Abstract
The development of bioconjugates is of pivotal importance in medicinal chemistry due to their potential applications as therapeutic agents to improve the targeting of specific diseases, decrease toxicity, or control drug release. In this work we achieved the synthesis and characterization of three novel opioid peptides fluorescently labeled, analogues of cyclic biphalin derivatives, namely 1D, 1C, and 2C. Among them, compound 1D, containing a dansyl-maleimide motif, exhibited an excellent binding affinity and functional potency for the δ-opioid receptor (DOR). 1D also demonstrated a strong fluorescence emission spectrum ranging from 300 to 700 nm. These features could be highly desirable for medical and biological applications needed for targeting the DOR, including in vivo imaging, and as a lead for the design of fluorescent probes.
Keywords: Biphalin, opioids, fluorescent probes, GPCRs, bioconjugates
Bioconjugate peptides occupy an important position in the fluorescent probes field, due to their potential application in supramolecular structure design, targeting of biological tissues, and diagnostic medicine.1 Peptides containing a fluorescent dye could be valuable tools for monitoring intracellular reactions and molecular signaling; also, fluorescent ligands could be used for pharmacokinetic studies, among other potential applications. Peptide bioconjugates are advantageous, due to their high receptor specificity correlated to the amino acid sequence, low toxicity, and low disposal procedures compared to radiolabeled compounds.2 Peptide conjugates also could be used in fluorescent microscopy. Studies in living cells require the management of selective probes to investigate the mechanism of internalization of membrane receptors, including the opioid receptors.3 In recent studies opioid peptides such as Dermorphin, deltorphin, TIPP, and endomorphin have been conjugated to the fluorescent dyes BODIPY TR and Alexa Fluor 488.4 Most of these peptides were biologically active, showing mixed μ/δ-opioid receptor (MOR/DOR) binding affinity and selectivity. They have been used in internalization study for the MOR and DOR, showing real-time visual tracking of receptor–ligand complexes in living cells, antagonist (naloxone)-sensitivity, and temperature-dependence.5 Leong et al. conjugated Endomorphin-1 (EM-1) to tetramethylrhodamine (TAMRA) to analyze the cell biology of the MOR in keratinocytes, revealing a rapid and complete internalization in the endoplasmic reticulum (ER) under resting conditions.6 Furthermore, fluorophore-conjugated opioid receptor ligands with peptidic or nonpeptidic structures may help to localize receptors at low density, allowing analysis of the receptor ligand-interaction of OR-containing cell populations. Simple labeling methods involve the anchorage of fluorescent dyes through succinimidyl ester or maleimide functional groups with primary amines or thiol groups.7,8 Maleimides may quench fluorescence in the conjugated form because the C=C bond undergoes selective addition reaction for thiols, but when they are linked to chromophores such as pyrene, phtalimide, and naphthopyranone, the fluorescence emission results increased. The application of maleimide in peptides and protein bioconjugation is highly desirable due to the prompt N-functionalization of maleimide and the possibility to insert the fluorescent molecule by a selective reaction with two cysteine residues present in the peptides. It also allows establishing a precise distance between the fluorescent core and the peptide by the addition of specific linkers.9 Biphalin has been used as model scaffold in the design of bioconjugated opioid peptides.10 Diverse hydrophilic/hydrophobic components and biochemical markers have been attached to biphalin in order to study the biological properties as opioid ligand and to explore its possible application as fluorescent probe.11−16 In 2002 Lipkowski et al. demonstrated that one biphalin pharmacophore could be replaced by hydrophobic groups without loss of receptor affinity, with the aim to develop a useful tool for pharmacokinetic and pharmacodynamic studies in vivo;17 the first biphalin analogue so designed contained a dansyl moiety, which expressed high receptor binding affinity for MOR and DOR and antinociceptive activity in vivo.17
The same fluorescent moiety has been applied to the derivatization of the DALDA sequence to give three potent MOR agonists.18 Further replacement of the dansyl group of one biphalin branch with amino-coumarin resulted in an increased affinity for the MOR and high antinociceptive activity comparable to that of biphalin.19 These studies establish proof-of-concept for the use of biphalin as a bioconjugate scaffold for targeting the MOR and DOR.
