Abstract
Rhodesain is an enzyme essential for the life of Trypanosoma brucei rhodesiense, a parasite causing a rapid-onset form of Human African Trypanosomiasis. RK-52 is a synthetic inhibitor of rhodesain, characterized by an impressive ksecond value (ksecond = 67000 × 103 M–1 min–1) and by a picomolar affinity toward the trypanosomal protease (Ki = 38 pM). Differently, curcumin, the golden multitarget nutraceutical obtained from Curcuma longa L., was proven to inhibit rhodesain noncompetitively with an IC50 of 7.75 μM. In the present study, we carried out studies of a combination of RK-52 and curcumin toward rhodesain, by applying the Chou and Talalay approach, which led us to obtain a combination index <1 for the most relevant fa values, which means a potent synergistic effect for the reduction of rhodesain activity from 40% to 99%.
Keywords: Rhodesain, Trypanosoma, drug combination studies, curcumin, synthetic inhibitor, synergism
Human African Trypanosomiasis (HAT) is a parasitic disease occurring in sub-Saharan Africa, inducing mortality despite a significant decrease of novel cases recently having been observed.1 HAT is induced by two subspecies of Trypanosoma: T. brucei gambiense, common in western and central Africa, able to cause a chronic HAT, and T. brucei rhodesiense, widespread in eastern and southern Africa, responsible for a rapid-onset high death rate HAT.2 In the hemolymphatic stage of HAT, the parasite invades the bloodstream and then the lymph nodes and spleen inducing illness, muscle aches, and fatigue. If the parasite crosses the blood–brain barrier (BBB) a neurological stage occurs characterized by sleep disorders and death.3 At present HAT therapy is based on only a few available drugs: pentamidine and suramin for the first-stage HAT, while the second-stage HAT is treated with eflornithine, melarsoprol, and nifurtimox. However, melarsoprol shows a high neurotoxicity, while eflornithine, whose use is safer, is active only against T. b. gambiense.4 Thus, the first-line therapy for the treatment of the second-stage HAT is based on the coadministration of nifurtimox and eflornithine; more recently, in 2018, fexinidazole was introduced in therapy because of its activity against T. brucei gambiense.5−7 Thus, new molecular targets have to be identified to develop innovative effective drugs for HAT therapy.
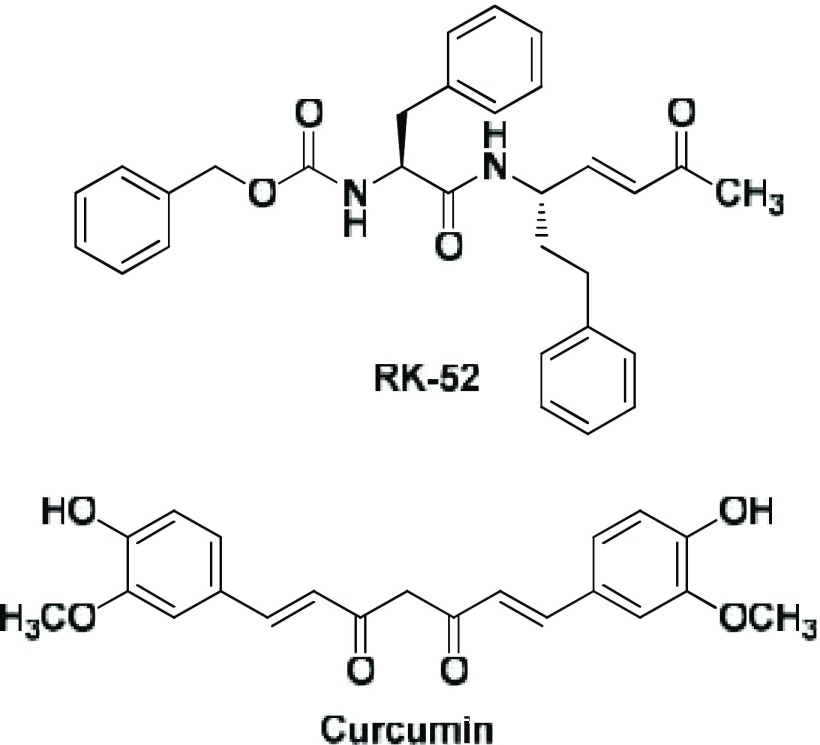
In this research area rhodesain, the key cysteine protease for the survival of T. brucei rhodesiense, is certainly a promising target to treat rhodesiense trypanosomiasis, the most lethal form of HAT.8,9 Rhodesain is essential to permit the parasite to cross the BBB reaching the central nervous system, thus inducing the second-stage HAT; it takes part in the turnover of variant surface glycoproteins of the trypanosome coat10 and in evasion of the host immune system by degrading host immunoglobulins.11 Moreover, rhodesain is predominant also in the lysosomes where it exerts its protease activity; thus, it is reasonable to consider the trypanosomal protease a promising target for HAT treatment.12 In this context, in the past decade our research team has been working on the discovery of new rhodesain inhibitors as a novel strategy for HAT therapy,13−20 leading to the identification of a potent irreversible rhodesain inhibitor, i.e. RK-52 (ksecond = 67000 × 103 M–1 min–1) (Figure 1), endowed with a picomolar affinity toward trypanosomal protease (Ki = 38 pM).21
Figure 1.
