Abstract
The Hu family of RNA-binding proteins plays a crucial role in post-transcriptional processes; indeed, Hu–RNA complexes are involved in various dysfunctions (i.e., inflammation, neurodegeneration, and cancer) and have been recently proposed as promising therapeutic targets. Intrigued by this concept, our research efforts aim at identifying small molecules able to modulate HuR–RNA interactions, with a focus on subtype HuR, upregulated and dysregulated in several cancers. By applying structure-based design, we had already identified racemic trans-BOPC1 as promising HuR binder. In this Letter, we accomplished the enantio-resolution, the assignment of the absolute configuration, and the recognition study with HuR of enantiomerically pure trans-BOPC1. For the first time, we apply DEEP (differential epitope mapping)-STD NMR to study the interaction of BOPC1 with HuR and compare its enantiomers, gaining information on ligand orientation and amino acids involved in the interaction, and thus increasing focus on the in silico binding site model.
Keywords: RNA-binding protein, HuR−RNA complexes, molecular modeling, chiral HPLC, DEEP-STD NMR
RNA-binding proteins (RBPs) are considered relevant targets to modulate gene expression and therefore to face several pathologies such as neurodegeneration and cancer.1−8 While most approaches focus on RNA interference-based oligonucleotides targeting different RNA-binding domains (RBDs),9−13 a more recent strategy involves the identification of small molecules able to modulate ribonucleoprotein (RNP) complexes. This strategy is intrinsically challenging, because RBPs generally lack a defined small-molecule binding site. In this context, in 2018, H3B-8800,14 a small molecule acting as an inhibitor of SF3b, a protein with critical functions in pre-mRNA splicing, reached the first stage of clinical phase for selected types of leukemia. This is the first rationally designed small molecule able to target RBPs to reach the clinic, and therefore, it represents a milestone in the story of RBP modulation, confirming that this strategy represents a way to develop new effective therapeutic agents.
Within our medicinal chemistry research group, we have been working in this field for several years, particularly studying the Hu protein family, a RBP class with a pivotal role in the stabilization of various RNAs.2,15−18 Among Hu proteins, HuR is the most studied, as it is an intriguing target for discovering new anticancer drugs.19,20 Nevertheless, to date little is known about the features of the HuR–small molecule interaction, even for proven interferers.21−23 In this context, to supply the lack of structural data and with the aim of discovering new HuR ligands able to modulate HuR–RNA binding, we exploit highly informative NMR methodologies combined with in silico studies.24−28 Briefly, according to a structure-based approach, we have focused on a pocket-like region of HuR formed by the RNP1 and RNP2 portions of RRM1 and 2 (RNA-recognition motif-type domain). Within the HuR RRM1/2–RNAc-fos cocrystal (PDB 4ED5),29 this region corresponds to the binding site of RNA–uridine residues 8 and 9 (U8–U9); thus, their position and key interactions represented the starting point (anchor) to our design of new HuR ligands. New compounds characterized by different cores were designed, synthesized, and subjected to a combined STD (saturation transfer difference) NMR and in silico investigation of their interactions with HuR.28
N-(2-(Benzylamino)ethyl)-2-(3,5-dimethoxyphenyl)-1-isobutyl-6-oxopiperidine-3-carboxamide, from now on BOPC1 (see Scheme 1), was effective in binding the target protein HuR. BOPC1 is characterized by the presence of two chiral centers, and in our preliminary experiments, it was tested as the trans-racemate. Since it is well recognized that enantiomers of chiral bioactive molecules may have a different interaction with the target, prior to the design of new derivatives, it is mandatory to study trans-BOPC1 in its enantiomerically pure forms. In this Letter, we accomplished the enantio-resolution of racemic trans-BOPC1, the assignment of the absolute configuration of the separated enantiomers, and their recognition study with HuR.
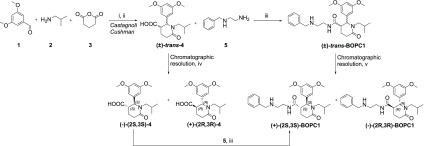
Scheme 1. Synthetic Pathway of BOPC1.
(i) 1,2, toluene, MS 4 Å, rt, 4 h; (ii) 3, p-xylene, reflux, 12 h, yield 60%; (iii) TBTU, DIPEA, THF, rt, 12 h, yield 71%; (iv) Chiralpak IA column, IPA:DEA:TFA 100:0.1:0.3 (v/v/v), flow rate 2 mL/min, injection volume 1 mL, concentration 10 mg/mL; (v) Chiralpak IC column, n-hexane:IPA:DEA 75:25:0.1 (v/v/v), flow rate 2 mL/min, injection volume 1 mL, concentration 10 mg/mL.
