Summary
Background
Optimal treatment regimens for AIDS-associated Kaposi sarcoma, a frequent contributor to morbidity and mortality among people with HIV, have not been systematically evaluated in low-income and middle-income countries, where the disease is most common. In this study, we aimed to investigate optimal treatment strategies for advanced stage disease in areas of high prevalence and limited resources.
Methods
In this open-label, non-inferiority trial, we enrolled people with HIV and advanced stage AIDS-associated Kaposi sarcoma attending 11 AIDS Clinical Trials Group sites in Brazil, Kenya, Malawi, South Africa, Uganda, and Zimbabwe. Eligible participants were randomly assigned (1:1:1) with a centralised computer system to receive either intravenous bleomycin and vincristine or oral etoposide (the investigational arms), or intravenous paclitaxel (the control arm), together with antiretroviral therapy (ART; combined efavirenz, tenofovir disoproxil fumarate, and emtricitabine). The primary outcome was progression-free survival (PFS) at week 48, using a 15% non-inferiority margin to compare the investigational groups against the active control group. Safety was assessed in all eligible treated study participants. The study was registered with ClinicalTrials.gov, NCT01435018.
Findings
334 participants were enrolled between Oct 1, 2013, and March 8, 2018, when the study was closed early due to inferiority of the bleomycin and vincristine plus ART arm, as per the recommendations of the Data and Safety Monitoring Board (DSMB). The etoposide plus ART arm also closed due to inferiority in March, 2016, following a DSMB recommendation. Week-48 PFS rates were higher in the paclitaxel plus ART arm than in both investigational arms. The absolute differences in PFS were –30% (95% CI –52 to –8) for the comparison of paclitaxel plus ART (week 48 PFS 50%, 32 to 67; n=59) and etoposide plus ART (20%, 6 to 33; n=59), and –20% (–33% to –7%) for the comparison of paclitaxel plus ART (64%, 55 to 73; n=138) and bleomycin and vincristine plus ART (44%, 35 to 53; n=132). Both CIs overlapped the non-inferiority margin. The most common adverse events, in 329 eligible participants who began treatment, were neutropenia (48 [15%]), low serum albumin (33 [10%]), weight loss (29 [9%]), and anaemia (28 [9%]), occurring at similar frequency across treatment arms.
Interpretation
Non-inferiority of either investigational intervention was not shown, with paclitaxel plus ART showing superiority to both oral etoposide plus ART and bleomycin and vincristine plus ART, supporting its use in treating advanced AIDS-associated Kaposi sarcoma in resource-limited settings.
Introduction
AIDS-associated Kaposi sarcoma is an HIV-associated neoplasm caused by the Kaposi sarcoma-associated herpesvirus.1 Although the incidence of this disease has decreased worldwide since the introduction of potent antiretroviral therapy (ART),2 it remains widespread and will probably continue to be an important contributor to morbidity and mortality in places where the prevalence of HIV and Kaposi sarcoma-associated herpesvirus coinfection is high.3 In 2018, Kaposi sarcoma was estimated to be the second most common cancer diagnosis in Malawi (accounting for 20·5% of all cancer diagnoses) and Uganda (13% of all cancer diagnoses), and the third most common in Zimbabwe (9% of all cancer diagnoses), regardless of HIV status.4 Importantly, AIDS-associated Kaposi sarcoma develops at all levels of immunosuppression, suggesting that improved ART coverage will not eradicate the disease. The reasons for the continued high incidence in these settings probably include high rates of Kaposi sarcoma-associated herpesvirus and HIV co-infection, suboptimal ART coverage, and high rates of infectious diseases, such as malaria, that could influence the acquisition and control of Kaposi sarcoma-associated herpesvirus infection.5
ART is considered to be essential for optimal management of all patients with AIDS-associated Kaposi sarcoma.6 For advanced stage, symptomatic disease, which is a frequent presentation in the low-income and middle-income countries where AIDS-associated Kaposi sarcoma is most common, a consensus also exists that effective management requires the addition of chemotherapy.6 However, optimal chemotherapy regimens have not been systematically evaluated in these settings. Data on AIDS-associated Kaposi sarcoma chemotherapy from countries with high prevalence are largely descriptive.7–15 Of the few prospectively randomised trials,16–19 only one compared different chemotherapy regimens, but without concomitant ART.19
In high-resource settings, pegylated liposomal doxorubicin and paclitaxel have been shown to be highly effective in inducing regression of advanced AIDS-associated Kaposi sarcoma.20–26 Treatment guidelines published in the USA by the National Comprehensive Cancer Network27 designate these drugs as the preferred, first-line, systemic chemotherapy for AIDS-associated Kaposi sarcoma, but they are used infrequently in areas with resource limitations because of their low availability and high cost. In such settings, the combination of bleomycin and vincristine, alone or in combination with non-liposomal doxorubicin, is commonly used due to its lower cost and wider availability. Although not widely used in either setting, orally administered etoposide has been shown to induce disease regression without excessive toxicity.15,19,28,29 Etoposide is the only orally bioavailable chemotherapeutic agent with demonstrated activity against AIDS-associated Kaposi sarcoma and can be easily incorporated into outpatient treatment regimens, making it a potentially attractive option where cancer treatment infrastructure is lacking.30 Randomised clinical trials to compare these treatment approaches together with ART have never been done.
We sought to investigate the optimal treatment strategy for advanced AIDS-associated Kaposi sarcoma at clinical trials sites in five sub-Saharan African countries and Brazil. During early development of the trial, we intended pegylated liposomal doxorubicin plus ART to be the active control, but a worldwide shortage of pegylated liposomal doxorubicin led us to substitute paclitaxel plus ART as the active control. We chose bleomycin and vincristine plus ART and oral etoposide plus ART as the investigational regimens. Recognising that clinical efficacy is not the only criterion on which to base the choice of treatment, and that the investigational regimens also offered potential advantages over the active control in terms of their cost, availability, adverse event profiles, and ease of administration, we designed the study to test whether bleomycin and vincristine plus ART or oral etoposide plus ART, or both, were non-inferior to paclitaxel plus ART.
Methods
Study design
We used a randomised, open-label, active-controlled, clinical trial (A5263/AMC-066) to evaluate three regimens of chemotherapy with ART for the treatment of advanced AIDS-associated Kaposi sarcoma. Full details of the study are available online. The study was done at 11 AIDS Clinical Trials Group (ACTG) sites in Brazil, Kenya, Malawi, South Africa, Uganda, and Zimbabwe, all of which obtained approval from local and national ethics committees. A four-step trial design enabled participants to receive one or more of the alternative chemotherapy regimens in the event that the initial randomised regimens proved ineffective or intolerable (appendix p 6). Before a change in study step, an independent endpoint review committee (IERC) consisting of eight Kaposi sarcoma experts, who were masked to treatment assignment, reviewed and confirmed site-reported Kaposi sarcoma progressions, including suspected Kaposi sarcoma-associated immune reconstitution inflammatory syndrome. Participants whose Kaposi sarcoma initially responded to step 1 chemotherapy but later progressed could receive another course of the same chemotherapy in step 2 if they had IERC-confirmed partial or complete response of Kaposi sarcoma in step 1 lasting at least 12 weeks before IERC-confirmed progression. Participants showing IERC-confirmed Kaposi sarcoma progression or intolerance to the chemotherapy regimen of step 1 or step 2, or both, could enter step 3 (randomised assignment) and later step 4 (the remaining regimen).
Participants
Eligible participants were people with HIV who were aged 18 years or older and had advanced, biopsy-confirmed Kaposi sarcoma but had not previously received local or systemic chemotherapy or radiotherapy. ART experience was initially limited to 28 days immediately prior to study entry, but in January, 2015 (16 months after the first participant enrolled), a protocol amendment increased the permissible duration of prior ART to 42 days. Advanced Kaposi sarcoma was defined as stage T1, including one or more of the following features: symptomatic tumour-associated oedema, tumour ulceration, extensive oral Kaposi sarcoma, and visceral (non-nodal) Kaposi sarcoma.31
Additional inclusion criteria included Karnofsky performance status of at least 60, at least five bi-dimensionally measurable cutaneous marker lesions or a total marker lesion surface area of at least 700 mm2, an absolute neutrophil count of at least 1000 cells per μL, at least 8∙0 mg/dL haemoglobin, at least 100 000 platelets per μL, a creatinine clearance of at least 60 mL/min, a maximum of five times the upper limit of normal (ULN) of aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase, and a maximum of 1·5 times ULN of bilirubin.
