Abstract
Aims
Peripheral T cell lymphomas represent approximately 10%–15% of non-Hodgkin lymphomas and are characterised by an aggressive clinical courses and poor outcomes. Ligands provided by constituents of the tumour microenvironment engage receptors expressed by malignant T cells, promoting tumour growth and chemotherapy resistance. In addition to stimulatory receptors that promote the growth and survival of malignant T cells, recent studies suggest that homologous inhibitory receptors may have an opposing effect and function as tumour suppressors. For example, recent data suggest that programmed cell death 1 blockade may lead to increased lymphoma growth. Therefore, the identification of alternative checkpoint receptors in T cell lymphoproliferative neoplasms is an important and clinically relevant question.
Methods
The checkpoint receptors T cell immunoglobulin-3 (TIM-3), V-domain Ig-containing suppressor of T cell activation (VISTA) and lymphocyte-activation gene 3 (LAG-3) play fundamental roles in peripheral tolerance, and their ligands are exploited by many solid tumours to evade host immunity. However, their expression in T cell lymphoproliferative neoplasms has not been evaluated. In this study, we evaluated the expression of TIM-3, VISTA and LAG-3 in a cohort of peripheral T cell lymphomas cases by immunohistochemistry and flow cytometric analysis.
Results
Our results demonstrate that TIM-3, VISTA and LAG-3 expression is rarely identified within a large cohort of T cell lymphomas and its tumour microenvironment.
Conclusions
Our data suggest that immune-regulatory roles for TIM-3, VISTA and LAG-3 may be predominant in lymphomas subsets different than the ones analysed in the current study. However, a potential role for these checkpoint receptors as tumour suppressors in T cell lymphomas remains to be elucidated.
INTRODUCTION
A role for the T cell receptor (TCR) in T cell lymphoma pathogenesis has been inferred from the observation that many T cell lymphomas disproportionally use specific TCR variable genes and retain TCR expression, despite the aberrant loss of other T cell antigens that are not required for TCR signalling (reviewed by Wilcox1). Elucidation of the genetic landscape in the most common peripheral and cutaneous T cell lymphomas has further demonstrated that gain-of-function mutations and translocations involving kinases, and other second messengers, required for TCR signalling in conventional (non-malignant) T cells are recurrently observed in many T cell lymphomas (reviewed by Wilcox, Elenitoba-Johnson1,2). Functional studies performed ex vivo in primary specimens demonstrate that TCR signalling leads to significant changes in gene expression, induces proliferation and confers resistance to chemotherapy3 and thus complements the molecular and genomic data implicating the TCR in T cell lymphoma biology. At least in conventional T cells, the TCR (or ‘signal 1’) collaborates with signalling input provided by second, costimulatory receptors, the ligands for which are frequently provided by professional antigen-presenting cells, most of which are abundant constituents of the tumour microenvironment (TME) among T cell lymphomas.4,5 Recent studies suggest that costimulatory receptors (‘signal 2’) may play a similarly important role in T cell lymphomagenesis, particularly in specific subsets6,7 (reviewed by Wilcox1). Unrestrained TCR and costimulatory receptor signalling, while required for fully competent T cell immunity, would lead to significant autoimmunity, if not held in check by coinhibitory, checkpoint receptors.8 Many tumours, expressing ligands for these inhibitory receptors, exploit these checkpoints to evade and suppress host antitumour immunity. The once nascent field of checkpoint blockade has spawned a revolution in immunooncology and immunotherapy.
Ligands for checkpoint receptors, most notably programmed death-ligand 1 (PD-L1), are highly expressed in many T cell lymphomas,9 and effector T cells infiltrating these lymphomas may be restrained by checkpoint receptors.10–12 Therefore, checkpoint blockade is a rational therapeutic strategy in these aggressive lymphomas (reviewed by Phillips13), and is supported by the available, although limited, clinical data.14,15 However, a recently reported study, performed in a specific murine T cell lymphoma model, suggests that the checkpoint receptor programmed cell death 1 (PD-1) may function as a tumour suppressor,16 and rapid progression following PD-1 blockade has been observed in adult T cell leukaemia/lymphoma patients.17 Therefore, the extent to which PD-1, and alternative checkpoint receptors, function as tumour suppressors remains an open question. While PD-1 expression in T cell lymphomas is well characterised,18–21 the extent to which additional checkpoint receptors are expressed in specific T cell lymphoma subsets, and their TME is unknown. This gap in knowledge is significant and clinically relevant, as trials targeting alternative checkpoint receptors are planned or ongoing.
T cell immunoglobulin-3 (TIM-3), expressed by Thl cells, cytotoxic T cells, regulatory T cells and myeloid-derived cells (including dendritic cells and monocytes), suppresses T cell immunity on bindings its ligands galectin-9, phosphatidylserine, HMGB1 or Ceacam-1 (reviewed by Anderson22) and has been implicated in B cell lymphomas.23 Lymphocyte-activation gene 3 (LAG-3) is inducibly expressed on activated T cells and similarly impairs T cell proliferation and effector functions. Despite evidence implicating alternative ligands, major histocompatibility complex (MHC) class II has long been considered the canonical ligand for LAG-3, although fibrinogen-like protein 1 was recently demonstrated to be an additional, high-affinity ligand for LAG-3. V-domain Ig-containing suppressor of T cell Activation (VISTA) is highly expressed on myeloid cells, including macrophages, and naive and regulatory T cells. In contrast to TIM-3 and LAG-3, VISTA functions as both a ligand and receptor. The context-dependent role of specific TIM-3, LAG-3 and VISTA ligands, and the signalling cascades triggered by ligand binding, are incompletely understood and remain areas of active investigation. Nonetheless, the therapeutic potential of antagonistic and agonistic antibodies specific for these receptors suggests that improved understanding of their potential role in T cell lymphomagenesis is needed. Therefore, we sought to characterise the expression of these alternative checkpoint receptors in peripheral T cell lymphomas (PTCLs).
