Abstract
The scope of the acid-mediated 3-component synthesis of thiadiazines was investigated. A selective functionalization of the six-membered heterocyclic core structure was accomplished by sequential alkylations, saponifications, and coupling reactions. Several new analogs of a dihydropyrimidinone Hsp70 chaperone agonist, MAL1-271, showed promising activity in a cell based model of Huntington’s disease.
Keywords: Thiadiazine, Hsp70, MAL1-271, Huntington’s disease, molecular chaperone
Sulfamide-based heterocycles are attractive synthetic targets in medicinal chemistry; while they have a wide variety of biological activities, they have been relatively neglected in SAR studies, in part due to a dearth of synthetic methods, and therefore cyclic sulfamides still offer considerable opportunities in patent space.1−4 In addition to their function as urea bioisosteres,5 agents containing these building blocks have been shown to exhibit antibacterial,6,7 opioid receptor like-1 receptor (ORL1, NOP),8 colony stimulating factor-1 (CSF-1, implied in rheumatoid arthritis and metastatic bone cancer),9 and 11β-HSD1 (a target for type 2 diabetes) inhibitory activities.10 A subclass of sulfamide-containing heterocycles, 1,2,6-thiadiazine 1,1-dioxides, has been shown to act as cannabinoid agonists and antagonists11 and display modest antimicrobial activity,12 smooth muscle relaxation,13 and sedative effects.14 Additionally, the structurally related 2,1,3-benzothiadiazine 2,2-dioxides, such as the commercial herbicide bentazon, have demonstrated herbicidal activity.15
The preparation of 1,2,6-thiadiazine 1,1-dioxides was first realized using an acid-mediated condensation of sulfamide and monoketones16,17 or β-diketones.18 Alternatively, functionalized thiadiazines have been prepared by base-mediated intramolecular cyclizations of sulfaminomethylene derivatives,19,20 condensation with substituted sulfamides and ethyl 3,3-diethoxypropanoate (1),21 condensation of sulfamide imines and 1,22 and the intramolecular Friedel–Crafts acylation of sulfamide iminium species.23 More recently, thiadiazines were prepared by joining an N,N’-dibenzylated sulfamide with 2-(acetoxymethyl)buta-2,3-dienoate24 and by a silver- and gold-catalyzed hydroamination of propargyl sulfamides;25 but, overall, there is a surprising lack of 1,2,6-thiadiazine 1,1-dioxides with carboxylic acid substituents in the 4-position in the literature.
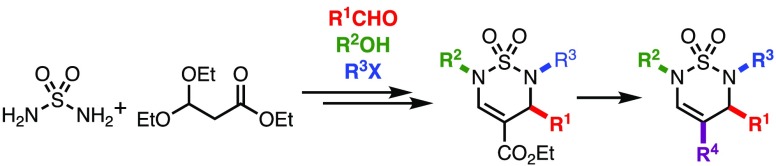
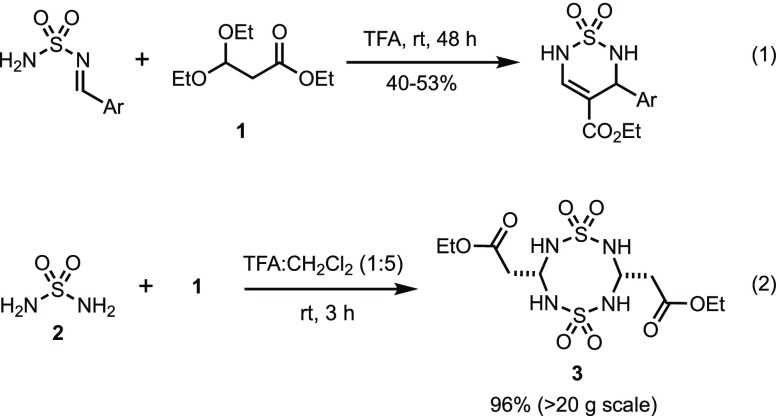
As part of our interest in the synthesis of novel heterocyclic compounds by multicomponent condensations (MCCs),26,27 we envisioned 1,2,6-thiadiazine 1,1-dioxides to become readily available by a Biginelli-like MCC and represent versatile scaffolds wherein the core heterocycle could be functionalized at several positions. Specifically, we wanted to explore if thiadiazine 1,1-dioxides could serve as bioisosteric analogs of Biginelli dihydropyrimidinones such as MAL1-271, an agonist of Hsp70 that reduces protein aggregation associated with neurodegenerative diseases.28 The thiadiazine 1,1-dioxide scaffold offers an attractive option to expand the hydrogen bond acceptor carbonyl moiety in the planar pyrimidinone urea moiety into three dimensions, as well as facilitate alkylation reactions for structure–activity relationship (SAR) purposes. We envisioned that a variety of novel thiadiazines could be prepared by selective N-alkylations29 followed by functional group interconversions of the 4-carboxylate ester. To test this hypothesis, we set out to synthesize the thiadiazine 1,1-dioxide core, initially using literature conditions.21,22 However, the use of neat TFA as a solvent required long reaction times and gave inconsistent yields in our hands (Scheme 1, eq 1). As a result, we initiated a search for optimal thiadiazine formation conditions. After considerable experimentation, we found that condensation of sulfamide (2) with 1 in a 1:5 mixture of TFA and CH2Cl2 resulted in the formation of stable, crystalline 8-membered ring dimer 3(30) after 3 h at room temperature (Scheme 1, eq 2). The unusual 8-membered ring structure and cis-configuration of dithiatetrazocane 3 was assigned based on an X-ray structure analysis (Figure 1). Notably, there are very few compounds of similar connectivity in the literature.31−33
Scheme 1. Literature Precedent for Thiadiazine 1,1-Dioxide Formation (Eq 1) and Preparation of Dithiatetrazocane 3 (Eq 2).
Figure 1.
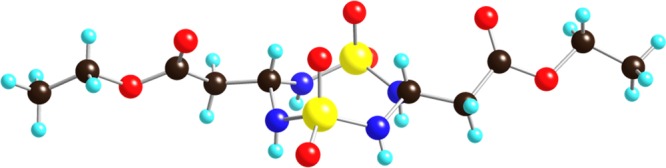
X-ray structure of 1,1,5,5-tetraoxido-1,5,2,4,6,8-dithiatetrazocane-3,7-diyl)diacetate 3 (CCDC 1972400).