Pregnant and breastfeeding women were excluded, as were individuals with less than 90% oxygen saturation or more than 4% exercise desaturation, or both, people with grade 3 or higher peripheral neuropathy, and individuals with active infections who had received less than 14 days of antimicrobial treatment.
Participants provided written informed consent. A model informed consent document was provided in English and translated into local languages at each trial site.
Randomisation and masking
Randomisation was done by a centralised computer system maintained by the ACTG Data Management Centre using permuted blocks of six in an open-label method. Masking was not feasible because of the different chemotherapy administration procedures. In step 1, participants were randomly assigned equally (1:1:1) to receive bleomycin and vincristine plus ART, etoposide plus ART, or paclitaxel plus ART. Participants eligible for step 2 received the same regimen as in step 1. Participants eligible for step 3 were randomly assigned equally (1:1) to the two remaining regimens not used in step 1. All randomisation was stratified by screening CD4 cell count (either <100 or ≥100 cells per μL) and country. No randomisation occurred in steps 2 or 4.
Procedures
Etoposide (VePesid, Bristol-Myers Squibb, New York, NY, USA) was given as one 50 mg capsule taken orally twice per day on days 1–7 of each 21-day cycle, with escalation as tolerated on subsequent cycles up to a maximum of 100 mg taken twice per day. A cycle could be delayed for a maximum of 14 days for toxicity management. Up to eight etoposide cycles were administered (two cycles during dose titration; six at maximum dose).
Bleomycin and vincristine and paclitaxel were each administered intravenously on day 1 of each of six 21-day cycles. Bleomycin (Hospira, Lake Forest, IL, USA) was administered intravenously over 10 min at a dose of 15 units per m2 body surface area. Vincristine (Hospira) was administered as an intravenous bolus at a fixed dose of 2 mg. Paclitaxel (Accord Healthcare, Barnstaple, UK) was administered as a 1-h infusion at a dose of 100 mg/m2 of body surface area using a non-PVC administration set and an in-line filter (≤0∙22 μm) following administration of a standard premedication regimen, which contained dexamethasone with H1-receptor and H2-receptor antagonists. Concurrent with chemotherapy, all participants received efavirenz (600 mg), tenofovir disoproxil fumarate (300 mg), and emtricitabine (200 mg), either as a single co-formulated tablet taken once per day (Atripla, Merck, Kenilworth, NJ, USA) or as tenofovir disoproxil fumarate and emtricitabine (Truvada, Gilead, Foster City, CA, USA) plus efavirenz taken daily (Stocrin, Merck).
Clinical and laboratory evaluations were done at screening, entry, and every 3 weeks until week 48, and then at increasing intervals until week 96, with up to 5 years of follow-up for observing late haematological toxicity (ie, myelodysplasia, leukaemia) in participants who received etoposide (full study details available online). Kaposi sarcoma response was categorised as complete response, partial response, stable disease, or progression of disease, as previously described,26,31 with additional refinements, including circumferential measurements of tumour-associated leg oedema. Signs, symptoms, and laboratory results were graded according to the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events.32 Chemotherapy doses were delayed, modified, or discontinued for graded adverse events, and if adverse events recurred after dose reduction or resulted in a delay of treatment by more than 3 weeks, chemotherapy was discontinued. The use of recombinant methionyl human granulocyte colony stimulating factor (filgrastim; G-CSF) to manage neutropenia was permitted, when available.
Outcomes
The primary outcome measure was progression-free survival (PFS), defined as an absence of IERC-confirmed Kaposi sarcoma progression, death, entry to an additional step, or loss to follow-up prior to week 48. Secondary outcomes evaluating superiority of paclitaxel plus ART included the composite of Kaposi sarcoma progression or death rate at week 48, week-48 death rate, tumour response rate and duration, time to death, and time to death or progression. The week-48 progression rate and time to progression were not analysed individually because of the competing risk of death. HIV virological failure was defined as two successive measurements of plasma HIV-1 RNA of at least 1000 copies per mL at weeks 12–24 or plasma HIV-1 RNA of at least 400 copies per mL at week 24 or later.
Suspected Kaposi sarcoma-associated immune reconstitution inflammatory syndrome was defined as progressive disease within 12 weeks of ART initiation together with an increase in CD4 cell count of at least 50 cells per μL above the entry value or a decrease in plasma HIV RNA of at least 0∙5 log10 copies per mL below the entry value, or both.
Statistical analysis
The study was designed to evaluate whether there was sufficient evidence to conclude that bleomycin and vincristine plus ART or etoposide plus ART, or both, were non-inferior to paclitaxel plus ART. Non-inferiority was defined as showing that the 48-week PFS rate in each investigational arm was within 15% of the PFS rate in the paclitaxel plus ART arm. After consultation with the site clinicians, the non-inferiority margin was based on a combination of clinical judgment and statistical reasoning. Two pairwise comparisons with 5% type 1 error were planned. With the 15% non-inferiority margin, the planned sample size of 706 gave 88% power to show non-inferiority, assuming that the true PFS rate in each arm was 65% and that the one-sided significance level was 2∙5%. This sample size was inflated for 10% loss to follow-up and for two planned, interim efficacy analyses at 33% and 67% of statistical information; alpha levels were determined by Lan-DeMets spending function (corresponding to the O’Brien-Fleming boundary).32,33 Formal stopping rules were not predetermined. Following premature closure of the etoposide plus ART arm, and to address accrual being slower than anticipated, the planned sample size was recalculated as 446, which included the 60 already assigned to etoposide plus ART. The 386 people randomly assigned to bleomycin and vincristine plus ART and paclitaxel plus ART provided 80% statistical power to evaluate non-inferiority.
The National Institute of Allergy and Infectious Diseases (NIAID) Complications and Co-Infections Data and Safety Monitoring Board (DSMB) monitored the study at least once per year and received summaries of study conduct, safety, and efficacy.
Because of observed differences in PFS rates between the treatment arms, an early, unplanned, interim efficacy analysis of the primary outcome was done at the second interim review (1 year after the first). From this review onwards, at the request of the DSMB, comparisons of PFS rates were provided at all reviews. CIs were determined by the proportion of statistical information available at the review and PFS rates were determined by Kaplan-Meier methods with Greenwood’s formula for the variance.34,35 Before each investigational arm was closed, two formal comparisons of etoposide plus ART with paclitaxel plus ART were done (0・0012 total alpha spent) and five of bleomycin and vincristine plus ART with paclitaxel plus ART were done (0∙0104 total alpha spent).
At the fourth review in March, 2016, the DSMB recommended that the etoposide plus ART arm be discontinued because of inferior PFS compared with the paclitaxel plus ART arm. The PFS difference (etoposide–paclitaxel) provided at this review was –39∙4% (99・9% CI –79∙9 to 1∙0). Immediately after this review, participants in the etoposide plus ART arm were offered the opportunity to switch treatments. Following DSMB recommendations, accrual to the bleomycin and vincristine plus ART and paclitaxel plus ART arms continued (with a reduced permutated block size of four for randomisation), and no data from these arms were released.
At the seventh DSMB review in March, 2018, the board recommended stopping the study and concluded that the primary objective of non-inferiority could not be demonstrated. This conclusion was based on predictive interval plot simulations, which showed almost 0% probability of showing non-inferiority given current trends. The DSMB also concluded that paclitaxel plus ART was superior to bleomycin and vincristine plus ART, based on the comparison of the 48-week PFS rates; the PFS difference was –20% (99·2% CI –37∙5 to –2∙5). Furthermore, the predictive interval plots showed that the results would be unlikely to change if the study continued, because 99% of the simulated intervals excluded 0%. Study accrual was permanently stopped, and the study was amended to allow all remaining participants who had not yet reached study week 72 to receive paclitaxel if appropriate.
