Abstract
Background
African Traditional Medicine (ATM) is used for the healthcare of about 80% of the rural populations of the continent of Africa. The practices of ATM make use of plant-products, which are known to contain plant-based secondary metabolites or natural products (NPs), likely to play key roles in drug discovery, particularly as lead compounds. For various reasons, including resistance of strains of Plasmodium to known anti-malarial drugs, local African populations often resort to plant-based treatments and/or a combination of this and standard anti-malarial regimens. Emphasis has been laid in this review to present the anti-malarial virtue of the most recently published phytochemicals or natural products, which have been tested by in vitro and in vivo assays.
Methods
The data was based on the current version of the African Compound Libraries, which are constantly being updated based on inputs from journal articles and student theses (M.Sc/Ph.D) from African University libraries. Emphasis was laid on data published after 2012. In order to carry out the original data collection, currently being included in the African Compounds Database, individual journal websites were queried using the country names in Africa as search terms. Over 40,000 articles “hits” were originally retrieved, then reduced to about 9000 articles. The retained articles/theses was further queried with the search terms “malaria”, “malarial”, “plasmodium”, “plasmodial” and a combination of them, resulting in over 500 articles. Those including compounds with anti-malarial activities for which the measured activities fell within the established cut off values numbered 55, which were all cited in the review as relevant references.
Results and discussion
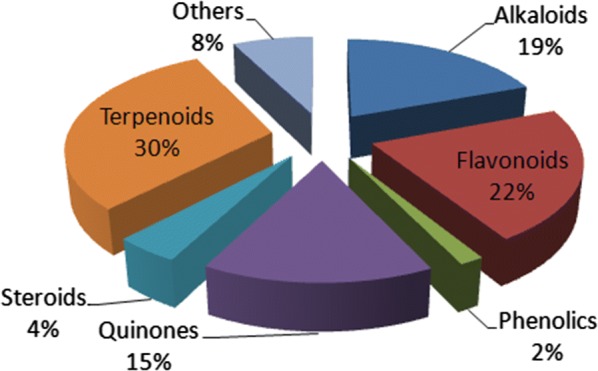
Pure compounds derived from African medicinal plants with demonstrated anti-malarial/antiplasmodial properties with activities ranging from “very active” to “weakly active” have been discussed. The majority of the 187 natural products were terpenoids (30%), followed by flavonoids (22%), alkaloids (19%) and quinones (15%), with each of the other compound classes being less than 5% of the entire compound collection. It was also observed that most of the plant species from which the compounds were identified were of the families Rubiaceae, Meliaceae and Asphodelaceae. The review is intended to continue laying the groundwork for an African-based anti-malarial drug discovery project.
Keywords: Africa, Malaria, Medicinal plants, Natural products, Traditional medicine
Background
Malaria is an endemic disease in most tropical countries (Africa, Asia, and Latin America), with about half of the world’s population at risk of infection according to the World Health Organization (WHO) [1]. According to the latest World Malaria Report, released in December 2019, there were 228 million cases of malaria in 2018, and the estimated number of malaria deaths stood at 405,000. The causative agents for malaria infections are Plasmodium protozoans (i.e. Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale, and Plasmodium vivax), although most severe infections are caused by P. falciparum [2–4]. Most deaths are recorded among African children below the age of 5 years [1–4]. This calls for an urgent need for new anti-malarial therapies for any one of the following reasons:
The development of resistance against insecticides (e.g. dichlorodiphenyltrichloroethane, DDT) by the disease vectors (female anopheline mosquitoes) [5–7].
The inefficacy of chemoprophylaxis, which has often resulted in poor results [1, 8–10].
The development of resistance by Plasmodium protozoans against most of the drugs currently used to treat malaria (e.g. chloroquine, artemisinin and its derivatives) [11–13].
Plants are known to be a rich reservoir of bioactive secondary metabolites (or natural products, NPs), for example, the anti-malarial drugs quinine and artemisinin (AT) are both of plant origin [14]. The benefits of plants containing bioactive anti-malarial compounds, particularly the bitter principles (alkaloids and terpenoids), include their use in the preparation of traditional remedies against malaria, fever, and inflammation [15]. In fact, more than 80% of the local populations of most tropical countries, including African populations, are dependent on medicinal plants for the treatment of most diseases, including malaria, despite the current wide availability of standard malaria treatments for populations in the rural areas, as well as those in cities [16, 17]. It has become of interest to summarize the major findings regarding the most promising secondary metabolites with proven in vitro and in vivo potencies, so as to pave the way for further development with compounds from African sources as leads for anti-malarial drug discovery. Recent reviews have either emphasized plants used in specific countries or regions for the treatment of malaria [18–21], secondary metabolites from selected plant species [22] or families of species [23], plants used as repellents against the mosquito vectors [24], or reports on the analysis of components of improved traditional preparations against malaria [25, 26].
Previous reviews have described the in vitro and in vivo potencies of compounds that have been isolated from African floral matter published data before 2013 [27, 28]. These reviews had previously described over 500 NPs, within the major NP classes, including alkaloids, terpenoids, flavonoids, coumarins, phenolics, polyacetylenes, xanthones, quinones, steroids, and lignans. These compounds were described in the literature as exhibiting from weak to very good in vitro anti-malarial activities, based on well-established cut-off values [29–31]. Besides, a cheminformatic analysis of the aforementioned dataset, with a focus on molecular descriptors related to “drug-likeness”, drug metabolism and pharmacokinetics (DMPK), and some rules of thumb such as the Lipinski “Rule of Five” [32], showed that over 50% of the anti-malarial compounds had physicochemical properties that fell within the range of “drug-like” molecules [33].
The present review focuses on compounds with tested activities against various malaria parasites derived from a literature survey from 2013 to 2019 [29–31]. A total of 187 NPs belonging to diverse classes, including alkaloids, flavonoids, phenolics, flavonoids, steroids, and terpenoids are described. These compounds have been identified from 45 plant species belonging to 23 families. It is hoped that the results summarized will help for lead compound identification and for further anti-malarial drug discovery. The review describes the NPs with potential anti-malarial properties from African medicinal plants, arranged alphabetically according to the main NP compound classes.
Materials and methods
Data collection
In this review, an attempt has been made to document the anti-malarial activities of NPs derived from African medicinal plants. The data was based on the current version of the African compound libraries [34–37], which are constantly being updated based on inputs from journal articles and student theses (M.Sc/Ph.D.) available in African University libraries. Emphasis was laid on data published after 2012. The original data collection, now being included in the African Compounds Database (http://www.african-compounds.org), was conducted from querying individual journal websites using the country names in Africa and search terms. The list of journals visited have been included in Additional file 1. The “hit” articles were retrieved, i.e. those for which plant materials were collected from Africa were then hand-picked by reading through the “Materials and methods“ section to ensure that the plant materials were from Africa. Student theses were also randomly collected as made available from university libraries, constituting a small portion of the data. The folder containing the retained articles/theses was further queried with the search terms “malaria”, “malarial”, “plasmodium”, “plasmodial” and a combination of them. Those for which compounds further showed anti-malarial activities published between 2013 and 2019 and for which the measured activities fell within the established cut-off values were selected and included as relevant references. The compounds displaying anti-malarial properties were classified according to their NP classes and source species of origin. This represented 187 compounds from 45 species belonging to 23 plant families.
Data analysis
The collected data was arranged into spreadsheets according to plant sources, compound classes, activity cut-offs and plasmodial strains tested. All activity data was converted to IC50 values in μM.
Throughout the text, the term antiplasmodial is referred to as that which counters the growth of parasites of the genus Plasmodium, while anti-malarial is referred to as an agent which prevents or counteracts the progress of the disease caused by the parasite or that which treats the disease (i.e. by killing the parasites in the host). Very often the two terms are used interchangeably in the literature surveyed.
Test methodologies
From the literature collected, a broad range plasmodial strains were tested, including those summarized in Table 1.
Table 1.
Summary of testing methodologies and parasite strains described in this report
| Murine (in vivo) models | Strains | Parasite name | Origin | Assay description | References |
|---|---|---|---|---|---|
| CQ-sensitive | NK 173 | Plasmodium berghei | Not reported | Not reported | |
| ANKA | P. berghei | Not reported | Not reported | [38–40] | |
| Plasmodium vinckei petteri | Not reported | Not reported | |||
| In vitro models | |||||
| CQ-sensitive | 3D7 | P. falciparum | Not reported | Parasite lactate dehydrogenase (pLDH) assay | [41, 42] |
| Not reported | Parasite growth inhibition assay | [43] | |||
| Not reported | Translation inhibitory assay | [44] | |||
| D6 | P. falciparum | Sierra Leone | Incorporated G-3H hypoxanthine assay | [41, 42, 45, 46] | |
| Parasite lactate dehydrogenase (pLDH) assay | [47, 48] | ||||
| Non-radioactive Malaria SYBR Green I assay | [49, 50] | ||||
| Modified non-radioactive Malaria SYBR Green I assay | [42, 49, 51] | ||||
| D10 | P. falciparum | Not reported | pLDH assay | [42, 52] | |
| F32 | P. falciparum | Tanzania | Not reported | ||
| FCA20 | P. falciparum | Ghana | Not reported | ||
| K1 | P. falciparum | Thailand | Modified [3H]-hypoxanthine incorporation assay and [3H]-hypoxanthine incorporation assay | [53–56] | |
| NF54 | P. falciparum | Not reported | [3H]-hypoxanthine incorporation assay | [53–56] | |
| CQ-resistant | Dd2 | P. falciparum | Not reported | Non-radioactive Malaria SYBR Green I assay | [49, 50] |
| FcM29 | P. falciparum | Cameroon | Not reported | ||
| FcB1 | P. falciparum | Colombia | [3H]-hypoxanthine incorporation assay | [41] | |
| K1 | P. falciparum | Thailand | Modified [3H]-hypoxanthine incorporation assay and [3H]-hypoxanthine incorporation assay | [53–56] | |
| W2 | P. falciparum | Indochina | Modified non-radioactive Malaria SYBR Green I assay | [42, 49, 51] | |
| Incorporated G-3H hypoxanthine assay | [45, 46] | ||||
| Non-radioactive Malaria SYBR Green I assay | [49, 50] | ||||
| NF54 | P. falciparum | [3H]-hypoxanthine incorporation assay | [41] | ||
| CQ- and pyrimethamine-resistant | K1 | P. falciparum | Thailand | Modified [3H]-hypoxanthine incorporation assay and [3H]-hypoxanthine incorporation assay | [53–55] |
| NF54 | P. falciparum | Thailand | [3H]-hypoxanthine incorporation assay | [54] | |
| Multidrug-resistant | Dd2 | P. falciparum | Not reported | [3H]-hypoxanthine incorporation assay | [53–56] |
| K1 | P. falciparum | Thailand | Parasite lactate dehydrogenase (pLDH) assay | [52, 57] | |
| NF54 | P. falciparum | Not reported | [3H]-hypoxanthine incorporation assay | [53–56] | |
| W2 | P. falciparum | Indochina | [3H]-hypoxanthine incorporation assay | [45, 46] | |
| W2mef | P. falciparum | Not reported | [3H]-hypoxanthine incorporation assay | [53–56] | |
Promising anti-malarial compounds derived from the African flora
Alkaloids
Table 2 summarizes the most promising alkaloids derived from the African flora, published since the earlier review [27], while the chemical structures are shown in Figs. 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10, arranged alphabetically according to their respective sub-classes (i.e. aporphines, furoquinolines, indoles, indolosesquiterpenes, Naphthylisoquinolines, protoberberines, pyridinones and others). Several of them had tested positive against CQ-sensitive and CQ-resistant strains of P. falciparum in vitro.
Table 2.
Summary of alkaloids
| Compound subclass | Isolated metabolites | Plasmodial strain (activities) | Plant species (Family), Taxon IDb | Part of the plant studied | Place of harvest (Locality, Country) | Author, references |
|---|---|---|---|---|---|---|
| Aporphines | 1 to 2 | K1 (8.24 and 2.90 μM, respectively) | Annickia kummeriae (Annonaceae), NCBI:txid225831 | Leaves | Amani Nature Reserve, Tanzania | Malebo et al. [58] |
| Furoquinolines | 3a to 6 | FcB1 (from 162.47 to 298.16 μM) | Teclea nobilis (Rutaceae), NCBI:txid1220089 | Fruits and leaves | Kamwenge district, Uganda | Lacroix et al. [59] |
| 7 | Dd2 (IC50 = 35 μM) | Melicope madagascariensis (Rutaceae), NCBI:txid1487113 | Stem bark | Antsasaka forest of Moramanga, Madagascar | Rasamison et al. [60] | |
| Indoles | 8a to 13 and 15 | 3D7 (from 0.41 to 110.58 μM) | Strychnos icaja (Loganiaceae), NCBI:txid1040889 | Stem bark | Bertoua, Cameroon | Tchinda et al. [61] |
| 14 | FCA20 (0.617 μM) | Strychnos icaja (Loganiaceae), NCBI:txid1040889 | Roots | Kasongo-Lunda, DR Congo | Beaufay et al. [62], Frédérich et al. [63] | |
| W2 (0.085 μM) | ||||||
| Indolosesquiterpenes | 16a and 17a | NF54 (7.6 μM and 29.1 μM, respectively) | Polyalthia oliveri (Annonaceae), NCBI:txid105756 | Stem bark | Mount Kala, Cameroon | Kouam et al. [64] |
| Naphthylisoquinolines | 18a and 19a | NF54 (0.043 and 0.055 μM, respectively) | Ancistrocladus sp. (Ancistrocladaceae), NCBI:txid 63071 | Leaves | Mbandaka, DR Congo | Lombe et al. [65] |
| 20a, 21`a, 22a and 23 to 25 | NF54 (from 0.090 to 6.54 μM) | Ancistrocladus ileboensis (Ancistrocladaceae), NCBI:txid1367080 | Leaves and root bark | Bambange, DR Congo | Li et al. [66] | |
| K1 (0.228 μM for compound 19) | ||||||
| 26a, 27a, 28a and 29a | NF54 (from 0.84 to 22.2 μM) | Ancistrocladus ealaensis (Ancistrocladaceae), NCBI:txid714098 | Twigs and leaves | Mbandaka, DR Congo | Tshitenge et al. [67] | |
| K1 (from 1.4 to 8.2 μM) | ||||||
| Protoberberines | 30 to 33 | K1 (from 0.22 to 0.71 μM) | Annickia kummeriae (Annonaceae), NCBI:txid225831 | Leaves | Amani Nature Reserve, Tanzania | Malebo et al. [58] |
| 34 | K1 (IC50 = 318.66 μM) | Polyalthia longifolium var. pendula (Annonaceae), NCBI:txid235806 | Stem | Tikrom, near Kumasi, Ghana | Gbedema et al. [68] | |
| Pyridinones | 35 | K1 (IC50 = 81.28 μM) | Polyalthia longifolium var. pendula (Annonaceae), NCBI:txid235806 | Stem | Tikrom, near Kumasi, Ghana | Gbedema et al. [68] |
| Others | 36 | K1 (IC50 = 32.12 μM) | Canthium multiflorum (Rubiaceae), NCBI:txid58501 | Aerial part | Obala, along River Sanaga, Cameroon | Kouam et al. [69] |
aCompounds identified for the first time in the cited publications
bIdentification number of the source species, derived from the NCBI Taxonomy database
Fig. 1.
Aporphine alkaloids (1 and 2)
Fig. 2.
Furoquinoline alkaloids (3 to 7)
Fig. 3.
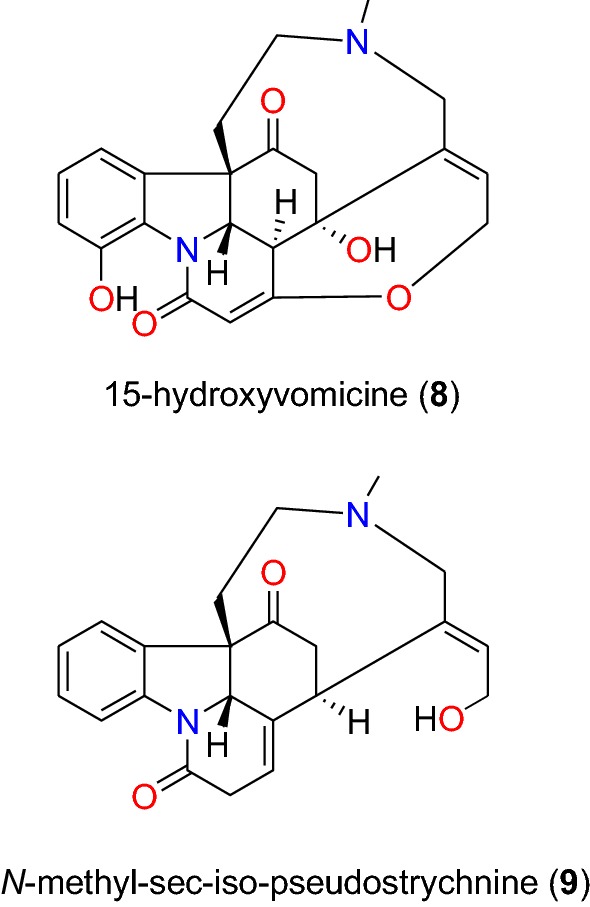
Indole alkaloids (8 and 9)
Fig. 4.
Indole alkaloids (10 to 15)
Fig. 5.
Indolosesquiterpene alkaloids (16 and 17)
Fig. 6.
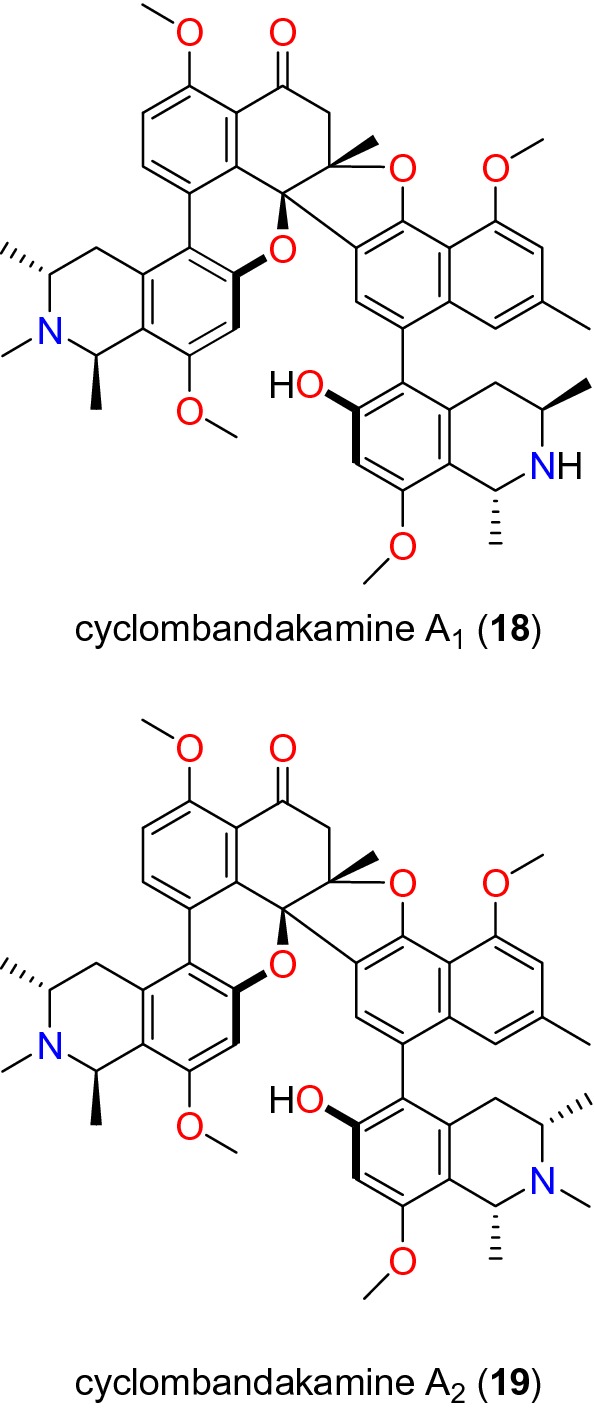
Naphthylisoquinoline alkaloids I (18 and 19)
Fig. 7.