Structures of RK-52 and curcumin.
In the same field, we demonstrated also that curcumin (Figure 1), the golden multitarget nutraceutical obtained from Curcuma longa L., possesses an antitrypanosomal activity due to rhodesain inhibition (IC50 value of 7.75 μM).22
In this study, we now discuss studies of a combination of RK-52 and curcumin, by applying the Chou and Talalay approach,23,24 to evaluate if additive or synergistic effects occur in rhodesain inhibition.
RK-52 was synthesized as previously reported by our group.21 Curcumin was purchased from Sigma-Aldrich.
Rhodesain was obtained as previously described by Caffrey et al.25 Curcumin and RK-52 were then separately tested in three independent experiments, each performed in duplicate.
Both compounds were subjected to detailed assays at 7 concentrations, starting from the minimum dose required for the enzyme inhibition to that necessary to fully suppress rhodesain activity. More specifically, we used 0.5 μM, 0.1 μM, 0.05 μM, 0.025 μM, 0.010 μM, 0.005 μM, and 0.0025 μM for RK-52, while 100 μM, 80 μM, 60 μM, 40 μM, 20 μM, 10 μM, and 5 μM have been used for curcumin.
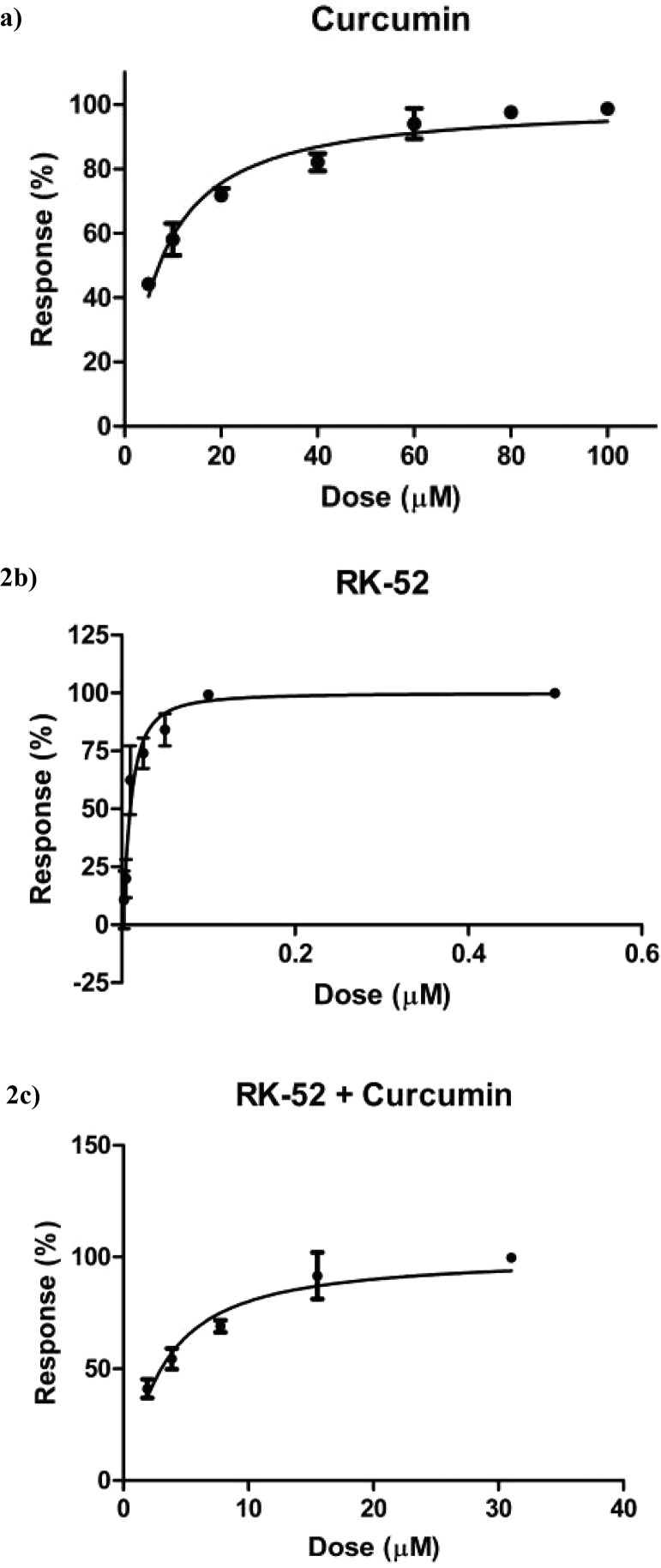
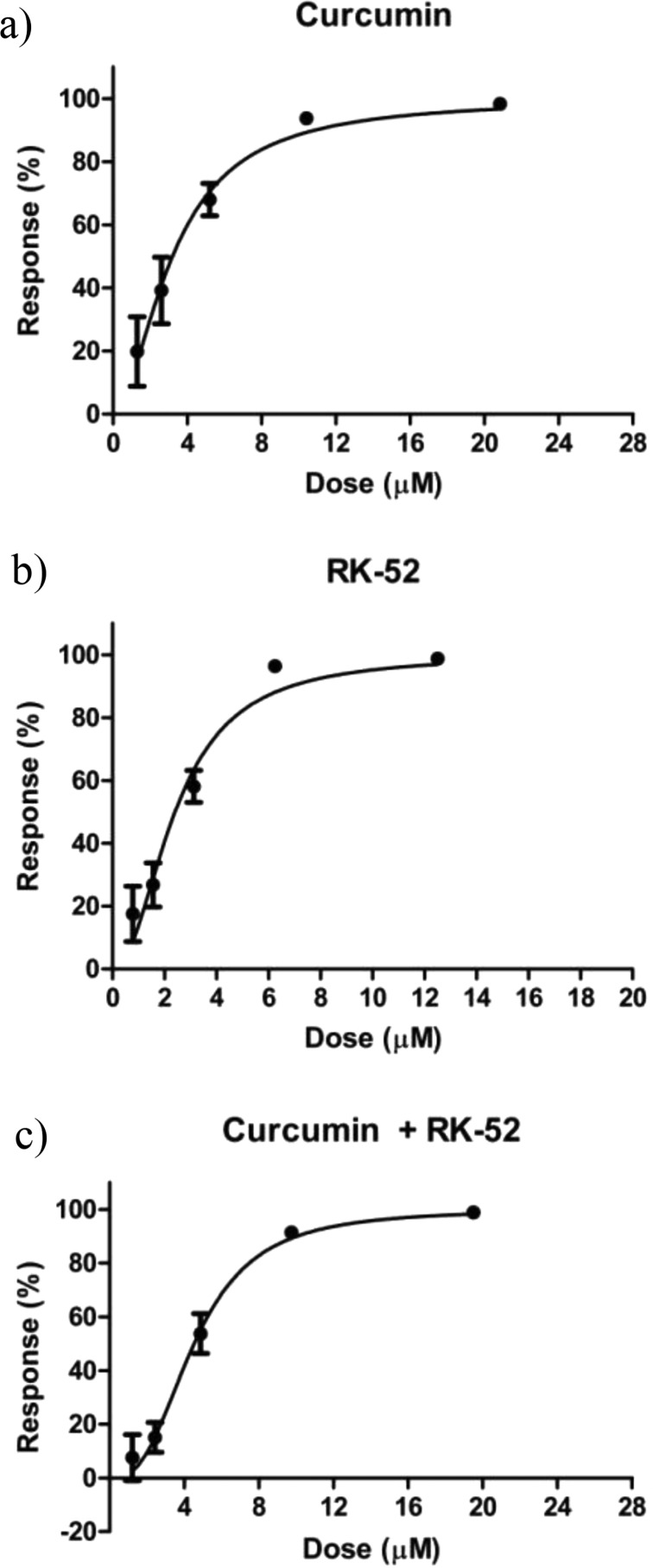
Biological evaluation against rhodesain was carried out using Cbz-Phe-Arg-AMC as fluorogenic substrate. IC50 values have been obtained from dose response-curves shown in Figure 2: 7.75 ± 1.53 μM for curcumin22 and 0.009 ± 0.0008 μM for RK-52. Subsequently, five data points for both compounds (1/4 × IC50, 1/2 × IC50, IC50, 2 × IC50, and 4 × IC50, Table 1) have been selected, with the aim to verify the possible synergistic, additive, or antagonist effect in the combination assay of the inhibitors. In this case, the combination of RK-52 and curcumin provided an IC50 value of 3.05 ± 0.21 μM.
Figure 2.
Dose response curves against rhodesain of curcumin22 (a), RK-52 (b), and RK-52 + curcumin in combination (c). Each experiment was performed three times, each in duplicate.