All treatment comparisons of primary and secondary outcomes were two-sided with 5% significance without adjustment for multiple comparisons. Because the investigational arms ended early, nominal 95% CIs were calculated for these comparisons without adjustment for prior alpha spending. As with the interim analyses, PFS rates were estimated using Kaplan-Meier methods with Greenwood’s formula for the variance.34,35 Two-sided 95% CIs for PFS rate differences were determined overall and with adjustments for each stratification factor; stratum weights were determined by the inverse of the stratum-specific variance. Estimates of secondary event rates were calculated in a similar way. Time-to-event analyses were conducted with Kaplan-Meier methods to estimate median survival time in each arm, and Cox proportional hazards regression was used to compare the risk of events between arms, adjusting for stratification factors. Objective responses (partial or complete response) were compared between arms with logistic regression, adjusting for stratification factors, and the median duration of objective response was determined by Kaplan-Meier methods. Descriptive summaries of changes in CD4 cell counts were made with medians and IQRs, and with HIV-1 RNA levels with percentages (<200, 200–1000, >1000 copies per mL) by study week. HIV-1 RNA suppression (<200 copies per mL) was compared across study weeks and treatment arms with percentages and corresponding 95% CIs in a post-hoc analysis. All eligible participants who started the randomised treatment were included in the analysis, and all treatment comparisons were of the initially randomised regimens.
Analyses comparing etoposide plus ART with paclitaxel plus ART used data collected until March 10, 2016, and analyses comparing bleomycin and vincristine plus ART with paclitaxel plus ART used data collected until March 13, 2018; these dates correspond to when each investigational arm was closed. Descriptive safety summaries of adverse events include all step 1 data until March 13, 2018, and detailed safety summaries according to treatment step were also made.
All analyses were conducted in SAS 9.4. The study was registered with ClinicalTrials.gov, NCT01435018.
Role of the funding source
NIAID funded the study through the ACTG, with additional funding from the National Cancer Institute through the AIDS Malignancy Consortium (AMC). The funders oversaw the development and monitoring of the study but had no role in the conduct, analyses, and conclusions of the study. The corresponding author had final responsibility for the decision to submit for publication.
Results
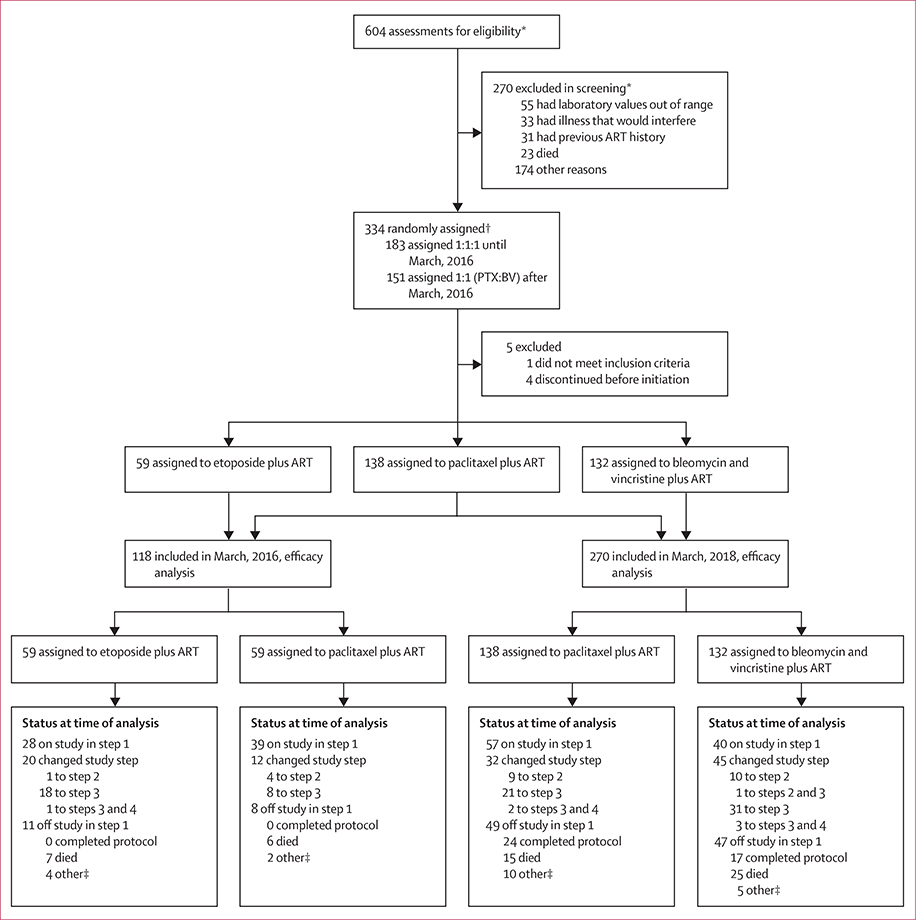
This three-arm, open-label, randomised, non-inferiority trial (A5263/AMC-066) enrolled 334 participants between Oct 1, 2013, and March 8, 2018 (table 1). The median study follow-up was 62 weeks (IQR 37–103; maximum 206 weeks) when study enrolment was closed. Of those enrolled, four participants never began the study chemotherapy and discontinued study participation, and one was found to be ineligible after randomisation (figure 1, table 1)
Table 1:
Baseline characteristics
| March, 2016 |
March, 2018 |
|||
|---|---|---|---|---|
| Etoposide plus, ART(n=59) | Paclitaxel plus ART (n=59)* | Bleomycin and vincristine plus ART (n=132) | Paclitaxel plus ART (n=138)* | |
| Set | ||||
| Female | 14(24%) | 11(19%) | 31(23%) | 31(22%) |
| Male | 45(76%) | 48(81%) | 101(77%) | 107(78%) |
| Age in years | 35(31–42) | 35(30–39) | 35(30–41) | 35(31–40) |
| Race | ||||
| Black or African | 58(98%) | 57(97%) | 130(98%) | 136(99%) |
| White | 1(2%) | 2(3%) | 2(2%) | 2(1%) |
| Hispanic or Latino ethnicity | 2(3%) | 5(8%) | 5(4%) | 7(5%) |
| Country of residence | ||||
| Brazil (one site) | 2(3%) | 5(8%) | 5(4%) | 7(5%) |
| Kenya (three sites) | 2(3%) | 2(3%) | 27(20%) | 29(21%) |
| Malawi (two sites) | 27(46%) | 27(46%) | 54(41%) | 56(41%) |
| SouthAfrica (three sites) | 0 | 0 | 7(5%) | 8(6%) |
| Uganda (one site) | 10(17%) | 9(15%) | 7(5%) | 9(7%) |
| Zimbabwe (one site) | 18 (31%) | 16(27%) | 32(24%) | 29(21%) |
| Previous ART exposure in days | ||||
| None | 53(90%) | 47(80%) | 79(60%) | 76(55%) |
| 1–28 | 5(8%) | 11(19%) | 29(22%) | 35(25%) |
| 29–42 | 1(2%) | 1(2%) | 24(18%) | 27(20%) |
| CD4 cell count (cells per mL) | 216(99–357) | 228(125–384) | 232(134–369) | 231(127–341) |
| CD4 cell count <100 cells | 15(25%) | 12(20%) | 25(19%) | 27(20%) |
| per μL | ||||
| UN RNA (log10 copies per mL) | 4.9(3.9–5.3) | 5.0 (3.9–5.4) | 4.6 (2.7–5.2) | 4.0(2.7–5.2) |
| HN RNA <400 copies per mL | 4(7%) | 4(7%) | 29(22%) | 38(28%) |
| KPS ≥90 | 32(54%) | 24(41%) | 79(60%) | 76(55%) |
Data are n (%) or median (IQR).
The 138 participants in the paclitaxel plus ART arm at the time of the March, 2018, analysis include the 59 participants enrolled as of March, 2016, plus 79 participants enrolled subsequently. ART=antiretroviral therapy. KPS=Karnofsky performance score.
Figure 1: Trial profile.
ART=antiretroviral therapy. *Potential participants could be screened more than once and be excluded for multiple reasons; these numbers reflect discrete screening events. †Participants were randomly assigned equally (1:1:1) to the three study arms until March, 2016, when the etoposide plus ART arm was closed, after which participants were randomly assigned equally (1:1) to the two remaining arms (paclitaxel plus ART and bleomycin and vincristine plus ART). ‡Other includes the participant having withdrawn consent, the participant not being willing to adhere to requirements, the participant not being able to get to the clinic, site closure, the participant being unable to continue because of severe debilitation, or the site being unable to contact the participant.
Self-reported adherence to ART and oral etoposide was high. Perfect adherence to ART, based on a 30-day recall, was reported by at least 87% of participants (286 of 329) across all study weeks and was similar across treatment arms. Although overall adherence to the prescribed etoposide dose was high (87%), only 39 (66%) of the 59 participants randomly assigned to receive etoposide were able to have the dose escalated to the planned maximum of 200 mg per day.