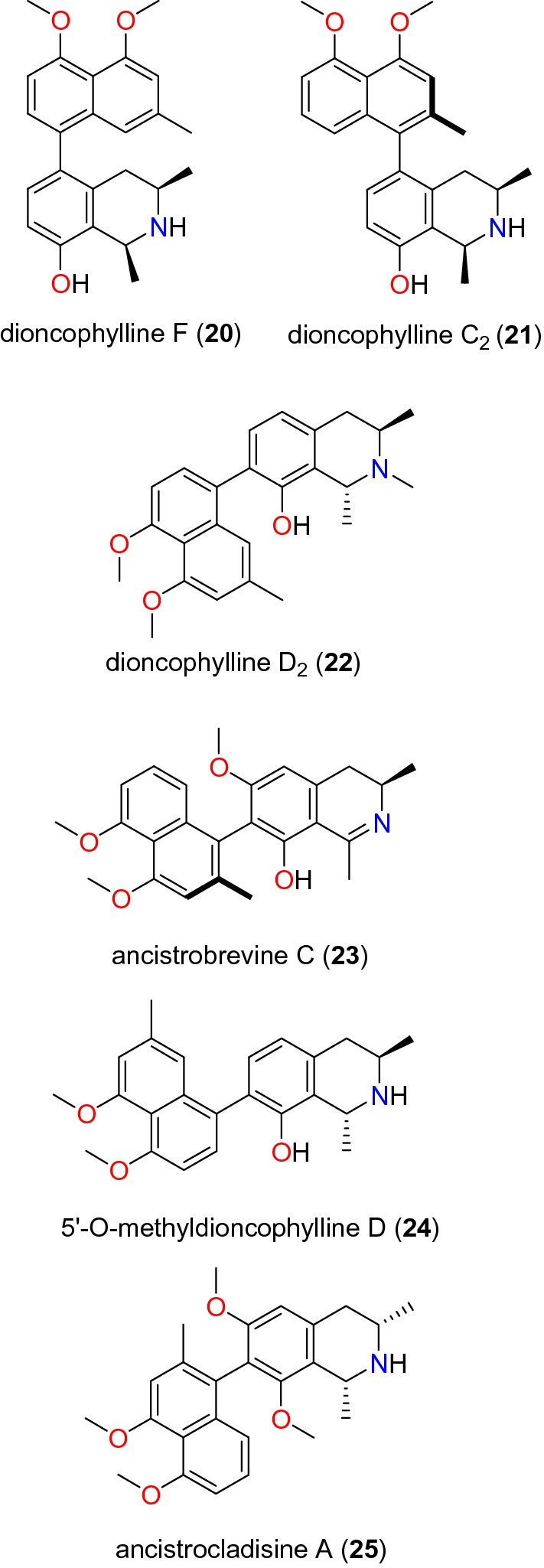
Naphthylisoquinoline alkaloids II (20 to 25)
Fig. 8.
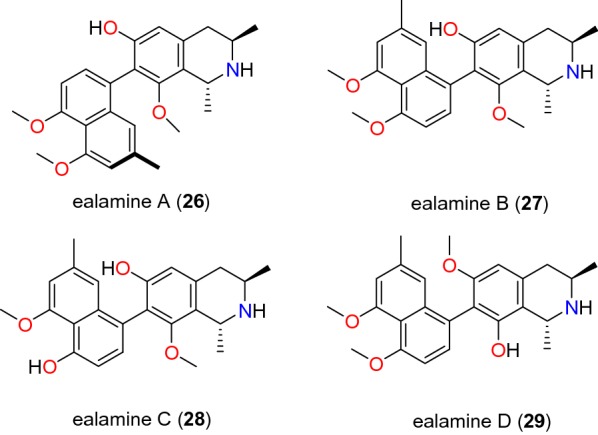
Naphthylisoquinoline alkaloids III (26 to 29)
Fig. 9.
Protoberberine alkaloids (30 to 34)
Fig. 10.
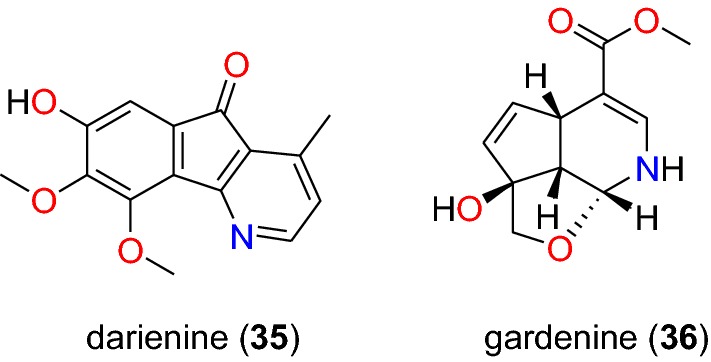
Other alkaloids (35 and 36)
Aporphines
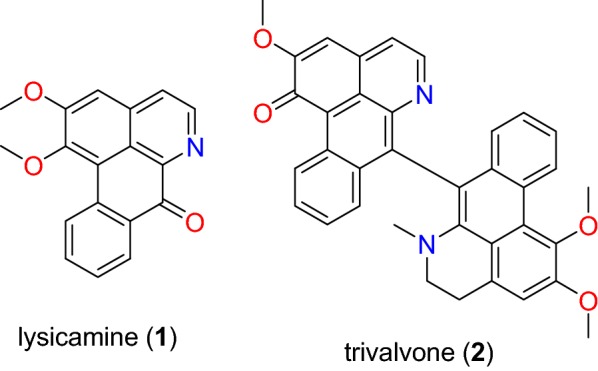
The aporphine alkaloids lysicamine (1), trivalvone (2) (Fig. 1) were identified from the leaves of Annickia kummeriae (Annonaceae) from Tanzania, along with four other (protoberberine) alkaloids. Plants from the genus Annickia (formerly Enantia) are popularly known in West and Central Africa for use in the treatment of malaria [70–72]. The study by Maleba et al. [58] showed that compounds 1 and 2 showed respective activities of 8.23 and 2.90 μM against the CQ-resistant K1 strain of P. falciparum.
Furoquinolines
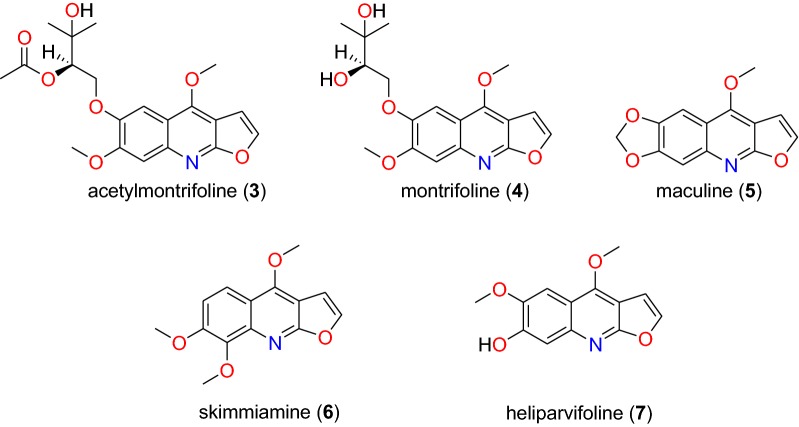
Four furoquinoline alkaloids (3 to 6) (Fig. 2) were isolated from the fruits and leaves of Teclea nobilis (Rutaceae) and tested on the chloroquine (CQ)-resistant FcB1/Colombia strain of P. falciparum by Lacroix et al. [59]. This species from Uganda has been used to treat a range of ailments from pain and fever to malaria [73]. The isolated compounds, including the novel acetylmontrifoline (3), and the known montrifoline (4), maculine (5), and skimmianine (6), were less potent than the reference drug CQ, showing inhibition against the tested parasite strain at < 300 μM [59].
Rasamison et al. also isolated seven furoquinolines from the stem bark of the Madagascan species, Melicope madagascariensis (Rutaceae), of which only compound 7 (6-methoxy-7-hydroxydictamnine, commonly called heliparvifoline) exhibited weak anti-malarial activity against the CQ-resistant strain, Dd2, with IC50 = 35 μM, the other compounds tested being inactive [60].
Indoles
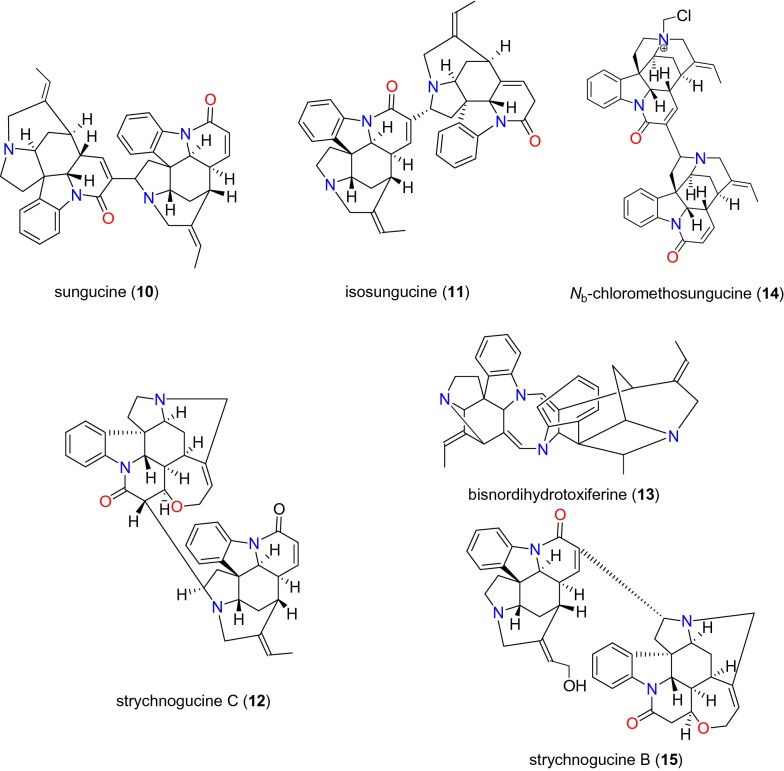
Strychnos icaja (Loganiaceae) is found all over Central Africa [74]. In Cameroon, for example, the roots are used by a Pygmy tribe to treat malaria. From their stem bark, six indole alkaloids (8 to 13) (Figs. 3 and 4), were isolated and evaluated against the CQ-sensitive 3D7 strain of P. falciparum by Tchinda et al. [61], with IC50 values ranging from 0.40 to 110 μM. These include 15-hydroxyvomicine (8), N-methyl-sec-iso-pseudostrychnine (9), sungucine (10), isosungucine (11), strychnogucine C (12), bisnordihydrotoxiferine (13), along with the chlorinated indole, Nb-chloromethosungucine (14).
Strychnogucine B (15) (Fig. 4), which was previously isolated from the roots of the same species by Frédérich et al. [63] was further investigated by Beaufay et al. [62] and the compound now displayed further inhibition against the CQ-sensitive FCA 20/Ghana and CQ-resistant W2/Indochina strains, with IC50 values of 0.617 and 0.085 μM, respectively.
Indolosesquiterpenes
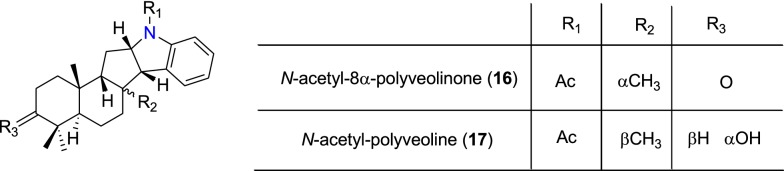
The bioactivity-guided screening of the stem bark of Polyalthia oliveri (Annonaceae) led Kouam et al. to isolate two indolosesquiterpene alkaloids, named N-acetyl-8α-polyveolinone (16) and N-acetyl-polyveoline (17) (Fig. 5) [64]. This species is used in folk medicine for the treatment of malaria [75]. Both compounds were tested against CQ-sensitive NF54 strain and compound 16 showed moderate antiplasmodial activity with IC50 = 7.6 μM, while compound 17 inhibited the strain weakly with an IC50 value of 29.1 μM [75].
Naphthylisoquinolines
These are compounds characterized by a chiral biarylaxis linkage between the naphthalene and the isoquinoline alkaloids, mainly isolated from plants of the genus Ancistrocladus (Acistrocladaceae), and the closely related genera Triphyophyllum, Dioncophyllum and Habropetalum (Dioncophyllaceae). Cyclombandakamines A1 (18) and A2 (19) (Fig. 6) are naphthoisoquinoline alkaloids isolated from the leaves of Ancistrocladus sp. (Ancistrocladaceae) by Lombe et al. [65]. These compounds displayed significant inhibitory activities against the NF54 strain of P. falciparum with IC50 values of 0.043 and 0.055 μM, respectively [65]. Li et al. [66] also investigated the twigs and leaves of Ancistrocladus ileboensis (Ancistrocladaceae) from DR Congo. Among the tested compounds with promising anti-malarial activities were dioncophylline F (20) and dioncophylline C2 (21), in addition to the 7,8′-coupled dioncophylline D2 (22), ancistrobrevine C (23), 5′-O-methyldioncophylline D (24), and ancistrocladisine A (25) (Fig. 7). Compound 20 showed activities against both the NF54 and K1 strains (with IC50 values of 0.090 and 0.045 μM, respectively), compounds 21 to 25 were only tested against the K1 strain, with IC50 values ranging from 0.107 to 6.51 μM [66].
Among the compounds identified from Ancistrocladaceae, Tshitenge et al. also isolated four naphthylisoquinolines, named ealamines A–D (26 to 29, Fig. 8) from the twigs and leaves of Ancistrocladus ealaensis (Ancistrocladaceae) harvested in Mbandaka, DR Congo [67]. These compounds were tested against CQ-sensitive NF54 and CQ- and pyrimethamine-resistant K1 strains of P. falciparum. The activities against the CQ-sensitive NF54 strain showed IC50 values of 6.3, 4.9, 0.84 and 22.2 μM, respectively. Meanwhile, compounds 26, 27 and 29 inhibited the CQ- and pyrimethamine-resistant K1 strain with IC50 values of 1.6, 1.4, and 8.2 μM, respectively [67].
Protoberberines
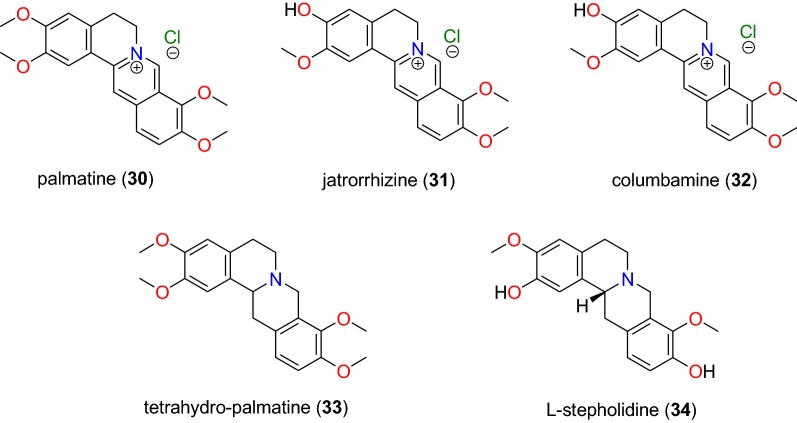
Maleba et al. [58] showed that against the CQ-resistant K1 strain of P. falciparum protoberberine alkaloids are a subclass of promising anti-malarials. The in vitro testing of compounds 30 to 33 (Fig. 9) showed that compound 30 (palmatine) was the most active, with an IC50 value of 0.23 μM. Jatrorrhizine (31) exhibited an IC50 of 0.71 μM, whereas a mixture of compound 31 and columbamine (32) inhibited the plasmodial strain with an IC50 value of 0.14 μg/mL, and a mixture of compound 26 and tetrahydro-palmatine (33) inhibited the parasite strain with IC50 = 0.098 μg/mL, probably explaining the synergistic activity of this plant extract. This justifies its use in African Traditional Medicine for the treatment of malaria [58].
Extracts of Polyalthia longifolium (Annonaceae), used in orally consumed preparations in traditional medicine in Ghana, was investigated in order to identify anti-malarial compounds [68]. The protoberberine l-stepholidine (34, Fig. 9) was identified from the stem of species among the isolated compounds [68], but this compound had only a weak antiplasmodial activity against the K1 strain of P. falciparum.
Pyridinones
Gbedema et al. also isolated darienine (35, Fig. 10), a known alkaloid with anti-malarial activity [68]. This compound exhibited varying degrees of antiplasmodial activity against the K1 strain of P. falciparum with an IC50 value of 81.28 μM.
Other alkaloids
Gardenine (36, Fig. 10), obtained from the investigation of crude extract of the aerial parts of Canthium multiflorum (Rubiaceae), harvested from Cameroon, exhibited antiplasmodial activity against the K1 strain of P. falciparum, with an IC50 value of 32.12 μM and weak cytotoxicity against L6 cell lines [69].
Flavonoids
Flavonoids (mainly chalcone, flavanone, isoflavone, and retonoid sub-classes) (Figs. 11, 12, 13 and 14) were previously seen as a promising class of NPs exhibiting anti-malarial and antiplasmodial activities [28].
Fig. 11.
Flavanones and flavones (37 to 49)
Fig. 12.
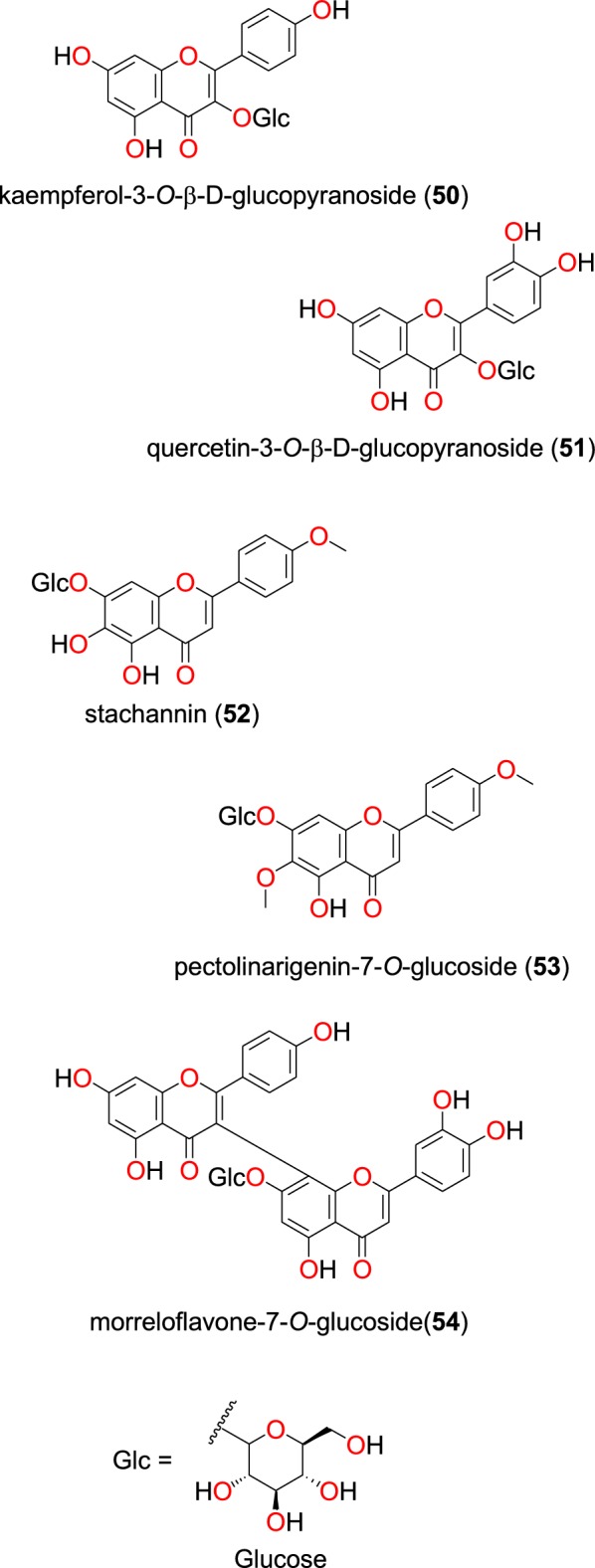
Glycoflavonoids (50 to 54)
Fig. 13.
Isoflavones (55 to 74)
Fig. 14.
