Abstract
In ϒ-proteobacteria and Actinomycetales, cysteine biosynthetic enzymes are indispensable during persistence and become dispensable during growth or acute infection. The biosynthetic machinery required to convert inorganic sulfur into cysteine is absent in mammals; therefore, it is a suitable drug target. We searched for inhibitors of Salmonella serine acetyltransferase (SAT), the enzyme that catalyzes the rate-limiting step of l-cysteine biosynthesis. The virtual screening of three ChemDiv focused libraries containing 91 243 compounds was performed to identify potential SAT inhibitors. Scaffold similarity and the analysis of the overall physicochemical properties allowed the selection of 73 compounds that were purchased and evaluated on the recombinant enzyme. Six compounds displaying an IC50 <100 μM were identified via an indirect assay using Ellman’s reagent and then tested on a Gram-negative model organism, with one of them being able to interfere with bacterial growth via SAT inhibition.
Keywords: Adjuvant therapies, Antimicrobial resistance, Cysteine biosynthesis, Nonessential targets, Serine acetyltransferase
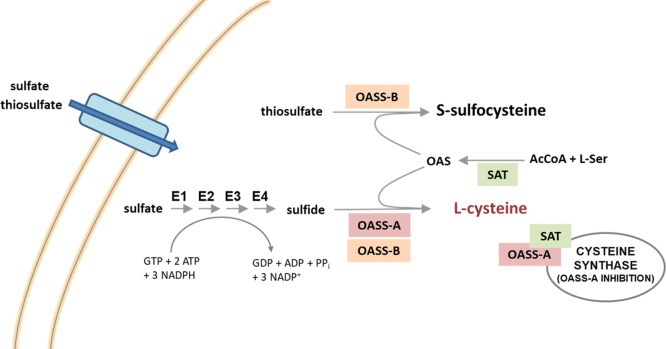
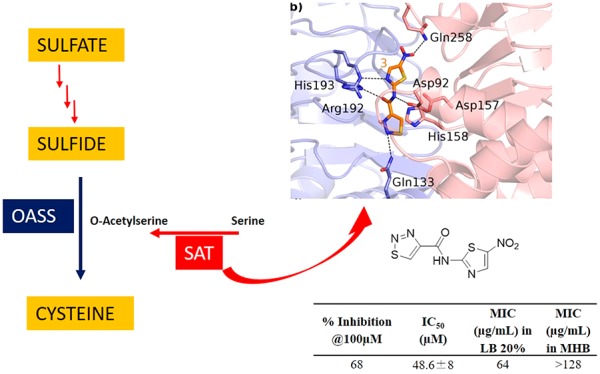
Antimicrobial resistance (AMR) is one of the most threatening insults to public health worldwide. Features related to this upset are worrying, in terms of both loss of human lives and costs for hospitalization.1 Experts estimate that 10 million people will die every year due to AMR by 2050, unless proper measures are adopted to contain the emergence.2 AMR refers to the ability of microorganisms (not necessarily bacteria, but also fungi, viruses, and protozoa) to nullify the therapeutic effects of antimicrobials, and it can affect anyone, at any age, in any country. In general, the misuse of prescribed medicines, incorrect prescriptions, and poor prevention and control measures account for the development of AMR.3−5 The search for novel druggable molecular targets different from those already exploited by the current antibacterial arsenal is crucial to prevent the onset of cross-resistance for the newest molecules under investigation. Along with this conventional approach, adjuvant strategies aimed at engaging nonessential targets, such as those related to bacterial virulence or persistence after colonization of the host, are gaining growing interest.6−9 It has been speculated that targeting those enzymes, which are dispensable during the normal bacterial life cycle but that become essential during host invasion, might have a lower impact on AMR because they facilitate the clearing action of the immune system without stimulating the rise of resistance. In addition, by decreasing the bacterial fitness, adjuvants act as antibiotic activity enhancers. The majority of pathogens spend part of their life cycle in extremely challenging environments like macrophages or the gastric mucosa, where survival and persistence require a fine metabolic adaptation. In many cases, this adaptation is made possible through a series of sulfur-containing biomolecules such as Fe–S clusters, thiamine, glutathione, and biotin, which ensure bacterial survival, especially due to their detoxifying properties and reducing power.10−12 In this regard, blocking the synthesis of cysteine, which is the most relevant building block for the above-mentioned biomolecules, holds great promise as an adjuvant strategy to undermine bacterial fitness and promote eradication of the infection. Cysteine metabolism has been indicated as a promising drug target in ϒ-proteobacteria (like Salmonella enterica serovar Typhimurium) and Actinomycetales (Mycobacterium tuberculosis)10,13 because it has been shown that suppression or reduction of the cysteine biosynthesis led to decreased bacterial fitness and infectivity.14,15 In both vegetative and swarm cell populations, the inhibition of cysteine biosynthesis is associated with an unpaired oxidative stress response, enhancing the antibacterial activity of common antibiotics and, in some cases, restoring the activity of obsolete antibacterials.14,15 Finally, whereas mammals lack the biosynthetic machinery that leads to the de novo synthesis of cysteine from inorganic sulfur, bacteria and plants possess highly conserved enzymes that enable its biosynthesis through the reductive sulfate assimilation pathway (Figure 1).10,16
Figure 1.
