Abstract
Background:
The composition of bacteria within the vaginal microbiome has garnered a lot of recent attention and has been associated with reproductive health and disease. Despite the common occurrence of yeast (primarily Candida) within the vaginal microbiome, there is still an incomplete picture of relationships between yeast and bacteria (especially lactobacilli), as well as how such associations are governed. Such relationships could be important to a more holistic understanding of the vaginal microbiome and its connection to reproductive health.
Objective:
To perform molecular characterization of clinical specimens to define associations between vaginal bacteria (especially Lactobacillus species) and Candida colonization. In vitro studies were conducted to test the two most common dominant Lactobacillus species (Lactobacillus crispatus and Lactobacillus iners) in their ability to inhibit Candida growth and to examine the basis for such inhibition.
Study Design:
A nested cross-sectional study of reproductive age women from the Contraceptive CHOICE Project was conducted. Vaginal swabs from 299 women were selected to balance race and BV status, resulting in similar representation of black and white women in each of the three Nugent score categories [normal (0–3), intermediate (4–6), and bacterial vaginosis (7–10)]. Sequencing of the 16S ribosomal gene (V4 region) was used to determine the dominant Lactobacillus species present (primarily L. iners and L. crispatus), defined as >50% of the community. Subjects without dominance by a single Lactobacillus species were classified as Diverse. A Candida-specific qPCR targeting the internally transcribed spacer 1 (ITS1) was validated using vaginal samples collected from a second cohort of women and used to assess Candida colonization. 255 nonpregnant women with sufficient bacterial biomass for analysis were included in the final analysis. Generalized linear models were employed to evaluate associations between Lactobacillus dominance, sociodemographic and risk characteristics and vaginal Candida colonization. In separate in vitro studies, the potential of cell-free supernatants from L. crispatus and L. iners cultures to inhibit Candida growth was evaluated.
Results:
Forty-two women (16%) were vaginally colonized with Candida. Microbiomes characterized as Diverse (38%), L. iners-dominant (39%), and L. crispatus-dominant (20%) were the most common. The microbiome, race and Candida colonization co-varied with a higher prevalence of Candida among black women and L. iners-dominant communities compared to white women and L. crispatus-dominant communities. L. iners-dominant communities were more likely to harbor Candida than L. crispatus-dominant communities (OR = 2.85, 95% CI: 1.03 to 7.21; Fisher’s Exact, p = 0.048). In vitro, L. crispatus produced greater concentrations of lactic acid and exhibited significantly more pH-dependent growth inhibition of C. albicans, suggesting a potential mechanism for the clinical observations.
Conclusion:
In nonpregnant women, L. iners-dominant communities were significantly more likely to harbor Candida than L. crispatus-dominant communities, suggesting that Lactobacillus species have different relationships with Candida. In vitro experiments indicate that L. crispatus may impede Candida colonization more effectively than L. iners through a greater production of lactic acid.
Keywords: vaginal microbiome, Candida, Lactobacillus, race, pH
Introduction
The human vagina is a dynamic ecosystem that hosts microbes from diverse taxa. Profiling 16S ribosomal gene diversity has expanded our understanding of the vaginal microbiome, allowing exploration of links between bacterial composition and reproductive outcomes. Vaginal microbial communities can be clustered into five common community types.1 Four of these are dominated by a single Lactobacillus species: L. crispatus, L. gasseri, L. iners, or L. jensenii. The final community type (often described as “Diverse”) has few lactobacilli and exhibits greater representation of anaerobic bacteria such as Gardnerella vaginalis, Atopobium vaginae and Prevotella spp.1 The prevalence of these community types varies with race and ethnicity; black and Hispanic women more frequently host L. iners-dominant and Diverse communities than white women, who more frequently host L. crispatus-dominant communities.1,2 Diverse communities often harbor bacterial taxa that are abundant during bacterial vaginosis (BV), a condition diagnosed by clinical (Amsel) criteria or by Nugent scoring,3 a 0–10 scale generated by scoring bacterial morphotypes in Gram-stained vaginal smears (0–3, normal; 4–6, intermediate; 7–10, BV). BV is associated with increased risks of sexually transmitted infections and adverse reproductive outcomes.4
Candida (most commonly C. albicans) is a common member of the vaginal microbiome (found in ~30% of women5). The prevalence of non-albicans species among women with vaginal Candida varies, ranging from ~10–30%.5–9 Vaginal Candida colonization may lead to vulvovaginal candidiasis (VVC), characterized by an aggressive host response to Candida overgrowth.10 However, Candida colonization is frequently asymptomatic and not all women colonized with Candida go on to experience VVC.5 Vaginal Candida colonization has also been linked to other adverse reproductive outcomes.8,11–16
Several prior studies have examined relationships between vaginal bacteria and Candida. A few of these studies implicate an abundance of lactobacilli with a greater likelihood of harboring Candida.5,6,17 Other studies suggest there may be co-occurrence of Candida with some BV-associated bacteria,18–21 and specifically that Candida may be correlated with the simultaneous presence of both lactobacilli and BV-associated bacteria.19–21 An important limitation is that prior studies, whether using molecular or culture-based techniques, have not distinguished between lactobacilli at the species level. This is a significant limitation, which if resolved, may shed light on why some women are so prone to Candida colonization and candidiasis.
Taken together with the prior studies above, several considerations led us to hypothesize that L. iners in particular may support the co-occurrence of Candida, especially compared to L. crispatus. L. iners is unique among the lactobacilli in being prevalent within less stable Nugent intermediate and BV communities1,22,23 and in producing a cytolytic toxin.24,25 Furthermore, L. iners dominance has been associated with other negative health outcomes such as increased risks of Chlamydia trachomatis infection,26 incident BV,27 defects in vaginal mucus that compromise antiviral barrier function,28 and cytokine signatures linked with HIV risk.29 We performed two types of studies to test our hypothesis that L. iners may preferentially support Candida colonization 1) a molecular evaluation of clinical specimens, and 2) in vitro growth inhibition studies.