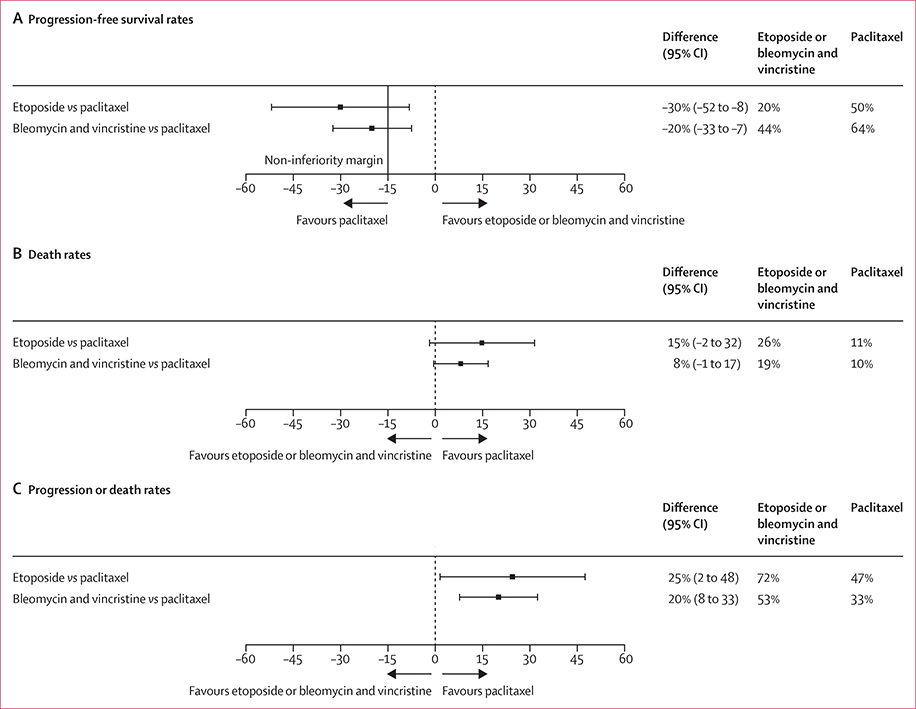
Non-inferiority was not able to be shown for either investigational arm compared with paclitaxel plus ART on the basis of the 15% threshold (figure 2). Week-48 PFS rates were higher in the paclitaxel plus ART arm than in each of the investigational arms. In the comparison of paclitaxel and etoposide, the PFS rate was 50% (95% CI 32 to 67) for paclitaxel plus ART (n=59) but only 20% (6 to 33) for etoposide plus ART (n=59; difference –30; 95% CI –52 to –8). Comparing paclitaxel with bleomycin and vincristine, the PFS rate was 64% (55 to 73) for paclitaxel plus ART (n=138) but only 44% (35 to 53) for bleomycin and vincristine plus ART (n=132; difference –20; –33 to –7). The results were identical when weighted for stratification factors (appendix p 7). Thus, paclitaxel plus ART was superior to both etoposide plus ART and bleomycin and vincristine plus ART with respect to zero difference, because 0% was excluded from the 95% CIs (figure 2).
Figure 2:
Comparison of 48-week progression-free survival rates (A), 48-week death rates (B), and 48-week composite progression or death rates (C) according to initial randomised treatment
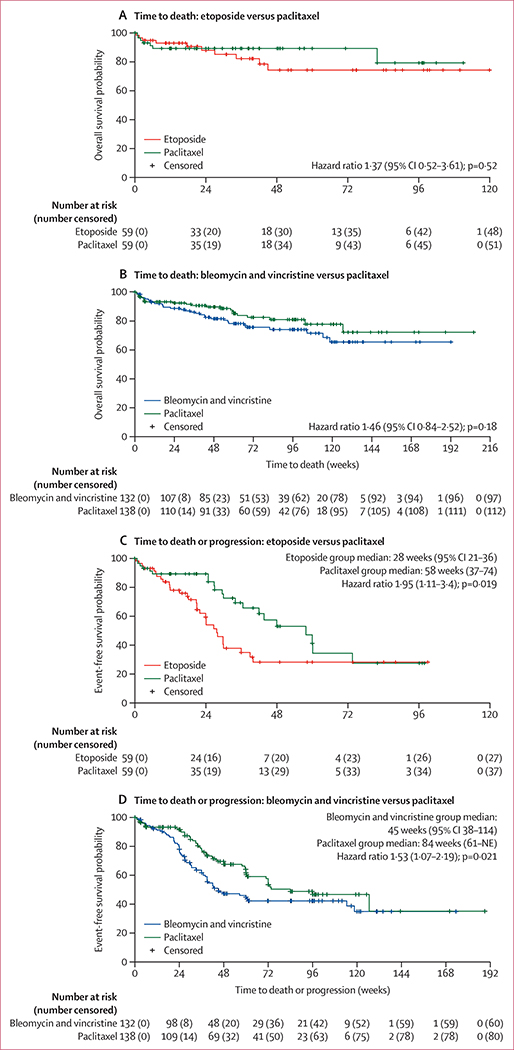
Although the differences in the death rates failed to reach statistical significance, the rates of IERC-confirmed Kaposi sarcoma progression or death, and the rates of death by week 48 were higher in the etoposide plus ART and bleomycin and vincristine plus ART arms than in the paclitaxel plus ART arm (figure 2). Similarly, although the time to death analysis did not show a significant difference between arms, the time to death and the time to progression or death analyses showed higher risk for both etoposide plus ART and bleomycin and vincristine plus ART than for paclitaxel plus ART (figure 3).
Figure 3: Time to death and time to death or progression according to randomised treatment.
Kaplan-Meier plots of the time to death (A and B) and composite of time to death or progression (C and D) summarised by treatment comparison. Hazard ratio with 95% CI and p value are from Cox proportional-hazards regression analysis. NE=not estimable.
The objective responses in step 1 favoured the paclitaxel plus ART arm. In the etoposide plus ART arm, 18 (31%) of 59 participants showed an objective response compared with 34 (58%) of 59 participants in the paclitaxel plus ART arm (odds ratio [OR] 0∙3; 95% CI 0∙1–0∙7; p=0∙0032). Among participants showing an objective response, the median response duration was longer for paclitaxel plus ART (54 weeks, 23–NE [not estimable]) than for etoposide plus ART (24 weeks, 9–NE). Although similar objective response proportions were observed in the bleomycin and vincristine plus ART group (80 [61%] of 132 participants) and the paclitaxel plus ART group (91 [66%] of 138 participants; OR 0∙8; 95% CI 0∙5–1∙3; p=0∙43), the median duration of response was longer for paclitaxel plus ART (87 weeks, 57–NE) than it was for bleomycin and vincristine plus ART (59 weeks, 32–NE). Complete responses were rare, occurring in only three (2%) of 132 participants in the bleomycin and vincristine plus ART arm and two (1%) of 138 participants in the paclitaxel plus ART arm.
Suspected Kaposi sarcoma-related immune reconstitution inflammatory syndrome, as defined by the protocol, was uncommon. Overall, only eight (2%) of 329 participants met the predefined criteria: two (2%) of 132 participants on the bleomycin and vincristine plus ART arm, six (10%) of 59 on the etoposide plus ART arm, and none on the paclitaxel plus ART arm.
