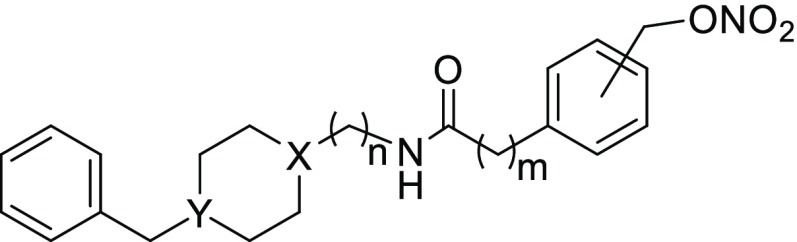
Abstract
We report the development of molecular hybrids in which a nitrate group serving as nitric oxide (NO) donor is covalently joined to σ receptor ligands to give candidates for double-targeted cancer therapy. The compounds have been evaluated in radioligand binding assay at both σ receptors and selected compounds tested for NO release. Compounds 9, 15, 18, 19, and 21 were subjected to MTT test. Compound 15 produced a significant reduction of MCF-7 and Caco-2 cellular viability with comparable IC50 as doxorubicin, being also not toxic for fibroblast HFF-1 cells. Compound 15 has shown a σ1 receptor antagonist/σ2 receptor agonist profile. Two derivatives of compound 15 lacking the nitrate group did not induce a reduction of MCF-7 cellular viability, suggesting a potential synergistic effect between the σ receptors and the NO-mediated events. Overall, the combination of NO donor and σ receptors ligands provided compounds with beneficial effects for the treatment of cancer.
Keywords: Sigma receptors, nitric oxide, nitric oxide donors, cancer, bifunctional ligands
Sigma (σ) receptors represent a unique receptor class involved in various biological and pathological conditions.1 Two σ receptors subtypes are recognized and termed sigma-1 (σ1) and sigma-2 (σ2), having different structures and biological functions.2,3 The σ1 receptor is a 25.3 kDa polypeptide that has been cloned in several species and recently crystallized revealing a trimeric architecture.4,5 The σ1 receptor has been identified as a ligand-operated chaperone protein localized in the mitochondria-associated endoplasmic reticulum (ER) membranes (MAM). At the MAM, the σ1 receptor forms a complex with another chaperone, the immunoglobulin heavy-chain binding protein (BiP). The σ1 receptor-BiP complex is silent and only activated when cells are stimulated by σ1 receptor agonists like (+)-pentazocine [(+)-PTZ] or undergo prolonged stress.6 In contrast, haloperidol, methamphetamine, and NE-100 can preserve the σ1 receptor-BiP complex in a silent state.7
The σ2 receptor is a poorly understood protein whose identification has been controversial. In 2017, Alon and co-workers identified the σ2 receptor as an endoplasmic reticulum-resident transmembrane protein (TMEM97) playing a role in the cholesterol homeostasis and the sterol transporter Niemann–Pick disease type C1.8 Despite the challenges in identifying its true identity, σ2 receptor and its ligands, as determined in radioligand binding assay using [3H]DTG,9,10 have earned a growing scientific interest due to its involvement in several pathological states.11,12 The overexpression of the σ2 receptor has been found in several cancer cells and proliferating tumors such as lung, colorectal, and breast.13 Due to a 10-fold higher density in proliferating tumor cells than in quiescent tumor cells, the σ2 receptor also represents a significant clinical biomarker for determining the proliferative status of solid tumors.14,15 A vast number of data support the use of σ1 receptor antagonists and σ2 receptor agonists for the treatment of cancer due to their antiproliferative effects.16−20 Rimcazole and haloperidol, as well as other σ receptors ligands endowed with opportune functional profile, have been shown to inhibit cell proliferation of several cancer cell lines.21,22