Methods
Study design:
This nested cross-sectional study uses samples and questionnaire data collected by the Contraceptive CHOICE Project (CHOICE)30 according to Washington University IRB-approved protocol 201108155. In total, 9256 women from the St. Louis-area gave informed consent from August 2007 through September 2011. For this nested study, 299 women enrolled from 08/2008–06/2009 were selected based on power calculations made from preliminary data. Women enrolled in the CHOICE study were between the ages of 14 and 45, reported sexual activity in the past six months or anticipated sexual activity with a male partner and were seeking contraception. Women with a history of tubal ligation or hysterectomy were excluded. All women underwent a pregnancy test. Vaginal swab specimens were self-collected in the vast majority of cases, then stored at −80℃ until analysis. Of the swabs used in the final analysis, one was collected by a clinician and the collection method was missing for five samples.
Women who completed a baseline survey (including Sociodemographic data) and had a vaginal swab available were eligible for inclusion. Samples from all participants underwent Nugent scoring to determine BV status.3,31,32 Unfortunately, vaginal pH and data regarding menstrual cycle and recent sexual activity was only available for a subset of women and were inadequate for analysis. Overall, the distribution of self-reported race/ethnicity of women in the CHOICE study were representative of the St. Louis region; few women reported a race other than “black or African-American” (hereafter referred to as “black”) or “white.” Due to small numbers of other groups, only women who reported “black” or “white” race were eligible for inclusion in this sub-study.
Composition of the vaginal microbiota has been previously associated with race.1 To test whether Candida was associated with vaginal niches occupied by particular bacterial communities, we sought a strategy to avoid inadequate representation of less common community types in the different demographic groups so that we would be powered to ask whether Candida is associated with particular microbial patterns. We used frequency matching to similarly represent black and white women in each of the three Nugent categories. We used a normal:intermediate:BV ratio of 2:2:1 to ensure that we had samples represented across the Nugent spectrum, while balancing the practical reality that relatively few BV specimens were available from white women. Of the 299 subjects selected, 35 were pregnant at the time of swab collection and excluded from final analysis. Additionally, 9 specimens were excluded due to low bacterial biomass. See Supplemental Methods.
Microbiome analysis and Candida colonization status:
DNA was extracted from eluted vaginal swabs and 16S ribosomal profiling of the V4 hypervariable region was performed as described in the Supplemental Methods. The microbiome was classified based on the dominant Lactobacillus species present, defined as 50% relative abundance or greater and referred to as, “L. crispatus-, L. iners-, L. gasseri-, or L. jensenii-dominant” microbiomes. Communities without a single Lactobacillus species reaching 50% were referred to as Diverse communities. A pan-Candida qRT-PCR33 that amplifies the internally transcribed spacer 1 (ITS1) was used to determine Candida colonization status using isolated DNA as template. Prior to analysis we validated this assay among vaginal specimens collected from a second cohort of women enrolled at a different site. See Supplemental Methods for details.
Candida growth inhibition:
Candida strains were grown in yeast extract-peptone-dextrose (YPD) media. C. albicans strain SC5314 was obtained from the American Type Culture Collection. Vaginal strains of Candida (C. albicans: BAT8133, BAT8135, BAT8143, BAT8152, BAT8154, BAT3353A; C. glabrata: BAT8139, BAT3353B) were isolated from women as described in the Supplemental Methods. L. crispatus (MV-1A-US, JV-V01, MV-3A-US, 125–2-CHN) and L. iners (UP II 143-D, Lactin V09V1-C, LEAF 2032-Ad, LEAF 3008-A) strains were obtained from BEI resources and cultured in De Man, Rogosa and Sharpe (MRS) media for 48 hours to make cell free supernatants (CFS). All Candida growth inhibition experiments were conducted in 96-well plates. Each well contained a 1:1 ratio of CFS and YPD inoculated with ~106 C. albicans colony-forming units (CFU)/mL. YPD was buffered with 300 mM sodium bicarbonate and 300 mM HEPES sodium salt for neutralization assays. For lactic acid growth inhibition assays, fresh MRS was supplemented with racemic lactic acid. A micro pH electrode was used to measure pH of each mixture and lactate was measured with a colorimetric assay. Protonated lactic acid concentrations were calculated using lactate molarity and pH using the Henderson-Hasselbalch equation (pKa = 3.9). See Supplemental Methods for more details about Candida growth inhibition experiments.
Statistical analysis:
Statistical analyses and data representation were completed in R (v3.5.1) and Prism (v7). Fisher’s Exact Tests (Fisher) were used to assess for associations between cohort characteristics and race, with odds ratios (OR) determined by a conditional maximum likelihood estimate. Unless otherwise noted, we used an extension of the generalized linear model (GLM) method that included race as a potentially confounding covariate to test for associations between cohort characteristics and Candida colonization status, using the exponent of the coefficient from the logistic regression to calculate ORs. Note that because Candida colonization incidence is >10% the odds ratios may not be an accurate approximation of the relative risk; see34 for conversion between the two.
We used type-II analysis of variance (ANOVA-II) with Wald test and Tukey’s Honestly Significant Different Test (Tukey) to evaluate significance in these models. In instances where multiple statistical tests were performed, we relied on GLM accounting for race. Mann-Whitney tests were used to test for associations with Candida abundance and effect size (r) was calculated from the Z value. Statistical tests for in vitro experiments included one-way ANOVA with Tukey’s correction for multiple comparisons and Mann-Whitney tests as appropriate. Regardless of the statistical method used, P-values < 0.05 were considered significant.
