Scn1a +/− vehicle‐treated animals compared to both WT vehicle‐treated or Scn1a +/− CBD‐treated animals (Figure 5b). No difference in rearing events was observed between the WT vehicle‐treated and Scn1a +/− CBD‐treated groups (P = .55).
Figure 5.
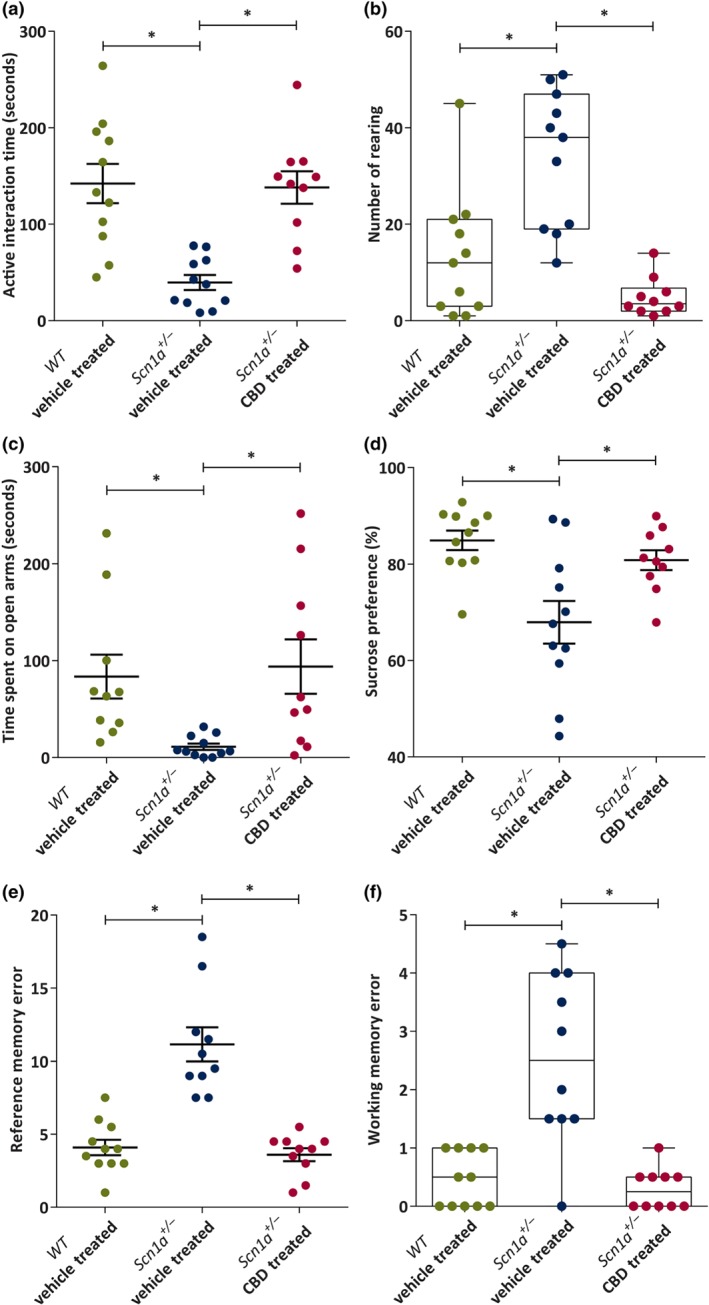
Dot plot or box and whisker plots showing the effect of chronic administration of CBD to Scn1a +/− mice on active social interaction, rearing, anxiety‐like and depression‐like behaviours, and cognition. (a) Mean time (seconds) spent on active interaction in social interaction (SI) test by the wild‐type (WT) vehicle‐treated (n = 11), Scn1a +/− vehicle‐treated (n = 11), and Scn1a +/− CBD‐treated (n = 10) groups. CBD significantly increased the active interaction time compared to the Scn1a +/− vehicle‐treated group. (b) Median number of rearing made in social interaction test. CBD treatment significantly reduced the number of rearing compared to the Scn1a +/− vehicle‐treated group. (c) Mean time (second) spent on open arms in elevated plus maze (EPM) test. CBD treatment significantly increased the time spent on the open arms of EPM compared to the Scn1a +/− vehicle‐treated group. (d) Mean sucrose preference (%) in sucrose preference test. CBD significantly increased the sucrose preference compared to the Scn1a +/− vehicle‐treated group. (e) Mean reference memory errors (RMEs) in WT vehicle‐treated (n = 11), Scn1a +/− vehicle‐treated (n = 10) and Scn1a +/− CBD‐treated (n = 10) groups in radial arm maze (RAM) test. CBD significantly improved RME compared to the Scn1a +/− vehicle‐treated group. (f) Median working memory errors (WMEs). CBD treatment significantly improved working memory errors compared to the Scn1a +/− vehicle‐treated group. All the data are expressed as mean ± SEM except rearing and working memory errors which are expressed as median, min to max and interquartile range (IQR); all data except rearing and working memory errors were analysed by one‐way ANOVA with Holm–Sidak's post hoc test; rearing and working memory errors data obtained from social interaction test and radial arm test respectively were analysed by Kruskal–Wallis post hoc test followed by Dunn's multiple comparison test; *P < .05
3.2.5. CBD reduced anxiety‐like behaviour in Scn1a +/− mice
The anxiety of the animals was assessed by the amount of the time spent on the open arms of an elevated plus maze. The time spent on the open arms differs significantly among the groups. The Scn1a +/− vehicle‐treated animals (n = 11; Figure 5c) spent significantly less time on the open arms compared to both WT vehicle‐treated (n = 11) and Scn1a +/− CBD‐treated (n = 10) animals. The time spent on the open arms was not different between WT vehicle‐treated and Scn1a +/− CBD‐treated groups (P = .73).