Table 1. Five Selected Doses for the Combination Experiments of Curcumin and RK-52.
| Five selected doses
(μM) |
|||||
|---|---|---|---|---|---|
| 0.25 × IC50 | 0.50 × IC50 | IC50 | 2 × IC50 | 4 × IC50 | |
| Curcumin | 1.93 | 3.87 | 7.75 | 15.5 | 31 |
| RK-52 | 0.00225 | 0.0045 | 0.009 | 0.018 | 0.036 |
| RK-52 + Curcumin | 0.00225 + 1.93 | 0.0045 + 3.87 | 0.009 + 7.75 | 0.018 + 15.5 | 0.036 + 31 |
The median effect equation described by Chou states that fa/fu = (D/Dm)m, where fa and fu represent the affected and the unaffected fractions, respectively, of the target enzyme, by the dose D; Dm corresponds to IC50, able to produce the median effect, while m represents the Hill-type coefficient, which means the sigmoidal trend (or S-shaped) of the dose-reponse curve.23,24
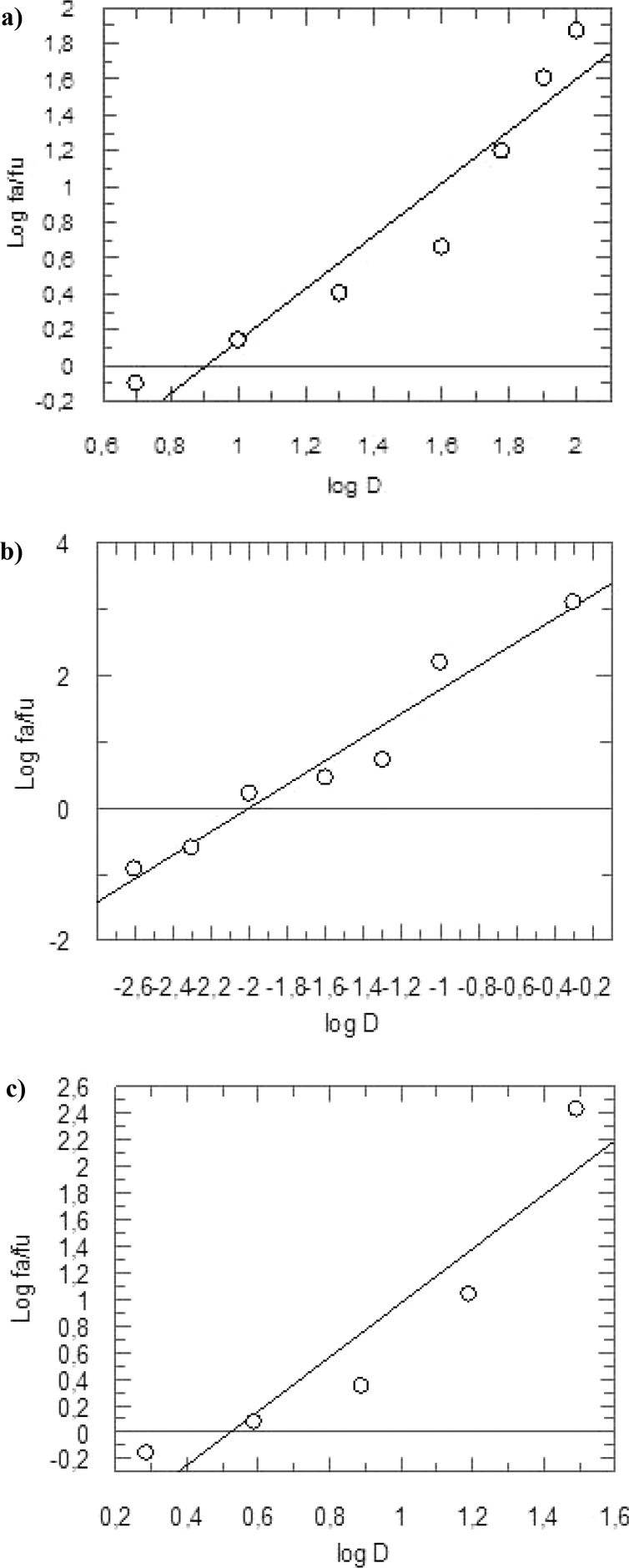
The median effect plot was generated plotting the three dose–response curves as log (fa/fu), on the y-axis, with respect to log (D), on the x-axis (Figure 3). This allowed us to obtain the IC50 values of the three different evaluations, together with the related m coefficients. In detail, curcumin showed IC50 = 7.75 μM and m1 = 1.4681, RK-52 IC50 = 0.009 μM and m2 = 1.7841 while for the combination assay we found IC50 = 3.05 μM and m1,2 = 2.0305, with a molar ratio RK-52/curcumin of 1:861.
Figure 3.
Median effect plot for curcumin22 (a), RK-52 (b), and the combination RK-52 + curcumin (1:861) (c). fa and fu are the affected and the unaffected fraction, respectively, of rhodesain activity at the doses D.
In order to determine the inhibitory effect given by curcumin and RK-52, the multiple drug effect evaluation reported in the literature by Chou and Talalay was used.23,24 In this contest, the combination index (CI) provides the nature of the inhibition toward the target enzyme, when two drugs are evaluated simultaneously. In particular, three effects have been demonstrated for CI > 1, CI = 1, and CI < 1, which correspond to an antagonistic, additive, and synergistic impact, respectively.