As a first step, we investigated the binding mode of the two trans-configured enantiomers in the previously considered HuR binding pocket, through molecular modeling simulations. The results of the in silico analysis, performed on the crystal structure of the HuR RRM1/2 domains, according to the computational approach recently we had published,27,28 clearly suggested a stereoselective binding mode (see Figure 1). In fact, though the two enantiomers bind to similar regions of the HuR binding pocket, they do it in a different way, and in order to evaluate the ligand binding free energy (ΔG bind) for BOPC1 enantiomers, the MM-GBSA calculations were performed [(2R,3R)-BOPC1MMGBSA value −41.27 kcal/mol and (2S,3S)-BOPC1MMGBSA value −53.37 kcal/mol]. Particularly, docking analysis showed that the aromatic rings of both BOPC1 enantiomers may establish different interactions with HuR.
Figure 1.
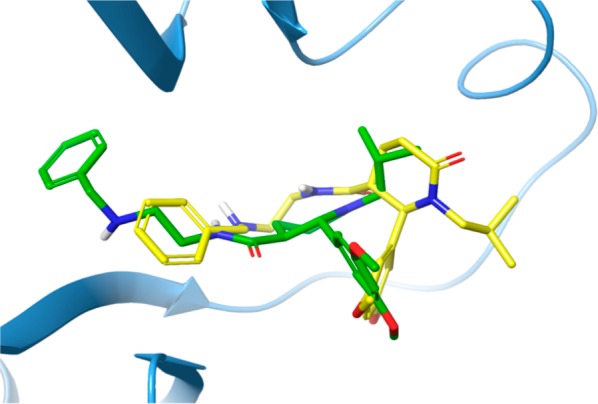
3D representation of (2R,3R)- and (2S,3S)-BOPC1 predicted binding modes overlapped in the HuR binding pocket. The protein is shown as light-blue cartoon, and the (2S,3S)-BOPC1 and the (2R,3R)-BOPC1 enantiomers are represented as green and yellow sticks, respectively.
Prompted by these results, we prepared (2R,3R)- and (2S,3S)-BOPC1 and investigated their interaction with HuR.
The synthetic strategy adopted for preparing BOPC1 is outlined in Scheme 1. The intermediate trans-2-(3,5- dimethoxyphenyl)-1- isobutyl-6-oxopiperidine-3- carboxylic acid was obtained as a racemate, by a Castagnoli–Cushman reaction, under the experimental conditions we had already described, with slight modifications. The subsequent amidation reaction provided racemic trans-BOPC1 (Scheme 1).28
To obtain enantiomerically pure BOPC1 in sufficient amounts for the subsequent studies, we selected chiral high-performance liquid chromatography (HPLC), an effective approach for the resolution of chiral compounds on both analytical and preparative scale.30−34 Upon screening various chiral stationary phases (CSPs) and eluents, we obtained a baseline HPLC separation of the two trans-BOPC1 enantiomers using the immobilized derivatized-cellulose Chiralcel IC as CSP eluting with n-hexane/isopropyl alcohol/DEA 75/25/0.1 (v/v/v). The good enantioselectivity and efficiency of the methodology allowed for an effective scale-up at the semipreparative level. By using a semipreparative IC column, we resolved 10 mg of sample for each HPLC run, finally isolating about 20 mg of enantiomers with an enantiomeric excess >98%.
To assign the absolute configuration to trans-BOPC1 enantiomers, we followed a multistep strategy, consisting in (i) chiral resolution of the key intermediate 4, (ii) assignment of the absolute configuration to (+)-trans-4 and (−)-trans-4, and (iii) synthesis of enantiomeric BOPC1, starting from enantiomerically pure 4.
Once again, the chiral resolution of the key intermediate (±)-trans-4 was performed via enantioselective HPLC. In this case a good resolution was obtained using the immobilized amylose chiral Chiralpak IA column under reverse phase elution conditions, eluting with isopropyl alcohol as mobile phase. Both enantiomers of trans-4 were obtained in a 50-mg scale, a suitable amount for further studies and with an enantiomeric excess >90%.