Nitric oxide (NO) is a short-lived gas with a dichotomous role in tumor biology. NO operates as a tumor progressor or suppressor based on its concentration, duration of exposure, and cell sensitivity.23 For instance, μM concentrations of NO result in antitumor effects, while pM–nM concentrations promote cytoprotective effects. In this view, the modulation of NO levels seems to have benefits in the treatment of cancer. However, NO is a reactive and unstable gas; thus, the use of NO donors (NODs) is preferred since these agents are stable chemical compounds able to release NO under specific chemical or enzymatical conditions. In order to ensure a high and effective release of cytotoxic amounts of NO, the choice of the releasing agent is crucial. Among the different classes of donors, organic nitrates have been reported to release adequate amounts of NO.16,24
The cytotoxic activity may be carried out by NODs alone, or they may be conjugated or embedded in chemical compounds that possess additional antitumoral action for double-targeted cytotoxic activity.25,26 On the basis of the aforementioned, the purpose of the present work is the development, biological evaluation, and molecular modeling studies of a series of molecular hybrids where the cytotoxic activity of the NOD is conjugated with that of σ receptors for optimal effect.16
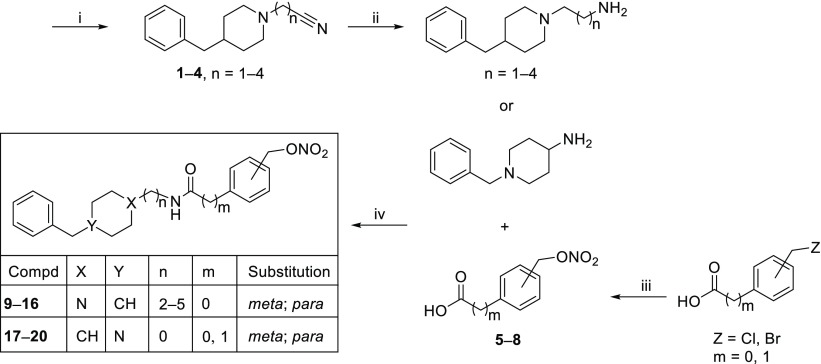
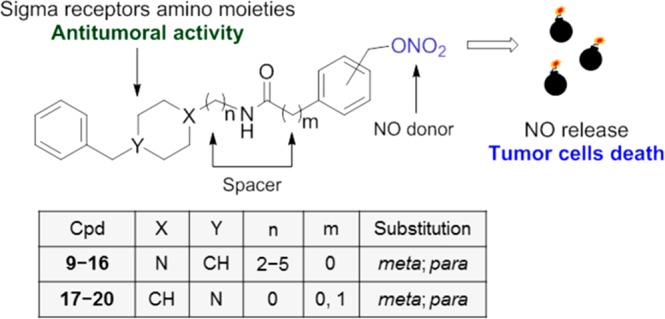
Compounds 9–20 were synthesized according to the steps illustrated in Scheme 1. Starting from commercially available 4-benzylpiperidine, intermediates 1–4 were obtained with opportune nitrile compounds and then converted into the corresponding amine derivatives by reaction with LiAlH4.6,16 The amine intermediates underwent condensation with [(nitrooxy)methyl]benzoic acid (5, 6) or para- or meta-[(nitrooxy)methyl]phenylacetic acid (7, 8) previously prepared via nitration with AgNO3 to give the final compounds 9–16. Compounds 17–20 were obtained through condensation of 1-benzylpiperidin-4-amine with the same acids 5–8.
Scheme 1. Synthetic Strategy for the Preparation of Target Compounds.
Reagents and conditions: (i) 4-benzylpiperidine, Br[(CH2)1–4]CN, NaI, K2CO3, DMF, 60 °C, overnight; (ii) LiAlH4, THF, rt, N2; (iii) AgNO3, CH3CN, rt; (iv) EDC, HOBT, CH3CN, rt, 5 h.
The synthesized compounds were evaluated for affinity at both σ1 and σ2 receptors through radioligand binding assay (Table 1). Within the series bearing a 4-benzylpiperidine (9–16), the meta-substituted compound 9 has shown a preferential σ1 receptor affinity with Ki value of 49 nM, and 330 nM for the σ2 receptor. The elongation to three-carbon chain (10) increased the σ2 receptor affinity by more than 3-fold with Kiσ1 value of 64 nM and Kiσ2 of 93 nM. The increased σ2 receptor affinity has also been maintained in compound 11 with a Ki value of 75 nM, although in the four-carbon chain, the σ1 receptor affinity (148 nM) is reduced. Further elongation of the chain to five carbons takes back σ1 receptor preferential affinity with a Ki value of 50 nM over the σ2 receptor (Ki value of 142 nM).
Table 1. σ1 and σ2 Binding Assays for Compounds 9–20.
