Abstract
Background
Treatment of recurrent glioblastoma (GBM) with bevacizumab can induce MRI changes that confound the determination of progression. We sought to determine the value of [18F]-fluorodeoxyglucose (FDG) positron emission tomography (PET) in GBM patients receiving bevacizumab at the time of suspected progression and, thereby, its utility as a potential prognostic adjunct in progressive disease.
Methods
This retrospective study included patients who underwent brain FDG PET within 4 weeks of receiving bevacizumab for recurrent GBM with suspected progression. Volumes-of-interest were placed over the reference lesion with measurement of maximum standardized uptake value (SUVmax), peak standardized uptake value (SUVpeak), metabolic tumor volume, total lesion glycolysis (TLG), and tumor-to-normal contralateral white matter ratios (TNR-WM). Tumors were additionally categorized as non-avid or avid based on qualitative FDG uptake. Associations between baseline variables and overall survival (OS) were examined using univariable and multivariable Cox proportional hazards regression, with P < .05 considered significant.
Results
Thirty-one patients were analyzed. Qualitative FDG uptake was significantly associated with OS (P = .03), with a median OS of 9.0 months in non-avid patients versus 4.5 months in avid patients. SUVmax, SUVpeak, TNR-WM, and TLG were significantly associated with OS (P < .001, TLG: P = .009). FDG avidity and SUVmax remained significantly associated with OS (P = .046 and .048, respectively) in the multivariable analysis including age, KPS, and MGMT status. Dichotomizing patients using an SUVmax cutoff of 15.3 was associated with OS (adjusted P = .048).
Conclusion
FDG PET is a promising imaging tool to further stratify prognosis in recurrent GBM patients on antiangiogenic therapy.
Keywords: bevacizumab, FDG, glioblastoma, positron emission tomography
Key Points.
FDG PET is negatively associated with overall survival in recurrent GBM patients on bevacizumab, including chronic treatment.
SUVmax can dichotomize these patients by overall survival.
Importance of the Study.
The ability of bevacizumab to induce changes in contrast-enhanced MRI appearance that hamper determination of GBM progression is well known. Several recent studies have evaluated alternative radiographic measures of progressive disease in this setting, many of which are not widely available and with no clear consensus. To our knowledge, the present study is the first evaluation of the prognostic value of FDG PET at the time of suspected progression in recurrent GBM patients receiving antiangiogenic therapy. We found that FDG avidity was associated with inferior overall survival in these patients and observed an association with survival when patients were dichotomized by an optimal SUVmax cutoff. Importantly, our cohort included many patients on chronic bevacizumab, mirroring its typical clinical use. These results suggest the utility of FDG PET, a broadly accessible technique, as an adjunct in further stratifying prognosis in recurrent GBM patients on antiangiogenic therapy.
Glioblastoma (GBM) is the most common malignant primary brain tumor, and recurrence is virtually inevitable. A hallmark of the disease is an extensive network of rapidly growing and abnormally tortuous blood vessels stimulated by potent angiogenic signals including vascular endothelial growth factor (VEGF). Bevacizumab, a recombinant humanized monoclonal antibody targeting VEGF-A, is approved by the Food and Drug Administration (FDA) to treat recurrent GBM. Through its direct antiangiogenic actions, bevacizumab reduces tumor vasculature and permeability while promoting the normalization of remaining tumor vasculature. Because these changes may occur independent of actual antitumor effects, the decreased contrast enhancement on MRI may confound the detection of recurrent tumors.1 The unchanged tumor viability is supported by the lack of convincing improvement in overall survival (OS) with the addition of bevacizumab in recurrent GBM despite enhanced radiographic response rates.2
To address this limitation of conventional contrast-enhanced MRI in assessing tumor viability in the context of antiangiogenic therapy, the Response Assessment in Neuro-Oncology (RANO) group suggested the incorporation of T2 or FLAIR signal hyperintensity as an ancillary indicator of treatment response independent of contrast enhancement.3 However, a multitude of processes beyond non-enhancing tumor progression, such as radiation injury, peritumoral edema, or ischemia, can also lead to an increase in T2/FLAIR signal hyperintensity.4 Moreover, there is no consensus provided as to a quantitative degree of increase in the T2/FLAIR signal that constitutes progression. Several alternative imaging techniques have thus been proposed to assess progressive disease, including perfusion imaging,5–8 diffusion-weighted imaging,9,10 radiomics,11,12 and metabolic imaging with different positron emission tomography (PET) radiotracers.13–15
While several of these approaches have shown some preliminary promise, their overwhelming focus has been on early assessment within the first month of antiangiogenic therapy. The median progression-free survival (PFS) in recurrent GBM patients on bevacizumab is approximately 4 months,2 however, and few studies have examined the utility of ancillary imaging techniques in detecting GBM progression during such prolonged treatment with bevacizumab.16 In addition, many of the explored techniques require specialized amino acid radiotracers or complex radiographic analyses that are not widely available.18F-Fluorodeoxyglucose (FDG) PET represents an accessible and FDA-approved imaging modality that has been used to differentiate radiation necrosis from tumor recurrence of enhancing brain lesions on MRI, distinguish glioma from CNS lymphoma, and diagnose opportunistic infections.17 In this retrospective study, we evaluated the prognostic value of FDG PET imaging at the time of suspected progression in recurrent GBM patients on bevacizumab.