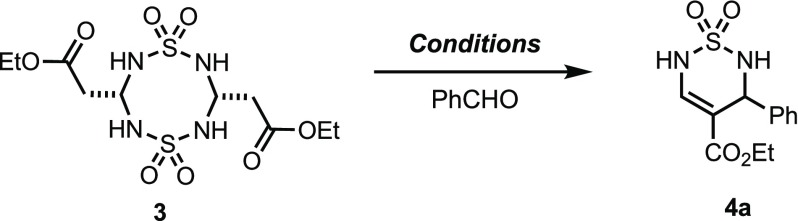
Condensation of 3 with benzaldehyde in a 1:1 mixture of TFA and CH2Cl2 provided the desired thiadiazine 4a in 61% yield (Table 1, entry 1). We also explored alternative acidic conditions that were milder and provided 4a in a higher yield. Polyphosphate ester (PPE),34 BF3·Et2O, triflamide, anhydrous HCl, methanesulfonic acid, and TFA:CH2Cl2 (1:5) yielded thiadiazine 4a in lower or comparable yields (entries 2–7). When the quantity of TFA was reduced to 10 mol equiv, product was obtained in 59% yield (entry 8). Further reduction of TFA to 2.5 mol equiv was sufficient to obtain 4a in 57% yield if the reaction concentration was increased to 0.5 M and the mixture was heated to 40 °C for 30 h (entry 9). Due to the limited solubility of the sulfamide dimer 3 in CH2Cl2 and our desire to increase the reaction rate, the solvent was changed to hexafluoroisopropanol (HFIP). We envisioned this non-nucleophilic alcohol with its remarkable hydrogen bond donor/acceptor capabilities would increase the dissolution of 3 and stabilize ionic intermediates, thus improving the conversion rate and product yield. However, the use of HFIP as a solvent in the presence of 2.5 equiv of TFA provided a modest decrease of the reaction time while producing 4a in comparable yields (entry 10).
Table 1. Optimization of Thiadiazine 4a Formation from 3.
| Entry | Conditions | Yielda |
|---|---|---|
| 1 | TFA:CH2Cl2 (1:1), rt, 30 min | 61% |
| 2 | PPE, THF, reflux, 40 min | 45% |
| 3 | BF3·OEt2 (2 equiv), CH2Cl2, rt, 6 h | 65% |
| 4 | 10% Tf2NH, CH2Cl2, rt, 2.5 h | 42% |
| 5 | 4 M HCl (10 equiv), dioxane, rt, 14 h | 47%b |
| 6 | MeSO3H (5.7 equiv), CH2Cl2, 0 °C, 40 min | 39% |
| 7 | TFA:CH2Cl2 (1:5), rt, 3 h | 61% |
| 8 | TFA (10 equiv), CH2Cl2, rt, 60 h | 59% |
| 9 | TFA (2.5 equiv), CH2Cl2, 40 °C, 30 h | 57%c |
| 10 | TFA (2.5 equiv), HFIP, 35–40 °C, 17 h | 66%d |
Isolated yield after chromatography on SiO2.
Isolated in 85% purity.
Reaction was performed at 0.51 M.
Reaction was performed at 0.50 M.
Reaction in the absence of TFA led to the recovery of 81% of 3.
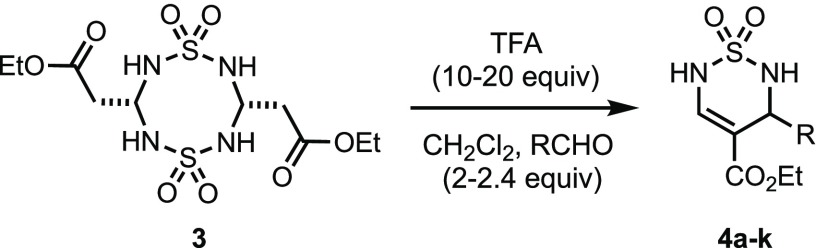
Based on these optimizations, we selected 10–20 mol equiv of TFA in a solution of CH2Cl2 for further investigations of the scope of compatible aldehydes in the thiadiazine 1,2-dioxide formation with 3 (Table 2). Aliphatic aldehydes (entries 2–3), as well as electron deficient (entries 4–8) and electron-rich aryl aldehydes (entries 9–10), provided the cyclocondensation products 4a–4j in 40–70% yield. The heterocyclic thiophene-3-carboxaldehyde provided 4k in a modest 30% yield (entry 11). Other heterocyclic aldehydes (furans, quinolines, and pyridines) resulted in the formation of complex mixtures and were not further analyzed.