Recently our research group published the first cyclic biphalin analogue incorporating a fluorescein-maleimide moiety with the aim to identify a good fluorescent ligand for in vivo and in vitro assays, concerning receptor binding and penetration of biological barriers, e.g. the blood brain barrier (BBB).20 This novel compound exhibited modest affinity for MOR and DOR and was able to partially stimulate their G protein activation. In this study we sought to improve on this initial work. We designed and synthesized three novel cyclic biphalin analogues incorporating different fluorescent-labeled moieties in order to find a good candidate for probe development on opioid receptors for both in vitro and in vivo studies.
Results and Discussion
Commercially available dansyl chloride and 7-amino-4-methylcoumarin were functionalized with tert-butyl N-(2-aminoethyl)carbamate and Boc-β-Ala-OH, respectively, following the reactions depicted in Scheme 1. Dansyl chloride was functionalized with tert-butyl N-(2-aminoethyl)carbamate following the procedure described by Youziel et al. so as to give the N-Boc protected compound in 93% yield after reaction workup.21 7-Amino-4-methylcoumarin was also functionalized with Boc-β-Ala-OH following the procedure reported by Heltweg et al. to obtain a crude intermediate compound pure on silica gel TLC plate, in 63% yield after reaction workup.22
Scheme 1. Functionalization of 7-Amino-4-methylcoumarin and Dansylchloride with Boc-β-Ala-OH and tert-Butyl N-(2-Aminoethyl)carbamate, Respectively.
Reagents and Conditions: (i) tert-Butyl N-(2-aminoethyl)carbamate, EDC·HCl, HOBt, DIPEA in DMF, rt, overnight; (ii) TFA:DCM = 1:1, rt, 1 h; (iii) 3,4-dibromofuran-2,5-dione, AcOH at rt for 6 h then reflux for 3 h; (iv) Boc-β-Ala-OH, POCl3, pyridine at −15 °C, 1 h.
These N-Boc protected intermediates were deprotected with a mixture of TFA:DCM = 1:1 at rt and promptly reacted with 3,4-dibromofuran-2,5-dione. Maleic anhydride was converted in the organic scaffold 3,4-dibromofuran-2,5-dione, following the procedure reported by Dubernet et al.23 The fluorescent scaffolds were conjugated to 3,4-dibromofuran-2,5-dione following the procedure reported by Stefanucci et al.20 so as to give three fluorescent-maleimide moieties in good overall yields (98% yield for 1, 40% yield for 2, 71% yield for 3) (see SI). The cyclic biphalin fluorescent analogs 1C, 2C, and 1D were prepared following the well-established procedure reported by us in solution (Scheme 2).20
Scheme 2. Synthesis of Novel Cyclic Peptides 1C, 2C, and 1D.
Reagents and conditions. (ii) TFA:DCM = 1:1, rt, 1 h; (v) TCEP, DMF, 1 for compound 1D, 2 for compound 2C, and 3 for 1C, rt, overnight.
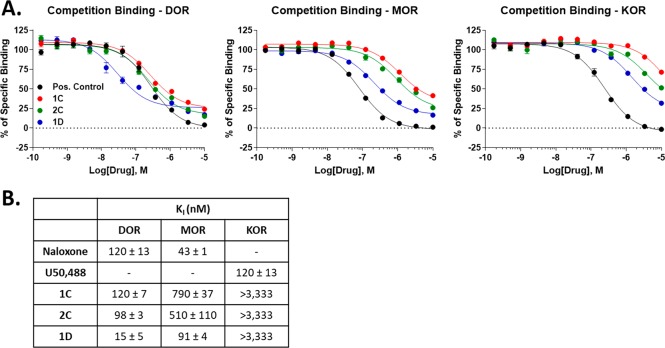
Compound 4 was prepared following the procedure previously described by our research group24 and was submitted to in situ reduction with the tris(2-carboxyethyl)phosphine hydrochloride solution (TCEP) reagent in the presence of the desired fluorescent probe to afford the N-Boc protected cyclic compounds.20 They were then treated with a mixture of TFA/DCM in solution at rt to give the final products 1D, 1C, and 2C as TFA salts in good overall yields (75% yield for 1D, 61% yield for 1C, and 58% yield for 2C) and excellent purity after purification by RP-HPLC (see SI). The three novel cyclic peptides were then tested for their ability to bind the MOR, DOR, and Kappa OR (KOR) in vitro using radioligand displacement binding assays. All 3 compounds showed varying modest to strong binding affinity for MOR and DOR, while all 3 showed very poor binding to the KOR (Figure 1). All compounds also showed a higher affinity for DOR over MOR, with 1D achieving a strong ∼15 nM Ki with all 3 compounds showing ∼5–8-fold selectivity for DOR > MOR. Notably, the compounds show incomplete competition at the MOR, which is consistent with an allosteric binding mode.