| |
Ki ± SDa (nM) |
||||||
|---|---|---|---|---|---|---|---|
| compd | X | Y | n | m | substitution | σ1 | σ2 |
| 9 | N | CH | 2 | 0 | meta | 49 ± 1 | 330 ± 9 |
| 10 | N | CH | 3 | 0 | meta | 64 ± 0.7 | 93 ± 3 |
| 11 | N | CH | 4 | 0 | meta | 148 ± 1 | 75 ± 2 |
| 12 | N | CH | 5 | 0 | meta | 50 ± 0.9 | 142 ± 13 |
| 13 | N | CH | 2 | 0 | para | 89 ± 2 | 2673 ± 44 |
| 14 | N | CH | 3 | 0 | para | 145 ± 12 | 230 ± 18 |
| 15 | N | CH | 4 | 0 | para | 250 ± 9 | 89 ± 2 |
| 16 | N | CH | 5 | 0 | para | 93 ± 2 | 70 ± 3 |
| 17 | CH | N | 0 | 0 | meta | 24 ± 0.6 | 19 ± 0.2 |
| 18 | CH | N | 0 | 1 | meta | 19 ± 1 | 320 ± 4 |
| 19 | CH | N | 0 | 0 | para | 22 ± 0.8 | 270 ± 5 |
| 20 | CH | N | 0 | 1 | para | 170 ± 6 | 2514 ± 24 |
| haloperidol | 2.5 ± 0.4 | 16 ± 2 | |||||
| (+)-PTZ | 4.7 ± 0.7 | 1465 ± 224 | |||||
| DTG | NDb | 18 ± 1 | |||||
Each value is the mean ± SD of at least two experiments performed in triplicate.
Not determined.
At the same time, the para substitution of the nitrate group gives compounds with generally lower affinity with respect to both receptor subtypes. It is observed an improvement of the σ2 receptor affinity with the linker elongation. Indeed, compound 13 has a Kiσ2 of 2673 nM, while compound 16 has a Kiσ2 of 70 nM. In terms of σ1 receptor affinity, compounds 9–16 show a mixed behavior with affinity ranging from 89 nM for compound 13 to 249 nM for compound 15. Compounds bearing the 1-benzylpiperidin-4-amine (17–20) have shown worthy σ1 receptor affinities and a general lower affinity at the σ2 receptor. In particular, compounds 17–19 have a σ1 receptor affinity in the low double digits nM range (Kiσ1 of 24, 19, and 22 nM, respectively), while only compound 17 shows σ2 receptor affinity in the same range (Kiσ2 of 19 nM). The other compounds show a lower affinity to the σ2 receptor, which leads to a >10 times selectivity with respect to the σ1 receptor.
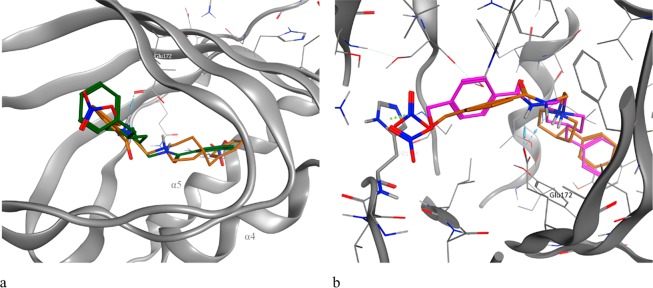
A docking study was conducted to identify and evaluate the key molecular interactions involved in the receptor/ligand recognition. The crystal structure of the σ1 receptor PD144418 (PDB code 5HK1)4 reveals an occluded and elongated binding cavity in a similar β-barrel fashion, with the highly conserved Glu172 amino acid residue located near the center of the cavity, forming a salt bridge with the ligands. Since all compounds possess a tertiary amine, we performed the study considering the N-protonated structures (pH = 7.4). Therefore, the formation of the salt bridge was used as a filter for the docking poses. The calculated free binding energies (ΔG) and Ki values to the catalytic site of the σ1 receptor for compounds 9–20 and haloperidol are reported in Table S1.
Active analysis of the site showed that the orientation changes according to the length of the ligands and the protonated N-position. Moreover, the position of the protonated piperidine ring is rather characteristic, being close to the carboxyl function of Glu172. The docking studies conducted upon the σ2 receptor were performed by using its homology model previously built by us.28 In this case, the salt bridge interactions between the ligands and the residues Asp29 and Asp56 were considered. In the 4-benzylpiperidine series with the nitrate group in the meta position (9–12), compound 9 showed a preferential affinity for the σ1 receptor with a Ki value of 49 nM.
Compound 9 is placed inside the receptor site with the piperidinium proton engaged in a salt bridge interaction with Glu172 and a hydrogen bond with N–H amide. The nitrate group is positioned on the opposite side helices α4 and α5, showing polar interactions with the residues Asp126 and His154. The optimal position of the piperidine ring favors the π–π stacking of the hydrogen in position 4 of the piperidine system with the residue Tyr103. The benzyl group is involved in hydrophobic interactions with the Leu182 and Met93 residues. The elongation to the four-carbon chain (11) decreases the affinity for the σ1 receptor while increasing the affinity for the σ2 receptor. Indeed, derivative 11 has an inverted orientation with respect to derivative 9, with the nitrate group toward the helix α5 to allow the accommodation of the aliphatic chain within the receptor pocket (Figure 1a).
Figure 1.