Materials and Methods
Patient Selection
This study was approved by the local institutional review board with a waiver of informed consent. Participants were retrospectively identified according to the following criteria: (1) pathological diagnosis of WHO grade IV GBM, (2) pathological or clinical/radiographic diagnosis of recurrence, (3) age at least 18 years, (4) no known mutation in IDH1 or IDH2, and (5) imaged with18F-FDG PET while on bevacizumab therapy (defined as ≤4 weeks from the last dose) between January 2009 and January 2019. PET scans were performed as part of routine clinical care at the time of suspected progression based on RANO criteria. An experienced neuro-oncologist extracted patient data from hospital medical records including demographics, tumor and treatment characteristics, and OS. In this study, OS was defined as the time from the date of the FDG PET scan to the date of death (n = 29) or last follow-up (n = 2). Patients treated with bevacizumab at an initial diagnosis under NCT0078275616 were excluded from the analysis.
18F-Fluorodeoxyglucose PET/CT Protocol
Before the injection of 18F-FDG, all patients were required to fast for at least 6 h. If the plasma glucose level was less than 200 mg/dL, the patient was injected intravenously with 370 MBq of a radiotracer. After approximately 60–90 min uptake time, patients were scanned while in the supine position on PET/CT scanners (GE Discovery series VCT, 690, 710, GE Healthcare). Cross-calibration between the dose calibrator and PET scanners was performed monthly. Low-dose CT images obtained during PET/CT were used for attenuation correction of the PET emission scan and for anatomical orientation. PET/CT images were reconstructed using an ordered-subsets expectation maximization algorithm and a Gaussian filter using the standard manufacturer-supplied reconstruction software. The acquisition and reconstruction parameters were harmonized to minimize differences in standardized uptake values (SUVs) between scanners and keep them within 10%, as tested using measurements of the IEC image quality phantom. A spiral CT was acquired using a full helical acquisition at 1 s/rotation,150 mA, 120–140 kV; slice thickness, 3.75 mm. Immediately upon completion of the CT, a 10-min 3D PET scan was acquired. CT and PET data were reconstructed using a 30-cm field of view.
Image Interpretation, Lesion Detection, and Data Analysis
A nuclear medicine physician defined 3-dimensional volumes-of-interest (VOIs) for the lesion and normal centrum semiovale white matter on a GE Advantage workstation using the PET VCAR application of the Volume Viewer software package (v. 12.3 Ext 4, GE Healthcare 2015). Lesion location on PET imagery was confirmed by fusing PET and MRI brain axial datasets using the Neuro Registration application of the Volume Viewer software package.
For quantitative assessment, tracer VOI-based measurements of FDG uptake were quantified using SUV parameters normalized to patients’ body weight, including SUVmax the maximum voxel value in the VOI; SUVpeak the highest average SUV in any 1 cc spherical subregion of the VOI (automatically identified by the software); and SUVmean the average SUV of all voxels in the VOI. Metabolic tumor volume (MTV) (volume encompassed by a 42% isocontour around the voxel with the highest PET uptake) and total lesion glycolysis (TLG) (calculated by multiplying MTV by SUVmean) were also determined. Brain tumor volume was quantified by adapting the semiautomated delineation technique and thresholding value reported by colleagues at our institution for body PET/CT.18 Additional VOIs were then drawn in the contralateral normal white matter at the centrum semiovale. Comparative lesion uptake was quantified using target-to-normal white matter (TNR-WM) ratios defined as SUVmax(lesion)/SUVmean (white matter).