3.2.6. CBD reduced depression‐like behaviour in Scn1a +/− mice
Depression‐like behaviour is inversely correlated with sucrose preference (Murray, Boss‐Williams, & Weiss, 2013). In the present study, sucrose preference differed significantly among the groups. Scn1a +/− vehicle‐treated animals (n = 11; Figure 5d) had a reduced preference for sucrose in comparison to both WT vehicle‐treated (n = 11) or Scn1a +/− CBD‐treated (n = 10 animals. Sucrose preference was similar in between the WT vehicle‐treated and Scn1a +/− CBD‐treated groups (P = .36).
3.2.7. CBD improved cognition in Scn1a +/− mice
The reference memory and WM function in the animals were assessed using an eight‐arm radial arm maze test. A significant difference in the number of reference memory error was observed among the groups. The Scn1a +/− vehicle‐treated group (n = 10) made significantly more reference memory error compared to both WT vehicle‐treated (n = 11) and Scn1a +/− CBD‐treated (n = 10) groups (Figure 5e). No difference in reference memory error was observed between the WT vehicle‐treated and Scn1a +/− CBD‐treated groups (P = .65).
Further, working memory errors were significantly different among the groups . The Scn1a +/− vehicle‐treated group made significantly more working memory errors compared to both WT vehicle‐treated and Scn1a +/− CBD‐treated groups (Figure 5f). The working memory errors was not differed in Scn1a +/− CBD‐treated group compared to the WT vehicle‐treated group.
4. DISCUSSION
We demonstrate for the first time that chronic administration of CBD extends the survival and improves neonatal welfare of Scn1a −/− mice. To our knowledge, CBD is the only drug to date which exerts such an effect in this severe model of Dravet syndrome. Given the extreme severity and early mortality observed in this model, it is not possible to study intricate behavioural co‐morbidities in the Scn1a −/− mice. We therefore tested CBD using the Scn1a +/− mouse model of Dravet syndrome to assess its effect on survival and behavioural co‐morbidities. Here, CBD prevented premature mortality and had a disease‐modifying action, improving social deficits, reducing anxiety‐like and depression‐like behaviours and improving memory in the Scn1a +/− mice.
In Study I, using the 129S Scn1a −/− knockout model, CBD did not produce any adverse effect on neonatal welfare in WT mice suggesting CBD is safe and well tolerated in healthy neonates. Indeed, CBD actually improved neonatal welfare scores in these mice indicating that CBD provided significant protection against the severe motor deficit and seizure associated deterioration of overall health in this model. In the Scn1a −/− mice, CBD also improved other welfare parameters such as OT (indicator of pain) and BC score (overall health). Vehicle‐treated Scn1a −/− mice died prematurely as expected with this severe epilepsy phenotype (Kalume et al., 2007; Yu et al., 2006) and CBD treatment significantly extended their survival. This result is remarkable as other AEDs, such as https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3364, have failed to extend survival in this model with only hand‐feeding previously observed to extend survival (Yu et al., 2006). No differences in weight (see Data S1) between CBD‐treated Scn1a −/− mice and vehicle‐treated Scn1a −/− mice were seen and CBD induced extension of survival is therefore unlikely to be due to increased feeding. We speculate that the extension of survival is due to the anticonvulsant and disease‐modifying properties of CBD, which also lacks the sedating effect of other anticonvulsants used in this model. Indeed, a previous study has established the anticonvulsant effect of CBD in a similar, but less severe, model with haploinsufficiency of NaV1.1 channels (Kaplan et al., 2017), a model which we adopted in Study II.
Given the positive results with CBD in the Scn1a −/− model of Dravet syndrome, we then conducted a comprehensive investigation into survival and co‐morbidities associated with Dravet syndrome using the haploinsufficiency model of Dravet syndrome, the hybrid Scn1a +/− heterozygote model. In this model, chronic CBD treatment significantly increased the survival of Scn1a +/− mice in comparison to their vehicle‐treated counterparts; as tonic‐clonic seizures (persisting for less than a minute), were the reason for death in all Scn1a +/− mice, we believe the lower mortality in CBD‐treated group is evidence that CBD provides protection against seizure related premature mortality. This result is therefore consistent with a previous study using this model where the anticonvulsant effect of CBD was demonstrated (Kaplan et al., 2017). Sudden unexpected death in epilepsy (SUDEP) in humans often occurs during sleep and is a common form of death in childhood epilepsies such as Dravet (Purnell, Thijs, & Buchanan, 2018). However, our data indicate that the vast majority of animals died during the active phase (dark cycle) indicating that death in this model may not be a predictor of sudden unexpected death in epilepsy in humans.
Seizures and premature mortality are not the only concern in Dravet syndrome, co‐morbidities such as motor deficits, including abnormal gait are frequently reported (Aljaafari, Fasano, Nascimento, Lang, & Andrade, 2017; Rilstone, Coelho, Minassian, & Andrade, 2012). Several AEDs used in Dravet syndrome such as https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2804, valproic acid, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2624 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7472 can also have adverse motor effects in epileptic patients (Bainbridge et al., 2017; Ristić et al., 2006; Zaccara, Perucca, Loiacono, Giovannelli, & Verrotti, 2013). In this study, CBD had no effect on motor function in either WT or Scn1a +/− mice indicating that, unlike other AEDs used in treatment of Dravet, CBD does not produce any motor deficits as measured by the accelerating rotorod, static beam or gait analysis.