The CI for mutually exclusive drugs was calculated as follows:
where (D50)1 and (D50)2 represent the IC50 values for curcumin and RK-52 when they are evaluated alone, while (D)1 and (D)2 are the concentrations of both compounds able to produce 50% of rhodesain inhibition when they are used in combination.
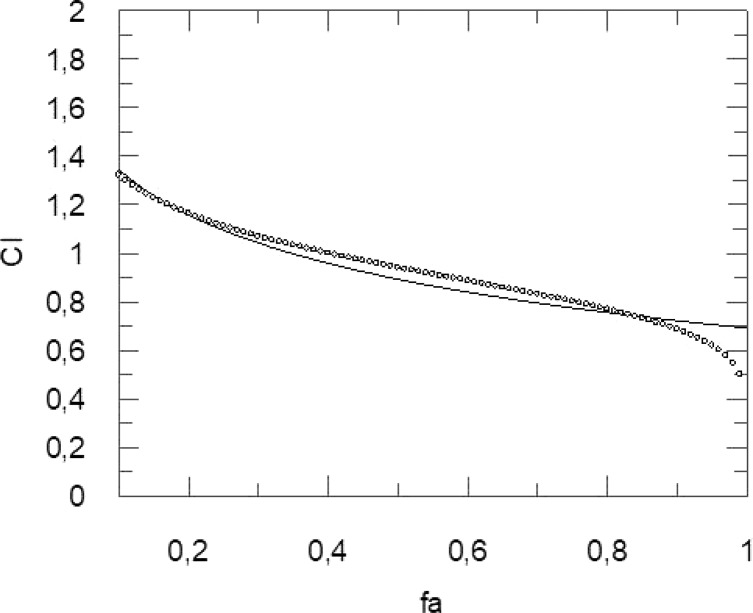
Grafit software was used to determine the CI (Figure 4). The obtained values show a clear synergistic effect when the most significant fa is ranging from 0.40 to 0.90, while for fa = 0.10 and fa ranging from 0.20 to 0.30, an antagonist and additive effect was recorded, respectively (Table 2).
Figure 4.
Combination index (CI) (y-axis) vs the fraction affected (x-axis), i.e. the reduction of rhodesain activity obtained by the combination RK-52–curcumin (1:861).
Table 2. Obtained Values of CI for the Combination RK-52–Curcumin (1:861) to Reduce Rhodesain Activity.
| Fraction affected (fa) | Combination index (CI) | Diagnosis of combined effect |
|---|---|---|
| 0.10 | 1,3227 | Antagonism |
| 0.20 | 1,1628 | Additive |
| 0.30 | 1,0694 | Additive |
| 0.40 | 0,9996 | Synergism |
| 0.50 | 0,9405 | Synergism |
| 0.60 | 0,8856 | Synergism |
| 0.70 | 0,8302 | Synergism |
| 0.80 | 0,7684 | Synergism |
| 0.90 | 0,6860 | Synergism |
| 0.99 | 0,4527 | Synergism |
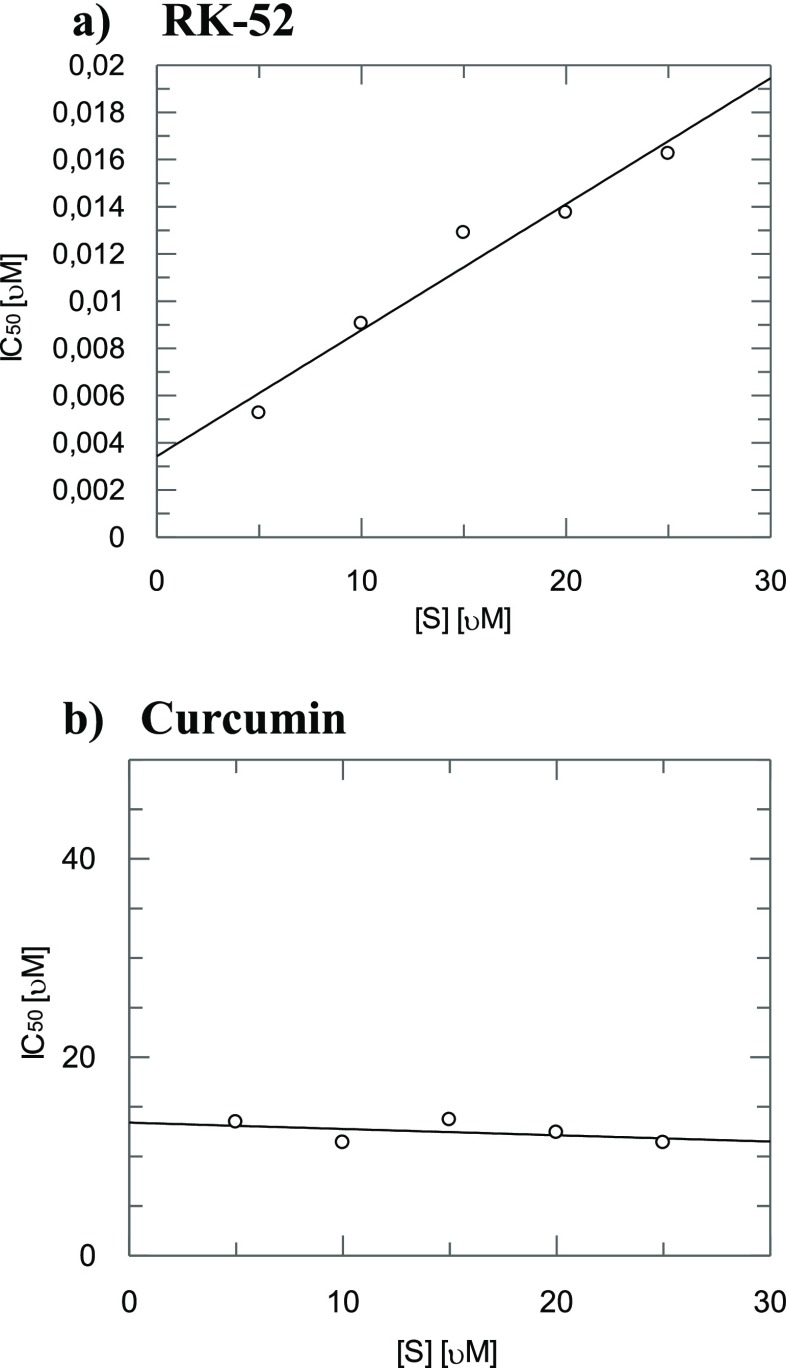
Finally, since a synergism at all the relevant fa values was observed between the two rhodesain inhibitors we further investigated if the inhibition is competitive with respect to the substrate by determining the IC50 in dependence of five substrate concentration (5, 10, 15, 20, and 25 μM).
In the case of RK-52 (Figure 5a) we found linearly increasing IC50 values with increasing substrate concentration proving that RK-52 binds to the active site. On the contrary, in the case of curcumin (Figure 5b) with increasing substrate concentration no variation of IC50 was observed thus suggesting that curcumin binds to an allosteric site.
Figure 5.
IC50 values of inhibition of rhodesain activity in dependence of the substrate concentration (5, 10, 15, 20, and 25 μM) for RK-52 (a) and curcumin (b).