Results
Description of the clinical cohort:
Two-hundred fifty-five non-pregnant women of reproductive age were included in our analysis. In this cohort, 53% of women identified as “white” and 47% identified as “black”. Forty-four (17%) women had BV, while 109 (43%) and 102 (40%) had intermediate and normal vaginal flora respectively. About half of the women (54%) reported using public assistance or having trouble meeting daily needs and were classified as having low socioeconomic status. Body mass index (BMI) was calculated and categorized using standard methods and definitions. Most women (64.3%) reported at least one prior pregnancy. Seventy-two women (28.2%) reported vaginal douching in the last 180 days. Race was found to be associated with socioeconomic status (p < 0.0001), BMI (p = 0.003), gravidity (p < 0.0001) and vaginal douching (p < 0.0001). A summary of demographic data and cohort characteristics by race is presented in Supplemental Table 1.
Forty-two (16%) women were vaginally colonized with Candida. Of these, most (90%) were colonized by C. albicans. C. glabrata was less common (~10%). Sequencing of the vaginal microbiome revealed that fifty-two women (20%) had L. crispatus-dominant microbiomes, 99 (39%) had L. iners-dominant microbiomes and 98 (38%) had microbiomes that were not dominated by a single Lactobacillus species (Diverse). We were not powered to test associations between Candida and microbiomes dominated by Lactobacillus jensenii or gasseri since few women (n=6) exhibited these microbiomes. Black women were more likely than white women to have L. iners-dominant communities (46.7% vs 31.9% Fisher’s Exact; OR = 1.87, 95% CI: 1.10 to 3.14, p = 0.020) and less likely to have L. crispatus-dominant communities (11.9% vs. 22.1% Fisher’s Exact; OR = 0.380, 95% CI: 0.185 to 0.747, p = 0.003).
Associations between Candida and cohort characteristics:
Forty-two (16%) women were vaginally colonized with Candida. Of these, most (90%) were colonized by C. albicans. C. glabrata colonization was less common (~10%). Table 1 contains a summary of Candida status by sociodemographic and other cohort characteristics. Only race was significantly correlated with vaginal Candida; black women were more likely to be colonized compared to white women (OR =2.05, 95% CI: 1.03 to 4.25, Fisher’s Exact, p = 0.042). Based on these findings, race was considered to be a potential confounder and incorporated into subsequent analyses using generalized linear models (GLM) to evaluate factors associated with Candida colonization.
Table 1:
Characteristics of subjects with vaginal Candida compared with those without vaginal Candida.
| Characteristics | Total Cohort | Candida Positive | Candida Negative | P-value |
|---|---|---|---|---|
| Total Number of Subjects | 255 | 42 (16.5) | 213 (83.5) | |
| Age | 0.811 | |||
| < 20 | 28 (11.0) | 6 (14.3) | 22 (10.3) | |
| 20 to 29 | 178 (69.8) | 29 (69.0) | 149 (70.0) | |
| 30 to 39 | 44 (17.3) | 7 (16.7) | 37 (17.4) | |
| 40 + | 5 (2.0) | 0 (0.0) | 5 (2.3) | |
| Race | 0.042 | |||
| Black | 120 (47.1) | 26 (61.9) | 94 (44.1) | |
| White | 135 (52.9) | 16 (38.1) | 119 (55.9) | |
| Nugent-defined Vaginal Flora | 0.833 | |||
| Normal | 102 (40.0) | 15 (35.7) | 87 (40.8) | |
| Intermediate | 109 (42.7) | 19 (45.2) | 90 (42.3) | |
| BV | 44 (17.3) | 8 (19.0) | 36 (16.9) | |
| Socioeconomic Status (SES) | 1 | |||
| Low SES | 138 (54.1) | 23 (54.8) | 115 (54.0) | |
| Not Low SES | 117 (45.9) | 19 (45.2) | 98 (46.0) | |
| Body Mass Index (kg/m2) | 0.127 | |||
| Underweight (< 18.5) | 15 (5.9) | 3 (7.1) | 12 (5.6) | |
| Normal Weight (18.5 – 24.9) | 103 (40.4) | 19 (45.2) | 84 (39.4) | |
| Overweight (25 – 30) | 48 (18.8) | 11 (26.2) | 37 (17.4) | |
| Obese (> 30) | 78 (30.6) | 7 (16.7) | 71 (33.3) | |
| Not Documented | 11 (4.3) | 2 (4.8) | 9 (4.2) | |
| Current Birth Control Method | 0.320 | |||
| Estrogen + Progestina | 72 (28.2) | 16 (38.1) | 56 (26.3) | |
| Progestinb | 12 (4.7) | 1(2.4) | 11 (5.2) | |
| Non-Hormonalc | 171 (67.1) | 25 (59.5) | 146 (68.5) | |
| Vaginal Douching in Last 180 Days | 0.323 | |||
| Yes | 72 (28.2) | 8 (19.0) | 64 (30.0) | |
| No | 182 (71.4) | 34 (81.0) | 148 (69.5) | |
| Don’t Know | 1 (0.4) | 0 (0.0) | 1 (0.5) | |
| Gravidity | 0.160 | |||
| None | 91 (35.7) | 15 (35.7) | 76 (35.7) | |
| 1 | 58 (22.7) | 6 (14.3) | 52 (24.4) | |
| 2 | 47 (18.4) | 6 (14.3) | 41 (19.2) | |
| 3+ | 59 (23.1) | 15 (35.7) | 44 (20.7) | |
| Community Type | 0.113 | |||
| L. crispatus-dominant | 52 (20.4) | 5 (11.9) | 47 (22.1) | |
| L. iners-dominant | 99 (38.8) | 23 (54.8) | 76 (35.7) | |
| L. jensenii-dominant | 3 (1.2) | 1 (2.4) | 2 (0.9) | |
| L. gasseri-dominant | 3 (1.2) | 0 (0.0) | 3 (1.4) | |
| Diverse | 98 (38.4) | 13 (31.0) | 85 (39.9) |
Values are n (%). Fisher’s Exact Tests were used to determine p-values for each set of variables without adjusting for race. Note that p-values given in the text use GLM (accounting for race as a potential confounder).