In addition to motor dysfunction, Dravet syndrome is linked to several neuropsychiatric co‐morbidities. For example, social deficits, a feature common in autism, are often observed in Dravet syndrome (Berkvens et al., 2015; Li et al., 2011; Wolff, Casse‐Perrot, & Dravet, 2006). We investigated the effect of CBD on the social behaviour of the Scn1a +/− mice. Vehicle‐treated Scn1a +/− mice had significant social deficits compared to their WT counterparts and CBD‐treated Scn1a +/− mice spent significantly more time in active social interaction compared to their vehicle‐treated counterparts. These results are in line with the previous studies conducted in this model where social interactions were impaired but restored by stimulation of https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=72 receptors (Han et al., 2012) and by a single dose of CBD (Kaplan et al., 2017). While this is not the first study to demonstrate that CBD can restore social interaction in a model of Dravet syndrome, we are the first to demonstrate this at doses shown to have anticonvulsant effects. We also demonstrate a chronic CBD dosing regimen, similar to that used in Dravet syndrome therapy (Devinsky et al., 2017, 2018), improves social interactions. Interestingly, CBD has been demonstrated to reduce https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2424‐induced (Malone, Jongejan, & Taylor, 2009) and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2403‐induced (Gururajan, Taylor, & Malone, 2012) social deficits in rats, indicating this may be a generalised and reproducible effect of CBD.
In addition to impaired social behaviour, anxiety and depression are major problems for patients with Dravet syndrome (Chen et al., 2018; Jain, Subendran, Smith, & Widjaja, 2018; Wang et al., 2018). Although AEDs such as valproate, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2622, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7149, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5483 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7254 have been reported to improve these behavioural co‐morbidities, a number of others have been shown to progressively deteriorate them (Chen, Choi, et al., 2017). Anxiety/depression has been previously reported the Dravet syndrome Scn1a +/− mouse model (Han et al., 2012) and we observed anxiety‐like behaviour in the elevated plus maze in vehicle‐treated Scn1a +/− mice, with animals spending significantly less time on the open arm compared to the WT animals; CBD normalised this behaviour. Interestingly, Scn1a +/− mice also displayed more rearing behaviours compared to the WT animals in the social interaction tests which could be interpreted as a higher level of anxiety compelling them to them try and escape the test situation (Mines, Yuskaitis, King, Beurel, & Jope, 2010). Again, CBD treatment reduced this anxiety‐like behaviour in Scn1a +/− mice. Together, this illustrates that chronic CBD treatment has anxiolytic potential in this model of Dravet syndrome. This is the first study to suggest an anxiolytic effect of CBD in an epilepsy model. This is consistent with reports of anxiolytic effects of CBD in a chronic unpredicted stress model in mice using both elevated plus maze and novelty supressed feeding test (Campos et al., 2013; Campos & Guimarães, 2009; Fogaca, Campos, Coelho, Duman, & Guimaraes, 2018) where an action of CBD to inhibit https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=507, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=57 was implicated respectively. Anxiolytic effects of CBD have also been observed in a mouse model of neuropathic pain where inhibition of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1 receptors was implicated (De Gregorio et al., 2019).
To investigate depression‐like behaviours, we employed the conventional sucrose preference test in the Scn1a +/− mice (Serova et al., 2017). This task is based on the assumption that depression‐like behaviour in rodents inversely correlates with sucrose preference (Murray et al., 2013). Here, vehicle‐treated Scn1a +/− mice showed typical depression‐like behaviour, that is, a reduced preference to sucrose over water when compared to the WT mice with chronic CBD‐treatment normalising sucrose preference. An antidepressant‐like action of CBD has previously been documented in genetic (Shoval et al., 2016), olfactory bulbectomy (Linge et al., 2016) and chronic unpredictable stress (Campos et al., 2013) models of depression, but we are the first to demonstrate such effect of CBD in any epilepsy model. Although previous studies in naïve rodents have proposed that CBD exerts an antidepressant action via modulating the https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5483 neurotransmission or endocannabinoid signalling (Campos et al., 2013; Linge et al., 2016), the underlying pathology of depression in epilepsy is complex and would necessitate a detailed mechanistic study to gain a better understanding on any antidepressant effect of CBD.
Cognitive deficit is also a frequently reported co‐morbidity in Dravet syndrome patients (Acha, Perez, Davidson, & Carreiras, 2015; Olivieri et al., 2016; Villeneuve et al., 2014); several domains of cognitive functions including visual attention, executive functions, and verbal, visual and working memories can be impaired in these patients (Acha et al., 2015; Pascalicchio et al., 2007; Roebling et al., 2009). Some AEDs including phenobarbital, phenytoin and topiramate negatively impact cognition in people with epilepsy (Chen, Chi Chow, & Lee, 2001; Mei, Montenegro, Guerreiro, & Guerreiro, 2006; Wandschneider et al., 2017). Here, vehicle‐treated Scn1a +/− mice made significantly more reference memory errors and working memory errors compared to the WT mice in the radial arm maze indicating the presence of spatial memory deficits. We showed that CBD improved both the reference and WM function of Scn1a +/− mice compared to their vehicle‐treated counterparts, to our knowledge, the first report of improving cognitive deficiencies in a model of Dravet syndrome. We have previously reported a similar reversal of cognitive deficits in a rat model of temporal lobe epilepsy (Patra et al., 2019), which may suggest that CBD has a more generalised role in protecting cognitive function in epilepsy, perhaps related to reduction in seizure frequency or severity. Further to this spatial learning memory deficits in a mouse model of Alzheimer's disease were attenuated by CBD in a CB2 receptor‐independent manner (Martin‐Moreno et al., 2011).