Reductive sulfate assimilation pathway in E. coli and Salmonella. OASS and SAT are highlighted. The enzymes that catalyze sulfate reduction are: E1, ATP sulfurylase; E2, APS kinase; E3, PAPS sulfotransferase; and E4, NADPH sulfite reductase.
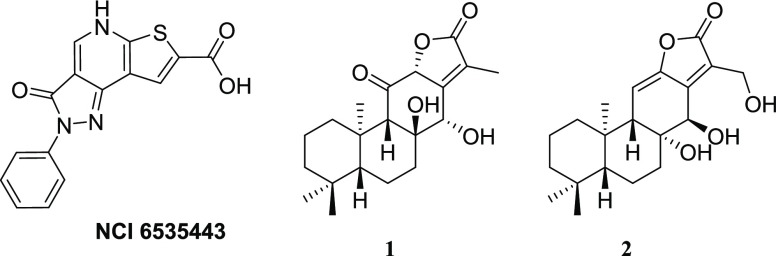
Sulfate and thiosulfate, the most abundant forms of extracellular sulfur, enter the cell through specific transporters. Sulfate is then activated to be reduced, and the entire process requires the consumption of one mole of GTP, two moles of ATP, and three moles of NADPH for each mole of cysteine produced. In E. coli and Salmonella, after the activation of sulfate by ATP with the formation of adenosine 5′-phosphosulfate (APS), 3′-phosphoadenosine-5′-phosphosulfate (PAPS) is synthesized. Afterward, PAPS is reduced to bisulfide that is incorporated into cysteine by O-acetylserine sulfhydrylase (OASS) in the last step of cysteine biosynthesis.17,18 This enzyme, present in two isoforms, OASS-A and OASS-B, depends on pyridoxal 5′-phosphate and catalyzes a β-replacement reaction on O-acetylserine (OAS), synthesized by serine acetyltransferase (SAT).19 It was demonstrated that the inhibition of OASS was able to revert the bacterial resistance to fluoroquinolones and tetracycline.14,15 This prompted us to undertake a medicinal chemistry campaign aimed at discovering potent and selective OASS inhibitors, which culminated with the release of the most potent inhibitor of StOASS known so far for both isoforms.20 Despite the remarkable inhibitory activity, it was impossible to evaluate the efficacy for bacterial cells likely due to the scarce permeability of the compound through the bacterial cell wall. After a thorough investigation of the structure–activity relationships for these OASS inhibitors,21−24 we have deemed it to be of interest to investigate the effects of SAT inhibition, which is the upstream enzyme of the cysteine synthase complex.25−28 It is notable that the inhibition of SAT is expected to exert partially distinct effects with respect to those brought about by OASS inhibition. Indeed, the inhibition of OASS is expected to induce the accumulation of OAS, which is converted to N-acetylserine (NAS), a natural inducer of the biosynthetic operon,29 and cysteine depletion. Conversely, the inhibition of SAT is expected to deplete the cell not only of cysteine but also of the natural inducer. SAT catalyzes the rate-limiting step of l-cysteine biosynthesis, where the acetyl group of acetyl-CoA is transferred to the hydroxyl group of l-serine to generate OAS. From the structural point of view, SAT is a dimer of trimers, with an α-helical N-terminal domain and a carboxyl-terminal left-handed β-helix domain ending with a flexible, disordered C-terminal sequence that is fundamental for its function and regulation. The C-terminal peptide binds OASS-A at the active site, and it is responsible for its competitive inhibition. Even though the exploitation of SAT inhibition holds promise as an antibacterial adjuvant strategy, only a limited amount of literature about potential inhibitors is reported. In one case, after a virtual screening (VS) based on the E. coli SAT crystal structure, a compound with an ICSAT50 of 72 μM (Figure 2), was identified.30 However, in the following studies, the compound was found to inhibit E. histolytica proliferation at a concentration of 0.61 μM, ruling out the possibility that the inhibition of SAT was the main mechanism of action. In another case, a small library of natural products was screened for their inhibitory activity toward methicillin-resistant Staphylococcus aureus (MRSA) SAT, and six compounds inhibiting SAT activity (IC50 ranging from 29.83 to 203.13 μM) were found.26 Interestingly, in this case, two of the hits (Figure 2, compounds 1 and 2) showed an inhibitory effect on MRSA growth (MIC at 12.5 and 25 μg/mL). Inspired by these encouraging preliminary data, we herein report the VS of a library of compounds to find potential inhibitors of SAT. As the most relevant result, we were able to identify a hit compound showing weak antibacterial properties, which is consistent with the inhibition of SAT.
Figure 2.