Retonoids (75 to 77)
Flavanones and flavones
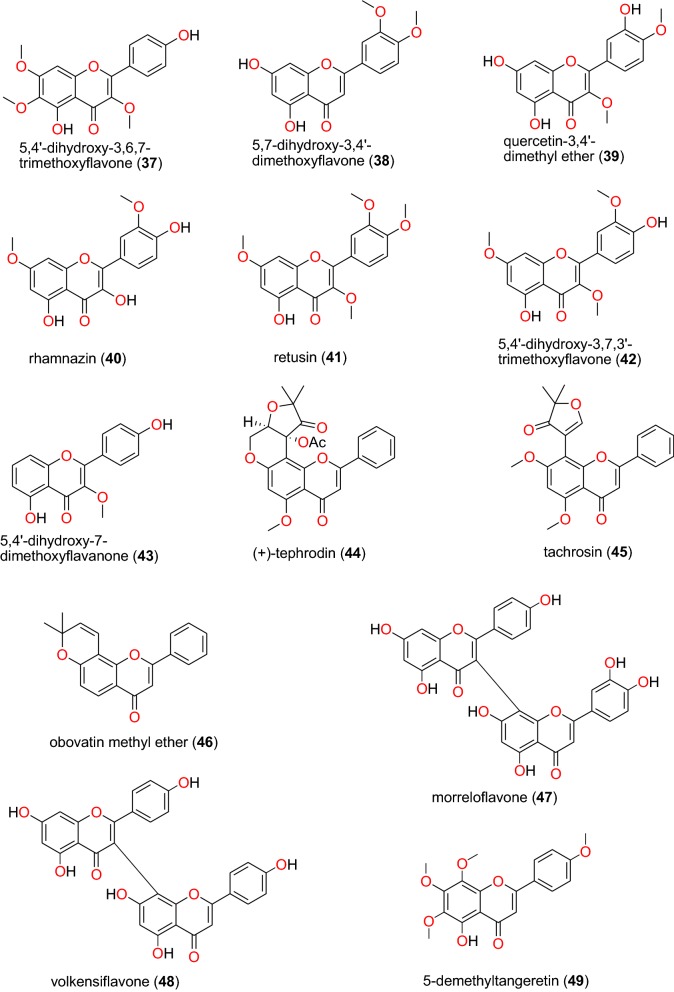
The most promising recently published anti-malarial flavanones and flavones derived from the African flora have been summarized in Table 3. These include 5,4′-dihydroxy-3,6,7-trimethoxyflavone (37), 5,7-dihydroxy-3,4′-dimethoxyflavone (38), quercetin-3,4′-dimethyl ether (39), rhamnazin (40), retusin (41), 5,4′-dihydroxy-3,7,3′-trimethoxyflavone (42), 5,4′-dihydroxy-7-dimethoxyflavanone (43), (+)-tephrodin (44), tachrosin (45), obovatin methyl ether (46), morreloflavone (47), volkensiflavone (48), and 5-demethyltangeretin (49), whose chemical structures of are shown in Fig. 11. The compounds were isolated from the species Senecio roseiflorus (Compositae-Asteraceae) [76], Tephrosia villosa (Leguminosae) [77], Allanblackia floribunda (Guttiferae) [78], and Peperomia vulcanica (Piperaceae) [79].
Table 3.
Summary of flavonoids
| Compound subclass | Isolated metabolites | Plasmodial strain (activities) | Plant species (Family), Taxon IDb | Part of the plant studied | Place of harvest (locality, country) | Author, references |
|---|---|---|---|---|---|---|
| Flavanones and flavones | 37 to 43 | D6 (from 11.26 to 56.31 μM) | Senecio roseiflorus (Compositae-Asteraceae), NCBI:txid1886451 | Leaves | Mount Kenya Forest, Meru, Kenya | Kerubo et al. [76] |
| W2 (from 15.48 to 87.50 μM) | ||||||
| 44 to 46 | D6 (from 11.30 to 14.00 μM) | Tephrosia villosa (Leguminosae-Fabaceae), NCBI:txid62125 | Roots | Manyani, TaitaTaveta County, Kenya | Muiva-Mutisya et al. [77] | |
| W2 (from 13.10 to 20.40 µM) | ||||||
| 47 and 48 | F32 (from 2.18 to 21.13 μM) | Allanblackia floribunda (Guttiferae- Clusiaceae), NCBI:txid469914 | Whole plant | Mount Kala, Cameroon | Azebaze et al. [78] | |
| FcM29 (from 1.75 to 22.59 μM) | ||||||
| 49 | W2mef (19.37 μM) | Peperomia vulcanica (Piperaceae), NCBI:txid1719589 | Whole plant | Mount Cameroon, Cameroon | Ngemenya et al. [79] | |
| Dd2 (3.18 μM) | ||||||
| Glycoflavonoids | 50 and 51 | D6 (97.1 and 42.9 μM, respectively) | Ekebergia capensis (Meliaceae), NCBI:txid124949 | Leaves | Gakoe Forest, Kiambu County, Kenya | Irungu et al. [80] |
| W2 (105.8 μM) | ||||||
| 52 and 53 | Gardenia ternifolia (Rubiaceae), NCBI:txid1237590; Crossopteryx febrifuga (Rubiaceae), NCBI:txid170354; and Lantana camara (Verbenaceae), NCBI:txid126435 | Stem barks and leaves | Kinshasa, DR Congo | Tshitenge et al. [26] | ||
| 54 | F32 (15.98 and 40.36 μM, respectively, at 24 h and 72 h) | Allanblackia floribunda (Guttiferae- Clusiaceae), NCBI:txid469914 | Whole plant | Mount Kala, Cameroon | Azebaze et al. [78] | |
| FcM29 (11.69 and 33.24 µM , respectively, at 24 h and 72 h) | ||||||
| Isoflavones | 55 to 70 | W2 (from 14.9 to 53.1 μM) | Millettia oblata ssp. teitensis (Leguminosae-Fabaceae), NCBI:txid53625 | Stem bark | Taita Hill Forest, Kenya | Derese et al. [81] |
| 63a | D6 (from 13.3 to 48.7 μM) | |||||
| 71a to 74 | 3D7 and Dd2 (70 to 90% inhibition at 40 μM) | Millettia dura (Leguminosae-Fabaceae), NCBI:txid62119 | Root bark | Kisarawe, Tanzania | Marco et al. [82] | |
| Retonoids | 75 to 77 | D6 (18.71 µM for compound 75 and 9.60 μg/mL for a mixture of compounds 76 and 77) | Tephrosia villosa (Leguminosae-Fabaceae), NCBI:txid62125 | Roots | Manyani, Taita Taveta County, Kenya | Muiva-Mutisya et al. [77] |
| W2 (28.64 µM for compound 75 and 22.60 μg/mL for a mixture of compounds 76 and 77) |
aCompounds identified for the first time in the cited publications
bIdentification number of the source species, derived from the NCBI Taxonomy database
Compounds 37 to 43 were derived from leaves of Senecio roseiflorus and have shown good to moderate antiplasmodial activities against D6 and W2 strains. The activities in terms of IC50 values ranged from 11.25 to 56.31 µM for the D6 strain, while for the W2 strain, this ranged from 15.47 to 87.50 µM [76]. Compounds 44 to 46, were derived from roots of Tephrosia villosa and exhibited anti-malarial activities against both the D6 and W2 strains with respective IC50 values from 11.30 to 14.00 µM for the D6 strain and from 13.10 to 20.40 µM for the W2 strain [77].
Azebaze et al. investigated the antiplasmodial activities of whole plant extracts of Allanblackia floribunda from Cameroon [78]. The biflavonoids 47 and 48 were isolated from the plant extract and exhibited in vitro antiplasmodial activities against the F32 and FcM29 strains. The IC50 values at 24 h and 72 h against the both strains were 21.13 and 6.03 µM; 22.59 and 8.61 µM for compound 47 and 1.83 and 2.18 µM; 1.44 and 1.75 µM for compound 48, respectively [78]. Additionally, 5-demethyltangeretin (49) was isolated from the whole plant of Peperomia vulcanica by Ngemenya et al. [79]. This compound showed antiplasmodial activity against the multidrug-resistant W2mef and Dd2 strains of P. falciparum, with respective IC50 values of 19.36 and 3.18 µM.
Glycoflavonoids
The glycoflavonoids kaempferol-3-O-β-d-glucopyranoside (50), and quercetin-3-O-β-d-glucopyranoside (51), Fig. 12, were isolated from the leaves of Ekebergia capensis (Meliaceae) from Kenya by Irungu et al. [80]. Both compounds were observed to possess moderate activities against the D6 and W2 strains of P. falciparum. The IC50 values of compounds 50 and 51 were, respectively, 97.1 and 42.9 µM against the D6 strain, while both compounds measured an IC50 value of 105.8 µM against the W2 strain.
Tshitenge et al. investigated the anti-malarial constituents of the medicinal plant-based SIROP KILMA, with constitutive plants composed from Gardenia ternifolia (Rubiaceae), Crossopteryx febrifuga (Rubiaceae), and Lantana camara (Verbenaceae) [26]. The authors identified two flavonoid glycosides; stachannin (52) and pectolinarigenin-7-O-glucoside (53) [26]. The flavone glycoside morreloflavone-7-O-glucoside (54) was isolated by Azebaze et al. from Allanblackia floribunda (Guttiferae), harvested in Cameroon. This compound presented antiplasmodial activities against the F32 and FcM29 strains with IC50 values of 15.98 and 11.69 µM; 40.36 and 33.24 µM at 24 h and 72 h, respectively [78].
Isoflavones
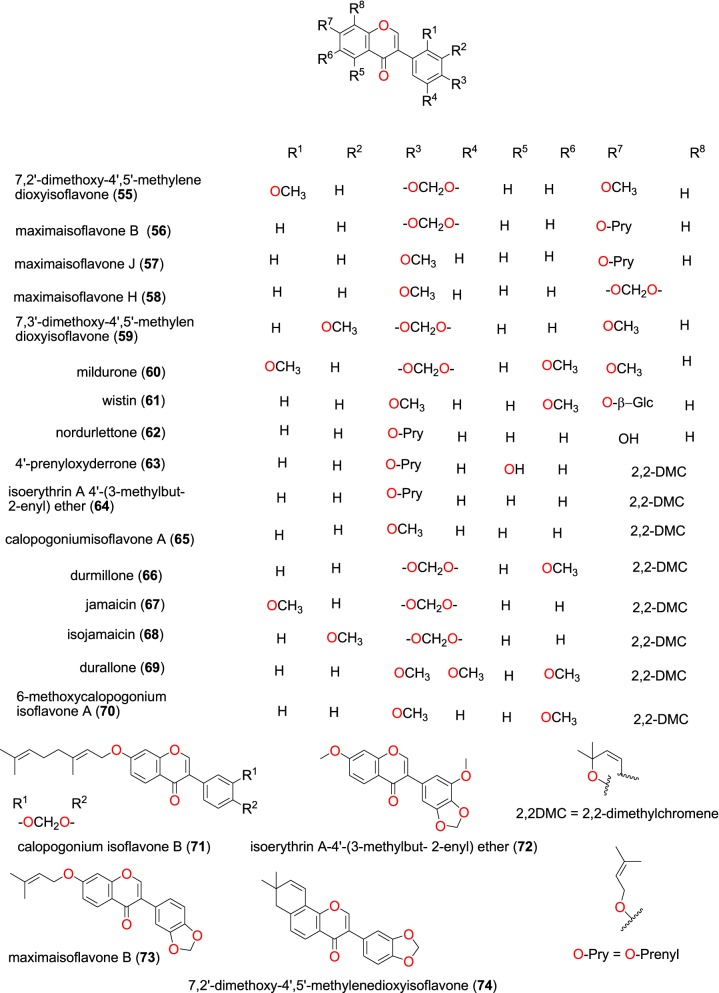
Studies by Derese et al. on the stem bark of Millettia oblata (Leguminosae) harvested from Kenya led to the isolation of 7,2′-dimethoxy-4′,5′-methylenedioxyisoflavone (55), maximaisoflavone B (56), maximaisoflavone J (57), maximaisoflavone H (58), 7,3′-dimethoxy-4′,5′-methylendioxyisoflavone (59), mildurone (60), wistin (61) nordurlettone (62), 4′-prenyloxyderrone (63), isoerythrin A 4′-(3-methylbut-2-enyl) ether (64), calopogoniumisoflavone A (65), durmillone (66), jamaicin (67), isojamaicin (68), durallone (69), and 6-methoxycalopogonium isoflavone A (70) (Fig. 13) [81]. The plant extracts and isolated compounds were tested in vitro against the P. falciparum W2 and D6 strains. All the plant extracts had IC50 values ranging from 10.0 to 25.4 μg/mL. The compounds showed good to moderate antiplasmodial activities with the following pairs of IC50 values; 45.6 and 47.5; 42.0 and 36.0; 29.7 and 35.7; 38.8 and 45.6; 48.4 and 37.7; 44.1 and 35.9; 23.2 and 22.3; 28.9 and 25.1; 14.9 and 13.3; 21.6 and 19.3; 51.5 and 45.8; 25.1 and 37.3; 38.6 and 41.0; 38.9 and 48.7; 50.0 and 32.7; 53.1 and 34.8 μM against the W2 and D6 strains, respectively [81].
Marco et al. obtained calopogonium isoflavone B (71) and isoerythrin A-4′-(3-methylbut-2-enyl) ether (72) maximaisoflavone B (73) and 7,2′-dimethoxy-4′,5′-methylenedioxyisoflavone (74) from the root bark of Millettia dura (Leguminosae) harvested in Tanzania [82]. These compounds showed marginal activities (70 to 90% inhibition at 40 μM) against the 3D7 and Dd2 strains of P. falciparum.
Retonoids
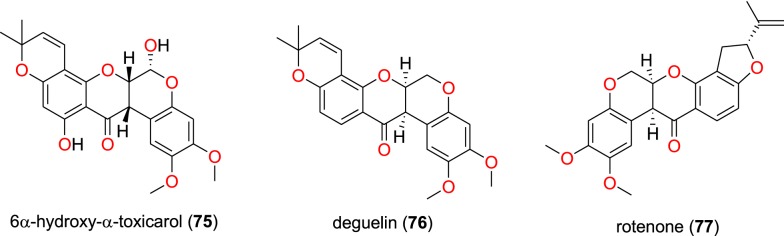
Muiva-Mutisya et al. also isolated the retonoids 6α-hydroxy-α-toxicarol (75), deguelin (76), rotenone (77) (Fig. 14), from the root extract of Tephrosia villosa (Leguminosae) [77]. The mixture of compounds 76 and 77 exhibited anti-malarial activities with IC50 values of 9.60 and 22.60 µg/mL against the CQ-sensitive D6 and CQ-resistant W2, respectively. Meanwhile, the activities of compound 75 against the same strains were 18.71 and 28.64 µM, respectively [77].
Phenolics and quinones
Summaries of the phenolics and quinones with most promising anti-malarial properties have been shown in Table 4 (according to their subclasses), with chemical structures shown in Figs. 15, 16, 17, 18, 19, 20 and 21.
Table 4.
Summary of phenolics and quinones
| Compound subclass | Isolated metabolites | Plasmodial strain (activities) | Plant species (Family), Taxon IDb | Part of the plant studied | Place of harvest (locality, country) | Author, references |
|---|---|---|---|---|---|---|
| Ellagic acid derivative (phenolics) | 78 | D6 (8.01 µM) | Terminalia brownii (Combretaceae), NCBI:txid1548809 | Stem bark | Machakos County, Kenya | Machumi et al. [84] |
| W2 (8.01 µM) | ||||||
| Phenolic glycosides (phenolics) | 79 and 80 | Gardenia ternifolia (Rubiaceae), NCBI:txid1237590; Crossopteryx febrifuga (Rubiaceae), NCBI:txid170354; and Lantana camara (Verbenaceae), NCBI:txid126435 | Stem barks and leaves | Kinshasa, DR Congo | Tshitenge et al. [26] | |
| Anthraquinones (quinones) | 81a, 82 to 92 | D6 (from 0.47 to 23.25 μM) | Kniphofia foliosa (Asphodelaceae), NCBI:txid214838 | Rhizomes | Addis Ababa, Ethiopia | Induli et al. [85] |
| 89 | W2 (from 0.35 to 18.42 μM ) | |||||
| 93 | D6 (7.73 μM) | Kniphofia foliosa (Asphodelaceae), NCBI:txid214838 | Roots | Gedo, Ethiopia | Abdissa et al. [86] | |
| W2 (2.22 μM) | ||||||
| 89a | D6 (9.40 μM) | Kniphofia foliosa (Asphodelaceae), NCBI:txid214838 | Roots | Gedo, Ethiopia | Abdissa et al. [86] | |
| W2 (14.58 μM) | ||||||
| 82 | 3D7 (0.7 μM) | Kniphofia foliosa (Asphodelaceae), NCBI:txid214838 | Leaves | Addis Ababa, Ethiopia | Feilcke et al. [89] | |
| 90a and 91a | K1 (0.17 and 0.26 μm, respectively) | Bulbine frutescens (Asphodelaceae), NCBI:txid210954 | Roots | Chiromo Campus Garden, Kenya | Bringmann et al. [87] | |
| 94 to 96 | D6 (19.66 to 82.80 μM) | Aloe pulcherrima (Asphodelaceae), NCBI:txid25641 | Roots | Saka Chokorsa, Ethiopia | Abdissa et al. [88] | |
| W2 (64.46 to 141.95 μM) | ||||||
| 86 | 3D7 (1.9 μM) | Kniphofia foliosa (Asphodelaceae), NCBI:txid214838 | Leaves | Addis Ababa, Ethiopia | Feilcke et al. [89] | |
| 97a | NF54 (weak activity) | Diospyros canaliculata (Ebenaceae), NCBI:txid13492 | Stem bark | Kribi, Cameroon | Lenta et al. [90] | |
| Anthrones (quinones) | 98 to 101 | Suppression of parasitaemia from 36.8 to 66.8% at doses of 100 to 400 mg/kg /day | Aloe percrassa (Asphodelaceae), NCBI:txid1593100 | Leaf latex | Edagahamus, Ethiopia | Geremedhin et al. [91] |
| Naphthohydroquinones (quinones) | 102a, 103a, 104a, 105a and 106a | D6 (from 19.59 to 36.03 μM) | Pentas bussei (syn: Rhodopentas bussei, Rubiaceae), NCBI:txid387051 | Roots | Mombasa, Kenya | Endale et al. [92] |
| W2 (60.08 to 144.43 μM) | ||||||
| Other quinones | 107 | W2mef (52.25 μM) | Peperomia vulcanica (Piperaceae), NCBI:txid1719589 | Whole plant | Mount Cameroon, Cameroon | Ngemenya et al. [79] |
| 108a | D6 (19.28 μM) | Neoboutonia macrocalyx (Euphorbaceae), NCBI:txid316724 | Stem bark | Kibale National Park, Uganda | Namukobe et al. [93] | |
| W2 (14.17 μM) |
aCompounds identified for the first time in the cited publications
bIdentification number of the source species, derived from the NCBI Taxonomy database
Fig. 15.
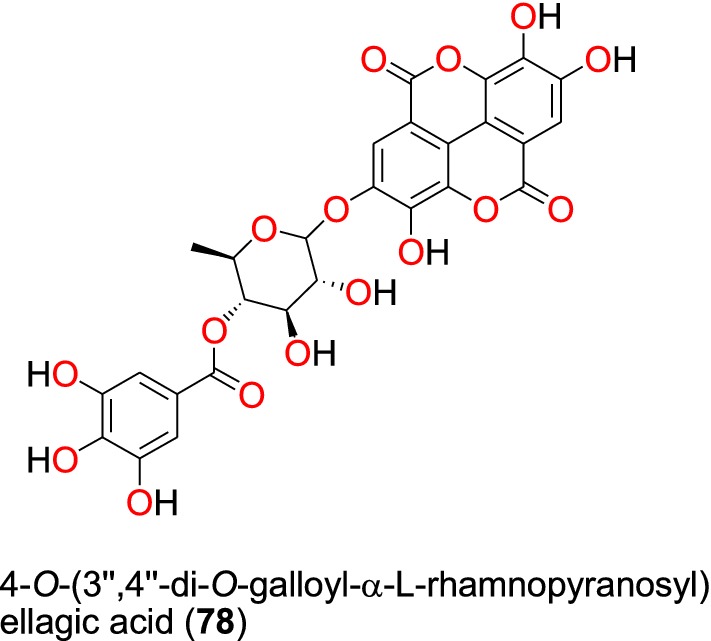
Ellagic acid derivative (78)
Fig. 16.
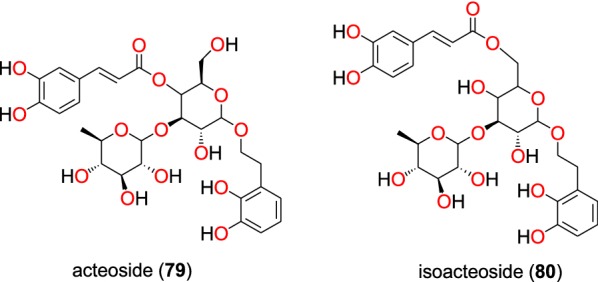
Phenolic glycosides (79 and 80)
Fig. 17.
Anthraquinones (81 to 92)
Fig. 18.
Anthraquinones (93 to 97)
Fig. 19.