As a first step of the empirical approach to assign the absolute configuration of enantiomerically pure trans-4, we used the enantiomers of the structurally related compound trans-6 (Figure 2) as a reference. Briefly, we compared both the electronic circular dichroism (ECD) spectra and the elution order, under the same chiral chromatographic conditions, of (+)-trans-4 and(−)-trans-4 with the structurally related (+)-(2R,3R)-6 and (−)-(2S,3S)-6, for which we have already assigned the absolute configuration by X-ray crystallographic analysis.35 The ECD spectra of trans-4 and the reference compound trans-6, recorded in acetonitrile, are shown in Figure 2a. As expected, the enantiomeric forms of trans-4 exhibited specular patterns. The findings of the ECD measurements allowed us to define a parallelism between absolute configuration and CD band signs. Briefly, comparable Cotton effects (CEs) for (+)-4 and (+)-(2R,3R)-6 compounds are evident in two ranges of wavelengths: negative CEs between 190 and 200 nm and positive CEs between 210 and 230 nm attributable to 1La and 1Lb electronic transitions of benzene. The sign of the CEs of 1La is consistently opposite that at the longer wavelength (1Lb). For compound (2R,3R)- 6 a twisting of the 1Lb band is observed, in accordance with related literature.36 Based on these considerations, the (2R,3R) absolute configuration of (+)- 6 may also be proposed for the first eluted (+)-trans-4. Naturally, a reversed sign of the ellipticity is expected from the second eluted (2S,3S)- enantiomer. The analysis performed on the Chiralpak IA column under the same elution conditions (n-hexane:IPA:DEA:TFA 75:25:0.1:0.3) confirmed the same elution order for the enantiomers of both compounds (see Figure 2b).
Figure 2.
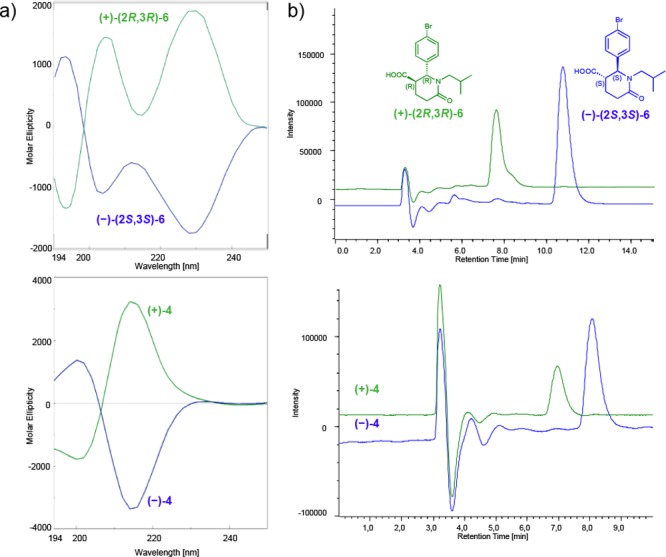
(a) ECD traces in acetonitrile and (b) chromatographic profiles at 220 nm (Chiralpak IA, mobile phase n-hexane:IPA:DEA:TFA 75:25:0.1:0.3, flow rate 0.5 mL/min) of (+)-(2R,3R)-6 and (−)-(2S,3S)-6 (top), and (+)-trans-4 and (−)-trans-4 (bottom). For (+) enantiomers the traces are reported in green, for (−) in blue.
Once it was determined the absolute configuration of the two enantiomers of the key intermediate was trans-4, the stereochemical assignment was extended to the stereoisomers of trans-BOPC1 by a chemical correlation method (see Scheme 1). The reaction of connection between the acid intermediate and amine 5 is a stereoconservative process. So, the absolute configuration of each single stereoisomer of trans-BOPC1 is determined by the stereochemistry of the enantiomerically pure key intermediate used in its synthesis. The stereochemical course of reactions was monitored by the enantioselective HPLC conditions described above. The absolute configurations of the two stereoisomers of trans-BOPC1 were then assigned as follows: first eluted isomer (+)-trans-BOPC1 as (2S,3S), and second eluted isomer (−)-trans-BOPC1 (2R,3R).
Once the absolute configuration of enantiomerically pure trans-BOPC1 was defined, for the study of their interaction with HuR, we employed STD NMR, a leading ligand-based NMR technique used to characterize ligand–macromolecule interactions as they occur in solution37 and identify ligand moieties important for binding the target.38 The experiment is based on the NOE effect and exploits the transfer of the magnetization from the macromolecule, selectively irradiated, to the ligand. The regions of the ligand closer to the macromolecule receive the magnetization more efficiently than those which are farther, producing differences in signal intensity; data processing into binding epitope maps can elucidate the structural features of the binding event. In particular, we compared the binding modes of the two enantiomers and elucidated additional binding site information by applying differential epitope mapping (DEEP) by STD NMR. This very recent technique can gain additional data to the simple STD experiment thanks to the application of differential conditions. Namely, it can identify the type of protein residues contacting the ligand through the generation of differential epitope maps and, if the 3D structure of the protein is known, it helps in orienting the ligand in the binding pocket, with a higher precision compared to the single STD experiment.39,40 In particular, we exploited the two enantiomers for interaction studies with the target HuR according to a DEEP-STD protocol for both comparison of the two enantiomers and to build a differential solvent epitope map. First of all, STD NMR spectra were recorded for both enantiomers of trans-BOPC1 in a 100% D2O buffer. Absolute and relative STD% for each enantiomer are stated in S2.2 of the Supporting Information, along with a schematic representation of each enantiomer epitope mapping.