Several hypotheses have been proposed to explain the underlying pathology of memory impairment in epilepsy and seizure is a contributory factor for cognitive decline during the developmental process (Ben‐Ari & Holmes, 2006; Khan, Zhao, Miller, & Holmes, 2010). This is consistent with the Dravet model used here as seizures exhibited from an early age. Seizure‐related disruption of neural plasticity and cognition has also been demonstrated in other animal models of epilepsies (Lenck‐Santini & Scott, 2015; Schubert, Siegmund, Pape, & Albrecht, 2005; Zhou, Lippman Bell, Sun, & Jensen, 2011). However, removing the cause of seizures does not always restore cognitive deficits in people with epilepsy (Helmstaedter, Kurthen, Lux, Reuber, & Elger, 2003). Strikingly, a caregiver‐reported quality of life in childhood epilepsy survey stated an improved memory function in the patients with refractory childhood epilepsy following CBD treatment (Rosenberg, Louik, Conway, Devinsky, & Friedman, 2017). This is consistent with the present study and illustrates the possibility that memory impairment associated with epilepsy/Dravet syndrome may be improved with chronic CBD treatment. We believe these findings warrant further study in the Scn1a +/− model of Dravet Syndrome to try and ascertain the molecular mechanisms underlying this effect.
5. CONCLUSION
We have established for the first time that the pharmaceutical formulation of CBD (Epidiolex®; Epidyolex®; therefore, results cannot be directly extrapolated to other CBD formulations) improves neonatal welfare and extends survival in Scn1a +/− mice. This study is also the first to demonstrate that chronic administration of CBD prevents premature mortality and improves several behavioural co‐morbidities, including impaired cognition and social interaction, associated with the Scn1a +/− mouse Dravet syndrome model. These effects are without the detrimental effects on motor function that are often seen with current pharmacotherapy for this disorder. In light of the recent FDA approval of CBD for treatment of Dravet syndrome in the United States and by the EMA in the EU, we believe that the results obtained from the present indicate that CBD may produce improvements in Dravet syndrome therapy related to survival and co‐morbidities in addition to seizure control.
CONFLICT OF INTEREST
B.J.W. and M.B. are employees of GW Pharmaceuticals.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
AUTHORS CONTRIBUTION
P.H.P. designed the study, conducted experiments, analysed and interpreted the data, and wrote the manuscript; E.S. conducted experiments; M.B. and B.J.W. designed the experiments; C.M.W. and A.J.M. supervised the project, designed the experiments, analysed and interpreted the data and wrote and revised the manuscript.
Supporting information
Figure S1. Example result from PCR used for genotyping animals produced via breeding of the 129S‐Scn1a tm1Kea/Mmjax transgenic mouse line. Mouse tail DNA was amplified with primers for the wild type (WT) and mutated (Scn1a −/−) Scn1a alleles. The presence of a 357 bp band indicates the WT allele, and a 200 bp band indicates the Scn1a −/− allele. ‐ve: negative control; +ve: positive control.
Figure S2. Plots showing chronic administration of CBD to wild type (WT) and Scn1a−/− mice on different welfare scores and weight change. A. Natural activity (NA) scores of WT vehicletreated, WT CBD‐treated, Scn1a−/− vehicle‐treated and Scn1a−/− CBD‐treated mice from postnatal day 8 (P8) onwards (n = 10/group). CBD had no effect on NA score of WT mice. CBD significantly improved the NA score in Scn1a−/− mice from P12 onwards compared to the vehicle‐treated Scn1a−/− mice. B. Reflex/response to touch (RT) scores. CBD significantly improved the RT score in WT mice from P8 to P9.5 compared to the vehicle‐treated WT mice. CBD significantly improved the RT score in Scn1a−/− mice from P12 to P14.5 and on P16 compared to the vehicle‐treated Scn1a −/− mice. C. Orbital tightening (OT) scores. CBD had no effect on OT score of WT mice. CBD significantly improved the OT score in Scn1a −/− mice from P13 onwards compared to the vehicle‐treated Scn1a −/− mice. D. Body condition (BC) scores. CBD had no effect on BC score of WT mice. CBD significantly improved the BC score in Scn1a −/− mice from P13.5 to P15.15 compared to the vehicle‐treated Scn1a −/− mice. E.Weight. CBD had no effect on weight in WT mice compared to the vehicle‐treated WT mice. No threeway interaction of treatment×genotype×time was observed on weight. Data are expressed as mean ± SEM; data were analysed by a three‐way ANOVA with Bonferroni's post hoc test; #p < 0.05; *p < 0.05; # represents WT vehicle‐treated vs WT CBD‐treated; * represents Scn1a −/− vehicle‐treated vs Scn1a −/− CBD‐treated.
Table S1. Welfare assessment sheet for dam; adapted from (Wolfensohn and Lloyd, 2007).
Table S2. Welfare assessment sheet for neonatal mice; adapted from (Langford et al., 2010; Ullman‐Culleré & Foltz, 1999; Wolfensohn & Lloyd, 2007)
Data S1 Supporting information
Data S2 Supporting information
Data S3 Supporting information
ACKNOWLEDGEMENTS
The study is sponsored by the GW Pharmaceuticals Ltd. Pabitra H. Patra is a PhD student sponsored by the GW Pharmaceuticals and the University of Reading.
Patra PH, Serafeimidou‐Pouliou E, Bazelot M, Whalley BJ, Williams CM, McNeish AJ. Cannabidiol improves survival and behavioural co‐morbidities of Dravet syndrome in mice. Br J Pharmacol. 2020;177:2779–2792. 10.1111/bph.15003
Claire Michelle Williams and Alister James McNeish contributed equally.
Contributor Information
Claire Michelle Williams, Email: claire.williams@reading.ac.uk.
Alister James McNeish, Email: a.mcneish@reading.ac.uk.
