Abstract
Betulinic acid is validated as a new self-assembly inducer for the formation of nanoparticles (NPs) in combination with different drugs. The target compounds are characterized by the presence of anticancer drugs acting on tubulin dynamics and of a linker that could be a carbon chain or a triazole-based one. Nanoparticles formed are characterized and their biological activity is evaluated.
Keywords: Self-assembled nanoparticles, cancer, betulinic acid, cabazitaxel, podophyllotoxin, thiocolchicine
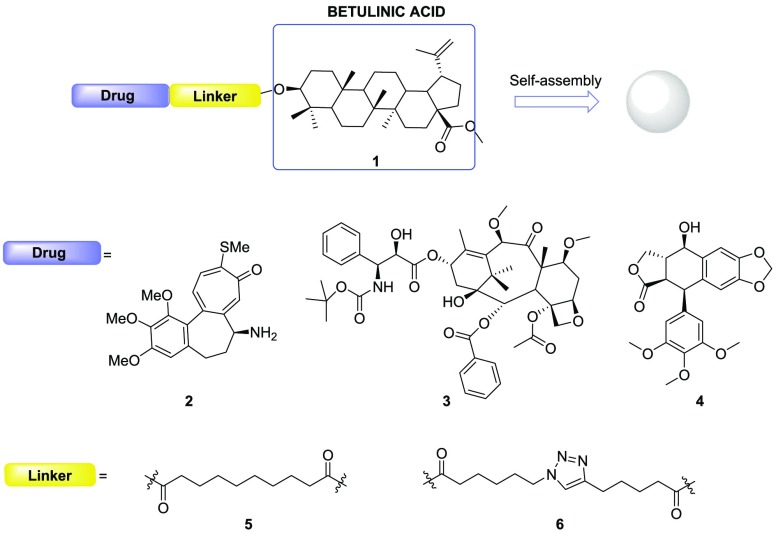
For several years we have been interested in the use of nanotechnology to improve the properties of both anticancer and neuroprotective drugs.1−8 We designed conjugates able to spontaneously assemble in water forming nanoparticles that can release the drug in cellular media,2 fluorescent hetero-NPs,3,4 and hetero-NPs bearing two different drugs.4−6 These compounds present the general structure of a drug conjugated through a linker to a self-assembly inducer, that was either squalene,2−5 4-(1,2-diphenylbut-1-en-1-yl)aniline,7 or 20-hydroxyecdisone.6 The choice of the self-assembly inducer is important for the formation of nanoparticles, and here is where we set our interest. In fact, the possibility to have a moiety that not only is able to induce the aggregation but also possesses some biological activity toward the same target could be useful to further improve the pharmacological properties of the drug.
Our goal was to identify a pharmacologically active compound that will be able to act as a self-assembly inducer in the formation of nanoparticles. Our previous results regarding the use of 20-hydroxyecdysone as a self-assembly inducer moved us to consider betulinic acid, a natural product derived from plane tree bark, that has shown beneficial properties for tumor therapy. In fact, the pentacyclic triterpenoid betulinic acid exhibits a wide range of biological and medicinal properties such as antivenom, anti-HIV, antibacterial, antimalarial, anti-inflammatory, anthelmintic, antinociceptive, anti-HSV-1, and anticancer activities.9 It has been reported to induce different forms of cell death, such as apoptosis, necrosis, and autophagy, in different types of cancers. In addition, nontumor cells, such as fibroblasts and lymphocytes, resulted less affected by betulinic acid than tumor cells.10 The use of betulinic acid in nanoformulations is really interesting,11 also because other pentacyclic triterpenoids were previously used as self-assembly inducers, like oleanolic acid and ursolic acid. We decided to consider betulinic acid for its possible dual activity (selective cytotoxic compound and self-assembly inducer) and to incorporate it in conjugated compounds using as second building block well-known tubulin binders (Figure 1). Synthesis of the planned compounds, their ability to form self-assembled nanoparticles and their ability to affect ovarian carcinoma cell viability are here reported.