For qualitative assessment, lesions were considered non-FDG-avid if tracer uptake was less than, equivalent to, or only mildly higher than normal white matter uptake, by subjective visual analysis of PET imagery (these categories of uptake were combined due to small sample size); lesions with higher tracer uptake were considered FDG-avid, noting that viable GBM is consistently reported as more FDG-avid than white matter in other clinical settings.19 These determinations were made independently by 2 nuclear medicine physicians, with a 97% concordance rate. The single discordant case was discussed, and a consensus was reached.
Statistics
Kaplan–Meier survival curves were generated to examine the OS experience of the study cohort. Log-rank test was used to compare OS between FDG-avid and non-avid patients. Univariable Cox proportional hazards regression was used to examine associations between FDG PET parameters and OS, with a false discovery rate (FDR) adjustment applied. Multivariable Cox proportional hazards regression was used to analyze the association between FDG avidity or SUVmax and OS while controlling for other patient and tumor characteristics (age, Karnovsky Performance Status [KPS], and MGMT promoter methylation status). A significance level of .05 was used throughout.
Maximally selected rank statistics were used to identify potentially “optimal” cutoff values for SUVmax. We considered nine cutoff values (representing the 10th through 90th percentile values) for each variable. Kaplan–Meier survival curves and log-rank tests were used to explore associations between these cutoff values and OS. We adjusted the log-rank P-values to account for the testing of multiple cutoff candidates.20 All statistical computations were performed, and all output was generated using SAS Software Version 9.4 (The SAS Institute).
Results
Patient Baseline Characteristics
Thirty-one patients with recurrent GBM were included in the study (22 males and 9 females, age 36–81 years, median 54 years) (Table 1). Median KPS was 80, with a range of 40–100. Four tumors (12.9%) showed MGMT promoter methylation. Median time from initiation of bevacizumab treatment to FDG PET was 4.3 months, with a range of 0–17.4 months. This is in keeping with the median PFS for bevacizumab documented in the literature. A more detailed description of sample characteristics for each patient, including the RANO criteria for the progressive disease that were met, is included in Supplementary Table 1.
Table 1.
Patient Clinical and Radiographic Characteristics
| All Patients | FDG Non-avid | FDG Avid | ||
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Clinical characteristics | ||||
| Sample size | 31 | 7 (22.6) | 24 (77.4) | |
| Age at diagnosis | Median (range) | 54 (36–81) | 53 (36–64) | 54 (44–81) |
| Sex | Male | 22 (71) | 5 (71.4) | 17 (70.8) |
| Female | 9 (29) | 2 (28.6) | 7 (29.2) | |
| KPS | Median (range) | 80 (40–100) | 80 (60–90) | 80 (40–100) |
| MGMT status | Methylated | 4 (12.9) | 0 (0) | 4 (16.7) |
| Unmethylated | 20 (64.5) | 5 (71.4) | 15 (62.5) | |
| Unknown | 7 (22.6) | 2 (28.6) | 5 (20.8) | |
| No. of prior recurrences | Median (range) | 1 (0–4) | 1 (0–3) | 1 (0–4) |
| Time from most recent radiation (months) | Median (range) | 12.0 (1.3–51.5) | 6.3 (1.4–16.8) | 12.6 (1.3–51.5) |
| Time from bevacizumab initiation (months) | Median (range) | 4.3 (0–17.4) | 4.3 (0.1–17.4) | 4.7 (0–12.7) |
| PET parameters | ||||
| SUVmax | Median (range) | 9.7 (2.7–52.8) | 5.5 (2.7–8.7) | 11.7 (6.8–52.8) |
| SUVpeak | Median (range) | 7.4 (4.0–35.3) | 5.2 (4.0–6.2) | 7.8 (5.0–35.3) |
| MTV | Median (range) | 5.1 (0.2–47.0) | 3.2 (0.2–11.7) | 7.2 (1.1–47.0) |
| TLG | Median (range) | 26.8 (0.3–469.1) | 13.3 (0.3–58.4) | 66.0 (7.7–469.1) |
| TNR-WM | Median (range) | 3.4 (1.2–17.6) | 2.1 (1.2–3.0) | 3.9 (2.4–17.6) |
FDG PET Measurements
FDG PET measurements are summarized in Table 1. The median SUVmax for the cohort was 9.7 (range, 2.7–52.8). The median SUVpeak was 7.4 (range, 4.0–35.3), with non-measurable SUVpeak in 4 patients due to small lesion sizes. To ensure intra- and inter-individual comparability, SUVmax was also normalized to FDG avidity in contralateral white matter (TNR-WM), yielding a median of 3.4 (range, 1.2–17.6). The median MTV was 5.1 (range, 0.2–47.0) and median TLG was 26.8 (range, 0.3–469.1). Boxplots displaying the spread of individual data points for each PET parameter are included in Supplementary Figure 1.