Chemical structures of some SAT inhibitors reported in the literature.26,28
Results and Discussion
Virtual Screening
A crucial step of a VS is the selection of the chemical library. In general, to cross the bacterial membrane, antibiotics must possess a series of peculiar physicochemical characteristics, such as molecular weight (MW), LogD, and polar surface area (PSA), which are poorly represented in commercial chemical libraries.31−33 Therefore, compound structures were retrieved from the ChemDiv anti-infective (8523), antibacterial (5460), and antiviral (77 260) focused library collections, for a total of 91 243 compounds. Ligand 3D structures were prepared along the initial stage of the Schrödinger virtual screening workflow (vsw), where compounds were (i) filtered according to the Lipinski’s rule of five, (ii) removed when the stereochemistry was undefined, (iii) protonated at pH 7.4 using “epik”, and (iv) clustered into conformers generated using the enhanced sampling methods. The structure of EcSAT in complex with cysteine (1T3D) and the structure of HiSAT in complex with CoA (1SSM) were used as protein structures for the VS. (See the Experimental Procedures in the Supporting Information). The VS identified a pool of 1409 compounds, which was reduced to 1391 hits after visual inspection. The analysis of commercial antibacterial products with activity toward Gram-negative pathogens suggests an average ClogD of −2.8 and a PSA of 165 Å as preferred characteristics.32 Therefore, 37 molecules with PSA <200 and ClogD <2 were initially cherry-picked to undergo activity assays.
The analysis of the remaining 1354 compounds was carried out using ChemGPS software34,35 to evaluate the scaffold similarity and purchase scaffolds as diverse as possible. Principal component (PC) analysis was carried out, and in Figure 3, the representation of the 1354 molecules is depicted using three PCs: PC1 (shape, size, and polarizability), PC2 (aromaticity and conjugation), and PC3 (lipophilicity, polarity, and H-bond capacity). This process allowed for the selection of 36 compounds with diverse chemical structures. At the end of the VS process, 73 compounds were purchased and evaluated for their ability to inhibit SAT in biochemical assay.
Figure 3.
Graphical representation of the principal components of the 1354 compounds. PC1 (shape, size, and polarizability) is represented on the x axis, PC2 (aromaticity and conjugation) is on the y axis, and PC3 (lipophilicity, polarity, and H-bond capacity) is represented on the z axis.
Determination of StSAT Inhibition
Whereas the StSAT 3D structure has not yet been solved, our group has a long-standing expertise in the characterization of cysteine biosynthetic enzymes from Salmonella, as previously reported.11,17,23 Because our ultimate goal is to develop molecules that are effective in blocking cysteine biosynthesis in Salmonella, we chose to test the compounds identified in the VS on StSAT. The very high overall sequence identity between the two proteins (i.e., EcSAT and StSAT, with 100% identity of the active site residues) supports the use of StSAT in the activity assays. SAT catalyzes the transfer of the acetyl group of acetyl-CoA to serine, with the release of CoASH. SAT activity can be determined by measuring the decrease in absorbance at 232 nm due to the hydrolysis of the thioester bond of acetyl-CoA.36,37 However, this methodology is not suitable for screening of compound libraries because most organic molecules display high absorbance at 232 nm.
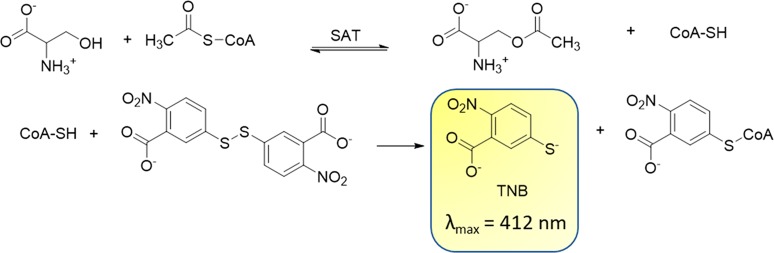
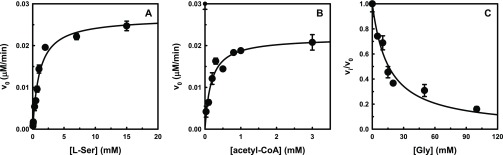
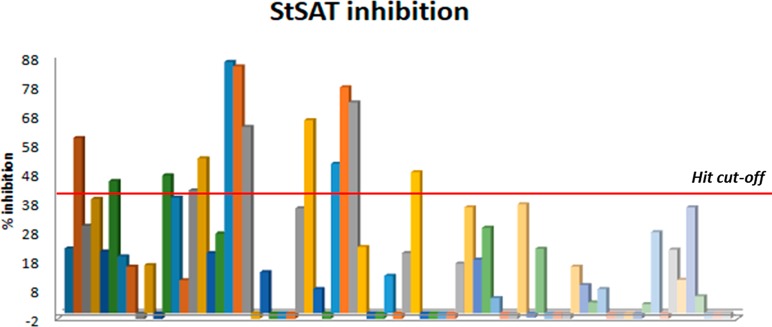
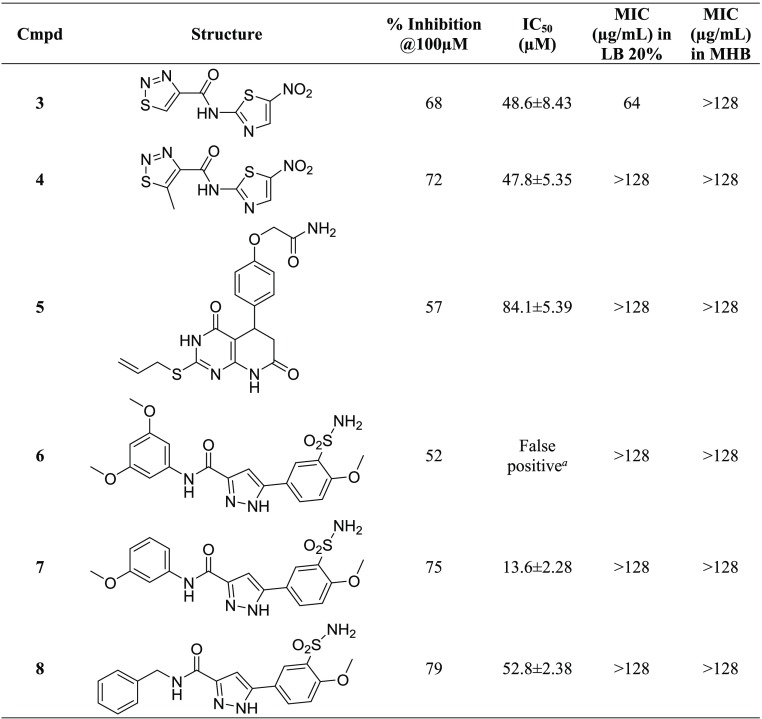
