Abstract
Novel diterpenoids were isolated from the extracts of Fabiana densa var. ramulosa and found to display a selective activity against Gram-positive bacterial strains with negligible cytotoxicity toward human keratinocytes. This study highlighted the role played by the acidic group at C18 of the tetracyclic ent-beyerene scaffold for antibacterial effects and how the length and flexibility of the alkyl chain between the two carbonyl groups are crucial factors to increase the antimicrobial activity of the molecules, supporting the development of natural products from F. densa and their derivatives for treatment of microbial infections.
Keywords: Natural products, plant secondary metabolites, diterpenes, Gram-positive bacteria, antimicrobial activity, cytotoxicity
Nowadays, a significant portion of the commercial drugs occurs in nature or is derived from natural products by means of chemical transformations or de novo synthesis. One of the difficulties in developing new drugs is the decline of natural products research, which started near the end of the 20th century, in favor of the development of enormous synthetic libraries of small molecules by combinatorial approaches. Nowadays, this trend is changing and a renewed interest in nature as a source of hit and lead compounds for drug discovery is emerging.1,2 Particularly, we are entering a New Golden Age of natural products drug discovery.3 However, a tremendous wide discrepancy between their historical significance and their occurrence in modern drug development still exists. Recently, a predictive structural and biodiversity-based analysis assessed that taxon’s abundance considerably affects the development of a natural product into a drug, and most plants of interest today are usually indigenous only to biodiversity-rich countries especially in the tropics and subtropics.4 In addition, the isolation of bioactive chemical constituents faces a number of technical challenges including the variability of the source material, the difficulty in isolating the active constituents, and the costs of collection and storage.5 A unique in-house library of about 1 000 natural compounds, mostly isolated from several plants used in traditional medicine of South America and collected over the years, is available at the Organic Chemistry Laboratory of the Department of Chemistry and Technology of Drugs (Sapienza University of Rome, Italy). Besides natural products from different classes, the library has been recently enlarged with natural products from commercially available sources and synthetic and semisynthetic derivatives. All components of our collection are incorporated into a virtual library, showing a satisfactory chemical diversity. Therefore, this in-house library offers a unique chance to identify unexpected new scaffolds for the development of therapeutically relevant molecules. Furthermore, it has been successfully screened in silico and in vitro for the identification of hit and lead compounds in recent early stage drug discovery projects.6−9 In order to further enrich the library with novel natural products, we investigated the Fabiana densa Remy var. ramulosa Wedd., a native shrub of Chile commonly called “checal”, “tolilla” or “tola-checal”. The genus Fabiana (Solanaceae family) grows in South America, especially along arid mountainous area between 16° and 51° latitude and between 1 000 and 4 900 m over sea level, featuring needlelike leaves and profuse tiny tubular flowers.10,11 This genus comprises 15 species: ten are present in Argentina, seven in Chile, four in Bolivia and one in Perú. However, the latest revision of the vascular flora of Chile indicates a total of eight species of the Fabiana genus, including F. densa J. Remy.12 Fabiana spp. extracts have long been employed as fuel (firewood), as incense, and as ash in many rituals and in traditional South American medicine,10 but