Figure 1.
Opioid receptor binding affinity of novel compounds. All compounds were competed against 3H-diprenorphine using membranes from CHO cells expressing the human opioid receptors (DOR, MOR, or KOR). Data reported as the mean ± SEM of N = 3 independent experiments. (A) Concentration–response curves shown for all 3 compounds and positive control (naloxone for MOR and DOR; U50,488 for KOR) at each receptor. All compounds show competition at each receptor, with affinity DOR > MOR > KOR. The compounds show incomplete competition at the MOR, which is consistent with an allosteric binding mode. (B) Affinity values (Ki) shown for each compound at each receptor derived from the curves in A.
These profiles of MOR/DOR binding are somewhat similar to the biphalin parent scaffold, and also suggest that these ligands could be effective labeling agents for the DOR and/or MOR. We next measured the functional activity of all compounds at the opioid receptors using the same CHO cell lines used above for the binding. We used 35S-GTPγS coupling, which we’ve used extensively to characterize the functional activity of our compounds.20,25
We first found that all 3 compounds were partial agonists at the DOR (EMAX 47–62%), with modest potencies (EC50 40–250 nM, Figure 2). These potencies were all about 2-fold worse than the binding affinities measured in Figure 1, suggesting that these ligands have somewhat poor intrinsic efficacy at the DOR. The reverse was true for the MOR, where all 3 compounds had improved potency vs binding affinity, suggesting good intrinsic efficacy, while all 3 were near-full to full agonists (EMAX 79–120%). Lastly, all 3 compounds were weak potency partial agonists at KOR, as suggested by the poor affinities above; however, they did show complete curves with improved potency vs affinity, suggesting good intrinsic efficacy at KOR. These results altered the binding selectivity profile at the different opioid receptors found in Figure 1, with all compounds now nearly equi-potent at MOR/DOR with little to no selectivity between them, at least at the functional level. These results should be kept in mind for evaluating these compounds in vivo. For the purposes of binding and labeling, for which the fluorescent moieties would be most useful, these compounds will likely be DOR-preferring. However, for any functional studies, they will likely be nonselective between MOR and DOR, similar to the parent scaffold biphalin. Taken together, these results show that the fluorescent moieties do not prevent effective binding and activation of the opioid receptors.
Figure 2.
Opioid receptor functional activity of novel compounds. All compounds were used to stimulate 35S-GTPγS accumulation using membranes from CHO cells expressing the human opioid receptors (DOR, MOR, or KOR). Data reported as the mean ± SEM of N = 3 independent experiments. (A) Concentration–response curves shown for all 3 compounds and positive control (DAMGO for MOR, SNC80 for DOR, U50,488 for KOR) at each receptor. All compounds show partial to full agonist activity at each receptor, with potency DOR = MOR > KOR. (B) Potency (EC50) and efficacy (EMAX) values shown for each compound at each receptor derived from the curves in A.