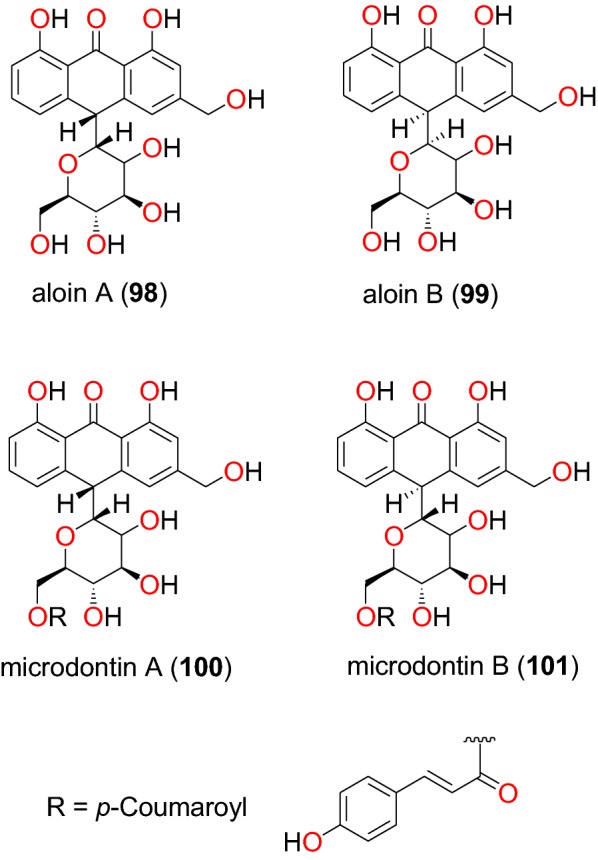
Anthrones (98 to101)
Fig. 20.
Naphthohydroquinones (102 to 106)
Fig. 21.
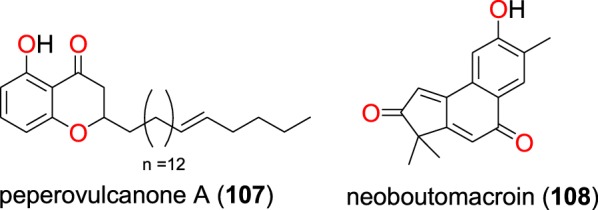
Other quinones (107 and 108)
Ellagic acid derivatives
The plant Terminalia brownii (Combretaceae) is used as a remedy for malaria in Eastern and Central Africa, although the detailed mode of preparation is not fully described in the literature [83]. The phenolic compound, 4-O-(3″,4″-di-O-galloyl-α-l-rhamnopyranosyl) ellagic acid (78) (Fig. 15), obtained from the stem bark of this plant harvested in Kenya was found to be active against chloroquine-sensitive (D6) and chloroquine-resistant (W2) strains of P. falciparum [77]. According to Machumi et al. [84], the IC50 value obtained against both strains was equal to 8.01 µM.
Phenolic glycosides
In addition to flavonoid glycosides, Tshitenge et al. [26] identified two phenolic glycosides as anti-malarial constituents of the medicinal plant-based SIROP KILMA: acteoside (79) and isoacteoside (80) (Fig. 16).
Anthraquinones
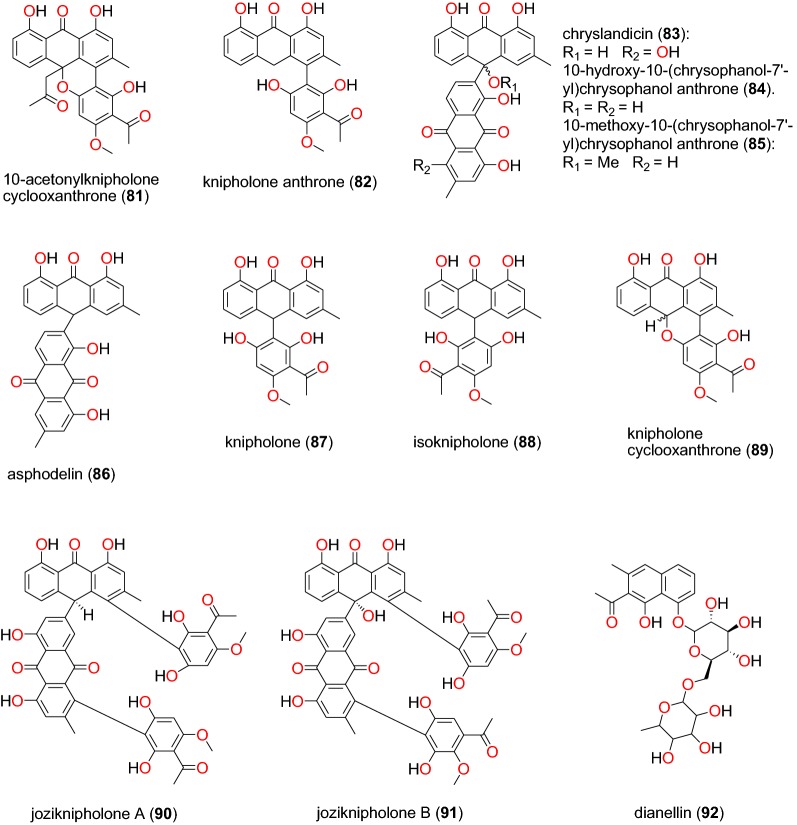
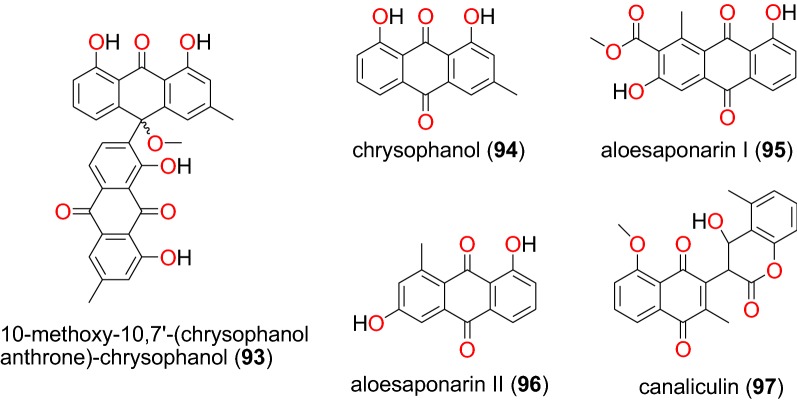
The study by Induli et al. of the rhizomes of Kniphofia foliosa (Asphodelaceae), growing in Ethiopia, led to the identification of several anthraquinones [85]: the novel 10-acetonylknipholone cyclooxanthrone (81), along with the known knipholone anthrone (82), chryslandicin (83), 10-hydroxy-10-(chrysophanol-7′-yl)-chrysophanol anthrone (84), 10-methoxy-10-(chrysophanol-7′-yl)chrysophanol anthrone (85), asphodelin (86), knipholone (87), isoknipholone (88) knipholone cyclooxanthrone (89), joziknipholone A (90), joziknipholone B (91) and dianellin (92) (Fig. 17). According to the authors, the IC50 values obtained for the plant extracts ranged from 3.4 to 8.9 μg/mL and from 3.4 to 8.9 μg/mL against the D6 and W2 strains of P. falciparum, respectively. The obtained compounds had IC50 values ranging from 0.47 to 23.25 μM and from 0.35 to 18.42 μM against the respective strains [85]. It is known that knipholone cyclooxanthrone (89) was actually isolated for the first time by Abdissa et al. and was also shown to exhibit antiplasmodial activities against the W2 and D6 strains with IC50 values of 14.58 and 9.42 μM, respectively [86].
The roots of the same plant, also harvested in Ethiopia, led Abdissa et al. to isolate a dimeric anthraquinone, 10-methoxy-10,7′-(chrysophanol anthrone)-chrysophanol (93) [86]. Compound 93 showed antiplasmodial activities against the W2 and D6 strains with IC50 values of 2.22 and 7.73 μM, respectively [86]. The investigations of Bringmann et al. led to the isolation of new dimeric phenylanthraquinones; joziknipholone A (90) and joziknipholone B (91) from the roots of Bulbine frutescens (Asphodelaceae) harvested in Kenya [87]. The authors also tested the two compounds against the K1 strain of P. falciparum and obtained remarkable activities, IC50 values of 0.17 and 0.26 μM, respectively [87]. Knipholone anthrone (82) was tested again from the leaves of the Ethiopian medicinal plant Kniphofia foliosa (Asphodelaceae) by Feilcke et al. [89]. The activity of this compound in several biological assays was described by the authors and showed antiplasmodial activity against 3D7 strain with IC50 value 0.7 μM.
The medicinal plant Aloe pulcherrima (Asphodelaceae) is one of the endemic Aloe species traditionally used for the treatment of malaria and wound healing in Central, Southern, and Northern Ethiopia, although the detailed mode of usage is not properly described in the literature [88]. Three compounds, chrysophanol (94), aloesaponarin I (95) and aloesaponarin II (96) (Fig. 18), were isolated from the acetone root extracts by Abdissa et al. [88]. The evaluation of their in vitro anti-malarial activities revealed moderate activity against D6 and W2 strains with IC50 values ranging from 19.66 to 82.80 μM and from 64.46 to 141.95 μM, respectively [88]. Knipholone (86) was also tested again from the leaves of Kniphofia foliosa (Asphodelaceae) by Feilcke et al. [89], showing significant antiplasmodial activity against the P. falciparum 3D7 strain, with an IC50 value of 1.9 μM.
Lenta et al. investigated the dichloromethane-methanol (1:1) extract of the stem bark of Diospyros canaliculata (Ebenaceae) harvested in Cameroon and obtained a new coumarinyl naphtoquinone, named canaliculin (97) [90]. The compound only exhibited weak activity against P. falciparum NF54 strain, unfortunately, along with pronounced toxicity [90].
Anthrones
The plant Aloe percrassa (Asphodelaceae), is an indigenous species used in Ethiopian folk medicine to treat malaria, wounds and gastric problems [91]. Aloin A (98) Aloin B (99) microdontin A (100) microdontin B (101) (Fig. 19), are four anthrones derived from the leaf latex of Aloe percrassa by Geremedhin et al. [91]. The anti-malarial activities of the mixtures of Aloin A/B and microdontin A/B were lower than the latex. The mixtures were shown to have suppressed parasitaemia from 36.8 to 66.8% at doses of 100 to 400 mg/kg/day. This suggested that the compounds within the two mixtures may have acted synergistically.
Naphthohydroquinones
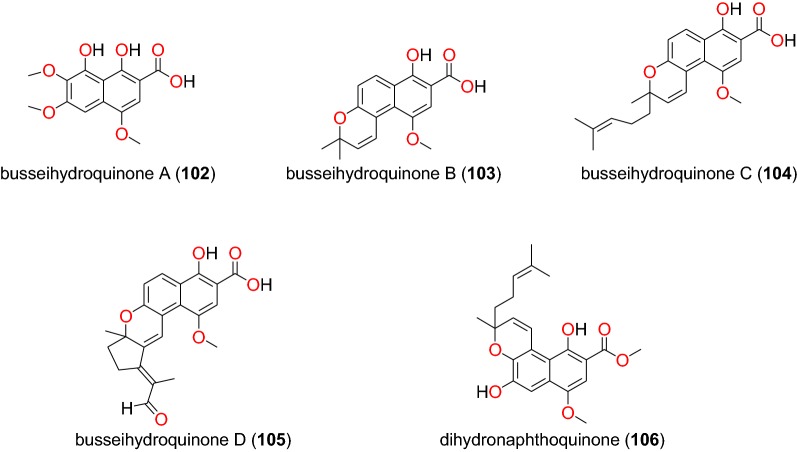
The plant species Pentas bussei (Rubiaceae) is frequently used in traditional medicine to treat malaria in Kenya, particularly the boiling of the roots and stems for oral consumption [92]. The roots of this species led Endale et al. to obtain five new naphthohydroquinones, called busseihydroquinone A (102) busseihydroquinone B (103) busseihydroquinone C (104) busseihydroquinone D (105) and the homoprenylated naphthoquinone named dihydronaphthoquinone (106) (Fig. 20). These compounds exhibited marginal activities against the D6 and W2 strains with IC50 values ranged from 19.59 to 36.03 μM and from 60.08 to 144.43 μM, respectively [92].
Other quinones
Peperovulcanone A (107), derived from the crude extracts of the whole plant of Peperomia vulcanica (Piperaceae), harvested from Cameroon, was shown to be active against the W2mef strain of P. falciparum with an IC50 value of 52.25 μM [79]. The new compound named, neoboutomacroin (108), was derived from extracts of the stem bark of Neoboutonia macrocalyx (Euphorbiaceae) from Uganda by Namukobe et al. [93]. Compound 108 displayed good antiplasmodial activity with IC50 values of 19.28 and 14.17 μM against the D6 and W2 strains, respectively.
Steroids
Ergostane phytosterols
A summary of bioactive steroids has been provided in Table 5. The novel steroids; 6α-methoxy-4,24(28)-ergostadiene-7α,20S-diol (109), 6α-methoxy-4,24(28)-ergostadien-7α-ol (110) (Fig. 21), along with the known steroid 7,20S-dihydroxyergosta-4,24(28)-dien-3-one (111) (Fig. 22), were isolated from the stem bark of Antrocaryon klaineanum (Anacardiaceae) by Douanla et al. [94]. The crude extracts and the isolated compounds were evaluated in vitro against the 3D7 and W2 strains of P. falciparum. While the crude extract showed moderate activity (IC50 = 16.7 µg/mL) against 3D7, the three steroids exhibited potent activity against both strains with IC50 values of 22.0, 11.2 and 21.3 µM, respectively, against the same strain.
Table 5.
Summary of steroids
| Compound subclass | Isolated metabolites | Plasmodial strain (activities) | Plant species (Family), Taxon IDb | Part of the plant studied | Place of harvest (City, Country) | Author, references |
|---|---|---|---|---|---|---|
| Ergostane phytosterols | 109a, 110a, and 111 | 3D7 (IC50 values range from 11.2 to 22.0 µM) | Antrocaryon klaineanum (Anacardiaceae), NCBI:txid289695 | Stem bark | Mount Kala, Cameroon | Douanla et al. [94] |
| W2 ( IC50 values range from 11.2 to 22.0 µM) | ||||||
| 112 | W2mef (IC50 value = 53.45 µM) | Peperomia vulcanica (Piperaceae), NCBI:txid1719589 | Whole plant | Mount Cameroon, Cameroon | Ngemenya et al. [79] | |
| 113 | W2 (IC50 value = 153.79 µM) | Polyalthia longifolium var. pendula (Annonaceae), NCBI:txid235806 | Stem | Tikrom, near Kumasi, Ghana | Gbedema et al. [68] | |
| 113 | W2 (IC50 value = 172.9 µM) | Turraea robusta (Meliaceae), NCBI:txid1899148 | Stem bark | Nairobi, Kenya | Irungu et al. [95] | |
| D6 (IC50 value = 68.3 µM) | ||||||
| Phytosterol glucosides | 114 to 116 | D6 and W2 (from weak to moderate activities) | Turraea nilotica (Meliaceae), NCBI:txid992803 | Stem bark | Nairobi, Kenya | Irungu et al. [95] |
aCompounds identified for the first time in the cited publications
bIdentification number of the source species, derived from the NCBI Taxonomy database
Fig. 22.
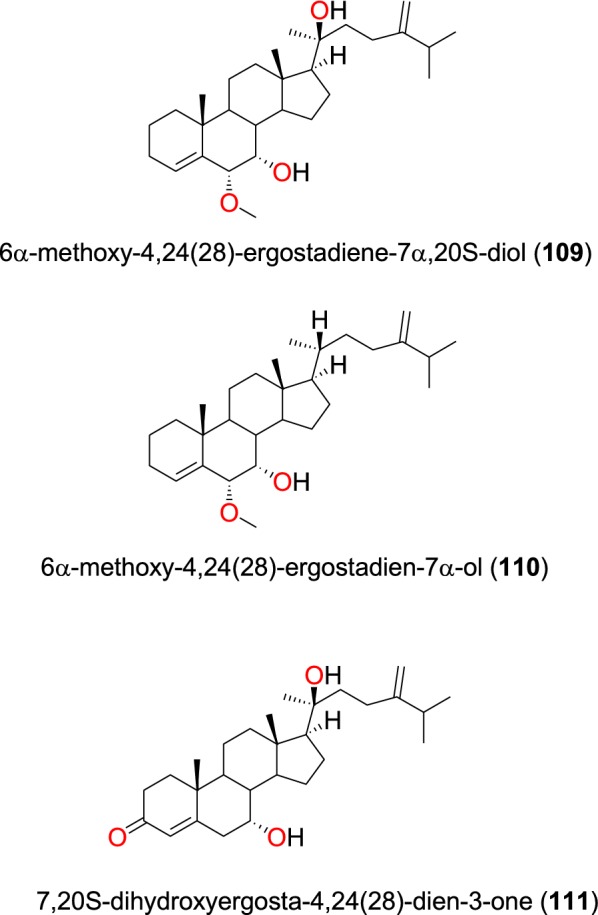
Ergostane phytosterols (109 to 111)
The known steroid stigmasterol (112) (Fig. 23), obtained from the whole plant of Peperomia vulcanica (Piperaceae), also showed antiplasmodial activity against the W2mef strain with an IC50 value of 53.45 µM [79]. β-stigmasterol (113) was also isolated from the stem of the Polyalthia longifolium (Annonaceae) harvested in Ghana [68]. This compound exhibited weak antiplasmodial activity against the K1, D6 and W2 strains of P. falciparum with IC50 values of 153.79, 68.3 and 172.9 µM, respectively [68, 94].
Fig. 23.
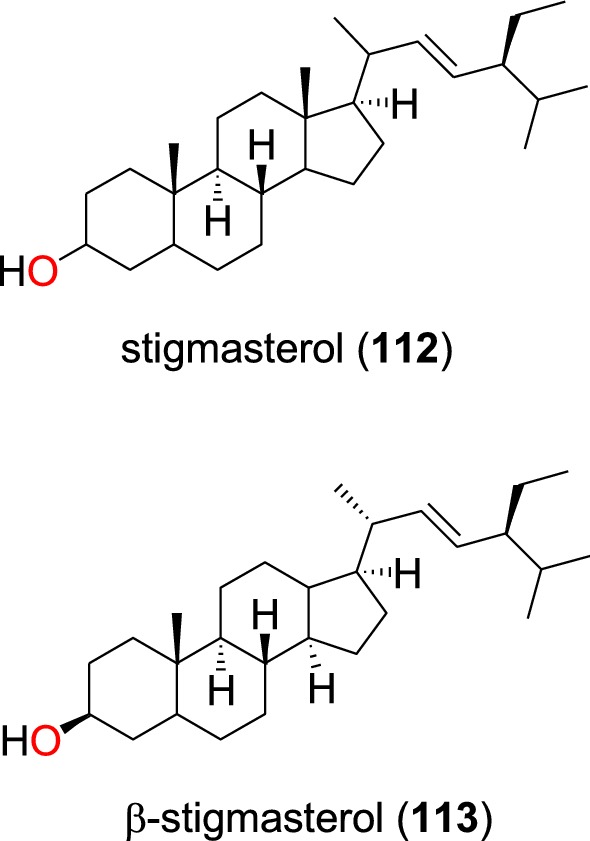
Ergostane phytosterols (112 and 113)
Phytosterol glucosides
The known steroid glycosides; sitosterol-3-O-β-d-glucopyranoside acetate (114), stigmasterol-3-O-β-d-glucopyranoside acetate (115), sitosterol-3-O-β-d-glucopyranoside (116) (Fig. 24), as well as a mixture of β-sitosterol and stigmasterol (112) were identified from the leaves of Turraea nilotica (Meliaceae) [95]. The glycosides only showed weak to moderate antiplasmodial activities against the D6 and W2 strains.
Fig. 24.
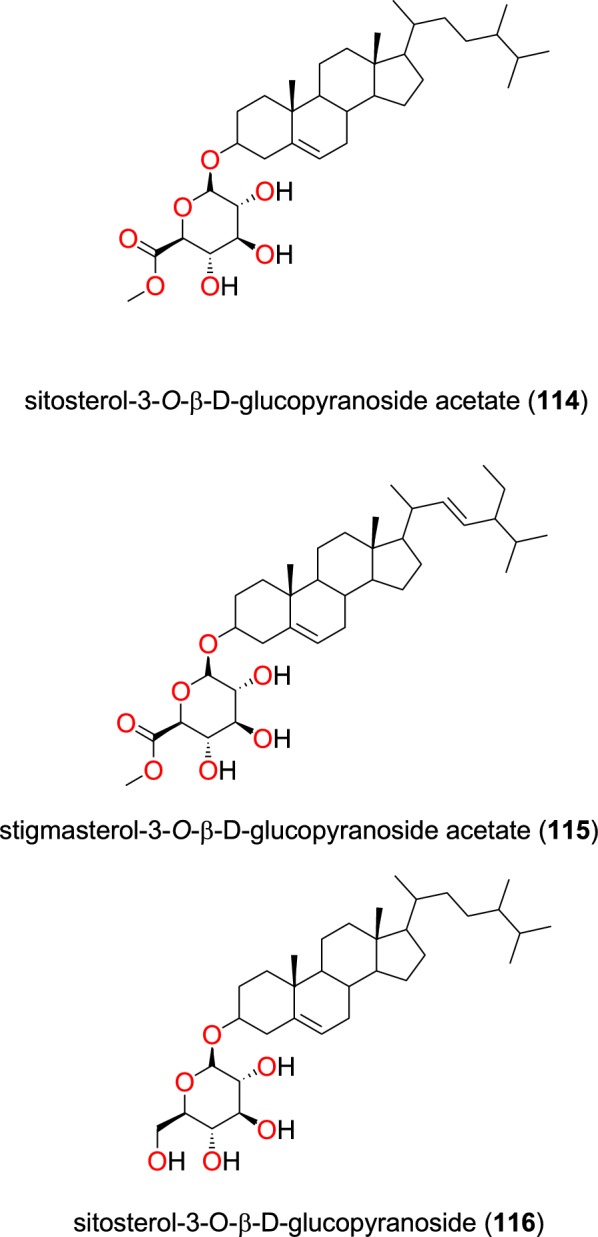
Phytosterol glucosides (114 to 116)
Terpenoids
The summary of the most promising diterpenoids and sesquiterpenoids has been provided in Table 6, while those of triterpenoids have been shown in Table 7.