there is no evidence to validate their folk use. A moderated diuretic activity of F. patagonica extract was reported by Alvarez et al., and it was suggested to be associated with the presence of oleanolic acid, which was isolated as the major metabolite.13 The antimicrobial activity of tinctures and aqueous extracts of Argentinean species occurring in the highlands (including F. bryoides, F. densa, and F. punensis) was reported against a panel of sensitive and multidrug-resistant Gram-positive and Gram-negative bacteria.14 The effectiveness of these plants as inhibitors of inflammatory mediators along with their free radical scavenging properties and genotoxic effects were investigated by Cuello and colleagues by studying the aqueous and ethanolic extracts of four Fabiana species growing in mountainous area of Argentina (F. bryoides, F. punensis, F. densa, and F. patagonica).11 An integrative overview of the traditional uses, chemistry, bioactivity, and chemical profiling of the crude F. imbricate, the most common species in central Chile, was recently provided.15 Notably, so far no studies have been reported on Fabiana densa Remy var. ramulosa, except for the work of Erazo and co-workers. This research group described the isolation of two diterpenoids, i.e., the succinoyl and the oxaloyl esters of the ent-beyer-15-en-18-ol (previously identified in Baccharis tola(16)) from F. densa resinous exudate.17 The antimicrobial activity of these compounds was evaluated against a small panel of Gram-negative and Gram-positive bacteria including Staphylococcus aureus, an important pathogen which resulted to be the most sensitive one. This finding supports the usage of F. densa for the development of new antimicrobial drugs. Presently, the rapid spread of antibiotic resistance in both Gram-negative and Gram-positive bacteria, represents a major threat to public health. Indeed, in 2017, the World Health Organization (WHO) published a list of 12 bacterial species for which new drugs are urgently needed.18 The promising antimicrobial activity of these noncommon diterpenes along with the limited knowledge of the chemical composition of small molecules belonging to F. densa prompted us to a more comprehensive study of this plant species. Here we report the isolation and structural elucidation of novel chemical constituents of F. densa and a characterization of their antimicrobial and cytotoxic activities. Among them, three dimeric diterpenes and a new diterpenoid were identified and described for the first time.
Results and Discussion
Isolation of Novel Constituents from the Aerial Parts of Fabiana densa var. ramulosa
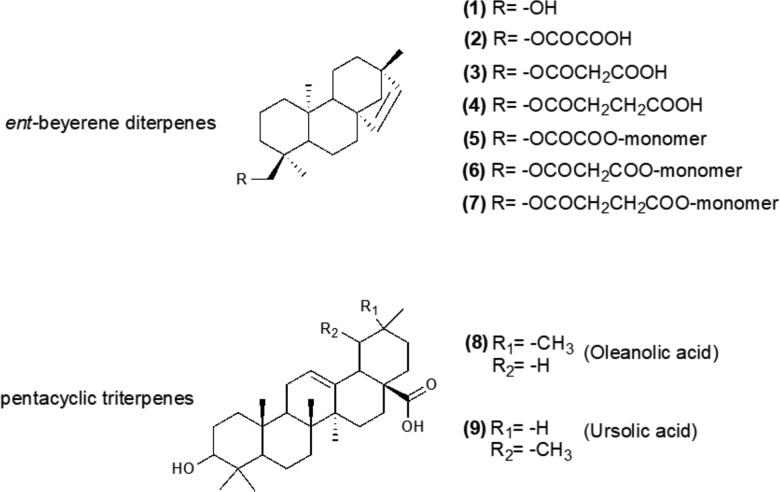
Aerial parts of F. densa were collected and botanically identified by the Department of Pharmacology and Toxicology of the University of Chile. Further investigations were carried out in our laboratories at Sapienza University. At first, the leaves were properly treated, dried, and separated from foreign materials such as soil, pebbles, and other matters unsuitable for the solid–liquid extraction process. Different from the extraction procedure reported by Erazo et al. by air-dried ground leaves, the aerial parts of this plant were macerated for 24 h with acetone that, due to its polarity, is able to extract polar as well as less polar organic compounds. Extensive purification of the obtained resinous extract was performed by silica gel chromatography with an eluting mixture of increasing polarity. The following products were isolated according to the following order: 5 (oil, 0.45% yield), 6 (oil, 0.48% yield), 7 (oil, 0.25% yield), 1 (white powder, 10% yield), 8 (white powder, 2% yield), 9 (white powder, 1% yield), 4 (brown powder, 20% yield), 3 (oil, 10% yield), and 2 (white powder, 5% yield) (Chart 1).
Chart 1. Chemical Structures of ent-Beyerene Diterpenes (1–7) and Pentacyclic Triterpenes (8 and 9).