Because the transfer of the acetyl group of acetyl-CoA to serine occurs with the release of the free thiol CoASH, we used an indirect assay for the determination of SAT activity. 5,5′-Dithiobis(2-nitrobenzoic acid) (DTNB, also known as Ellman’s reagent)) has a highly oxidizing disulfide bond that is stoichiometrically reduced by free thiols originating from a mixed disulfide and a molecule of 5-thio-2-nitrobenzoic acid (TNB) (Scheme 1). The product TNB at pH higher than 7 absorbs strongly (ε = 14 000 M–1 cm–1)38 and was thus used to follow the catalytic activity of the enzyme.16 The method was optimized and validated under our experimental conditions through the determination of the kinetic constants for l-Ser and acetyl-CoA and the IC50 for the known inhibitor glycine (Figure 4). The results obtained agree with data in the literature determined using the direct assay.39 In particular, the IC50 for glycine is in very good agreement with the Ki of 13 ± 4 mM previously reported.25 The method was adapted and validated to a 96-well plate format to allow higher throughput. At the primary screening phase, the inhibitory activity of the 73 compounds from VS was determined at 100 μM (Figure 5). The obtained values for assay quality parameters, Z′, SW, and S/B, were robust enough to conclude that this assay can be used for screening procedures.40,41 Inhibitory effects were first assessed by kinetic measurements with 27 s intervals because the total number of compounds allowed such measurements. Nevertheless, the 180 s time point was selected as the optimal endpoint measurement for calculation of the % inhibition because it provided a higher robustness of the parameters Z′, SW, and S/B. Thirteen compounds, displaying a percentage of inhibition ≥40% in the primary screen, were retested at 100 μM, and, after the confirmatory testing, dose–response experiments were performed for six compounds displaying a percentage of inhibition >50% at 100 μM concentration. These six compounds (Table 1) displayed micromolar IC50 values toward StSAT, with compound 7 being the most potent inhibitor with an IC50 of 14 μM (Table 1). Being aware that many Gram-negative antibacterial drug discovery programs fail because of the scarce aptitude for numerous small molecules to cross the cellular membrane, before establishing the most promising hit compound to move forward, we decided to investigate the in-cell antimicrobial activity of compounds 3–8.
Scheme 1. Indirect Assay of SAT Activity.
CoA released by SAT activity spontaneously reacted with DTNB with the release of one molecule of TNB for each molecule of CoA produced.
Figure 4.
Validation of the indirect assay for the screening of potential SAT inhibitors. (A) Initial reaction rate as a function of l-Ser concentration. The line through data points represents the fitting to the Michaelis–Menten equation with kcat = 3813 ± 169 min–1, Km = 1.07 ± 0.15 mM, and kcat/Km = 3563 min–1 mM–1. (B) Initial reaction rate as a function of acetyl-CoA concentration. The line through data points represents the fitting to the Michaelis–Menten equation with kcat = 3128 ± 186 min–1, Km = 0.17 ± 0.04 mM, and kcat/Km = 18 400 min–1 mM–1. (C) Relative activity as a function of glycine concentration. The line through data points represents the fitting to a hyperbolic equation with IC50 = 15 ± 1 mM.
Figure 5.
Determination of the inhibitory potential of the 73 purchased compounds against StSAT at time point 180s.
Table 1. StSAT Inhibition, IC50StSAT, and MIC Values against E. coli of the Hits Derived from Virtual Screening.
Changing the dose between 200 and 50 μM always caused the same inhibition. At higher concentration, the compound was not soluble.
Inhibitory Effects on E. coli Growth
The inhibitory activity of compounds 1–6 against the Gram-negative E. coli ATCC25922 was determined by using broth microdilution assays. As mentioned, testing SAT inhibitors in a rich medium would be of poor significance for the purpose of this study. In fact, the cysteine present in the growth medium would be transported inside the cells, thus reversing the inhibition of its biosynthesis. Therefore, minimum inhibitory concentrations (MICs) were evaluated both in conventional Müller-Hinton broth (MHB) and in minimal media (LB 20%) with a limited amount of nutrients, where cysteine is absent, and thus the effect of inhibiting its biosynthesis would lead to growth-inhibiting effects. Compound 3 was able to interfere with bacterial growth in LB20% (Table 1), although at a fairly high concentration of 64 μg/mL. In addition, it can be speculated that SAT inhibition is indeed accountable for the activity of compound 3, as in rich medium, MHB, the MIC was not reached even at the highest concentration tested (128 μg/mL). Results were confirmed upon the resynthesis of compound 3, previously used from a commercial source.