Figure 1.
Structure of the designed conjugate compounds.
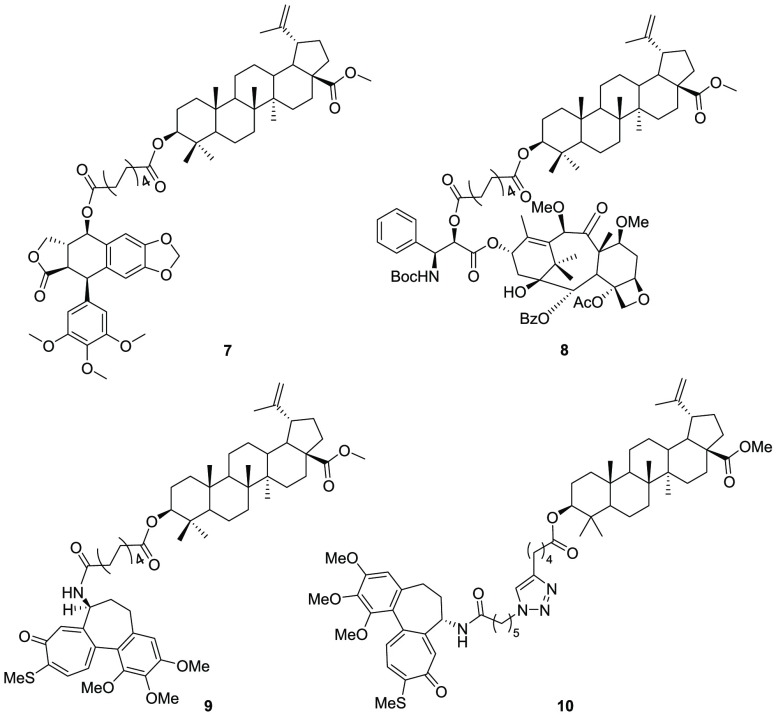
To assess the capability of betulinic acid to act as a self-assembly inducer, we conjugated it to three different drugs, all interacting with microtubules as either stabilizers or destabilizers: N-desacetyl thiocolchicine (2), cabazitaxel (3), and podophyllotoxin (4).
All these moieties were attached to betulinic acid through sebacic acid as a linker. Moreover, an additional triazole-based linker was used with N-desacetyl thiocolchicine to verify its influence in the formation and in the biological properties of nanoparticles.12 (Figure 2)
Figure 2.
Structure of the conjugates.
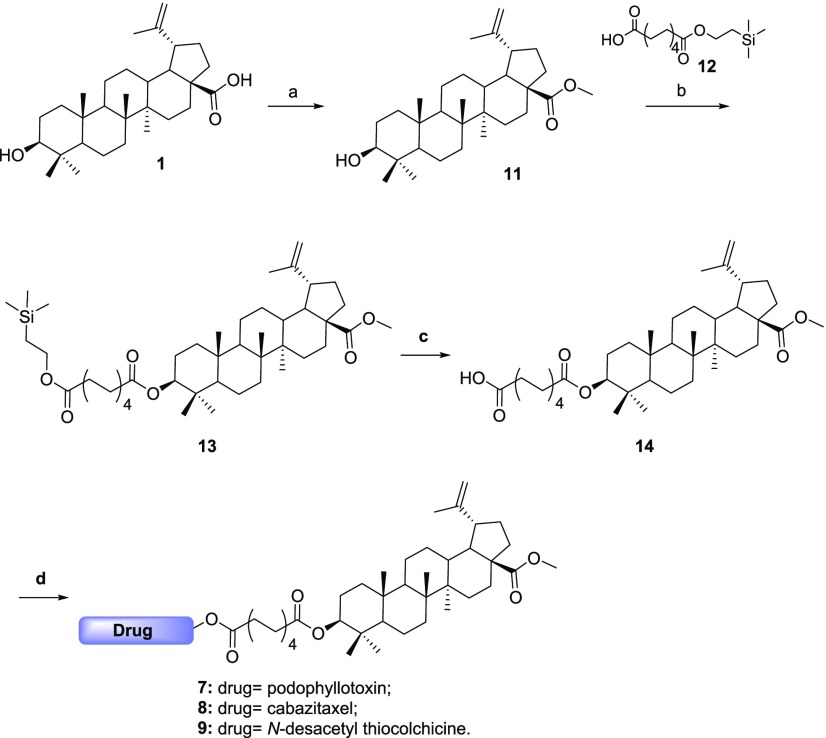
For this synthesis, betulinic acid needed to be modified, as its acidic moiety could give side reaction in the conjugation steps. It was reacted with trimethylsilyl diazomethane to form its methyl ester 11, that was subsequently used for the synthesis of the two series of conjugates. When sebacic acid was used as a linker, compound 