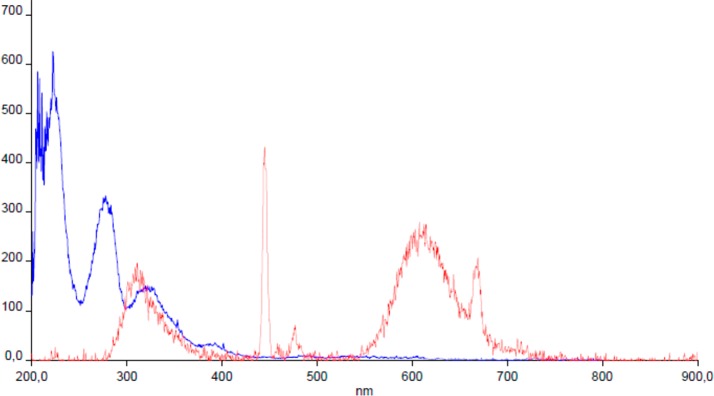
After establishing the molecular pharmacology of our compounds, we finally sought to evaluate their potential as fluorescent labeling tools. We thus measured the excitation and emission spectra of our highest affinity cyclic peptide 1D, using an Edinburg Instruments FLS-920 fluorimeter in 100% methanol matrix at rt, with a sample concentration of 10–2 μM (Figure 3). The fluorescence of the flurescent-conjugated molecule is strongly dependent on the polarity of the solvent and chemical surroundings. The fluorescence emission spectrum of our novel compound 1D shows an emission band ranging from 300 to 700 nm, with a maximum at 450 nm in methanol. In agreement with the findings of Guy et al. a maximum at 605 nm is present in the spectra, which represents the fluorescence emission of N-substituted dansylamide.26 Two strong emission bands are present around 300 and 450 nm associable to tyrosine residues and conjugated hydrazine into the cyclic peptide.27,28 These results establish that 1D is indeed a fluorescent ligand, with potential for use as a labeling and imaging agent. The good separation between excitation and emission spectra means that the compound would show little bleed through or crosstalk if the excitation and emission gates were correctly chosen. In conclusion three novel chemical entities have been synthesized and characterized with the aim to find a useful fluorescent bioconjugate of cyclic biphalin able to bind opioid receptors. Among them, compound 1D exhibits the best affinity profile, being able to bind MOR/DOR and to stimulate the G protein coupled receptors with high affinity and potency. This compound represents an ideal candidate for further future work to define the pharmacokinetic properties of cyclic biphalin compounds, and a valuable tool for binding studies, cellular uptake, and tissue distribution measurement of opioid receptors.
Figure 3.
Adsorption (blue)/emission (red) spectrum of compound 1D in 100% methanol. Y axis: Absorbance in A.U.; X axis: wavelength in nm unit.
Experimental Procedures
General Information
Amino acids and reagents were acquired from Sigma-Aldrich (Milano, Italy), and HPLC grade solvents from VWR (Milano, Italy). The three novel cyclic peptides as TFA salts were purified by RP-HPLC on a Waters XBridgeTM Prep BEH130 C18, 5.0 μm, 250 mm × 10 mm column; flow rate of 7 mL/min; Waters Binary pump 1525 (Waters, MA, USA); eluent: linear gradient of H2O/ACN 0.1% TFA from 5 to 90% ACN in 35 min. Nα-Boc-protected products were identified by NMR analysis on a Varian Mercury 300 MHz instrument and mass spectrometry ESI-LRMS (Thermo Finnigan, NJ, USA). The purity of the final compounds as TFA salts was calculated using analytical reverse phase high performance liquid chromatography (RP-HPLC; C18-bonded 4.6 × 150 mm) at a flow rate of 1 mL/min, gradient eluent of H2O/ACN 0.1% TFA from 5 to 95% ACN in 26 min, and was found to be ≥90% pure. The identity of all final compounds was determined by 1H NMR and ESI-LRMS. Fluorescence measurement was performed using an Edinburg Instruments FLS-920 fluorometer. The fluorescence measure was done in 100% methanol matrix at rt; sample concentration was 10–2 μM, with wavelength ranging from 200 to 900 nm in 10 min.