Binding Modes of Compound 3
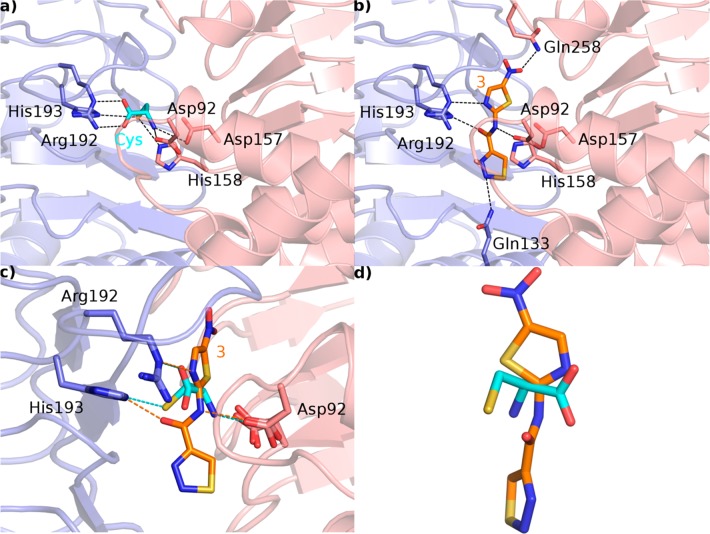
The binding modes of the substrate cysteine and compound 3 are reported in Figure 6. Cysteine is bound inside the EcSAT active site through a network of H bonds with Asp92, Asp157, His158, Arg192, and His193 (Figure 6a, dashed black lines). Interestingly, 3 establishes: (i) similar interaction with Asp92, Asp157, Arg192, and His193; (ii) a hydrophobic interaction with His158; and (iii) a further H-bond interaction with Gln133 and Gln258 (Figure 6b). The comparison of the binding modes of both cysteine and 3 reveals how compound 3 is able to mimic the interactions observed for cysteine (Figure 6c,d).
Figure 6.
(a) X-ray crystal structure of EcSAT in complex with Cys (PDB code: 1T3D). The two monomeric chains of SAT are depicted as blue and pink transparent cartoons, the amino acid side chains are depicted as sticks and are color-coded depending on the chain they belong to, the feedback inhibitor Cys is depicted as cyan sticks, and relevant H bonds are depicted as dashed black lines. (b) Binding mode of 3 (orange sticks) into the SAT active site. The color code is the same as that reported in panel a. (c) Superposition of the SAT-Cys and SAT-3 complexes. Highlighted with dashed lines are H bonds conserved in both complexes (cyan dashed lines for Cys; orange dashed lines for 3). (d) Superposition of 3 (orange sticks) and Cys (cyan sticks).
In summary, AMR is currently one of the most serious threats to public health, and developing strategies for its containment is a crucial pharmaceutical challenge. Adjuvant strategies, such as targeting the biosynthesis of molecular building blocks that are crucial for bacterial adaptation upon host invasion, represent an intriguing approach to face the onset of bacterial resistance. Among these strategies, the inhibition of the biosynthesis of cysteine, an amino acid involved in the synthesis of detoxifying biomolecules such as glutathione, holds promise for further investigation. The VS of three focused libraries from ChemDiv containing 91 243 compounds was carried out to deliver an inhibitor of bacterial SAT, an enzyme responsible for the penultimate step of cysteine biosynthesis. The process led to the disclosure of 73 virtual hit compounds that were purchased and tested against StSAT. A screening methodology using Ellman’s reagent to indirectly measure SAT activity was developed in a 96-well plate format, yielding six compounds displaying an IC50 <100 μM that were further evaluated for growth inhibitory effects against E. coli. To rule out off-target effects, MIC assays were carried out in both complete and minimal media, and we were pleased to notice that compound 3 retained an encouraging inhibitory activity toward Gram-negative E. coli in the cell-based assay.
Although we are aware that 2-aminothiazoles may cause a false-positive, and taking into consideration the call made by some editors of the ACS journals with regard to the early identification of PAINS (pan assay interference compounds), we would like to point out that derivatives 3–8 were not recognized as PAINS using the software “False Positive Remover” (http://www.cbligand.org/PAINS/) nor as aggregators according to the software “Aggregator Advisor” (http://advisor.bkslab.org/).
Acknowledgments
This work was funded under the MSCA-ITN-2014-ETN project INTEGRATE (grant number 642620). P.T. thanks the Academy of Finland (grant no. 277001) for financial support. The “Centro Interdipartimentale Misure “G. Casnati” is kindly acknowledged for the contribution to the analytical determination of the molecules synthesized. This Letter is dedicated to the truly brilliant memory of Prof. Maurizio Botta, who has inspired generations of researchers with his vision and sympathy.
