Abstract
Alcohol-related liver disease (ALD), which includes a range of disorders of different severity and is one of the most prevalent types of liver disease worldwide, has recently regained increased attention. Among other reasons, the realisation that any alcohol intake, regardless of type of beverage represents a health risk, and the new therapeutic strategies tested in recently published or undergoing clinical trials spur scientific interest in this area.
In April 2019, Gut convened a round table panel of experts during the European Association for the Study of the Liver International Liver Congress in Vienna to discuss critical and up-to-date issues and clinical trial data regarding ALD, its epidemiology, diagnosis, management, pathomechanisms, possible future treatments and prevention. This paper summarises the discussion and its conclusions.
INTRODUCTION
Alcohol-related liver disease (ALD) is one of the most prevalent types of liver disease worldwide. According to the latest WHO estimates, alcoholic beverage consumption globally resulted in 3 million alcohol-attributable deaths (5.3% of all deaths worldwide in 2016) and 5.1% of the global disease burden in 2016. Furthermore, it is estimated that alcohol-attributable liver cirrhosis (AC) caused 607000 deaths and 22.2 million disability adjusted life years (DALYs) in 2016.1
Alcohol can interfere with lipid metabolism and induce fat deposition in the liver through different mechanisms, including inhibition of mitochondrial β-oxidation and increased fatty acid synthesis, resulting in the accumulation of triglycerides, phospholipids and cholesterol esters.2 Hepatic inflammation, histologically referred to as alcoholic steatohepatitis (ASH), may ensue in some individuals, with histological signs of hepatocellular injury and ballooning. While initially involving innate immunity, it may be followed by an adaptive immune response triggered by neoantigens derived from alcohol metabolism.2 In a subset of patients, heavy alcohol consumption resulting in chronic inflammation and injury leads to extracellular matrix deposition and fibrosis. In ALD pericellular and perisinusoidal matrix accumulation with a ‘chicken-wire’ appearance is a common pattern of fibrosis. Advanced fibrosis results in severe derangement of hepatic architecture, with predominant fibrous tissue over the parenchymal component and impairment of blood flow, characteristics of the cirrhotic stage. Chronic liver injury, oxidative stress, inflammation and fibrosis, together with the carcinogenic action of alcohol metabolites may ultimately lead to DNA mutations, the neoplastic transformation of hepatocytes and development of hepatocellular carcinoma (HCC).
Chronic alcohol abuse over months or years results in most patients developing an alcoholic fatty liver. Up to a third of the individuals who continue with heavy alcohol drinking progress to ASH, and up to 20% will develop AC. About 2% of cirrhotic patients develop primary HCC. In patients with severe ASH an acute condition characterised by jaundice and liver failure, acute alcoholic hepatitis (AH), may occur. Among patients surviving acute AH, 70% will progress to cirrhosis. In addition, cirrhotic individuals may develop acute AH (acute-on-chronic condition) with severe mortality rates.2 (figure 1)
Figure 1.
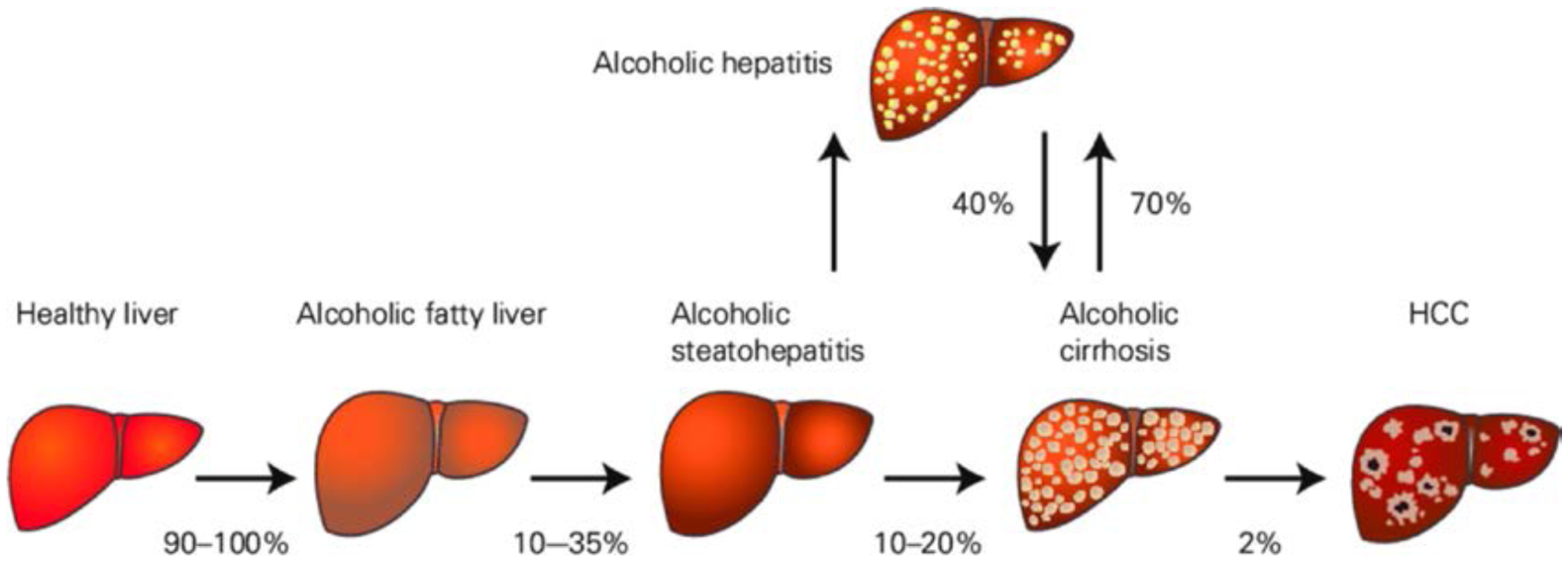
The natural course of alcohol-related liver disease. HCC, hepatocellular carcinoma. Adapted from.2 Published with permission from Springer nature.
ALD may be observed even in the absence of clinical or biological manifestations, therefore it is often diagnosed at a very late stage.
Recently, ALD has gained an increased interest not only among researchers, but also among socioeconomic and community health stakeholders. A major genetic study debunking the apparent protective effects of moderate alcohol consumption against stroke and demonstrating the direct and uniform association between increased alcohol intake and cardiovascular events,3 and a number of clinical trials recently published or currently underway have regained the attention of the scientific community.
In April 2019, Gut convened a round table panel of experts during the European Association for the Study of the Liver (EASL) International Liver Congress in Vienna to discuss critical and up-to-date issues and clinical trial data regarding ALD, covering
epidemiology,
diagnosis,
management of AH,
acute-on-chronic liver failure,
transplant,
animal models,
mechanisms and targets of disease,
the gut-liver axis in ALD,
future therapeutic targets.
industry’s influence on the topic of alcohol consumption.
global strategies to reduce the harmful use of alcohol.
This paper summarises the discussion and key messages.
EPIDEMIOLOGY
Global burden
The global burden of disease caused by the harmful use of alcohol is enormous. Worldwide more than half million individuals died in the year 2016, due to alcohol-related cirrhosis, with an associated loss of 22.2 million DALYs in the same year.1 In fact, the alcohol-attributable fraction (ie, the contribution alcohol has as a risk factor to disease or death) regarding global deaths or DALYs, is about 50% for cirrhosis, and 10% for liver cancer, according to the WHO report on Alcohol and Health.1 However, the death rates associated with digestive disease, evaluated by the WHO (mostly liver cirrhosis), very much vary among different regions of the world, from less than 1.9 to more than 17 per 100 000 individuals. It is of note that the calculation of the alcohol-attributable fraction was not based on International Classification of Diseases (ICD) coding, since there is a strong interaction between other factors such as viral hepatitis or obesity and metabolic risk factors.4
Geographical differences
Interestingly, the burden of alcohol-attributable digestive deaths (liver cirrhosis, pancreatitis and other digestive diseases) is highest in Africa and the Western Pacific (16.9 and 10.8 deaths per 100 000, respectively), while the contribution of alcohol to digestive diseases is highest in Europe and the Western Pacific (30.5% and 28.9%, respectively).1 This may be partly explained by the socioeconomic status modifying the effect of alcohol consumption and the structure of mortality and morbidity in different regions.
Europe
Data from the HEPAHEALTH project that aggregates by broad categories of liver disease for the most recent years available, shows that alcohol is a large contributor to the mortality rate of many European countries.5 Mortality from unknown causes also appears frequently, probably due to reluctance to put an alcohol aetiology in death certificates. This makes it difficult to evaluate the true prevalence.
Prevalence
The prevalence of advanced ALD probably ranges between 2% and 5% in at-risk populations, with substantial differences according to age, gender and drinking history. The first pivotal population-based study, the Dionysos Study, investigated 6534 adult Italians from two municipalities. While 21% reported drinking above 30 g/day, evidence of cirrhosis was found in only 2%.6 This was however before elastography appeared as a highly accurate method for assessment of advanced fibrosis.
In a primary care population of 20 868 patients from the UK, 7% exhibited hazardous drinking. Four hundred and one of these patients underwent liver stiffness measurement, with 19% having values above 8.0 kPa and 3% were diagnosed with cirrhosis.7 Similarly, in a Danish biopsy-controlled study including 128 asymptomatic patients from primary care with a history of excess drinking, 2% had severe fibrosis and 4% had cirrhosis.8 A recent study investigated a background population in Catalonia, Spain, and found that 9% reported risk drinking behaviour. Of these, 7% had liver stiffness above 9.0 kPa.9 Global annual alcohol consumption is steadily increasing, from 5.9 L per-capita in 1990 to 6.5 L in 2017, particularly in middle-income countries.10 As alcohol consumption is projected to further increase to 7.6 L in 2030, in parallel with an increasing prevalence of current drinkers and a decrease in lifetime abstainers, the burden of ALD will most likely also continue to rise.
Mortality
Recent years have seen an increase in mortality due to ALD. In the USA, alcohol exceeds hepatitis C virus as the primary aetiology for liver related deaths due to a continued, linear increase in age-standardised mortality since 2007, with annual increases of 3.4%11 (figure 2). It is also of concern that the increase in mortality in the USA seems to affect younger age groups. The percental increase in mortality due to AC is higher than for cirrhosis in general in age groups 45 years and upwards, but the steepest incline in mortality of AC is in the age group 25 to 34 years, growing more than 10% annually.12
Figure 2.
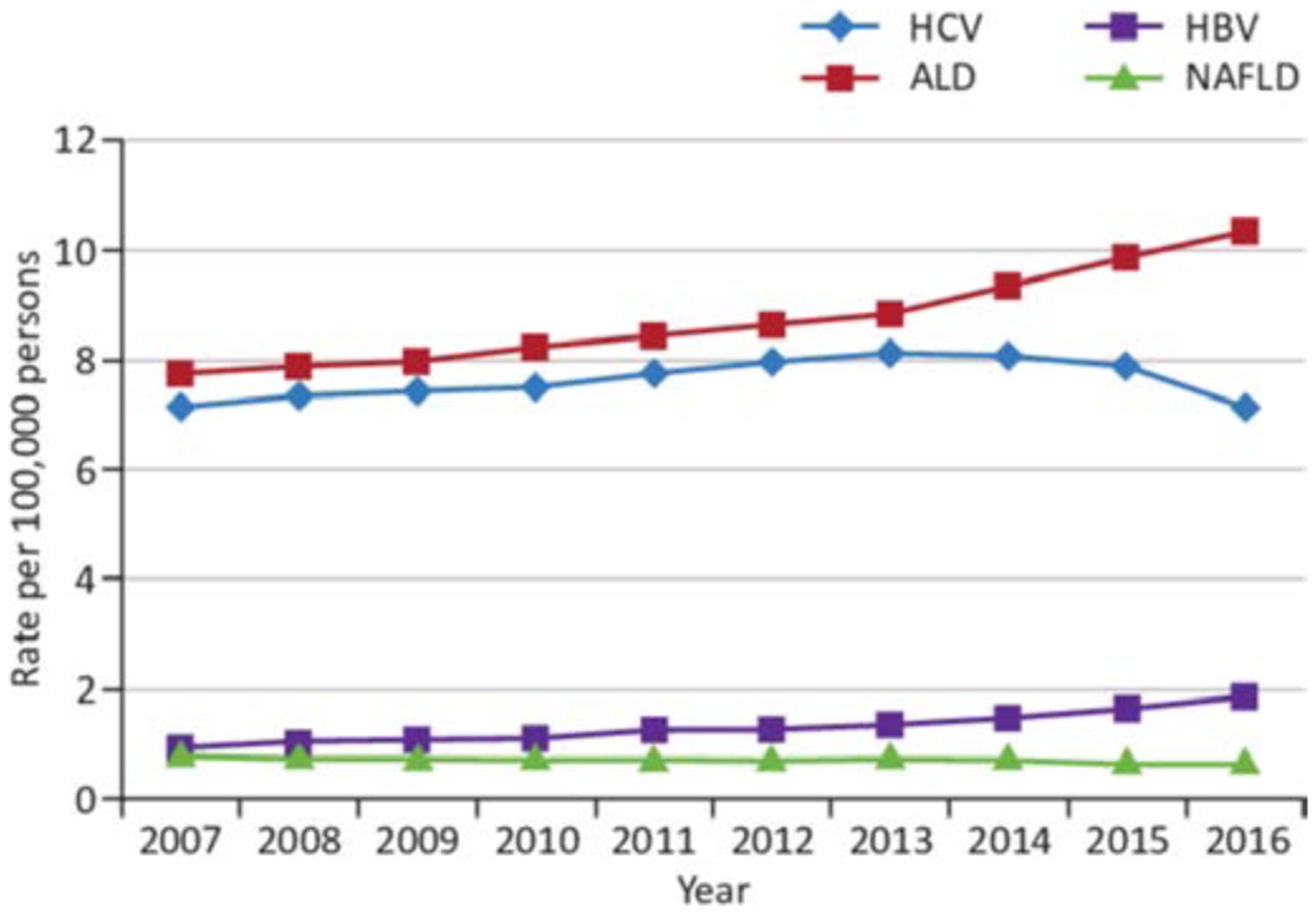
Annual age-standardised overall mortality rates for chronic liver disease in the USA from 2007 through to 2016.11 ALD, alcohol-related liver disease; HCV, hepatitis C virus; HBV, hepatitis B virus; NAFLD, non-alcoholic fatty liver disease. Published with permission from Elsevier.