MATERIAL AND METHODS
Immunohistochemistry
Immunohistochemistry was performed with rabbit monoclonal antibody against LAG-3 (Abeam, clone EPR20261), VISTA (Cell Signaling, clone D5L5T) and TIM-3 (Cell Signaling, clone D5D5R). Immunohistochemical stains were performed at the histolab from Michigan State University (further details included in online supplementary data).
Tumour scoring
Scoring of tumour cells and its surrounding microenvironment was performed in cases collected from the archives of the University of Michigan, assembled in tumour microarrays. All the cases were rerevised and classified according to the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (2008 edition). Due to recent updates in PTCL classification, only PTCL-NOS (non-otherwise specified) cases graded positive for any of the markers tested (VISTA, TIM-3 and LAG-3) were rerevisited for T cell follicular helper (TFH) markers (PD-1, BCL-6 and CD10); the cases with a immunohistochemical profile consistent with TFH-PTCL were not included in the current study. For VISTA, TIM-3 and LAG-3 grading, a z-score was calculated for all the cases analysed. TIM-3 was considered positive if more than 13% of tumour cells were positive (z-score >1); VISTA was considered positive if more than 15% of tumour cells were positive (z-score >1). The number of histiocytes or lymphocytes surrounding the tumour cells was annotated from at least 10 high power fields per case; only the cases that featured on average more than five positive cells per high power field were annotated. Identification of tumour cells was performed by morphological assessment or by immunohistochemical interpretation with specific tumour markers (when applicable): PTCL-NOS (GATA3 or TBX21) or ALCL (CD30).5 Tumour-associated macrophages were identified with positive CSF1R immunostaining.24 Morphology analysis and interpretation of immunohistochemical data were performed independently by two haematopathologists (CAMZ and NB).
Flow cytometry and purification of primary T cell lymphoma specimens
Antibodies for flow cytometric analysis were purchased from BD Pharmigen: LAG-3 (CD223, clone T47-530), TIM-3 (CD366, clone 7D3) and VISTA (clone MIH65). Antibody concentrations and incubations were performed as indicated by the manufacturer instructions using an appropriate isotype control. T cell lymphoma cells and benign peripheral blood T cell lymphocytes were purified as previously described.3
RESULTS
TIM-3 is expressed in a minor subset of peripheral T cell lymphomas
In order to evaluate the expression of TIM-3 in PTCL and the TME, TIM-3 expression was first evaluated in benign (reactive) lymphoid tissues. TIM-3 expression was observed in lymphocytes and histiocytes surrounding the germinal centre but was notably absent from the germinal centre (online supplementary figure 1). As checkpoint receptors may be expressed in specific PTCL subsets, depending on their ‘cell of origin’, TIM-3 was evaluated in a PTCL cohort, including peripheral T cell lymphoma nonotherwise specified (PTCL and NOS), angioimmunoblastic T cell lymphoma (AITL), anaplastic large cell lymphoma (ALCL), enteropathy-associated T cell lymphoma (EATL) and extranodal natural killer T cell lymphoma (ENKTCL). TIM-3 expression was detected in lymphoma cells in a minority of the cases analysed (figure 1), being observed in 8% of PTCL, NOS (n=37) and in a single EATL case (table 1). TIM-3 expression was absent or minimally expressed (ie, expression in ≤5% of lymphoma cells) in the remaining cases. Among TIM-3 positive cases, the proportion of positive lymphoma cells was variable and ranged from 5% to 70% (table 1). The only case with more than 50% expression was an EATL case. TIM-3 was also expressed by morphologically benign, infiltrating lymphocytes and macrophages in a minority of cases (figure 1 and table 2). While the density of these TIM-3 expressing cells was variable, a high-density (≥20 positive cells per high power field) of TIM-3 + cells was rarely observed (table 2). No cases were identified in which lymphoma cells and constituents of the TME concurrently expressed TIM-3. TIM-3 was also evaluated by flow cytometry in normal donor CD3+ T cells (figure 2B), in a panel of PTCIVcutaneous T-cell lymphoma (CTCL) cell lines (n = 6; Figure 2E,F) and primary specimens isolated from patients with leukaemic involvement (PTCL and NOS: patient A; Sezary syndrome: patient B and C; figure 2A,C). With the exception of a single CTCL cell line (Mac-1), TIM-3 expression was dim or negative in cell lines (figure 2A–F). Activation with CD3/CD28 beads led to TIM-3 upregulation in Sezary cells from one patient (figure 2C).
Figure 1.