Glossary
Abbreviations
- AMR
antimicrobial resistance
- APS
adenosine 5′-phosphosulfate
- DTNB
5,5′-dithiobis(2-nitrobenzoic acid)
- MHB
Müller-Hinton broth
- MIC
minimum inhibitory concentration
- MRSA
methicillin-resistant S. aureus
- NAS
N-acetylserine
- OASS
O-acetylserine sulfhydrylase
- PAINS
pan assay interference compounds
- PAPS
3′-phosphoadenosine-5′-phosphosulfate
- PSA
polar surface area
- SAT
serine acetyltransferase
- TNB
5-thio-2-nitrobenzoic acid
- VS
virtual screening.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.9b00627.
Description of methods used to set up the virtual screening, protein expression and purification, determination of StSAT inhibition, and evaluation of MIC (PDF)
Author Present Address
◆ A.B.: Experimental Therapeutics Program, IFOM – The FIRC Institute for Molecular Oncology Foundation, Via Adamello 16, 20139 Milano, Italy.
Author Contributions
J.M.: synthesis, virtual screening, set up of the StSAT assay, and preliminary microbiological investigation; N.F.: data collection and data analysis; S.R.: data collection and analysis and manuscript revision; G.A.: data analysis and manuscript revision; P.T.: supervision of StSAT screening and MIC determination and manuscript revision; A.B.: computational analysis and manuscript revision; S.B.: study design, resources, and manuscript revision; A.M.: resources, study design and supervision, and manuscript revision; MP: supervision and manuscript writing and revision; BC: study design, supervision, and manuscript writing and revision; G.C.: resources, supervision, and manuscript revision.
The authors declare no competing financial interest.
Supplementary Material
References
- Antimicrobial Resistance. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed January 16, 2020).
- de Kraker M. E. A.; Stewardson A. J.; Harbarth S. Will 10 Million People Die a Year Due to Antimicrobial Resistance by 2050?. PLOS Med. 2016, 13 (11), e1002184 10.1371/journal.pmed.1002184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell K. M. G.; Hodgkinson J. T.; Sore H. F.; Welch M.; Salmond G. P. C.; Spring D. R. Combating Multidrug-Resistant Bacteria: Current Strategies for the Discovery of Novel Antibacterials. Angew. Chem., Int. Ed. 2013, 52 (41), 10706–10733. 10.1002/anie.201209979. [DOI] [PubMed] [Google Scholar]
- Blair J. M. A.; Webber M. A.; Baylay A. J.; Ogbolu D. O.; Piddock L. J. V. Molecular Mechanisms of Antibiotic Resistance. Nat. Rev. Microbiol. 2015, 13 (1), 42–51. 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- Antibiotics: Mode of Action and Mechanisms of Resistance: Art Science. https://www.scribd.com/document/202262261/Antibiotic-Action-and-Resistance (accessed September 3, 2019).
- Taylor P. L.; Wright G. D. Novel Approaches to Discovery of Antibacterial Agents. Anim. Health Res. Rev. 2008, 9 (2), 237–246. 10.1017/S1466252308001527. [DOI] [PubMed] [Google Scholar]
- Alekshun M. N.; Levy S. B. Targeting Virulence to Prevent Infection: To Kill or Not to Kill?. Drug Discovery Today: Ther. Strategies 2004, 1 (4), 483–489. 10.1016/j.ddstr.2004.10.006. [DOI] [Google Scholar]
- Shallcross L. J.; Howard S. J.; Fowler T.; Davies S. C. Tackling the Threat of Antimicrobial Resistance: From Policy to Sustainable Action. Philos. Trans. R. Soc., B 2015, 370 (1670), 20140082. 10.1098/rstb.2014.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato G. Strategies to Overcome Antimicrobial Resistance (AMR) Making Use of Non-Essential Target Inhibitors: A Review. Int. J. Mol. Sci. 2019, 20 (23), 5844. 10.3390/ijms20235844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanini B.; Pieroni M.; Raboni S.; Bettati S.; Benoni R.; Pecchini C.; Costantino G.; Mozzarelli A. Inhibitors of the Sulfur Assimilation Pathway in Bacterial Pathogens as Enhancers of Antibiotic Therapy. Curr. Med. Chem. 2014, 22 (2), 187–213. 10.2174/0929867321666141112122553. [DOI] [PubMed] [Google Scholar]
- Spyrakis F.; Singh R.; Cozzini P.; Campanini B.; Salsi E.; Felici P.; Raboni S.; Benedetti P.; Cruciani G.; Kellogg G.; et al. Isozyme-Specific Ligands for O-Acetylserine Sulfhydrylase, a Novel Antibiotic Target. PLoS One 2013, 8, e77558 10.1371/journal.pone.0077558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noji M.; Takagi Y.; Kimura N.; Inoue K.; Saito M.; Horikoshi M.; Saito F.; Takahashi H.; Saito K. Serine Acetyltransferase Involved in Cysteine Biosynthesis from Spinach: Molecular Cloning, Characterization and Expression Analysis of CDNA Encoding a Plastidic Isoform. Plant Cell Physiol. 2001, 42 (6), 627–634. 10.1093/pcp/pce078. [DOI] [PubMed] [Google Scholar]