Underreporting of ALD
ALD cannot be measured reliably using usual death registries since the assessment of whether a liver disease is due to alcohol use or other risk factors is dependent on sociocultural factors, particularly stigma. It was found in a seminal study by Puffer and Griffith published in the 1960s that after triangulating data on death certificates with data from hospital records and interviews with doctors or family members the number of deaths with AC more than doubled, with recoding from cirrhosis that does not mention alcohol.13 There is evidence of persistent underreporting of ALD. It is therefore suggested that the estimate should be based on measures that have less bias such as the alcohol-attributable fractions of cirrhosis.14
Hazardous drinking
Hazardous drinking is the mainstay of ALD. Hazardous drinking is broadly defined as alcohol consumption in a quantity or pattern that puts the patient at risk for adverse health outcomes. For liver-related events, there is no clear threshold for how much alcohol constitutes a significantly increased risk, or for a minimum duration of excessive drinking.15 In spite of the lacking threshold effect, several studies and meta-analyses denote 30 g of alcohol daily as the limit marking a clinically significant elevated risk of progressing to advanced liver disease.16 The EASL guideline on non-alcoholic fatty liver disease (NAFLD) uses a threshold for daily alcohol ingestion of 30 g for men and 20 g for women to delimit ALD from NAFLD17 while the American Association for the Study of Liver Diseases NAFLD guidance18 refrains from defining a limit of drinking for discriminating between ALD and NAFLD. They do however suggest using >21 drinks/week for men and >14 drinks/week for women over a 2 year period as exclusion criteria for non-alcoholic steatohepatitis clinical trials. A similar criterium could be used in « reverse » for inclusion of patients in ALD trials, but more often ALD trials include patients drinking in excess of 30 g/day, or even 60 g/day. The unclear definition of what constitutes hazardous drinking therefore has obvious consequences for the patient characteristics of future clinical trials in ALD and their comparability.
Less evidence exists regarding lifetime exposure and the importance of abstinence periods.
Early-life excess drinking increases the risk of severe liver disease later in life. In 18- to 19-year-old Swedish males, who reported drinking more than 30 g/day (4% of participants), the cumulative incidence of severe liver disease was 3% during 38±5 years of follow-up. The crude HRs increased from 5.0 (2.4 to 10.6) in those who drank 31 to 40 g/day, to 11.1 (5.2 to 23.4) in those who drank >60 g/day.16 However, the main limitation of this and other studies is the inability to adjust for lifetime changes in alcohol use. Patients with an alcohol use disorder (AUD) may illustrate the effect of prolonged, heavy drinking. A Danish case-control study investigated 14 091 men and 4911 women who attended alcohol rehabilitation from 1977 to 2013. Liver-related mortality in these patients was 14% for both sexes, with corresponding relative risks of 5.6 for men and 11.7 for women, when compared with population controls.19
The UK Million Women Study confirmed that cirrhosis incidence increases with the amount of alcohol consumed (relative risk (RR) 3.4 (2.9 to 4.1) in those drinking more than 16 g/day vs 8 to 16 g/week). The study also found that cirrhosis incidence among women who consumed more than 56 g of alcohol weekly (corresponding to seven drinks) doubled with daily consumption versus non-daily consumption, and not drinking with meals (RR 2.5, 2.0 to 3.1).15
Genetic factors
The heritability for AC is well known.20 The genetic disposition is probably polygenic, as evidenced by a three times higher concordance of AC in monozygotic twins compared with dizygotic, irrespective of concordance for alcoholism, in a single twin study.21 There is however far more evidence on the genetic aspects of alcohol dependence than on ALD, with the most recent meta-analysis on twin studies suggesting 49% heritability.22 As an example, single polymorphisms in the genes encoding alcohol dehydrogenase and acetaldehyde dehydrogenase protects against AUDs by a slower metabolism of acetaldehyde, causing unpleasant symptoms such as flushing and nausea after alcohol consumption. These gene variants will invariably, although indirectly, also protect against ALD.
While several individual genetic variants have been identified for AC, they are rarely independently validated and reproduced.23 This may be because controls are consistently difficult to match correctly with regards to alcohol exposure, drinking pattern and additional lifestyle risk factors. The only polymorphism that has been consistently correlated with cirrhosis and HCC in patients with ALD is the patatin-like phospholipase domain-containing 3 (PNPLA3) rs738409 polymorphism.24 Effect size is small however, with ORs between 1.5 and 2.2. The same PNPLA3 polymorphism may also be associated with reduced long-term survival after an episode of severe AH (sAH), but this finding remains to be repeated.25 Additional promising polymorphisms include HSD17B13, TM6SF2, MBOAT7 and SERPINA1.26
The heterozygous carriage of the alpha1-antitrypsin Pi*Z variant was associated with an increased risk for alcohol misusers to develop cirrhosis, with an adjusted OR of 5.8.27
Other risk factors
The interaction of other risk factors with alcohol concerning the risk of developing liver disease has been neglected in recent years. However, several studies have shown an additive effect of overweight or obesity with alcohol consumption in the risk of developing liver disease or in fibrosis severity.28,29 Trembling et al found in a large cohort of postmenopausal women that those who were overweight or obese and drank the most alcohol had the highest risk of developing liver related events. However also abstainers had an increased risk.30 This last observation highlights the controversial issue of moderate amounts of alcohol having the potential to be protective as several studies suggest.31,32
However, a recent study from the Global Burden of Disease Study, found that risk of all-cause mortality rises with increasing levels of consumption, and that there is no safe lower level of consumption.33
Another important cofactor in ALD is iron; in fact, iron overload is frequently present, in the absence of a specific iron overload disease. However, since haemochromatosis is one of the most common genetic conditions, it should always be considered a cofactor for ALD.34
A prospective cohort study found that there was a synergism between obesity and alcohol in increasing the risk of HCC.35 The reason for the interaction between overweight and alcohol on the risk of liver disease is not fully explained but may be caused by the effect of many adipose tissue-induced systemic inflammatory pathways.36 In fact, a Scottish study demonstrated that the harm to health as well as the rates of alcohol-induced hospitalisation or death are strongly predicted by social status.37
The adjustment for drinking pattern or binge drinking did not make a significant difference. However, controlling for body mass index and cigarette smoking (CS) attenuated the difference, suggesting the strong association of alcohol abuse with CS and obesity. In fact, patients with alcohol abuse have an elevated prevalence of heavy CS.38 Also, there is evidence that CS may exacerbate the pathogenic effects of alcohol on the liver and heavy CS aggravates the harmful effect of alcohol excess in humans, signifying that both factors may have a synergistic effect.39
Alcohol consumption was shown to be associated, with faster development and earlier mortality in patients with chronic hepatitis B or hepatitis C virus infection.40
Regarding coffee consumption, a recent study in Norway including 219 279 men and women aged 30 to 67 years, found an inverse correlation between coffee consumption and ALD.41 Also, a prospective analysis of 14 208 participants aged 45 to 64 years from the Atherosclerosis Risk in Communities study found that coffee drinkers may be at lower risk for liver-related hospitalisation.42
Economic burden and late diagnosis
With regards to the economic burden of ALD, a recent study in the USA has shown that in 1 240 152 patients admitted between 2002 and 2014 with cirrhosis, AC accounts for more than half of the cirrhosis charges.43 The elevated mortality and costs of ALD is at least partially due to the fact that it is often detected at very advanced stages of the disease, compared with other liver diseases.44
Diagnosis of ALD
The diagnosis of ALD should begin with the assessment of alcohol use in all patients including the levels and patterns of alcohol consumption, signs of AUD and advanced liver disease. ALD is usually suspected in patients with a regular alcohol consumption larger than 20 g/day in females and larger than 30 g/day in males, together with clinical and/or biological abnormalities suggesting liver injury.
When it comes to screening risky drinking and alcohol dependence, AUDIT (Alcohol Use Disorders Inventory Test) is considered to be the most valuable questionnaire.45 It includes 10 items that explore alcohol consumption using three questions, alcohol dependence also using three questions and alcohol-related problems with further four questions. A score over 8 indicates harmful use of alcohol. Its shorter version includes only the first three questions of AUDIT. This version is reliable for rapid screening of alcohol use.46
Among the serum markers to detect alcohol consumption are carbohydrate deficient transferrin and gamma-glutamyl transferase (GGT). Their predictive values have been validated to detect alcohol consumption higher than 50 g/day within the 2 or 3 previous weeks. However, those markers are less useful in patients with liver disease as liver injury is a confounding factor that affects their diagnostic value to detect heavy drinking.45 For example, the GGT level is elevated in patients with extensive fibrosis regardless of the cause, even in patients with a long period of abstinence.
Alcohol-related liver injury may be diagnosed even in the absence of clinical or biological symptoms. The degree of steatosis and liver fibrosis needs to be established. Liver biopsy is still considered the gold standard for the definite diagnosis and for assessing the fibrosis stage of ALD. However, as it is an invasive procedure, it is associated with side effects and therefore not always recommended.
In the last 15 years, a number of non-invasive tests have been developed to assess the degree of liver fibrosis and steatosis on which the round table discussion was focussing regarding diagnosis.
Non-invasive methods depend either on
a physical methodology based on the measurement of liver stiffness (transient elastography (TE)), or
a biological methodology based on the quantification of serum biomarkers.
In ALD, TE has been shown to reliably detect advanced fibrosis and cirrhosis and can therefore act as a prognostic marker of clinical outcomes.47 A meta-analysis to determine specific diagnostic cut-off values for liver stiffness in alcohol-related fibrosis has analysed biopsy-proven FibroScan studies on liver stiffness in ALD. It highlights the link between liver stiffness and the histological features of asymptomatic and non-severe AH. Aspartate aminotransferase (AST) levels and cholestasis had a significant effect on liver stiffness and must therefore be considered when interpreting liver stiffness cut-offs.48
Non-invasive biological serum tests can be grouped into two classes :
patented methods such as FibroTest, FibroMeter and enhanced liver fibrosis (ELF) test.
non-patented methods of which the best known are APRI (AST to Platelet Ratio Index), FIB-4 (Fibrosis-4) and Forns index.49
In a recent prospective study of 10 liver fibrosis markers, the two most commonly used blood-based assessment tools for liver fibrosis FibroTest and ELF have been found to have a comparable high performance for the diagnosis of advanced fibrosis in ALD, with a lower performance for non-patented methods. No conclusion can be reached regarding FibroMeter, another patented method, as this method was not evaluated. Diagnostic accuracy of ELF, FibroTest and liver stiffness measurement was very similar.8
New serum biomarkers are under investigation to non-invasively diagnose more severe forms of ALD and to predict the prognosis of patients.
A recent study comparing TE with TE plus FibroTest found no improvement in diagnostic accuracy for the combination.50
Although ultrasound is still used for screening for steatosis and portal hypertension, new methods such as controlled attenuation parameter have recently been developed to detect steatosis.51 MRI techniques are accurate and repeatable, however they are often not available and expensive to use.
Due to the progress in the development of non-invasive methods for the diagnosis of cirrhosis, it can be expected and would be desirable, that the next decade will bring large-scale screening for cirrhosis.
Management of ALD
Patients with ALD should be managed by specialists in liver disease as well as in addiction. The basic principles of management in patients with ALD include the achievement of complete abstinence from alcohol use and the management of complications of cirrhosis such as ascites, spontaneous bacterial peritonitis, encephalopathy and oesophageal varices. In addition, extrahepatic complications of alcohol use, such as neuropathy, proximal myopathy, pancreatitis or pancreatic exocrine insufficiency, should be sought and addressed. Protein and calorie dietary deficiency, often associated with sarcopaenia are commonly present and should be treated by a specialist dietician where available.
Many patients with ALD can stop drinking without much difficulties, but those dependent should receive adequate therapy to enable them to become abstinent. Concerning liver disease, it is important to exclude liver diseases of other origin and to treat relevant comorbidities such as obesity and type 2 diabetes mellitus.
There are currently no specific treatments for patients with ALD and there are very few trials in this patient population.
Management of alcoholic hepatitis
Next to HCC, AH is the most serious manifestation of ALD as it can lead to severe complications and early death. It is a clinical entity characterised by rapid development of jaundice and liver-related complications in a patient with heavy long-term alcohol use.2 The severity of AH is assessed by the Maddrey’s discriminant function (mDF) or by the Model for End-Stage Liver Disease (MELD) score. SAH, commonly defined by a mDF ≥32 or MELD score >20, occurs predominantly on a background of cirrhosis, and is associated with poor short-term prognosis.45
Given the risk of complications, patients with suspected AH should be admitted to hospital for management.
In patients with confounding factors (eg, suspicion of drug-induced liver injury, atypical laboratory tests, suspicion of ischaemic hepatitis, etc) a transjugular biopsy can help to establish a definite diagnosis.52 The AH Histological Score also has a prognostic value for these patients.53
Recent studies found that biomarkers such as cytokeratin-18 fragments, soluble cluster of differentiation 14, lipopolysaccharide binding protein (LBP), osteopontin (a multifunctional phosphoprotein involved in neutrophil activation) and circulating levels of macrophage activation sCD163 and sCD206t can indicate severity and predict clinical outcomes in AH helping the management of the disease.54,55
General support includes providing adequate calorie and protein intake, as well as vitamins (ie, thiamine, folate and pyridoxine) and preventing/treating withdrawal syndrome in patients with alcohol dependence.
Patients with AH are prone to develop acute kidney injury (AKI) that heavily impacts short-term prognosis.56 A scoring system (AKI-AH) is useful for predicting AKI.57
Development of AKI is favoured by the existence of systemic inflammatory response syndrome (SIRS) that could be due to bacterial infections or sterile inflammation.58 While waiting for culture results, serum procalcitonin may be a useful biomarker to suspect sepsis-induced SIRS. Since infection is a major prognostic factor in these patients, a complete infection screen is mandatory at admission and if patients develop fever, SIRS criteria or deterioration in liver or renal function. A recent study showed that the risk of developing an infection was lower in patients with AH receiving antibiotics after gastrointestinal bleeding.59 Clinical trials evaluating the specific role of antibiotic prophylaxis (eg, amoxicillin/clavulanic acid) in patients with AH are currently taking place.
In the absence of contraindications, corticosteroids are currently used in patients with sAH.60
The short-term survival benefit of corticosteroid treatment has been confirmed in a recent meta-analysis that included the Steroids or Pentoxifylline for Alcoholic Hepatitis (STOPAH) trial.61 However, the survival benefit associated with corticosteroids is modest and transient as the benefit does not persist beyond 28 days. Moreover, not all patients benefit from this therapy. This can be partly explained by the fact that the prognosis for patients with sAH is heterogeneous. At one end of the spectrum, patients with sAH who have spontaneous improvement in liver function in the days following admission have a better prognosis than patients without spontaneous improvement.62 The benefit of corticosteroids has never been demonstrated in this subgroup of patients. A clinical trial evaluating this question is ongoing (NCT03160651). At the other end of the spectrum, AH patients with (multi)organ failure have a very poor prognosis.