(a) 3D superposition of the best-docked pose for 9 (orange) and 11 (green) bound to the σ1 receptor orthosteric site. The different lengths of the aliphatic linker reverse the orientation of the ligand inside the receptor. (b) Top-scored docking poses for 9 (orange) and 13 (magenta) bound to the σ1 receptor orthosteric site. The different position of the nitrate group allows better interaction with His154 (green dotted line).
The para substitution of the nitrate group (13–16) decreases the affinity with respect to the ligands substituted in the meta position. This is probably due to a lower cation−π interaction with the His154 residue (Figure 1b). An elongation of the aliphatic portion of the ligands leads to a better affinity for the σ2 receptor. The most similar compounds in the series (11 and 16) show comparable interactions within the σ2 receptor pocket. Both form the salt bridge with the residue Asp56 while the benzyl portion in position 4 to the piperidine ring shows aliphatic interactions with the residues Leu70, Met108, Pro113, and Val146. The nitrate group establishes electrostatic interactions with the Arg36 residue, discriminating against the different affinity between the different isomers. A detailed description of the interactions with σ2 receptor is reported in the Molecular Docking section in the Supporting Information.
Compound 18 showed the preeminent affinity to the σ1 receptor. On this compound, a molecular dynamics (MD) simulation was performed (Supporting Information). An elongation of the aliphatic portion of the ligands leads to a better affinity for σ2 receptor.
Once the affinity profiles of the synthesized compounds at σ receptors were evaluated, we determined the ability of these compounds to release NO. Nitrite content was measured by the Griess method incubating the compounds (100 μM), at 37 °C, in Tris-HCl buffer for 30 min (Figure 2).
Figure 2.
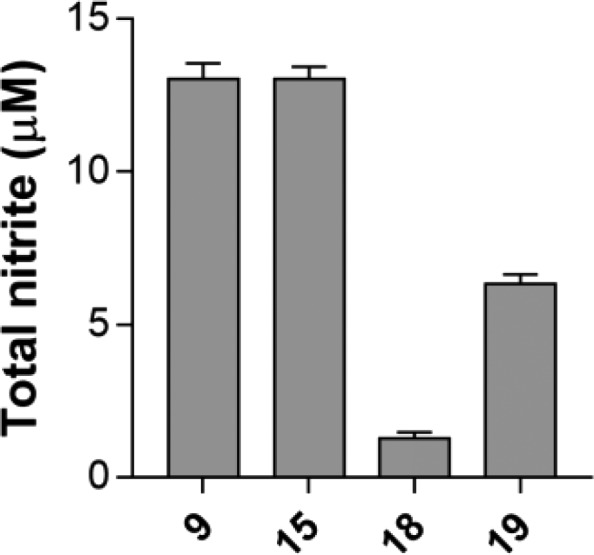
Total nitrite content measured using Griess reagent.
For this assay, we selected the compounds based on their affinity profile against σ receptors. In particular, we evaluated compounds 9, 11, 15, and 17–19, having shown good affinity at both receptors or prevalence for one receptor subtype. Compounds 9 and 15 were able to release a significant amount of NO in the μM range (9, 13.0 ± 0.5 μM; 15, 13.0 ± 0.4 μM). The amount of NO released by compound 18 was 1.3 ± 0.2 μM, while compound 19 produced 6.3 ± 0.3 μM NO. Negligible NO amounts were detected for compounds 11 and 17 (data not shown).
After having obtained the desired chemical tools, we evaluated their activity in the appropriate cell lines. We evaluated those compounds that, in previous experiments, have been demonstrated to possess the desired profile (compounds 9, 15, 18, and 19). Two cancer cell lines were selected, Caco-2 and MCF-7 cells, for their expression of both σ receptors. Although MCF-7 cells are reported to exclusively express the σ2 subtypes, a Western blot analysis has shown the presence of the σ1 isoform in our in-house cell line (Figure S4).29 The toxicity against human fibroblast HFF-1 cells was also evaluated. Doxorubicin (21) was used as the standard cytotoxic compound. Results showed a reduction of cellular viability on both Caco-2 and MCF-7 cell lines for compounds 9 and 15 (Table 2). These compounds were those with a higher rate of NO release. Measured IC50 values were better with respect to compound 21 for MCF-7 cells, with IC50 of 36 μM for compound 9 and 26 μM for compound 15. None of the synthesized compounds, nor compound 21, were demonstrated to be toxic for the human fibroblasts HFF-1 at the maximal tested concentration. Compounds 18 and 19 have been shown to be nontoxic for the three evaluated cell lines (IC50 > 100 μM), probably due to the lower rate of NO release and to an unsuitable functional profile at σ receptors.