Curcumin, RK-52, and the combination RK-52 + curcumin were also tested against T. brucei brucei (Figure 6). In these assays we obtained IC50 values of 3.12 ± 0.43 μM, 2.33 ± 0.29 μM, and 4.64 ± 0.35 μM for curcumin, RK-52, and RK-52 + curcumin in combination (molar ratio 1:1.34), respectively.
Figure 6.
Dose response curves for the inhibition of Trypanosoma brucei brucei growth for curcumin (a), RK-52 (b), and RK-52 + curcumin in combination (c). Each experiment was performed three times, each in duplicate.
The comparable activity of curcumin with respect to RK-52 against T. b. brucei, with respect to the very different activity against rhodesain, could be explained considering that the antitrypanosomal activity of curcumin might be due, in addition to the inhibition of rhodesain, to the interaction with other proteases that can be found in the trypanosomal intracellular environment.
We have also to consider that the activity against T. b. brucei is also related to the capability of a drug to cross the cellular membranes, which is certainly more easy for low molecular weight molecules such as curcumin, with respect to RK-52.
All in all, in this study starting from the activities of RK-52 and curcumin against rhodesain, we investigated the activity of their combination, obtaining for the most significant fa values (from 0.40 to 0.90) a synergistic effect for rhodesain inhibition. The combination curcumin + RK-52 showed an IC50 value againt T. b. brucei of 4.64 μM; thus, it is reasonable in future studies to further investigate the overall reduction of toxicity of the combination with respect to the single inhibitors, which is normally the main reason for the use of drug combinations.
Acknowledgments
RE would like to thank Annika Wagner, Ute A. Hellmich of Mainz University for performing assays against Trypanosoma brucei.
Glossary
Abbreviations
- HAT
Human African Trypanosomiasis
- BBB
blood–brain barrier
- VSGs
variant surface glycoproteins
- Cbz
carbobenzyloxy
- Phe
phenylalanine
- Arg
arginine
- AMC
7-amino-4-methyl-coumarin
- fa
fraction affected
- fu
fraction unaffected
- CI
combination index
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.9b00635.
Rhodesain inhibition assays; antitrypanosomal assays (PDF)
Author Contributions
All authors approved the content of the manuscript.