11 was conjugated to a monoprotected sebacic acid to give product 13 that, upon deprotection by TBAF, gave us the desired betulinic acid-linker 14. This moiety was then conjugated, under the same conditions, to the three above-mentioned drugs, leading to final compounds 7, 8, and 9 (Scheme 1).
Scheme 1. Synthesis of Conjugates with Sebacic Linker.
Reaction conditions: (a) (CH3)3SiCHN2, MeOH/PhCH3, rt, 30 h, 95%; (b) DCC, DMAP, CH2Cl2, rt, overnight, 92%; (c) TBAF, THF, rt, 20 h, 93%; (d) drug, DCC, DMAP, CH2Cl2, rt, 26 h, 66–95%.
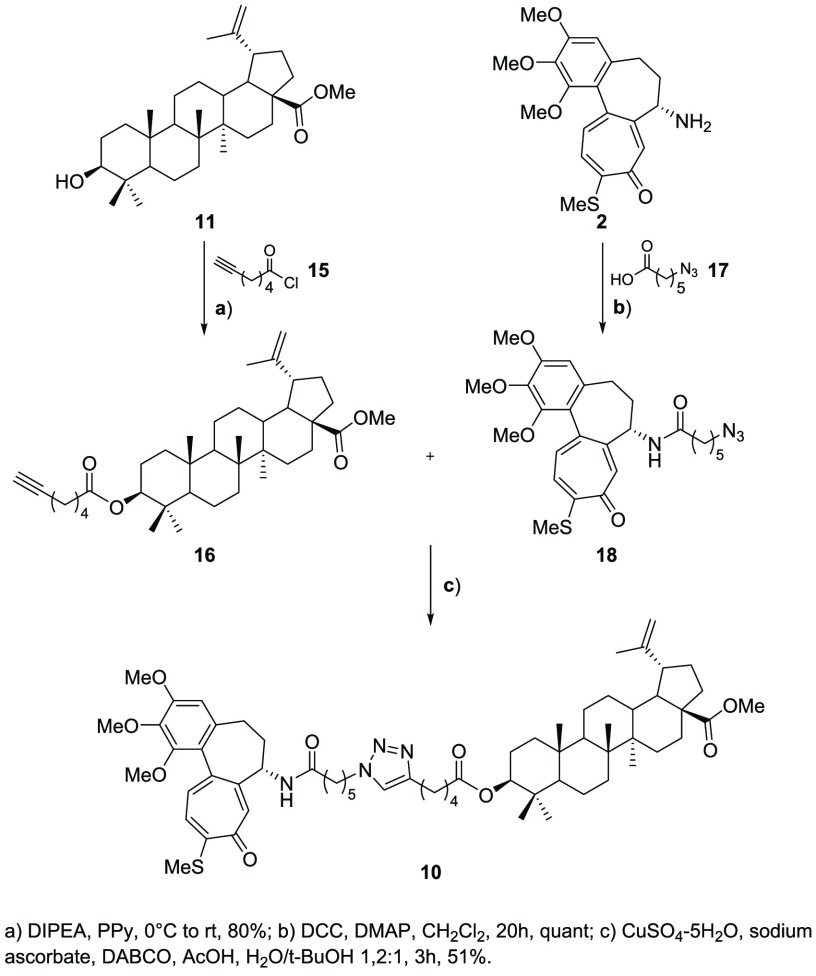
For what regards the conjugate bearing the triazole moiety, instead, compound 11 was reacted with acyl chloride 15 (prepared following a procedure already reported in the literature13) to give the corresponding ester. Meanwhile, the chosen drug, i.e. N-desacetyl thiocolchicine, was conjugated to 17, thus obtaining azide 18. Having in our hands the azide and the alkyne moieties, we performed a 1,3-dipolar cycloaddition to obtain triazole 10 as the final compound (Scheme 2).