Table 2. MTT Test on MCF-7, Caco-2, and HFF-1 for Compounds 21, 9, 15, 18, and 19.
| IC50 ± SD (μM)a |
|||
|---|---|---|---|
| compd | MCF-7 | Caco-2 | HFF-1 |
| 21 | 44 ± 0.3 | 21 ± 0.3 | >100b |
| 9 | 36 ± 0.2 | 59 ± 0.5 | >100b |
| 15 | 26 ± 0.4 | 28 ± 0.2 | >100b |
| 18 | >100b | >100b | >100b |
| 19 | >100b | >100b | >100b |
Each value is the mean ± SD of at least two experiments performed in quadruplicate.
Cell viability reduction lower than 50% at 100 μM.
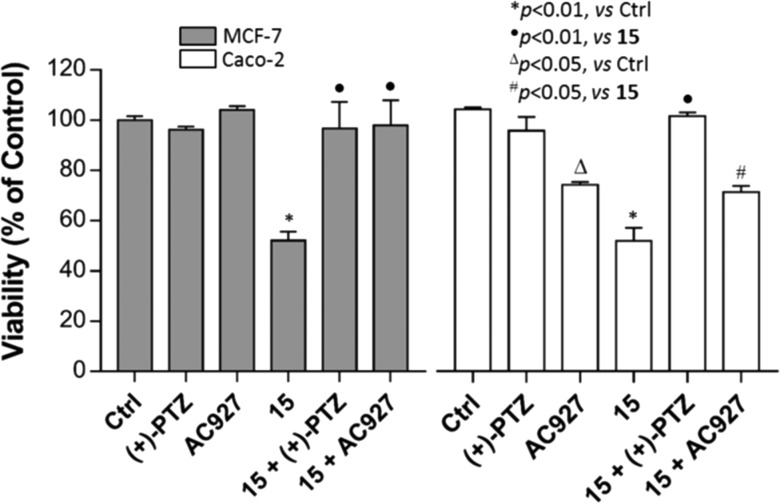
In order to determinate the precise mechanism for the reduction of cellular viability, compound 15 (IC50 concentration) was evaluated in combination with the σ1 receptor agonist (+)-PTZ (1 μM) and the σ2 receptor antagonist 1-phenethylpiperidine (AC927, 1 μM) by MTT.30
Incubation of compound 15 with (+)-PTZ or AC927 wholly restored the loss of cell viability induced by 15 alone (Figure 3). This outlines a σ1 and σ2 receptors involvement in the observed cellular events and a σ1 receptor antagonist/σ2 receptor agonist functional profile for compound 15.
Figure 3.
Effects of compound 15 in combination with the selective σ1 receptor agonist (+)-PTZ and σ2 receptor antagonist AC927 on MCF-7 and Caco-2 viability by MTT test.
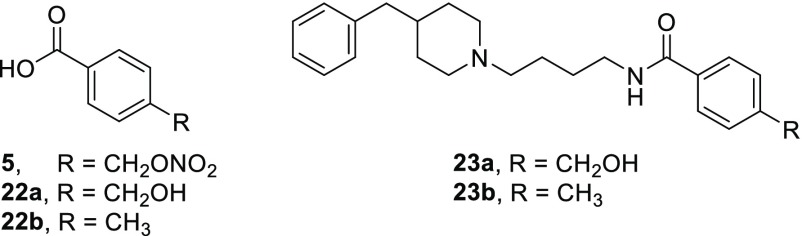
To dig into the dual mechanism of the prepared compounds, we tested fragments 5 and 22a,b and synthesized two derivatives of compound 15 lacking the nitrate function, compounds 23a and 23b, as negative control (Table 3 and Scheme S1).31,32
Table 3. Binding Assays and MTT Viability Test on MCF-7 for Compounds 22a,b, 23a,b, and 5.
|
Ki ± SDa (nM) |
IC50 ± SD (μM)b | ||
|---|---|---|---|
| compd | σ1 | σ2 | MCF-7 |
| 5 | >10000c | >10000c | >100d |
| 22a | >10000c | >10000c | >100d |
| 22b | >10000c | >10000c | >100d |
| 23a | 711 ± 127 | 2600 ± 730 | >100d |
| 23b | 62 ± 3 | 127 ± 16 | 87 ± 0.3 |
Each value is the mean ± SD of at least two experiments performed in triplicate.
Each value is the mean ± SD of at least two experiments performed in quadruplicate.
Radioligand displacement lower than 50% at 10 μM.
Cell viability reduction lower than 50% at 100 μM.