Structural Elucidation of Novel Constituents from Fabiana densa var. ramulosa
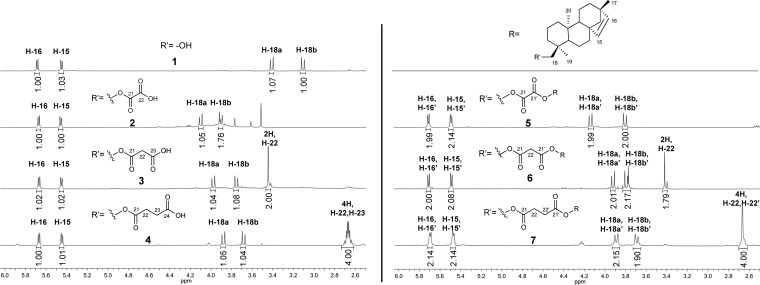
The structures of all compounds were unambiguously confirmed through nuclear magnetic resonance (NMR) spectroscopy and by electrospray ionization-high-resolution mass spectrometry (ESI-HRMS). Most of the isolated molecules belong to the family of tetracyclic ent-beyerene diterpenes, and only 8 and 9 are pentacyclic triterpenoids (Chart 1). Regarding the diterpenes derivatives, in addition to the succinoyl 4 and the oxaloyl 2 esters (already extracted from F. densa(17)), we isolated the alcohol 1 and a new diterpene, the malonoyl ester 3. The 1H NMR spectral data of 1, 2, and 4 showed the peculiar signals of the ent-beyerene scaffold, i.e., a pair of doublets for the olefinic protons H-15 and H-16, two doublets for the diastereotopic protons H-18a and H-18b, and three intense singlets for the three methyl groups (Figure 1). The 1H NMR spectra of 4 revealed a multiplet centered at 2.67 ppm integrated for four protons, which was attributed to H-22 and H-23 of the succinate side chain (Figure 1). Comparing the 13C NMR spectra, the only difference was the presence of signals at 158.65 and 157.77 ppm in the case of 2 and 177.44 and 172.28 ppm in the case of 4, attributed to the oxalate and succinate carbonyl groups, respectively (Figures S4 and S8). Accordingly, the 1H NMR spectral data of compound 3 were almost coincident with those of the ent-beyerene scaffold of 1, 2, and 4, except for the signals (a pair of doublets) of H-18a and H-18b which were shifted at 3.98 and 3.75 ppm and a singlet at 3.45 ppm integrated for two protons, which was attributed to the protons H-22 of the malonate side chain (Figure 1). Comparison of the 13C NMR spectral data of 3 with those of alcohol diterpene 1 evidenced two characteristic signals at 169.30 and 167.81 ppm, which were assigned to the carbonyl groups at C-23 and C-21 of the malonate function, respectively (Figure S6). Taking into account the spectral information on 2, 3, and 4, structural elucidation of the new compounds 5, 6, and 7 was carried out. With respect to compound 5, the NMR spectral data were coincident with those of oxaloyl ester 2 (Figure 1), except for the signal at 158.08 ppm in the 13C NMR spectrum which was assigned to the equivalent carbonyl groups at C-21 and C-21′ of the oxalate bridge, thus suggesting a dimeric structure (Figure S10). NMR structural elucidation was confirmed by the ESI-high-resolution mass spectrum, which revealed the diagnostic peak at 653.45408 m/z attributed to the [M + Na]+ sodium adduct. From a visual inspection of the 6 and 71H NMR spectra, the integrals of signals located in the region from 2.5 to 6.0 ppm suggested dimeric structures featuring two diterpene