Two recent randomised controlled trials63,64 and a recent Meta-analysis of individual data61 confirmed that pentoxifylline is ineffective.
Serum levels of lipopolysaccharide (LPS) and a patient’s baseline hepatic gene expression could predict the response to corticosteroids, although they are not used in clinical practice.58,65
For patients not responding to corticosteroids, as assessed by the Lille model for AH, there are no effective targeted therapies. However, several encouraging pilot studies have yielded to larger clinical trials testing new pathophysiologically-orientated approaches.
Besides improving short-term survival, the most important determinant of long-term outcome in patients surviving an episode of AH is complete alcohol abstinence that needs to be promoted and assisted.
As non-severe forms of acute AH often respond to alcoholic abstinence, healthcare professionals should focus more on their diagnosis.52
In the last years, increasing number of hospitals are offering early liver transplantation (LT) for highly selected patients with AH non-responding to medical therapy.66,67
Figure 3 shows an algorithm for the management of AH.
Figure 3.
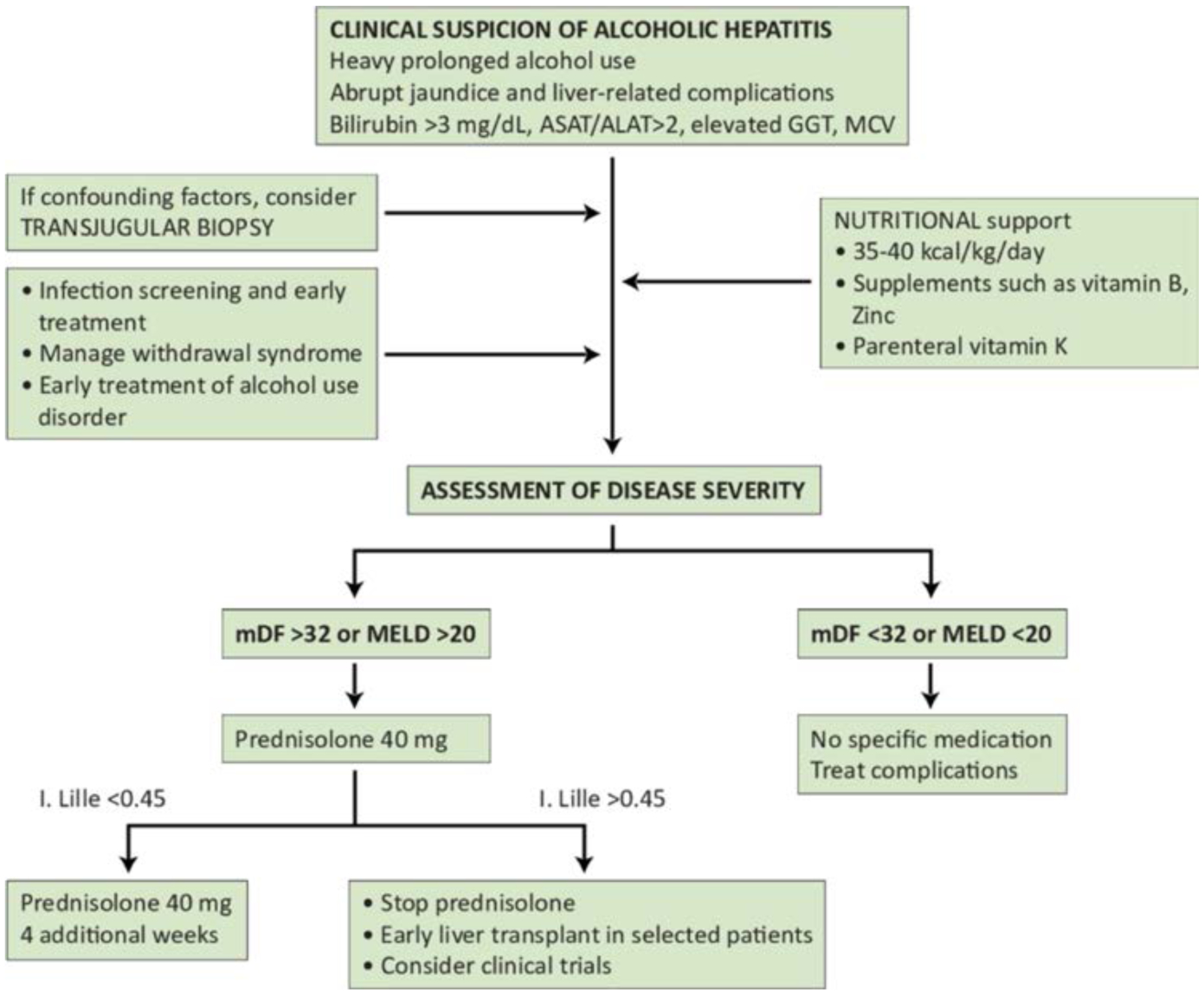
Algorithm for the management of alcoholic hepatitis (provided by Ramon Bataller, original figure). ASAT/ALAT, aspartate amino transferase/alanine amino transferase ratio; GGT, gamma-glutamyl transferase; MCV, mean corpuscular volume; mDF, Maddrey’s discriminant function; MELD, Model for End-Stage Liver Disease.
Acute-on-chronic liver failure
Acute-on-chronic liver failure (ACLF) is an entity that occurs in patients with cirrhosis and is characterised by acute deterioration, organ failure and a high risk of short-term mortality. The EASL-Chronic Liver Failure (CLIF) consortium proposed a definition and diagnostic criteria for ACLF based on a large multicentre European cohort including all patients with decompensated cirrhosis, the CANONIC study.68 According to this study, the grade of ACLF was defined by the number of organ failures determined by the CLIF Consortium Organ Failure score (an adapted and simplified version of the sequential organ assessment score), and the presence of kidney and/or neurological dysfunction.69 In the CANONIC study, AC with active alcohol consumption represented about 25% of the cases of ACLF. Interestingly, although liver biopsy results were not reported, laboratory features and the prescription of corticosteroids in a significant proportion of this group of patients highly suggest AH is a frequent cause of ACLF. Moreover, patients with AC and active alcohol consumption more often have ACLF grade 2 and 3 than other patients. In a prospective cohort of patients with sAH, the 28 day cumulative incidence of death in patients without ACLF or with ACLF grades 1, 2 or 3 was 10%, 31%, 58% and 72%, respectively.70 (figure 4).
Figure 4.
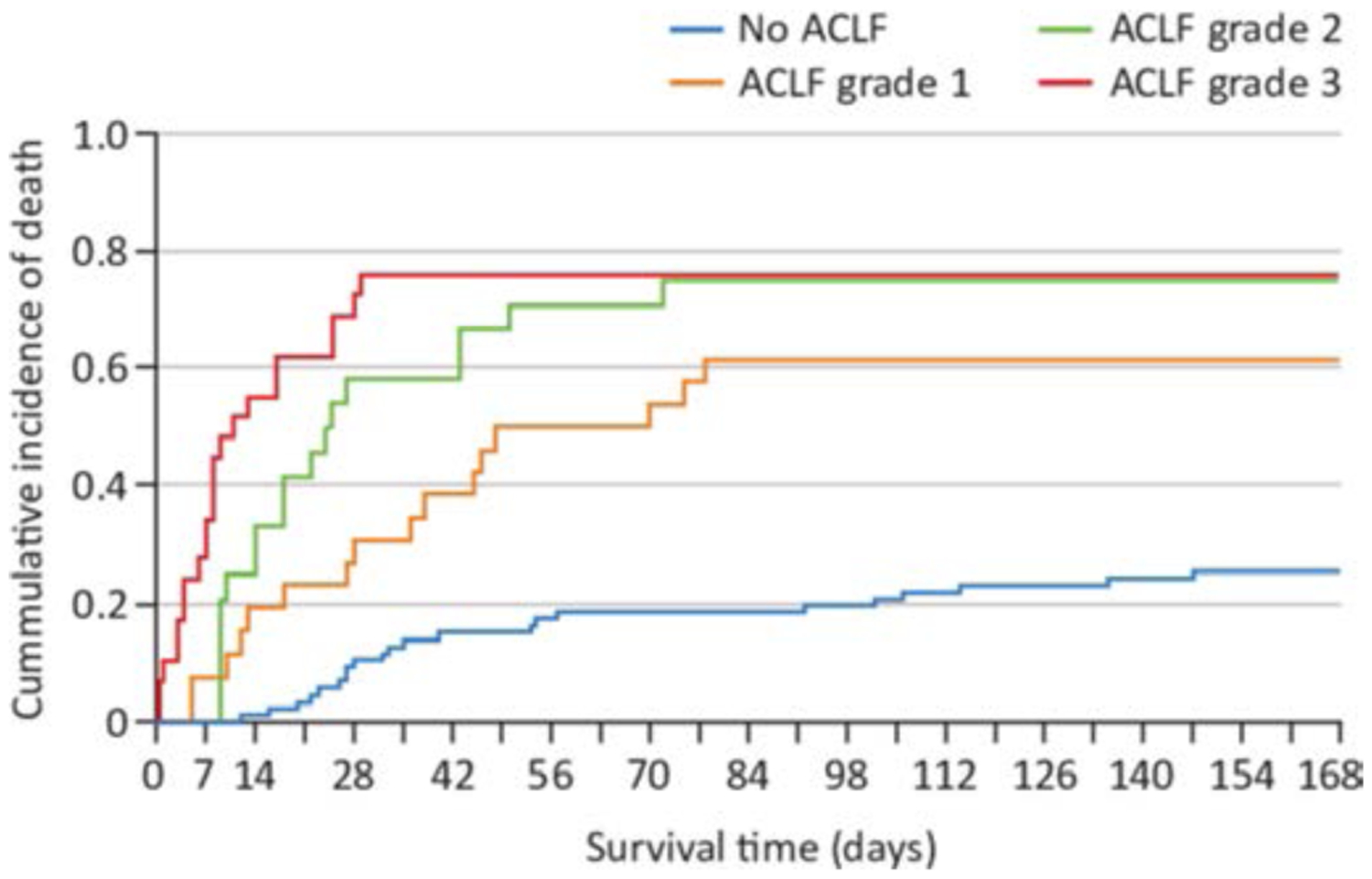
Cumulative incidence of death in patients with severe alcoholic hepatitis and without acute-on-chronic liver failure (ACLF) or with ACLF grades 1, 2 or 3.70 Published with permission from Elsevier.
If corticosteroid administration improves short-term survival in patients with sAH and ACLF is currently unknown, as the majority of these patients are excluded from clinical trials. In a Belgian cohort of sAH patients, the probability of response to corticosteroids using the Lille model was reduced in patients with ACLF and progressively reduced among grades of ACLF (77% for patients with sAH and without ACLF, 52% for ACLF-1, 42% for ACLF-2 and 8% for ACLF-3).70 (figure 5). In a subanalysis of the STOPAH trial, a decreased chance of response using the Lille model and greater mortality with higher ACLF grades were also reported.71 However, the survival benefit of corticosteroids is maintained in responders (using the Lille model), irrespective of ACLF grade. Therefore, the optimal therapeutic strategy in patients with sAH and ACLF is still a matter of debate and needs further investigation.
Figure 5.
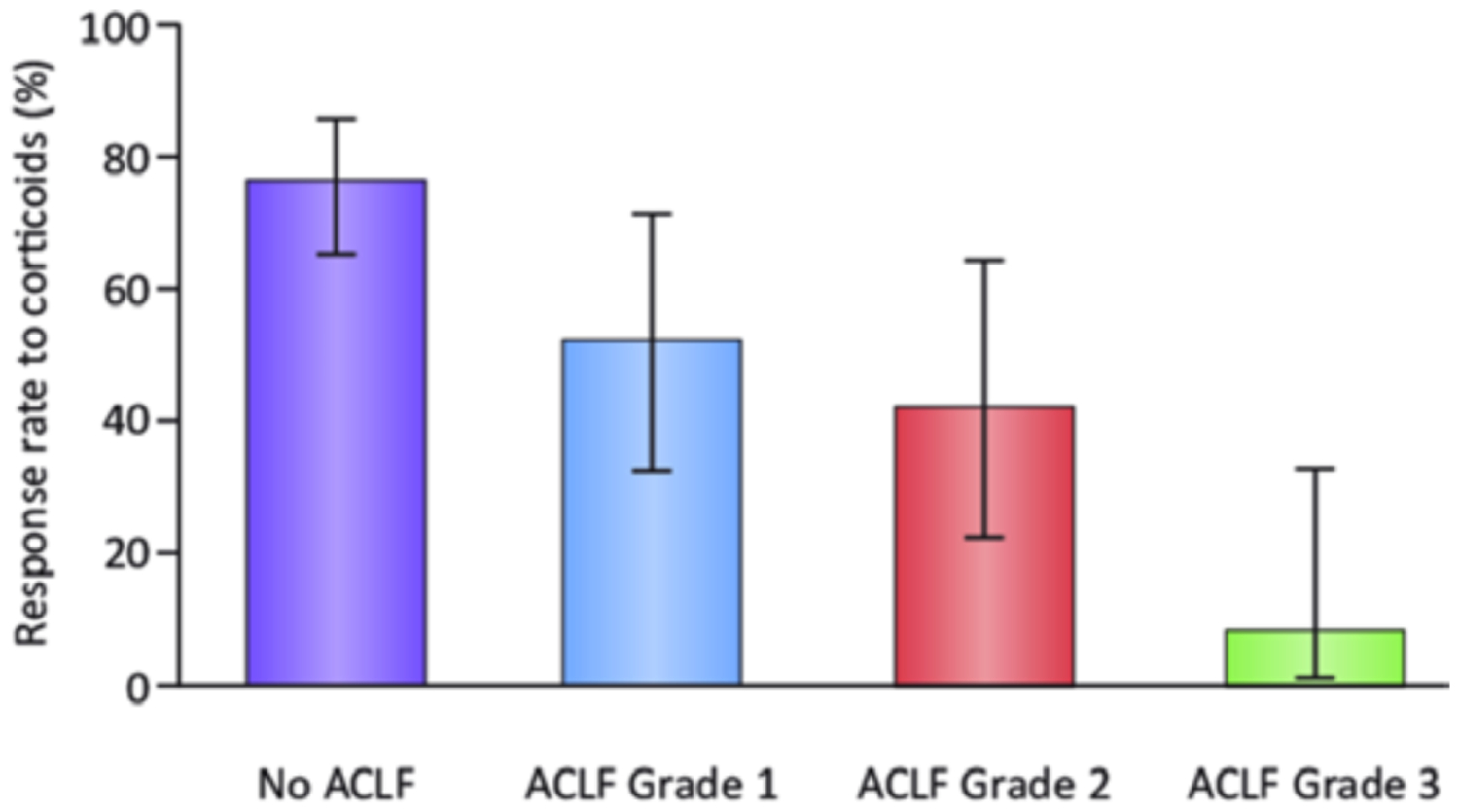
Response rate to corticosteroids using the Lille model in patients with severe alcoholic hepatitis and without acute-on-chronic liver failure (ACLF) and among grades of ACLF.70 Published with permission from Elsevier.