REFERENCES
- Acha, J. , Perez, A. , Davidson, D. J. , & Carreiras, M. (2015). Cognitive characterization of children with Dravet syndrome: A neurodevelopmental perspective. Child Neuropsychology, 21, 693–715. [DOI] [PubMed] [Google Scholar]
- Akiyama, M. , Kobayashi, K. , Yoshinaga, H. , & Ohtsuka, Y. (2010). A long‐term follow‐up study of Dravet syndrome up to adulthood. Epilepsia, 51, 1043–1052. 10.1111/j.1528-1167.2009.02466.x [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , … Sharman, J. L. (2019). The concise guide to pharmacology 2019/20: Ion channels. British Journal of Pharmacology, 176, S142–S228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljaafari, D. , Fasano, A. , Nascimento, F. A. , Lang, A. E. , & Andrade, D. M. (2017). Adult motor phenotype differentiates Dravet syndrome from Lennox‐Gastaut syndrome and links SCN1A to early onset parkinsonian features. Epilepsia, 58, e44–e48. 10.1111/epi.13692 [DOI] [PubMed] [Google Scholar]
- Anderson, L. L. , Hawkins, N. A. , Thompson, C. H. , Kearney, J. A. , & George, A. L. Jr. (2017). Unexpected efficacy of a novel sodium channel modulator in Dravet syndrome. Scientific Reports, 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge, J. , Backer, M. D. , Eckhardt, K. , Tennigkeit, F. , Bongardt, S. , Sen, D. , … Faught, E. (2017). Safety and tolerability of lacosamide monotherapy in the elderly: A subgroup analysis from lacosamide trials in diabetic neuropathic pain. Epilepsia Open, 2, 415–423. 10.1002/epi4.12079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Ari, Y. , & Holmes, G. L. (2006). Effects of seizures on developmental processes in the immature brain. Lancet Neurology, 5, 1055–1063. 10.1016/S1474-4422(06)70626-3 [DOI] [PubMed] [Google Scholar]
- Berkvens, J. J. , Veugen, I. , Veendrick‐Meekes, M. J. , Snoeijen‐Schouwenaars, F. M. , Schelhaas, H. J. , Willemsen, M. H. , … Aldenkamp, A. P. (2015). Autism and behavior in adult patients with Dravet syndrome (DS). Epilepsy & Behavior, 47, 11–16. 10.1016/j.yebeh.2015.04.057 [DOI] [PubMed] [Google Scholar]
- Campos, A. C. , & Guimarães, F. S. (2009). Evidence for a potential role for TRPV1 receptors in the dorsolateral periaqueductal gray in the attenuation of the anxiolytic effects of cannabinoids. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 33, 1517–1521. 10.1016/j.pnpbp.2009.08.017 [DOI] [PubMed] [Google Scholar]
- Campos, A. C. , Ortega, Z. , Palazuelos, J. , Fogaca, M. V. , Aguiar, D. C. , Diaz‐Alonso, J. , … Galve‐Roperh, I. (2013). The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: involvement of the endocannabinoid system. The International Journal of Neuropsychopharmacology, 16, 1407–1419. 10.1017/S1461145712001502 [DOI] [PubMed] [Google Scholar]
- Chen, B. , Choi, H. , Hirsch, L. J. , Katz, A. , Legge, A. , Buchsbaum, R. , & Detyniecki, K. (2017). Psychiatric and behavioral side effects of antiepileptic drugs in adults with epilepsy. Epilepsy & Behavior, 76, 24–31. [DOI] [PubMed] [Google Scholar]
- Chen, M. , Yan, H.‐h. , Shu, S. , Pei, L. , Zang, L.‐K. , Fu, Y. , … Bi, L. L. (2017). Amygdalar endothelin‐1 regulates pyramidal neuron excitability and affects anxiety. Scientific Reports, 7, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Chi Chow, J. , & Lee, I. (2001). Comparison the cognitive effect of anti‐epileptic drugs in seizure‐free children with epilepsy before and after drug withdrawal. Epilepsy Research, 44, 65–70. [DOI] [PubMed] [Google Scholar]
- Chen, Y. Y. , Huang, S. , Wu, W. Y. , Liu, C. R. , Yang, X. Y. , Zhao, H. T. , … Xiao, B. (2018). Associated and predictive factors of quality of life in patients with temporal lobe epilepsy. Epilepsy & Behavior, 86, 85–90. 10.1016/j.yebeh.2018.06.025 [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio, D. , McLaughlin, R. J. , Posa, L. , Ochoa‐Sanchez, R. , Enns, J. , Lopez‐Canul, M. , … Gobbi, G. (2019). Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety‐like behavior in a model of neuropathic pain. Pain, 160, 136–150. 10.1097/j.pain.0000000000001386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky, O. , Cross, J. H. , Laux, L. , Marsh, E. , Miller, I. , Nabbout, R. , … Cannabidiol in Dravet syndrome study group (2017). Trial of cannabidiol for drug‐resistant seizures in the Dravet syndrome. The New England Journal of Medicine, 376, 2011–2020. 10.1056/NEJMoa1611618 [DOI] [PubMed] [Google Scholar]
- Devinsky, O. , Patel, A. D. , Thiele, E. A. , Wong, M. H. , Appleton, R. , Harden, C. L. , … Sommerville, K. (2018). Randomized, dose‐ranging safety trial of cannabidiol in Dravet syndrome. Neurology, 90, e1204–e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravet, C. , Bureau, M. , Oguni, H. , Fukuyama, Y. , & Cokar, O. (2005). Severe myoclonic epilepsy in infancy: Dravet syndrome. Advances in Neurology, 95, 71–102. [PubMed] [Google Scholar]
- FDA . 2018. FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. [Online] Available from https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm611046.htm. [Accessed].