Women who reported the oral contraceptive pill or the birth control ring;
Women who reported the levonorgestrel-containing intrauterine device or depot medroxyprogesterone acetate;
Women who reported condoms, rhythm/natural family planning, abstinence, withdrawal or nothing.
Associations between Candida and cohort characteristics
Candida colonization rates did not differ based on Nugent-defined BV status (GLM; ANOVA-II, p = 0.897). We did not find any association between a woman’s socioeconomic status and vaginal Candida colonization. Candida colonization did not differ significantly among underweight (20% Candida), normal weight (18%) and overweight (23%) women. However, obese women were less likely to be colonized compared to non-obese women (GLM; OR = 0.322, 95% CI: 0.123 to 0.744; Tukey’s HSD, p = 0.013, see Supplement for comment). Women reporting current use of hormonal contraceptives containing estrogen and progestin were Candida-colonized at higher rates than women reporting non-hormonal methods, although this did not reach statistical significance (GLM; OR = 1.77, 95% CI: 0.858 to 3.58; Tukey’s HSD, p = 0.237, see Supplement for details). Women who reported vaginal douching in the last 180 days were less likely to be Candida positive compared to women who reported no vaginal douching (GLM; OR = 0.364, 95% CI: 0.143 to 0.838; Tukey’s HSD, p = 0.047).
Relationships between Candida colonization and the vaginal microbiome:
Next, we investigated relationships between Candida colonization and dominant members of the vaginal microbiome based on 16S ribosomal gene profiling. Candida prevalence did not differ between Lactobacillus dominated (50% or greater Lactobacillus) and non-Lactobacillus dominated microbiomes (GLM; ANOVA-II, p = 0.327). Although the absolute abundance of Candida as measured by qPCR did not differ within L. iners-dominant communities compared to other community types (Mann-Whitney, r = 0.046, p = 0.617), L. iners-dominant communities were more likely to harbor Candida than non-L. iners-dominant communities (GLM; OR = 2.00, 95% CI: 1.02 to 3.98; Tukey’s HSD, p = 0.045; see supplemental Table 2). Further analysis specifically showed that L. iners-dominant communities were more likely to be colonized than L. crispatus-dominant communities (OR = 2.85, 95% CI: 1.03 to 7.21; Fisher’s Exact, p = 0.048). Among Candida positive women, higher levels of Candida (by qRT-PCR) were observed among black women compared to white women, although not statistically significant (Mann-Whitney test, r = 0.173, p = 0.131).
In vitro studies: inhibition of Candida growth by lactobacilli:
Both L. crispatus and lactic acid have been shown to thwart the growth of C. albicans.35–37 Next, we compared the inhibitory potential of L. crispatus and L. iners on Candida growth in vitro. C. albicans was cultured together with cell free supernatants (CFS) from L. crispatus and L. iners (8 strains total), followed by Candida CFU enumeration. Compared to L. iners CFS, L. crispatus CFS resulted in lower pH (pH = 4.0 vs. pH = 4.6, p < 0.0001) and correspondingly higher levels of protonated lactic acid in CFS-YPD (55 mM vs. 11 mM, p < 0.0001) (Figure 2). Buffering CFS-YPD to a neutral pH reduced levels of protonated lactic acid to below appreciable levels, ablated Candida growth inhibition, and eliminated the difference in C. albicans growth observed between L. crispatus and L. iners (Figure 2). Further, lactic acid was sufficient to inhibit Candida growth. In particular, significantly more growth inhibition was observed at 49 mM protonated lactic acid compared to 11 mM, levels comparable to the L. crispatus and L. iners CFS-YPD respectively. Similar findings were seen using vaginal isolates of C. albicans. In contrast, C. glabrata exhibited only modest growth inhibition (Figure 2). Together, these data suggest that lactic acid is both necessary and sufficient for growth inhibition of C. albicans in vitro.
Figure 2: In vitro inhibition of Candida by Lactobacillus CFS and lactic acid.