The fragments 5 and 22a,b have shown no significant viability reduction on MCF-7 and no affinity for both σ receptors. Compound 23a showed a loss of affinity at both σ receptors, while compound 23b retained a similar affinity profile at both σ receptors with respect to compound 9 or 15. When evaluated on the MCF-7 cell line, compound 23a induced lower than 50% viability reduction at 100 μM, while compound 23b had an IC50 of 87 μM. Overall compound 23b, while maintaining a similar profile against σ receptors, showed a lower ability in reducing MCF-7 viability, thus sustaining a possible synergistic effect between σ receptors and the NO-mediated events.
In conclusion, this contribution reports the development of novel hybrid compounds able to release NO and to bind σ receptors as candidates for double-targeted cancer therapy. The compounds have been evaluated for their affinity at σ receptors and ability to release NO. Four compounds showed the desired profile with compounds 9 and 15 also able to induce a marked loss of viability in MCF-7 and Caco-2 cell lines while not being toxic for healthy human fibroblast HFF-1.
In cellular experiments involving the use of selective σ receptors agonist and antagonist, compound 15 has shown a σ1 receptor antagonist/σ2 receptor agonist functional profile. The elimination of the nitrate function of compound 15 as in compounds 23a,b determined the loss of its ability to reduce MCF-7 viability sustaining a possible synergistic effect between the σ receptors and the NO-mediated events. Molecular docking studies have shown that the length of the aliphatic linker has an essential role in the orientation of the ligands inside the receptor pocket and for σ1/σ2 selectivity. The nitrate group stabilizes the ligand/receptor complex to cation−π interaction with His154 residue in the σ1 receptor, also confirmed by the MD simulation, and with Phe71 and Pro113 residues in the σ2 receptor. Overall, the combination of NO donor and σ receptors ligands provided compounds with potential beneficial effects in the treatment of neoplastic disorders.
Acknowledgments
The authors thank Prof. Giuseppina Immè, Dr. Roberto Catalano, and Nunzio Giudice from the Department of Physics and Astronomy, University of Catania, for technical and instrumental support of the Beckman LS6500 liquid scintillation counter.
Glossary
Abbreviations
- σ
sigma
- σ1
sigma-1
- σ2
sigma-2
- ER
endoplasmic reticulum
- [3H]-DTG
[3H]-1,3 di-o-tolylguanidine
- NO
nitric oxide
- NOD
nitric oxide donor
- AC927
1-phenetylpiperidine
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.9b00661.
General synthetic methods and spectral data of final compounds, procedures for in vitro biological assays, and computational methods (PDF)
Author Contributions
∥ E.A. and M.D. contributed equally.
This work was financially supported by Università degli Studi di Catania, “Piano per la Ricerca 2016–2018”, Grants 57722172105 and 57722172114.
The authors declare no competing financial interest.
This paper was originally published ASAP on April 15, 2020. Due to a production error, additional corrections were needed to Tables 2 and 3. The corrected version was reposted on April 15, 2020.
Supplementary Material
References
- Kim F. J. Introduction to Sigma Proteins: Evolution of the Concept of Sigma Receptors. Handb. Exp. Pharmacol. 2017, 244, 1–11. 10.1007/164_2017_41. [DOI] [PubMed] [Google Scholar]
- Schmidt H. R.; Kruse A. C. The Molecular Function of sigma Receptors: Past, Present, and Future. Trends Pharmacol. Sci. 2019, 40, 636–654. 10.1016/j.tips.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F.; Wunsch B. Medicinal Chemistry of sigma1 Receptor Ligands: Pharmacophore Models, Synthesis, Structure Affinity Relationships, and Pharmacological Applications. Handb. Exp. Pharmacol. 2017, 244, 51–79. 10.1007/164_2017_33. [DOI] [PubMed] [Google Scholar]