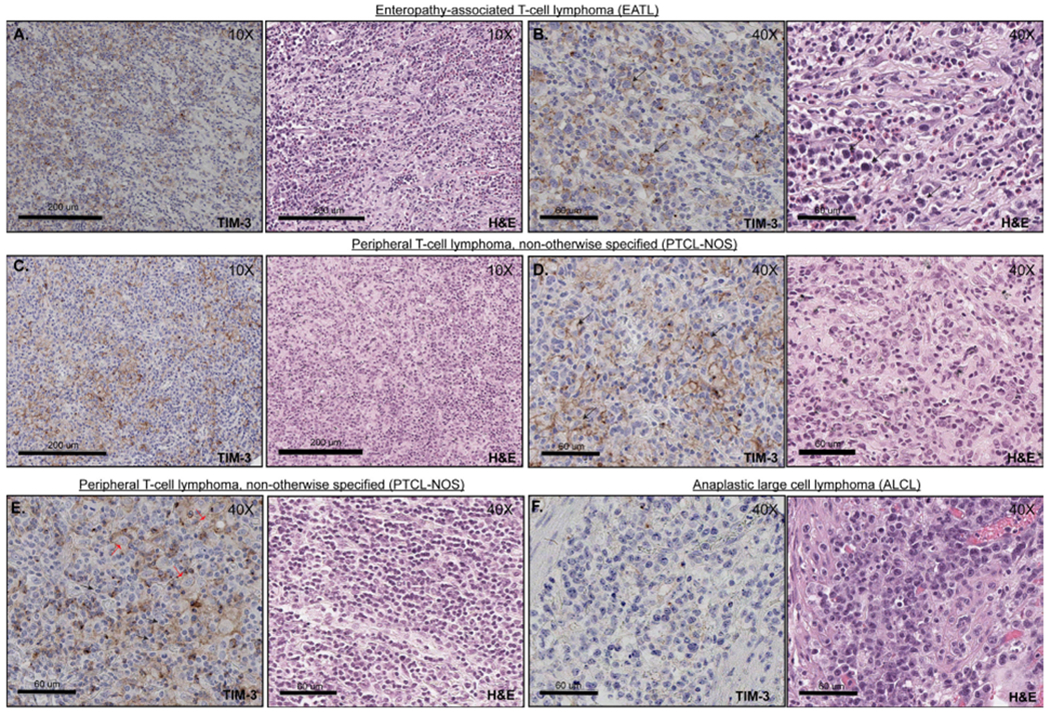
TIM-3 expression in PTCL. (A) and (B) Positive membranous expression of TIM-3 among T cell lymphoma tumour cells (EATL). Right side panels (B) represent higher magnification images (40×). The tumour cells are medium to large in size, with condensed chromatin, irregular nuclear contours and high nuclear to cytoplasmic (N:C) ratios. (C) and (D) Positive membranous expression of TIM-3 within T cell lymphoma cells (PTCL-NOS). Right side panels (D) represent higher magnification images (40×). The tumour cells are medium in size with irregular nuclear contours, open chromatin and prominent nucleoli. (E) PTCL-NOS with TIM-3 expression within lymphoma-associated histiocytes and lymphocytes. Corresponding H&E image demonstrates that lymphoma cells are small to medium in size with scant cytoplasm, irregular nuclear contours and clumped chromatin (black arrows). In contrast, histiocytes have plump nuclei with variable amounts of cytoplasm (red arrows). (F) Representative image of an ALCL with negative expression of TIM-3. ALCL, anaplastic large cell lymphoma; EATL, enteropathy-associated T cell lymphoma; PTCL, peripheral T cell lymphoma; TIM-3, T cell immunoglobulin-3.
Table 1.
| TIM-3 | % of positive cells | VISTA | % of positive cells | |||||
|---|---|---|---|---|---|---|---|---|
| Positive cases | Number of cases | Positive cases | Number of cases | |||||
| n (%) | 5%–15% | 15%–50% | >50% | n (%) | 5%–15% | 15%–50% | >50% | |
| PTCL, NOS | 3/37 (8) | 1 | 3 | 0 | 2/37 (5) | 1 | 6 | 0 |
| ALCL | 0/11 (0) | 0 | 0 | 0 | 0/11 (0) | 0 | 0 | 0 |
| AITL | 0/6 (0) | 0 | 0 | 0 | 2/6 (33) | 0 | 2 | 0 |
| EATL | 1/3 (33) | 0 | 0 | 1 | 2/3 (66) | 0 | 0 | 2 |
| ENKTCL | 0/3 (0) | 0 | 0 | 0 | 0/3 (0) | 0 | 0 | 0 |
AITL, angioimmunoblastic T cell lymphoma; ALCL, anaplastic large cell lymphoma; EATL, enteropathy-associated T cell lymphoma; ENKTCL, extranodal natural killer T cell lymphoma ; PTCL, peripheral T cell lymphoma; TIM-3, T cell immunoglobulin-3; VISTA, V-domain Ig-containing suppressor of T cell activation.
Table 2.
| TIM-3 | Number of positive cells per HPF | VISTA | Number of positive cells per HPF | |||||
|---|---|---|---|---|---|---|---|---|
| Positive cases | Number of cases | Positive cases | Number of cases | |||||
| n (%) | 5–10 (1+) | 10–20 (2+) | >20 (3+) | n (%) | 5–10 (1+) | 10–20 (2+) | >20 (3+) | |
| PTCL, NOS | 2/37 (5) | 0 | 0 | 2 | 1/37 (2) | 1 | 0 | 0 |
| ALCL | 2/11 (18) | 2 | 0 | 0 | 0/11 (0) | 0 | 0 | 0 |
| AITL | 0/6 (0) | 0 | 0 | 0 | 0/6 (0) | 0 | 0 | 0 |
| EATL | 0/3 (0) | 0 | 0 | 0 | 0/3 (0) | 0 | 0 | 0 |
| ENKTCL | 0/3 (0) | 0 | 0 | 0 | 0/3 (0) | 0 | 0 | 0 |
AITL, angioimmunoblastic T cell lymphoma; ALCL, anaplastic large cell lymphoma; EATL, enteropathy-associated T cell lymphoma; ENKTCL, extranodal natural killer T cell lymphoma; HPF, high power field; PTCL, peripheral T cell lymphoma; TIM-3, T cell immunoglobulin-3; VISTA, V-domain Ig-containing suppressor of T cell activation.