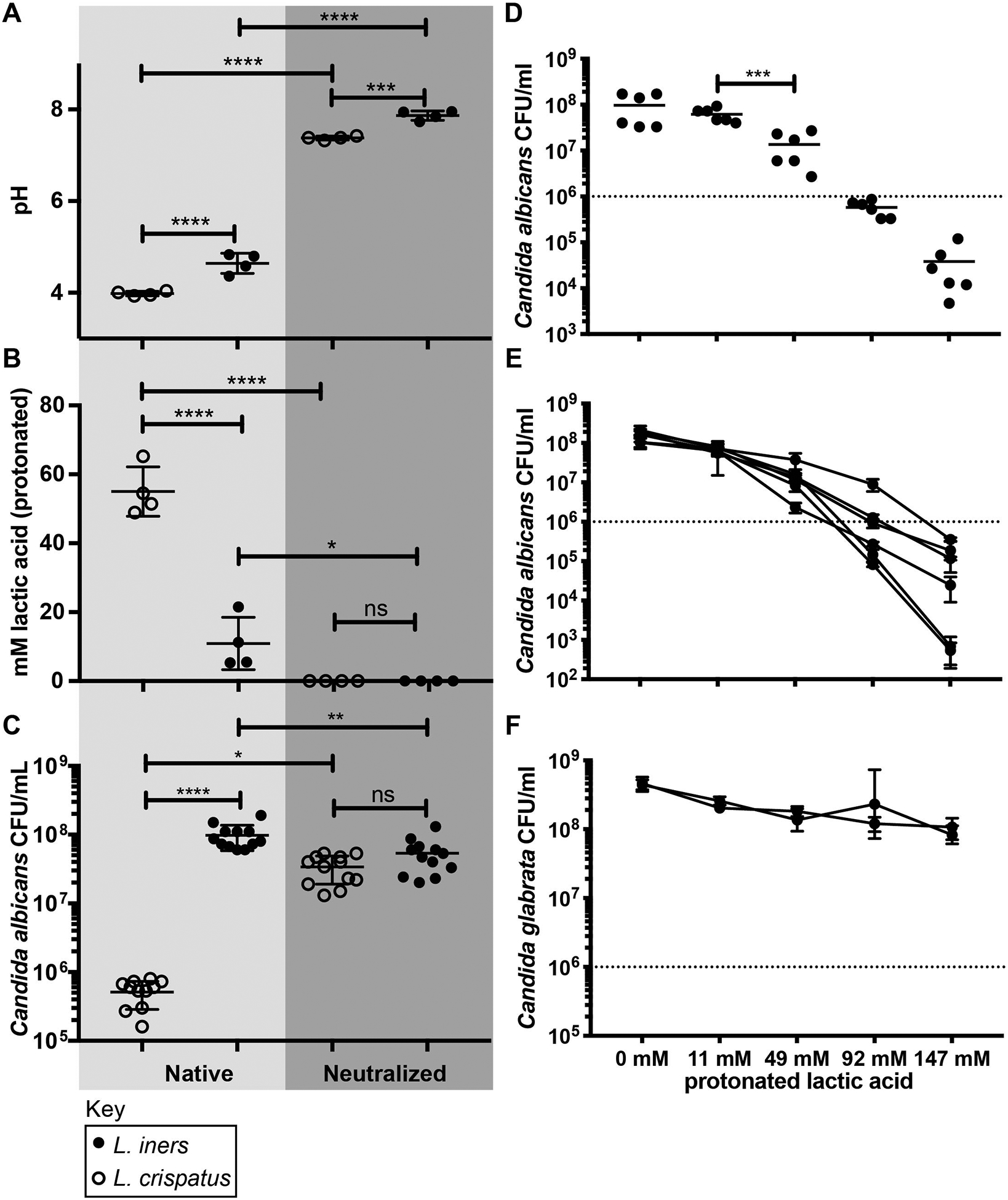
A-B, Characterization of Candida growth medium supplemented with Lactobacillus CFS (YPD-CFS) in native and buffered states from four L. crispatus and four L. iners strains, prior to Candida inoculation. A, pH of YPD-CFS; B, Concentration of protonated lactic acid in YPD-CFS; C, Growth inhibition of Candida laboratory strain SC5314, showing three technical replicates for each Lactobacillus YPD-CFS. Analysis by one-way ANOVA with Tukey’s correction for multiple comparisons. D-F, Characterization of the inhibitory effect of lactic acid supplemented medium on Candida growth. Three technical replicates from two biological experiments are shown. D, Growth inhibition of SC5314 by lactic acid showing Mann-Whitney test comparison of 11 mM to 49 mM protonated lactic acid; E, Lactic acid growth inhibition of 6 vaginal C. albicans isolates; F, Lactic acid growth inhibition of 2 vaginal C. glabrata isolates. Data points in panel D reflect 6 replicates from two experiments for each condition. Error bars in E-F show the standard deviation from the mean of three replicates for each isolate. Approximate starting inoculum for growth assays is indicated by a dashed line. Statistical significance: ns (not significant), *p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.0001.
Comment
Principal Findings:
We demonstrate that Candida colonization is associated with characteristics of the vaginal microbiome (dominance of L. iners compared to L. crispatus). Results in clinical specimens are consistent with in vitro data, which show that L. crispatus produces a pH-dependent factor that inhibits C. albicans growth more effectively compared to secreted factors of L. iners grown under the same conditions.
Results:
As a relatively common vaginal microbial community member, Candida may influence reproductive health. Previous studies suggested vaginal Lactobacillus colonization as a risk factor for Candida colonization or VVC,5,6,17 but seem inconsistent with other reports of Candida-bacteria associations.18–21 Here we provide more taxonomic resolution, showing that that not all Lactobacillus-dominant communities are equally associated with Candida colonization.
Clinical Implications:
Clinicians often group all lactobacilli together. This study adds to the growing body of evidence suggesting that L. iners-dominant communities are more permissive to vaginal colonization with potential pathogens, including Candida.
Research Implications:
Of interest, black race was associated with obesity and vaginal douching as in prior studies. But surprisingly, the correlation between Candida and black race cannot be accounted for by obesity or douching because obese women and those who douche were actually less likely to be colonized with Candida (OR = 0.322 and 0.364 respectively). The literature contains inconsistent reports regarding the role of Lactobacillus colonization as a risk factor for Candida colonization or VVC.5,6,17,18–21 We show that that not all Lactobacillus-dominant communities are equally associated with Candida. In vitro data provide one possible explanation, showing that L. iners strains do not produce the same magnitude of lactic acid compared to L. crispatus strains. An alternative, albeit not mutually exclusive explanation, is that vaginal Candida colonization may shift the microbiome to favor L. iners.
Interestingly, we observed similar rates of Candida colonization in L. crispatus-dominant and Diverse communities. With fewer lactic acid producing bacteria present, the vaginal pH of women with Diverse microbiome is less acidic.1 These findings indicate that Diverse communities resist Candida by lactic acid-independent mechanisms.