Liver transplant in ALD
ALD represents the second most common indication for LT worldwide,72 accounting for about 30% of all primary transplants in Europe and about 25% in the USA.73,74 Over the last two decades there has been a constant improvement in post-transplant survival rates, which are comparable with those of LT for other aetiologies.73 However, ALD is still sometimes considered a controversial indication to LT mainly due to the perception that it is a self-inflicted disease,75 and due to the potential risk of alcohol relapse after LT with a negative impact on the new graft. Although most transplant programmes still require a 6 month period of abstinence as a mandatory criterion to consider a patient suitable for LT, the role of the pre-transplant length of abstinence as predictor of alcohol relapse after LT has never been demonstrated. This period is becoming an instrument to assess patients who could recover their liver function after a prolonged alcohol abstinence and therefore could avoid unnecessary LT. Conversely, a multidisciplinary assessment, involving several stakeholders such as a transplant hepatologist, transplant surgeon, psychologist, psychiatrist and addiction specialist is becoming mandatory to properly evaluate the presence of risk factors for alcohol relapse after LT.45,76,77
The rates of alcohol relapse ranged from 11.5% to 49%, although this was rarely a consideration when looking for reasons for graft failure in ALD patients.78 Relapse directly leading to graft dysfunction ranged from 0% to 17%, whereas relapse directly causing mortality ranged from 0% to 5%. About 10% of patients relapse into heavy drinking usually within the first year after LT.79
Although there is evidence that a shorter prelisting abstinence period corresponds to a shorter period before post-transplant relapse, the ideal period of abstinence pre-transplant is still debatable.80
Moreover, regular testing for detecting any alcohol use during the evaluation process or while the patients are on the waiting list is mandatory. Lastly, the role of donor/recipient gender mismatch should be explored in LT for ALD, as data reported worse outcomes when grafts from female donors are used in male recipients.81
LT for sAH
With regards to early LT for sAH not responding to medical therapy, there is increasing evidence that if LT is performed in selected patients it represents an effective treatment. Post-transplant outcomes are good67,82,83 with survival rates that are significantly higher when compared with patients with sAH not responding to medical therapy and not transplanted82 and similar to those of ALD cirrhotic LT patients.83 Despite the good results reported, some ethical and social issues persist regarding the role of early LT for patients with sAH. These mainly rely on the potential reduction of organ donations because of the public perception that a graft is offered to patients who were actively drinking just before admission to the waiting list, with an increased risk of post-transplant alcohol relapse. However, available data shows that, when a strict selection process is applied to this special population of patients, relapse rates are comparable to those reported in ALD patients who underwent LT after 6 month of pre-transplant abstinence.67,82,83 Further studies are ongoing to confirm these preliminary results.84 Lastly, a strong variability exists in terms of access to LT for sAH, with differences that are evident also at a national level with a potential inequity among patients with the same disease. This issue needs to be resolved through the development of international and national consensus on criteria for listing and transplanting patients with sAH.85 In Italy, patients with sAH can be considered potential candidates for LT only if strict criteria are met, as recently published in a position paper.77 The first Italian experience was reported by Germani et al86 who evaluated patients admitted for sAH at the Multivisceral Transplant Unit of Padua University Hospital (between January 2013 to June 2018). Out of 25 patients with sAH, 18 (72%) were non-responders to medical therapy and underwent the selection process. Among these, nine patients were placed on the waiting list and seven out of nine underwent LT. Six-month survival after LT was significantly higher in patients with sAH who underwent LT compared with patients who were considered not suitable for LT (100% vs 43%; p=0.03). At a median follow-up of 2.5 years, one patient experienced alcohol relapse.
Further studies should identify the main predictors of relapse of drinking in these patients in order to optimise patient selection and management.
LT in patients with ACLF
Due to the very poor prognosis for these patients, the option of LT for patients with ACLF (particularly grade 2 and 3) is frequently considered. Although some groups have recently reported acceptable 1 year survival post-LT (including patients with ACLF-3),87 this topic is highly controversial. In addition to the need for strict selection criteria to minimise the risk of alcohol relapse after LT, the question of objective limits beyond which the patient must be considered too sick for LT remains unanswered. Future studies are needed to better define LT selection criteria for those patients without any effective medical therapeutic option.
LT in patients with HCC associated with ALD
HCC is one of the main causes of cancer-related death and its mortality is increasing worldwide. In Europe, alcohol abuse accounts for about half of liver cancer cases and it will become the leading cause of HCC in the future. LT for patients with HCC represents the best possible treatment in case of tumour recurrence or progression despite locoregional or surgical treatments. Long-term results after LT for HCC associated with ALD are good. However, cardiovascular disease and other de novo malignancies can significantly hamper patients’ survival and should be carefully considered by the transplant team.88
Animal models
Over the last five decades many animal models for the study of the pathogenesis of ALD have been developed and are useful to study the different stages of ALD (see figure 6). Recently, one of the major advances in the field of ALD research was the introduction of binge alcohol intake into chronically ethanol-fed mice, causing neutrophilia, hepatic neutrophil infiltration and significant liver injury.89 This is useful for the study of the early stage of ASH. Briefly, mice were fed chronically the Lieber-DeCarli ethanol diet for 10 days, followed by gavaging a single dose of ethanol.89 This model was orignially called the NIAAA model89 and later was also called the Gao-binge model.90,91 Due to easy, short and reproducible feeding protocol, this model has been now widely used to study early stages of ASH and mild AH.92 Binge ethanol intake was later also introduced into long-term (up to 12 weeks) chronic ethanol-fed mice,93 causing more severe steatohepatitis than the 10 day chronic-plus-binge ethanol feeding model. This long-term chronic-plus-binge ethanol feeding induced mild fibrosis whereas the 10 day chronic-plus-binge ethanol model only caused fibrogenic responses without significant fibrosis. Moreover, high-fat diet feeding plus binge ethanol intake caused severe steatohepatitis with significant neutrophil infiltration and mild fibrosis94–97 whereas high-fat diet feeding only caused steatosis in mice. This high-fat diet feeding plus binge ethanol intake is a very useful model to study the synergistic effect of obesity and binge drinking on liver injury. Finally, weekly binges were also recently introduced into mice that are chronically fed ethanol, high-fat and high cholesterol diets via intragastric infusion.98 This complex model shifted macrophge infiltration into neutrophil infiltration, causing more severe liver inflammation (neutrophil infiltration), injury and fibrosis than chronic-plus-binge ethanol feeding, representing a good model to study moderate AH.98 The limitation to use this complex hybrid model includes its technical difficulty, and its requirement for intensive medical care and expensive equipment.99
Figure 6.
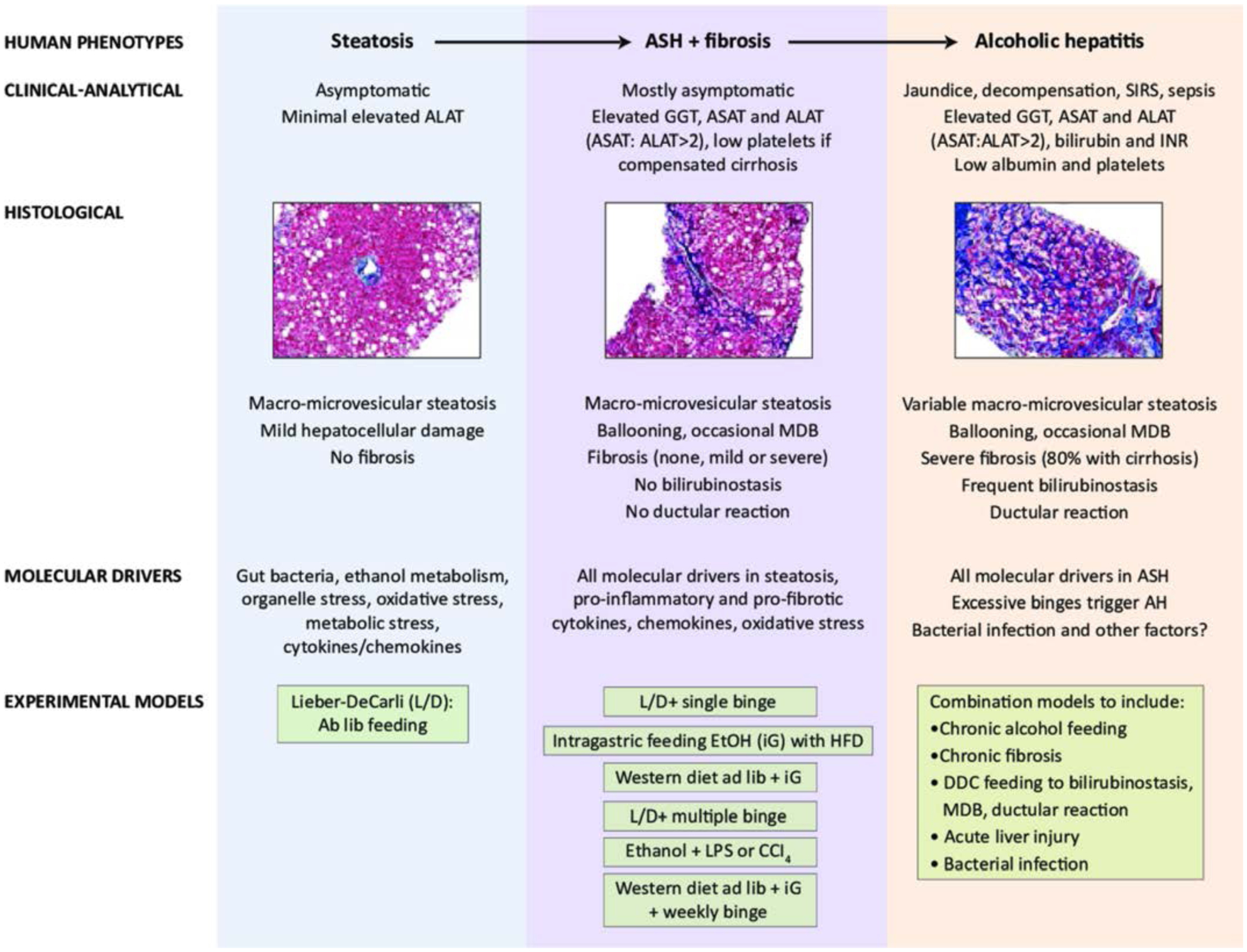
Experimental models for various stages of alcohol-related liver disease (ALD), representing steatosis, subclinical alcoholic steatohepatitis (ASH), and alcoholic hepatitis (AH) in patients. Ab lib, Ab libitum; ALAT, alanine aminotransferase; ASAT, aspartate transaminase; CCI, carbon tetrachloride; DDC,3,5-diethoxycarbonyl-1,4-dihydrocollidine; EtOH,ethanol; GGT, γ-glutamyl transferase; HFD, high fat diet; iG, intragastric infusion of ethanol diet; INR, international normalised ratio; L/D, Lieber-DeCarli ethanol diet; LPS, lipopolysaccharide; MDB, Mallory-Denk body; SIRS, systemic inflammatory response syndrome. Modified from99 with permission from Wiley.
By using these newly developed models with binge ethanol intake, many novel mechanisms underlying alcohol-induced steatohepatitis have been recently identified, including neutrophils, natural killer T cells, micro ribonucleic acids (miRNAs), autophagy, extracellular vesicles and others.92 The chronic-plus-binge ethanol feeding model has also been used to examine several potential therapeutic targets for the treatment of ALD, including recombinant interleukin (IL)-22,100 engineered bacteria that produce IL-22101 and an IL-1 antagonist.102 Both IL-22 and the IL-1 antagonist are currently being evaluated in clinical trials as therapeutic targets for the treatment of patients with sAH.
Although the chronic-plus-binge ethanol feeding model and hybrid feeding model are useful for the study of ASH, they do not generate the full features and complications observed in sAH, such as cirrhosis, jaundice, renal failure, bacterial infections and others. Due to the fast ethanol metabolism and short-term feeding periods, rodents are unlikely to be able to develop severe forms of ALD including sAH by consuming alcohol alone. Thus, investigators have tried combinations of alcohol feeding and other toxins (such as LPS, carbon tetrachloride (CCl4), 3,5-diethoxycarbonyl-1,4-dihydrocollidine, concanavalin A and others) to induce more severe forms of liver injury.103–106 Moreover, chronic CCl4 administration plus ethanol feeding induced liver cancer development in mice,107 which has been used to study the pathogenesis of alcohol-associated liver cancer. However, these combination models have not been well accepted as ALD models in the field since the majority of phenotypes observed in these models are caused by toxins other than alcohol. Moreover, many administration observed in severe ALD were due to the secondary effects of severe damage and inflammation and were not due to the primary effects of alcohol-induced toxicity; thus these combination models are probably still useful to study the mechanisms underlying severe ALD and to test therapeutic targets for the treatment of severe ALD.
Mechanisms and targets of disease
The pathophysiology of ALD is complex and the key elements are related first, to the direct effects of ethanol and its metabolites on the liver and other organs and second, to immune cell activation and inflammation triggered by the alcohol’s effects.
The metabolism of high concentrations of alcohol results not only in acetaldehyde, that has cellular toxic effects, but also in the production of reactive oxygen species that disturb mitochondrial energy transport and other intracellular signalling pathways.108 In disrupting cellular homeostasis, alcohol was shown to induce endoplasmic reticulum (ER) stress, unfolded protein response, mitochondrial, Golgi and lysosomal stress responses and injuries.