Table 6.
Summary of diterpenoids and sesquiterpenoids
| Compound subclass | Isolated metabolites | Plasmodial strain (activities) | Plant species (Family), Taxon IDb | Part of the plant studied | Place of harvest (Locality, Country) | Author, references |
|---|---|---|---|---|---|---|
| Clerodane diterpenes | 117 to 119 | K1 (IC50 values range from 9.59 to 18.41 µM) | Polyalthia longifolium var. pendula (Annonaceae), NCBI:txid235806 | Stem | Tikrom, near Kumasi, Ghana | Gbedema et al. [68] |
| Daphnane diterpenoids | 120 | FcB1 (IC50 value = 19.02 µM) | Neoboutonia macrocalyx (Euphorbaceae), NCBI:txid316724 | Stem bark | Kibale National Park, Uganda | Namukobe et al. [96] |
| 121 and 122 | D6 (IC50 values = 65.14 and 6.96 µM, respectively) | Neoboutonia macrocalyx (Euphorbaceae), NCBI:txid316724 | Stem bark | Kibale National Park, Uganda | Namukobe et al. [93] | |
| W2 (IC50 values = 57.82 and 4.10 µM, respectively) | ||||||
| Iridoid diterpenoid | 123 | K1 (IC50 value = 171.68 µM) | Canthium multiflorum (Rubiaceae), NCBI:txid58501 | Aerial part | Obala, along River Sanaga, Cameroon | Kouam et al. [69] |
| Labdane diterpenoids | 124 | Suppression of Plasmodium berghei at doses of 25, 50 and 100 mg/kg with chemosuppression values of 50.13, 65.58 and 73.16%, respectively. | Otostegia integrifolia (syn: Rydingia integrifolia, Lamiaceae), NCBI:txid483857 | Leaves | Chancho, Central Ethiopia | Endale et al. [97] |
| Norcassane furanoditerpene | 125 | 3D7 (IC50 value = 2.20 μM) | Caesalpinia bonducella (Caesalpiniaceae), NCBI:txid53845 | Roots | Dar es Salaam Region, Tanzania | Nondo et al. [98] |
| Dd2 (IC50 value = 4.16 μM) | ||||||
| Sesquiterpenoids | 126a, 127a, 128a, 129a, and 130a | W2 (IC50 values range from 1.71 to 2.63 µM) | Salacia longipes (Celastraceae), NCBI:txid662028 | Seeds | Mount Kala, Cameroon | Mba’ning et al. [99] |
| 131a | NF54 (IC50 value = 15.69 μM) | Scleria striatinux (Cyperaceae), NCBI:txid1916803 | Rhizomes | Oku, Cameroon | Nyongbela et al. [100] | |
| K1 (IC50 value = 13.54 μM) |
aCompounds identified for the first time in the cited publications
bIdentification number of the source species, derived from the NCBI Taxonomy database
Table 7.
Summary of triterpenoids
| Compound subclass | Isolated metabolites | Plasmodial strain (activities) | Plant species (Family), Taxon IDb | Part of the plant studied | Place of harvest (Locality, Country) | Author, references |
|---|---|---|---|---|---|---|
| Acyclic triterpenes | 132 and 133 | D6 (IC50 values = 27.1 and 56.1 µM, respectively) | Ekebergia capensis (Meliaceae), NCBI:txid124949 | Leaves | Gakoe forest, Kiambu County, Kenya | Irungu et al. [80] |
| W2 (IC50 values = 66.9 and 64.3 µM, respectively) | ||||||
| Apotirucallane triterpenoids | 134a, 135a, 136a, 137a, 138a, 139a, and 140 to 142 | NF54 (IC50 values range from 0.67 to 19.3 µM) | Entandrophragma congoense (Meliaceae), NCBI:txid2590899 | Bark | Nkomokui, Cameroon | Happi et al. [101] |
| Cycloartane triterpenes | 143 to 150 | FcB1 (all IC50 values < 11 μM, the lowest value being 1.48 μM) | Neoboutonia macrocalyx (Euphorbaceae), NCBI:txid316724 | Stem bark | Kibale National Park, Uganda | Namukobe et al. [96] |
| aAll new | ||||||
| Lanostane triterpene | 151a | D6 (IC50 value = 257.8 nM) | Ganoderma sp. (Ganodermataceae), NCBI:txid5314 | Whole organism | Egypt | Wahba et al. [102] |
| W2 (IC50 value = 2000.0 nM) | ||||||
| Limonoids | 152 | D6 (IC50 value = 84.7 µM) | Ekebergia capensis (Meliaceae), NCBI:txid124949 | Leaves | Gakoe forest, Kiambu County, Kenya | Irungu et al. [80] |
| W2 (IC50 value = 150.2 µM) | ||||||
| 153 to 157 | D6 (IC50 values range from 2.4 to 36.6 µM) | Turraea robusta (Meliaceae), NCBI:txid1899148 | Root bark | Nairobi, Kenya | Irungu et al. [95] | |
| W2 (from 1.1 to 40.5 µM) | ||||||
| Oleanane triterpenes | 158 to 161 | D6 (IC50 values range from 38.8 to 205.0 µM) | Ekebergia capensis (Meliaceae), NCBI:txid124949 | Leaves | Gakoe forest, Kiambu County, Kenya | Irungu et al. [80] |
| W2 (IC50 values range from 76.7 to 179.4 µM) | ||||||
| 160 and 162 | 3D7 (IC50 values = 59.4 and 32.4 µM, respectively) | Keetia leucantha (Rubiaceae), NCBI:txid 43504 | Twigs | Adjarra-Ouémé, Benin Republic | Bero et al. [103] | |
| 162, 163 and 164 | D10 (IC50 values range from 3.81 to 15.54 μM) | Mimusops caffra (Sapotaceae), NCBI:txid362720 | Leaves | Durban, KwaZulu-Natal Province, South Africa | Simelane et al. [104] | |
| Tirucallane-type triterpenoids | 165a , 166a and 167 | NF54 (IC50 values range from 2.4 to 6.1 µM) | Entandrophragma congoense (Meliaceae), NCBI:txid2590899 | Bark | Nkomokui, Cameroon | Happi et al. [105] |
| Protolimonoids | 168 to 170 | D6 (IC50 values range from 36.8 to 48.2 µM) | Turraea nilotica (Meliaceae), NCBI:txid992803 | Stem bark | Nairobi, Kenya | Irungu et al. [95] |
| W2 (IC50 values range from 37.2 to 77.0 µM) | ||||||
| Other triterpenoids (hopane-type and cycloartane-type) | 171 | NF54 (IC50 value = 112.94 μM) | Diospyros canaliculata (Ebenaceae), NCBI:txid13492 | Stem bark | Kribi, Cameroon | Lenta et al. [90] |
| 172 | NF54 (IC50 value = 97.73 μM) | Erythrina caffra (Papilionaceae), NCBI:txid3842 | Stem bark | Pietermaritzburg, South Africa | Chukwujekwu et al. [106] | |
| 173 | FcB1( IC50 value = 2.15 µM) | Neoboutonia macrocalyx (Euphorbaceae), NCBI:txid316724 | Stem bark | Kibale National Park, Uganda | Namukobe et al. [96] |
aCompounds identified for the first time in the cited publications
bIdentification number of the source species, derived from the NCBI Taxonomy database
Clerodane diterpenes
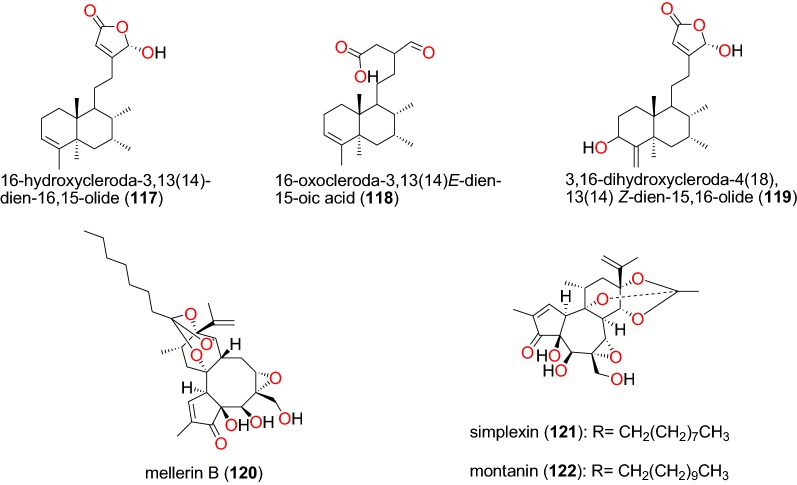
The ethanolic extract of Polyalthia longifolium var. pendula, which is traditionally used to treat malaria in Ghana (the traditional preparation not properly described in the literature) displayed in vitro antiplasmodial activity against the multidrug-resistant, K1 strain with an IC50 value of 22.04 μg/mL. Spectroscopic analysis of compounds obtained from this extract led to the identification of three known clerodane diterpenes; 16-hydroxycleroda-3,13(14)-dien-16,15-olide (117), 16-oxocleroda-3,13(14)E-dien-15-oic acid (118), and 3,16-dihydroxycleroda-4(18),13(14) Z-dien-15,16-olide (119) (Fig. 25) [68]. The compounds showed activities with IC50 values varying from 9.59 to 18.41 µM.
Fig. 25.
Clerodane and daphnane diterpenoids (117 to 122)
Daphnane diterpenes
The daphnane diterpenoid mellerin B (120) was isolated from the stem bark of Neoboutonia macrocalyx (Euphorbaceae) and potently inhibited the CQ-resistant FcB1/Colombia strain of P. falciparum, with an IC50 value 19.02 µM [96]. Chemical investigation of the stem bark of Neoboutonia macrocalyx (Euphorbiaceae) also yielded simplexin (121) and montanin (122) (Fig. 25), which showed antiplasmodial activities against the D6 and W2 strains, with IC50 values of 65.14 and 57.82 µM, respectively, and 6.96 and 4.10 µM, respectively [93].
Iridoids, labdanes, and norcassane furanoditerpenes
From the aerial part of Canthium multiflorum (Rubiaceae) harvested in Cameroon, Kouam et al. also isolated the known iridoid, garjasmine (123) (Fig. 26) [69]. This compound only showed weak inhibition against the K1 strain of P. falciparum, with an IC50 value of 171.68 µM [69].
Fig. 26.
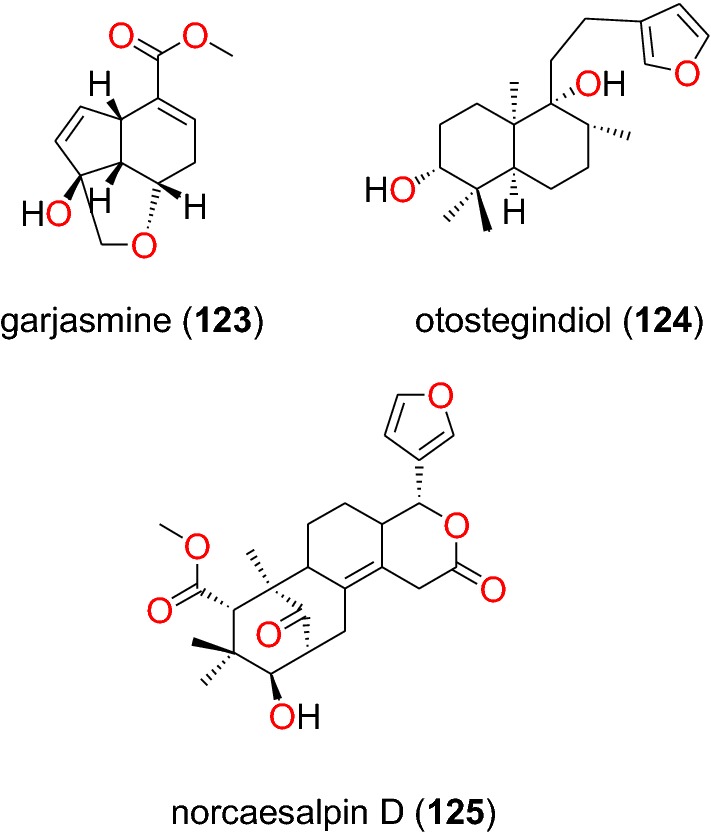
Iridoid, labdanes and norcassane diterpenoids (123 to 125)
The leaves of Otostegia integrifolia (Lamiaceae) are used in Ethiopian folk medicine for the treatment of several diseases including malaria [97]. The known labdane diterpenoid, otostegindiol (124) (Fig. 26) was isolated from the methanol leaf extract of the species by Endale et al. [97]. The isolated compound 125 displayed a significant (p < 0.001) anti-malarial activity at doses of 25, 50 and 100 mg/kg with chemosuppression values of 50.13, 65.58 and 73.16%, respectively. The previously reported norcassane furanoditerpene, norcaesalpin D (125), was isolated from the roots of Caesalpinia bonducella (Caesalpiniaceae) from Tanzania by Nondo et al. [98]. This compound was active with an IC50 value of 2.20 and 4.16 µM against the 3D7 and Dd2 strains, respectively [98].
Sesquiterpenoids
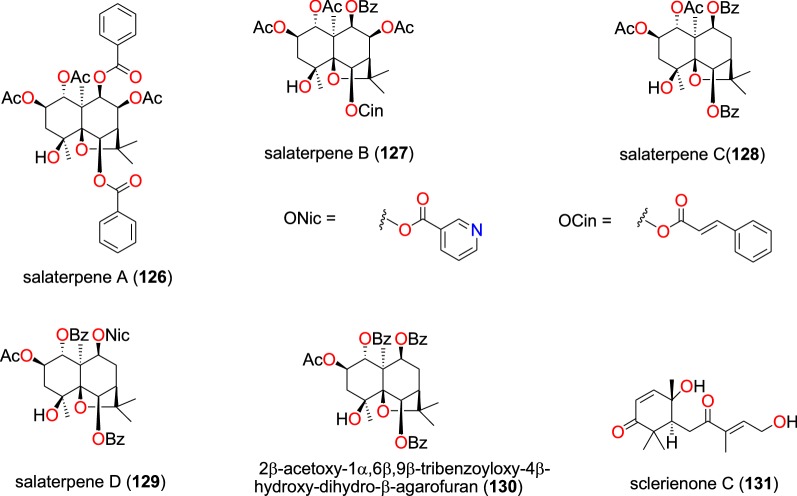
The novel sesquiterpenoids salaterpenes A–D (126 to 129), and 2β-acetoxy-1α,6β,9β-tribenzoyloxy-4β-hydroxy-dihydro-β-agarofuran (130) (Fig. 27), were isolated from the seeds of Salacia longipes (Celastraceae), harvested in Cameroon by Mba’ning et al. [99]. The investigation of their potential for anti-malarial drug discovery demonstrated that these compounds inhibited the W2 strain of P. falciparum with IC50 values varying from 1.71 to 2.63 µM [99].
Fig. 27.
Sesquiterpenes (126 to 131)
Nyongbela et al. [100] isolated the new sesquiterpene sclerienone C (131) from the rhizomes of Scleria striatonux (Cyperaceae), harvested from Cameroon. According to the authors, this compound exhibited antimicrobial and antiplasmodial activities with IC50 values against the NF54 and K1 strains of 15.69 and 13.54 μM, respectively [100].
Acyclic triterpenes
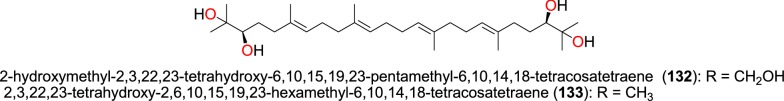
The previously reported acyclic triterpenes; 2-hydroxymethyl-2,3,22,23-tetrahydroxy-6,10,15,19,23-pentamethyl-6,10,14,18-tetracosatetraene (132) and 2,3,22,23-tetrahydroxy-2,6,10,15,19,23-hexamethyl-6,10,14,18-tetracosatetraene (133) were isolated from the leaves of the Ekebergia capensis (Meliaceae) harvested in Kenya [80]. The compounds (Fig. 28) exhibited selective antiplasmodial activity against the W2 strain, with IC50 values of 27.1 and 56.1 µM, and against the D6 66.9 and 64.3 µM, respectively [80].
Fig. 28.
Acyclic triterpenes (132 and 133)
Apotirucallane triterpenoids
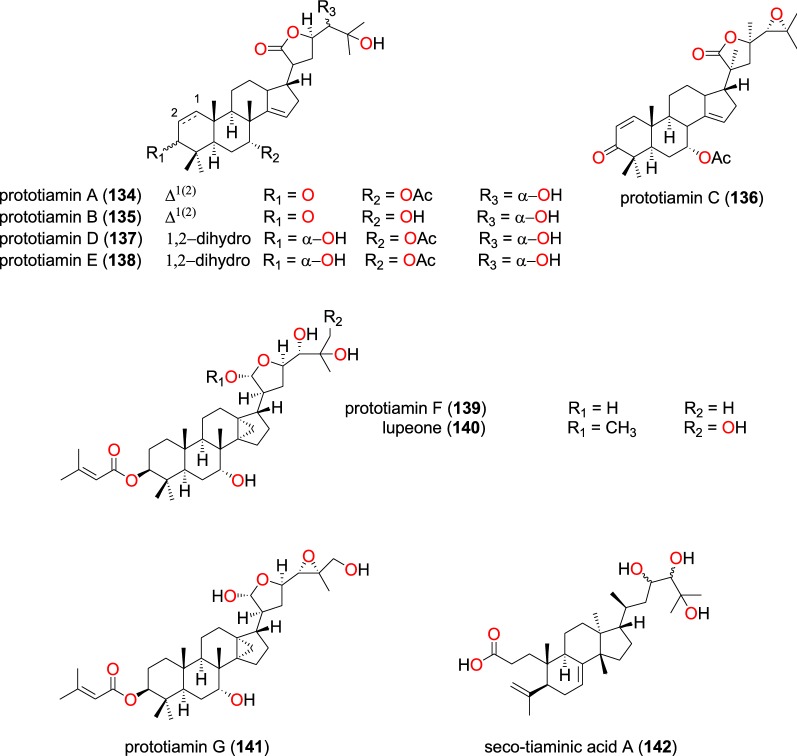
Phytochemical investigations of the root barks of Entandrophragma congoense (Meliaceae) harvested from Kenya, led Happi et al. to the isolation of the novel apotirucallane triterpenoids with antiplasmodial activities; prototiamins A–F (134–139) (Fig. 29), as well as the known lupeone (140), prototiamin G (141) and seco-tiaminic acid A (142) [101]. The obtained compounds (134–142) were also evaluated against the CQ-sensitive strain NF54. Compound 134 displayed strong selectivity for the NF54 strain against rat skeletal myoblast L6 cells (with a selectivity index of 104.7), while 136 and 138 had selective indices of 12 and 13, respectively. Compounds 135, 137, 139, and 140 were active against P. falciparum, with IC50 values ranging from 1.3 to 2.0 μM, and were less selective, while compound 142 inhibited the strain with an IC50 value of 19.3 μM.
Fig. 29.