Nonetheless, absolute STD values coming from different STD spectra cannot be directly compared, due to possible differences in sample preparation or instrument setting. For this reason, in order to obtain a comparison between the interaction of the two separate enantiomers with HuR, we exploited the DEEP equation reported in S2.1 of the Supporting Information.41 The experiment of (2S,3S)-BOPC1 was chosen as exp1 (since the global saturation is larger than that of (2R,3R)-BOPC1), and the ratio and average ratio of STD intensities over all protons was calculated affording “enantio DEEP-STD” values for each proton. The raw and processed data and ΔSTD factors are shown in S2.3 of the Supporting Information and represented schematically in Figure 3. We recorded positive ΔSTD factors for the protons of ring B and its methoxy group, while negative ΔSTD factors were observed for protons 3, 9–10, and 16. These differences suggest that the two enantiomers interact with the same protons but different orientation into the binding site. Positive ΔSTD factors suggest which moieties are most involved in the interaction of (2S,3S)-BOPC1 with HuR, while negative ΔSTD factors are used for (2R,3R)-BOPC1 and HuR.
Figure 3.
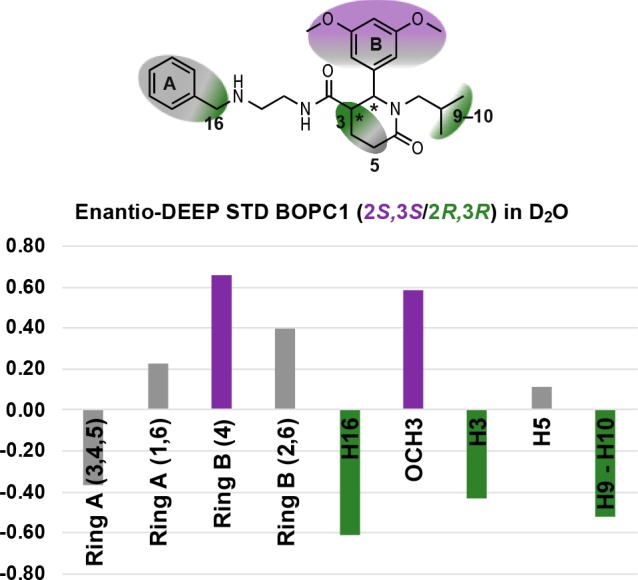
Enantioapplication of the DEEP-STD experiment. The STD spectra of (2S,3S)-BOPC1 and (2R,3R)-BOPC1 in D2O are compared thanks to the equation exploited in the differential approach. Stronger binding components are reported in purple for (2S,3S)-BOPC1 and dark green for (2R,3R)-BOPC1. Gray indicates no significant difference in moiety contribution for the two enantiomers tested.
We then performed differential solvent experiments on both enantiomers by studying their interaction with HuR in H2O:D2O 90:10 and comparing each experiment with the respective one in D2O, following the literature procedure (S2.1).41 Briefly, DEEP STD negative values (obtained considering D2O/H2O ratios) suggest an interaction with exchanging protons of the protein (so, interaction mediated by polar groups). The presence of signals with positive DEEP STD values indicates the involvement in a binding with apolar residues of the protein. The experiment highlighted that for (2S,3S)-BOPC1, ring A points toward apolar residues, along with protons 5 and 3, while protons 16 and 9–10 are projected toward polar residues. A different interaction was observed for (2R,3R)-BOPC1: ring A is still interacting with hydrophobic residues, along with 16, while the signals of ring B and its methoxy groups show vicinity to polar residues (all raw and processed data are reported in S2.5 and S2.6 of the Supporting Information).
The data thus obtained were exploited to confirm and refine ligand orientation in the HuR binding pocket. In accordance with experimental data, ring B of (2R,3R)-BOPC1 (MMGBSA value −41.27 kcal/mol) establishes only an interaction mediated by polar group namely an H-bond with Asn25. Conversely, ring B of the (2S,3S)-BOPC1 (MMGBSA value −53.37 kcal/mol) can form a stacking interaction with Tyr63 and three H-bonds, with Arg153 and with Asn25; this is mirrored by low DEEP-STD values for this moiety indicating no prevalence of either polar or apolar interactions. Combined DEEP-STD and in silico results are shown in Figure 4.
Figure 4.