It has been shown that innate immune system activation is a central driver in ALD and particularly in AH where pro-inflammatory cell activation and pro-inflammatory cytokine production contribute to disease severity as well as to clinical symptoms and prognosis. The direct effects of alcohol and its metabolites in hepatocytes result in ER stress that induces the activation of the stimulator of interferon inducible genes leading to phosphorylation of Interferon Regulatory Factor 3 (IRF3).109 In addition to being involved in type-1 interferon induction, phosphorylated IRF3 interacts with mitochondrial apoptotic molecules, thus leading to hepatocyte damage and the release of sterile damage-associated molecular patterns (DAMPs) such as uric acid, ATP, high mobility group box 1 protein (HMGB1).110,111
Gut-liver axis in ALD
Alcohol also affects the gut at multiple levels. ALD is associated with changes in the intestinal microbiota. The importance of intestinal dysbiosis for the development of ALD was shown by faecal microbiota transfer. Mice transplanted with stool from patients with sAH develop more severe liver disease when compared with mice transplanted with stool from patients with less severe disease following feeding of an oral Lieber-DeCarli diet for 28 days.112 Bacterial overgrowth of the luminal and mucosa-associated microbiota in the small intestine occurs in patients with early stages of ALD.113,114 Changes in the bacterial taxa are dependent on the stage of liver disease as patients with early disease have a different microbiota composition when compared with patients with AC or AH.115 Published reports are not consistent with respect to changes in bacterial taxa, which is likely due to different methods that are being applied in each study and different countries of patient enrolment.115,116 Development of intestinal dysbiosis is not occurring in all patients with AUD.117 Taxonomic changes in fungi are largely characterised by a decrease in fungal diversity and overgrowth of Candida species in patients with AUD and AH. This appears to be mostly independent from the stage of liver disease.118,119 Although increased intestinal permeability is present in about 50% of patients with AUD and early stages of liver disease,117 paracellular tight junction disruption is more common in cirrhosis and AH.54
Dysbiosis is closely linked to increased intestinal permeability.117 Dysbiosis-induced intestinal immune dysregulation connects the gut microbiota and translocation of microbial products in preclinical models.120 Alcohol affects intestinal permeability by other mechanisms, including disruption of the circadian rhythm and a direct effect of the ethanol metabolite acetaldehyde on tight junctions.121 In addition, translocation of viable bacteria occurs independently from changes in tight junctions.114,122 Both, translocation of microbial products such as bacterial LPS or fungal β-D-glucan, and of viable bacteria are required for the development of ethanol-induced liver disease in preclinical models. Microbial translocation contributes to hepatic inflammation, hepatocyte injury and fibrosis. In patients with AC or AH, bacterial translocation contributes to systemic inflammation, decompensation and mortality.54
The intestinal microbiota contributes to ALD not only by microbial translocation. Changes in microbial metabolism affect the intestinal metabolites, including bile acids,123,124 short-chain fatty acids,125–127 saturated long-chain fatty acids,128 indole derivatives101 and possibly other molecules. Figure 7 shows the contribution of intestinal microbiota to ALD.
Figure 7.
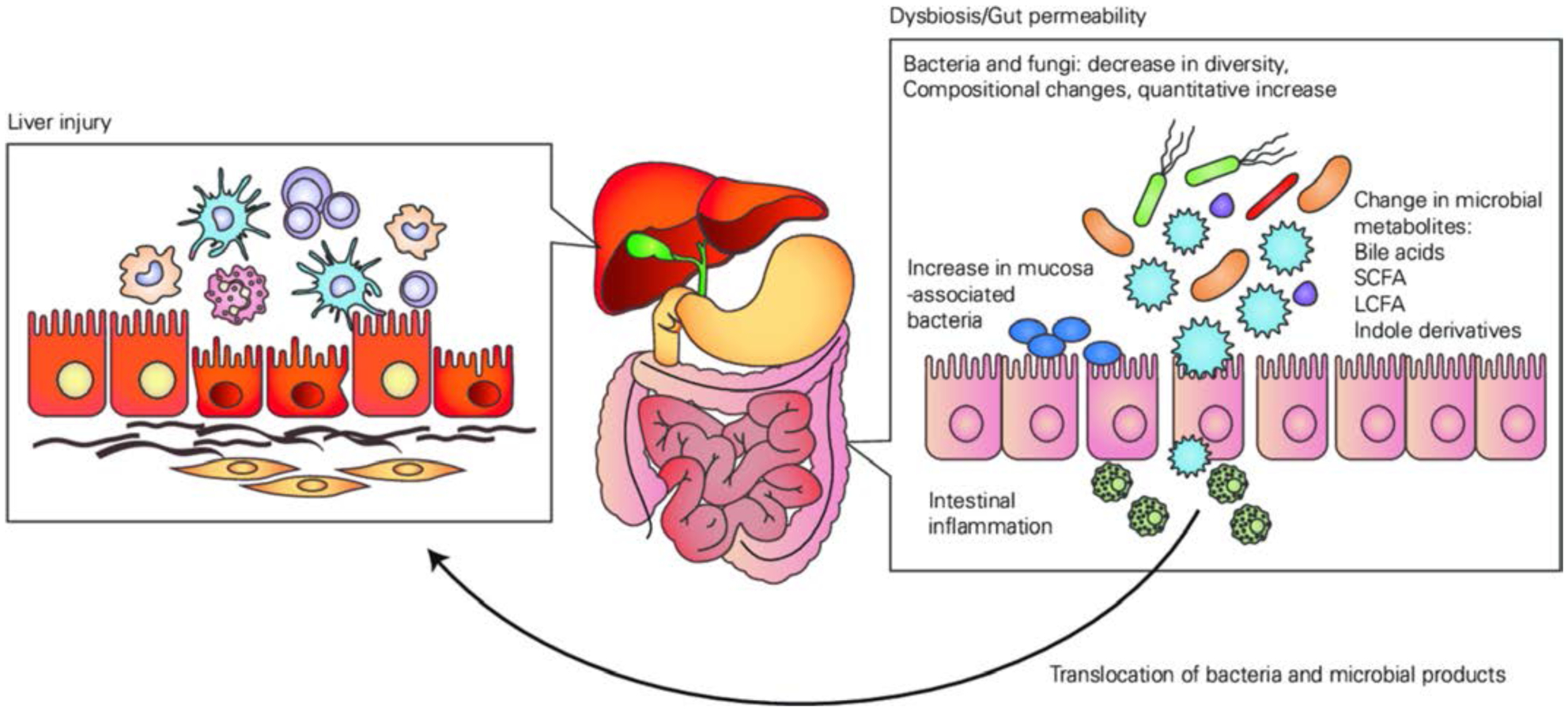
Contribution of intestinal microbiota to alcohol-relatedliver disease. LCFA, long-chain fatty acids; SCFA, short-chain fatty acids.
Bile acid homeostasis is disrupted in patients with ALD. Systemic total bile acids are elevated in 40% of patients with pre-cirrhotic ALD, while 81% of patients with AC have increased serum bile acids.129 Patients with AH have increased total, and absolute and proportional conjugated bile acid levels.123 Fibroblast growth factor (FGF19) messenger ribonucleic acid (mRNA) expression is increased in the terminal ileum of actively drinking patients with cirrhosis when compared with patients with cirrhosis of non-alcoholic aetiology.130
Serum FGF19 levels are markedly increased in patients with AH, which is partly explained by de novo induction of FGF19 mRNA expression in the liver. FGF19 inhibits bile acid synthesis, and synthesis of bile acids is hence significantly decreased in patients with AH and patients with AUD compared with controls.123
There is also evidence that in ALD, the antibacterial potency of mucosa-associated invariant T cells (MAIT) cells may be reduced as a result of contact with microbial products and microbiota. So, it is possible that the ‘leaky’ gut seen in ALD leads to MAIT cell dysfunction, thus increasing susceptibility to infection in these patients.131
Immune activation in the liver
Intestinal bacterial overgrowth and changes in the composition of the bacterial and fungal microbiota due to chronic alcohol use132 together with inflammation in the intestinal wall and reduced expression of tight junction proteins result in an increased gut permeability permitting pathogen-associated molecular patterns (PAMPs) such as LPS and bacterial DNA to enter the portal and systemic circulation.108
These PAMPs are sensed by the different pattern recognition receptors on cell surfaces or intracellular resulting in activation of pro-inflammatory innate immune activation pathways. Different Toll-like receptors (TLR) are involved in the recognition of the PAMPs and DAMPs leading to the common pro-inflammatory pathway of nuclear factor kappa-light-chain-enhancer of activated B cells activation, chemokines and pro-inflammatory cytokines (tumour necrosis factor alpha, IL-6, pro-IL-1ß). The intracellular inflammasome complex, particularly nucleotide-binding oligomerisation domain-like receptor P3, activated by uric acid and ATP, lead to caspase-1 activation that results in the secretion of bioactive IL-1ß.102,108
This provides an amplification loop for increasing pro-inflammatory cytokine production with additional effects on the liver promoting hepatocyte damage, fibrosis and inhibiting regeneration.133 These pathways are activated primarily in recruited macrophages and activated Kupffer cells in the liver. In addition, there is a major recruitment of neutrophil leukocytes to the liver in AH.
Based on animal models, neutrophils show an in vivo activated phenotype after chronic alcohol exposure but cannot properly respond to new stimulation.134
Preclinical studies indicate that strategies that interrupt inflammatory cascade activation may have benefits in ALD and AH.
Future therapeutic targets for the treatment of ALD
In recent years advances in the understanding of the molecular mechanisms of ALD have yielded the identification of new therapeutic targets in the treatment of ALD. Several encouraging pilot studies have led to larger clinical trials testing new pathophysiologically-oriented approaches. The focus of emerging therapeutics in preclinical testing is on targeting inflammation, gut permeability and dysbiosis and on hepatoprotective agents.
Preclinical studies indicate that strategies which interrupt inflammatory cascade activation may have benefits in ALD and AH. For example, inhibition of the IL-1 pathway with administration of the IL-1 receptor antagonist anakinra attenuated alcohol-induced steatosis, liver damage, inflammation and early fibrosis in mice.102,133 Anakinra and the IL-1β specific monoclonal antibody, canakinumab, are now being evaluated in clinical trials in the UK and USA.
Although mice do not replicate all of the typical features of ALD including steatosis, inflammation and fibrosis, the inflammatory components were eliminated in mice after administration of a small molecular inhibitor of CCR2 and CCR5, cenicriviroc.135
Another potential target is spleen tyrosine kinase (Syk). Dampening TLR-mediated pathways by inhibition of Syk was proven to reduce steatosis liver damage, inflammation and fibrosis in a mouse model of ALD.136 This has not been tested in humans yet. Targeting TLR 7/8 improves host anti-infective response in AC.137,138
Apoptosis signal regulating protein 1 (ASK-1) is a member of the mitogen activated protein kinase family of signal transducters. It activates c-Jun N-terminal kinase and p38 map kinase in response to oxidative stress or ER stress. A recent phase 2 trial (NCT02854631) evaluated the efficacy of selonsertib, a novel inhibitor of ASK-1 in patients with severe AH. Unfortunately, the results of this study were negative.
Increasing evidence suggest a role for miRNAs and extracellular vesicles in the pathomechanisms of ALD.54,136,139 Chronic alcohol was shown to reduce liver and hepatocyte expression of miRNA-122, the most abundant microRNA essential for hepatocyte functions. Replacement of miR-122 in hepatocytes in vivo alleviated alcohol-induced liver injury, steatosis and fibrosis.139 The inflammation-related miRNA-155 is increased by alcohol in immune cells and miR-155 deficient mice were protected from features of ALD suggesting miR-155 as another potential therapeutic target.140
Another approach is the inhibition of CYP2E1 to decrease oxidative stress and the generation of reactive oxygen species (ROS) that contributes to inflammation.141
Granulocyte colony-stimulating factor (G-CSF) has been shown to increase hepatocyte proliferation and to increase the number of circulating CD34 positive cells. Initial trials from India suggest a potential benefit for the treatment of patients with AH.142–144 It remains to be determined whether the effect of G-CSF in AH is related to its effects on neutrophil leukocytes or linked to recruitment of bone marrow-derived steam cells in the circulation and the liver.
Hepatoprotective agents also can provide benefits in ALD, protecting against cell damage and improving liver regeneration. As previously mentioned, one example is cytokine IL-22 which is currently in clinical trials for the treatment of AH.145
Another excellent target for therapy is intestinal dysbiosis. As referred above, transfer of human microbiomes from patients with ALD or healthy controls impacts the susceptibility of mice models to AH. As proof of principle, small clinical trials using faecal microbiota transfer have shown benefit in mortality for patients with sAH not eligible for treatment with steroids146 and in improving hepatic encephalopathy in patients with decompensated AC.147 Other more targeted approaches might include restoring intestinal eubiosis using prebiotics, probiotics, synbiotics or antibiotics; restoring the function of altered microbial metabolism using bio-engineered bacteria or supplementing metabolites or altering host metabolism that is disrupted during dysbiosis.115 Moreover, precisely editing the instestinal microbiota with bacteriophages may also represent a promising therapeutic approach. This has been recently demonstrated using bacteriophages that specifically target Enterococcus faecalis, a bacterium that accumulates in the gut of patients with AH and that releases a hepatotoxic exotoxin. Administration of E. faecalis-specific bacteriophages abolished ethanol-induced liver injury in humanised mice.148
Finally, the farnesoid X receptor (FXR)-bile acid axis/FXR agonists may be a promising therapeutic target for ALD.124 In mouse models of liver disease FXR agonists have been shown to improve intestinal mucosal integrity leading to reduced microbial translocation and also to reduced hepatic stellate cell activation which might result in lowering of portal pressure.149
To date this therapeutic target has not been evaluated in humans with AH. More studies are needed to further examine the different approaches in alcohol-induced hepatotoxicity and steatosis.
New targeted therapies from clinical trials might be available within the next 5 to 10 years.
Figure 8 is summarising the future therapeutic targets discussed.
Figure 8.