Figure 2.
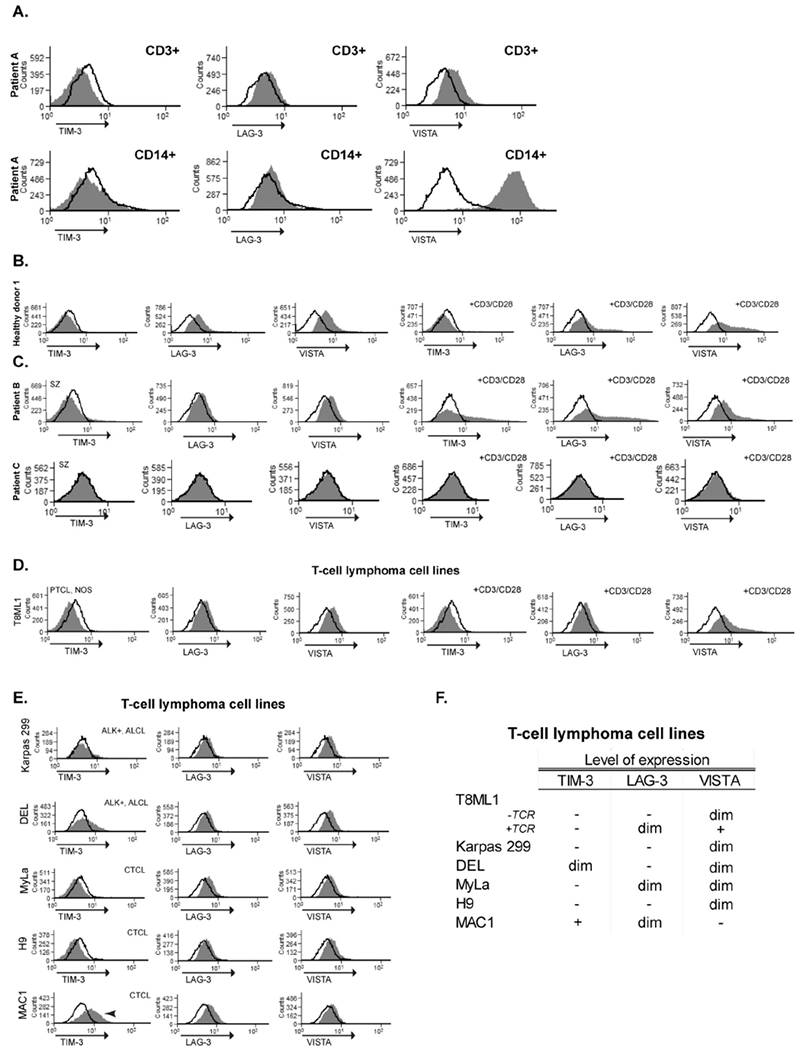
TIM-3, LAG-3 and VISTA expression evaluated by flow cytometry. (A) Patient A was obtained from the peripheral blood of an adult male with leukaemic involvement by PTCL, NOS. Both monocytes (CD14+) and T cell lymphoma cells (CD3+, 99% lymphoma cells) were gated for further analysis, as indicated. (B and C) Purified CD3+ lymphocytes from a healthy donor and purified CD4+ T cells from Sezary patients (patients B and C; ≥85% lymphoma cells). Expression of TIM-3, LAG-3 and VISTA was evaluated in resting conditions and on activation with anti-CD3/CD28 beads (24 hours), as indicated. (D) PTCL, NOS cell lines (T8ML1) were similarly evaluated in resting conditions and after CD3/CD28 activation. (E) Expression of TIM-3, LAG-3 and VISTA by different T cell lymphoma lines. (F) Table summarises the level of expression of the proteins analysed within the different T cell lymphoma lines. ALCL, Anaplastic large cell lymphoma; CTCL, Cutaneous T-cell lymphoma; LAG-3, lymphocyte-activation gene 3; PTCL, peripheral T cell lymphoma; TCR, T-cell receptor; TIM-3, T cell immunoglobulin-3; VISTA, V-domain Ig-containing suppressor of T cell activation.
VISTA is expressed within few peripheral T cell lymphomas cases and its TME
In a similar manner, VISTA was first evaluated within benign lymphoid tissue. VISTA was expressed by lymphocytes and rarely observed in histiocytes within the interfollicular regions. VISTA expression by lymphocytes within primary or secondary germinal centres was not observed (online supplementary figure 1). VISTA expression was evaluated across a cohort of T cell lymphoma cases and was detected in 5% of PTCL-NOS cases (n=37), 66% cases of EATL (n=3) and 33% of AITL cases (n = 6) (figure 3 and table 1). VISTA was most highly expressed in EATL, as the majority of lymphoma cells expressed VISTA. In contrast, a minority of cells expressed VISTA among the positive PTCL, NOS and AITL cases examined. VISTA expression was absent or minimally expressed (expression in ≤15% of lymphoma cells) in ALCL and ENKTCL (table 1). VISTA expression within the TME was rarely observed (table 2), and coexpression of VISTA by both lymphoma cells and the TME was not observed. VISTA was evaluated by flow cytometry in normal donor CD3 + T cells (figure 2B), in a panel of PTCIVCTCL cell lines (figure 2E,F) and in primary specimens isolated from patients with leukaemic involvement (figure 2A,C). VISTA expression was negative or dim in these cells but was inducibly expressed on CD3/CD28 activation (figure 2A–F). As anticipated, VISTA was highly expressed by peripheral blood monocytes (figure 2).