- Bhave D. P.; Muse W. B. III; Carroll K. S. Drug Targets in Mycobacterial Sulfur Metabolism. Infect. Disord.: Drug Targets 2007, 7 (2), 140–158. 10.2174/187152607781001772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull A. L.; Surette M. G. L-Cysteine Is Required for Induced Antibiotic Resistance in Actively Swarming Salmonella Enterica Serovar Typhimurium. Microbiology 2008, 154 (11), 3410–3419. 10.1099/mic.0.2008/020347-0. [DOI] [PubMed] [Google Scholar]
- Turnbull A. L.; Surette M. G. Cysteine Biosynthesis, Oxidative Stress and Antibiotic Resistance in Salmonella Typhimurium. Res. Microbiol. 2010, 161 (8), 643–650. 10.1016/j.resmic.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Kredich N. M.; Tomkins G. M. The Enzymic Synthesis of L-Cysteine in Escherichia Coli and Salmonella Typhimurium. J. Biol. Chem. 1966, 241 (21), 4955–4965. [PubMed] [Google Scholar]
- Spyrakis F.; Felici P.; Bayden A. S.; Salsi E.; Miggiano R.; Kellogg G. E.; Cozzini P.; Cook P. F.; Mozzarelli A.; Campanini B. Fine Tuning of the Active Site Modulates Specificity in the Interaction of O-Acetylserine Sulfhydrylase Isozymes with Serine Acetyltransferase. Biochim. Biophys. Acta, Proteins Proteomics 2013, 1834 (1), 169–181. 10.1016/j.bbapap.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Mozzarelli A.; Bettati S.; Campanini B.; Salsi E.; Raboni S.; Singh R.; Spyrakis F.; Kumar V. P.; Cook P. F. The Multifaceted Pyridoxal 5′-Phosphate-Dependent O-Acetylserine Sulfhydrylase. Biochim. Biophys. Acta, Proteins Proteomics 2011, 1814 (11), 1497–1510. 10.1016/j.bbapap.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Kredich N. M.; Becker M. A.; Tomkins G. M. Purification and Characterization of Cysteine Synthetase, a Bifunctional Protein Complex, from Salmonella Typhimurium. J. Biol. Chem. 1969, 244 (9), 2428–2439. [PubMed] [Google Scholar]
- Pieroni M.; Annunziato G.; Beato C.; Wouters R.; Benoni R.; Campanini B.; Pertinhez T. A.; Bettati S.; Mozzarelli A.; Costantino G. Rational Design, Synthesis, and Preliminary Structure-Activity Relationships of α-Substituted-2-Phenylcyclopropane Carboxylic Acids as Inhibitors of Salmonella Typhimurium O-Acetylserine Sulfhydrylase. J. Med. Chem. 2016, 59 (6), 2567–2578. 10.1021/acs.jmedchem.5b01775. [DOI] [PubMed] [Google Scholar]
- Annunziato G.; Pieroni M.; Benoni R.; Campanini B.; Pertinhez T. A.; Pecchini C.; Bruno A.; Magalhães J.; Bettati S.; Franko N.; et al. Cyclopropane-1,2-Dicarboxylic Acids as New Tools for the Biophysical Investigation of O-Acetylserine Sulfhydrylases by Fluorimetric Methods and Saturation Transfer Difference (STD) NMR. J. Enzyme Inhib. Med. Chem. 2016, 31 (sup4), 78–87. 10.1080/14756366.2016.1218486. [DOI] [PubMed] [Google Scholar]
- Magalhães J.; Annunziato G.; Franko N.; Pieroni M.; Campanini B.; Bruno A.; Costantino G. Integration of Enhanced Sampling Methods with Saturation Transfer Difference Experiments to Identify Protein Druggable Pockets. J. Chem. Inf. Model. 2018, 58 (3), 710–723. 10.1021/acs.jcim.7b00733. [DOI] [PubMed] [Google Scholar]
- Magalhães J.; Franko N.; Annunziato G.; Welch M.; Dolan S. K.; Bruno A.; Mozzarelli A.; Armao S.; Jirgensons A.; Pieroni M.; et al. Discovery of Novel Fragments Inhibiting O-Acetylserine Sulphhydrylase by Combining Scaffold Hopping and Ligand-Based Drug Design. J. Enzyme Inhib. Med. Chem. 2018, 33 (1), 1444–1452. 10.1080/14756366.2018.1512596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães J.; Franko N.; Annunziato G.; Pieroni M.; Benoni R.; Nikitjuka A.; Mozzarelli A.; Bettati S.; Karawajczyk A.; Jirgensons A.; et al. Refining the Structure-Activity Relationships of 2-Phenylcyclopropane Carboxylic Acids as Inhibitors of O-Acetylserine Sulfhydrylase Isoforms. J. Enzyme Inhib. Med. Chem. 2019, 34 (1), 31–43. 10.1080/14756366.2018.1518959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu L. S.; Cook P. F. Kinetic Mechanism of Serine Transacetylase from Salmonella Typhimurium. Biochemistry 1994, 33 (9), 2667–2671. 10.1021/bi00175a040. [DOI] [PubMed] [Google Scholar]