- Schmidt H. R.; Zheng S.; Gurpinar E.; Koehl A.; Manglik A.; Kruse A. C. Crystal structure of the human sigma1 receptor. Nature 2016, 532, 527–530. 10.1038/nature17391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim F. J.; Pasternak G. W. sigma1 Receptor ligand binding: an open-and-shut case. Nat. Struct. Mol. Biol. 2018, 25, 992–993. 10.1038/s41594-018-0146-1. [DOI] [PubMed] [Google Scholar]
- Hayashi T.; Su T. P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 2007, 131, 596–610. 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Morales-Lazaro S. L.; Gonzalez-Ramirez R.; Rosenbaum T. Molecular Interplay Between the Sigma-1 Receptor, Steroids, and Ion Channels. Front. Pharmacol. 2019, 10, 419. 10.3389/fphar.2019.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon A.; Schmidt H. R.; Wood M. D.; Sahn J. J.; Martin S. F.; Kruse A. C. Identification of the gene that codes for the sigma2 receptor. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 7160–7165. 10.1073/pnas.1705154114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastasi G.; Miceli C.; Pittala V.; Modica M. N.; Prezzavento O.; Romeo G.; Rescifina A.; Marrazzo A.; Amata E. S2RSLDB: a comprehensive manually curated, internet-accessible database of the sigma-2 receptor selective ligands. J. Cheminf. 2017, 9, 3. 10.1186/s13321-017-0191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezzavento O.; Arena E.; Sanchez-Fernandez C.; Turnaturi R.; Parenti C.; Marrazzo A.; Catalano R.; Amata E.; Pasquinucci L.; Cobos E. J. (+)-and (−)-Phenazocine enantiomers: Evaluation of their dual opioid agonist/sigma1 antagonist properties and antinociceptive effects. Eur. J. Med. Chem. 2017, 125, 603–610. 10.1016/j.ejmech.2016.09.077. [DOI] [PubMed] [Google Scholar]
- Huang Y. S.; Lu H. L.; Zhang L. J.; Wu Z. Sigma-2 receptor ligands and their perspectives in cancer diagnosis and therapy. Med. Res. Rev. 2014, 34, 532–566. 10.1002/med.21297. [DOI] [PubMed] [Google Scholar]
- Crawford K. W.; Bowen W. D. Sigma-2 receptor agonists activate a novel apoptotic pathway and potentiate antineoplastic drugs in breast tumor cell lines. Cancer Res. 2002, 62, 313–322. [PubMed] [Google Scholar]
- van Waarde A.; Rybczynska A. A.; Ramakrishnan N. K.; Ishiwata K.; Elsinga P. H.; Dierckx R. A. Potential applications for sigma receptor ligands in cancer diagnosis and therapy. Biochim. Biophys. Acta, Biomembr. 2015, 1848, 2703–2714. 10.1016/j.bbamem.2014.08.022. [DOI] [PubMed] [Google Scholar]
- Zeng C.; McDonald E. S.; Mach R. H. Molecular Probes for Imaging the Sigma-2 Receptor: In Vitro and In Vivo Imaging Studies. Handb. Exp. Pharmacol. 2016, 244, 309–330. 10.1007/164_2016_96. [DOI] [PubMed] [Google Scholar]
- McDonald E. S.; Doot R. K.; Young A. J.; Schubert E. K.; Pryma D. A.; Farwell M. D.; Tchou J.; Nayak A.; Ziober A.; Feldman M. D.; DeMichele A.; Clark A. S.; Shah P. D.; Lee H.; Carlin S. D.; Mach R. H.; Mankoff D. A. Breast Cancer (18)F-ISO-1 Uptake as a Marker of Proliferation Status. J. Nucl. Med. 2019, 10.2967/jnumed.119.232363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amata E.; Dichiara M.; Arena E.; Pittala V.; Pistara V.; Cardile V.; Graziano A. C. E.; Fraix A.; Marrazzo A.; Sortino S.; Prezzavento O. Novel Sigma Receptor Ligand-Nitric Oxide Photodonors: Molecular Hybrids for Double-Targeted Antiproliferative Effect. J. Med. Chem. 2017, 60, 9531–9544. 10.1021/acs.jmedchem.7b00791. [DOI] [PubMed] [Google Scholar]
- Olivieri M.; Amata E.; Vinciguerra S.; Fiorito J.; Giurdanella G.; Drago F.; Caporarello N.; Prezzavento O.; Arena E.; Salerno L.; Rescifina A.; Lupo G.; Anfuso C. D.; Marrazzo A. Antiangiogenic Effect of (+/-)-Haloperidol Metabolite II Valproate Ester (+/-)-MRJF22 in Human Microvascular Retinal Endothelial Cells. J. Med. Chem. 2016, 59, 9960–9966. 10.1021/acs.jmedchem.6b01039. [DOI] [PubMed] [Google Scholar]