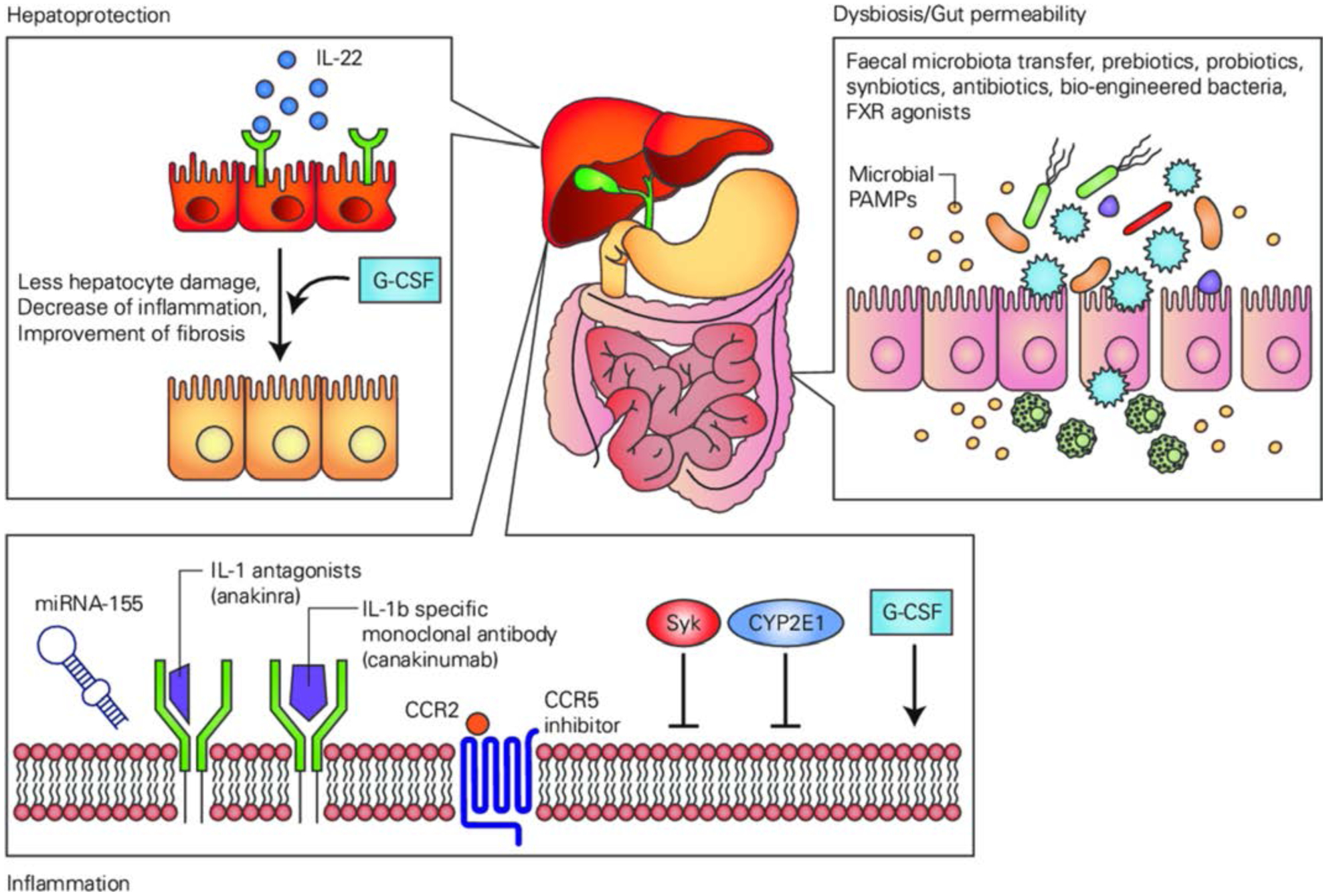
Overview of future therapeutic targets targeting hepatoprotection, inflammation and intestinal dysbiosis and gut permeability. FXR,farnesoid X receptor; G-CSF,granulocyte-colony stimulating factor; IL, interleukin; miRNA, micro ribonucleic acid; PAMPs,pathogen-associated molecular patterns; Syk,spleen tyrosine kinase.
Public health policies on alcohol consumption and the influence of industry
The root of ALD is obviously harmful alcohol use. A number of stakeholders seek to influence alcohol policy discourse. There is a major need to examine in detail the role of all these parties, especially non-governmental organisations. The term non-governmental organisation can be confusing as it has, for example, been used by the United Nations to include business interests.150
Actors in the alcohol field include global alcohol producers, retailers (particularly supermarket chains) and pubs, paid lobbyists and so-called independent charities funded by alcohol industry. These sit alongside independent public health bodies, and it is in the industry’s interest to blur the distinctions. There are finally arms-length bodies where the influence and control by governments may be difficult to assess.
It is essential to consider all types of actors and scrutinise their potential for conflicts of interest to arise and distort public health policies. The potential conflicts arise also in areas such as the dissemination of public health messaging and the funding of research.
The most overt and egregious examples of untoward influence in developing alcohol policy occur in the developing countries - for example in Africa where it is documented that global producers supported countries in developing their alcohol policies.151
Within western countries there are many examples of subtle influence through so called corporate social responsibility - examples include:
Covert access of global companies to government decision-makers.152
The ability of global producers to capture organisations like international football tournaments.153
The use of ‘front organisations’ like the International Centre for Alcohol Policy, the more recent International Alliance for Responsible Drinking and the European Policy Centre.154
The commissioning of non-peer reviewed reports such as that produced by the Institute of Economic Affairs to counteract minimum unit price.155
Voluntary partnerships with industry like the Responsibility Deal Alcohol Network in the UK and the European Commission’s European Alcohol and Health Forum.
There are also organisations in unclear positions, often at the behest of governments to offload their financial responsibility, for example Drinkaware in the UK. These are funded by voluntary donations from industry but may be registered as charities. They do not overtly push industry agendas but tend to support less effective harm-reduction policies, such as education and can lack transparency in their declaration of interests. Also, and very importantly, the perception that the funding is coming from industry and hence ‘tainted’ discourages independent researchers from applying for grants while giving other research bodies an excuse not to fund the area. Governments must bear large responsibility for situations like this - they allow industry actors to ‘help’ and give prominence to views of those where there is a clear conflict of interest in the name of ‘balance’. If we worked in an environment of complete transparency, these conflicts might be manageable, but this is not practical in the real world. The trail of inappropriate influence is long and complex and is supported by resources that the truly independent charities and public health organisations can never match. It is only through vigilance that their impact can be minimised.
Global strategies on alcohol consumption and related harm
Health services and health professionals play an important role in reducing the harmful use of alcohol not only at individual, but also at population level by promoting effective public health measures. In 2010 the World Health Assembly endorsed the Global strategy to reduce the harmful use of alcohol that is the only global policy framework for alcohol control.156 The strategy defines the harmful use of alcohol as the drinking that causes detrimental health and social consequences for the drinker, the people around the drinker and society at large, as well as the patterns of drinking that are associated with increased risk of adverse health outcomes. This translates into the following diagnostic categories of the 11th revision of the International Classification of Diseases (ICD-11)157: episode of harmful use of alcohol (6C40.0), harmful pattern of use of alcohol (6C40.1), alcohol dependence (6C40.2) as well as hazardous alcohol use (QE10). The ICD-11 includes several diagnostic categories under the umbrella term ‘alcoholic liver disease’ (DB94) that has some negative connotations in its name and can be in future renamed as ‘alcohol-related liver disease’.
In the UN Political Declaration on prevention and control of non-communicable diseases (NCDs) in 2011158 and in the WHO Global NCD Action Plan 2013–2020159 the harmful use of alcohol is included as one of the key risk factors for major NCDs. In the context of defining the most cost-effective interventions for reducing the harmful use of alcohol, the WHO recommends (a) increase excise taxes on alcoholic beverages, (b) enact and enforce bans or comprehensive restrictions on exposure to alcohol advertising (across multiple types of media), (c) enact and enforce restrictions on physical availability of retailed alcohol (via reduced hours of sale), (d) enact and enforce drink-driving laws and blood alcohol concentration limits via sobriety checkpoints, (e) provide brief psychosocial interventions for persons with hazardous and harmful alcohol use.160
As alcohol consumption is causally linked to numerous health conditions1,14 and in all cases of liver disease, health professionals should be attentive to drinking patterns in their patients, using whenever possible the available screening instruments, and provide brief intervention for hazardous and harmful drinking. If alcohol dependence is diagnosed, treatment interventions may include pharmacological and psychosocial interventions or their combination and/or referral to treatment programmes for AUD.161 For pharmacological treatment of alcohol dependence three medications are recommended (disulfiram, naltrexone and acamprosate), and several other medications (including nalmefene, baclofen, gabapentin, topiramate) are being explored for treatment of AUDs in clinical trials with so far inconsistent results.162,163 Effective psychosocial interventions include cognitive-behavioural therapy, motivational enhancement therapy, family therapy, 12-step facilitation, mutual help groups.161,162
Acknowledging the important role of effective treatment options for AUDs and other prevention interventions and treatment interventions delivered by health services, the most effective strategies to reduce the harmful use of alcohol at population level are strongly linked to population-based measures. In 2018 the WHO launched a new ‘SAFER’ initiative to boost the implementation of the global strategy to reduce the harmful use of alcohol and support the implementation of high-impact and cost-effective interventions at country level.164
A systemic analysis for the Global Burden of Disease Study 2016 found the risk of all-cause mortality, and of cancers specifically rises with increasing levels of alcohol consumption, and the level of alcohol consumption that minimises health loss is zero. These results suggest that alcohol control policies might need to be revised worldwide, refocusing on efforts to lower overall population-level consumption.33
CONCLUSION
The round table panel discussed a broad variety of important and timely aspects regarding the topic of ALD in the areas of epidemiology, diagnosis, management, pathomechanisms and future therapeutic targets, as well as industry’s influence and global public health strategy on alcohol consumption and related harm.
The panel agreed that the commonly used name alcoholic liver disease is stigmatised and should change to alcohol-related liver disease.
The panel recognised the accumulating evidence indicating that any level of alcohol consumption, regardless the type of beverage, represents a health risk.
Experimental models of ALD have been refined over the past years. Although some of these models are useful to investigate mechanisms and targets in early disease stages, they do not reproduce the more salient features of advanced ALD. The panel concluded that the lack of relevant models in this area is an important limitation and that more efforts should be made in this direction.
The panel concluded that more research regarding associated risk factors for the development and progression of ALD, and on how both short-term and long-term fluctuations in drinking influence the risk of liver damage should be conducted.
Efforts should be undertaken to find a biomarker as a proxy measure for alcohol use for clinical and research purposes. The panel found that healthcare professionals should focus more on the diagnosis of non-severe AH.
The optimal therapeutic strategy in patients with sAH and ACLF needs further investigation.
More studies are needed to identify novel targets for treatment and to further examine the different new therapeutic approaches.
Hepatologists should get better training in the identification and management of AUDs and referral and cooperation with addiction specialists needs to be improved.
Finally, in the area of public health, governments and related bodies need clearer definitions and terms of engagement with industry actors. Also, minimum pricing and taxation are important in reducing the harmful use of alcohol.
Key messages from the round table discussion regarding epidemiology.
Improve the codification systems of death certificates.
Set up selected representative sentinel centres for in-depth evaluation of the cause of liver disease for admitted patients to define attributable fractions for cirrhosis and hepatocellular carcinoma in each country.
Undertake more translational research on the associated risk factors regarding the development and progression of alcohol-related liver disease.
Undertake population studies with long-term clinical follow-up of patients including accurate non-invasive assessment of liver disease severity.
Assess how both short-term and long-term fluctuations in drinking influence risk of liver damage.
Map genetic susceptibility as genetic risk scores, beyond single nucleotide polymorphisms.
Key messages from the round table discussion regarding diagnosis of alcohol-related liver disease.
In all cases of liver disease alcohol consumption should be assessed in terms of its level and patterns.
More trials are needed to confirm advantages and limitations of the different non-invasive diagnostic techniques and their combinations.
More validated trials should be undertaken to find a biomarker as a proxy measure for alcohol use for clinical and research purposes.
Key message from the round table regarding management of alcoholic hepatitis.
Healthcare professionals should focus more on the diagnosis of non-severe alcoholic hepatitis.
Key message from the round table regarding management of ACLF.
Although the optimal therapeutic strategy in patients with severe alcoholic hepatitis and acute-on-chronic liver failure (ACLF) needs further investigation, we do not recommend the use of corticosteroids in patients with ACLF grade 2 and 3.
Key messages from the round table discussion regarding transplant in patients with alcohol-related liver disease.
Future studies are needed to better define liver transplantation selection criteria for patients without any effective medical therapeutic option.
Further studies should identify the main predictors of relapse of drinking in order to optimise patient selection and management.
Key messages from the round table discussion regarding animal models.
Despite their limitations, these newly developed mouse models with ethanol binge intake are useful for the study of the early stages of alcohol-relatedliver disease (ALD).
Combination models are needed to generate more severe forms of liver injury and fibrosis to study the pathogenesis of severe ALD and to test therapeutic targets for severe ALD therapy.
Key messages from the round table discussion regarding gut-liver axis in ALD.
The gut microbiota contributes to alcohol-relatedliver disease (ALD) by different mechanisms such as gut barrier dysfunction and changes in microbial metabolites.
Intestinal dysbiosis is an excellent target for therapy.
Key messages from the round table discussion regarding new therapeutic targets.
More studies are needed to further examine the different approaches in alcohol-induced hepatotoxicity and steatosis.
Despite new therapy developments, it is also possible to optimise the use of existing therapies. Important are the right patient selection and to avoid infection. Also the treatment of alcohol use disorders shouldn’t be forgotten.
Key message from the round table discussion with regards to industry’s influence.
Governments and related bodies need clear definitions and terms of engagement with industry actors.
Key messages from the round table discussion with regards to global policies on alcohol consumption:
The name alcoholic liver disease is stigmatising and should change to alcohol-related liver disease.
Pricing policies and taxation are important as well as other population-based measures shown to be effective in reducing the harmful use of alcohol and summarised in the WHO-led SAFER initiative.
Referral and cooperation with addiction specialists needs to be improved.
Hepatologists should have improved training in identification and management of hazardous alcohol use and alcohol use disorders.
Acknowledgements
Dr Christiane Rehwagen has organised the roundtable and has provided medical writing support for this manuscript. Mark Thursz would like to acknowledge NIHR Imperial BRC. Matias Avila would like to acknowledge the Hepacare Project from ‘la Caixa’.
Funding The event was solely funded by Gut.
Footnotes
Patient consent for publication Not required.
Provenance and peer review Commissioned; externally peer reviewed.