- Fogaca, M. V. , Campos, A. C. , Coelho, L. D. , Duman, R. S. , & Guimaraes, F. S. (2018). The anxiolytic effects of cannabidiol in chronically stressed mice are mediated by the endocannabinoid system: Role of neurogenesis and dendritic remodeling. Neuropharmacology, 135, 22–33. 10.1016/j.neuropharm.2018.03.001 [DOI] [PubMed] [Google Scholar]
- Genton, P. , Velizarova, R. , & Dravet, C. (2011). Dravet syndrome: The long‐term outcome. Epilepsia, 52, 44–49. [DOI] [PubMed] [Google Scholar]
- Gururajan, A. , Taylor, D. A. , & Malone, D. T. (2012). Cannabidiol and clozapine reverse MK‐801‐induced deficits in social interaction and hyperactivity in Sprague‐Dawley rats. Journal of Psychopharmacology (Oxford, England), 26, 1317–1332. [DOI] [PubMed] [Google Scholar]
- Han, S. , Tai, C. , Westenbroek, R. E. , Yu, F. H. , Cheah, C. S. , Potter, G. B. , … Catterall, W. A. (2012). Autistic‐like behaviour in Scn1a+/− mice and rescue by enhanced GABA‐mediated neurotransmission. Nature, 489, 385–390. 10.1038/nature11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … Bryant, C. (2017). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkin, L. A. , McMahon, J. M. , Iona, X. , Dibbens, L. , Pelekanos, J. T. , Zuberi, S. M. , … Scheffer, I. E. (2007). The spectrum of SCNIA‐related infantile epileptic encephalopathies. Brain, 130, 843–852. 10.1093/brain/awm002 [DOI] [PubMed] [Google Scholar]
- Hawkins, N. A. , Anderson, L. L. , Gertler, T. S. , Laux, L. , George, A. L. Jr. , & Kearney, J. A. (2017). Screening of conventional anticonvulsants in a genetic mouse model of epilepsy. Annals of Clinical Translational Neurology, 4, 326–339. 10.1002/acn3.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter, C. , Kurthen, M. , Lux, S. , Reuber, M. , & Elger, C. E. (2003). Chronic epilepsy and cognition: A longitudinal study in temporal lobe epilepsy. Annals of Neurology, 54, 425–432. 10.1002/ana.10692 [DOI] [PubMed] [Google Scholar]
- Jain, P. , Subendran, J. , Smith, M. L. , & Widjaja, E. (2018). Care‐related quality of life in caregivers of children with drug‐resistant epilepsy. Journal of Neurology, 265, 2221–2230. 10.1007/s00415-018-8979-4 [DOI] [PubMed] [Google Scholar]
- Kalume, F. , Yu, F. H. , Westenbroek, R. E. , Scheuer, T. , & Catterall, W. A. (2007). Reduced sodium current in Purkinje neurons from Nav1.1 mutant mice: Implications for ataxia in severe myoclonic epilepsy in infancy. The Journal of Neuroscience, 27, 11065–11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, J. S. , Stella, N. , Catterall, W. A. , & Westenbroek, R. E. (2017). Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proceedings of the National Academy of Sciences of the United States of America, 114, 11229–11234. 10.1073/pnas.1711351114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, O. I. , Zhao, Q. , Miller, F. , & Holmes, G. L. (2010). Interictal spikes in developing rats cause long‐standing cognitive deficits. Neurobiology of Disease, 39, 362–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford, D. J. , Bailey, A. L. , Chanda, M. L. , Clarke, S. E. , Drummond, T. E. , Echols, S. , … Matsumiya, L. (2010). Coding of facial expressions of pain in the laboratory mouse. Nature Methods, 7, 447–449. [DOI] [PubMed] [Google Scholar]
- Lenck‐Santini, P. P. , & Scott, R. C. (2015). Mechanisms responsible for cognitive impairment in epilepsy. Cold Spring Harbor Perspectives in Medicine, 5, a022772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, B. M. , Liu, X. R. , Yi, Y. H. , Deng, Y. H. , Su, T. , Zou, X. , & Liao, W. P. (2011). Autism in Dravet syndrome: Prevalence, features, and relationship to the clinical characteristics of epilepsy and mental retardation. Epilepsy & Behavior, 21, 291–295. [DOI] [PubMed] [Google Scholar]
- Linge, R. , Jiménez‐Sánchez, L. , Campa, L. , Pilar‐Cuéllar, F. , Vidal, R. , Pazos, A. , … Díaz, Á. (2016). Cannabidiol induces rapid‐acting antidepressant‐like effects and enhances cortical 5‐HT/glutamate neurotransmission: role of 5‐HT1A receptors. Neuropharmacology, 103, 16–26. 10.1016/j.neuropharm.2015.12.017 [DOI] [PubMed] [Google Scholar]
- Malone, D. T. , Jongejan, D. , & Taylor, D. A. (2009). Cannabidiol reverses the reduction in social interaction produced by low dose Delta(9)‐tetrahydrocannabinol in rats. Pharmacology, Biochemistry, and Behavior, 93, 91–96. [DOI] [PubMed] [Google Scholar]
- Marini, C. , Scheffer, I. E. , Nabbout, R. , Suls, A. , De Jonghe, P. , Zara, F. , & Guerrini, R. (2011). The genetics of Dravet syndrome. Epilepsia, 52, 24–29. [DOI] [PubMed] [Google Scholar]