Figure 3.
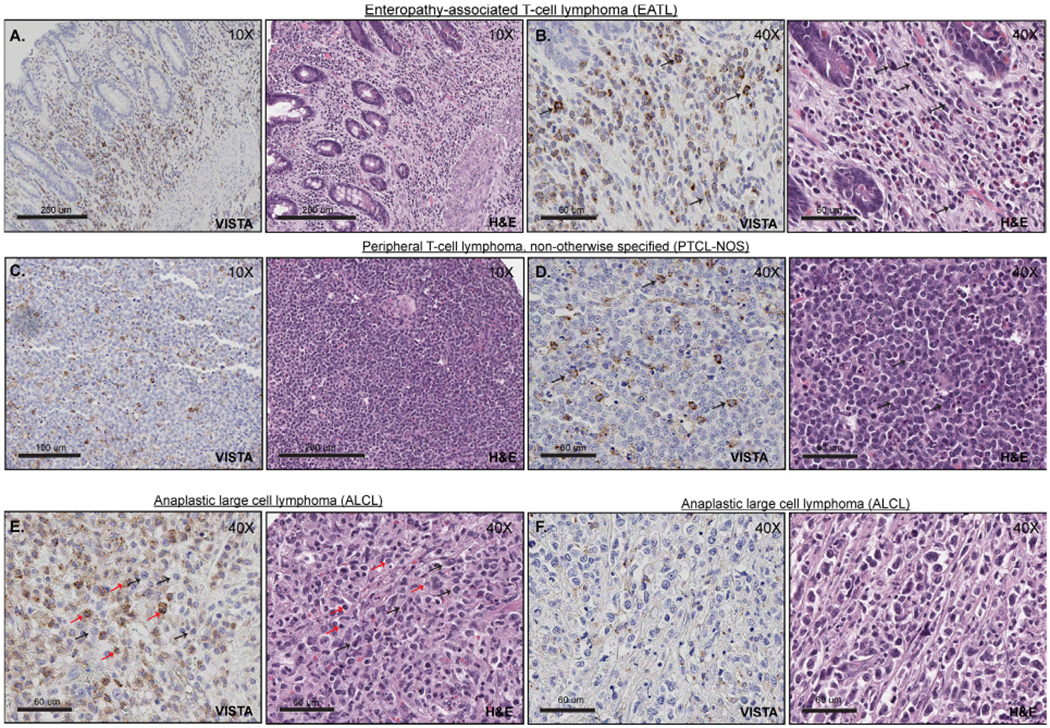
VISTA expression in PTCL. (A and B) Positive expression of VISTA within T cell lymphoma tumour cells (EATL), at low (A) and high magnification (B, 40×). The tumour cells are medium to large in size, with variable amount of cytoplasm, irregular nuclear contours and condensed chromatin (black arrows). (C and D) Positive expression of VISTA within T cell lymphoma tumour cells (PTCL-NOS), at low (C) and high magnification (D, 40×). Tumour cells (black arrows) are small to medium in size with scant cytoplasm, clumped chromatin, irregular nuclear contours and prominent nucleoli. (E) VISTA expression within lymphoma-associated histiocytes and lymphocytes (ALCL). Lymphoma cells are medium to large in size with scant cytoplasm, irregular nuclear contours, clumped chromatin and prominent nucleoli (black arrows). In contrast, reactive lymphocytes are small with plump nuclei (red arrows). (F) Representative image of T cell lymphoma (ALCL) with negative expression of VISTA. ALCL, anaplastic large cell lymphoma; PTCL, peripheral T cell lymphoma; VISTA, V-domain Ig-containing suppressor of T cell activation.
LAG-3 expression is not detected in peripheral T cell lymphomas or its TME
In order to evaluate LAG-3 expression, reactive lymphoid tissue was analysed with a monoclonal antibody directed against LAG-3. LAG-3 expression was expressed by lymphocytes within germinal centres (online supplementary figure 1). Among the cohort of peripheral lymphomas evaluated (PTCL-NOS n=41, ALCL n=11, EATL n=3, ENKTCL n=3 and AITL n=6), LAG-3 expression was not observed (online supplementary figure 1). LAG-3 was also evaluated by flow cytometry in normal donor CD3+ Tcells, in a panel of PTCL/CTCL cell lines (n = 6) and primary specimens isolated from patients with leukaemic involvement. LAG-3 expression was inducible on CD3/CD28 activation in normal donor T cells and in Sezary cells but was otherwise negative or dim.