Chemistry
The organic scaffold 3,4-dibromofuran-2,5-dione was prepared starting from the commercially available maleic anhydride following the procedure reported by Dubernet et al.23 The fluorescent intermediates reported in Scheme 1 were synthesized in solution as described by Youziel et al.21 and Heltweg et al.22, respectively. Compounds 1–3 were prepared starting from the treatment of dibromomaleic anhydride with the appropriate fluorescent intermediates as TFA salts or 7-amino-4-methylcoumarin at room temperature (rt), followed by reflux in AcOH to give the desired fluorescent maleimides in good yields. All linear intermediate peptides were obtained by solution phase peptide synthesis using the EDC·HCl/HOBt hydrate/N,N-diisopropylethylamine (DIPEA) in the dimethylformamide (DMF) coupling method. Deprotection of the Nα terminus of peptides from Boc-protecting group was performed with a mixture of TFA in DCM 1:1 at rt. The intermediate TFA salts were used for subsequent reactions without further purification. Nα-Boc-protected intermediates were isolated by silica gel column chromatography when required. The cyclic biphalin intermediate 4 was synthesized in solution, following the well-established procedure reported by us.24 Then this intermediate was treated with tris(2-carboxyethyl)phosphine hydrochloride solution (TCEP) reagent in DMF at rt and the fluorescent-probes 1–3 were added in situ to promptly react with the thiol groups, so as to form the cyclic compounds 1C, 1D, and 2C after N-Boc deprotection.20 The fluorescent cyclic intermediates were obtained in good yields after an easy workup and were used as such for the following reaction without further purification. All final biphalin fluorescent analogs were deprotected with a mixture of TFA:DCM = 1:1, purified by RP-HPLC, and characterized as TFA salts. 1H NMR, ESI-LRMS, and analytical RP-HPLC were performed for all final compounds; details of the experimental procedures are reported in the SI.
Competition-Binding Assays
Opioid receptor-expressing CHO cells were used as previously reported.19 The cells were grown using 50:50 DMEM/F12 culture media with 10% heat-inactivated fetal bovine serum, 1X penicillin/streptomycin, with propagation cultures maintained with 500 μg/mL G418 (all culture reagents from ThermoFisher, Gibco brand). The cells were grown in a 37 °C humidified incubator with 5% CO2. Cells were grown and pellets harvested for both binding and G protein coupling assays as reported.20 The competition binding assay was also performed as reported.20 Briefly, 20–25 μg of cell membrane protein was combined with concentration curves of experimental compound or positive control and a fixed concentration (1.2–4.6 nM) of 3H-diprenorphine (PerkinElmer) in a 200 μL reaction volume. The reactions were incubated for 1 h at rt, and terminated by rapid filtration onto GF/B filter plates (PerkinElmer) using a Brandel cell harvester. The plates were dried, 40 μL of Microscint PS (PerkinElmer) was added, and the data was collected using a MicroBeta2 96 well format scintillation counter (PerkinElmer). The data was analyzed using GraphPad Prism 8.2 with a 1-site competition binding model using the previously measured KD of the 3H-diprenorphine in each cell line. The data was further normalized to radioligand alone (100%) or in the presence of 10 μM naloxone (nonspecific binding, 0%). The resulting affinity (Ki) values were reported as the mean ± SEM of N = 3 independent experiments.
35S-GTPγS Coupling Assay
The cells were grown and harvested as above; the assay protocol used was also reported previously in Stefanucci et al.20,25 15 μg of cell membrane protein was combined with concentration curves of experimental or positive control agonist and 0.1 nM 35S-GTPγS (PerkinElmer) in a 200 μL volume. Reactions were incubated at 30 °C for 1 h and then harvested and data collected as above. The data was normalized to the stimulation caused by positive control agonist (100%) or vehicle treatment (0%). GraphPad Prism 8.2 was used to analyze the data using a 3-variable nonlinear regression model (Hill Slope defined as 1). The resulting potency (EC50) and efficacy (EMAX) values were reported as the mean ± SEM of N = 3 independent experiments.