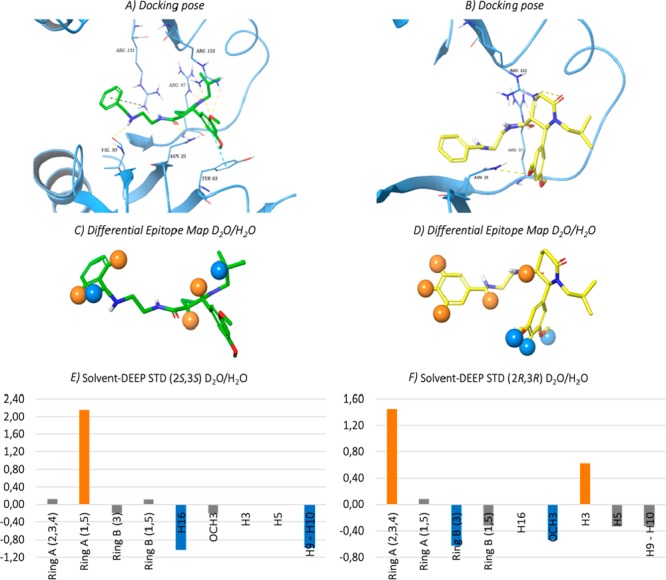
Selected docking pose of the (a) (2S,3S)-BOPC1 and (b) (2R,3R)-BOPC1 in the HuR binding pocket. The protein residues involved in crucial contacts with the ligands are reported as light-blue carbon sticks. Hydrogen bonds, pi–pi stacking and pi–cation interactions are reported, respectively, as dashed yellow, light-blue, and green lines. (c,d) Graphic representation of the solvent DEEP-STD experiment on the (2S,3S)-BOPC1–HuR and (2R,3R)-BOPC1–HuR complexes. Blue spheres indicate ligand contacts with protein side chains carrying slowly exchanging protons; orange spheres indicate ligand contacts with apolar residues. (e,f) Solvent DEEP-STD histograms of (2S,3S)-BOPC1–HuR and (2R,3R)-BOPC1–HuR complexes; blue and orange bars represent contacts with polar and apolar residues, respectively; gray bars indicate no significant difference in type of amino acid contacts for the two solvent conditions tested. For protons 5 and 3 (e) and 16 (f) the DEEP-STD values could not be calculated due to absence of their signal in the H2O experiment. Nonetheless, the contribution of apolar residues (D2O experiment) for these protons is still prevalent.
The DEEP-STD protocol, applied in the present Letter to study for the first time the interaction of trans-BOPC1 enantiomers with HuR, gains information on the orientation of the compounds in the binding site and elucidates the type of amino acids involved in the binding event. The two binding modes reported in Figure 4 for the two trans-BOPC1 enantiomers are the best agreement between best docking poses and DEEP-STD data. Given the precious information originated from the collected spectroscopic and computational data, and with the increased focus on the in silico binding site model in hands, we plan to design and synthesize a focused library of novel oxopiperidine-3-carboxamides, to gain complete structure–activity relationships, and to identify leads with improved affinity toward HuR, potentially active against different types of cancer.
Acknowledgments
FV, SDV, and SC gratefully acknowledge Donatella Potenza for the fruitful scientific discussion and for her contribution to the development of the STD technique and its application to this project; all authors thankfully recognize Alessandro Provenzani and his team for expressing, purifying, and providing native HuR protein utilized for interaction studies. FV acknowledges University of Milano for the grant PSR 2018 (“Piano di Sostegno per la Ricerca” -linea 2). SA and GC acknowledge the PRIN 2017 research project “Novel anticancer agents endowed with multitargeting mechanism of action” (201744BN5T).
Glossary
Abbreviations
- RBP
RNA-binding protein
- RBD
RNA-binding domains
- RNP
ribonucleoprotein
- RRM
RNA recognition motif-type
- STD-NMR
saturation transfer difference-NMR
- BOPC1
N-(2-(benzylamino)ethyl)-2-(3,5-dimethoxyphenyl)-1-isobutyl-6-oxopiper dine-3-carboxamide
- ECD
electronic circular dichroism
- HPLC
high-performance liquid chromatography
- CSP
chiral stationary phases
- CE
Cotton effects
- MMGBSA
molecular mechanism generalized Born suface area
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.9b00659.
Synthesis, enantiomeric resolution by chiral HPLC, and compound characterization. We report all protocols in SI, S1. Interaction studies with HuR STD and DEEP-STD NMR studies were carried out as reported in SI, S2. Molecular modeling molecular dynamics simulations and docking studies were performed as stated in SI, S3. (PDF)
Author Contributions
The manuscript was written through contributions of all authors.
The authors declare no competing financial interest.