DISCUSSION
Improved understanding of the genetic landscape among T cell lymphomas demonstrates that both costimulatory and checkpoint receptors may be exploited by malignant T cells. Recurrent mutations in the costimulatory receptor CD28 involving both the extracellular and intracytoplasmic domains have been observed in PTCL.25,26 Extracellular domain mutations increase the binding affinity to CD28 ligands, whereas intracytoplasmic domain mutations enhance binding to downstream signalling intermediates, and collectively these mutations promote CD28 signalling. An alternative strategy used to promote CD28 signalling involves a novel in-frame fusion between the extracellular and transmembrane domain of CTLA-4 and the intracytoplasmic domain of CD28.27–29 As the CTLA-4 extracellular domain has a higher affinity for CD28 ligands, this novel fusion promotes ligand-dependent CD28 signalling. A patient harbouring this novel fusion transiently responded to CTLA-4 blockade.27 In contrast to CD28, which is widely expressed on naïve T cells, alternative costimulatory receptors are preferentially expressed by distinct T cell subsets. For example, inducible T cell costimulatory (ICOS) is highly expressed on follicular helper T (Tfh) cells and is required for their differentiation and maintenance.30–32 Not surprisingly then, ICOS is expressed by Tfh-derived PTCL and collaborates with the genetic landscape to promote their growth and survival.7,33 Despite the emerging role of costimulatory receptors (‘signal 2’) in T cell lymphomagenesis, the potential role of alternative, and inhibitory, CD28 family members in T cell lymphomagenesis has been little studied, at least until recently.
PD-1 (CD279) and its ligands, PD-L1 (B7-H1, CD274) and PD-L2 (B7-DC, CD273), play an important role in peripheral self-tolerance and are exploited by many tumours, including T cell lymphomas9 to create a TME that is particularly hostile for effector T cells and effective antitumour immunity. PD-1/PD-L1 checkpoint blockade has revolutionised the management of many cancers and is being studied in the T cell lymphomas (reviewed by Phillips13). The PD-1 cytoplasmic tail, by recruiting the protein tyrosine phosphatase SHP2, inhibits proximal TCR signalling,34 leading to increased PTEN phosphatase activity and potent inhibition of PI3K-Akt signalling.35–37 PD-1 is highly expressed in the majority of most PTCL, including PTCL, NOS and Tfh-derived PTCL and other T cell lymphomas.11,20,21,33,38,39 Its potential role as a tumour suppressor in T cell lymphomas was investigated using mice that transgenically express a novel ITK-SYK fusion protein. This novel translocation [t(5;9)(q33;q22)j, observed in ≈20% of selected Tfh-derived PTCL,40–43 fuses the pleckstrin homology domain of LTK with the SYK kinase domain, culminating in TCR-independent signalling and T cell lymphoma development when inducibly expressed in murine T cells.42 On ITK-SYK expression, T cells transiently expand and upregulate PD-1 before contracting, thus suggesting the presence of an intrinsic checkpoint that prevents unrestrained proliferation. Both genetic and antibody-based approaches were used to demonstrate that PD-1, perhaps not surprisingly, is a dominant checkpoint in this model.16 The extent to which these findings are generalisable to human PTCL remains uncertain, however, as PD-1 expression is prevalent in many PTCL, particularly those that are Tfh derived.11,20,21,33,38,39 Furthermore, PD-1 impairs TCR signalling and proliferation in large part by impairing PI3K/Akt signalling in a PTEN-dependent manner. Not surprisingly then, pharmacological PI3K inhibition significantly impaired the expansion of PD-1-deficient T cells in this model. This is pertinent, as PTEN deletions, which presumably dissociate PI3K/Akt signalling from PD-1 regulation, are prevalent in many PTCL.44 Of course, the extent to which PD-1 suppresses T cell lymphomagenesis may be context dependent, as the available clinical data addressing this question may suggest.14,15,17 In contrast to PD-1, the expression and function of alternative checkpoint receptors in T cell lymphomas, including TIM-3, LAG-3 and VISTA, have not been previously described. This is a clinically important question, as antibodies blocking these alternative checkpoints are clinically available. Therefore, we examined each of these checkpoints in a PTCL cohort.
In stark contrast to PD-1, these alternative checkpoints were rarely expressed in most PTCL that we examined. However, these data should be interpreted with caution, as these checkpoints, in contrast to PD-1, may not be ontologically linked to the cell of origin of the PTCL subtypes that we examined. For example, two molecularly and clinically distinct subsets of PTCL, NOS were recently described, raising the possibility that TIM-3 and VISTA may be preferentially expressed in one of these subsets.5,45 TIM-3, for example, is preferentially expressed on Thl cells and cytotoxic T cells,46 raising the possibility that it may be preferentially expressed by PTCL, NOS that highly express the transcription factor T-bet and its gene targets, including interferon-γ. Alternatively, if these alternative checkpoints are bone fide tumour suppressors in T cell lymphomas, then minimal expression may be predicted. Of course, suitable model systems will be needed to directly interrogate each of these checkpoints and determine their function, if any, in the T cell lymphomas. As in conventional T cells, TCR activation may induce the expression of these checkpoints, but it is possible that this may only occur in specific niches or in a subset of malignant T cells. Similarly, VISTA may be inducibly expressed in a p53-dependent manner in the setting of genotoxic stress.47 Consequently, the prevalence of p53 deletions in many T cell lymphomas, including a subset of PTCL, NOS,44,48 may contribute to the minimal VISTA expression we observed. In addition, we cannot exclude the possibility that immunohistochemistry may be insufficiently sensitive to detect low level expression of these checkpoints, as this has been observed with other T cell antigens,49 and even low-level expression may be functionally relevant. While most antibodies targeting checkpoint receptors and their ligands are antagonistic, agonistic antibodies targeting these receptors may have utility in the T cell lymphomas. For example, agonistic VISTA antibodies are profoundly immunosuppressive.50,51 Nonetheless, as both the repertoire of checkpoint receptors, and the clinically available antibodies targeting them, continues to expand, further studies to elucidate their role, and therapeutic potential, in the T cell lymphomas seem warranted.