Apotirucallane triterpenoids (134 to 142)
Cycloartane triterpenes
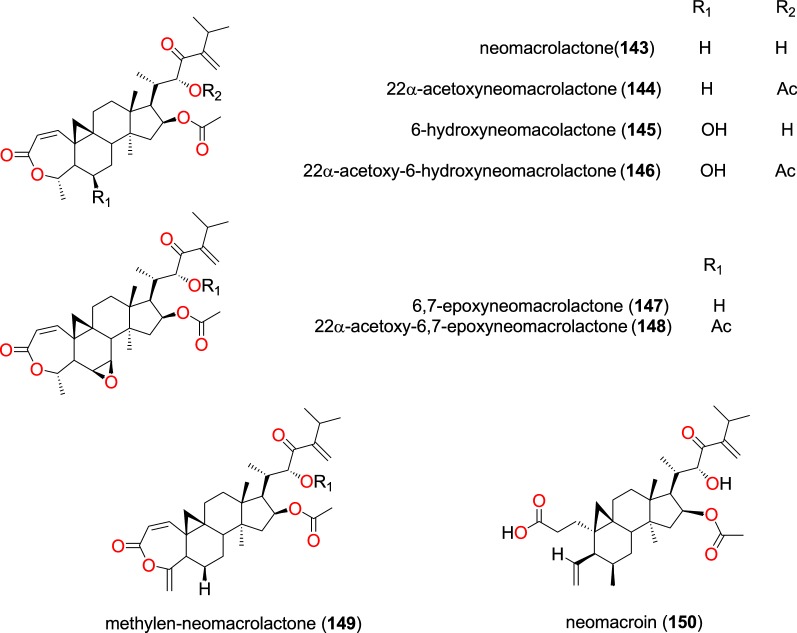
The plant species Neoboutonia macrocalyx (Euphorbiaceae) is traditionally used to treat malaria in Southwestern Uganda around Kibale National Park, where the stem bark is widely used [96]. The investigation of the stem bark of this plant by Namukobe et al. led to the isolation nine new cycloartane triterpenes, among which eight; neomacrolactone (143), 22α-acetoxyneomacrolactone (144), 6-hydroxyneomacolactone (145), 22α-acetoxy-6-hydroxyneomacrolactone (146), 6,7-epoxyneomacrolactone (147), 22α-acetoxy-6,7-epoxyneomacrolactone (148), 4-methylen-neomacrolactone (149), and neomacroin (150), Fig. 30, displayed anti-malarial properties [96]. The obtained compounds were also evaluated for antiplasmodial activity against the FcB1/Colombia strain and for cytotoxicity against the KB (nasopharyngeal epidermoid carcinoma) and MRC-5 (human diploid embryonic lung) cells. Compounds (143–147, 149,150) exhibited antiplasmodial activities with IC50 of < 11 μM [96].
Fig. 30.
Cycloartane triterpenes (143 to 150)
Lanostane triterpene and limonoids
Ganoderic acid AW1 (151) (Fig. 31), a new lanostane triterpene, was isolated from the whole organism of the Ganoderma sp. (Ganodermataceae) collected from Egypt [102]. This compound exhibited good anti-malarial activity against the D6 strain of P. falciparum with an IC50 value of 257.8 nM with no cytotoxicity up to the concentration of 9 μM. The compound also tested positive against the W2 strain with an IC50 value of 2000 nM [102].
Fig. 31.
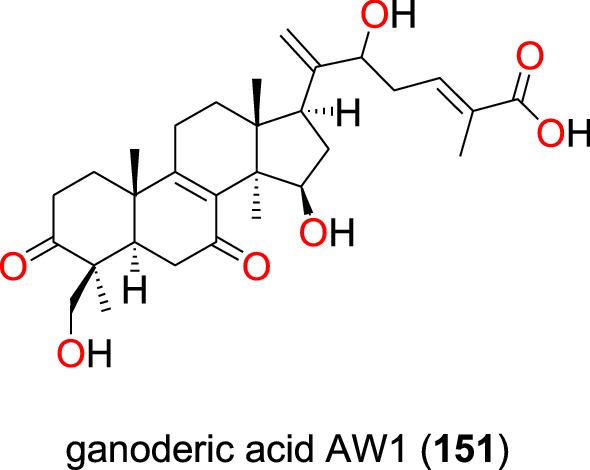
Lanostane triterpene (151)
The known limonoid, proceranolide (152) (Fig. 32) was found in the leaves of Ekebergia capensis (Meliaceae) by Irungu et al. [80]. The isolated compound was then evaluated in vitro against the D6 and W2 strains of P. falciparum. This compound exhibited weak antiplasmodial activity against the D6 and W2 strains with IC50 values of 84.7 and 150.2 µM, respectively [80].
Fig. 32.
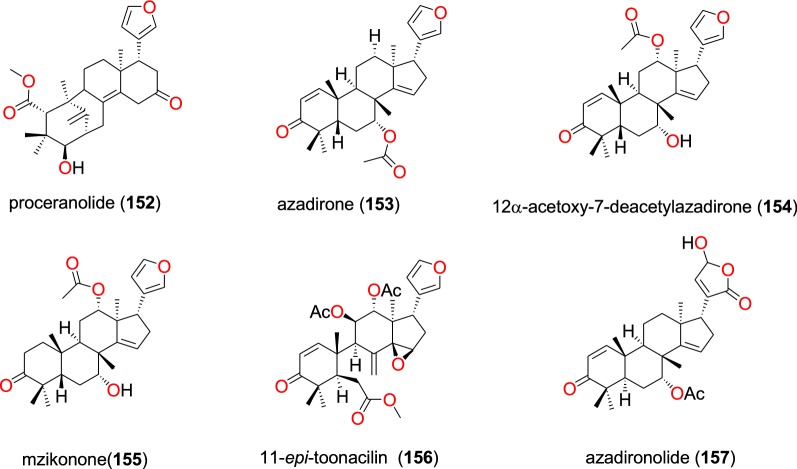
Limonoids (152 to 157)
Additionally, three known limonoids; azadirone (153), 12α-acetoxy-7-deacetylazadirone (154), mzikonone (155), 11-epi-toonacilin (156) and azadironolide (157), which were isolated from the stem bark of Turraea nilotica (Meliaceae), all showed potent antiplasmodial activity against the D6 and W2 strains with IC50 values ranged from 2.4 to 36.6 µM and from 1.1 to 40.5 µM, respectively [95].
Oleanane triterpenes
The known oleanonic acid (158), 3-epi-oleanolic acid (159), oleanolic acid (160) and ekeberin A (161) (Fig. 33) were also isolated from the leaves of Ekebergia capensis by Irungu et al. [80]. The four oleanane triterpenes potently inhibited the D6 and W2 strains of P. falciparum with IC50 values ranging from 38.8 to 205.0 µM and from 76.7 to 179.4 µM, respectively, against both strains.
Fig. 33.
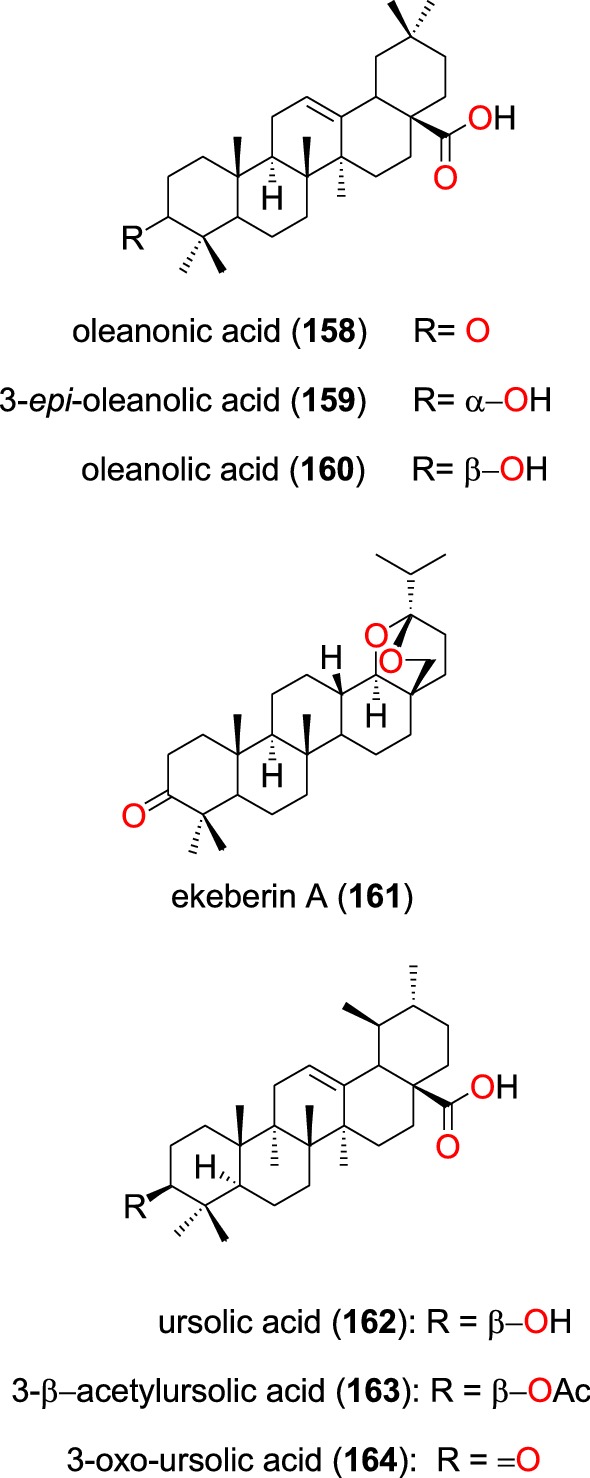
Oleanane triterpenes (158 to 164)
Bero et al. also isolated the known ursolic acid (162) and oleanolic acid (160) from the twigs of Keetia leucantha (Rubiaceae). The authors re-tested the compounds, showing them to have in vitro activities on the 3D7 strain of P. falciparum with IC50 values of 32.4 and 59.4 µM, respectively [104]. From the leaves of Mimusops caffra (Sapotaceae) growing in South Africa, ursolic acid acetate (163) and 3-oxo-ursolic acid (164), as well as the known compound 162 were isolated by Simelane et al. [104]. These three compounds showed promising in vitro activities against the D10 strain with IC50 values ranging from 3.81 to 15.54 μM [104].
Tirucallane-type triterpenoids
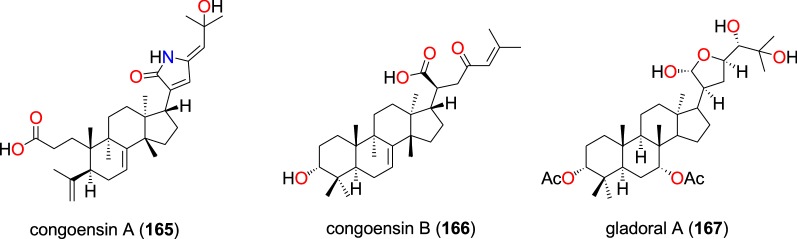
Two new tirucallane-type triterpenoids, namely congoensin A (165) and congoensin B (166), along with the known tirucallane-type triterpenoid gladoral A (167) (Fig. 34) were isolated from the bark of Entandrophragma congoënse (Meliaceae) harvested from Cameroon by Happi et al. [105]. These compounds exhibited activities against the NF54 strain with IC50 values ranging from 2.4 to 6.1 µM [105].
Fig. 34.
Tirucallane-type triterpenoids (165 to 167)
Protolimonoids
Irungu et al. [95] also examined the stem bark of Turraea nilotica (Meliaceae) growing in Kenya. Three known potent anti-malarial protolimonoids; niloticin (168), hispidol B (169) and piscidinol A (170) were isolated (Fig. 35). These compounds exhibited activities against the D6 strain with IC50 values ranging from 36.8 to 48.2 µM and against the W2 strain, with IC50 values ranging from 37.2 to 77.0 µM [95].
Fig. 35.
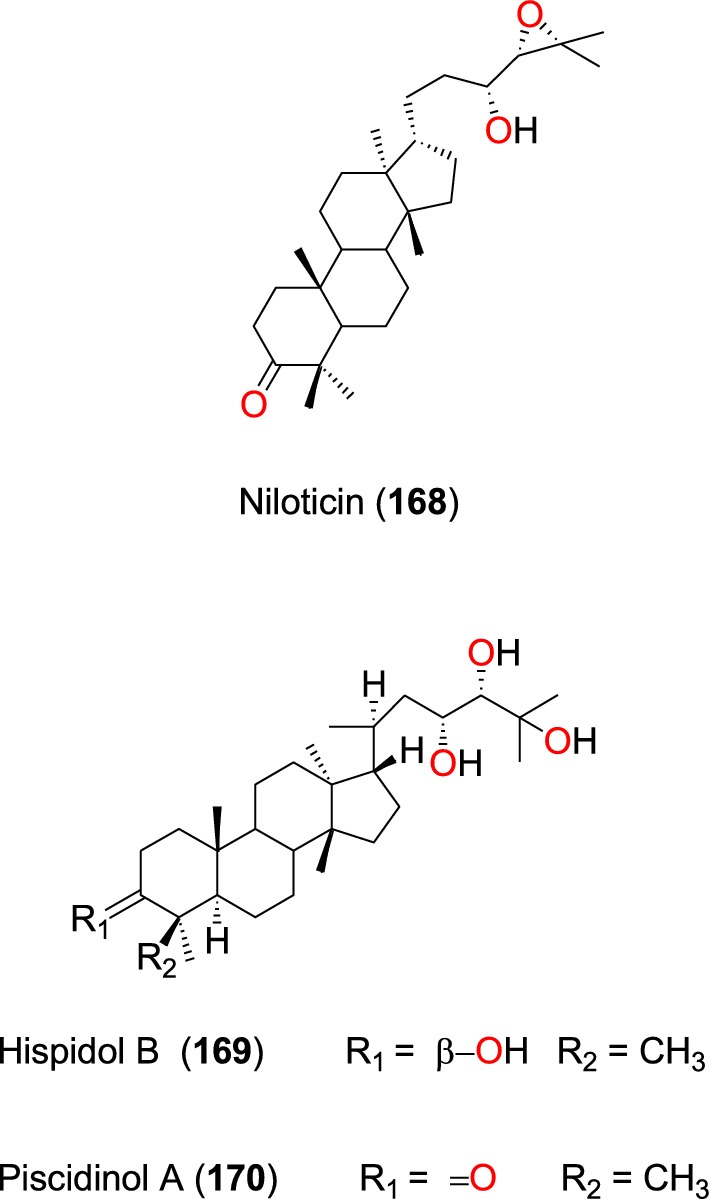
Protolimonoids (168 to 170)
Other triterpenoids
The known hopane type triterpenoids; betulin (171) and lupeol (172) (Fig. 36) were isolated from the stem bark of Diospyros canaliculata (Ebenaceae) and Erythrina caffra (Papilionaceae), respectively [90, 106]. These triterpenoids only exhibited weak activities against the NF54 strain, with IC50 values of 112.94 and 97.73 μM, respectively [90, 106]. The cycloartane-type triterpenoid 22-de-O-acetyl-26-deoxyneoboutomellerone (173) was isolated from the stem bark of Neoboutonia macrocalyx (Euphorbaceae) [96]. The compound potently inhibited the CQ-resistant FcB1/Colombia strain of P. falciparum, with IC50 value of 2.15 µM [96].
Fig. 36.
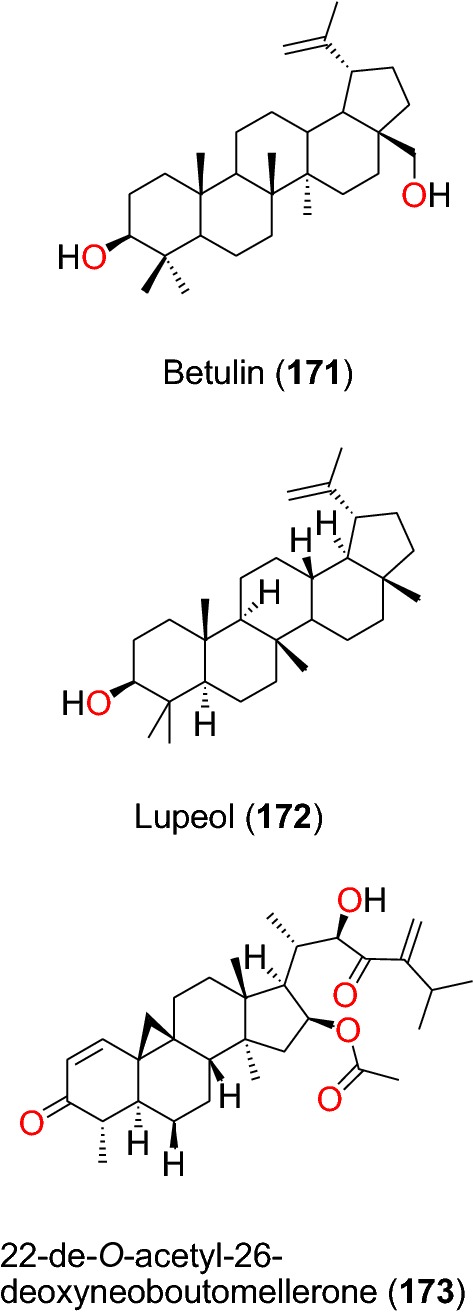
Other triterpenes (171 and 173)
Other compound classes
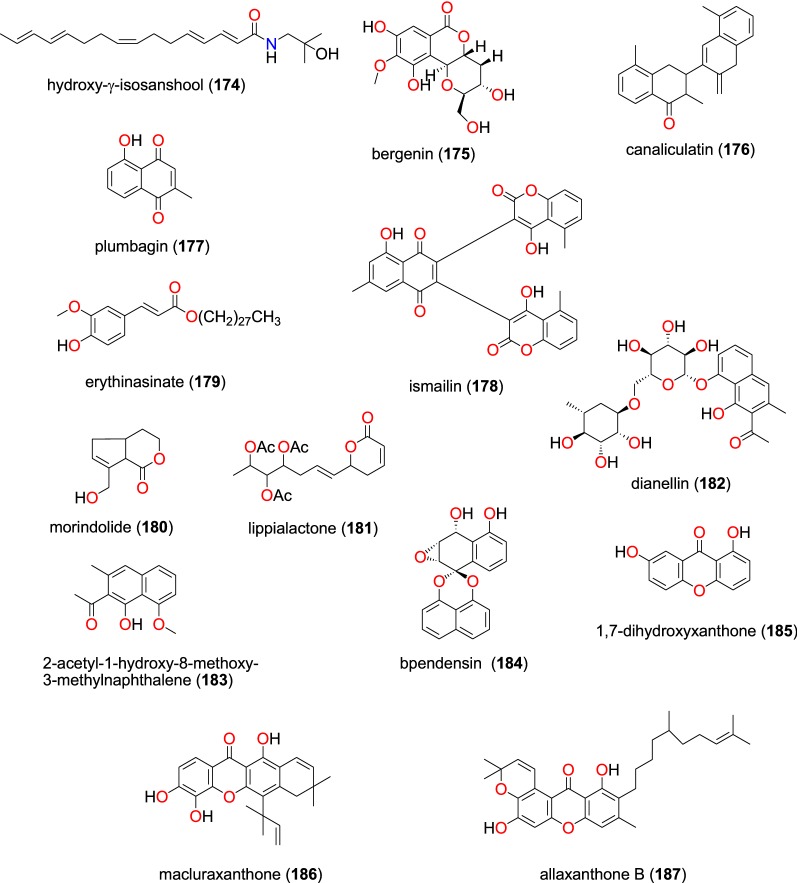
These are summarized in Table 8. The amide hydroxy-γ-isosanshool (174) and the coumarin bergenin (175), Fig. 37, obtained from the leaves of Zanthoxylum heterophyllum (Rutaceae) and Diospyros conocarpa (Ebenaceae), respectively [107, 108]. While the amide showed and activity against the 3D7 strain with IC50 = 39.04 µM [107], and percentage viability of compound 175 was recorded as 101.15 against the same plasmodial strain [108]. Lenta et al. also isolated three known coumarins; canaliculatin (176), plumbagin (177) and ismailin (178) from the stem bark of Diospyros canaliculata (Ebenaceae) harvested from Cameroon [90]. The compounds were shown to be active against the NF54 strain of P. falciparum with IC50 values ranging from 2.17 to 60.09 µM [90].
Table 8.
Summary of other compound classes
| Compound subclass | Isolated metabolites | Plasmodial strain (activities) | Plant species (Family), Taxon IDa | Part of the plant studied | Place of harvest (Locality, Country) | Author, references |
|---|---|---|---|---|---|---|
| Amide | 174 | 3D7 (IC50 value = 39.04 µM) | Zanthoxylum heterophyllum (Rutaceae), NCBI:txid1908418 | Leaves | Langevin, Reunion Island | Ledoux et al. [107] |
| Coumarins | 175 | 3D7 (viability percentage = 101.15) | Diospyros conocarpa (Ebenaceae), NCBI:txid13492 | Leaves, trunk, and roots | Ntouessong and Nkoemvone, Cameroon | Fouokeng et al. [108] |
| 176, 177 and 178 | NF54 (IC50 values vary from 2.17 to 60.09 µM) | Diospyros canaliculata (Ebenaceae), NCBI:txid13492 | Stem bark | Kribi, Cameroon | Lenta et al. [90] | |
| Ester | 179 | NF54 (IC50 value = 42.59 µM) | Erythrina caffra (Papilionaceae), NCBI:txid3842 | Stem bark | Pietermaritzburg, South Africa | Chukwujekwu et al. [106] |
| Lactones | 180 | NF54 (IC50 value = 109.99 µM) | Vangueria infausta spp. infausta (Rubiaceae), NCBI:txid164485 | Roots | Mutale Municipality, Limpopo Province, South Africa | Bapela [109] |
| 181 | D10 (IC50 value = 24.70 µM) | Lippia javanica (Verbenaceae), NCBI:txid925357 | Leaves | Thathe Vondo village, Limpopo Province, South Africa | Ludere et al. [110] | |
| Naphthalene derivatives | 182 and 183 | D6 (IC50 value = 10.52 µM for compound 182) | Kniphofia foliosa (Asphodelaceae), NCBI:txid214838 | Rhizomes | Addis Ababa, Ethiopia | Induli et al. [85] |
| W2 (IC50 value = 6.32 µM for compound 182) | ||||||
| 3D7 (IC50 value = 67.32 µM for compound 183) | ||||||
| 182 | D6 (IC50 value = 10.48 µM) | Kniphofia foliosa (Asphodelaceae), NCBI:txid214838 | Roots | Gedo, Ethiopia | Abdissa n [86] | |
| W2 (IC50 value = 6.28 µM) | ||||||
| Spirobisnaphthalene | 184 | NF54 (IC50 value = 73.28 µM) | Entandrophragma congoense (Meliaceae), NCBI:txid2590899 | Bark | Nkomokui, Cameroon | Happi et al. [101] |
| Xanthones | 185 to 187 | F32/24h (IC50 values range from 1.16 to 70.33 µM) | Allanblackia floribunda (Guttiferae- Clusiaceae), NCBI:txid469914 | Whole plant | Mount Kala, Cameroon | Azebaze et al. [78] |
| F32/72h (from 0.91 to 50.23 µM) | ||||||
| FCM29/24h (from 0.83 to 17.93 µM) | ||||||
| FCM29/24h (from 0.68 to 67.22 µM) |
aIdentification number of the source species, derived from the NCBI Taxonomy database
Fig. 37.