moieties linked by an intramolecular malonate and succinate bridge, respectively (Figure 1). With regard to the product 6, the 1H NMR spectral data were almost coincident with those of the malonoyl ester 3 (Figure 1). However, the singlet of the protons H-22, belonging to the intramolecular malonate bridge, was integrated in a 1:1 ratio with respect to the characteristic signals of the diterpene scaffold. The dimeric structure was confirmed by the 13C NMR, which showed only one signal at 166.84 ppm for both the carbonyl groups at C-21 and C-21′ of the malonate bridge (Figure S12), and by the ESI-high-resolution mass spectrum which revealed the diagnostic peak at 667.46911 m/z attributed to the [M + Na]+ sodium adduct. Regarding dimer 7, a comparison of its NMR spectral data with those of the monomeric diterpene 4 was revealed by the following points. In the proton spectrum, a singlet centered at 2.66 ppm integrated for four protons was assigned to the equivalent protons H-22 and H-22′ (Figure 1). In addition, this signal was integrated in 1:2 ratio with the characteristic signals of diterpene scaffold, suggesting a dimeric structure also for 7. Only one signal at 172.45 ppm for both the carbonyl groups at C-21 and C-21′ of the succinate bridge in the carbon spectrum (Figure S14) and a diagnostic peak at 681.48580 m/z in the ESI-high-resolution mass spectrum, which was attributed to the [M + Na]+ sodium adduct, confirmed the dimeric structure. The pentacyclic triterpenes 8 and 9 are widely distributed in the plant kingdom. In particular, triterpene 8 was isolated in high yields from the Chilean medicinal plants belonging to the Fabiana genus: Fabiana imbricata R. et P. and F. patagonica Speg.13,19 In this work, triterpenes 8 and 9 were isolated for the first time as constituents of F. densa var. ramulosa. Chemical identity of these compounds was confirmed by NMR (Figures S15–S18) and ESI-MS experiments and proved to be in agreement with the literature data.20,21
Figure 1.
1H NMR (400 MHz, CDCl3) spectra of compounds 1–7. Region from 2.5 to 6.0 ppm.
Semisynthesis of Diterpene Esters 2, 3, and 4
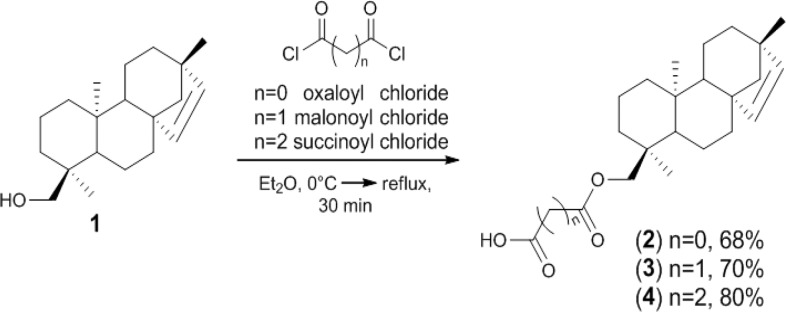
In order to obtain the monomeric diterpenes in higher yield, the alcohol 1 was used as a starting material. In particular, the esterification of diterpene 1 with the corresponding acyl chloride was carried out in Et2O at reflux for 30 min (Scheme 1). The corresponding oxaloyl 2, malonoyl 3, and succinoyl 4 esters were obtained in 68%, 70%, and 80% yields, respectively. The semisynthetic approach, based on the employment of 1 as a suitable platform featuring the ent-beyerene scaffold, provided a mild and cost-effective procedure to prepare several diterpene derivatives.
Scheme 1. Semisynthesis of Compounds 2, 3, and 4 from 1.