- Tesei A.; Cortesi M.; Zamagni A.; Arienti C.; Pignatta S.; Zanoni M.; Paolillo M.; Curti D.; Rui M.; Rossi D.; Collina S. Sigma Receptors as Endoplasmic Reticulum Stress “Gatekeepers” and their Modulators as Emerging New Weapons in the Fight Against Cancer. Front. Pharmacol. 2018, 9, 711. 10.3389/fphar.2018.00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresta G.; Dichiara M.; Gentile D.; Prezzavento O.; Marrazzo A.; Rescifina A.; Amata E. Morphing of Ibogaine: A Successful Attempt into the Search for Sigma-2 Receptor Ligands. Int. J. Mol. Sci. 2019, 20, 488. 10.3390/ijms20030488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amata E.; Rescifina A.; Prezzavento O.; Arena E.; Dichiara M.; Pittala V.; Montilla-Garcia A.; Punzo F.; Merino P.; Cobos E. J.; Marrazzo A. (+)-Methyl (1R,2S)-2-{[4-(4-Chlorophenyl)-4-hydroxypiperidin-1-yl]methyl}-1-phenylcyclopropa necarboxylate [(+)-MR200] Derivatives as Potent and Selective Sigma Receptor Ligands: Stereochemistry and Pharmacological Properties. J. Med. Chem. 2018, 61, 372–384. 10.1021/acs.jmedchem.7b01584. [DOI] [PubMed] [Google Scholar]
- Happy M.; Dejoie J.; Zajac C. K.; Cortez B.; Chakraborty K.; Aderemi J.; Sauane M. Sigma 1 Receptor antagonist potentiates the anti-cancer effect of p53 by regulating ER stress, ROS production, Bax levels, and caspase-3 activation. Biochem. Biophys. Res. Commun. 2015, 456, 683–688. 10.1016/j.bbrc.2014.12.029. [DOI] [PubMed] [Google Scholar]
- Spruce B. A.; Campbell L. A.; McTavish N.; Cooper M. A.; Appleyard M. V.; O’Neill M.; Howie J.; Samson J.; Watt S.; Murray K.; McLean D.; Leslie N. R.; Safrany S. T.; Ferguson M. J.; Peters J. A.; Prescott A. R.; Box G.; Hayes A.; Nutley B.; Raynaud F.; Downes C. P.; Lambert J. J.; Thompson A. M.; Eccles S. Small molecule antagonists of the sigma-1 receptor cause selective release of the death program in tumor and self-reliant cells and inhibit tumor growth in vitro and in vivo. Cancer Res. 2004, 64, 4875–4886. 10.1158/0008-5472.CAN-03-3180. [DOI] [PubMed] [Google Scholar]
- Sakuma S.; Ikeda Y.; Inoue I.; Yamaguchi K.; Honkawa S.; Kohda T.; Minamino S.; Fujimoto Y. Nitric oxide represses the proliferation of Caco-2 cells by inducing S-G2/M cell cycle arrest. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 205–211. [PMC free article] [PubMed] [Google Scholar]
- Wang P. G.; Xian M.; Tang X.; Wu X.; Wen Z.; Cai T.; Janczuk A. J. Nitric oxide donors: chemical activities and biological applications. Chem. Rev. 2002, 102, 1091–1134. 10.1021/cr000040l. [DOI] [PubMed] [Google Scholar]
- Huang Z.; Fu J.; Zhang Y. Nitric Oxide Donor-Based Cancer Therapy: Advances and Prospects. J. Med. Chem. 2017, 60, 7617–7635. 10.1021/acs.jmedchem.6b01672. [DOI] [PubMed] [Google Scholar]
- Ding Q. G.; Zang J.; Gao S.; Gao Q.; Duan W.; Li X.; Xu W.; Zhang Y. Nitric oxide donor hybrid compounds as promising anticancer agents. Drug Discoveries Ther. 2016, 10, 276–284. 10.5582/ddt.2016.01067. [DOI] [PubMed] [Google Scholar]
- Floresta G.; Amata E.; Barbaraci C.; Gentile D.; Turnaturi R.; Marrazzo A.; Rescifina A. A Structure- and Ligand-Based Virtual Screening of a Database of “Small” Marine Natural Products for the Identification of “Blue” Sigma-2 Receptor Ligands. Mar. Drugs 2018, 16, 384. 10.3390/md16100384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim F. J.; Maher C. M. Sigma1 Pharmacology in the Context of Cancer. Handb. Exp. Pharmacol. 2017, 244, 237–308. 10.1007/164_2017_38. [DOI] [PubMed] [Google Scholar]
- Bucolo C.; Campana G.; Di Toro R.; Cacciaguerra S.; Spampinato S. Sigma1 recognition sites in rabbit iris-ciliary body: topical sigma1-site agonists lower intraocular pressure. J. Pharmacol. Exp. Ther. 1999, 289, 1362–1369. [PubMed] [Google Scholar]
- Pisani L.; Iacobazzi R. M.; Catto M.; Rullo M.; Farina R.; Denora N.; Cellamare S.; Altomare C. D. Investigating alkyl nitrates as nitric oxide releasing precursors of multitarget acetylcholinesterase-monoamine oxidase B inhibitors. Eur. J. Med. Chem. 2019, 161, 292–309. 10.1016/j.ejmech.2018.10.016. [DOI] [PubMed] [Google Scholar]
- Wold S.; Sjöström M.; Eriksson L. PLS-regression: a basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. 10.1016/S0169-7439(01)00155-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.