Qualitative assessment of FDG PET scans was also performed, and patient lesions were divided into FDG non-avid and FDG avid subgroups. Seven patients (22.6%) were categorized as non-avid and 24 patients (77.4%) were categorized as avid. These patients had similar clinical characteristics, including median age at diagnosis, gender, KPS, number of prior recurrences, and time from initiation of bevacizumab therapy (Table 1). All 4 MGMT-methylated tumors were FDG avid, despite the increased incidence of pseudoprogression described in these tumors.21
Association With OS
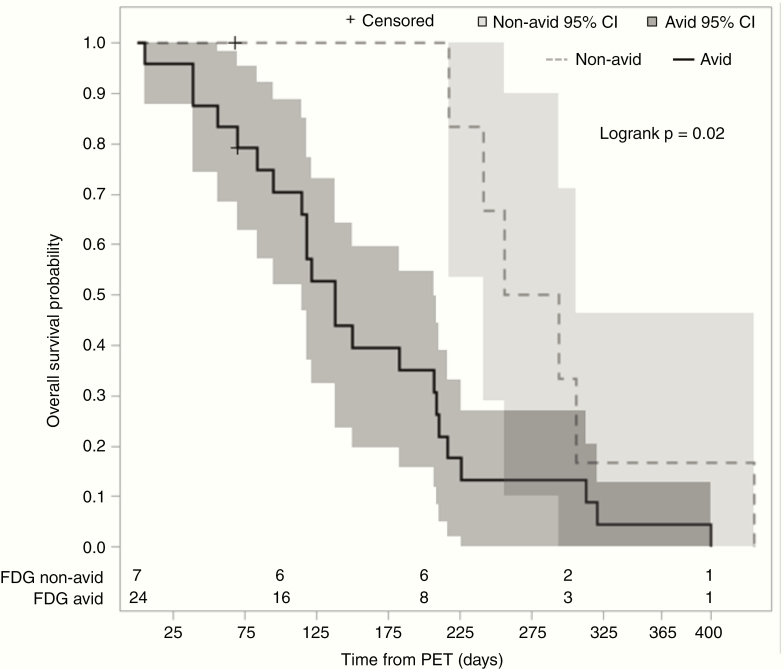
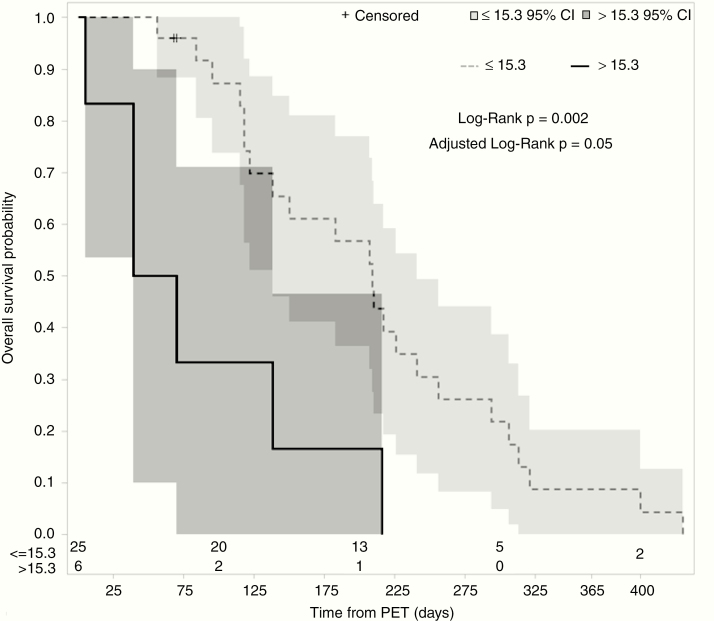
Median OS from the time of PET scan for all patients was 6.0 months (95% confidence interval [CI] 3.9–7.1). When stratified by qualitative FDG avidity, the non-avid cohort had a longer median OS of 9.0 months as compared with the avid cohort at 4.5 months. Log-rank analysis demonstrated a difference in OS between the 2 groups (P = .02, Figure 1). Univariable Cox regression analysis demonstrated a significant association of qualitative FDG avidity with inferior OS (hazard ratio [HR] 2.97; 95% CI 1.10–7.99; P = .03) (Table 2). Higher SUVmax, SUVpeak, and TNR-WM were also associated with an inferior OS on univariable Cox regression analysis (P < .001), as was TLG (P = .009) (Table 2). These associations remained significant after FDR adjustment. MTV, however, was not significantly associated with OS (P = .59). In multivariable Cox regression analysis including FDG avidity, SUVmax, age at diagnosis, KPS, and MGMT status, only FDG avidity (HR 3.49, 95% CI 1.02–11.92, P = .046) and SUVmax (HR 1.05, 95% CI 1.00–1.11, P = .048) were significantly associated with OS (Table 3). We also sought to determine an optimal cutoff for SUVmax that maximized difference in OS between the 2 groups using maximally selected rank statistics. The optimal cutoff was 15.3, with a median OS of 6.9 months (95% CI 4.5–7.9 months) in patients with SUVmax not more than 15.3 as compared with 1.8 months (95% CI 1.3–4.5 months) in patients with SUVmax more than 15.3 (adjusted P = .048, Figure 2).