Antimicrobial Activity
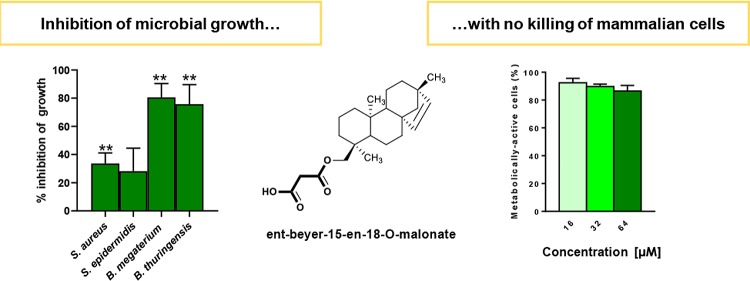
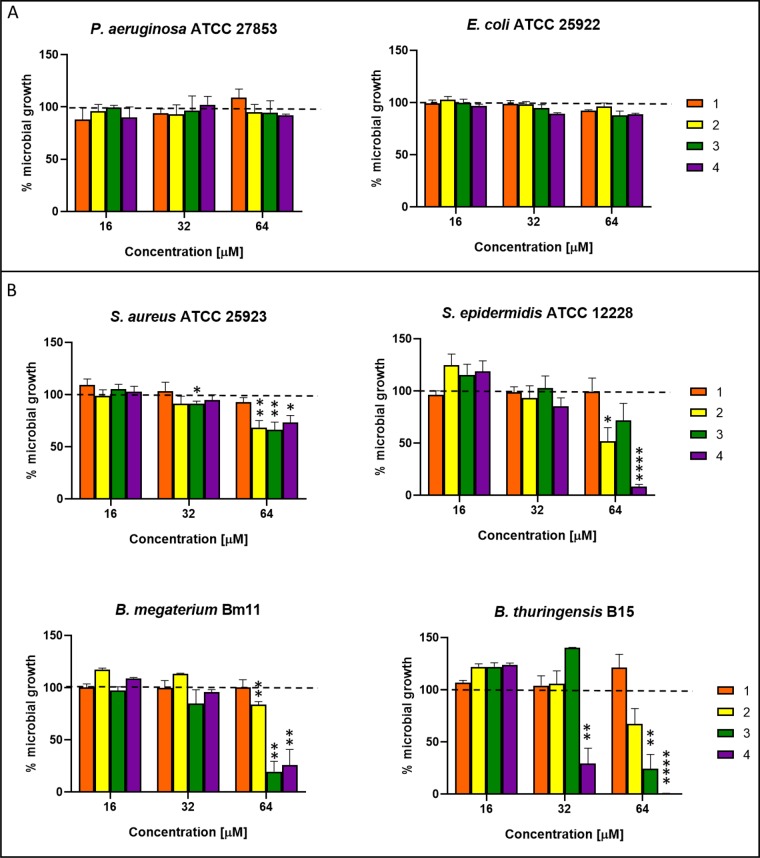
The newly identified molecules from F. densa var. ramulosa (compounds 3, 5, 6, 7) as well as the diterpenoids previously isolated from this plant species (compounds 1, 2, and 4) were analyzed for their capability to inhibit the growth of a panel of Gram-negative and Gram-positive bacterial reference strains. Ursolic and oleanolic acids (compounds 8 and 9), already identified in other plant species and largely investigated for their antimicrobial activity,15,22,23 were not studied in this work. As reported in Figure 2, compound 1 resulted to be inactive against all the tested bacterial strains. In comparison, compound 2 displayed a selective activity against Gram-positive bacterial strains, causing approximately 50% inhibition of growth of Staphylococcus epidermidis ATCC 12228 and Bacillus thuringensis B15 at the highest concentration used (64 μM), while compound 4 induced more than 80% inhibition of S. epidermidis and Bacillus megaterium Bm11 growth, and complete inhibition of bacterial growth in the case of B. thuringensis. A weaker effect (∼40% growth inhibition) was instead exhibited by the two compounds (2 and 4) against S. aureus ATCC 25923. Interestingly, the new diterpene, i.e., the malonoyl ester (compound 3), which was tested for the first time in this study, was found to be active against all the Gram-positive species, although the inhibitory effect was stronger against Bacillus spp. (>70% inhibition at 64 μM). In contrast, the dimeric compounds 5, 6, and 7 were devoid of efficacy against all the tested microorganisms and therefore are not shown.
Figure 2.
Effect of compounds 1, 2, 3, and 4 at different concentrations, on the growth of a panel of Gram-negative (A) and Gram-positive (B) bacterial strains. The data are expressed as percentage of bacterial growth compared to the control (vehicle-treated bacteria) and represent the mean of three independent experiments ± standard error of the mean (SEM). Dotted line indicates bacterial growth of control samples. The level of statistical significance of each treated group versus control is indicated as follows: *, p < 0.05; **, p < 0.01, ****, p < 0.0001.