Scheme 2. Synthesis of Thiocolchicine–Triazole Conjugate.
Synthesized all the desired conjugates, we evaluated the successful formation of nanoparticles by dynamic light scattering (DLS) measurements (Table 1). All the compounds gave a stable and monodisperse suspension of NPs, characterized by hydrodynamic diameters (HD) in the range of 320–560 nm and a negative Z-potential (<−25.0 mV).
Table 1. Hydrodynamic Diameter and Z-Potential of Nanoformulated Compounds 7, 8, 9, and 10.
| Compound | Polidispersity index (PI) | Hydrodinamic Diameters (nm) | Z-Potential (mV) |
|---|---|---|---|
| 7 | 0.088 ± 0.022 | 322 ± 11 | –34.02 ± 0.48 |
| 8 | 0.126 ± 0.010 | 503 ± 104 | –35.53 ± 0.51 |
| 9 | 0.074 ± 0.012 | 558 ± 49 | –41.03 ± 0.65 |
| 10 | 0.173 ± 0.015 | 329 ± 59 | –25.66 ± 3.84 |
The ability of the building blocks (desacetylthiocolchicine, podophyllotoxin, and cabazitaxel) of conjugates and of the NPs to affect cell viability was assayed by trypan blue exclusion test on ovarian carcinoma cell line, A2780. The obtained results, expressed as GI50 values (μM), are shown in Table 2.
Table 2. Cell Growth Inhibition of A2780 Cells in the Presence of Tested Compounds and NPs.
| Compound | A2780 cells (GI50 μM)a |
|---|---|
| N-Desacetylthiocolchicine (2) | 0.0121 ± 0.0003 |
| 10 | 2.6 ± 0.1 |
| NP-10 | 3.0 ± 0.1 |
| 9 | 14.5 ± 0.6 |
| NP-9 | 9.5 ± 1.2 |
| Podophyllotoxin (4) | 0.0085 ± 0.0005 |
| 7 | 16.9 ± 0.3 |
| NP-7 | 17.0 ± 3.2 |
| Cabazitaxel (3) | 0.000327 ± 0.000023 |
| 8 | 0.35 ± 0.01 |
| NP-8 | 12.0 ± 2.3 |
| Betulinic methyl ester (11) | 17.6 ± 1.7 |
Values are the mean ± SD of at least three independent experiments.
All starting drugs are very effective in inducing cytotoxicity, as expected, with GI50 values in the nanomolar range. Otherwise, 11, the methyl ester of betulinic acid, appears significantly less active. Interestingly, the antiproliferative capacity is similarly maintained both by the conjugates as monomers and by the corresponding NPs, even though to a lesser extent than native drugs. Indeed, for all NPs GI50 values in the micromolar range were obtained. In particular, 10 and 9 exert the highest cytotoxicity, while 8 formulated as a nanoparticle demonstrates the most pronounced decrease in biological effect with respect to the starting cabazitaxel, by about 5 orders of magnitude. This result may be attributed to a slower disaggregation of the nanoparticles or cleavage of the ester moiety. Moreover, the difference in antiproliferative effect between 10 and 9 is noteworthy. In detail, 10 shows a GI50 value about three times lower than that of 9, which highlights the crucial role played by the linker and, in particular, by the insertion of the triazole moiety.