Supplementary Material
Take home messages.
Expression of the checkpoint receptors lymphocyte-activation gene 3 (LAG-3), V-domain Ig-containing suppressor of T cell activation (VISTA) and T cell immunoglobulin-3 (TIM-3) is rarely detected in T cell lymphomas and within its tumour microenvironment.
A role for LAG-3, VISTA and TIM-3 as checkpoint immune-regulators may occur in different types of lymphomas than the ones analysed in the current study.
A potential role for LAG-3, VISTA and TIM-3 as tumour suppressors remains to be elucidated in peripheral T cell lymphomas.
Acknowledgments
Funding Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number K08-CA218460 (CMZ) and R01CA217994 (RAW).
Footnotes
Handling editor Mary Frances McMullin.
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement All data relevant to the study are included in the article or uploaded as supplementary information.
REFERENCES
- 1.Wilcox RA. A three-signal model of T-cell lymphoma pathogenesis. Am J Hematol 2016;91:113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elenitoba-Johnson KSJ, Wilcox R. A new molecular paradigm in mycosis fungoides and Sézary syndrome. Semin Diagn Pathol 2017;34:15–21. [DOI] [PubMed] [Google Scholar]
- 3.Wang T, Lu Y, Polk A, et al. T-Cell receptor signaling activates an ITK/NF-κB/GATA-3 axis in T-cell lymphomas facilitating resistance to chemotherapy. Clin Cancer Res 2017;23:2506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilcox RA, Wada DA, Ziesmer SC, et al. Monocytes promote tumor cell survival in T-cell lymphoproliferative disorders and are impaired in their ability to differentiate into mature dendritic cells. Blood 2009;114:2936–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang T, Feldman AL, Wada DA, et al. Gata-3 expression identifies a high-risk subset of PtCl, NOS with distinct molecular and clinical features. Blood 2014;123:3007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohr J, Guo S, Huo J, et al. Recurrent activating mutations of CD28 in peripheral T-cell lymphomas. Leukemia 2016;30:1062–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortes JR, Ambesi-Impiombato A, Couronné L, et al. Rhoa G17V induces T follicular helper cell specification and promotes lymphomagenesis. Cancer Cell 2018;33:259–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fritz JM, Lenardo MJ. Development of immune checkpoint therapy for cancer. J Exp Med 2019;216:1244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilcox RA, Feldman AL, Wada DA, et al. B7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disorders. Blood 2009;114:2149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samimi S, Benoit B, Evans K, et al. Increased programmed death-1 expression on CD4+ T cells in cutaneous T-cell lymphoma: implications for immune suppression.Arch Dermatol 2010;146:1382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wada DA, Wilcox RA, Harrington SM, et al. Programmed death 1 is expressed in cutaneous infiltrates of mycosis fungoides and Sezary syndrome. Am J Hematol 2011;86:325–7. [DOI] [PubMed] [Google Scholar]
- 12.Querfeld C, Leung S, Myskowski PL, et al. Primary T cells from cutaneous T-cell lymphoma skin explants display an exhausted immune checkpoint profile. Cancer Immunol Res 2018;6:900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips T, Devata S, Wilcox RA. Challenges and opportunities for checkpoint blockade in T-cell lymphoproliferative disorders. J Immunother Cancer 2016;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol 2016;34:2698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barta SK, Zain J, MacFarlane AW, et al. Phase II study of the PD-1 inhibitor pembrolizumab for the treatment of relapsed or refractory mature T-cell lymphoma. Clin Lymphoma Myeloma Leuk 2019;19:356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wartewig T, Kurgyis Z, Keppler S, et al. Pd-1 is a haploinsufficient suppressor of T cell lymphomagenesis. Nature 2017;552:121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratner L, Waldmann TA, Janakiram M, et al. Rapid progression of adult T-cell leukemia-lymphoma after PD-1 inhibitor therapy. N Engl J Med 2018;378:1947–8. [DOI] [PubMed] [Google Scholar]
- 18.Dorfman DM, Brown JA, Shahsafaei A, et al. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am J Surg Pathol 2006;30:802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kantekure K, Yang Y, Raghunath P, et al. Expression patterns of the immunosuppressive proteins PD-1/CD279 and PD-L1/CD274 at different stages of cutaneous T-cell lymphoma/mycosis fungoides. Am J Dermatopathol 2012;34:126–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roncador G, Garcia Verdes-Montenegro J-F, Tedoldi S, et al. Expression of two markers of germinal center T cells (SAP and PD-1) in angioimmunoblastic T-cell lymphoma. Haematologica 2007;92:1059–66. [DOI] [PubMed] [Google Scholar]
- 21.Yu H, Shahsafaei A, Dorfman DM. Germinal-Center T-helper-cell markers PD-1 and CXCL13 are both expressed by neoplastic cells in angioimmunoblastic T-cell lymphoma. Am J Clin Pathol 2009;131:33–41. [DOI] [PubMed] [Google Scholar]
- 22.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized functions in immune regulation. Immunity 2016;44:989–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Z-Z, Grote DM, Ziesmer SC, et al. Il-12 upregulates Tim-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. J Clin Invest 2012;122:1271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martín-Moreno AM, Roncador G, Maestre L, et al. Csf1R protein expression in reactive lymphoid tissues and lymphoma: its relevance in classical Hodgkin lymphoma. PLoS One 2015;10:e0125203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rohr J, Guo S, Hu D, et al. Cd28 mutations in peripheral T-cell lymphomagenesis and progression. Blood 2014;124:1681. [Google Scholar]