Cytotoxicity
On the basis of the results obtained from the antimicrobial assays, we studied the effect of compounds 1, 2, 3, and 4 on the viability of mammalian cells that can be easily infected and colonized by Staphylococcus spp., such as keratinocytes.24,25 Therefore, human immortalized keratinocytes (HaCaT cells) were incubated for 24 h with the selected molecules at a concentration range from 16 to 64 μM, and the percentage of metabolically active cells was evaluated by the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (Figure 3).
Figure 3.
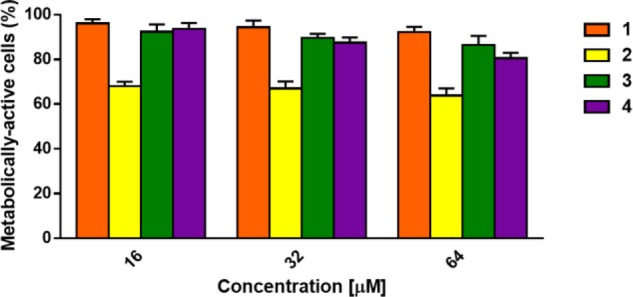
Effect of compounds 1, 2, 3, and 4 at different concentrations, on the viability of HaCaT cells. The percentages of metabolically active cells, calculated with respect to the vehicle-treated cells, are the mean of three independent experiments ± SEM.
Compound 1 which was inactive against microbial strains (Figure 2) was also harmless to HaCaT cells at all concentrations tested. In parallel, compounds 2 and 4 caused about 36% and 20% reduction of cell viability at the highest concentration of 64 μM. Interestingly, after treatment with compound 3, the reduction in the percentage of metabolically active cells at 64 μM was only 14%, suggesting this compound as the less toxic molecule among those showing significant antimicrobial activities.
Conclusions
Based on previous evidence on the antimicrobial activity of noncommon diterpenes from Fabiana densa var. ramulosa, we performed a more comprehensive study of this plant species. In this work, we described the isolation and structural elucidation of novel chemical constituents of F. densa and, among them, three dimeric diterpenes and a new diterpenoid (3) were identified and reported for the first time. Interestingly, a thorough characterization of their antimicrobial and cytotoxic activities indicated diterpene 3 as the less toxic molecule among those showing significant antimicrobial activities, whereas the dimeric diterpenes were not active toward any of the tested microorganisms. These results indicated a key role played by the acidic group at C18 of the tetracyclic ent-beyerene scaffold for the antibacterial effects and highlighted how the length and flexibility of the alkyl chain between the two carbonyl groups are crucial factors for the biological activity of the molecule. Importantly, these studies should assist the optimization of new diterpene-based drugs for the development of new anti-infective agents.
Acknowledgments
This work was supported from PON (Piano Operativo Nazionale) Grant ARS01_00432 PROGEMA, “Processi Green per l’Estrazione di Principi Attivi e la Depurazione di Matrici di Scarto e Non”, 03/2018–09/2020 and PRIN 2017-“Targeting Hedgehog pathway: Virtual screening identification and sustainable synthesis of novel Smo and Gli inhibitors and their pharmacological drug delivery strategies for improved therapeutic effects in tumors” by the Italian Ministry of Education, University and Research (MIUR) and Sapienza University of Rome. This work was also supported by MIUR–Dipartimenti di Eccellenza– L. 232/2016 and Sapienza University of Rome (Project RM11816436113D8A). The authors acknowledge networking contribution by the COST Action CM1407 “Challenging Organic Syntheses Inspired by Nature–From Natural Products Chemistry to Drug Discovery”.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications Web site. The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.9b00605.
Chemistry experimental section and the biological assays (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Dedication
This manuscript is dedicated to the memory of Professor Maurizio Botta (University of Siena, Department of Biotechnology, Chemistry and Pharmacy). During his praiseworthy scientific career, he synthesized and characterized an extraordinarily large number of small bioactive molecules for the development of new therapeutic agents for cancer therapy and/or treatment of microbial infections, thus providing a priceless worldwide contribution in the field of medicinal chemistry and drug discovery.