Supplementary Material
References
- Tang A. Y. RNA Processing-Associated Molecular Mechanisms of Neurodegenerative Diseases. J. Appl. Genet. 2016, 57, 323–333. 10.1007/s13353-015-0330-5. [DOI] [PubMed] [Google Scholar]
- Kotta-Loizou I.; Giaginis C.; Theocharis S.. Clinical Significance of HuR Expression in Human Malignancy. Med. Oncol.. Published online 13 August 2014. 10.1007/s12032-014-0161-y. [DOI] [PubMed] [Google Scholar]
- Wang J.; Guo Y.; Chu H.; Guan Y.; Bi J.; Wang B. Multiple Functions of the RNA-Binding Protein HuR in Cancer Progression, Treatment Responses and Prognosis. Int. J. Mol. Sci. 2013, 14, 10015–10041. 10.3390/ijms140510015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Melo D.; Droppelmann C. A.; Volkening K.; Strong M. J. RNA-Binding Proteins as Molecular Links between Cancer and Neurodegeneration. Biogerontology 2014, 15, 587–610. 10.1007/s10522-014-9531-2. [DOI] [PubMed] [Google Scholar]
- Wurth L.; Gebauer F. RNA-Binding Proteins, Multifaceted Translational Regulators in Cancer. Biochim. Biophys. Acta, Gene Regul. Mech. 2015, 1849, 881–886. 10.1016/j.bbagrm.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Dejong E.; Luy B.; Marino J. RNA and RNA-Protein Complexes as Targets for Therapeutic Intervention. Curr. Top. Med. Chem. 2002, 2, 289–302. 10.2174/1568026023394245. [DOI] [PubMed] [Google Scholar]
- Talman V.; Pascale A.; Jäntti M.; Amadio M.; Tuominen R. K. Protein Kinase C Activation as a Potential Therapeutic Strategy in Alzheimer’s Disease: Is There a Role for Embryonic Lethal Abnormal Vision-like Proteins?. Basic Clin. Pharmacol. Toxicol. 2016, 119, 149–160. 10.1111/bcpt.12581. [DOI] [PubMed] [Google Scholar]
- Talman V.; Amadio M.; Osera C.; Sorvari S.; Boije Af Gennäs G.; Yli-Kauhaluoma J.; Rossi D.; Govoni S.; Collina S.; Ekokoski E.; et al. The C1 Domain-Targeted Isophthalate Derivative HMI-1b11 Promotes Neurite Outgrowth and GAP-43 Expression through PKCα Activation in SH-SY5Y Cells. Pharmacol. Res. 2013, 73, 44–54. 10.1016/j.phrs.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Kim D. H.; Behlke M.; Rossi J. J. Designing and Utilization of SiRNAs Targeting RNA Binding Proteins. Methods Mol. Biol. 2008, 488, 367–381. 10.1007/978-1-60327-475-3_24. [DOI] [PubMed] [Google Scholar]
- Jacobsen A.; Wen J.; Marks D. S.; Krogh A. Signatures of RNA Binding Proteins Globally Coupled to Effective MicroRNA Target Sites. Genome Res. 2010, 20, 1010–1019. 10.1101/gr.103259.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X.; Wu Y.; Hartley R. S. MicroRNA-125a Represses Cell Growth by Targeting HuR in Breast Cancer. RNA Biol. 2009, 6, 575–583. 10.4161/rna.6.5.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeyev A. V.; Eastmond D. L.; Liebhaber S. A. Targeting a KH-Domain Protein with RNA Decoys. RNA 2002, 8, 1160–73. 10.1017/S135583820202808X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abil Z.; Zhao H. Engineering Reprogrammable RNA-Binding Proteins for Study and Manipulation of the Transcriptome. Mol. BioSyst. 2015, 11, 2658–2665. 10.1039/C5MB00289C. [DOI] [PubMed] [Google Scholar]
- Seiler M.; Yoshimi A.; Darman R.; Chan B.; Keaney G.; Thomas M.; Agrawal A. A.; Caleb B.; Csibi A.; Sean E.; et al. H3B-8800, an Orally Available Small-Molecule Splicing Modulator, Induces Lethality in Spliceosome-Mutant Cancers. Nat. Med. 2018, 24, 497–504. 10.1038/nm.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman M. N.; Lou H. Diverse Molecular Functions of Hu Proteins. Cell. Mol. Life Sci. 2008, 65, 3168–3181. 10.1007/s00018-008-8252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone-Bizzozero N.; Bird C. W. Role of HuD in Nervous System Function and Pathology. Front. Biosci., Scholar Ed. 2013, 5, 554–563. 10.2741/S389. [DOI] [PubMed] [Google Scholar]