- 26.Yoo HY, Sung MK, Lee SH, et al. A recurrent inactivating mutation in RhoA GTPase in angioimmunoblastic T cell lymphoma. Nat Genet 2014;46:371–5. [DOI] [PubMed] [Google Scholar]
- 27.Sekulic A, Liang WS, Tembe W, et al. Personalized treatment of Sézary syndrome by targeting a novel CTLA4 : CD28 fusion. Mol Genet Genomic Med 2015;3:130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Ni X, Covington KR, et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet 2015;47:1426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ungewickell A, Bhaduri A, Rios E, et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet 2015;47:1056–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi YS, Kageyama R, Eto D, et al. Icos receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor BCL6. Immunity 2011;34:932–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol 2011;29:621–3. [DOI] [PubMed] [Google Scholar]
- 32.Wilcox RA, Ansell SM, Lim MS, et al. The B7 homologues and their receptors in hematologic malignancies. Eur J Haematol 2012;88:465–75. [DOI] [PubMed] [Google Scholar]
- 33.Manso R, González-Rincón J, Rodríguez-Justo M, et al. Overlap at the molecular and immunohistochemical levels between angioimmunoblastic T-cell lymphoma and a subgroup of peripheral T-cell lymphomas without specific morphological features. Oncotarget 2018;9:16124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, et al. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med 2012;209:1201–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parry RV, Chemnitz JM, Frauwirth KA, et al. Ctla-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 2005;25:9543–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patsoukis N, Brown J, Petkova V, et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal 2012;5:ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patsoukis N, Li L, Sari D, et al. Pd-1 increases PTEN phosphatase activity while decreasing PTEN protein stability by inhibiting casein kinase 2. Mol Cell Biol 2013;33:3091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaja F, Tabanelli V, Agostinelli C, et al. Cd38, Bcl-2, PD-1, and PD-1L expression in nodal peripheral T-cell lymphoma: possible biomarkers for novel targeted therapies? Am J Hematol 2017;92:E1–2. [DOI] [PubMed] [Google Scholar]
- 39.Cetinözman F, Jansen PM, Willemze R. Expression of programmed death-1 in primary cutaneous CD4-positive small/medium-sized pleomorphic T-cell lymphoma, cutaneous pseudo-T-cell lymphoma, and other types of cutaneous T-cell lymphoma. Am J Surg Pathol 2012;36:109–16. [DOI] [PubMed] [Google Scholar]
- 40.Dierks C, Adrian F, Fisch P, et al. The ITK-SYK fusion oncogene induces a T-cell lymphoproliferative disease in mice mimicking human disease. Cancer Res 2010;70:6193–204. [DOI] [PubMed] [Google Scholar]
- 41.Hussain A, Mohammad DK, Gustafsson MO, et al. Signaling of the ITK (interleukin 2-inducible T cell kinase)-SYK (spleen tyrosine kinase) fusion kinase is dependent on adapter SLP-76 and on the adapter function of the kinases SYK and ZAP70. J. Biol. Chem 2013;288:7338–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pechloff K, Holch J, Ferch U, et al. The fusion kinase ITK-SYK mimics a T cell receptor signal and drives oncogenesis in conditional mouse models of peripheral T cell lymphoma. J Exp Med 2010;207:1031–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Streubel B, Vinatzer U, Willheim M, et al. Novel t(5;9)(q33;q22) fuses ITK to SYK in unspecified peripheral T-cell lymphoma. Leukemia 2006;20:313–8. [DOI] [PubMed] [Google Scholar]
- 44.Heavican TB, Bouska A, Yu J, et al. Genetic drivers of oncogenic pathways in molecular subgroups of peripheral T-cell lymphoma. Blood 2019;133:1664–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iqbal J, Wright G, Wang C, et al. Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood 2014;123:2915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monney L, Sabatos CA, Gaglia JL, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002;415:536–41. [DOI] [PubMed] [Google Scholar]
- 47.Yoon KW, Byun S, Kwon E, et al. Control of signaling-mediated clearance of apoptotic cells by the tumor suppressor p53. Science 2015;349:1261669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watatani Y, Sato Y, Miyoshi H, et al. Molecular heterogeneity in peripheral T-cell lymphoma, not otherwise specified revealed by comprehensive genetic profiling. Leukemia 2019;26. [DOI] [PubMed] [Google Scholar]
- 49.Kim YH, Tavallaee M, Sundram U, et al. Phase II investigator-initiated study of Brentuximab Vedotin in mycosis fungoides and Sézary syndrome with variable CD30 expression level: a Multi-Institution collaborative project. JCO 2015;33:3750–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flies DB, Han X, Higuchi T, et al. Coinhibitory receptor PD-1H preferentially suppresses CD4(+) T cell-mediated immunity. J Clin Invest 1966;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flies DB, Higuchi T, Chen L. Mechanistic assessment of PD-1H Coinhibitory Receptor–Induced T cell tolerance to allogeneic antigens. J.i 2015;194:5294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