Other classes (174 to 187)
The known ester erythinasinate (179) was isolated from the stem bark of Erythrina caffra (Papilionaceae) collected in South Africa by Chukwujekwu et al. [106] and inhibited the NF54 strain with an IC50 value of 42.59 µM. The antiplasmodial activities of two lactones: morindolide (180) and lippialactone (181), obtained from roots of Vangueria infausta spp. infausta (Rubiaceae) and the leaves of Lippia javanica (Verbenaceae), respectively, were also evaluated [109, 110]. Compound 180 only inhibited the NF54 strain weakly, with an IC50 value of 109.99 µM, while compound 181 inhibited the D10 strain moderately with an IC50 value of 24.70 µM [109].
Two naphthalene derivatives; dianellin (182) and 2-acetyl-1-hydroxy-8-methoxy-3-methylnaphthalene (183) isolated from the rhizomes of Kniphofia foliosa (Asphodelaceae) harvested in Ethiopia both inhibited the D6, W2, and 3D7 strains of P. falciparum with IC50 ranging from 6.32 to 67.32 µM [85, 86]. The spirobisnaphthalene bipendensin (184) was isolated from the bark of Entandrophragma congoense (Meliaceae) collected in Cameroon by Happi et al. [101], this naphthalene derivative inhibiting the NF54 strain with an IC50 value of 73.28 µM.
Three xanthones; 1,7-dihydroxyxanthone (185), macluraxanthone (186) and allaxanthone B (187) were obtained from Allanblackia floribunda (Guttiferae) by Azebaze et al. [78]. The three compounds exhibited antiplasmodial activities against the F32 and FCM29 strains with IC50 values ranging from 0.91 to 70.33 µM for the first strain and from 0.68 to 67.22 µM against the second [78].
Novel compounds identified and principal compound classes
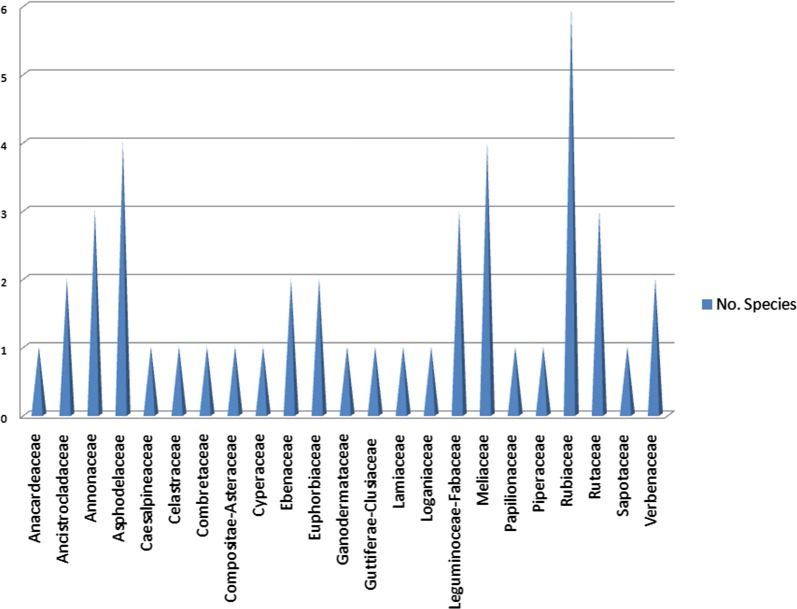
It was observed that 53 out of the 187 compounds (about 28%) were described in the literature for the very first time. Besides, from Fig. 38, the majority of the NPs were terpenoids (30%), followed by flavonoids (22%), alkaloids (19%) and quinones (15%), the rest of the compound classes, each representing only less than 5% of the entire compound collection. It was also observed that most of the plant species from which the compounds were identified were of the families Rubiaceae, Meliaceae, and Asphodelaceae (Fig. 39).
Fig. 38.
Pie chart showing the distribution of the 187 NPs by compound classes
Fig. 39.
Bar chart showing the distribution of the number of plant species of origin by their families
Compound distribution by plant families
A classification of the compounds by class into the plant families showed that most of the plant families represented their typical (chemotaxonomic) compound classes, often seen in the literature for species harvested from the African continent [34, 111–114]. As an example, for the collected data (Fig. 40), all the 26 compounds from the Leguminoceae-Fabaceae were flavonoids, while 23 out of the 25 anti-malarial NPs from the Asphodelaceae were quinones. It was also noted that 27 out of the 34 compounds from the Meliacious species were terpenoids, just like the Euphorbiaceous species that included 12 terpenoids out of 13 compounds identified within the family. Meanwhile, all the 12 compounds from the Ancistrocladaceae were alkaloids, just like the Loganiaceae and Annonaceae for which all 8 compounds and 9 out of the 12 identified compounds were, respectively, alkaloids. On the contrary, the compounds from the Rubiaceous species were distributed among different classes, the majority being phenolics and quinones.
Fig. 40.
An attempted chemotaxomic distribution of compound by their classes sorted by the families of the plant species from which they were identified
The most active compounds
Raw data retrieved from the literature showed activities reported in diverse units. A classification of the compounds by potencies (after all measured IC50 values were converted to μM), and taking a cut off of 10 μM for the most promising secondary metabolites most likely to be lead compounds. The most active compounds within this range for at least one plasmodial strain, i.e. 25 out of 66 NPs were alkaloids ~ (38%), while 23 of them were terpenoids ~ (35%) and 11 were quinones ~ (17%). Taking a cut off IC50 value of at most 1 μM left us with 19 compounds, 14 of them being alkaloids. Besides, the majority of the 187 NPs were terpenoids (30%), followed by flavonoids (22%), alkaloids (19%) and quinones (15%), the rest of the compound classes only represent a negligible part of the current collection.
Conclusions
In this review, an attempt has been made to document the anti-malarial/antiplasmodial activities of NPs derived from African medicinal plants in their various compound classes and source species, published between 2013 and 2019. A description of the in vitro and available in vivo activities for 187 compounds is shown, as well as their classification into the various known NP compound classes and plant families of origin. From the collected data, the most active compounds belong to the same compound classes as the malarial drugs of natural origin, e.g. the alkaloid class for quinine and the terpenoid class for artemisinin. A previous report from Titanji et al. [115] had shown that plant-derived alkaloids from African medicinal plants have a great potential for anti-malarial drug development.
Although recently published reviews have described the activities of anti-malarial secondary metabolites of terrestrial and marine origins, input data from African sources has not been the focal point. Tajuddeen and van Heerden recently published a review of 1524 natural compounds from around the world, which have been assayed against at least one strain of Plasmodium, out of which 39% were described as new NPs, with 29% having IC50 values ≤ 3.0 µM against at least one of the tested plasmodial strains [116]. However, the study was limited to the period between 2010 and 2017 and did not include data from 2018 to 2019. Although the ability of NPs to block the transmission of malaria is still in the early stage, the current review, along with the previous studies that covers data for antiplasmodial compounds from African flora [27, 28], could serve as the baseline data for the discovery of new anti-malarial compounds from Africa.
Supplementary information
Additional file 1. List of journals consulted in building the initial data collection.
Acknowledgements
The authors heartily that Dr. David J. Newman for proofreading the original manuscript.
Abbreviations
- ATM
African Traditional Medicine
- AT
Artemisinin
- CQ
Chloroquine
- NP
Natural product
- WHO
World Health Organization
Authors’ contributions
FNK conceived the idea. BDB, FNK, and PAO participated in the data collection. BDB, FNK and PAO contributed to the data analysis, the discussion of results and the conception of the paper under the supervision of LCOO, WS, KF and LLL. BDB and FNK wrote the first draft of the paper. All authors read and approved the final manuscript.
Funding
FNK acknowledges an equipment donation by the Alexander von Humboldt Foundation and a return fellowship to Germany, accompanied by BDB. Funding by Bundesministerium für Forschung und Entwicklung (BmBF, Germany) and German Academic Exchange Service (DAAD) through the PhytoSustain/Trisustain project.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Boris D. Bekono and Fidele Ntie-Kang contributed equally and should be regarded as joint first authors
Contributor Information
Fidele Ntie-Kang, Email: ntiekfidele@gmail.com.
Wolfgang Sippl, https://pc.pharmazie.uni-halle.de/medchem/sipple/.
Luc C. O. Owono, Email: lcowono@yahoo.fr
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12936-020-03231-7.
References
- 1.WHO. World malaria report 2018. Geneva: World Health Organization; 2018. https://www.who.int/malaria/publications/world_malaria_report/en/. Accessed 27 Nov 2019.
- 2.Mosnier E, Roux E, Cropet C, Lazrek Y, Moriceau O, Gaillet M, et al. Prevalence of Plasmodium spp. in the Amazonian border context (French Guiana-Brazil): associated factors and spatial distribution. Am J Trop Med Hyg. 2019;102:130–141. doi: 10.4269/ajtmh.19-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haeseleer C, Martiny D, Van Laethem Y, Cantinieaux B, Martin C. Reactivation of Plasmodium infection during a treatment with infliximab: a case report. Int J Infect Dis. 2019;91:101–103. doi: 10.1016/j.ijid.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Musyoka KB, Kiiru JN, Aluvaala E, Omondi P, Chege WK, Judah T, et al. Prevalence of mutations in Plasmodium falciparum genes associated with resistance to different antimalarial drugs in Nyando, Kisumu County in Kenya. Infect Genet Evol. 2019;78:104121. doi: 10.1016/j.meegid.2019.104121. [DOI] [PubMed] [Google Scholar]
- 5.Sumarnrote A, Overgaard HJ, Marasri N, Fustec B, Thanispong K, Chareonviriyaphap T, et al. Status of insecticide resistance in Anopheles mosquitoes in Ubon Ratchathani province, Northeastern Thailand. Malar J. 2017;16:299. doi: 10.1186/s12936-017-1948-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaumeau V, Cerqueira D, Zadrozny J, Kittiphanakun P, Andolina C, Chareonviriyaphap T, et al. Insecticide resistance in malaria vectors along the Thailand–Myanmar border. Parasit Vectors. 2017;10:165. doi: 10.1186/s13071-017-2102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rakotoson JD, Fornadel CM, Belemvire A, Norris LC, George K, Caranci A, et al. Insecticide resistance status of three malaria vectors, Anopheles gambiae (s.l.), An. funestus and An. mascarensis, from the south, central and east coasts of Madagascar. Parasit Vectors. 2017;10:396. doi: 10.1186/s13071-017-2336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanscheid T, Schlagenhauf P, Grobusch MP. Atovaquone/proguanil for malaria chemoprophylaxis—could a difference in susceptibility during hepatic development explain the need to continue drug intake for 7 days post-exposure? Travel Med Infect Dis. 2019;20:101527. doi: 10.1016/j.tmaid.2019.101527. [DOI] [PubMed] [Google Scholar]
- 9.Schlagenhauf P, Grobusch MP, Leder K, Toovey S, Patel D. Complex choices: which malaria chemoprophylaxis can be recommended for the pregnant traveller? Travel Med Infect Dis. 2019;20:101525. doi: 10.1016/j.tmaid.2019.101525. [DOI] [PubMed] [Google Scholar]
- 10.Haston JC, Hwang J, Tan KR. Guidance for using tafenoquine for prevention and antirelapse therapy for malaria—United States, 2019. MMWR. 2019;68:1062–1068. doi: 10.15585/mmwr.mm6846a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassett MR, Riegel BE, Callaghan PS, Roepe PD. Analysis of Plasmodium vivax chloroquine resistance transporter mutant isoforms. Biochemistry. 2017;56:5615–5622. doi: 10.1021/acs.biochem.7b00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang M, Wang C, Otto TD, Oberstaller J, Liao X, Adapa SR, et al. Uncovering the essential genes of the human malaria parasite Plasmodium falciparum by saturation mutagenesis. Science. 2018;360:eaap7847. doi: 10.1126/science.aap7847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parobek CM, Parr JB, Brazeau NF, Lon C, Chaorattanakawee S, Gosi P, et al. Partner-drug resistance and population substructuring of artemisinin-resistant Plasmodium falciparum in Cambodia. Genome Biol Evol. 2017;9:1673–1686. doi: 10.1093/gbe/evx126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinheiro LCS, Feitosa LM, Gandi MO, Silveira FF, Boechat N. The development of novel compounds against malaria: quinolines, triazolpyridines, pyrazolopyridines and pyrazolopyrimidines. Molecules. 2019;24:E4095. doi: 10.3390/molecules24224095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okello D, Kang Y. Exploring antimalarial herbal plants across communities in Uganda based on electronic data. Evid Based Complement Altern Med. 2019;2019:3057180. doi: 10.1155/2019/3057180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2013;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodeker C, Bodeker G, Ong CK, Grundy CK, Burford G, Shein K. WHO global atlas of traditional, complementary and alternative medicine. Geneva: World Health Organization; 2005. [Google Scholar]
- 18.Alebie G, Urga B, Worku A. Systematic review on traditional medicinal plants used for the treatment of malaria in Ethiopia: trends and perspectives. Malar J. 2017;16:307. doi: 10.1186/s12936-017-1953-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esmaeili S, Ghiaee A, Naghibi F, Mosaddegh M. Antiplasmodial activity and cytotoxicity of plants used in traditional medicine of Iran for the treatment of fever. Iran J Pharm Res. 2015;14:103–107. [PMC free article] [PubMed] [Google Scholar]
- 20.Mukungu N, Abuga K, Okalebo F, Ingwela R, Mwangi J. Medicinal plants used for management of malaria among the Luhya community of Kakamega East sub-County, Kenya. J Ethnopharmacol. 2016;194:98–107. doi: 10.1016/j.jep.2016.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appiah KS, Oppong CP, Mardani HK, Omari RA, Kpabitey S, Amoatey CA, et al. Medicinal plants used in the Ejisu-Juaben Municipality, Southern Ghana: an ethnobotanical study. Medicines (Basel) 2019;6:1. doi: 10.3390/medicines6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salehi B, Zakaria ZA, Gyawali R, Ibrahim SA, Rajkovic J, Shinwari ZK, et al. Piper species: a comprehensive review on their phytochemistry, biological activities and applications. Molecules. 2019;24:1364. doi: 10.3390/molecules24071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan W-H, Xu X-Y, Shi N, Tsang SW, Zhang H-J. Antimalarial activity of plant metabolites. Int J Mol Sci. 2018;19:1382. doi: 10.3390/ijms19051382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Youmsi RDF, Fokou PVT, Menkem EZ, Bakarnga-Via I, Keumoe R, Nana V, et al. Ethnobotanical survey of medicinal plants used as insects repellents in six malaria endemic localities of Cameroon. J Ethnobiol Ethnomed. 2017;13:33. doi: 10.1186/s13002-017-0155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karar MGE, Kuhnert N. Herbal drugs from Sudan: traditional uses and phytoconstituents. Pharmacogn Rev. 2017;11:83–103. doi: 10.4103/phrev.phrev_15_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tshitenge DT, Ioset KN, Lami JN, Ndelo-di-Phanzu J, Mufusama J-PKS, Bringmann G. Rational quality assessment procedure for less-investigated herbal medicines: case of a Congolese antimalarial drug with an analytical report. Fitoterapia. 2016;110:189–195. doi: 10.1016/j.fitote.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Onguéné PA, Ntie-Kang F, Lifongo LL, Ndom JC, Sippl W, Mbaze LM. The potential of anti-malarial compounds derived from African medicinal plants, part I: a pharmacological evaluation of alkaloids and terpenoids. Malar J. 2013;12:449. doi: 10.1186/1475-2875-12-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ntie-Kang F, Onguéné PA, Lifongo LL, Ndom JC, Sippl W, Mbaze LM. The potential of anti-malarial compounds derived from African medicinal plants, part II: a pharmacological evaluation of non-alkaloids and non-terpenoids. Malar J. 2014;13:81. doi: 10.1186/1475-2875-13-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahmoudi N, de Julian-Ortiz JV, Cicerone L, Galvez J, Mazier D, Danism M, et al. Identification of new antimalarial drugs by linear discriminant analysis and topological virtual screening. J Antimicrob Chemother. 2006;57:489–497. doi: 10.1093/jac/dki470. [DOI] [PubMed] [Google Scholar]
- 30.Willcox M, Bodeker G, Rasanaivo P. Traditional medicinal plants and malaria. Boca Raton: CRC Press; 2004. [Google Scholar]
- 31.Rasoanaivo P, Oketch-Rabah H. Preclinical considerations on anti-malarial phytomedicines. Part II, Efficacy evaluation. Antananarivo: Institut Malgache de Recherches Appliquées; 1998. [Google Scholar]
- 32.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 33.Onguéné PA, Ntie-Kang F, Mbah JA, Lifongo LL, Ndom JC, Sippl W, et al. The potential of anti-malarial compounds derived from African medicinal plants, part III: an in silico evaluation of drug metabolism and pharmacokinetics profiling. Org Med Chem Lett. 2014;4:6. doi: 10.1186/s13588-014-0006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ntie-Kang F, Mbah JA, Mbaze LM, Lifongo LL, Scharfe M, Ngo Hanna J, et al. CamMedNP: building the Cameroonian 3D structural natural products database for virtual screening. BMC Complement Altern Med. 2013;13:88. doi: 10.1186/1472-6882-13-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ntie-Kang F, Onguéné PA, Scharfe M, Owono LCO, Megnassan E, Mbaze LM, et al. ConMedNP: a natural product library from Central African medicinal plants for drug discovery. RSC Adv. 2014;4:409–419. [Google Scholar]
- 36.Ntie-Kang F, Zofou D, Babiaka SB, Meudom R, Scharfe M, Lifongo LL, et al. AfroDb: a select highly potent and diverse natural product library from African medicinal plants. PLoS ONE. 2013;8:e78085. doi: 10.1371/journal.pone.0078085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ntie-Kang F, Telukunta KK, Döring K, Simoben CV, Moumbock AFA, Malange YI, et al. NANPDB: a resource for natural products from Northern African sources. J Nat Prod. 2017;80:2067–2076. doi: 10.1021/acs.jnatprod.7b00283. [DOI] [PubMed] [Google Scholar]
- 38.Organization for Economic Growth and Development (OECD). OECD guidelines for the testing of chemicals: acute oral toxicity up and down-procedure (UDP). 2008; 1–27.