REFERENCES
- 1.World Health Organization. Global status report on alcohol and health 2018 Geneva, 2018. [Google Scholar]
- 2.Seitz HK, Bataller R, Cortez-Pinto H, et al. Alcoholic liver disease. Nat Rev Dis Primers 2018;4. [DOI] [PubMed] [Google Scholar]
- 3.Millwood IY, Walters RG, Mei XW, et al. Conventional and genetic evidence on alcohol and vascular disease aetiology: a prospective study of 500 000 men and women in China. The Lancet 2019;393:1831–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mokdad AA, Lopez AD, Shahraz S, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med 2014;12:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pimpin L, Cortez-Pinto H, Negro F, et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol 2018;69:718–35. [DOI] [PubMed] [Google Scholar]
- 6.Bellentani S, Saccoccio G, Costa G, et al. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut 1997;41:845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harman DJ, Ryder SD, James MW, et al. Obesity and type 2 diabetes are important risk factors underlying previously undiagnosed cirrhosis in general practice: a cross-sectional study using transient elastography. Aliment Pharmacol Ther 2018;47:504–15. [DOI] [PubMed] [Google Scholar]
- 8.Thiele M, Madsen BS, Hansen JF, et al. Accuracy of the enhanced liver fibrosis test vs FibroTest, elastography, and indirect markers in detection of advanced fibrosis in patients with alcoholic liver disease. Gastroenterology 2018;154:1369–79. [DOI] [PubMed] [Google Scholar]
- 9.Caballería L, Pera G, Arteaga I, et al. High prevalence of liver fibrosis among European adults with unknown liver disease: a population-based study. Clin Gastroenterol Hepatol 2018;16:1138–45. [DOI] [PubMed] [Google Scholar]
- 10.Manthey J, Shield KD, Rylett M, et al. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. The Lancet 2019;393:2493–502. [DOI] [PubMed] [Google Scholar]
- 11.Kim D, Li AA, Gadiparthi C, et al. Changing trends in Etiology-Based annual mortality from chronic liver disease, from 2007 through 2016. Gastroenterology 2018;155:1154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ 2018;362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puffer RR, Griffith GW. Patterns of urban mortality: report of the Inter-American investigation of mortality. Washington, DC: Pan American Health Organization, 1967. [Google Scholar]
- 14.Rehm J, Gmel GE, Gmel G, et al. The relationship between different dimensions of alcohol use and the burden of disease-an update. Addiction 2017;112:968–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpson RF, Hermon C, Liu B, et al. Alcohol drinking patterns and liver cirrhosis risk: analysis of the prospective UK Million women study. Lancet Public Health 2019;4:e41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagström H, Hemmingsson T, Discacciati A, et al. Alcohol consumption in late adolescence is associated with an increased risk of severe liver disease later in life. J Hepatol 2018;68:505–10. [DOI] [PubMed] [Google Scholar]
- 17.European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–402. [DOI] [PubMed] [Google Scholar]
- 18.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology 2018;67:328–57. [DOI] [PubMed] [Google Scholar]
- 19.Holst C, Tolstrup JS, Sørensen HJ, et al. Alcohol dependence and risk of somatic diseases and mortality: a cohort study in 19 002 men and women attending alcohol treatment. Addiction 2017;112:1358–66. [DOI] [PubMed] [Google Scholar]
- 20.Stickel F, Moreno C, Hampe J, et al. The genetics of alcohol dependence and alcohol-related liver disease. J Hepatol 2017;66:195–211. [DOI] [PubMed] [Google Scholar]
- 21.Hrubec Z, Omenn GS. Evidence of genetic predisposition to alcoholic cirrhosis and psychosis: twin concordances for alcoholism and its biological end points by zygosity among male veterans. Alcoholism Clin Exp Res 1981;5:207–15. [DOI] [PubMed] [Google Scholar]
- 22.Verhulst B, Neale MC, Kendler KS. The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychol Med 2015;45:1061–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stickel F, Hampe J. Genetic determinants of alcoholic liver disease. Gut 2012;61:150–9. [DOI] [PubMed] [Google Scholar]
- 24.Kolla BP, Schneekloth TD, Biernacka J, et al. Pnpla3 association with alcoholic liver disease in a cohort of heavy drinkers. Alcohol Alcohol 2018;53:357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atkinson SR, Way MJ, McQuillin A, et al. Homozygosity for rs738409:G in PNPLA3 is associated with increased mortality following an episode of severe alcoholic hepatitis. J Hepatol 2017;67:120–7. [DOI] [PubMed] [Google Scholar]
- 26.Abul-Husn NS, Cheng X, Li AH, et al. A Protein-Truncating HSD17B13 Variant and Protection from Chronic Liver Disease. N Engl J Med 2018;378:1096–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strnad P, Buch S, Hamesch K, et al. Heterozygous carriage of the alpha1-antitrypsin Pi*Z variant increases the risk to develop liver cirrhosis. Gut 2019;68:1099–107. [DOI] [PubMed] [Google Scholar]
- 28.Hart CL, Morrison DS, Batty GD, et al. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ 2010;340:c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yi S-W, Hong J-S, Yi J-J, et al. Impact of alcohol consumption and body mass index on mortality from nonneoplastic liver diseases, upper aerodigestive tract cancers, and alcohol use disorders in Korean older middle-aged men: prospective cohort study. Medicine 2016;95:e4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trembling PM, Apostolidou S, Gentry-Maharaj A, et al. Risk of chronic liver disease in post-menopausal women due to body mass index, alcohol and their interaction: a prospective nested cohort study within the United Kingdom collaborative trial of ovarian cancer screening (UKCTOCS). BMC Public Health 2017;17:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker U et al Lower risk for alcohol-induced cirrhosis in wine drinkers. Hepatology 2002;35:868–75. [DOI] [PubMed] [Google Scholar]
- 32.Dunn W, Sanyal AJ, Brunt EM, et al. Modest alcohol consumption is associated with decreased prevalence of steatohepatitis in patients with non-alcoholic fatty liver disease (NAFLD). J Hepatol 2012;57:384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griswold MG, Fullman N, Hawley C, et al. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the global burden of disease study 2016. The Lancet 2018;392:1015–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kowdley KV. Iron overload in patients with chronic liver disease. Gastroenterol Hepatol 2016;12:695–8. [PMC free article] [PubMed] [Google Scholar]
- 35.Loomba R, Yang H-I, Su J, et al. Synergism between obesity and alcohol in increasing the risk of hepatocellular carcinoma: a prospective cohort study. Am J Epidemiol 2013;177:333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engstler AJ, Aumiller T, Degen C, et al. Insulin resistance alters hepatic ethanol metabolism: studies in mice and children with non-alcoholic fatty liver disease. Gut 2016;65:1564–71. [DOI] [PubMed] [Google Scholar]
- 37.Katikireddi SV, Whitley E, Lewsey J, et al. Socioeconomic status as an effect modifier of alcohol consumption and harm: analysis of linked cohort data. The Lancet Public Health 2017;2:e267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grucza RA, Bierut LJ. Co-Occurring risk factors for alcohol dependence and habitual smoking: update on findings from the Collaborative study on the genetics of alcoholism. Alcohol Res Health 2006;29:172–8. [PMC free article] [PubMed] [Google Scholar]
- 39.Altamirano J, Bataller R. Cigarette smoking and chronic liver diseases. Gut 2010;59:1159–62. [DOI] [PubMed] [Google Scholar]
- 40.Marcellin P, Pequignot F, Delarocque-Astagneau E, et al. Mortality related to chronic hepatitis B and chronic hepatitis C in France: evidence for the role of HIV coinfection and alcohol consumption. J Hepatol 2008;48:200–7. [DOI] [PubMed] [Google Scholar]
- 41.Tverdal A, Skurtveit S, Selmer R, et al. Coffee and wine consumption is associated with reduced mortality from alcoholic liver disease: follow-up of 219,279 Norwegian men and women aged 30–67 years. Ann Epidemiol 2018;28:753–8. [DOI] [PubMed] [Google Scholar]
- 42.Hu EA, Lazo M, Selvin E, et al. Coffee consumption and liver-related hospitalizations and deaths in the ARIC study. Eur J Clin Nutr 2019;73:1133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barritt AS, Jiang Y, Schmidt M, et al. Charges for alcoholic cirrhosis exceed all other etiologies of cirrhosis combined: a national and state inpatient survey analysis. Dig Dis Sci 2019;64:1460–9. [DOI] [PubMed] [Google Scholar]
- 44.Shah ND, Ventura-Cots M, Abraldes JG, et al. Alcohol-Related liver disease is rarely detected at early stages compared with liver diseases of other etiologies worldwide. Clin Gastroenterol Hepatol 2019;17:2320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thursz M, Gual A, Lackner C, et al. EASL clinical practice guidelines: management of alcohol-related liver disease. J Hepatol 2018;69:154–81. [DOI] [PubMed] [Google Scholar]
- 46.Bradley KA, DeBenedetti AF, Volk RJ, et al. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcoholism Clin Exp Res 2007;31:1208–17. [DOI] [PubMed] [Google Scholar]
- 47.Mueller S, Seitz HK, Rausch V. Non-Invasive diagnosis of alcoholic liver disease. WJG 2014;20:14626–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen-Khac E, Thiele M, Voican C, et al. Non-Invasive diagnosis of liver fibrosis in patients with alcohol-related liver disease by transient elastography: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol 2018;3:614–25. [DOI] [PubMed] [Google Scholar]
- 49.Calès P, Oberti F, Michalak S, et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology 2005;42:1373–81. [DOI] [PubMed] [Google Scholar]
- 50.Voican CS, Louvet A, Trabut J-B, et al. Transient elastography alone and in combination with FibroTest ® for the diagnosis of hepatic fibrosis in alcoholic liver disease. Liver Int 2017;37:1697–705. [DOI] [PubMed] [Google Scholar]
- 51.Thiele M, Rausch V, Fluhr G, et al. Controlled attenuation parameter and alcoholic hepatic steatosis: diagnostic accuracy and role of alcohol detoxification. J Hepatol 2018;68:1025–32. [DOI] [PubMed] [Google Scholar]
- 52.Crabb DW, Bataller R, Chalasani NP, et al. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA alcoholic hepatitis consortia. Gastroenterology 2016;150:785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Altamirano J, Miquel R, Katoonizadeh A, et al. A Histologic Scoring System for Prognosis of Patients With Alcoholic Hepatitis. Gastroenterology 2014;146:1231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saha B, Tornai D, Kodys K, et al. Biomarkers of macrophage activation and immune danger signals predict clinical outcomes in alcoholic hepatitis. Hepatology 2019;70:1134–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bissonnette J, Altamirano J, Devue C, et al. A prospective study of the utility of plasma biomarkers to diagnose alcoholic hepatitis. Hepatology 2017;66:555–63. [DOI] [PubMed] [Google Scholar]
- 56.Altamirano J, Fagundes C, Dominguez M, et al. Acute kidney injury is an early predictor of mortality for patients with alcoholic hepatitis. Clin Gastroenterol Hepatol 2012;10:65–71. [DOI] [PubMed] [Google Scholar]
- 57.Sujan R, Cruz-Lemini M, Altamirano J, et al. A validated score predicts acute kidney injury and survival in patients with alcoholic hepatitis. Liver Transpl 2018;24:1655–64. [DOI] [PubMed] [Google Scholar]
- 58.Michelena J, Altamirano J, Abraldes JG, et al. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis. Hepatology 2015;62:762–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Conejo I, Augustin S, Pons M, et al. Alcohol consumption and risk of infection after a variceal bleeding in low-risk patients. Liver Int 2016;36:994–1001. [DOI] [PubMed] [Google Scholar]
- 60.Mathurin P, O’Grady J, Carithers RL, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut 2011;60:255–60. [DOI] [PubMed] [Google Scholar]
- 61.Louvet A, Thursz MR, Kim DJ, et al. Corticosteroids reduce risk of death within 28 days for patients with severe alcoholic hepatitis, compared with pentoxifylline or Placebo-a meta-analysis of individual data from controlled trials. Gastroenterology 2018;155:458–68. [DOI] [PubMed] [Google Scholar]
- 62.Louvet A, Artru F, Colin M, et al. Spontaneous improvement of liver function is not a strong predictor of outcome in patients with severe alcoholic hepatitis treated with corticosteroids. Hepatology 2012. Abstract 1694.