- Chen C.; Yan Q.; Tao M.; Shi H.; Han X.; Jia L.; Huang Y.; Zhao L.; Wang C.; Ma X.; et al. Characterization of Serine Acetyltransferase (CysE) from Methicillin-Resistant Staphylococcus Aureus and Inhibitory Effect of Two Natural Products on CysE. Microb. Pathog. 2019, 131, 218–226. 10.1016/j.micpath.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Johnson C. M.; Huang B.; Roderick S. L.; Cook P. F. Kinetic Mechanism of the Serine Acetyltransferase from Haemophilus Influenzae. Arch. Biochem. Biophys. 2004, 429 (2), 115–122. 10.1016/j.abb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Inoue K.; Noji M.; Saito K. Determination of the Sites Required for the Allosteric Inhibition of Serine Acetyltransferase by L-Cysteine in Plants. Eur. J. Biochem. 1999, 266 (1), 220–227. 10.1046/j.1432-1327.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Tyrrell R.; Verschueren K. H.; Dodson E. J.; Murshudov G. N.; Addy C.; Wilkinson A. J. The Structure of the Cofactor-Binding Fragment of the LysR Family Member, CysB: A Familiar Fold with a Surprising Subunit Arrangement. Structure 1997, 5 (8), 1017–1032. 10.1016/S0969-2126(97)00254-2. [DOI] [PubMed] [Google Scholar]
- Agarwal S. M.; Jain R.; Bhattacharya A.; Azam A. Inhibitors of Escherichia Coli Serine Acetyltransferase Block Proliferation of Entamoeba Histolytica Trophozoites. Int. J. Parasitol. 2008, 38 (2), 137–141. 10.1016/j.ijpara.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Tommasi R.; Brown D. G.; Walkup G. K.; Manchester J. I.; Miller A. A. ESKAPEing the Labyrinth of Antibacterial Discovery. Nat. Rev. Drug Discovery 2015, 14 (8), 529–542. 10.1038/nrd4572. [DOI] [PubMed] [Google Scholar]
- Brown D. G.; May-Dracka T. L.; Gagnon M. M.; Tommasi R. Trends and Exceptions of Physical Properties on Antibacterial Activity for Gram-Positive and Gram-Negative Pathogens. J. Med. Chem. 2014, 57 (23), 10144–10161. 10.1021/jm501552x. [DOI] [PubMed] [Google Scholar]
- O’Shea R.; Moser H. E. Physicochemical Properties of Antibacterial Compounds: Implications for Drug Discovery. J. Med. Chem. 2008, 51 (10), 2871–2878. 10.1021/jm700967e. [DOI] [PubMed] [Google Scholar]
- Larsson J.; Gottfries J.; Muresan S.; Backlund A. ChemGPS-NP: Tuned for Navigation in Biologically Relevant Chemical Space. J. Nat. Prod. 2007, 70 (5), 789–794. 10.1021/np070002y. [DOI] [PubMed] [Google Scholar]
- Rosén J.; Lövgren A.; Kogej T.; Muresan S.; Gottfries J.; Backlund A. ChemGPS-NP(Web): Chemical Space Navigation Online. J. Comput.-Aided Mol. Des. 2009, 23 (4), 253–259. 10.1007/s10822-008-9255-y. [DOI] [PubMed] [Google Scholar]
- Takagi H.; Yoshioka K.; Awano N.; Nakamori S.; Ono B. Role of Saccharomyces Cerevisiae Serine O-Acetyltransferase in Cysteine Biosynthesis. FEMS Microbiol. Lett. 2003, 218 (2), 291–297. 10.1111/j.1574-6968.2003.tb11531.x. [DOI] [PubMed] [Google Scholar]
- Yeon J. Y.; Yoo S. J.; Takagi H.; Kang H. A. A Novel Mitochondrial Serine O -Acetyltransferase, OpSAT1, Plays a Critical Role in Sulfur Metabolism in the Thermotolerant Methylotrophic Yeast Ogataea Parapolymorpha. Sci. Rep. 2018, 8 (1), 1–12. 10.1038/s41598-018-20630-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyer P.; Worek F.; Kiderlen D.; Sinko G.; Stuglin A.; Simeon-Rudolf V.; Reiner E. Molar Absorption Coefficients for the Reduced Ellman Reagent: Reassessment. Anal. Biochem. 2003, 312 (2), 224–227. 10.1016/S0003-2697(02)00506-7. [DOI] [PubMed] [Google Scholar]
- Baecker P. A.; Wedding R. T. Purification of Serine Acetyltransferase, a Component of a Multienzyme Complex, by Immunoadsorption and Selective Dissociation of the Complex. Anal. Biochem. 1980, 102 (1), 16–21. 10.1016/0003-2697(80)90310-3. [DOI] [PubMed] [Google Scholar]
- Inglese J.; Shamu C. E.; Guy R. K. Reporting Data from High-Throughput Screening of Small-Molecule Libraries. Nat. Chem. Biol. 2007, 3 (8), 438–441. 10.1038/nchembio0807-438. [DOI] [PubMed] [Google Scholar]
- Iversen P. W.; Eastwood B. J.; Sittampalam G. S.; Cox K. L. A Comparison of Assay Performance Measures in Screening Assays: Signal Window, Z’ Factor, and Assay Variability Ratio. J. Biomol. Screening 2006, 11 (3), 247–252. 10.1177/1087057105285610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.