- Martin‐Moreno, A. M. , Reigada, D. , Ramirez, B. G. , Mechoulam, R. , Innamorato, N. , Cuadrado, A. , & de Ceballos, M. L. (2011). Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: Relevance to Alzheimer's disease. Molecular Pharmacology, 79, 964–973. 10.1124/mol.111.071290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath, J. C. , & Lilley, E. (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. British Journal of Pharmacology, 172(13), 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei, P. A. , Montenegro, M. A. , Guerreiro, M. M. , & Guerreiro, C. A. (2006). Pharmacovigilance in epileptic patients using antiepileptic drugs. Arquivos de Neuro‐Psiquiatria, 64, 198–201. 10.1590/s0004-282x2006000200005 [DOI] [PubMed] [Google Scholar]
- Miller, A. R. , Hawkins, N. A. , McCollom, C. E. , & Kearney, J. A. (2014). Mapping genetic modifiers of survival in a mouse model of Dravet syndrome. Genes, Brain, and Behavior, 13, 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, J. (1991). Reaction time analysis with outlier exclusion: bias varies with sample size. The Quarterly Journal of Experimental Psychology, 43, 907–912. 10.1080/14640749108400962 [DOI] [PubMed] [Google Scholar]
- Mines, M. A. , Yuskaitis, C. J. , King, M. K. , Beurel, E. , & Jope, R. S. (2010). GSK3 influences social preference and anxiety‐related behaviors during social interaction in a mouse model of fragile X syndrome and autism. PLoS ONE, 5, e9706 10.1371/journal.pone.0009706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, R. , Boss‐Williams, K. A. , & Weiss, J. M. (2013). Effects of chronic mild stress on rats selectively bred for behavior related to bipolar disorder and depression. Physiology & Behavior, 119, 115–129. [DOI] [PubMed] [Google Scholar]
- Olivieri, G. , Battaglia, D. , Chieffo, D. , Rubbino, R. , Ranalli, D. , Contaldo, I. , … Guzzetta, F. (2016). Cognitive‐behavioral profiles in teenagers with Dravet syndrome. Brain Dev, 38, 554–562. 10.1016/j.braindev.2015.12.014 [DOI] [PubMed] [Google Scholar]
- Pascalicchio, T. F. , de Araujo Filho, G. M. , da Silva Noffs, M. H. , Lin, K. , Caboclo, L. O. , Vidal‐Dourado, M. , … Yacubian, E. M. (2007). Neuropsychological profile of patients with juvenile myoclonic epilepsy: A controlled study of 50 patients. Epilepsy & Behavior, 10, 263–267. 10.1016/j.yebeh.2006.11.012 [DOI] [PubMed] [Google Scholar]
- Patel, S. , & Hillard, C. J. (2001). Cannabinoid CB1 receptor agonists produce cerebellar dysfunction in mice. The Journal of Pharmacology and Experimental Therapeutics, 297, 629–637. [PubMed] [Google Scholar]
- Patra, P. H. , Barker‐Haliski, M. , White, H. S. , Whalley, B. J. , Glyn, S. , Sandhu, H. , … McNeish, A. J. (2019). Cannabidiol reduces seizures and associated behavioral comorbidities in a range of animal seizure and epilepsy models. Epilepsia, 60, 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell, B. S. , Thijs, R. D. , & Buchanan, G. F. (2018). Dead in the night: Sleep‐wake and time‐of‐day influences on sudden unexpected death in epilepsy. Frontiers in Neurology, 9 10.3389/fneur.2018.01079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilstone, J. J. , Coelho, F. M. , Minassian, B. A. , & Andrade, D. M. (2012). Dravet syndrome: Seizure control and gait in adults with different SCN1A mutations. Epilepsia, 53, 1421–1428. 10.1111/j.1528-1167.2012.03583.x [DOI] [PubMed] [Google Scholar]
- Ristić, A. J. , Vojvodić, N. , Janković, S. , Sindelić, A. , & Sokić, D. (2006). The frequency of reversible parkinsonism and cognitive decline associated with valproate treatment: A study of 364 patients with different types of epilepsy. Epilepsia, 47, 2183–2185. 10.1111/j.1528-1167.2006.00711.x [DOI] [PubMed] [Google Scholar]
- Roebling, R. , Scheerer, N. , Uttner, I. , Gruber, O. , Kraft, E. , & Lerche, H. (2009). Evaluation of cognition, structural, and functional MRI in juvenile myoclonic epilepsy. Epilepsia, 50, 2456–2465. [DOI] [PubMed] [Google Scholar]
- Rosenberg, E. C. , Louik, J. , Conway, E. , Devinsky, O. , & Friedman, D. (2017). Quality of life in childhood epilepsy in pediatric patients enrolled in a prospective, open‐label clinical study with cannabidiol. Epilepsia, 58, e96–e100. 10.1111/epi.13815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, E. C. , Patra, P. H. , & Whalley, B. J. (2017). Therapeutic effects of cannabinoids in animal models of seizures, epilepsy, epileptogenesis, and epilepsy‐related neuroprotection. Epilepsy & Behavior, 70, 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Mizuguchi M, & Ikeda K (2013). Social interaction test: A sensitive method for examining autism‐related behavioral deficits. Protoc Exch.