Glossary
Abbreviations
- DOR
δ-opioid receptor
- MOR
μ-opioid receptor
- KOR
k-opioid receptor
- EDC
1-ethyl-(3-(dimethylamino)propyl)-carbodiimide
- DAMGO
[DAla(2), N-Me-Phe-(4), Gly-ol(5)] enkephalin
- SNC80
(+)-4-[(αR)-α-((2S,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide
- TAMRA
tetramethylrhodamine
- ER
endoplasmic reticulum
- SEM
standard error of measurement
- HOBt
1-hydroxybenzotriazole
- DIPEA
N,N-Diisopropylethylamine
- DMF
dimethylformamide
- EtOAc
ethyl acetate
- RP-HPLC
reversed phase high performance liquid chromatography
- ACN
acetonitrile
- NMR
nuclear magnetic resonance
- ESI-LRMS
electrospray ionization low resolution mass spectrometry
- HRMS
high resolution mass spectrometry
- TFA
trifluoroacetic acid
- DCM
dichloromethane
- TLC
thin layer chromatography
- BBB
blood brain barrier
- CNS
central nervous system
- GTP
guanosine triphosphate
- DMSO
dimethylsulfoxide
- CHO
chinese hamster ovary
- DMEM/F12
Dulbecco’s modified eagle medium/nutrient mixture F-12
- EDTA
ethylenediaminetetraacetic acid
- PBS
phosphate buffered saline
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.9b00569.
Details of compound synthesis and characterization (PDF)
Author Contributions
AS designed and synthesized the novel compounds and cowrote and organized the manuscript. MPD helped in the purification and characterization of the novel compounds. GL and EN revised the entire manuscript for English language; GZ collaborated in the interpretation and rationalization of the data. JMS participated with and supervised GM in the performance of the in vitro experiments and cowrote the manuscript. AM cowrote and organized the manuscript and coordinated all the research units.
This study was funded in part by institutional funds from the University of Arizona to JMS.
The authors declare no competing financial interest.
Supplementary Material
References
- Benizri S.; Gissot A.; Martin A.; Vialet B.; Grinstaff M. W.; Barthélémy P. Bioconjugated oligonucleotides: Recent developments and therapeutic applications. Bioconjugate Chem. 2019, 30, 366–383. 10.1021/acs.bioconjchem.8b00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A.-C.; Chao C. C.; Takemori A. E.; Gekker G.; Hu S.; Peterson P. K.; Portoghese P. S. Arylacetamide-derived fluorescent probes: Synthesis, biological evaluation, and direct fluorescent labeling of κ opioid receptors in mouse microglial cells. J. Med. Chem. 1996, 8, 1729–1735. 10.1021/jm950813b. [DOI] [PubMed] [Google Scholar]
- Huynh A. S.; Estrella V.; Stark V. E.; Cohen A. S.; Chen T.; Casagni T. J.; Josan J. S.; Lloyd M. C.; Johnson J.; Kim J.; Hruby V. J.; Vagner J.; Morse D. L. Tumor targeting and pharmacokinetics of a near-Infrared fluorescent-labeled δ-opioid receptor antagonist agent, Dmt-Tic-Cy5. Mol. Pharmaceutics 2016, 13, 534–544. 10.1021/acs.molpharmaceut.5b00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arttamangkul S.; Alvarez-Maubecin V.; Thomas G.; Williams J. T.; Grandy D. K. Binding and internalization of fluorescent opioid peptide conjugates in living cells. Mol. Pharmacol. 2000, 58, 1570–1580. 10.1124/mol.58.6.1570. [DOI] [PubMed] [Google Scholar]
- Dado R. J.; Law P. Y.; Loh H. H.; Elde R. Immunofluorescent identification of a t-opioid receptor on primary afferent nerve terminals. NeuroReport 1993, 5, 341–344. 10.1097/00001756-199312000-00041. [DOI] [PubMed] [Google Scholar]