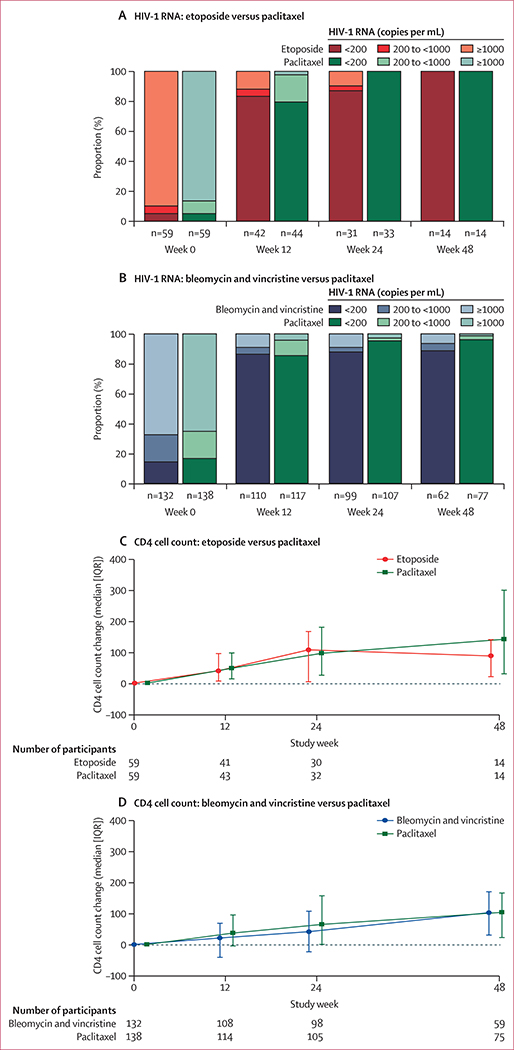
Plasma HIV-1 RNA suppression below 200 copies per mL at week 48 occurred in 55 (89%) of the 62 participants in the bleomycin and vincristine plus ART group, 74 (96%) of 77 participants in the paclitaxel plus ART group, and 14 (100%) of 14 participants in the etoposide plus ART group (figure 4). Among the subset of participants who had viral assays with a detection limit of 50 copies per mL, 51 (82%) of 62 participants on bleomycin and vincristine plus ART, 69 (90%) of 77 participants on paclitaxel plus ART, and 14 (100%) of 14 participants on etoposide plus ART suppressed below this limit. Virological failure by week 48 was uncommon, occurring in three (5%) of 59 participants receiving etoposide plus ART, eight (6%) of 132 participants receiving bleomycin and vincristine plus ART, and two (1%) of 138 participants receiving paclitaxel plus ART. In post-hoc analysis (appendix p 8), the proportion of participants with suppression (<200 copies per mL) within each arm and the corresponding 95% binomial CIs were examined at weeks 12, 24, and 48. The proportions with suppression were similar across the study weeks, indicating that differences in viral load suppression did not contribute to the primary findings. CD4 lymphocyte counts increased progressively with time in all treatment arms (figure 4), with the exception of week 48 in the etoposide plus ART arm.
Figure 4: HIV-1 RNA and CD4 cell count changes.
Proportion of participants in each HIV-1 RNA category by study week for etoposide vs paclitaxel (A) and bleomycin and vincristine vs paclitaxel (B); and median (IQR as error bars) change in CD4 cell counts summarised by study week for etoposide vs paclitaxel (C) and bleomycin and vincristine vs paclitaxel (D).
A detailed summary of all grade 3 or 4 events, new AIDS-defining events, and serious non-AIDS defining events according to treatment step was made (appendix pp 10–25). Grade 3 or higher clinical adverse events or laboratory abnormalities were reported in 162 (49%) of the 329 participants during step 1 (table 2), and were similar across treatment arms. The most frequent events were decreases in the absolute neutrophil count (n=48 [15%]), blood albumin (n=33 [10%]), weight (n=29 [9%]), and haemoglobin (n=28 [9%]). Sensory peripheral neuropathy assessments included self-reports of symptom severity in three domains: pain, aching, or burning in the feet and legs; pins and needles in the feet and legs; and numbness in the feet and legs. Most participants’ symptoms, as well as objective measures (vibratory sense and deep tendon reflexes), remained unchanged or improved during chemotherapy. However, pain scores were more variable, with more participants reporting pain increases in the bleomycin and vincristine plus ART group than in the paclitaxel plus ART group (17% vs 7%), and more in the etoposide plus ART group than in the paclitaxel plus ART group (7% vs 0%). New AIDS-defining events, all of which were infections, were documented in 46 (14%) of the 329 participants, the most common being bacterial pneumonias (n=24 [7%]) and pulmonary tuberculosis (n=11 [3%]). Serious non-AIDS-defining events were reported in 68 participants (21%), the majority of which (n=40 [12%]) were non-AIDS-defining bacterial infections. 22 participants (7%) were described as having sepsis, of which only one was reported as occurring in the setting of neutropenia, nine (3%) were clinical diagnoses only, and none were rigorously confirmed with multiple positive blood cultures. G-CSF use was reported in eight (6%) of the 132 participants in the bleomycin and vincristine plus ART arm, eight (14%) of the 59 participants in the etoposide plus ART arm, and 11 (8%) of the 138 participants in the paclitaxel plus ART arm. G-CSF use was inconsistent across sites because of differences in availability, cost constraints, and local practice.
Table 2:
Safety and adverse events during step 1 according to initially randomised treatment
| Bleomycin and vincristine plus ART (n=132) | Etoposide plus ART (n=59) | Paclitaxel plus ART (n=138) | Total (n=329) | |
|---|---|---|---|---|
| Any grade 3 of higher adverse event | 64(48%) | 34(58%) | 64(46%) | 162(49%) |
| Neutropenia (grade ≥ 3) | 19(14%) | 13(22%) | 16(12%) | 48(15%) |
| Serum albumin (grade ≥ 3) | 14(11%) | 9(15%) | 10(7%) | 33(10%) |
| Weight loss (grade ≥ 3) | 13(10%) | 5(8%) | 11(8%) | 29(9%) |
| Haemoglobin (grade ≥ 3) | 17(13%) | 3(5%) | 8(6%) | 28(9%) |
| AIDS-defining event | 18(14%) | 6(10%) | 22 (16%) | 46(14%) |
| Bacterial pneumona | 11(8%) | 3(5%) | 10(7%) | 24(7%) |
| Pulmonary tuberculosis | 5(4%) | 2(3%) | 4(3%) | 11(3%) |
| Serous non-AIDS-defining event | 28(21%) | 11(19%) | 29(21%) | 68(21%) |
| Bacterial infections | 17(13%) | 7(12%) | 16(12%) | 40(12%) |
| Data are n (%). ART=antiretroviral therapy. | ||||
Two study participants became pregnant (one each in the bleomycin and vincristine plus ART and paclitaxel plus ART arms) after having completed six cycles of step 1 chemotherapy. One of the pregnancies, which was detected at study week 27 in a woman whose last cycle of bleomycin and vincristine had been administered at study week 16, ended in spontaneous abortion at study week 29 (we presume this was a first trimester pregnancy because pregnancy tests needed to be negative before each dose of chemotherapy and the last dose was at week 16). The other pregnancy, which was detected at study week 119 in a woman whose last cycle of paclitaxel had been administered at study week 15, ended in a live birth at study week 149, without evidence of congenital abnormalities.
Discussion
Our prospectively randomised study was designed to evaluate non-inferiority of the investigational arms compared with the active control, paclitaxel plus ART. Because the 95% CIs of PFS rate differences overlapped with the prespecified 15% non-inferiority margin, the study failed to show non-inferiority of either investigational arm compared with paclitaxel plus ART. Although we had not planned a priori to consider inferiority of the investigational arms, the 95% CIs for each of the comparisons excluded zero. The clinical interpretation is that paclitaxel plus ART is a superior treatment to both oral etoposide plus ART and bleomycin and vincristine plus ART.
In addition to the unexpected finding that paclitaxel plus ART was superior to both investigational arms with respect to the primary outcome, PFS at 48 weeks, we found that participants who received paclitaxel as their initial chemotherapy showed a superior outcome compared with participants who received either etoposide or bleomycin and vincristine with respect to overall response rate, response duration, and the composite of time to death or progression. These findings, together with the overall safety profile, effective suppression of HIV viraemia, and progressive increase in CD4 cell count, support the use of paclitaxel plus ART as initial therapy for AIDS-related Kaposi sarcoma in this setting.
The assumption that the true PFS rate at 48 weeks was 65% was based on the findings of a randomised, USA-based trial26 that compared paclitaxel with pegylated liposomal doxorubicin as initial treatment for people with AIDS-related Kaposi sarcoma, two-thirds of whom had advanced stage disease. A nearly identical PFS rate of 64% was observed in the current trial for the paclitaxel plus ART arm. The objective response rates for participants who received paclitaxel in the two trials were also similar: 57% in the US study and 66% in the current trial. Notably, although the individual paclitaxel doses administered in the two studies were identical, the paclitaxel dose intensity was 50% higher in the US study, because the drug was administered every 2 weeks rather than every 3 weeks as in our trial. This reduced dose intensity was not associated with a poorer objective response rate or PFS, but the rate of grade 3 or 4 neutropenia was much lower: 12% in the current trial versus 58% in the US trial.26 The relatively low rates of severe neutropenia and bacterial sepsis observed with the dose and schedule of paclitaxel used in our study is reassuring, given the scarcity of haemopoietic colony stimulating factors in resource-limited settings. Furthermore, although bleomycin and vincristine is generally considered to be a relatively non-myelosuppressive regimen, the observed frequencies of grade 3 or more neutropenia and anaemia in our study were both lower in the paclitaxel plus ART arm than in the bleomycin and vincristine plus ART arm. Taken together, these observations provide evidence that the efficacy and safety of paclitaxel plus ART for treating AIDS-related Kaposi sarcoma in low-income and middle-income countries is similar to that observed in a high-income country.