Supplementary Material
References
- Harvey A. L.; Edrada-Ebel R.; Quinn R. J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discovery 2015, 14 (2), 111–29. 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- Khan R. A. Natural products chemistry: The emerging trends and prospective goals. Saudi Pharm. J. 2018, 26 (5), 739–753. 10.1016/j.jsps.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B. A New Golden Age of Natural Products Drug Discovery. Cell 2015, 163 (6), 1297–300. 10.1016/j.cell.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirkia V.; Heinrich M. Alkaloids as drug leads – A predictive structural and biodiversity-based analysis. Phytochem. Lett. 2014, 10, xlviii–liii. 10.1016/j.phytol.2014.06.015. [DOI] [Google Scholar]
- Kingston D. G. Modern natural products drug discovery and its relevance to biodiversity conservation. J. Nat. Prod. 2011, 74 (3), 496–511. 10.1021/np100550t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarello A.; Mori M.; Chiaradia-Delatorre L. D.; Menegatti A. C. O.; Monache F. D.; Ferrari F.; Yunes R. A.; Nunes R. J.; Terenzi H.; Botta B.; Botta M. Discovery of Mycobacterium tuberculosis protein tyrosine phosphatase B (PtpB) inhibitors from natural products. PLoS One 2013, 8 (10), e77081 10.1371/journal.pone.0077081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante P.; Alfonsi R.; Ingallina C.; Quaglio D.; Ghirga F.; D’Acquarica I.; Bernardi F.; Di Magno L.; Canettieri G.; Screpanti I.; Gulino A.; Botta B.; Mori M.; Di Marcotullio L. Inhibition of Hedgehog-dependent tumors and cancer stem cells by a newly identified naturally occurring chemotype. Cell Death Dis. 2016, 7 (9), e2376 10.1038/cddis.2016.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M.; Tottone L.; Quaglio D.; Zhdanovskaya N.; Ingallina C.; Fusto M.; Ghirga F.; Peruzzi G.; Crestoni M. E.; Simeoni F.; Giulimondi F.; Talora C.; Botta B.; Screpanti I.; Palermo R. Identification of a novel chalcone derivative that inhibits Notch signaling in T-cell acute lymphoblastic leukemia. Sci. Rep. 2017, 7 (1), 2213. 10.1038/s41598-017-02316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casciaro B.; Calcaterra A.; Cappiello F.; Mori M.; Loffredo M. R.; Ghirga F.; Mangoni M. L.; Botta B.; Quaglio D. Nigritanine as a New Potential Antimicrobial Alkaloid for the Treatment of Staphylococcus aureus-Induced Infections. Toxins 2019, 11 (9), 511. 10.3390/toxins11090511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaria A. S.; Peralta I. E.. Las especies de Fabiana Ruiz et PAv (solanaceae) que crecen en Chile. Chloris Chilensis 2013, 16 ( (1), ). [Google Scholar]
- Cuello S.; Alberto M. R.; Zampini I. C.; Ordonez R. M.; Isla M. I. Comparative study of antioxidant and anti-inflammatory activities and genotoxicity of alcoholic and aqueous extracts of four Fabiana species that grow in mountainous area of Argentina. J. Ethnopharmacol. 2011, 137 (1), 512–22. 10.1016/j.jep.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Rodriguez R.; Marticorena C.; Alarcón D.; Baeza C.; Cavieres L.; Finot V. L.; Fuentes N.; Kiessling A.; Mihoc M.; Pauchard A.; Ruiz E.; Sánchez P.; Marticorena A. Catálogo de las plantas vasculares de Chile. Gayana. Botánica 2018, 75, 1–430. 10.4067/S0717-66432018000100001. [DOI] [Google Scholar]