Table 2. Thiadiazine Formation with 3 and Various Aldehydes.
| Entry | R | 4a–j | Yielda |
|---|---|---|---|
| 1 | Ph | 4a | 66%b |
| 2 | Me | 4b | 59% |
| 3 | Et | 4c | 56% |
| 4 | 2,4-Cl2C6H3 | 4d | 70% |
| 5 | 4-NCC6H4 | 4e | 41% |
| 6 | 4-MeCO2C6H4 | 4f | 57% |
| 7 | 4-CF3C6H4 | 4g | 65% |
| 8 | 2-BrC6H4 | 4h | 45% |
| 9 | 4-AcOC6H4 | 4i | 48% |
| 10 | 3-MeOC6H4 | 4j | 62% |
| 11 | 3-thiophene | 4k | 30% |
Isolated yield after chromatography on SiO2.
Reaction was performed using TFA (2.5 equiv), HFIP, 35–40 °C, 17 h.
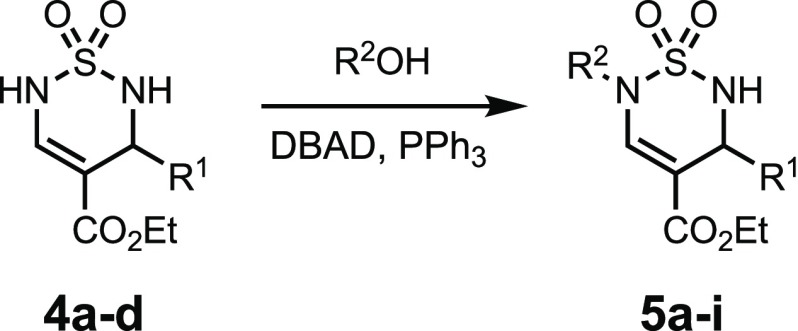
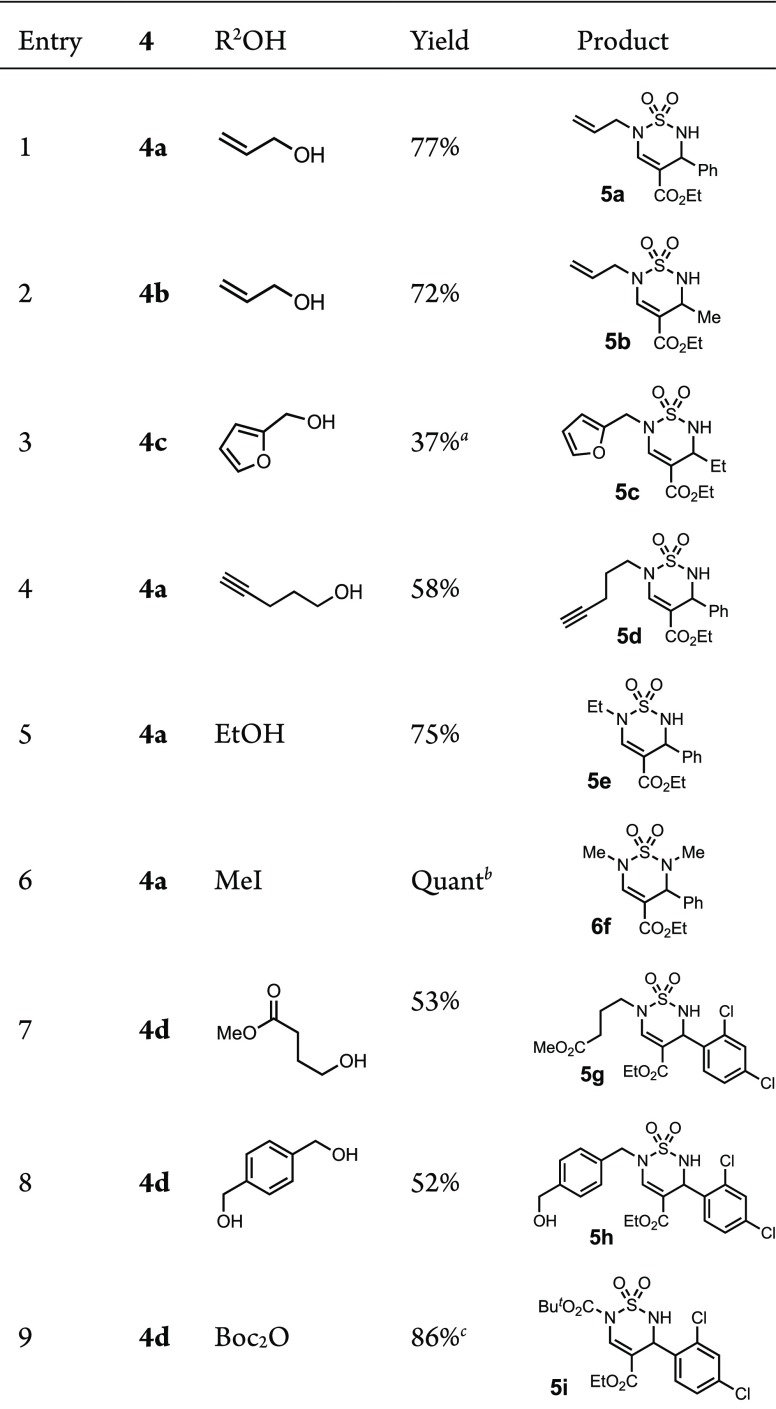
Next, we examined the possibility of regioselective sequential N-alkylation of the two sulfamide nitrogens by exploiting their inherent difference in acidity (pKa1 ca. 9.2 vs pKa2 ca. 9.5; that is, the vinylogous carbamate sulfamide N(6)-H is calculated to be slightly more acidic)35 as well as their steric environment. Treatment of thiadiazine 4a with NaH followed by allyl iodide led to a mixture of mono- and dialkylated products. In contrast, Mitsunobu36 conditions with allyl alcohol using DBAD led to a selective (N)6-monoalkylation of thiadiazines 4a and 4b in good yields (Table 3, entries 1–2). The regiochemistry was determined by NOESY correlations between the methylene hydrogens of the allyl group and the hydrogen of the thiadiazine alkene. Furanylmethanol required a change of the dialkylazodicarboxylate to DEAD, which simplified the purification (entry 3). Simple or functionalized alkyl alcohols also gave good conversions (entries 4, 5, and 7). While the yield was slightly lower with 1,4-phenylenedimethanol, monoalkylated product 5h was readily isolated (entry 8), and a Boc-protection was also highly selective and generated thiadiazine 1,2-dioxide 5i in 86% yield (entry 9). A symmetrical dialkylation was straightforward by treating 4a with an excess of MeI in the presence of K2CO3 to give 6f in excellent yield (entry 6).
Table 3. Regioselective N(6)-Alkylation of Thiadiazines 4a–d.
DEAD was used in place of DBAD.
4a was treated with MeI (5 equiv), K2CO3, and MeCN.
Reaction with 4d (1.1 equiv), Boc2O (1 equiv), and K2CO3 (2.5 equiv).
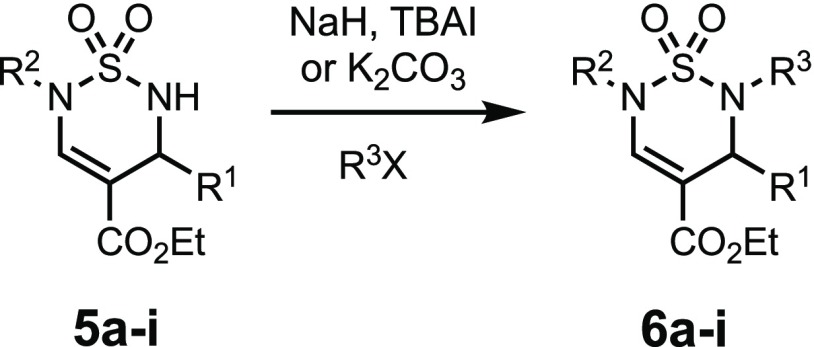
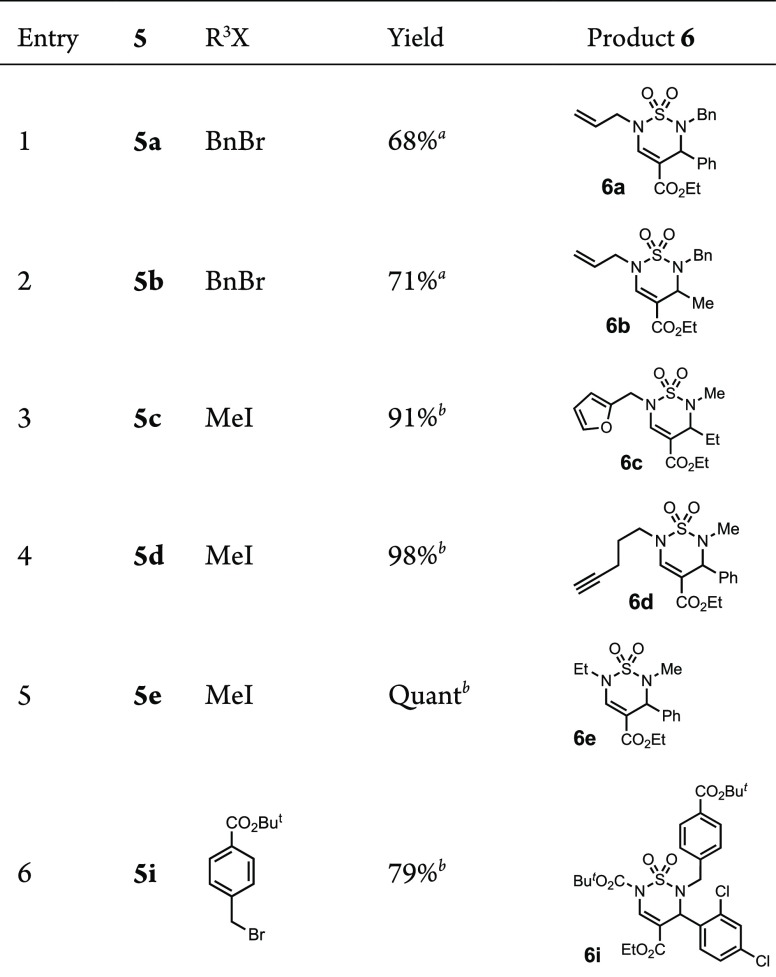
The alkylation of the thiadiazine N(2) amide was investigated next. Benzylations of 5a and 5b were accomplished in the presence of NaH and TBAI to provide 6a and 6b in 68 and 71% yield, respectively (Table 4, entries 1–2).