- Schubert, M. , Siegmund, H. , Pape, H. C. , & Albrecht, D. (2005). Kindling‐induced changes in plasticity of the rat amygdala and hippocampus. Learning & Memory, 12, 520–526. 10.1101/lm.4205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedy, J. , Urdzikova, L. , Jendelova, P. , & Sykova, E. (2008). Methods for behavioral testing of spinal cord injured rats. Neuroscience and Biobehavioral Reviews, 32, 550–580. 10.1016/j.neubiorev.2007.10.001 [DOI] [PubMed] [Google Scholar]
- Serova, L. , Mulhall, H. , & Sabban, E. (2017). NPY1 receptor agonist modulates development of depressive‐like behavior and gene expression in hypothalamus in SPS rodent PTSD model. Frontiers in Neuroscience, 11, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoval, G. , Shbiro, L. , Hershkovitz, L. , Hazut, N. , Zalsman, G. , Mechoulam, R. , & Weller, A. (2016). Prohedonic effect of cannabidiol in a rat model of depression. Neuropsychobiology, 73, 123–129. 10.1159/000443890 [DOI] [PubMed] [Google Scholar]
- Thiele, E. A. , Marsh, E. D. , French, J. A. , Mazurkiewicz‐Beldzinska, M. , Benbadis, S. R. , Joshi, C. , … Gunning, B. (2018). Cannabidiol in patients with seizures associated with Lennox‐Gastaut syndrome (GWPCARE4): A randomised, double‐blind, placebo‐controlled phase 3 trial. The Lancet, 391, 1085–1096. [DOI] [PubMed] [Google Scholar]
- Ullman‐Culleré, M. H. , & Foltz, C. J. (1999). Body condition scoring: A rapid and accurate method for assessing health status in mice. Laboratory Animal Science, 49, 319–323. [PubMed] [Google Scholar]
- Villeneuve, N. , Laguitton, V. , Viellard, M. , Lepine, A. , Chabrol, B. , Dravet, C. , & Milh, M. (2014). Cognitive and adaptive evaluation of 21 consecutive patients with Dravet syndrome. Epilepsy & Behavior, 31, 143–148. [DOI] [PubMed] [Google Scholar]
- Wandschneider, B. , Burdett, J. , Townsend, L. , Hill, A. , Thompson, P. J. , Duncan, J. S. , & Koepp, M. J. (2017). Effect of topiramate and zonisamide on fMRI cognitive networks. Neurology, 88, 1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. J. , Tan, G. , Deng, Y. , He, J. , He, Y. J. , Zhou, D. , & Liu, L. (2018). Prevalence and risk factors of depression and anxiety among patients with convulsive epilepsy in rural West China. Acta Neurologica Scandinavica, 138, 541–547. 10.1111/ane.13016 [DOI] [PubMed] [Google Scholar]
- Wecker, L. , Engberg, M. E. , Philpot, R. M. , Lambert, C. S. , Kang, C. W. , Antilla, J. C. , … Rowell, P. P. (2013). Neuronal nicotinic receptor agonists improve gait and balance in olivocerebellar ataxia. Neuropharmacology, 73, 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfensohn, S. , & Lloyd, M. (2007). Pain, stress and humane end points In Sarah Wolfensohn M. L. (Ed.), Handbook of laboratory animal management and welfare (pp. 59–73). Blackwell Publishing Ltd. [Google Scholar]
- Wolff, M. , Casse‐Perrot, C. , & Dravet, C. (2006). Severe myoclonic epilepsy of infants (Dravet syndrome): Natural history and neuropsychological findings. Epilepsia, 47(Suppl 2), 45–48. [DOI] [PubMed] [Google Scholar]
- Yu, F. H. , Mantegazza, M. , Westenbroek, R. E. , Robbins, C. A. , Kalume, F. , Burton, K. A. , … Catterall, W. A. (2006). Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nature Neuroscience, 9, 1142–1149. 10.1038/nn1754 [DOI] [PubMed] [Google Scholar]
- Zaccara, G. , Perucca, P. , Loiacono, G. , Giovannelli, F. , & Verrotti, A. (2013). The adverse event profile of lacosamide: A systematic review and meta‐analysis of randomized controlled trials. Epilepsia, 54, 66–74. 10.1111/j.1528-1167.2012.03589.x [DOI] [PubMed] [Google Scholar]
- Zhou, C. , Lippman Bell, J. J. , Sun, H. , & Jensen, F. E. (2011). Hypoxia‐induced neonatal seizures diminish silent synapses and long‐term potentiation in hippocampal CA1 neurons. The Journal of Neuroscience, 31, 18211–18222. 10.1523/JNEUROSCI.4838-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Example result from PCR used for genotyping animals produced via breeding of the 129S‐Scn1a tm1Kea/Mmjax transgenic mouse line. Mouse tail DNA was amplified with primers for the wild type (WT) and mutated (Scn1a −/−) Scn1a alleles. The presence of a 357 bp band indicates the WT allele, and a 200 bp band indicates the Scn1a −/− allele. ‐ve: negative control; +ve: positive control.
Figure S2. Plots showing chronic administration of CBD to wild type (WT) and Scn1a−/− mice on different welfare scores and weight change. A. Natural activity (NA) scores of WT vehicletreated, WT CBD‐treated, Scn1a−/− vehicle‐treated and Scn1a−/− CBD‐treated mice from postnatal day 8 (P8) onwards (n = 10/group). CBD had no effect on NA score of WT mice. CBD significantly improved the NA score in Scn1a−/− mice from P12 onwards compared to the vehicle‐treated Scn1a−/− mice. B. Reflex/response to touch (RT) scores. CBD significantly improved the RT score in WT mice from P8 to P9.5 compared to the vehicle‐treated WT mice. CBD significantly improved the RT score in Scn1a−/− mice from P12 to P14.5 and on P16 compared to the vehicle‐treated Scn1a −/− mice. C. Orbital tightening (OT) scores. CBD had no effect on OT score of WT mice. CBD significantly improved the OT score in Scn1a −/− mice from P13 onwards compared to the vehicle‐treated Scn1a −/− mice. D. Body condition (BC) scores. CBD had no effect on BC score of WT mice. CBD significantly improved the BC score in Scn1a −/− mice from P13.5 to P15.15 compared to the vehicle‐treated Scn1a −/− mice. E.Weight. CBD had no effect on weight in WT mice compared to the vehicle‐treated WT mice. No threeway interaction of treatment×genotype×time was observed on weight. Data are expressed as mean ± SEM; data were analysed by a three‐way ANOVA with Bonferroni's post hoc test; #p < 0.05; *p < 0.05; # represents WT vehicle‐treated vs WT CBD‐treated; * represents Scn1a −/− vehicle‐treated vs Scn1a −/− CBD‐treated.
Table S1. Welfare assessment sheet for dam; adapted from (Wolfensohn and Lloyd, 2007).
Table S2. Welfare assessment sheet for neonatal mice; adapted from (Langford et al., 2010; Ullman‐Culleré & Foltz, 1999; Wolfensohn & Lloyd, 2007)
Data S1 Supporting information
Data S2 Supporting information
Data S3 Supporting information