- Alvarez M. E.; Maria A. O.; Saad J. R. Diuretic activity of Fabiana patagonica in rats. Phytother. Res. 2002, 16 (1), 71–3. 10.1002/ptr.754. [DOI] [PubMed] [Google Scholar]
- Zampini I.C.; Cuello S.; Alberto M.R.; Ordonez R.M.; D’Almeida R.; Solorzano E.; Isla M.I. Antimicrobial activity of selected plant species from ″the Argentine Puna″ against sensitive and multi-resistant bacteria. J. Ethnopharmacol. 2009, 124 (3), 499–505. 10.1016/j.jep.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Schmeda-Hirschmann G.; Theoduloz C. Fabiana imbricata Ruiz et Pav. (Solanaceae), a review of an important Patagonian medicinal plant. J. Ethnopharmacol. 2019, 228, 26–39. 10.1016/j.jep.2018.09.020. [DOI] [PubMed] [Google Scholar]
- Martín A. S.; Rovirosa J.; Becker R.; Castillo M. Diterpenoids from Baccharis tola. Phytochemistry 1980, 19 (9), 1985–1987. 10.1016/0031-9422(80)83018-4. [DOI] [Google Scholar]
- Erazo S.; Zaldivar M.; Delporte C.; Backhouse N.; Tapia P.; Belmonte E.; Delle Monarche F.; Negrete R. Antibacterial diterpenoids from Fabiana densa var. ramulosa. Planta Med. 2002, 68 (4), 361–3. 10.1055/s-2002-26746. [DOI] [PubMed] [Google Scholar]
- WHO . Global Priority List of Antibiotic-resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics, 2017; http://apps.who.int/medicinedocs/documents/s23171en/s23171en.pdf (accessed October 24, 2019).
- Astudillo L.; Rodriguez J. A.; Schmeda-Hirschmann G. Gastroprotective activity of oleanolic acid derivatives on experimentally induced gastric lesions in rats and mice. J. Pharm. Pharmacol. 2002, 54 (4), 583–8. 10.1211/0022357021778718. [DOI] [PubMed] [Google Scholar]
- Silva M. G.; Vieira I. G.; Mendes F. N.; Albuquerque I. L.; dos Santos R. N.; Silva F. O.; Morais S. M. Variation of ursolic acid content in eight Ocimum species from northeastern Brazil. Molecules 2008, 13 (10), 2482–7. 10.3390/molecules13102482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoja E. E.; Ndukwe I. G. Isolation of oleanolic acid from chloroform extract of Borreria stachydea[(DC) Hutch. and Dalziel]. J. Nat. Prod. Plant Resour 2013, 3 (2), 57–60. [Google Scholar]
- Park S. N.; Lim Y. K.; Choi M. H.; Cho E.; Bang I. S.; Kim J. M.; Ahn S. J.; Kook J. K. Antimicrobial Mechanism of Oleanolic and Ursolic Acids on Streptococcus mutans UA159. Curr. Microbiol. 2018, 75 (1), 11–19. 10.1007/s00284-017-1344-5. [DOI] [PubMed] [Google Scholar]
- Wolska K. I.; Grudniak A. M.; Fiecek B.; Kraczkiewicz-Dowjat A.; Kurek A. Antibacterial activity of oleanolic and ursolic acids and their derivatives. Central European Journal of Biology 2010, 5 (5), 543–553. 10.2478/s11535-010-0045-x. [DOI] [Google Scholar]
- Kintarak S.; Whawell S. A.; Speight P. M.; Packer S.; Nair S. P. Internalization of Staphylococcus aureus by human keratinocytes. Infect. Immun. 2004, 72 (10), 5668–75. 10.1128/IAI.72.10.5668-5675.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong G.; Paulino F.; Wachtel S.; Parker D.; Wickersham M.; Zhang D.; Brown A.; Lauren C.; Dowd M.; West E.; Horst B.; Planet P.; Prince A. Methicillin-resistant Staphylococcus aureus adaptation to human keratinocytes. mBio 2015, 6 (2), e00289-1. 10.1128/mBio.00289-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.