Table 4. N(2)-Alkylation of Thiadiazines 5.
NaH, TBAI, THF.
K2CO3, MeCN.
N-Methylations of 5c–e were achieved with K2CO3 in MeCN and produced 6c–e in high yields (Table 4, entries 3–5). An ester-functionalized benzyl bromide was similarly and successfully introduced to generate the Boc-protected diester 6i (entry 6).
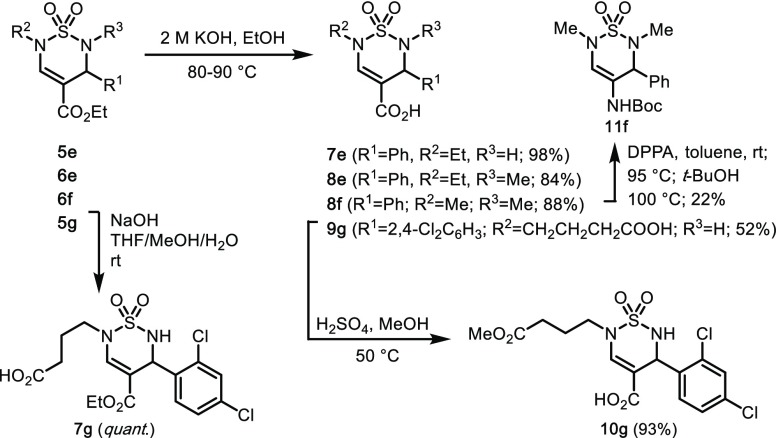
For additional chemical scaffold diversifications, we focused on selective conversions of the C(4)-esters (Scheme 2). Initial attempts at a Lewis acid mediated transesterification, or a mild hydrolysis using TMSOK or Bu3SnOH, were unsuccessful. Gratifyingly, ester hydrolysis was achieved by heating 5e, 6e, 6f, and 5g in 2 M KOH in EtOH to provide acids 7e, 8e, 8f, and 9g, respectively. Under milder conditions with NaOH in THF, MeOH, and water at room temperature, the aliphatic carboxylate in 5g was saponified selectively, and 7g was isolated in quantitative yield. Furthermore, diacid 9g could be selectively re-esterified to the monomethyl ester 10g under Fischer conditions, thus allowing for a regiospecific conversion of the carboxylate functional groups in diester 5g. Finally, a Curtius rearrangement of thiadiazine 8f with DPPA37 afforded the tert-butyl carbamate 11f, providing the first entry to this unprecedented thiadiazine 1,1-dioxide substitution pattern.
Scheme 2. Saponification of Mono- and Dialkylated Thiadiazines and Curtius Rearrangement of Carboxylate 8f.
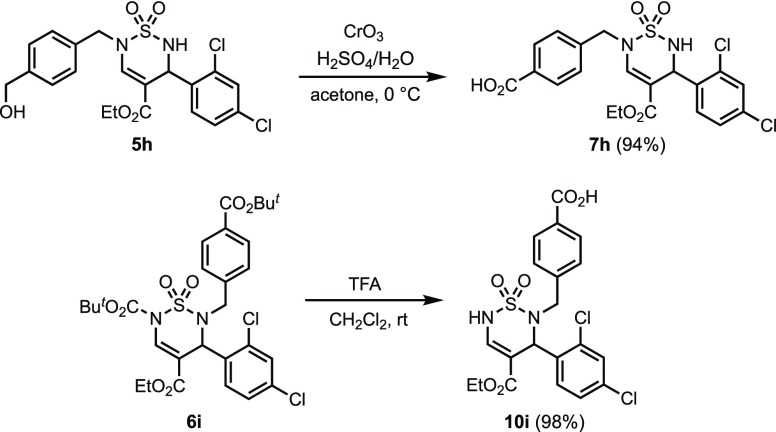
Jones oxidation of the side chain alcohol in 5h provided benzoic acid 7h in 94% yield, and treatment of 6i with TFA generated the regioisomeric benzoate 10i with concomitant removal of the Boc-group (Scheme 3). These transformations added additional versatility and valuable sites for diversifications to the collection of thiadiazine 1,1-dioxide building blocks.
Scheme 3. Selective Formations of Monoacid Thiadiazine 1,1-Dioxides.
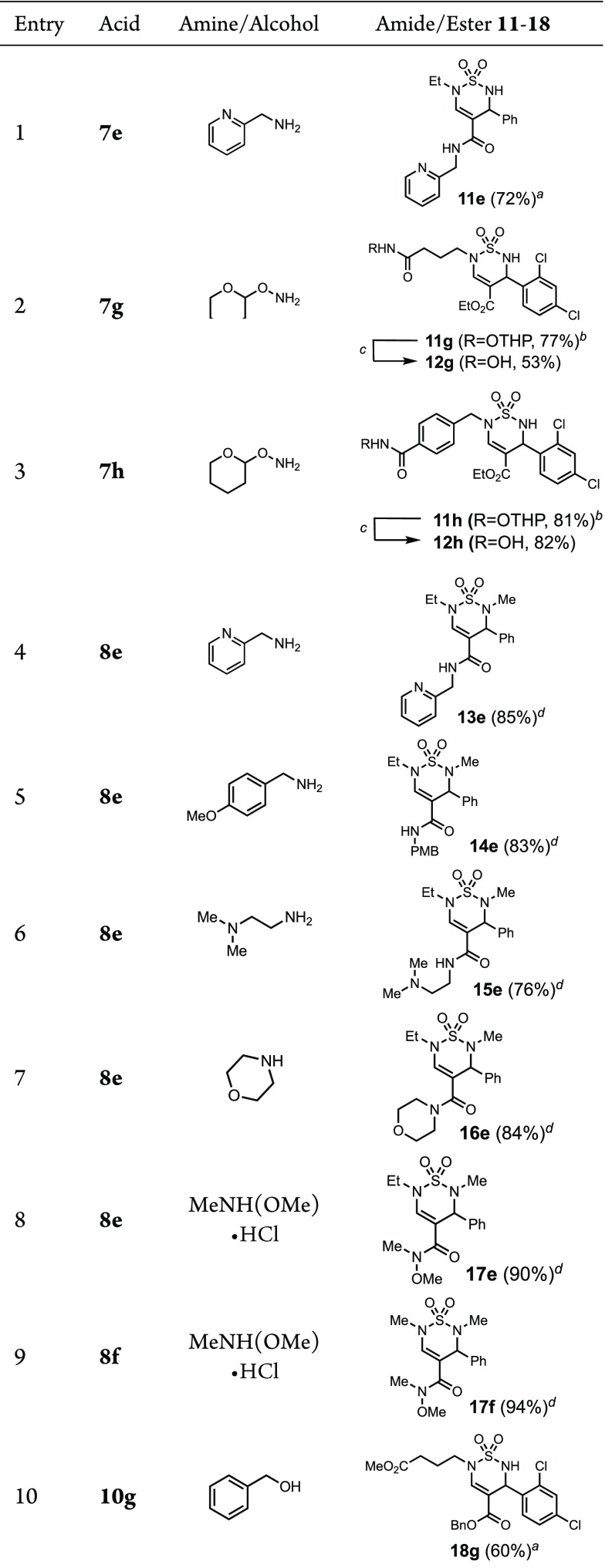
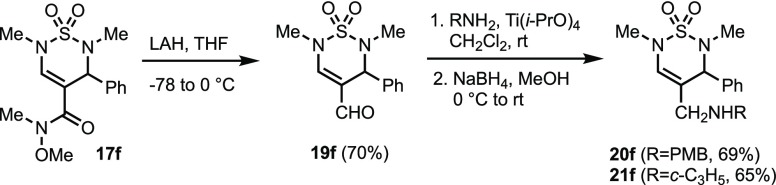
In order to demonstrate the utility of these building blocks for the preparation of bioactive screening samples, we generated a series of amide and ester analogs and subjected them to a representative biological assay. Amide bond formation using PyBOP and DIPEA, or EDCI, DMAP, and DIPEA, with pyridinyl methanamine proceeded in good yield with thiadiazines 7e