Additional studies are needed to evaluate potential mechanisms governing these relationships and apply these findings in clinical settings.
Strengths and Limitations:
Key strengths of our study design were the validation of a Candida-specific qPCR assay33 for laboratory testing for Candida colonization, offering flexibility in settings where archived frozen vaginal swabs are more practical. We acknowledge that the specimens selected for this study are not a naturalistic representation of vaginal microbiomes. Rather, the frequency matching of black and white women across the Nugent spectrum is a strength that enabled power to test associations between yeast and bacteria in different racial groups. Limitations include: 1) the sample size and number of Candida-positive women were relatively small, limiting power to model multiple potential confounders, 2) this cohort may not be representative of the U.S. population, 3) clinical data were not available to examine the relationship between Candida colonization and VVC, and 4) our in vitro findings may not be representative of in vivo relationships.
Conclusion:
These data suggest that L. iners-dominant vaginal communities may support the co-occurrence of Candida.
Supplementary Material
Figure 1: Heatmap of all samples in the cohort clustered by community type.
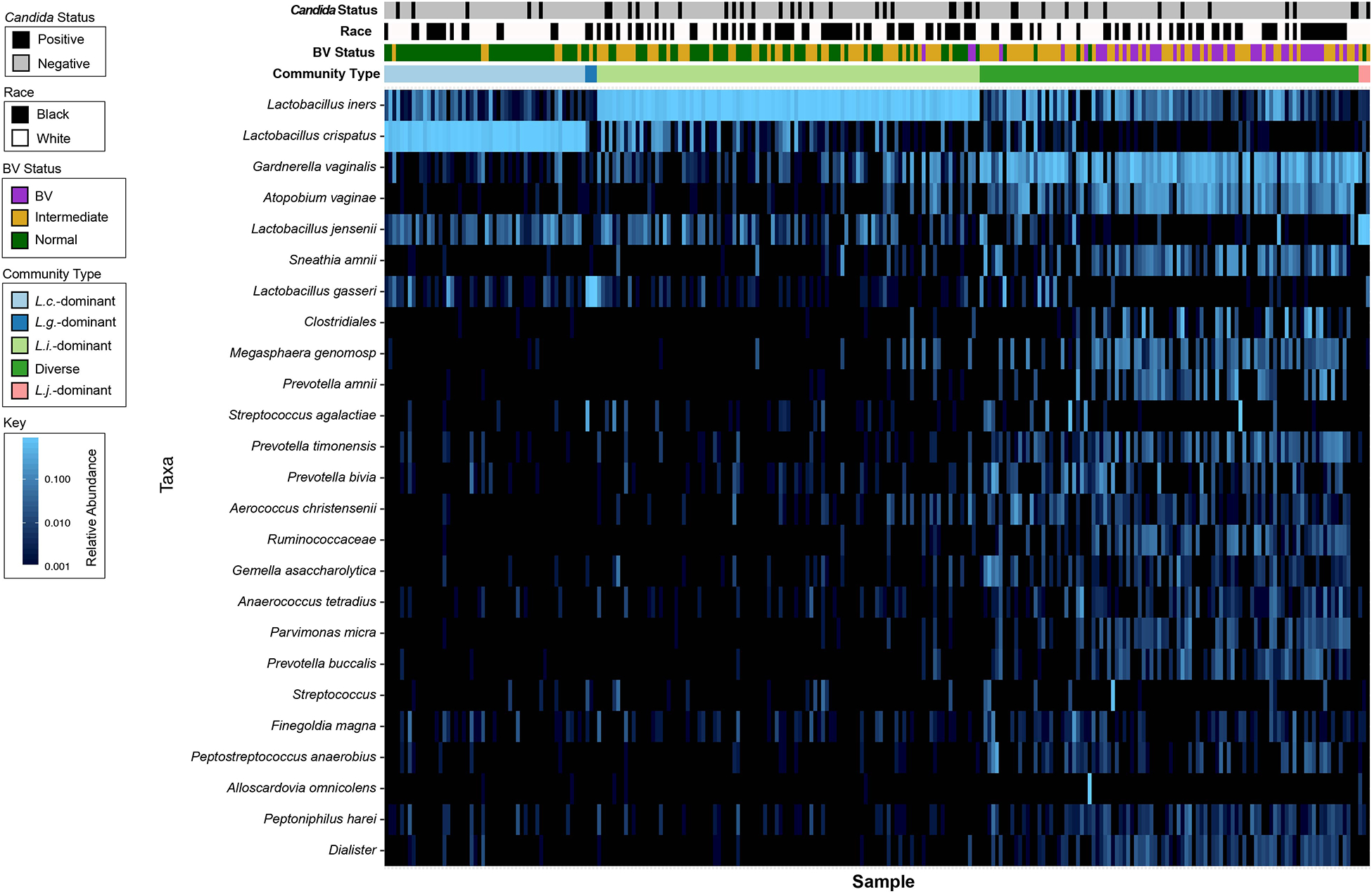
Heat map of samples clustered by community type showing the top 25 taxa observed across the cohort. The bars above the heatmap indicate community type, BV status by Nugent score, race and Candida status. In the heat map, light blue indicates the highest abundance, darker blues indicate lower abundance and black indicates very low abundance or not present. Black race (p = 0.037) and L. iners-dominant communities (p = 0.045) were associated with Candida colonization.
AJOG at a Glance:
The purpose of the study was to characterize the relationship between the composition of the vaginal microbiome and Candida colonization among non-pregnant women.
Women with Lactobacillus iners-dominant microbiomes were more likely to harbor Candida than women with Lactobacillus crispatus-dominant microbiomes. In vitro data suggests higher production of lactic acid by Lactobacillus crispatus compared to Lactobacillus iners may contribute to differential anti-Candida activity. Neutralization of pH eliminated the anti-Candida activity secreted by lactobacilli.