- Leong C.; Neumann C.; Ramasamy S.; Rout B.; Yi W. L.; Bigliardi-Qi M. Investigating endogenousμ-opioidreceptors in human keratinocytes as pharmacological targets using novel fluorescent ligand. PLoS One 2017, 12 (12), e0188607 10.1371/journal.pone.0188607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. E. B.; Felix F.; Schumacher C. P.; Lauren R.; Tedaldi M.; Papaioannou D.; Waksman G.; Caddick S.; Baker J. R. Protein modification, bioconjugation, and disulfide bridging using bromomaleimides. J. Am. Chem. Soc. 2010, 132, 1960–1965. 10.1021/ja908610s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin M. P.; Wilson P.; Mabire A. B.; Kiviaho J. K.; Raymond J. E.; Haddleton D. M.; O’Reilly R. K. Conjugation-induced fluorescent labeling of proteins and polymers using dithiomaleimides. J. Am. Chem. Soc. 2013, 135, 2875–2878. 10.1021/ja3105494. [DOI] [PubMed] [Google Scholar]
- Marculescu C.; Kossen H.; Morgan R. E.; Mayer P.; Fletcher S. A.; Tolner B.; Chester K. A.; Jones L. H.; Baker J. R. Aryloxymaleimides for cysteine modification, disulfide bridging and the dual functionalization of disulfide bonds. Chem. Commun. 2014, 50, 7139–7142. 10.1039/C4CC02107J. [DOI] [PubMed] [Google Scholar]
- Dvoracsko S.; Stefanucci A.; Novellino E.; Mollica A. The design of multitarget ligands for chronic and neuropathic pain. Future Med. Chem. 2015, 7, 2469–2483. 10.4155/fmc.15.156. [DOI] [PubMed] [Google Scholar]
- Stefanucci A.; Carotenuto A.; Macedonio G.; Novellino E.; Pieretti S.; Marzoli F.; Szücs E.; Erdei A. I.; Zádor F.; Benyhe S.; Mollica A. Cyclic biphalin analogues incorporating a xylene bridge: synthesis, characterization, and biological profile. ACS Med. Chem. Lett. 2017, 8, 858–863. 10.1021/acsmedchemlett.7b00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollica A.; Carotenuto A.; Novellino E.; Limatola A.; Costante R.; Pinnen F.; Stefanucci A.; Pieretti S.; Borsodi A.; Samavati R.; Zador F.; Benyhe S.; Davis P.; Porreca F.; Hruby V. J. Novel cyclic biphalin analogue with improved antinociceptive properties. ACS Med. Chem. Lett. 2014, 5, 1032–1036. 10.1021/ml500241n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollica A.; Costante R.; Stefanucci A.; Pinnen F.; Lucente G.; Fidanza S.; Pieretti S. Antinociceptive profile of potent opioid peptide AM94, a fluorinated analogue of biphalin with non-hydrazine linker. J. Pept. Sci. 2013, 19, 233–239. 10.1002/psc.2465. [DOI] [PubMed] [Google Scholar]
- Costante R.; Pinnen F.; Stefanucci A.; Mollica A. Potent biphalin analogs with μ/δ mixed opioid activity: in vivo and in vitro biological evaluation. Arch. Pharm. 2014, 347, 305–312. 10.1002/ardp.201300380. [DOI] [PubMed] [Google Scholar]
- Mollica A.; Pinnen F.; Costante R.; Locatelli M.; Stefanucci A.; Pieretti S.; Davis P.; Lai J.; Rankin D.; Porreca F.; Hruby V. J. Biological active analogues of the opioid peptide biphalin: Mixed ppp3-peptides. J. Med. Chem. 2013, 56, 3419–3423. 10.1021/jm301456c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollica A.; Pinnen F.; Feliciani F.; Stefanucci A.; Lucente G.; Davis P.; Porreca F.; Ma S. W.; Lai J.; Hruby V. J. New potent biphalin analogues containing p-fluoro-L-phenylalanine at the 4,4′ positions and non-hydrazine linkers. Amino Acids 2011, 40, 1503–1511. 10.1007/s00726-010-0760-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkowski A. W.; Misicka A.; Kosson D.; Kosson P.; Lachwa-From M.; Brodzik-Bienkowska A.; Hruby V. J. Biological properties of a new fluorescent biphalin fragment analogue. Life Sci. 2002, 70, 893–897. 10.1016/S0024-3205(01)01467-9. [DOI] [PubMed] [Google Scholar]