and 8e to give 11e and 13e (Table 5, entries 1 and 4). Hydroxamic acids 12g and 12h were obtained by coupling of carboxylic acids 7g and 7h, respectively, with THP-protected hydroxylamine in the presence of T3P and TEA, followed by cleavage of the THP group with Amberlyst-15 resin (entries 2 and 3). p-Methoxybenzylamine, N,N-dimethylethylenediamine, and morpholine yielded amides 14e, 15e, and 16e (entries 5–7). The formation of hydroxamic esters 17e and 17f and benzyl ester 18g also occurred in moderate to high yield (entries 8–10). Furthermore, methyl hydroxamate 17f was selectively reduced to the aldehyde 19f (Scheme 4). We anticipated that this aldehyde would allow access to secondary amines by reductive amination. While one-pot imine formation–reduction conditions were unsuccessful, sequential imine formation using Ti(i-PrO)4 followed by reduction with NaBH4 provided amines 20f and 21f in 69% and 65% overall yield from 19f.
Table 5. Amidation and Esterification of Acids 7, 8, and 10.
Coupling with EDCI, DMAP, DIPEA.
T3P and TEA.
Amberlyst-15, MeOH, rt.
coupling with PyBOP, DIPEA
Scheme 4. Reduction of Hydroxamide 17f and Reductive Amination of Aldehyde 19f.
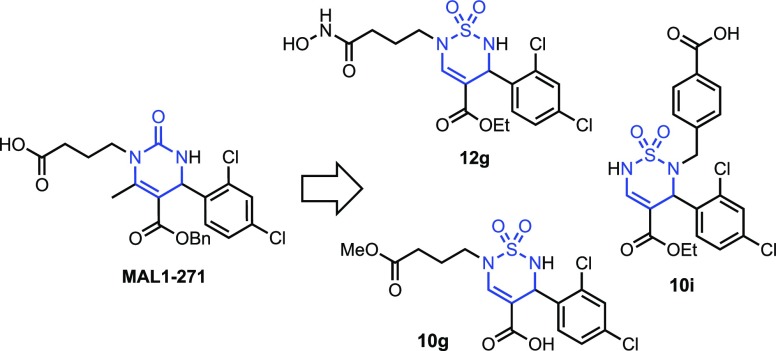
After developing a versatile strategy and reaction conditions for the preparation and sequential functionalization of thiadiazine 1,1-dioxides, we investigated our hypothesis that this heterocyclic core could be a suitable replacement for a dihydropyrimidine-2-one and show similar efficacy in a model of neurodegenerative disease.38,39 Therefore, ten structurally related analogs of the Biginelli product MAL1-271 were selected for a cell-based screen in a Huntington’s disease (HD) model (Figure 2).
Figure 2.
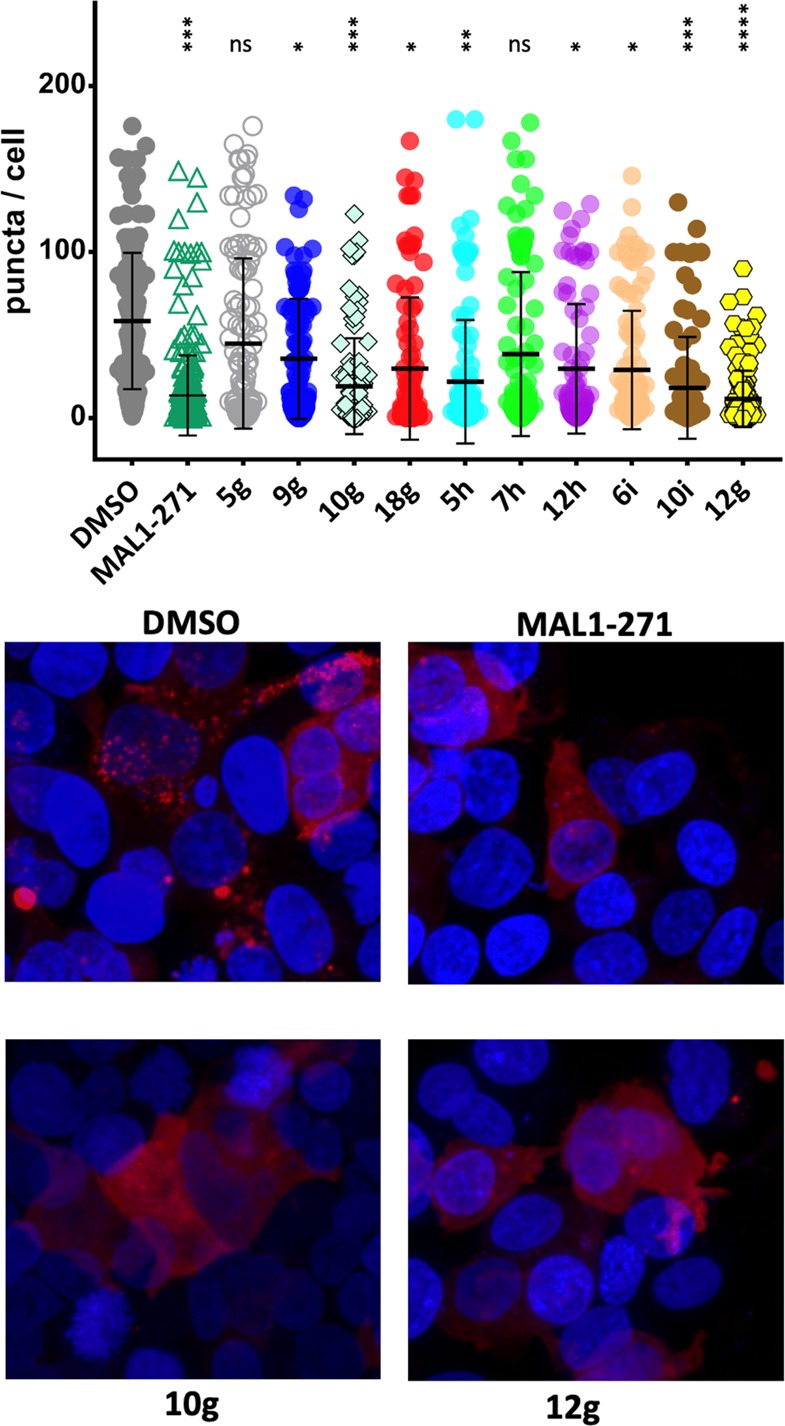
HEK293H cells were transfected with 4 μg of an HTT17Q-mCherry construct,41 and 24 h after transfection cells were treated with 10 μM compound or DMSO for 6 h. Top panel: number of puncta per cell. Statistically significant differences between control and treated samples are indicated by asterisks. * p < 0.05; ** p < 0.005; *** p < 0.0005; **** p < 0.00005 compared to the DMSO control. Bottom panel: representative cell images for negative control (DMSO), positive control (MAL1-271), and analogs 10g and 12g. Toxic aggregates are shown as red dots. See SI for additional information.