Overall, combined treatment with chemotherapy and effective ART was well tolerated in this group of individuals with advanced AIDS-associated Kaposi sarcoma, allaying concerns about the tolerability of chemotherapy in this population. The increased reports of pain, aching, or burning in the lower extremities from participants in both the etoposide and bleomycin and vincristine groups compared with the paclitaxel group could simply reflect symptoms associated with progressive oedema and Kaposi sarcoma lesions in the less effective regimens, rather than reflecting increasing neuropathic pain itself. Consistent with earlier findings when etoposide was administered together with ART to individuals with limited-stage AIDS-associated Kaposi sarcoma,17 Kaposi sarcoma-associated immune reconstitution inflammatory syndrome occurred infrequently during combined treatment with ART and each of the chemotherapeutic regimens tested in this study.
The choice of paclitaxel as the active control chemotherapeutic agent in this study merits comment. When we began designing this study more than a decade ago, we planned to use pegylated liposomal doxorubicin as the active control. Shortly before the trial was scheduled to begin in 2011, however, a worldwide shortage in pegylated liposomal doxorubicin led us to revise the study to substitute paclitaxel as the active control. Although this decision delayed the start of the study, the switch to paclitaxel was probably fortunate, because in the intervening years paclitaxel has become more widely available in resource-limited settings and its cost remains considerably lower than that for pegylated liposomal doxorubicin. Furthermore, unlike pegylated liposomal doxorubicin, paclitaxel is included in the WHO Model List of Essential Medicines. Even though its current high cost might limit the use of pegylated liposomal doxorubicin in resource-limited settings, it has a different adverse event profile to paclitaxel and could prove useful for people with HIV in whom paclitaxel proves ineffective or causes unacceptable toxicity. Additionally, studies to compare the efficacy, safety, and cost-effectiveness of pegylated liposomal doxorubicin and paclitaxel as first-line treatment in resource-limited settings are warranted.
The strengths of this study include a prospective, randomised design with frequent follow-up visits, during which participants were systematically evaluated using rigorous criteria for Kaposi sarcoma response, Kaposi sarcoma progression, and adverse events. A diverse population was enrolled from multiple sites in central, eastern, and southern Africa, and our findings should therefore apply broadly to countries in Africa that have a high burden of AIDS-associated Kaposi sarcoma. All participants had the diagnosis of Kaposi sarcoma confirmed by histopathology, using a quality assurance programme for local interpretation and centralised confirmation,36 and all progression events included in the primary outcome were confirmed by blinded independent review by a panel of expert clinicians.
The trial has several limitations. The conditions under which participants were treated are unlikely to be replicated in routine clinical practice in low-income and middle-income countries, where cancer therapeutic and diagnostic infrastructure are scarce.29 Study drugs, the laboratory tests to assess participant safety, the equipment and personnel required to safely store, prepare, and administer chemotherapy, and the supportive medications needed to safely administer paclitaxel were provided without charge, and participants were reimbursed for travel costs. Additionally, participation in the study was limited to individuals who had received limited or no previous ART, and we therefore have no direct data to support favouring paclitaxel over bleomycin and vincristine or etoposide in people with HIV with advanced AIDS-associated Kaposi sarcoma who have already had ART. We see no reason to believe that paclitaxel would be less effective in individuals who have already had ART whose HIV infection had already been successfully controlled, or in those with poorly controlled HIV who have alternative ART options that are likely to result in virological suppression. However, because HIV control is considered an essential component of AIDS-associated Kaposi sarcoma management, for those individuals who have exhausted available effective treatment options for HIV, the optimal management strategy for advanced Kaposi sarcoma is unclear. We also acknowledge that many potential participants were excluded from this study because of abnormal organ function or poor functional status, and the study provides no clear guidance on the best treatment options for the many people who present with advanced sequelae of HIV infection or disseminated Kaposi sarcoma (eg, neutropenia, poorly controlled secondary infections) in whom chemotherapy is contraindicated. Finally, the tools available to conclusively diagnose visceral involvement with Kaposi sarcoma were limited, in most cases, to chest x-rays and clinical assessments. Thus, although 31% of participants were assessed to have visceral involvement, 24% of which affected the lungs, the presence or absence of Kaposi sarcoma in visceral organs was not rigorously confirmed, the differential diagnosis for abnormal x-ray and clinical findings was broad, and we were unable to determine whether the results of treatment varied with the presence or absence of visceral Kaposi sarcoma.
Although our findings strongly suggest that using paclitaxel plus ART to treat advanced AIDS-associated Kaposi sarcoma in resource-limited settings improves outcomes compared with more widely used treatment regimens or those that are easier to administer, the overall costs of treatment with paclitaxel plus ART are likely to be higher than for the alternative regimens evaluated in this study. Not only is paclitaxel generally more costly than the alternative regimens, but its safe administration requires the use of specialised filters and pre-medications to prevent hypersensitivity reactions, further increasing the costs of treatment. Thus, it remains to be seen whether adopting paclitaxel plus ART as the standard of care would be a cost-effective use of resources. Efforts underway to provide greater access to essential cancer drugs, including paclitaxel, in Africa37 might increase the feasibility of its broader use in the region, but implementation will still require enhancement of the infrastructure and personnel dedicated to cancer care.
Supplementary Material
Research in context.
Evidence before this study
A consensus exists that effective management of advanced AIDS-associated Kaposi sarcoma requires treatment with a combination of antiretroviral therapy (ART) and chemotherapy. In high-resource countries, the preferred chemotherapeutic agents are pegylated liposomal doxorubicin and paclitaxel. These drugs are rarely used in countries with limited resources where AIDS-associated Kaposi sarcoma is most common because of their relatively high cost and low availability. Few prospective randomised trials have evaluated the optimal chemotherapy regimens to use for AIDS-associated Kaposi sarcoma management in areas of the world where the availability and affordability of chemotherapy drugs is low and the infrastructure to administer them safely is scarce. Most of the published data on treatment for advanced AIDS-associated Kaposi sarcoma in low-income and middle-income countries comes from retrospective, descriptive analyses of treatments with empirically chosen chemotherapy regimens, the most common of which is a combination of bleomycin and vincristine, which is widely available and relatively inexpensive. The only randomised, controlled study to compare different chemotherapy regimens directly in this setting was conducted in Zimbabwe in the 1990s, prior to the introduction of ART in that country.
Added value of this study
Our study is the first randomised controlled trial to compare different chemotherapy regimens in combination with effective ART for people with advanced AIDS-related Kaposi sarcoma in low-income and middle-income countries, where AIDS-related Kaposi sarcoma is a common complication of HIV infection. We showed that when combined with an effective ART regimen, intravenously administered paclitaxel was superior to both a widely used intravenous regimen of bleomycin and vincristine, and an easy-to-administer oral regimen of etoposide, with respect to progression-free survival, overall response rate, response duration, and a composite of time to progression or death. In addition, administration of paclitaxel with ART was associated with increases in CD4 lymphocyte counts, suppression of HIV viraemia, and an acceptable adverse event profile that contradicts commonly held concerns about the ability of people with HIV to tolerate cytotoxic chemotherapy and achieve effective HIV control.
Implications of all the available evidence
Our results provide support for preferring paclitaxel over a commonly used regimen of bleomycin and vincristine or an oral etoposide regimen as first-line chemotherapy for advanced AIDS-related Kaposi sarcoma in resource-limited settings. Making paclitaxel widely available as the initial treatment for advanced AIDS-related Kaposi sarcoma in these settings will require enhancements to the infrastructure and personnel dedicated to cancer care. Analysis of the cost-effectiveness of different Kaposi sarcoma treatments would further inform national strategies.