- 63.Thursz MR, Richardson P, Allison M, et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015;372:1619–28. [DOI] [PubMed] [Google Scholar]
- 64.Mathurin P, Louvet A, Duhamel A, et al. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis. JAMA 2013;310:1033–41. [DOI] [PubMed] [Google Scholar]
- 65.Sharma S, Maras JS, Das S, et al. Pre-therapy liver transcriptome landscape in Indian and French patients with severe alcoholic hepatitis and steroid responsiveness. Sci Rep 2017;7:6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Altamirano J, López-Pelayo H, Michelena J, et al. Alcohol abstinence in patients surviving an episode of alcoholic hepatitis: prediction and impact on long-term survival. Hepatology 2017;66:1842–53. [DOI] [PubMed] [Google Scholar]
- 67.Lee BP, Mehta N, Platt L, et al. Outcomes of early liver transplantation for patients with severe alcoholic hepatitis. Gastroenterology 2018;155:422–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moreau R, Jalan R, Gines P, et al. Acute-On-Chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426–37. [DOI] [PubMed] [Google Scholar]
- 69.Hernaez R, Solà E, Moreau R, et al. Acute-On-Chronic liver failure: an update. Gut 2017;66:541–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sersté T, Cornillie A, Njimi H, et al. The prognostic value of acute-on-chronic liver failure during the course of severe alcoholic hepatitis. J Hepatol 2018;69:318–24. [DOI] [PubMed] [Google Scholar]
- 71.Forrest EH, Atkinson SR, Richardson P, et al. Prevalent acute-on-chronic liver failure and response to corticosteroids in alcoholic hepatitis. J Hepatol 2018;69:1200–1. [DOI] [PubMed] [Google Scholar]
- 72.Mathurin P, Bataller R. Trends in the management and burden of alcoholic liver disease. J Hepatol 2015;62:S38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burra P, Senzolo M, Adam R, et al. Liver transplantation for alcoholic liver disease in Europe: a study from the ELTR (European liver transplant registry). Am J Transplant 2010;10:138–48. [DOI] [PubMed] [Google Scholar]
- 74.Singal AK, Guturu P, Hmoud B, et al. Evolving frequency and outcomes of liver transplantation based on etiology of liver disease. Transplantation Journal 2013;95:755–60. [DOI] [PubMed] [Google Scholar]
- 75.Neuberger J Transplantation for alcoholic liver disease: a perspective from Europe. Liver Transpl Surg 1998;4:S51–7. [PubMed] [Google Scholar]
- 76.Addolorato G, Bataller R, Burra P, et al. Liver transplantation for alcoholic liver disease. Transplantation 2016;100:981–7. [DOI] [PubMed] [Google Scholar]
- 77.Burra P, Belli LS, Ginanni Corradini S, et al. Common issues in the management of patients in the waiting list and after liver transplantation. Dig Liver Dis 2017;49:241–53. [DOI] [PubMed] [Google Scholar]
- 78.Cuadrado A, Fábrega E, Casafont F, et al. Alcohol recidivism impairs long-term patient survival after orthotopic liver transplantation for alcoholic liver disease. Liver Transpl 2005;11:420–6. [DOI] [PubMed] [Google Scholar]
- 79.European Association for the Study of Liver. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol 2012;57:399–420. [DOI] [PubMed] [Google Scholar]
- 80.Varma V, Webb K, Mirza DF. Liver transplantation for alcoholic liver disease. WJG 2010;16:4377–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Germani G, Ferrarese A, Adam R, et al. Influence of donor and recipient gender on liver transplantation outcomes in Europe: a ELTR study. J Hepatol 2016;64:S537–8. [DOI] [PubMed] [Google Scholar]
- 82.Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011;365:1790–800. [DOI] [PubMed] [Google Scholar]
- 83.Weeks SR, Sun Z, McCaul ME, et al. Liver Transplantation for Severe Alcoholic Hepatitis, Updated Lessons from the World’s Largest Series. J Am Coll Surg 2018;226:549–57. [DOI] [PubMed] [Google Scholar]
- 84.Validation of the Procedure of Early Liver Transplantation in Alcoholic Hepatitis Resisting to Medical Treatment (QuickTrans) (NCT01756794).. Available: https://clinicaltrials.gov
- 85.Thursz M, Allison M. Liver transplantation for alcoholic hepatitis: being consistent about where to set the bar. Liver Transpl 2018;24:733–4. [DOI] [PubMed] [Google Scholar]
- 86.Germani G, Romandini E, Ferrarese A, et al. Early liver transplantation for severe acute alcoholic hepatitis: pilot program in a single transplant centre in Italy. Transplantation 2017;49:e238–9. [Google Scholar]
- 87.Artru F, Louvet A, Ruiz I, et al. Liver transplantation in the most severely ill cirrhotic patients: a multicenter study in acute-on-chronic liver failure grade 3. J Hepatol 2017;67:708–15. [DOI] [PubMed] [Google Scholar]
- 88.Burra P, Zanetto A, Germani G. Liver transplantation for alcoholic liver disease and hepatocellular carcinoma. Cancers 2018;10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bertola A, Mathews S, Ki SH, et al. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc 2013;8:627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sanz-Garcia C, Poulsen KL, Bellos D, et al. The non-transcriptional activity of IRF3 modulates hepatic immune cell populations in acute-on-chronic ethanol administration in mice. J Hepatol 2019;70:974–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chao X, Wang S, Zhao K, et al. Impaired TFEB-Mediated Lysosome Biogenesis and Autophagy Promote Chronic Ethanol-Induced Liver Injury and Steatosis in Mice. Gastroenterology 2018;155:865–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao B, Xu M-J, Bertola A, et al. Animal models of alcoholic liver disease: pathogenesis and clinical relevance. Gene Expr 2017;17:173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu M-J, Cai Y, Wang H, et al. Fat-Specific protein 27/CIDEC promotes development of alcoholic steatohepatitis in mice and humans. Gastroenterology 2015;149:1030–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chang B, Xu M-J, Zhou Z, et al. Short-or long-term high-fat diet feeding plus acute ethanol binge synergistically induce acute liver injury in mice: an important role for CXCL1. Hepatology 2015;62:1070–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang W, Xu M-J, Cai Y, et al. Inflammation is independent of steatosis in a murine model of steatohepatitis. Hepatology 2017;66:108–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou Z, Xu M-J, Cai Y, et al. Neutrophil-Hepatic Stellate Cell Interactions Promote Fibrosis in Experimental Steatohepatitis. Cell Mol Gastroenterol Hepatol 2018;5:399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Torres‐Hernandez A, Wang W, Nikiforov Y, et al. γδ T cells Promote Steatohepatitis by Orchestrating Innate and Adaptive Immune Programming. Hepatology 2019. [DOI] [PubMed] [Google Scholar]
- 98.Khanova E, Wu R, Wang W, et al. Pyroptosis by caspase11/4-gasdermin-D pathway in alcoholic hepatitis in mice and patients. Hepatology 2018;67:1737–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mandrekar P, Bataller R, Tsukamoto H, et al. Alcoholic hepatitis: translational approaches to develop targeted therapies. Hepatology 2016;64:1343–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ki SH, Park O, Zheng M, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology 2010;52:1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hendrikx T, Duan Y, Wang Y, et al. Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice. Gut 2019;68:1504–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Petrasek J, Bala S, Csak T, et al. Il-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest 2012;122:3476–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Furuya S, Chappell GA, Iwata Y, et al. A mouse model of alcoholic liver fibrosis-associated acute kidney injury identifies key molecular pathways. Toxicol Appl Pharmacol 2016;310:129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Blaya D, Coll M, Rodrigo-Torres D, et al. Integrative microRNA profiling in alcoholic hepatitis reveals a role for microRNA-182 in liver injury and inflammation. Gut 2016;65:1535–45. [DOI] [PubMed] [Google Scholar]
- 105.Affò S, Dominguez M, Lozano JJ, et al. Transcriptome analysis identifies TNF superfamily receptors as potential therapeutic targets in alcoholic hepatitis. Gut 2013;62:452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gao Y, Zhou Z, Ren T, et al. Alcohol inhibits T-cell glucose metabolism and hepatitis in ALDH2-deficient mice and humans: roles of acetaldehyde and glucocorticoids. Gut 2019;68:1311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Seo W, Gao Y, He Y, et al. Aldh2 deficiency promotes alcohol-associated liver cancer by activating oncogenic pathways via oxidized DNA-enriched extracellular vesicles. J Hepatol 2019;71:1000–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol 2015;12:387–400. [DOI] [PubMed] [Google Scholar]
- 109.Petrasek J, Iracheta-Vellve A, Csak T, et al. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc Natl Acad Sci U S A 2013;110:16544–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Petrasek J, Iracheta-Vellve A, Saha B, et al. Metabolic danger signals, uric acid and ATP, mediate inflammatory cross-talk between hepatocytes and immune cells in alcoholic liver disease. J Leukoc Biol 2015;98:249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Iracheta-Vellve A, Petrasek J, Satishchandran A, et al. Inhibition of sterile danger signals, uric acid and ATP, prevents inflammasome activation and protects from alcoholic steatohepatitis in mice. J Hepatol 2015;63:1147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Llopis M, Cassard AM, Wrzosek L, et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut 2016;65:830–9. [DOI] [PubMed] [Google Scholar]
- 113.Bode JC, Bode C, Heidelbach R, et al. Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology 1984;31:30–4. [PubMed] [Google Scholar]
- 114.Wang L, Fouts DE, Stärkel P, et al. Intestinal REG3 lectins protect against alcoholic steatohepatitis by reducing mucosa-associated microbiota and preventing bacterial translocation. Cell Host Microbe 2016;19:227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sarin SK, Pande A, Schnabl B. Microbiome as a therapeutic target in alcohol-related liver disease. J Hepatol 2019;70:260–72. [DOI] [PubMed] [Google Scholar]
- 116.Allaband C, McDonald D, Vázquez-Baeza Y, et al. Microbiome 101: studying, analyzing, and interpreting gut microbiome data for clinicians. Clin Gastroenterol Hepatol 2019;17:218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leclercq S, Matamoros S, Cani PD, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A 2014;111:E4485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang A-M, Inamine T, Hochrath K, et al. Intestinal fungi contribute to development of alcoholic liver disease. J Clin Invest 2017;127:2829–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lang S, Duan Y, Liu J, et al. Intestinal fungal dysbiosis and systemic immune response to fungi in patients with alcoholic hepatitis. Hepatology 2019;65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen P, Stärkel P, Turner JR, et al. Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology 2015;61:883–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014;146:1513–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Llorente C, Jepsen P, Inamine T, et al. Gastric acid suppression promotes alcoholic liver disease by inducing overgrowth of intestinal Enterococcus. Nat Commun 2017;8:837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brandl K, Hartmann P, Jih LJ, et al. Dysregulation of serum bile acids and FGF19 in alcoholic hepatitis. J Hepatol 2018;69:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hartmann P, Hochrath K, Horvath A, et al. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology 2018;67:2150–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Couch RD, Dailey A, Zaidi F, et al. Alcohol induced alterations to the human fecal VOC metabolome. PLoS One 2015;10:e0119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cresci GA, Bush K, Nagy LE. Tributyrin supplementation protects mice from acute ethanol-induced gut injury. Alcohol Clin Exp Res 2014;38:1489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cresci GA, Glueck B, McMullen MR, et al. Prophylactic tributyrin treatment mitigates chronic-binge ethanol-induced intestinal barrier and liver injury. J Gastroenterol Hepatol 2017;32:1587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen P, Torralba M, Tan J, et al. Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology 2015;148:203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alm R, Carlson J, Eriksson S. Fasting serum bile acids in liver disease. A comparison with histological features. Scand J Gastroenterol 1982;17:213–8. [DOI] [PubMed] [Google Scholar]
- 130.Bajaj JS, Kakiyama G, Zhao D, et al. Continued alcohol misuse in human cirrhosis is associated with an impaired Gut-Liver axis. Alcohol Clin Exp Res 2017;41:1857–65. [DOI] [PubMed] [Google Scholar]
- 131.Riva A, Patel V, Kurioka A, et al. Mucosa-Associated invariant T cells link intestinal immunity with antibacterial immune defects in alcoholic liver disease. Gut 2018;67:918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Szabo G, Petrasek J. Gut-liver axis and sterile signals in the development of alcoholic liver disease. Alcohol Alcohol 2017;52:414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Iracheta-Vellve A, Petrasek J, Gyogyosi B, et al. Interleukin-1 inhibition facilitates recovery from liver injury and promotes regeneration of hepatocytes in alcoholic hepatitis in mice. Liver Int 2017;37:968–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bukong TN, Cho Y, Iracheta-Vellve A, et al. Abnormal neutrophil traps and impaired efferocytosis contribute to liver injury and sepsis severity after binge alcohol use. J Hepatol 2018;69:1145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ambade A, Lowe P, Kodys K, et al. Pharmacological inhibition of CCR2/5 signaling prevents and reverses alcohol-induced liver damage, steatosis, and inflammation in mice. Hepatology 2019;69:1105–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bukong TN, Iracheta-Vellve A, Saha B, et al. Inhibition of spleen tyrosine kinase activation ameliorates inflammation, cell death, and steatosis in alcoholic liver disease. Hepatology 2016;64:1057–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rolas L, Boussif A, Weiss E, et al. Nadph oxidase depletion in neutrophils from patients with cirrhosis and restoration via Toll-like receptor 7/8 activation. Gut 2018;67:1505–16. [DOI] [PubMed] [Google Scholar]
- 138.Albano E, Stickel F. Targeting Toll-like receptor 7/8 improves host anti-infective response in alcoholic cirrhosis. Gut 2018;67:1749–50. [DOI] [PubMed] [Google Scholar]
- 139.Satishchandran A, Ambade A, Rao S, et al. MicroRNA 122, Regulated by GRLH2, Protects Livers of Mice and Patients From Ethanol-Induced Liver Disease. Gastroenterology 2018;154:e237:238–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bala S, Csak T, Saha B, et al. The pro-inflammatory effects of miR-155 promote liver fibrosis and alcohol-induced steatohepatitis. J Hepatol 2016;64:1378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mueller S, Peccerella T, Qin H, et al. Carcinogenic etheno DNA adducts in alcoholic liver disease: correlation with cytochrome P-4502E1 and fibrosis. Alcohol Clin Exp Res 2018;42:252–9. [DOI] [PubMed] [Google Scholar]
- 142.Spahr L, Lambert J-F, Rubbia-Brandt L, et al. Granulocyte-Colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatology 2008;48:221–9. [DOI] [PubMed] [Google Scholar]
- 143.Singh V, Sharma AK, Narasimhan RL, et al. Granulocyte colony-stimulating factor in severe alcoholic hepatitis: a randomized pilot study. Am J Gastroenterol 2014;109:1417–23. [DOI] [PubMed] [Google Scholar]
- 144.Morgan TR. Is granulocyte colony stimulating factor a new treatment for alcoholic hepatitis? Clin Gastroenterol Hepatol 2018;16:1564–5. [DOI] [PubMed] [Google Scholar]
- 145.Kong X, Feng D, Mathews S, et al. Hepatoprotective and anti-fibrotic functions of interleukin-22: therapeutic potential for the treatment of alcoholic liver disease. J Gastroenterol Hepatol 2013;28:56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Philips CA, Pande A, Shasthry SM, et al. Healthy donor fecal microbiota transplantation in Steroid-Ineligible severe alcoholic hepatitis: a pilot study. Clin Gastroenterol Hepatol 2017;15:600–2. [DOI] [PubMed] [Google Scholar]
- 147.Bajaj JS, Kassam Z, Fagan A, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology 2017;66:1727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Duan Y, Llorente C, Lang S, et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 2019;575:505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Carr RM, Reid AE. Fxr agonists as therapeutic agents for non-alcoholic fatty liver disease. Curr Atheroscler Rep 2015;17:500. [DOI] [PubMed] [Google Scholar]
- 150.Conflicts of Interest Coalition.Statement of Concern at. Available: http://info.babymilkaction.org/sites/info.babymilkaction.org/files/COIC150_0.pdf
- 151.Babor TF, Robaina K, Jernigan D. The influence of industry actions on the availability of alcoholic beverages in the African region. Addiction 2015;110:561–71. [DOI] [PubMed] [Google Scholar]
- 152.Alcohol Gornall J. and public health. under the influence. BMJ 2014;348. [DOI] [PubMed] [Google Scholar]
- 153.Gornall J, Cup W. Festival of football or alcohol? BMJ 2014;2014. [DOI] [PubMed] [Google Scholar]
- 154.Miller D, Harkins C, Schlögl M, et al. Impact of market forces on addictive substances and behaviours. The web of influence of addictive industries. OUP, 2018.
- 155.Snowdon C Six reasons to reject minimum alcohol pricing. 28 November 2012. Blog, ADAM Smith Institute website (accessed May 2019). [Google Scholar]
- 156.World Health Organization. Global strategy to reduce the harmful use of alcohol. Geneva, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.World Health Organization. International classification of diseases 11th revision (ICD-11). available at. Available: https://icd.who.int/browse11/l-m/en [Accessed Apr 2019].
- 158.UN General Assembly. Political Declaration of the high-level meeting of the general assembly on the prevention and control of non-communicable diseases. New York: United nations, 2011. http://www.un.org/en/ga/ncdmeeting2011/ [Google Scholar]
- 159.World Health Organization. Global action plan for the prevention Nd control of NCDS 2013–2020. Geneva, 2013. Available: https://www.who.int/nmh/events/ncd_action_plan/en/ [Accesse April 2019]. [Google Scholar]
- 160.World Health Organization. Tackling NCDs: ‘best buys’ and other recommended interventions for the prevention and control of non-communicable diseases. Geneva, 2017. Available: http://www.who.int/iris/handle/10665/259232 [Google Scholar]
- 161.WHO, Geneva: mhGAP intervention guide for mental, neurological and substance use disorders in non-specialized health settings: mental health gap action programme (mhGAP) - version 2.0., 2016. Available: https://www.who.int/mental_health/mhgap/mhGAP_intervention_guide_02/en/ [PubMed] [Google Scholar]
- 162.Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder. JAMA 2018;320:815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Carvalho AF, Heilig M, Perez A, Ch P, et al. Alcohol use disorders. The Lancet 2019;394:781–92. [DOI] [PubMed] [Google Scholar]
- 164.World Health Organization. Safer: a world free from alcohol related harms. Geneva, 2018. Available: https://www.who.int/substance_abuse/safer/msb_safer_brochure.pdf?ua=1 [Google Scholar]