HD is an ultimately fatal neurodegenerative disorder that is caused by a polyglutamine repeat expansion in the Huntingtin protein (HTT). Studies in model systems indicate that Hsp70 overexpression reduces the cellular levels of toxic HTT aggregates, and in animals Hsp70 induction can even suppress some of the negative consequences of polyglutamine expansion.40 Based on the fact that MAL1-271 functions as an Hsp70 agonist, we examined ten diverse analogs, i.e. 5g, 5h, 6i, 7h, 9g, 10g, 10i, 12g, 12h, and 18g, for their ability to blunt the formation of toxic aggregates in HEK293 cells that express an HTT exon containing 17 glutamine repeats. Among these compounds, 5g, 9g, 10g, 12g, and 18g show a closer structural resemblance to MAL1-271 than 5h, 6i, 7h, 10i, and 12h. We discovered that several analogs reduced the number of cellular puncta/aggregates compared to the DMSO control. Cells were stained for confocal microscope imaging with 4′,6-diamidino-2-phenylindole (DAPI), a fluorescent dye with high affinity to adenine–thymine rich DNA regions. A bright spot detection tool was used to identify and quantify the number of protein aggregates (“dots”) per cell.
Compared to the MAL1-271 positive control, 5g, 9g, 18g, 5h, 7h, 12h, and 6i were less effective (p < 0.0001), whereas 10g and 10i were equally effective (Figure 2). Thiadiazine 12g exhibited even a slightly greater effect on aggregate suppression than MAL1-271 (p < 0.05). Interestingly, the chemotype of 10g and 12g is closely related to MAL1-271, but 10i represents a novel heterocycle substitution pattern that can serve as a starting point for new structure–activity studies (Figure 3).
Figure 3.
Structures of Hsp70 agonist MAL1-271 and thiadiazine 1,1-dioxide analogs that showed similar activity in the HD model assay. The respective Biginelli (dihydropyrimidinone) and thiadiazine scaffolds are highlighted in blue.
It is interesting to note that 12g is a hydroxamic acid analog of MAL1-271; in order to address the possibility that 12g or another analog exerted antiaggregation effects due to inhibition of a histone deacetylase (HDAC),42 we counter-screened actives 10g, 10i, 12g, and 12h (negative control) against HDAC 1–8 (Table 1 in the Supporting Information). None of the active compounds, in particular not even the hydroxamic acid 12g, displayed significant HDAC 1–6 inhibition at 0.1–1 μM concentrations. Only hydroxamic acid 12h showed 40% inhibition of HDAC 7 at 1 μM, and all compounds showed moderate inhibition (35–60%) of HDAC 8 at 1 μM concentration in the assay. The absence of a clear correlation between HDAC inhibition and activity in the HD assay for hydroxamates 12g and 12h suggests that the active hit compound 12g does not reduce cellular HTT aggregates due to direct HDAC inhibition. Moreover, HDAC6, which has been implicated in heat shock protein gene expression,43 was also not inhibited by hydroxamates 12g and 12h at 0.2 μM concentration. However, since the biochemical assays at higher concentrations were prevented by low aqueous solubility, we cannot exclude the possibility of some HDAC inhibition in HEK293H cells at 10 μM concentration.
In summary, we have developed a versatile strategy for the preparation and selective functionalization of thiadiazine 1,1-dioxides, a relatively rare heterocycle that has previously been underutilized in medicinal chemistry screening campaigns. In addition, we have demonstrated the utility of this scaffold as a potential biomimetic of the privileged Biginelli heterocycle, the dihydropyrimidinone. The identification of active analogs of the Hsp70 agonist dihydropyrimidinone MAL1-271, i.e. thiadiazines 10g, 10i, and 12g, in a relevant cell based biological assay highlights the potential application of thiadiazine 1,1-dioxides in hit identification in general, and specifically in Huntington’s disease and perhaps other neurodegenerative diseases associated with the accumulation of toxic protein aggregates.
Acknowledgments
The authors thank T. S. Maskrey, I. Mager, and A. Cervi (University of Pittsburgh) for project management (TSM) and technical assistance (TSM, IM, AC), S. J. Geib (University of Pittsburgh) for the X-ray analysis of 3, P. Needham (University of Pittsburgh) for technical assistance with the HD assay, and U. Pandey (University of Pittsburgh) and Alice Migazzi (University of Trento) for reagents for the HD assay.
Glossary
Abbreviations
- DAPI
4′,6-diamidino-2-phenylindole
- DBAD
di-tert-butyl azodicarboxylate
- DEAD
diethyl azodicarboxylate
- DIPEA
diisopropylethylamine
- DMAP
4-dimethylaminopyridine
- DPPA
diphenylphosphoryl azide
- EDCI
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide
- HD
Huntington’s disease
- HDAC
histone deacetylase
- HFIP
hexafluoroisopropanol
- PPE
polyphosphate ester
- HSF1
heat shock factor 1
- Hsp70
heat shock protein 70 kDa
- HTT
Huntingtin protein
- PyBOP
benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate
- TBAI
tetrabutylammonium iodide
- TEA
triethylamine
- T3P
propanephosphonic acid anhydride
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00018.
Experimental details and 1H and 13C NMR spectra for new synthetic intermediates and products. Assay information. (PDF)
Accession Codes
CCDC 1972400 contains the supplementary crystallographic data for compound 3 in this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: + 44 1223 336033.
Author Present Address
⊥ Instrumental Analysis Center, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240, China.
Author Contributions
∥ L. Terrab, C. J. Rosenker, and L. Johnstone contributed equally to this work and should be considered cofirst authors. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This work was supported in part by grants P50 GM067082, R35 GM131732, and P30 DK79307 from the National Institutes of Health and a generous donation from the Moravitz family.
The authors declare no competing financial interest.
Dedication
§ Dedicated to Prof. Maurizio Botta (University of Siena) in gratitude for his friendship and in appreciation of his scholarship.