Acknowledgments
This research was presented in part at the 22nd International AIDS Conference, July 23–27, 2018, in Amsterdam, Netherlands, and at the 17th International Conference on Malignancies in HIV/AIDS, Oct 21–22, 2019, Bethesda, MD, USA. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (awards UM1 AI068634, UM1 AI068636, and UM1 AI106701) and by the National Cancer Institute of the National Institutes of Health (award UM1CA121947). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We are grateful to the following clinical research sites (CRSs) and collaborators for their participation: Agnes Moses and Cecilia Kanyama, Malawi CRS (site 12001; grant UM1 AI069423-08); Pamela Mukwekwerere and Ivy Gudza, Parirenyatwa CRS (site 30313; grant UM1 AI069436-08); Felluna Chauwa and Godwin Ulaya, Blantyre CRS (site 30301; grant UM1 AI069518-08); Irene Kutto and Priscilla Cheruiyot, Moi University Clinical Research Centre (site 12601; grant UM1 AI108568); Clement D Okello and Annet Nakaganda, Uganda Cancer Institute ACTG CRS (site 31713; grant UM1 AI069501-08); Geoffrey K Koskei and Winnie C Keter, Kenya Medical Research Institute and Walter Reed Project Clinical Research Center (site 12501; grant UM1 AI108568-01); Juliana Netto and Tamiris Baião, Instituto de Pequisa Clínica Evandro Chagas CRS (site 12101; grant UM1 AI069476-08); Iveshni Govender and Jessica O’Connell-Maritz, Wits Helen Joseph CRS (site 11101; grant AI069463); Kevin Cain and John Okanda, Kisumu CRS (site 31460; grant UM1 AI069418-08); Lynne Cornelissen and Marije van Schalkwyk, Family Clinical Research Unit, Stellenbosch University (site 8950; grant UM1 AI069521-08); Rejoice Sikhosana and Minenhle Ngcobo, Durban International CRS (site 11201; grant UM1 AI069432-08). We extend our sincere thanks to the participants in this study, to the dedicated staff at each of the participating Clinical Trials Units, to Drs Ronald Mitsuyasu and Robert Yarchoan for their steadfast support of the study, to the Community Advisory Boards of the AIDS Clinical Trials Group and the AIDS Malignancy Consortium for their advocacy on behalf of the study, to Dr Jeannette Y Lee for helpful comments on an earlier version of the manuscript, to Dr Katherine Shin for her meticulous attention in assuring study drug availability at the trial sites, to the members of the IERC for their careful reviews of participant outcomes, to Dr Scott Evans for his inspiration in the statistical design of the study, to Dr William Wachsman for his advice on the use of haematopoietic colony-stimulating factors, to Dr Taylor Harrison for his contributions to the evaluation of peripheral neuropathy, and to the protocol specialists, Jennifer Rothenberg and Sean McCarthy. The site pathologists’ biopsy-associated Kaposi sarcoma diagnoses were confirmed by independent pathologists at Weill Cornell Medical College, Department of Pathology and Laboratory Medicine, New York, NY, USA. Site pathologists also participated in an external quality assurance programme sponsored by the Department of Allergy and Infectious Diseases. Oversight of this programme was provided by the ACTG Pathology External Review Committee (UM1-AI-06701). Pharmaceutical support in the form of drug donations was provided by Gilead, Merck, and Bristol-Myers Squibb. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, National Institute of Allergy and Infectious Diseases, or the National Institutes of Health.
Funding US National Institute of Allergy and Infectious Diseases and National Cancer Institute, National Institutes of Health.
Declaration of interests
CG and MNo are employed by the US National Institutes of Health. SEK received grant support through her institute from the National Cancer Institute (National Institutes of Health) and received non-financial support from Celgene, personal fees from Pfizer and Applied Clinical Intelligence, and royalties from Wolters-Kluwer, outside the submitted work. CBM, PM, RMM, SRC, MCH, WS, MNy, JK, BH, NM, VOO, RM, and TBC received grant support through their institutes from the National Institute of Allergy and Infectious Disease (National Institutes of Health). NWB, FMO, HB, and MZB received grant support through their institutes from both the National Cancer Institute and the National Institute of Allergy and Infectious Disease (National Institutes of Health). CBM also received personal fees from Northwestern University Infectious Diseases, outside the submitted work. TBC also received personal fees and non-financial support from ViiV Healthcare, and grants, personal fees, and non-financial support from Gilead Sciences, outside the submitted work. HB also received non-financial support from Bayer Pharmaceuticals, non-financial support from Roche Pharmaceuticals, non-financial support from ASCO Conquer Cancer Foundation, and personal fees and non-financial support from the International Atomic Energy Agency, outside the submitted work.
Footnotes
Data sharing
Individual participant data and a data dictionary defining each field in the set will be made available to investigators for work that was not initially proposed as part of the parent protocol on a case by case basis via an online request to the AIDS Clinical Trials Group. Completion of an ACTG Data Use Agreement might be required.
To request data from the AIDS Clinical Trials Group visit https://submit.actgnetwork.org/
For the full study details see https://clinicaltrials.gov/ct2/show/NCT01435018
See Online for appendix
For more on the WHO Model List of Essential Medicines see https://www.who.int/medicines/publications/essentialmedicines/en/
Contributor Information
Susan E Krown, AIDS Malignancy Consortium, New York, NY, USA.
Carlee B Moser, Center for Biostatistics in AIDS Research, Harvard T H Chan School of Public Health, Boston, MA, USA.
Patrick MacPhail, Clinical HIV Research Unit, Department of Internal Medicine, University of the Witwatersrand, Johannesburg, South Africa.
Roy M Matining, Center for Biostatistics in AIDS Research, Harvard T H Chan School of Public Health, Boston, MA, USA.
Catherine Godfrey, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
Stephanie R Caruso, Frontier Science Foundation, Amherst, NY, USA.
Mina C Hosseinipour, UNC Project-Malawi, Lilongwe, Malawi; Department of Medicine, University of North Carolina at Chapel Hill School of Medicine, Division of Infectious Diseases, Chapel Hill, NC, USA.
Wadzanai Samaneka, Parirenyatwa Clinical Research Site, Harare, Zimbabwe.
Mulinda Nyirenda, Johns Hopkins Research Project, University of Malawi College of Medicine, Blantyre, Malawi.
Naftali W Busakhala, Moi University School of Medicine, Eldoret, Kenya.
Fred M Okuku, Uganda Cancer Institute, Kampala, Uganda.
Josphat Kosgei, Kenya Medical Research Institute, USA Medical Directorate for Africa/Kenya, Kericho, Kenya.
Brenda Hoagland, Oswaldo Cruz Foundation, Evandro Chagas National Institute of Infectious Diseases, Rio de Janeiro, Brazil.
Noluthando Mwelase, Clinical HIV Research Unit, Department of Internal Medicine, University of the Witwatersrand, Johannesburg, South Africa.
Vincent O Oliver, Kenya Medical Research Institute, Centre for Global Health Research, Centers for Disease Control and Prevention, Kisumu CRS, HIV-Research Branch, Kisumu, Kenya.
Henriette Burger, Family Clinical Research Unit CRS, Stellenbosch University and Tygerberg Hospital, Cape Town, South Africa; Division of Radiation Oncology, Stellenbosch University and Tygerberg Hospital, Cape Town, South Africa.
Rosie Mngqibisa, Durban International Clinical Research Site, Enhancing Care Foundation, Durban, South Africa.
Mostafa Nokta, Office of HIV and AIDS Malignancy, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Thomas B Campbell, Division of Infectious Diseases, Department of Medicine, University of Colorado School of Medicine, Aurora, CO, USA.
Margaret Z Borok, Department of Medicine, University of Zimbabwe College of Health Sciences, Harare, Zimbabwe.
References
- 1.Cesarman E, Damania B, Krown SE, Martin J, Bower M, Whitby D. Kaposi sarcoma. Nat Rev Dis Primers 2019; 5: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semeere AS, Busakhala N, Martin JN. Impact of antiretroviral therapy on the incidence of Kaposi’s sarcoma in resource-rich and resource-limited settings. Curr Opin Oncol 2012; 24: 522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Judd A, Zangerle R, Touloumi G, et al. Comparison of Kaposi sarcoma risk in human immunodeficiency virus-positive adults across 5 continents: a multiregional multicohort study. Clin Infect Dis 2017; 65: 1316–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Cancer Observatory, International Agency for Research on Cancer. Population fact sheets. http://gco.iarc.fr/today/fact-sheetspopulations (accessed May 23, 2019).