- Antic D.; Keene J. D. Embryonic Lethal Abnormal Visual RNA-Binding Proteins Involved in Growth, Differentiation, and Posttranscriptional Gene Expression. Am. J. Hum. Genet. 1997, 61, 273–278. 10.1086/514866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikantan S.; Gorospe M. HuR Function in Disease. Front. Biosci., Landmark Ed. 2012, 17, 189–205. 10.2741/3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelmohsen K.; Gorospe M. Posttranscriptional Regulation of Cancer Traits by HuR. Wiley Interdiscip. Rev.: RNA 2010, 1, 214–229. 10.1002/wrna.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z.; Sanders A. J.; Ye L.; Wang Y.; Jiang W. G. Prognostic Value of the Human Antigen R (HuR) in Human Breast Cancer: High Level Predicts a Favourable Prognosis. Anticancer Res. 2011, 31, 303–10. [PubMed] [Google Scholar]
- Nasti R.; Rossi D.; Amadio M.; Pascale A.; Unver M. Y.; Hirsch A. K. H.; Collina S. Compounds Interfering with Embryonic Lethal Abnormal Vision (ELAV) Protein-RNA Complexes: An Avenue for Discovering New Drugs. J. Med. Chem. 2017, 60, 8257–8267. 10.1021/acs.jmedchem.6b01871. [DOI] [PubMed] [Google Scholar]
- Lal P.; Cerofolini L.; D’Agostino V. G.; Zucal C.; Fuccio C.; Bonomo I.; Dassi E.; Giuntini S.; Maio D. Di; Vishwakarma V.; et al. Regulation of HuR Structure and Function by Dihydrotanshinone-I. Nucleic Acids Res. 2017, 45, 9514–9527. 10.1093/nar/gkx623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni L.; Zucal C.; Maio D. Di; D’Agostino V. G.; Thongon N.; Bonomo I.; Lal P.; Miceli M.; Baj V.; Brambilla M.; et al. Interfering with HuR-RNA Interaction: Design, Synthesis and Biological Characterization of Tanshinone Mimics as Novel, Effective HuR Inhibitors. J. Med. Chem. 2018, 61, 1483–149. 10.1021/acs.jmedchem.7b01176. [DOI] [PubMed] [Google Scholar]
- Rossi D.; Amadio M.; Baraglia A. C.; Azzolina O.; Ratti A.; Govoni S.; Pascale A.; Collina S. Discovery of Small Peptides Derived from Embryonic Lethal Abnormal Vision Proteins Structure Showing RNA-Stabilizing Properties. J. Med. Chem. 2009, 52, 5017–5019. 10.1021/jm900741e. [DOI] [PubMed] [Google Scholar]
- Amadio M.; Pascale A.; Govoni S.; Laurini E.; Pricl S.; Gaggeri R.; Rossi D.; Collina S. Identification of Peptides with ELAV-like MRNA-Stabilizing Effect: An Integrated in Vitro/in Silico Approach. Chem. Biol. Drug Des. 2013, 81, 707–14. 10.1111/cbdd.12117. [DOI] [PubMed] [Google Scholar]
- Vasile F.; Rossi D.; Collina S.; Potenza D. Diffusion-Ordered Spectroscopy and Saturation Transfer Difference NMR Spectroscopy Studies of Selective Interactions between ELAV Protein Fragments and an MRNA Target. Eur. J. Org. Chem. 2014, 29, 6399–6404. 10.1002/ejoc.201403014. [DOI] [Google Scholar]
- Vasile F.; Della Volpe S.; Ambrosio F. A.; Costa G.; Unver M. Y.; Zucal C.; Rossi D.; Martino E.; Provenzani A.; Hirsch A. K. H.; et al. Exploration of Ligand Binding Modes towards the Identification of Compounds Targeting HuR: A Combined STD-NMR and Molecular Modelling Approach. Sci. Rep. Published online September 13, 2018, 10.1038/s41598-018-32084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Volpe S.; Nasti R.; Queirolo M.; Unver M. Y.; Jumde V. K.; Dömling A.; Vasile F.; Potenza D.; Ambrosio F. A.; Costa G.; et al. Novel Compounds Targeting the RNA-Binding Protein HuR. Structure-Based Design, Synthesis, and Interaction Studies. ACS Med. Chem. Lett. 2019, 10, 615–620. 10.1021/acsmedchemlett.8b00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Zeng F.; Liu Q.; Liu H.; Liu Z.; Niu L.; Teng M.; Li X. The Structure of the ARE-Binding Domains of Hu Antigen R (HuR) Undergoes Conformational Changes during RNA Binding. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2013, 69, 373–380. 10.1107/S0907444912047828. [DOI] [PubMed] [Google Scholar]