Supplementary Material
References
- Lawson A.; Tinkler R. B. Chemistry of Thiadiazole and Thiadiazine S-Oxides. Chem. Rev. 1970, 70, 593–618. 10.1021/cr60267a004. [DOI] [PubMed] [Google Scholar]
- Petersen H. Syntheses of Cyclic Ureas by α-Ureidoalkylation. Synthesis 1973, 1973, 243–292. 10.1055/s-1973-22190. [DOI] [Google Scholar]
- McDermott S. D.; Spillane W. J. Synthesis and Reactions of Sulfamides. A Review. Org. Prep. Proced. Int. 1984, 16, 49–77. 10.1080/00304948409356167. [DOI] [Google Scholar]
- Gazieva G. A.; Kravchenko A. N.; Lebedev O. V. Sulfamides in the Synthesis of Heterocyclic Compounds. Russ. Chem. Rev. 2000, 69, 221–230. 10.1070/RC2000v069n03ABEH000562. [DOI] [Google Scholar]
- Ghosh A. K.; Brindisi M.. Urea Derivatives in Modern Drug Discovery and Medicinal Chemistry. J. Med. Chem. 2019, 10.1021/acs.jmedchem.9b01541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. J.; Jung M.-H.; Yoo K. H.; Cho J.-H.; Oh C.-H. Synthesis and Antibacterial Activities of Novel Oxazolidinones Having Cyclic Sulfonamide Moieties. Bioorg. Med. Chem. Lett. 2008, 18, 5815–5818. 10.1016/j.bmcl.2008.09.034. [DOI] [PubMed] [Google Scholar]
- Kim S. J.; Cho J.-H.; Oh C.-H. Novel 1β-Methylcarbapenems Having Cyclic Sulfonamide Moieties: Synthesis and Evaluation of in-Vitro Biological Activity - Part II. Arch. Pharm. 2009, 342, 528–532. 10.1002/ardp.200800226. [DOI] [PubMed] [Google Scholar]
- Palin R.; Clark J. K.; Evans L.; Feilden H.; Fletcher D.; Hamilton N. M.; Houghton A. K.; Jones P. S.; McArthur D.; Montgomery B.; Ratcliffe P. D.; Smith A. R. C.; Sutherland A.; Weston M. A.; Wishart G. Rapid Access Towards Follow-up NOP Receptor Agonists Using a Knowledge Based Approach. Bioorg. Med. Chem. Lett. 2009, 19, 6441–6446. 10.1016/j.bmcl.2009.09.028. [DOI] [PubMed] [Google Scholar]
- Wilson K. J.; Illig C. R.; Chen J.; Wall M. J.; Ballentine S. K.; Des Jarlais R. L.; Chen Y.; Schubert C.; Donatelli R.; Petrounia I.; Crysler C. S.; Molloy C. J.; Chaikin M. A.; Manthey C. L.; Player M. R.; Tomczuk B. E.; Meegalla S. K. Reducing Ion Channel Activity in a Series of 4-Heterocyclic Arylamide FMS Inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 3925–3929. 10.1016/j.bmcl.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Kim S. H.; Bok J. H.; Lee J. H.; Kim I. H.; Kwon S. W.; Lee G. B.; Kang S. K.; Park J. S.; Jung W. H.; Kim H. Y.; Rhee S. D.; Ahn S. H.; Bae M. A.; Ha D. C.; Kim K. Y.; Ahn J. H. Synthesis and Biological Evaluation of Cyclic Sulfamide Derivatives as 11β-Hydroxysteroid Dehydrogenase 1 Inhibitors. ACS Med. Chem. Lett. 2012, 3, 88–93. 10.1021/ml200226x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano C.; Goya P.; Paez J. A.; Giron R.; Sanchez E.; Martin M. I. Discovery of 1,1-Dioxo-1,2,6-Thiadiazine-5-Carboxamide Derivatives as Cannabinoid-Like Molecules with Agonist and Antagonist Activity. Bioorg. Med. Chem. 2007, 15, 7480–7493. 10.1016/j.bmc.2007.07.056. [DOI] [PubMed] [Google Scholar]
- Bhatt N.; Vyas K.; Joshi K.; Nimavat K. Synthesis and Biological Screening of Alkyl 2-(3,5-dimethyl-1,1-dioxido-2H-1,2,6-thiadiazin-4-yl)benzoate. Asian J. Biochem. Pharm. Res. 2011, 1, 464–469. [Google Scholar]
- Castro A.; Martinez A.; Cardelus I.; Llenas J. Dioxides of Bicyclic Thiadiazines: A New Family of Smooth Muscle Relaxants. Bioorg. Med. Chem. 1995, 3 (2), 179–185. 10.1016/0968-0896(95)00012-6. [DOI] [PubMed] [Google Scholar]
- Houlihan W. J.Dioxobenzothiadiazines. US Patent 3278532, 1966.
- Lamberth C. Pyrimidine Chemistry in Crop Protection. Heterocycles 2006, 68, 561–603. 10.3987/REV-05-604. [DOI] [Google Scholar]
- Degering E. F.; Wilson J. E. Derivatives of Sulfamide. J. Org. Chem. 1952, 17, 339–341. 10.1021/jo01137a001. [DOI] [Google Scholar]
- Ouchi A.; Moeller T. The Condensation Reaction between Sulfamide and Monoketones. J. Org. Chem. 1964, 29, 1865–1867. 10.1021/jo01030a048. [DOI] [Google Scholar]
- Wright J. B. The Reaction of Sulfamide with Alpha- and Beta-Diketones. The Preparation of 1,2,5-Thiadiazole 1,1-Dioxides and 1,2,6-Thiadiazine 1,1-Dioxides. J. Org. Chem. 1964, 29, 1905–1909. 10.1021/jo01030a059. [DOI] [Google Scholar]
- Goya P.; Stud M. Synthesis of 4-Substituted 3-Hydroxy- and 3-Amino-6H-1,2,6-Thiadiazine 1,1-Dioxides. J. Heterocycl. Chem. 1978, 15, 253–256. 10.1002/jhet.5570150214. [DOI] [Google Scholar]
- Hansen H.; Koenig K. H.; Rohr W. Synthesis and Reactions of Substituted 3-Oxo-1,2,6-Thiadiazine 1,1-Dioxides. Liebigs Ann. Chem. 1979, 1979, 950–958. 10.1002/jlac.197919790705. [DOI] [Google Scholar]
- Lee C. H.; Kohn H. Functionalized 5,6-Dihydro-2H-1,2,6-Thiadiazine 1,1-Dioxides. Synthesis, Structure and Chemistry. J. Heterocycl. Chem. 1990, 27, 2107–2111. 10.1002/jhet.5570270747. [DOI] [Google Scholar]
- Lee C. H.; Lee Y. H.; Choi W. S.; Chung B. Y. Synthesis of 4-Carbethoxy-5-Aryl-5,6-Dihydro-2H-1,2,6-Thiadiazine 1,1-Dioxides. Bull. Korean Chem. Soc. 1992, 13, 462–463. [Google Scholar]
- Lee C.-H.; Jin G. F.; Lim H. W.; Yang E. H.; Lee J.-D.; Nakamura H.; Ban H. S.; Kang S. O. Facile Synthesis of 4-Substituted 3,4-Dihydro-1H-2,1,3-Benzothiadiazine 2,2-Dioxides. Heteroat. Chem. 2011, 22, 192–197. 10.1002/hc.20670. [DOI] [Google Scholar]