This study was economically supported by the “Fondo per il finanziamento delle attività base di ricerca” (FFABR 2017) financed by MIUR.
The authors declare no competing financial interest.
Supplementary Material
References
- Human African Trypanosomiasis; World Health Organization: Geneva, 2018; https://www.who.int/trypanosomiasis_african/en/ (March 20, 2019).
- Büscher P.; Cecchi G.; Jamonneau V.; Priotto G. Human African Trypanosomiasis. Lancet 2017, 390, 2397–2409. 10.1016/S0140-6736(17)31510-6. [DOI] [PubMed] [Google Scholar]
- MacLean L.; Reiber H.; Kennedy P. G.; Sternberg J. M. Stage Progression and Neurological Symptoms in Trypanosoma Brucei Rhodesiense Sleeping Sickness: Role of the CNS Inflammatory Response. PLoS Neglected Trop. Dis. 2012, 6, e1857 10.1371/journal.pntd.0001857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babokhov P.; Sanyaolu A. O.; Oyibo W. A.; Fagbenro-Beyioku A. F.; Iriemenam N. C. A Current Analysis of Chemotherapy Strategies for the Treatment of Human African Trypanosomiasis. Pathog. Global Health 2013, 107, 242–252. 10.1179/2047773213Y.0000000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansiime F.; Adibaku S.; Wamboga C.; Idi F.; Kato C. D.; Yamuah L.; Vaillant M.; Kioy D.; Olliaro P.; Matovu E. A Multicentre, Randomised, Non-Inferiority Clinical Trial Comparing a Nifurtimox-Eflornithine Combination to Standard Eflornithine Monotherapy for Late Stage Trypanosoma Brucei Gambiense Human African Trypanosomiasis in Uganda. Parasites Vectors 2018, 11, 105–115. 10.1186/s13071-018-2634-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priotto G.; Kasparian S.; Mutombo W.; Ngouama D.; Ghorashian S.; Arnold U.; Ghabri S.; Baudin E.; Buard V.; Kazadi-Kyanza S.; Ilunga M.; Mutangala W.; Pohlig G.; Schmid C.; Karunakara U.; Torreele E.; Kande V. Nifurtimox-Eflornithine Combination Therapy for Second-Stage African Trypanosoma Brucei Gambiense Trypanosomiasis: A Multicentre, Randomised, Phase III, Non-Inferiority Trial. Lancet 2009, 374, 56–64. 10.1016/S0140-6736(09)61117-X. [DOI] [PubMed] [Google Scholar]
- EMA/791484/2018 Committee for Medicinal Products for Human Use (CHMP). November 15, 2018.
- Ettari R.; Previti S.; Tamborini L.; Cullia G.; Grasso S.; Zappalà M. The Inhibition of Cysteine Proteases Rhodesain and TbCatB: A Valuable Approach to Treat Human African Trypanosomiasis. Mini-Rev. Med. Chem. 2016, 16, 1374–1391. 10.2174/1389557515666160509125243. [DOI] [PubMed] [Google Scholar]
- Ettari R.; Tamborini L.; Angelo I. C.; Micale N.; Pinto A.; De Micheli C.; Conti P. Inhibition of Rhodesain as a Novel Therapeutic Modality for Human African Trypanosomiasis. J. Med. Chem. 2013, 56, 5637–5658. 10.1021/jm301424d. [DOI] [PubMed] [Google Scholar]
- Overath P.; Chaudhri M.; Steverding D.; Ziegelbauer K. Invariant Surface Proteins in Bloodstream Forms of Trypanosoma Brucei. Parasitol. Today 1994, 10, 53–58. 10.1016/0169-4758(94)90393-X. [DOI] [PubMed] [Google Scholar]
- Lalmanach G.; Boulange A.; Serveau C.; Lecaille F.; Scharfstein J.; Gauthier F.; Authie E. Congopain from Trypanosoma Congolense: Drug Target and Vaccine Candidate. Biol. Chem. 2002, 383, 739–749. 10.1515/BC.2002.077. [DOI] [PubMed] [Google Scholar]
- Steverding D.; Sexton D. W.; Wang X.; Gehrke S. S.; Wagner G. K.; Caffrey C. R. Trypanosoma Brucei: Chemical Evidence That Cathepsin L Is Essential for Survival and a Relevant Drug Target. Int. J. Parasitol. 2012, 42, 481–488. 10.1016/j.ijpara.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Ettari R.; Previti S.; Cosconati S.; Maiorana S.; Schirmeister T.; Grasso S.; Zappalà M. Development of Novel 1,4-Benzodiazepine-Based Michael Acceptors as Antitrypanosomal Agents. Bioorg. Med. Chem. Lett. 2016, 26, 3453–3456. 10.1016/j.bmcl.2016.06.047. [DOI] [PubMed] [Google Scholar]