The maintenance of the cytotoxic effect by the NPs prompts us to investigate if the mechanism of action is also retained. For this purpose, cytofluorimetric analyses were performed on A2780 stained with propidium iodide and incubated with test agents, and the effect on cell cycle was examined. The cytograms of DNA content and the percentages of cells in the different phases of cell cycle are reported in Figure 3a–b and in Table 3 and Table 4. The obtained results indicate that the NPs are able to induce an increase in G2/M, thus acting, in accordance with the starting drugs, as microtubule-targeting agents.
Figure 3.
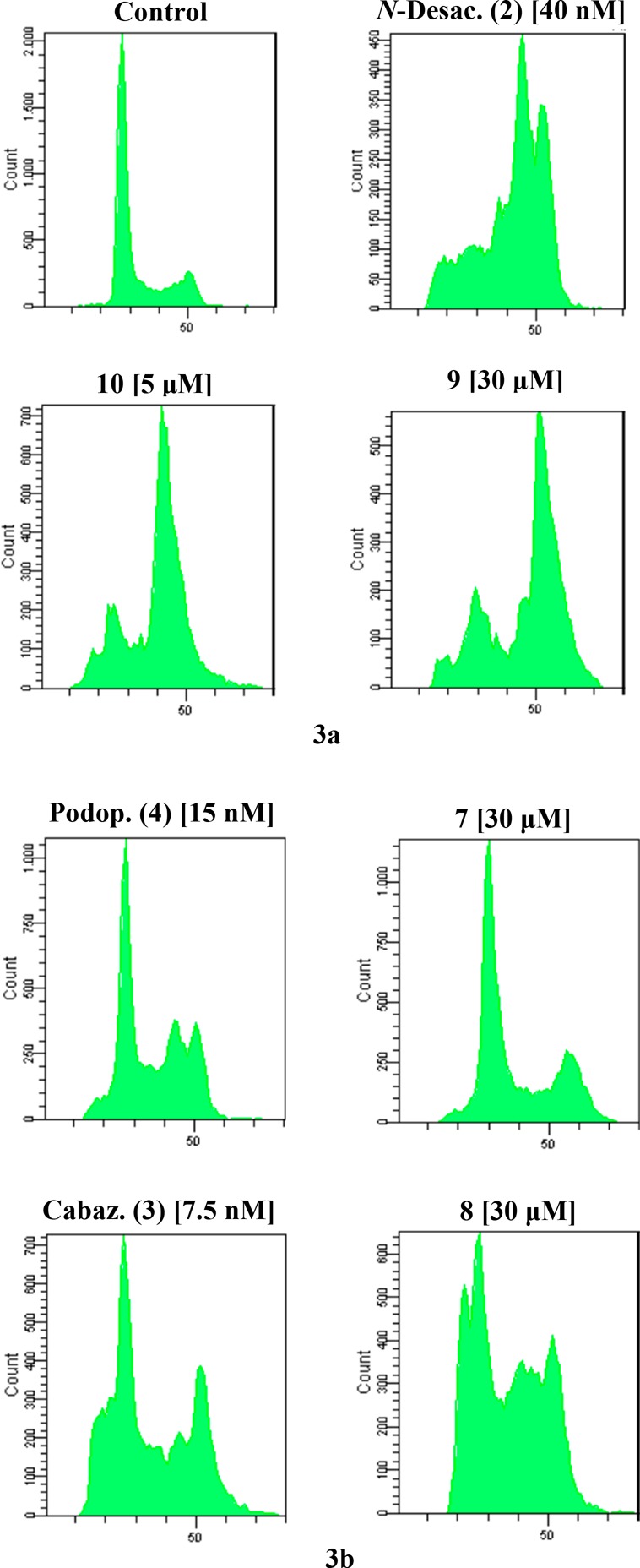
(a–b) Cell cycle distribution of A2780 cells incubated in the presence of the test agents for 24 h at the indicated concentrations.