- Li C.; Zhang Q.; Tong X. Amine-Catalyzed (3+n) Annulations of 2-(Acetoxymethyl)Buta-2,3-Dienoates with 1,n-Bisnucleophiles (n = 3–5). Chem. Commun. 2010, 46, 7828–7830. 10.1039/c0cc01966f. [DOI] [PubMed] [Google Scholar]
- Veguillas M.; Rosair G. M.; Bebbington M. W. P.; Lee A.-L. Silver Effect in Regiodivergent Gold-Catalyzed Hydroaminations. ACS Catal. 2019, 9, 2552–2557. 10.1021/acscatal.9b00249. [DOI] [Google Scholar]
- Maskrey T. S.; Frischling M. C.; Rice M. L.; Wipf P. A Five-Component Biginelli-Diels-Alder Cascade Reaction. Front. Chem. 2018, 6, 376. 10.3389/fchem.2018.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huryn D. M.; Brodsky J. L.; Brummond K. M.; Chambers P. G.; Eyer B.; Ireland A. W.; Kawasumi M.; LaPorte M. G.; Lloyd K.; Manteau B.; Nghiem P.; Quade B.; Seguin S. P.; Wipf P. Chemical Methodology as a Source of Small-Molecule Checkpoint Inhibitors and Heat Shock Protein 70 (Hsp70) Modulators. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 6757–6762. 10.1073/pnas.1015251108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrab L.; Wipf P.. Hsp70 and the Unfolded Protein Response as a Challenging Drug Target and an Inspiration for Probe Molecule Development. ACS Med. Chem. Lett. 2020, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For an example of a selective methylation, see:Goya P.; Martinez P.; Ochoa C.; Stud M. Regioselective N-Alkylation of 1,2,6-Thiadiazine 1,1-Dioxide Derivatives. J. Heterocycl. Chem. 1981, 18, 459–462. 10.1002/jhet.5570180304. [DOI] [Google Scholar]
- Goya P.; Stud M. Synthesis of 4-Substituted 3-Hydroxy- and 3-Amino-6H-1,2,6-Thiadiazine 1,1-Dioxides. J. Heterocycl. Chem. 1978, 15, 253–256. 10.1002/jhet.5570150214. [DOI] [Google Scholar]
- Dusemund J. Reaction of Sulfamide with Aldehydes and Acetals. Arch. Pharm. 1974, 307, 881–883. 10.1002/ardp.19743071113. [DOI] [PubMed] [Google Scholar]
- Lee C. H.; Kohn H. 3,7-Bis(Carboethoxy)Perhydro-1,5,2,4,6,8-Dithiatetrazocine 1,1,5,5-Tetroxide. Synthesis, Structure and Chemistry. Heterocycles 1988, 27, 2581–2588. 10.3987/COM-88-4618. [DOI] [Google Scholar]
- Lee C. H.; Kohn H. Intra- and Intermolecular α-Sulfamidoalkylation Reactions. J. Org. Chem. 1990, 55, 6098–6104. 10.1021/jo00312a013. [DOI] [Google Scholar]
- Cava M. P.; Lakshmikantham M. V.; Mitchell M. J. Synthesis of Caseadine Methyl Ether. J. Org. Chem. 1969, 34, 2665–2667. 10.1021/jo01261a039. [DOI] [Google Scholar]
- pKa and LogD values were calculated with Instant JChem 19.19.0, downloaded on 9/9/2019 (ChemAxon; http://www.chemaxon.com).
- Mitsunobu O.; Yamada M. Preparation of Esters of Carboxylic and Phosphoric Acid Via Quaternary Phosphonium Salts. Bull. Chem. Soc. Jpn. 1967, 40, 2380–2382. 10.1246/bcsj.40.2380. [DOI] [Google Scholar]
- Shioiri T.; Ninomiya K.; Yamada S. Diphenylphosphoryl Azide: A New Convenient Reagent for a Modified Curtius Reaction and for the Peptide Synthesis. J. Am. Chem. Soc. 1972, 94, 6203–6205. 10.1021/ja00772a052. [DOI] [PubMed] [Google Scholar]
- Chiang A. N.; Liang M.; Dominguez-Meijide A.; Masaracchia C.; Goeckeler-Fried J. L.; Mazzone C. S.; Newhouse D. W.; Kendsersky N. M.; Yates M. E.; Manos-Turvey A.; Needham P. G.; Outeiro T. F.; Wipf P.; Brodsky J. L. Synthesis and Evaluation of Esterified Hsp70 Agonists in Cellular Models of Protein Aggregation and Folding. Bioorg. Med. Chem. 2019, 27, 79–91. 10.1016/j.bmc.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick K.; Novoa J. A.; Hancock T.; Guerriero C. J.; Wipf P.; Brodsky J. L.; Segatori L. Chemical Induction of Hsp70 Reduces α-Synuclein Aggregation in Neuroglioma Cells. ACS Chem. Biol. 2013, 8, 1460–1468. 10.1021/cb400017h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. K.; Pratt W. B.; Lieberman A. P.; Osawa Y.. Targeting Hsp70 Facilitated Protein Quality Control for Treatment of Polyglutamine Diseases. Cell. Mol. Life Sci. 2019, 10.1007/s00018-019-03302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotti A.; Benner C.; Kerman B. E.; Gosselin D.; Lagier-Tourenne C.; Zuccato C.; Cattaneo E.; Gage F. H.; Cleveland D. W.; Glass C. K. Mutant Huntingtin Promotes Autonomous Microglia Activation Via Myeloid Lineage-Determining Factors. Nat. Neurosci. 2014, 17, 513–521. 10.1038/nn.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebzehnruebl F. A.; Raber K. A.; Urbach Y. K.; Schulze-Krebs A.; Canneva F.; Moceri S.; Habermeyer J.; Achoui D.; Gupta B.; Steindler D. A.; Stephan M.; Nguyen H. P.; Bonin M.; Riess O.; Bauer A.; Aigner L.; Couillard-Despres S.; Paucar M. A.; Svenningsson P.; Osmand A.; Andreew A.; Zabel C.; Weiss A.; Kuhn R.; Moussaoui S.; Blockx I.; Van der Linden A.; Cheong R. Y.; Roybon L.; Petersen A.; von Hoersten S. Early Postnatal Behavioral, Cellular, and Molecular Changes in Models of Huntington Disease Are Reversible by HDAC Inhibition. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, E8765–E8774. 10.1073/pnas.1807962115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet L.; Faure V.; Gilquin B.; Dufour-Guerin S.; Khochbin S.; Vourc’h C. HDAC6-Ubiquitin Interaction Controls the Duration of HSF1 Activation after Heat Shock. Mol. Biol. Cell 2014, 25, 4187–4194. 10.1091/mbc.e14-06-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.