Consideration of Candida as part of the vaginal microbiome may have utility for understanding different relationships between vaginal microbiome and adverse outcomes.
Acknowledgements:
We thank the Contraceptive CHOICE Project support staff and Jennifer Reed (née Bick, Washington University) for Nugent scoring. We appreciate Dr. Sharon Hillier for verifying Nugent scoring. We also thank Denise Spear WHNP-BC, Valerie Higginbotham WHNP-BC (St. Louis County Department of Public Health) and Courtney Amegashie (Washington University, NIH funding) for collection and swab processing and the St. Louis County Department of Public Health for facilitating sample collection used to validate the Candida qPCR assay. We thank Andrew Kau for advice (Washington University, in part NIH funding) on microbiome profiling and Deborah Frank (Washington University) for editorial input. Finally, we appreciate those who provided vaginal samples.
Source of funding: Funding for the Contraceptive CHOICE Project was provided by an anonymous foundation. This study was supported in part by a March of Dimes grant (Prematurity Research Center) and a pilot grant from the same (ALL). Funding was provided by a pilot grant from the Center for Women’s Infectious Disease Research (JCF) and by the NIH: R01 AI114635 (ALL and WGL), R01 AI127554 (WGL), and by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the NIH: F30HD094435 (BAT). The sponsors had no role in the study design, the collection, analysis or interpretation of data, or the decision to submit the article for publication. The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest: Dr. Peipert serves on advisory boards for Bayer and CooperSurgical, and has received research support from Merck, Bayer, and CooperSurgical/Teva. Although not directly related to the work, A. L. L. received personal (consulting) fees from companies involved in making diagnostics or treatments for bacterial vaginosis (Talis Biomedical Corporation, Tennor Therapeutics, and Toltec Pharmaceuticals) and both Dr. AL and WG Lewis have performed research sponsored by Metis Therapeutics and Metrodora Therapeutics. Remaining authors report no conflict of interest.
Paper presentation information: This manuscript was presented in part at the 14th ASM Conference on Candida and Candidiasis in Providence RI, April 15–19th 2018 and the American Physician Scientist Association 15th Annual Meeting in Chicago IL, April 5–7th 2019.
Condensation: Lactobacillus iners-dominant vaginal microbiomes are more likely to harbor vaginal Candida than Lactobacillus crispatus-dominant vaginal microbiomes.
References:
- 1.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4680–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fettweis JM, Brooks JP, Serrano MG, et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology. 2014;160(Pt 10):2272–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anahtar MN, Gootenberg DB, Mitchell CM, Kwon DS. Cervicovaginal Microbiota and Reproductive Health: The Virtue of Simplicity. Cell Host Microbe. 2018;23(2):159–168. [DOI] [PubMed] [Google Scholar]
- 5.Beigi RH, Meyn LA, Moore DM, Krohn MA, Hillier SL. Vaginal yeast colonization in nonpregnant women: a longitudinal study. Obstet Gynecol. 2004;104(5 Pt 1):926–930. [DOI] [PubMed] [Google Scholar]
- 6.Cotch MF, Hillier SL, Gibbs RS, Eschenbach DA. Epidemiology and outcomes associated with moderate to heavy Candida colonization during pregnancy. Vaginal Infections and Prematurity Study Group. Am J Obstet Gynecol. 1998;178(2):374–380. [DOI] [PubMed] [Google Scholar]
- 7.Brandolt TM, Klafke GB, Goncalves CV, et al. Prevalence of Candida spp. in cervical-vaginal samples and the in vitro susceptibility of isolates. Braz J Microbiol. 2017;48(1):145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Payne MS, Ireland DJ, Watts R, et al. Ureaplasma parvum genotype, combined vaginal colonisation with Candida albicans, and spontaneous preterm birth in an Australian cohort of pregnant women. BMC Pregnancy Childbirth. 2016;16(1):312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Payne MS, Cullinane M, Garland SM, et al. Detection of Candida spp. in the vagina of a cohort of nulliparous pregnant women by culture and molecular methods: Is there an association between maternal vaginal and infant oral colonisation? Aust N Z J Obstet Gynaecol. 2016;56(2):179–184. [DOI] [PubMed] [Google Scholar]
- 10.Fidel PL Jr., Barousse M, Espinosa T, et al. An intravaginal live Candida challenge in humans leads to new hypotheses for the immunopathogenesis of vulvovaginal candidiasis. Infect Immun. 2004;72(5):2939–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maki Y, Fujisaki M, Sato Y, Sameshima H. Candida Chorioamnionitis Leads to Preterm Birth and Adverse Fetal-Neonatal Outcome. Infect Dis Obstet Gynecol. 2017;2017:9060138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.To V, Gurberg J, Krishnamurthy S. Tubo-Ovarian Abscess Caused by Candida albicans in an Obese Patient. J Obstet Gynaecol Can. 2015;37(5):426–429. [DOI] [PubMed] [Google Scholar]
- 13.Menon S, Broeck DV, Rossi R, Ogbe E, Harmon S, Mabeya H. Associations Between Vaginal Infections and Potential High-risk and High-risk Human Papillomavirus Genotypes in Female Sex Workers in Western Kenya. Clin Ther. 2016;38(12):2567–2577. [DOI] [PubMed] [Google Scholar]
- 14.Holzer I, Farr A, Kiss H, Hagmann M, Petricevic L. The colonization with Candida species is more harmful in the second trimester of pregnancy. Arch Gynecol Obstet. 2017;295(4):891–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farr A, Kiss H, Holzer I, Husslein P, Hagmann M, Petricevic L. Effect of asymptomatic vaginal colonization with Candida albicans on pregnancy outcome. Acta Obstet Gynecol Scand. 2015;94(9):989–996. [DOI] [PubMed] [Google Scholar]