Table 3. Data Relative to Figure 3a.
| Control | N-Desac. (2) [40 nM] | 10 [5 μM] | 9 [30 μM] | |
|---|---|---|---|---|
| PreG0 | 0.6% | 15.5% | 6.5% | 6.2% |
| G0/G1 | 69.1% | 11.4% | 15.8% | 17.2% |
| S | 12.8% | 16.7% | 10% | 8.7% |
| G2/M | 17.2% | 55.8% | 67.1% | 67.4% |
Table 4. Data Relative to Figure 3b.
| Podop. (4) [15 nM] | 7 [40 μM] | Cabaz. (3) [7.5 nM] | 8 [30 μM] | |
|---|---|---|---|---|
| PreG0 | 9.8% | 4.2% | 17.4% | 12.4% |
| G0/G1 | 39.4% | 56.9% | 30.8% | 30.5% |
| S | 16.7% | 11.5% | 15.8% | 26.4% |
| G2/M | 33.2% | 27.3% | 35.5% | 30.5% |
Conclusions
A class of betulinic acid-based conjugate compounds of cabazitaxel, podophyllotoxin, and thiocolchicine were prepared. The obtained compounds self-assemble and form nanoassemblies that we characterized in this study. Both the conjugates as monomers and the relative nanoparticles were tested for their antiproliferative activity, obtaining good results even though all the products result less active than the native drugs. In particular, while the thiocolchicine-based conjugates maintain an interesting activity, especially the compound that presents the triazole ring-based linker, the one bearing cabazitaxel was the most affected by the conjugation. A reasonable explanation of that can be attributed to a sluggish disaggregation of said nanoparticles, to a limited interaction of the drug conjugate or to a partial hydrolysis of the ester bond that connects the native drug to the linker. We consider relevant that the studies regarding the biological mechanism inducing the detected cytotoxicity show an increase in G2/M, that confirms the maintenance of the activity of the native microtubules-tubulin binders cabazitaxel, podophyllotoxin, and thiocolchicine. The introduction of a proper self-immolative linker could secure the release of the native drugs and the improvement of the biological activity. The described results are a further demonstration of the easy obtainment of self-assembled nanoparticles by simple chemical functionalization of known anticancer drugs and of the possible modulation of their activity by varying the nature of the self-assembly inducer and linker.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.9b00668.
Experimental details regarding synthesis of conjugates, nanoparticles preparation and characterization, and biological evaluation (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Fumagalli G.; Marucci C.; Christodoulou M. S.; Stella B.; Dosio F.; Passarella D. Self Assembly Drug Conjugates for Anticancer Treatment. Drug Discovery Today 2016, 21, 1321–1329. 10.1016/j.drudis.2016.06.018. [DOI] [PubMed] [Google Scholar]
- Borrelli S.; Christodoulou M. S.; Ficarra I.; Silvani A.; Cappelletti G.; Cartelli D.; Damia G.; Ricci F.; Zucchetti M.; Dosio F.; Passarella D. New Class of Squalene-Based Releasable Nanoassemblies of Paclitaxel, Podophyllotoxin, Camptothecin and Epothilone A. Eur. J. Med. Chem. 2014, 85, 179–190. 10.1016/j.ejmech.2014.07.035. [DOI] [PubMed] [Google Scholar]
- Borrelli S.; Cartelli D.; Secundo F.; Fumagalli G.; Christodoulou M. S.; Borroni A.; Perdicchia D.; Dosio F.; Milla P.; Cappelletti G.; Passarella D. Self-Assembled Squalene-based Fluorescent Hetero-nanoparticles. ChemPlusChem 2015, 80, 47–49. 10.1002/cplu.201402239. [DOI] [Google Scholar]