- Rossi D.; Tarantino M.; Rossino G.; Rui M.; Juza M.; Collina S. Approaches for Multi-Gram Scale Isolation of Enantiomers for Drug Discovery. Expert Opin. Drug Discovery 2017, 12, 1253–1269. 10.1080/17460441.2017.1383981. [DOI] [PubMed] [Google Scholar]
- Rossi D.; Nasti R.; Collina S.; Mazzeo G.; Ghidinelli S.; Longhi G.; Memo M.; Abbate S. The Role of Chirality in a Set of Key Intermediates of Pharmaceutical Interest, 3-Aryl-Substituted-γ-Butyrolactones, Evidenced by Chiral HPLC Separation and by Chiroptical Spectroscopies. J. Pharm. Biomed. Anal. 2017, 144, 41–51. 10.1016/j.jpba.2017.01.007. [DOI] [PubMed] [Google Scholar]
- Marra A.; Rossi D.; Pignataro L.; Bigogno C.; Canta A.; Oggioni N.; Malacrida A.; Corbo M.; Cavaletti G.; Peviani M.; et al. Toward the Identification of Neuroprotective Agents: G-Scale Synthesis, Pharmacokinetic Evaluation and CNS Distribution of (R)-RC-33, a Promising SIGMA1 Receptor Agonist. Future Med. Chem. 2016, 8, 287–295. 10.4155/fmc.15.191. [DOI] [PubMed] [Google Scholar]
- Rossi D.; Nasti R.; Marra A.; Meneghini S.; Mazzeo G.; Longhi G.; Memo M.; Cosimelli B.; Greco G.; Novellino E.; et al. Enantiomeric 4-Acylamino-6-Alkyloxy-2 Alkylthiopyrimidines As Potential A3Adenosine Receptor Antagonists: HPLC Chiral Resolution and Absolute Configuration Assignment by a Full Set of Chiroptical Spectroscopy. Chirality 2016, 28, 434–440. 10.1002/chir.22599. [DOI] [PubMed] [Google Scholar]
- Rossi D.; Pedrali A.; Marra A.; Pignataro L.; Schepmann D.; Wünsch B.; Ye L.; Leuner K.; Peviani M.; Curti D.; et al. Studies on the Enantiomers of RC-33 as Neuroprotective Agents: Isolation, Configurational Assignment, and Preliminary Biological Profile. Chirality 2013, 25, 814–822. 10.1002/chir.22223. [DOI] [PubMed] [Google Scholar]
- Listro R.; Boiocchi M.; Della Volpe S.; Pignataro L.; Rossi D.; Rossino G.; Vasile F.; Collina S.. Assignment of the absolute configuration of a series of biologically-interesting 2,3-disubstituted-δ-lactams. Chirality, unpublished work. [Google Scholar]
- Toda M.; Inoue Y.; Mori T. Circular Dichroisms of Mono- and Dibromo[2.2]Paracyclophanes: A Combined Experimental and Theoretical Study. ACS Omega. 2018, 3, 22–29. 10.1021/acsomega.7b01642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M.; Meyer B. Characterization of Ligand Binding by Saturation Transfer Difference NMR Spectroscopy. Angew. Chem., Int. Ed. 1999, 38, 1784–1788. . [DOI] [PubMed] [Google Scholar]
- Viegas A.; Manso J.; Nobrega F. L.; Cabrita E. J. Saturation-Transfer Difference (STD) NMR: A Simple and Fast Method for Ligand Screening and Characterization of Protein Binding. J. Chem. Educ. 2011, 88, 990–994. 10.1021/ed101169t. [DOI] [Google Scholar]
- Sattin S.; Panza M.; Vasile F.; Berni F.; Goti G.; Tao J.; Moroni E.; Agard D.; Colombo G.; Bernardi A. Synthesis of Functionalized 2-(4-Hydroxyphenyl)-3-Methylbenzofuran Allosteric Modulators of Hsp90 Activity. Eur. J. Org. Chem. 2016, 20, 3349–3364. 10.1002/ejoc.201600420. [DOI] [Google Scholar]
- Guzzetti I.; Civera M.; Vasile F.; Arosio D.; Tringali C.; Piarulli U.; Gennari C.; Pignataro L.; Belvisi L.; Potenza D. Insights into the Binding of Cyclic RGD Peptidomimetics to A5β1 Integrin by Using Live-Cell NMR And Computational Studies. ChemistryOpen 2017, 6, 128–136. 10.1002/open.201600112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco S.; Tailford L. E.; Juge N.; Angulo J. Differential Epitope Mapping by STD NMR Spectroscopy To Reveal the Nature of Protein–Ligand Contacts. Angew. Chem., Int. Ed. 2017, 56, 15289–15293. 10.1002/anie.201707682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.