- 16.Babu G, Singaravelu BG, Srikumar R, Reddy SV, Kokan A. Comparative Study on the Vaginal Flora and Incidence of Asymptomatic Vaginosis among Healthy Women and in Women with Infertility Problems of Reproductive Age. J Clin Diagn Res. 2017;11(8):DC18–DC22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McClelland RS, Richardson BA, Hassan WM, et al. Prospective study of vaginal bacterial flora and other risk factors for vulvovaginal candidiasis. J Infect Dis. 2009;199(12):1883–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez-Fernandez D, Koski KG, Sinisterra OT, Del Carmen Pons E, Murillo E, Scott ME. Interactions among urogenital, intestinal, skin, and oral infections in pregnant and lactating Panamanian Ngabe women: a neglected public health challenge. Am J Trop Med Hyg. 2015;92(6):1100–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu MB, Xu SR, He Y, et al. Diverse vaginal microbiomes in reproductive-age women with vulvovaginal candidiasis. PLoS One. 2013;8(11):e79812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vahidnia A, Tuin H, Bliekendaal H, Spaargaren J. Association of sexually transmitted infections, Candida species, Gram-positive flora and perianal flora with bacterial vaginosis. New Microbiol. 2015;38(4):559–563. [PubMed] [Google Scholar]
- 21.Rathod SD, Klausner JD, Krupp K, Reingold AL, Madhivanan P. Epidemiologic features of Vulvovaginal Candidiasis among reproductive-age women in India. Infect Dis Obstet Gynecol. 2012;2012:859071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353(18):1899–1911. [DOI] [PubMed] [Google Scholar]
- 23.Fredricks DN. Molecular methods to describe the spectrum and dynamics of the vaginal microbiota. Anaerobe. 2011;17(4):191–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rampersaud R, Lewis EL, LaRocca TJ, Ratner AJ. Environmental pH modulates inerolysin activity via post-binding blockade. Sci Rep. 2018;8(1):1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rampersaud R, Planet PJ, Randis TM, et al. Inerolysin, a cholesterol-dependent cytolysin produced by Lactobacillus iners. J Bacteriol. 2011;193(5):1034–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Houdt R, Ma B, Bruisten SM, Speksnijder A, Ravel J, de Vries HJC. Lactobacillus iners-dominated vaginal microbiota is associated with increased susceptibility to Chlamydia trachomatis infection in Dutch women: a case-control study. Sex Transm Infect. 2018;94(2):117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muzny CA, Blanchard E, Taylor CM, et al. Identification of Key Bacteria Involved in the Induction of Incident Bacterial Vaginosis: A Prospective Study. J Infect Dis. 2018;218(6):966–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nunn KL, Wang YY, Harit D, et al. Enhanced Trapping of HIV-1 by Human Cervicovaginal Mucus Is Associated with Lactobacillus crispatus-Dominant Microbiota. MBio. 2015;6(5):e01084–01015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joag V, Obila O, Gajer P, et al. Impact of Standard Bacterial Vaginosis Treatment on the Genital Microbiota, Immune Milieu, and Ex Vivo Human Immunodeficiency Virus Susceptibility. Clin Infect Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Secura GM, Allsworth JE, Madden T, Mullersman JL, Peipert JF. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203(2):115 e111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis WG, Robinson LS, Perry J, et al. Hydrolysis of secreted sialoglycoprotein immunoglobulin A (IgA) in ex vivo and biochemical models of bacterial vaginosis. J Biol Chem. 2012;287(3):2079–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amegashie CP, Gilbert NM, Peipert JF, Allsworth JE, Lewis WG, Lewis AL. Relationship between nugent score and vaginal epithelial exfoliation. PLoS One. 2017;12(5):e0177797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Hung GC, Nagamine K, Li B, Tsai S, Lo SC. Development of Candida-Specific Real-Time PCR Assays for the Detection and Identification of Eight Medically Important Candida Species. Microbiol Insights. 2016;9:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1691. [DOI] [PubMed] [Google Scholar]
- 35.Krasner RI, Young G, Yudkofsky PL. Interactions of oral strains of Candida albicans and lactobacilli. J Bacteriol. 1956;72(4):525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang S, Wang Q, Yang E, Yan L, Li T, Zhuang H. Antimicrobial Compounds Produced by Vaginal Lactobacillus crispatus are able to Strongly Inhibit Candida albicans Growth, Hyphal Formation and Regulate Virulence-related Gene Expressions. Front Microbiol. 2017;8:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parolin C, Marangoni A, Laghi L, et al. Isolation of Vaginal lactobacilli and Characterization of Anti-Candida Activity. PLoS One. 2015;10(6):e0131220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kau AL, Planer JD, Liu J, et al. Functional characterization of IgA-targeted bacterial taxa from undernourished Malawian children that produce diet-dependent enteropathy. Sci Transl Med. 2015;7(276):276ra224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150):1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007;34(11):864–869. [DOI] [PubMed] [Google Scholar]
- 41.Brookheart RT, Lewis WG, Peipert JF, Lewis AL, Allsworth JE. Association between obesity and bacterial vaginosis as assessed by Nugent score. Am J Obstet Gynecol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Si J, You HJ, Yu J, Sung J, Ko G. Prevotella as a Hub for Vaginal Microbiota under the Influence of Host Genetics and Their Association with Obesity. Cell Host Microbe. 2017;21(1):97–105. [DOI] [PubMed] [Google Scholar]
- 43.Marrazzo JM, Fiedler TL, Srinivasan S, et al. Extravaginal reservoirs of vaginal bacteria as risk factors for incident bacterial vaginosis. J Infect Dis. 2012;205(10):1580–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