- Fumagalli G.; Mazza D.; Christodoulou M. S.; Damia G.; Ricci F.; Perdicchia D.; Stella B.; Dosio F.; Sotiropoulou P. A.; Passarella D. Cyclopamine–Paclitaxel-Containing Nanoparticles: Internalization in Cells Detected by Confocal and Super ResolutionMicroscopy. ChemPlusChem 2015, 80, 1380–1383. 10.1002/cplu.201500156. [DOI] [PubMed] [Google Scholar]
- Fumagalli G.; Stella B.; Pastushenko I.; Ricci F.; Christodoulou M. S.; Damia G.; Mazza D.; Arpicco S.; Giannini C.; Morosi L.; Dosio F.; Sotiropoulou P. A.; Passarella D. ACS Med. Chem. Lett. 2017, 8, 953–957. 10.1021/acsmedchemlett.7b00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli G.; Giorgi G.; Vágvölgyi M.; Colombo E.; Christodoulou M. S.; Collico V.; Prosperi D.; Dosio F.; Hunyadi A.; Montopoli M.; Hyeraci M.; Silvani A.; Lesma G.; Dalla Via L.; Passarella D. Heteronanoparticles by self-assembly of ecdysteroid and doxoru-bicin conjugates to overcome cancer resistance. ACS Med. Chem. Lett. 2018, 9, 468–471. 10.1021/acsmedchemlett.8b00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli G.; Christodoulou M. S.; Riva B.; Revuelta I.; Marucci C.; Collico V.; Prosperi D.; Riva S.; Perdicchia D.; Bassanini I.; García-Argáez A.; Dalla Via L.; Passarella D. Self-assembled 4-(1,2-diphenylbut-1-en-1-yl)aniline based Nanoparticles: Podophyllotoxin and Aloin as building blocks. Org. Biomol. Chem. 2017, 15, 1106–1109. 10.1039/C6OB02591A. [DOI] [PubMed] [Google Scholar]
- Colombo E.; Biocotino M.; Frapporti G.; Piccoli G.; Randazzo P.; Christodoulou M. S.; Polito L.; Seneci P.; Passarella D. Nanolipid-Trehalose Conjugates and Nano-Assemblies as Putative Autophagy Inducers. Pharmaceutics 2019, 11, 422–438. 10.3390/pharmaceutics11080422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordyjewska A.; Ostapiuk A.; Horecka A.; Kurzepa J. Betulin and betulinic acid: triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951. 10.1007/s11101-019-09623-1. [DOI] [Google Scholar]
- Zuco V.; Supino R.; Righetti S. C.; Cleris L.; Marchesi E.; Gambacorti-Passerini C.; Formelli F. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 2002, 175, 17–25. 10.1016/S0304-3835(01)00718-2. [DOI] [PubMed] [Google Scholar]
- Saneja A.; Arora D.; Kumar R.; Dubey R. D.; Panda A. K.; Gupta P. N. Therapeutic applications of betulinic acid nanoformulations. Ann. N. Y. Acad. Sci. 2018, 1421, 5–18. 10.1111/nyas.13570. [DOI] [PubMed] [Google Scholar]
- Bonandi E.; Christodoulou M. S.; Fumagalli G.; Perdicchia D.; Rastelli G.; Passarella D. 1,2,3-Triazole ring as bioisostere in medicinal chemistry. Drug Discovery Today 2017, 22, 1572–1581. 10.1016/j.drudis.2017.05.014. [DOI] [PubMed] [Google Scholar]
- Menchon G.; Prota A. E.; Lucena-Agell D.; Bucher P.; Jansen R.; Irschik H.; Muller R.; Paterson I.; Diaz J. F.; Altmann K.-H.; Steinmetz M. O. A fluorescence anisotropy assay to discover and characterize ligands targeting the maytansine site of tubulin. Nat. Commun. 2018, 9, 1–9. 10.1038/s41467-018-04535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.