- Berezowska I.; Lemieux C.; Chung N. N.; Zelent B.; Schiller P. W. Dansylated analogues of the opioid peptide [Dmt1] DALDA: in vitro activity profiles and fluorescence parameters. Acta Biochim Polym. 2004, 51, 107–113. 10.18388/abp.2004_3601. [DOI] [PubMed] [Google Scholar]
- Lukowiak M.; Kosson P.; Hennink W.; Lipkowski A. W. Synthesis and pharmacological properties of a new fluorescent opioid peptide analog. Pharmacol. Rep. 2009, 61, 727–731. 10.1016/S1734-1140(09)70126-4. [DOI] [PubMed] [Google Scholar]
- Stefanucci A.; Lei W.; Hruby V. J.; Macedonio G.; Luisi G.; Carradori S.; Streicher J. M.; Mollica A. Fluorescent-labeled bioconjugates of the opioid peptides biphalin and DPDPE incorporating fluorescein-maleimide linkers. Future Med. Chem. 2017, 9, 859–869. 10.4155/fmc-2016-0232. [DOI] [PubMed] [Google Scholar]
- Youziel J.; Akhbar A. R.; Aziz Q.; Smith M. E.; Caddick S.; Tinker A.; Baker J. R. Bromo- and thiomaleimides as a new class of thiol-mediated fluorescence ‘turn-on’ reagents. Org. Biomol. Chem. 2014, 12, 557–560. 10.1039/C3OB42141D. [DOI] [PubMed] [Google Scholar]
- Heltweg B.; Dequiedt F.; Marshall B. L.; Brauch C.; Yoshida M.; Nishino N.; Verdin E.; Jung M. Subtype selective substrates for histone deacetylases. J. Med. Chem. 2004, 47, 5235–5243. 10.1021/jm0497592. [DOI] [PubMed] [Google Scholar]
- Dubernet M.; Caubert V.; Guillard J.; Viaud-Massuard M. C. Synthesis of substituted bis(heteroaryl)maleimides. Tetrahedron 2005, 61, 4585–4593. 10.1016/j.tet.2005.03.016. [DOI] [Google Scholar]
- Mollica A.; Davis P.; Ma S. W.; Porreca F.; Lai J.; Hruby V. J. Synthesis and biological activity of the first cyclic biphalin analogues. Bioorg. Med. Chem. Lett. 2006, 16, 367–372. 10.1016/j.bmcl.2005.09.080. [DOI] [PubMed] [Google Scholar]
- Stefanucci A.; Lei W.; Pieretti S.; Dimmito M. P.; Luisi G.; Novellino E.; Nowakowski M.; Koźmiński W.; Mirzaie S.; Zengin G.; Streicher J. M.; Mollica A. Novel cyclic biphalin analogues by ruthenium-catalyzed ring closing metathesis: in vivo and in vitro biological profile. ACS Med. Chem. Lett. 2019, 10, 450–456. 10.1021/acsmedchemlett.8b00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J.; Caron K.; Dufresne S.; Michnick S. W.; Jeffrey S.; Keillor W. Convergent preparation and photophysical characterization of dimaleimidedansylfluorogens:Elucidation of the maleimide fluorescence quenching mechanism. J. Am. Chem. Soc. 2007, 129, 11969–11977. 10.1021/ja0738125. [DOI] [PubMed] [Google Scholar]
- Tang J.; Yin H.; Qiu J.; Tucker M. J.; DeGrado W. F.; Gai F. Using two fluorescent probes to dissect the binding, insertion, and dimerization kinetics of a model membrane peptide. J. Am. Chem. Soc. 2009, 131, 3816–3817. 10.1021/ja809007f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan K. R.; Shilajyan H. A. Fluorescence 2D and 3D spectra analysis of tryptophan, tyrosine and phenylalanine. Proc. of the Yerevan State Univ. Chemistry and Biology 2017, 51, 3–7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.