- Ettari R.; Previti S.; Cosconati S.; Kesselring J.; Schirmeister T.; Grasso S.; Zappalà M. Synthesis and Biological Evaluation of Novel Peptidomimetics as Rhodesain Inhibitors. J. Enzyme Inhib. Med. Chem. 2016, 31, 1184–1191. 10.3109/14756366.2015.1108972. [DOI] [PubMed] [Google Scholar]
- Ettari R.; Pinto A.; Previti S.; Tamborini L.; Angelo I. C.; La Pietra V.; Marinelli L.; Novellino E.; Schirmeister T.; Zappalà M.; Grasso S.; De Micheli C.; Conti P. Development of Novel Dipeptide-Like Rhodesain Inhibitors Containing the 3-Bromoisoxazoline Warhead in a Constrained Conformation. Bioorg. Med. Chem. 2015, 23, 7053–7060. 10.1016/j.bmc.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Ettari R.; Pinto A.; Tamborini L.; Angelo I. C.; Grasso S.; Zappalà M.; Capodicasa N.; Yzeiraj L.; Gruber E.; Aminake M. N.; Pradel G.; Schirmeister T.; De Micheli C.; Conti P. Synthesis and Biological Evaluation of Papain-Family Cathepsin L-Like Cysteine Protease Inhibitors Containing a 1,4-Benzodiazepine Scaffold as Antiprotozoal Agents. ChemMedChem 2014, 9, 1817–1825. 10.1002/cmdc.201402079. [DOI] [PubMed] [Google Scholar]
- Ettari R.; Tamborini L.; Angelo I. C.; Grasso S.; Schirmeister T.; Lo Presti L.; De Micheli C.; Pinto A.; Conti P. Development of Rhodesain Inhibitors with a 3-Bromoisoxazoline Warhead. ChemMedChem 2013, 8, 2070–2076. 10.1002/cmdc.201300390. [DOI] [PubMed] [Google Scholar]
- Ettari R.; Zappalà M.; Micale N.; Schirmeister T.; Gelhaus C.; Leippe M.; Evers A.; Grasso S. Synthesis of Novel Peptidomimetics as Inhibitors of Protozoan Cysteine Proteases Falcipain-2 and Rhodesain. Eur. J. Med. Chem. 2010, 45, 3228–3233. 10.1016/j.ejmech.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Ettari R.; Micale N.; Grazioso G.; Bova F.; Schirmeister T.; Grasso S.; Zappalà M. Synthesis and Molecular Modeling Studies of Derivatives of a Highly Potent Peptidomimetic Vinyl Ester as Falcipain-2 Inhibitors. ChemMedChem 2012, 7, 1594–1600. 10.1002/cmdc.201200274. [DOI] [PubMed] [Google Scholar]
- Bova F.; Ettari R.; Micale N.; Carnovale C.; Schirmeister T.; Gelhaus C.; Leippe M.; Grasso S.; Zappalà M. Constrained Peptidomimetics as Antiplasmodial Falcipain-2 Inhibitors. Bioorg. Med. Chem. 2010, 18, 4928–4938. 10.1016/j.bmc.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Previti S.; Ettari R.; Cosconati S.; Amendola G.; Chouchene K.; Wagner A.; Hellmich U. A.; Ulrich K.; Krauth-Siegel R. L.; Wich P. R.; Schmid I.; Schirmeister T.; Gut J.; Rosenthal P. J.; Grasso S.; Zappalà M. Development of Novel Peptide-Based Michael Acceptors Targeting Rhodesain and Falcipain-2 for the Treatment of Neglected Tropical Diseases (NTDs). J. Med. Chem. 2017, 60, 6911–6923. 10.1021/acs.jmedchem.7b00405. [DOI] [PubMed] [Google Scholar]
- Ettari R.; Previti S.; Maiorana S.; Allegra A.; Schirmeister T.; Grasso S.; Zappalà M. Drug Combination Studies of Curcumin and Genistein against Rhodesain of Trypanosoma Brucei Rhodesiense. Nat. Prod. Res. 2019, 33, 3577. 10.1080/14786419.2018.1483927. [DOI] [PubMed] [Google Scholar]
- Chou T. C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- Chou T. C.; Talalay P. Quantitative Analysis of Dose-Effect Relationships: The Combined Effects of Multiple Drugs or Enzyme Inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Caffrey C. R.; Hansell E.; Lucas K. D.; Brinen L. S.; Alvarez Hernandez A.; Cheng J.; Gwaltney S. L. 2nd; Roush W. R.; Stierhof Y. D.; Bogyo M.; Steverding D.; McKerrow J. H. Active Site Mapping, Biochemical Properties and Subcellular Localization of Rhodesain, the Major Cysteine Protease of Trypanosoma Brucei Rhodesiense. Mol. Biochem. Parasitol. 2001, 118, 61–73. 10.1016/S0166-6851(01)00368-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.