- 39.Dikasso D, Makonnen E, Debella A, Abebe D, Urga K, Makonnen W, et al. In vivo antimalarial activity of hydroalcoholic extracts from Asparagus africanus Lam. in mice infected with Plasmodium berghei. Ethiop J Health Dev. 2006;280:112–118. [Google Scholar]
- 40.Akuodor GC, Idris-Usman M, Anyalewechi N, Eucheria O, Ugwu CT, Akpan JL, et al. In vivo antimalarial activity of ethanolic leaf extract of Verbena hastata against Plasmodium berghei in mice. J Herb Med Toxicol. 2010;4:17–23. [Google Scholar]
- 41.Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 43.Duffy S, Avery VM. Development and optimization of a novel 384-well antimalarial imaging assay validated for high-throughput screening. Am J Trop Med Hyg. 2012;86:84–92. doi: 10.4269/ajtmh.2012.11-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Novac O, Guenier AS, Pelletier J. Inhibitors of protein synthesis identified by a high throughput multiplexed translation screen. Nucleic Acids Res. 2004;32:902–905. doi: 10.1093/nar/gkh235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kigondu EV, Rukunga GM, Keriko JM, Tonui WK, Gathirwa JW, Kirira PG, et al. Antiparasitic activity and cytotoxicity of selected medicinal plants from Kenya. J Ethnopharmacol. 2009;123:504–509. doi: 10.1016/j.jep.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Gathirwa JW, Rukunga GM, Njagi ENM, Omar SA, Mwitari PG, Guantai AN, et al. The in vitro antiplasmodial and in vivo antimalarial efficacy of combinations of some medicinal plants used traditionally for treatment of malaria by the Meru community in Kenya. J Ethnopharmacol. 2008;115:223–231. doi: 10.1016/j.jep.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 47.Samoylenko V, Jacob MR, Khan SI, Zhao J, Tekwani BL, Midiwo JO, et al. Antimicrobial, antiparasitic and cytotoxic spermine alkaloids from Albizia schimperiana. Nat Prod Commun. 2009;4:791–796. [PMC free article] [PubMed] [Google Scholar]
- 48.Makler MT, Ries JM, Williams JA, Bancroft JE, Piper RC, Gibbins BL, et al. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am J Trop Med Hyg. 1993;48:739–741. doi: 10.4269/ajtmh.1993.48.739. [DOI] [PubMed] [Google Scholar]
- 49.Smilkstein M, Sriwilaijaroen N, Kelly JX, Wilairat P, Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob Agents Chemother. 2004;48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Juma WP, Akala HM, Eyase FL, Muiva LM, Heydenreich M, Okalebo FA, et al. Terpurinflavone: antiplasmodial flavones from the stem of Tephrosia purpurea. Phytochem Lett. 2011;4:176–178. [Google Scholar]
- 51.Johnson JA, Dennull RA, Gerena L, Lopez-Sanchez M, Roncal NE, Waters NC. Assessment and continued validation of the malaria SYBR Green I-based fluorescence assay for use in malaria drug screening. Antimicrob Agents Chemother. 2007;51:1926–1933. doi: 10.1128/AAC.01607-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Makler MT, Hinrichs DJ. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am J Trop Med Hyg. 1993;48:205–210. doi: 10.4269/ajtmh.1993.48.205. [DOI] [PubMed] [Google Scholar]
- 53.Matile H, Pink JRL. Chapter 15: Plasmodium falciparum malaria parasite cultures and their use in immunology. In: Lefkovits I, Pernis B, editors. Immunological methods. San Diego: Academic Press; 1990. pp. 221–234. [Google Scholar]
- 54.Thaithong S, Beale GH, Chutmongkonkul M. Susceptibility of Plasmodium falciparum to five drugs: an in vitro study of isolates mainly from Thailand. Trans R Soc Trop Med Hyg. 1983;77:228–231. doi: 10.1016/0035-9203(83)90080-9. [DOI] [PubMed] [Google Scholar]
- 55.Ahmed SA, Gogal RM, Walsh JE. A new rapid simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J Immunol Methods. 1994;170:211–224. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 56.Ponnudurai T, Leeuwenberg AD, Meuwissen JH. Chloroquine sensitivity of isolates of Plasmodium falciparum adapted to in vitro culture. Trop Geogr Med. 1981;33:50–54. [PubMed] [Google Scholar]
- 57.Malik S, Khan S, Das A, Samantaray JC. Plasmodium lactate dehydrogenase assay to detect malarial parasites. Natl Med J India. 2004;17:237–239. [PubMed] [Google Scholar]
- 58.Malebo HM, Wenzler T, Cal M, Swaleh SM, Omolo MO, Hassanali A, et al. Anti-protozoal activity of aporphine and protoberberine alkaloids from Annickia kummeriae (Engl. & Diels) Setten & Maas (Annonaceae) BMC Complement Altern Med. 2013;13:48. doi: 10.1186/1472-6882-13-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lacroix D, Prado S, Kamoga D, Kasenene J, Bodo B. Absolute configuration of 2′(R)-acetylmontrifoline and 2′(R)-montrifoline, furoquinolines from the fruits of Teclea nobilis. Phytochem Lett. 2012;5:22–25. [Google Scholar]
- 60.Rasamison VE, Brodie PJ, Merino EF, Cassera MB, Ratsimbason MA, Rakotonandrasana S, et al. Furoquinoline alkaloids and methoxyflavones from the stem bark of Melicope madagascariensis (Baker) T.G. Hartley. Nat Prod Bioprospect. 2016;6:261–265. doi: 10.1007/s13659-016-0106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tchinda AT, Tamze V, Ngono ARN, Ayimele GA, Cao M, Angenot L, et al. Alkaloids from the stem bark of Strychnos icaja. Phytochem Lett. 2012;5:108–113. [Google Scholar]
- 62.Beaufay C, Ledoux A, Jansen O, Bordignon A, Zhao S, Teijaro CN, et al. In vivo antimalarial and antitrypanosomal activity of strychnogucine B, a bisindole alkaloid from Strychnos icaja. Planta Med. 2018;84:881–885. doi: 10.1055/a-0644-2723. [DOI] [PubMed] [Google Scholar]
- 63.Frédérich M, De Pauw M-C, Prosperi C, Tits M, Brandt V, Penelle J, et al. Strychnogucines A and B, two new antiplasmodial bisindole alkaloids from Strychnos icaja. J Nat Prod. 2001;64:12–16. doi: 10.1021/np000285t. [DOI] [PubMed] [Google Scholar]
- 64.Kouam SF, Ngouonpe AW, Lamshöft M, Talontsi FM, Bauer JO, Strohmann C, et al. Indolosesquiterpene alkaloids from the Cameroonian medicinal plant Polyalthia oliveri (Annonaceae) Phytochemistry. 2014;105:52–59. doi: 10.1016/j.phytochem.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 65.Lombe BK, Bruhn T, Feineis D, Mudogo V, Brun R, Bringmann G. Cyclombandakamines A1 and A2, oxygen-bridged naphthylisoquinoline dimers from a Congolese Ancistrocladus liana. Org Lett. 2017;19:1342–1345. doi: 10.1021/acs.orglett.7b00209. [DOI] [PubMed] [Google Scholar]
- 66.Li J, Seupel R, Feineis D, Mudogo V, Kaiser M, Brun R, et al. Dioncophyllines C2, D2, and F and related naphthylisoquinoline alkaloids from the Congolese liana Ancistrocladus ileboensis with potent activities against Plasmodium falciparum and against multiple myeloma and leukemia cell lines. J Nat Prod. 2017;80:443–458. doi: 10.1021/acs.jnatprod.6b00967. [DOI] [PubMed] [Google Scholar]
- 67.Tshitenge DT, Bruhn T, Feineis D, Schmidt D, Mudogo V, Kaiser M, et al. Ealamines A-H, a series of naphthylisoquinolines with the rare 7,8′-coupling site, from the Congolese liana Ancistrocladus ealaensis, targeting pancreatic cancer cells. J Nat Prod. 2019;82:3150–3164. doi: 10.1021/acs.jnatprod.9b00755. [DOI] [PubMed] [Google Scholar]
- 68.Gbedema SY, Bayor MT, Annan K, Wright CW. Clerodane diterpenes from Polyalthia longifolia (Sonn) Thw. var. pendula: Potential antimalarial agents for drug resistant Plasmodium falciparum infection. J Ethnopharmacol. 2015;169:176–182. doi: 10.1016/j.jep.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 69.Kouam SF, Ngouonpe AW, Bullach A, Lamshöft M, Kuigoua GM, Spiteller M. Monoterpenes with antibacterial activities from a Cameroonian medicinal plant Canthium multiflorum (Rubiaceae) Fitoterapia. 2013;91:199–204. doi: 10.1016/j.fitote.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 70.Betti JL. Medicinal plants sold in Yaoundé markets, Cameroon. Afr Study Monogr. 2002;23:47–64. [Google Scholar]
- 71.Bouquet A, Debray M. Plantes médicinales de la Côte d’Ivoire. Paris: Mémoires Office de la Recherche Scientifique et Technique d’Outre-Mer (O.R.S.T.O.M); 1974. p. 232. [Google Scholar]
- 72.Wafo P, Nyasse B, Fontaine C, Sondengam BL. Aporphine alkaloids from Enantia chlorantha. Fitoterapia. 1999;70:157–160. [Google Scholar]
- 73.Lacroix D, Prado S, Kamoga D, Kasenene J, Namukobe J, Krief S, et al. Antiplasmodial and cytotoxic activities of medicinal plants traditionally used in the village of Kiohima, Uganda. J Ethnopharmacol. 2011;133:850–855. doi: 10.1016/j.jep.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 74.Neuwinger HD. African ethnobotany: poisons and drugs: chemistry, pharmacology, toxicology. Boca Raton: CRC Press; 1996. [Google Scholar]
- 75.Boyom FF, Kemgne EM, Tepongning R, Ngouana V, Mbacham WF, Tsamo E, et al. Antiplasmodial activity of extracts from seven medicinal plants used in malaria treatment in Cameroon. J Ethnopharmacol. 2009;123:483–488. doi: 10.1016/j.jep.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 76.Kerubo LO, Midiwo JO, Derese S, Langat MK, Akala HM, Waters NC, et al. Antiplasmodial activity of compounds from the surface exudates of Senecio roseiflorus. Nat Prod Commun. 2013;8:175–176. [PubMed] [Google Scholar]
- 77.Muiva-Mutisya L, Macharia B, Heydenreich M, Koch A, Akala HM, Derese S, et al. 6α-Hydroxy-α-toxicarol and (+)-tephrodin with antiplasmodial activities from Tephrosia species. Phytochem Lett. 2014;10:179–183. [Google Scholar]
- 78.Azebaze AGB, Teinkela JEM, Nguemfo EL, Valentin A, Dongmo AB, Vardamides JC. Antiplasmodial activity of some phenolic compounds from Cameroonians Allanblackia. Afr Health Sci. 2015;15:835–840. doi: 10.4314/ahs.v15i3.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ngemenya MN, Metuge HM, Mbah JA, Zofou D, Babiaka SB, Titanji VPK. Isolation of natural product hits from Peperomia species with synergistic activity against resistant Plasmodium falciparum strains. Eur J Med Plants. 2015;5:77–87. [Google Scholar]
- 80.Irungu BN, Orwa JA, Gruhonjic A, Fitzpatrick PA, Landberg G, Kimani F, et al. Constituents of the roots and leaves of Ekebergia capensis and their potential antiplasmodial and cytotoxic activities. Molecules. 2014;19:14235–14246. doi: 10.3390/molecules190914235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Derese S, Barasa L, Akala HM, Yusuf AO, Kamau E, Heydenreich M, et al. 4′-Prenyloxyderrone from the stem bark of Millettia oblata ssp. teitensis and the antiplasmodial activities of isoflavones from some Millettia species. Phytochem Lett. 2014;31:69–72. [Google Scholar]
- 82.Marco M, Deyou T, Gruhonjic A, Holleran JP, Duffy S, Heydenreich M, et al. Pterocarpans and isoflavones from the root bark of Millettia micans and of Millettia dura. Phytochem Lett. 2017;21:216–220. [Google Scholar]
- 83.Mbwambo ZH, Moshi MJ, Masimba MJ, Kapingu MC, Nondo RSO. Antimicrobial activity and brine shrimp toxicity of extracts of Terminalia brownii roots and stem. BMC Complement Altern Med. 2007;7:9. doi: 10.1186/1472-6882-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Machumi F, Midiwo JO, Jacob MR, Khan SI, Tekwani BL, Zhang J, et al. Phytochemical, antimicrobial and antiplasmodial investigations of Terminalia brownii. Nat Prod Commun. 2013;8:761–764. [PMC free article] [PubMed] [Google Scholar]
- 85.Induli M, Gebru M, Abdissa N, Akala H, Wekesa I, Byamukama R, et al. Antiplasmodial quinones from the rhizomes of Kniphofia foliosa. Nat Prod Commun. 2013;8:1261–1264. [PubMed] [Google Scholar]
- 86.Abdissa N, Induli M, Akala HM, Heydenreich M, Midiwo JO, Ndakala A, et al. Knipholone cyclooxanthrone and an anthraquinone dimer with antiplasmodial activities from the roots of Kniphofia foliosa. Phytochem Lett. 2013;6:241–245. [Google Scholar]
- 87.Bringmann G, Mutanyatta-Comar J, Maksimenka K, Wanjohi JM, Heydenreich M, Brun R, et al. Joziknipholones A and B: the first dimeric phenylanthraquinones, from the roots of Bulbine frutescens. Chemistry. 2008;14:1420–1429. doi: 10.1002/chem.200701328. [DOI] [PubMed] [Google Scholar]
- 88.Abdissa D, Geleta G, Bacha K, Abdissa N. Phytochemical investigation of Aloe pulcherrima roots and evaluation for its antibacterial and antiplasmodial activities. PLoS ONE. 2017;12:e0173882. doi: 10.1371/journal.pone.0173882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feilcke R, Arnouk G, Raphane B, Richard K, Tietjen I, Andrae-Marobela K, et al. Biological activity and stability analyses of knipholone anthrone, a phenyl anthraquinone derivative isolated from Kniphofia foliosa Hochst. J Pharm Biomed Anal. 2019;174:277–285. doi: 10.1016/j.jpba.2019.05.065. [DOI] [PubMed] [Google Scholar]
- 90.Lenta BN, Ngamgwe RF, Kamdem LM, Ngatchou J, Tantangmo F, Antheaume C, et al. Compounds from Diospyros canaliculata (Ebenaceae) and their antiparasitic activities. Int Res J Pure Appl Chem. 2015;6:56. [Google Scholar]
- 91.Geremedhin G, Bisrat D, Asres K. Isolation, characterization and in vivo antimalarial evaluation of anthrones from the leaf latex of Aloe percrassa Todaro. J Nat Remedies. 2014;14:1–7. [Google Scholar]
- 92.Endale M, Ekberg A, Akala HM, Alao JP, Sunnerhagen P, Yenesew A, et al. Busseihydroquinones A-D from the roots of Pentas bussei. J Nat Prod. 2012;75:1299–1304. doi: 10.1021/np3002223. [DOI] [PubMed] [Google Scholar]
- 93.Namukobe J, Kiremire BT, Byamukama R, Kasenene JM, Akala HM, Kamau E, et al. Antiplasmodial compounds from the stem bark of Neoboutonia macrocalyx Pax. J Ethnopharmacol. 2015;162:317–322. doi: 10.1016/j.jep.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 94.Douanla PD, Tabopda TK, Tchinda AT, Cieckiewicz E, Frédérich M, Boyom FF, et al. Antrocarines A-F, antiplasmodial ergostane steroids from the stem bark of Antrocaryon klaineanum. Phytochemistry. 2015;117:521–526. doi: 10.1016/j.phytochem.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 95.Irungu BN, Adipo N, Orwa JA, Kimani F, Heydenreich M, Midiwo JO, et al. Antiplasmodial and cytotoxic activities of the constituents of Turraea robusta and Turraea nilotica. J Ethnopharmacol. 2015;174:419–425. doi: 10.1016/j.jep.2015.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Namukobe J, Kiremire BT, Byamukama R, Kasenene JM, Dumontet V, Guéritte F, et al. Cycloartane triterpenes from the leaves of Neoboutonia macrocalyx L. Phytochemistry. 2014;102:189–196. doi: 10.1016/j.phytochem.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 97.Endale A, Bisrat D, Animut A, Bucar F, Asres K. In vivo antimalarial activity of a labdane diterpenoid from the leaves of Otostegia integrifolia Benth. Phytother Res. 2013;27:1805–1809. doi: 10.1002/ptr.4948. [DOI] [PubMed] [Google Scholar]
- 98.Nondo RS, Moshi MJ, Erasto P, Masimba PJ, Machumi F, Kidukuli AW, et al. Anti-plasmodial activity of Norcaesalpin D and extracts of four medicinal plants used traditionally for treatment of malaria. BMC Complement Altern Med. 2017;17:167. doi: 10.1186/s12906-017-1673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mba’ning BM, Lenta BN, Noungoué DT, Antheaume C, Fongang YF, Ngouela SA, et al. Antiplasmodial sesquiterpenes from the seeds of Salacia longipes var. camerunensis. Phytochemistry. 2013;96:347–352. doi: 10.1016/j.phytochem.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 100.Nyongbela KD, Makolo FL, Hoye TR, Efange SMN. Isolation and characterization of Sclerienone C from Scleria striatinux. Nat Prod Commun. 2016;11:5–6. [PubMed] [Google Scholar]
- 101.Happi GM, Kouam SF, Talontsi FM, Lamshöft M, Zühlke S, Bauer JO, et al. Antiplasmodial and cytotoxic triterpenoids from the bark of the Cameroonian medicinal plant Entandrophragma congoënse. J Nat Prod. 2015;78:604–614. doi: 10.1021/np5004164. [DOI] [PubMed] [Google Scholar]
- 102.Wahba AE, El-Sayed AKA, El-Falal AA, Soliman EM. New antimalarial lanostane triterpenes from a new isolate of Egyptian Ganoderma species. Med Chem Res. 2019;28:2246–2251. [Google Scholar]
- 103.Bero J, Hérent MF, Schmeda-Hirschmann G, Frédérich M, Quetin-Leclercq J. In vivo antimalarial activity of Keetia leucantha twigs extracts and in vitro antiplasmodial effect of their constituents. J Ethnopharmacol. 2013;149:176–183. doi: 10.1016/j.jep.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 104.Simelane MBC, Shonhai A, Shode FO, Smith P, Singh M, Opoku AR. Anti-plasmodial activity of some Zulu medicinal plants and of some triterpenes isolated from them. Molecules. 2013;18:12313–12323. doi: 10.3390/molecules181012313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Happi GM, Kouam SF, Talontsi FM, Zühlke S, Lamshöft M, Spiteller M. Minor secondary metabolites from the bark of Entandrophragma congoense (Meliaceae) Fitoterapia. 2015;102:35–40. doi: 10.1016/j.fitote.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 106.Chukwujekwu JC, de Kock CA, Smith PJ, van Heerden FR, van Staden J. Antiplasmodial activity of compounds isolated from Erythrina caffra. S Afr J Bot. 2016;106:101–103. [Google Scholar]
- 107.Ledoux A, Maraetefau H, Jansen O, Etienne D, Quetin-Leclercq J, Clerc P, et al. Phytochemical profile and biological activity evaluation of Zanthoxylum heterophyllum leaves against malaria. Planta Med Lett. 2015;2:e10–e11. [Google Scholar]
- 108.Fouokeng Y, Feusso HMF, Teinkela JEM, Noundou XS, Wintjens R, Isaacs M, et al. In vitro antimalarial, antitrypanosomal and HIV-1 integrase inhibitory activities of two Cameroonian medicinal plants: Antrocaryon klaineanum (Anacardiaceae) and Diospyros conocarpa (Ebenaceae) S Afr J Bot. 2019;122:510–517. [Google Scholar]
- 109.Bapela MJ. NMR-based metaboliomic study of medicinal plants used against malaria and the isolated bioactive alkaloids. Ph.D thesis, University of Pretoria, South Africa. 2016.
- 110.Ludere MT, van Ree T, Vleggaar R. Isolation and relative stereochemistry of lippialactone, a new antimalarial compound from Lippia javanica. Fitoterapia. 2013;86:188–192. doi: 10.1016/j.fitote.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 111.Zofou D, Ntie-Kang F, Sippl W, Efange SMN. Bioactive natural products derived from the Central African flora against neglected tropical diseases and HIV. Nat Prod Rep. 2013;30:1098–1200. doi: 10.1039/c3np70030e. [DOI] [PubMed] [Google Scholar]
- 112.Ntie-Kang F, Lifongo LL, Mbaze LM, Ekwelle N, Owono LCO, Megnassan E, et al. Cameroonian medicinal plants: a bioactivity versus ethnobotanical survey and chemotaxonomic classification. BMC Complement Altern Med. 2013;13:147. doi: 10.1186/1472-6882-13-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lifongo LL, Simoben CV, Ntie-Kang F, Babiaka SB, Judson PN. A bioactivity versus ethnobotanical survey of medicinal plants from Nigeria, West Africa. Nat Prod Bioprospect. 2014;4:1–19. doi: 10.1007/s13659-014-0005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ntie-Kang F, Lifongo LL, Simoben CV, Babiaka SB, Sippl W, Mbaze LM. The uniqueness and therapeutic value of natural products from West African medicinal plants, part I: uniqueness and chemotaxonomy. RSC Adv. 2014;4:28728–28755. [Google Scholar]
- 115.Titanji VPK, Zofou D, Ngemenya MN. The antimalarial potential of medicinal plants used for the treatment of malaria in Cameroonian folk medicine. Afr J Trad Cam. 2008;5:302–321. [PMC free article] [PubMed] [Google Scholar]
- 116.Tajuddeen N, van Heerden FR. Antiplasmodial natural products: an update. Malar J. 2019;18:404. doi: 10.1186/s12936-019-3026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. List of journals consulted in building the initial data collection.
Data Availability Statement
Not applicable.