- 5.Nalwoga A, Cose S, Wakeham K, et al. Association between malaria exposure and Kaposi’s sarcoma-associated herpes virus seropositivity in Uganda. Trop Med Int Health 2015; 20: 665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Guidelines on the treatment of skin and oral HIV associated conditions in children and adults. 2014. https://www.who.int/maternal_child_adolescent/documents/skin-mucosal-and-hiv/en/ (accessed May 23, 2019). [PubMed]
- 7.Strother RM, Gregory KM, Pastakia SD, et al. Retrospective analysis of the efficacy of gemcitabine for previously treated AIDS-associated Kaposi’s sarcoma in western Kenya. Oncology 2010; 78: 5–11. [DOI] [PubMed] [Google Scholar]
- 8.Herce ME, Kalanga N, Wroe EB, et al. Excellent clinical outcomes and retention in care for adults with HIV-associated Kaposi sarcoma treated with systemic chemotherapy and integrated antiretroviral therapy in rural Malawi. J Int AIDS Soc 2015; 18: 19929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mwafongo AA, Rosenberg NE, Ng’ambi W, et al. Treatment outcomes of AIDS-associated Kaposi’s sarcoma under a routine antiretroviral therapy program in Lilongwe, Malawi: bleomycin/ vincristine compared to vincristine monotherapy. PLoS One 2014; 9: e91020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mtonga W, Mujajati A, Munkombwe D, et al. Therapeutic outcomes in AIDS-associated Kaposi’s sarcoma patients on antiretroviral therapy treated with chemotherapy at two tertiary hospitals in Lusaka, Zambia. Curr HIV Res 2018; 16: 231–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burger H, Ismail Z, Taljaard JJ. Establishing a multidisciplinary AIDS-associated Kaposi’s sarcoma clinic: Patient characteristics, management and outcomes. S Afr Med J 2018; 108: 1059–65. [DOI] [PubMed] [Google Scholar]
- 12.Rohner E, Kasaro M, Msadabwe-Chikuni SC, et al. Treatment and outcome of AIDS-related Kaposi sarcoma in South Africa, Malawi and Zambia: an international comparison. Pan Afr Med J 2017; 28: 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalya PL, Mbunda F, Rambau PF, et al. Kaposi’s sarcoma: a 10-year experience with 248 patients at a single tertiary care hospital in Tanzania. BMC Res Notes 2015; 8: 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bekolo CE, Soumah MM, Tiemtore OW, et al. Assessing the outcomes of HIV-infected persons receiving treatment for Kaposi sarcoma in Conakry-Guinea. BMC Cancer 2017; 17: 806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartsmann G, Sprinz E, Kromfield M, et al. Clinical and pharmacokinetic study of oral etoposide in patients with AIDS-related Kaposi’s sarcoma with no prior exposure to cytotoxic therapy. J Clin Oncol 1997; 15: 2118–24. [DOI] [PubMed] [Google Scholar]
- 16.Mosam A, Shaik F, Uldrick TS, et al. A randomized controlled trial of highly active antiretroviral therapy versus highly active antiretroviral therapy and chemotherapy in therapy-naive patients with HIV-associated Kaposi sarcoma in South Africa. J Acquir Immune Defic Syndr 2012; 60: 150–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosseinipour MC, Kang M, Krown SE, et al. As-needed versus immediate etoposide chemotherapy in combination with antiretroviral therapy for mild or moderate AIDS-associated Kaposi sarcoma in resource-limited settings: A5264/AMC-067. Clin Infect Dis 2018; 67: 251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Busakhala NW, Waako PJ, Strother MR, et al. Randomized phase IIA trial of gemcitabine compared with bleomycin plus vincristine for treatment of Kaposi’s sarcoma in patients on combination antiretroviral therapy in western Kenya. J Glob Oncol 2018; 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olweny CL, Borok M, Gudza I, et al. Treatment of AIDS-associated Kaposi’s sarcoma in Zimbabwe: results of a randomized quality of life focused clinical trial. Int J Cancer 2005; 113: 632–39. [DOI] [PubMed] [Google Scholar]
- 20.Northfelt DW, Dezube BJ, Thommes JA, et al. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: results of a randomized phase III clinical trial. J Clin Oncol 1998; 16: 2445–51. [DOI] [PubMed] [Google Scholar]
- 21.Goebel FD, Goldstein D, Goos M, Jablonowski H, Stewart JS. Efficacy and safety of Stealth liposomal doxorubicin in AIDS-related Kaposi’s sarcoma. Br J Cancer 1996; 73: 989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart S, Jablonowski H, Goebel FD, et al. Randomized comparative trial of pegylated liposomal doxorubicin versus bleomycin and vincristine in the treatment of AIDS-related Kaposi’s sarcoma. J Clin Oncol 1998; 16: 683–91. [DOI] [PubMed] [Google Scholar]
- 23.Krown SE, Northfelt DW, Osoba D, Stewart JS. Use of liposomal anthracyclines in Kaposi’s sarcoma. Semin Oncol 2004; 31 (suppl 13): 36–52. [DOI] [PubMed] [Google Scholar]
- 24.Gill PS, Tulpule A, Espina BM, et al. Paclitaxel is safe and effective in the treatment of advanced AIDS-related Kaposi’s sarcoma. J Clin Oncol 1999; 17: 1876–83. [DOI] [PubMed] [Google Scholar]
- 25.Welles L, Saville MW, Lietzau J, et al. Phase II trial with dose titration of paclitaxel for the therapy of human immunodeficiency virus-associated Kaposi’s sarcoma. J Clin Oncol 1998; 16: 1112–21. [DOI] [PubMed] [Google Scholar]
- 26.Cianfrocca M, Lee S, Von Roenn J, et al. Randomized trial of paclitaxel versus pegylated liposomal doxorubicin for advanced human immunodeficiency virus-associated Kaposi sarcoma: evidence of symptom palliation from chemotherapy. Cancer 2010; 116: 3969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reid E, Suneja G, Ambinder RF, et al. AIDS-related Kaposi sarcoma, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2019; 17: 171–89. [DOI] [PubMed] [Google Scholar]
- 28.Evans SR, Krown SE, Testa MA, Cooley TP, Von Roenn JH. Phase II evaluation of low-dose oral etoposide for the treatment of relapsed or progressive AIDS-related Kaposi’s sarcoma: an AIDS Clinical Trials Group clinical study. J Clin Oncol 2002; 20: 3236–41. [DOI] [PubMed] [Google Scholar]
- 29.Paredes J, Kahn JO, Tong WP, et al. Weekly oral etoposide in patients with Kaposi’s sarcoma associated with human immunodeficiency virus infection: a phase I multicenter trial of the AIDS Clinical Trials Group. J Acquir Immune Defic Syndr Hum Retrovirol 1995; 9: 138–44. [PubMed] [Google Scholar]
- 30.Stefan DC. Cancer care in Africa: an overview of resources. J Glob Oncol 2015; 1: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krown SE, Metroka C, Wernz JC. Kaposi’s sarcoma in the acquired immune deficiency syndrome: a proposal for uniform evaluation, response, and staging criteria. J Clin Oncol 1989; 7: 1201–07. [DOI] [PubMed] [Google Scholar]
- 32.National Institute of Allergy and Infectious Diseases. DAIDS adverse event grading tables. https://rsc.niaid.nih.gov/clinical-research-sites/daids-adverse-event-grading-tables (accessed May 23, 2019).
- 33.DeMets DL, Lan KK. Interim analysis: the alpha spending function approach. Stat Med 1994; 13: 1341–52. [DOI] [PubMed] [Google Scholar]
- 34.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979; 35: 549–56. [PubMed] [Google Scholar]
- 35.Greenwood M Reports on Public Health and Medical Subjects. vol 33 London: HM Stationery Office, 1926. [Google Scholar]
- 36.Ely S, Barouk S, Gapara M, et al. Pathology external quality assurance program for Kaposi’s sarcoma international clinical trials AMC-067/ A5264 and AMC-066/A5263. 15th International Conference on Malignancies in AIDS and Other Acquired Immunodeficiencies (ICMAOI) Bethesda, MD, USA Oct 26–27, 2015. [Google Scholar]
- 37.American Cancer Society. American Cancer Society and Clinton Health Access Initiative announce collaborations with Pfizer and Cipla to increase access to lifesaving cancer treatment in Africa. June 20, 2017. http://pressroom.cancer.org/2017-06-20-American-Cancer-Society-and-Clinton-Health-Access-Initiative-Announce-Collaborations-with-Pfizer-and-Cipla-to-Increase-Access-to-Lifesaving-Cancer-Treatment-in-Africa. (accessed May 23, 2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.