Figure 1.
Qualitative FDG avidity during bevacizumab treatment predicts overall survival. Kaplan–Meier survival curves for patients separated into FDG-avid (solid line, n = 24) and FGD-non-avid (dotted line, n = 7), with 95% confidence intervals shaded. The number of patients at risk at 100-day intervals are delineated at the bottom.
Table 2.
Univariable Cox Proportional Hazards Regression Analysis of PET Parameters With OS
| Variable | HR (95% CI) | P |
|---|---|---|
| SUVmax | 1.080 (1.034–1.128) | <.001* |
| SUVpeak | 1.115 (1.046–1.189) | <.001* |
| MTV | 1.008 (0.979–1.039) | .59 |
| TLG | 1.005 (1.001–1.008) | .009* |
| TNR-WM | 1.308 (1.135–1.507) | <.001* |
| FDG avidity | ||
| Non-avid | Ref. | |
| Avid | 2.968 (1.102–7.989) | .03* |
*Significant after FDR adjustment.
Table 3.
Multivariable Cox Proportional Hazards Regression Analysis of FDG Avidity and SUVmax With OS
| Variable | HR (95% CI) | P | |
|---|---|---|---|
| FDG avidity | Non-avid | Ref. | |
| Avid | 3.49 (1.02–11.92) | .046 | |
| Age at diagnosis | 0.98 (0.92–1.04) | .5 | |
| SUVmax | 1.05 (1.00–1.11) | .048 | |
| KPS | 0.99 (0.96–1.02) | .62 | |
| MGMT | Unmethylated | Ref. | |
| Methylated | 0.53 (0.13–2.15) | .37 | |
| Unknown | 2.71 (0.78–9.34) | .11 |
Figure 2.
SUVmax during bevacizumab treatment predicts overall survival. Kaplan–Meier survival curves for patients stratified by SUVmax: SUVmax more than 15.3 (solid line, n = 6) and SUVmax not more than 15.3 (dotted line, n = 25), with 95% confidence intervals shaded. The number of patients at risk at 100-day intervals are delineated at the bottom.
Histopathological Correlation
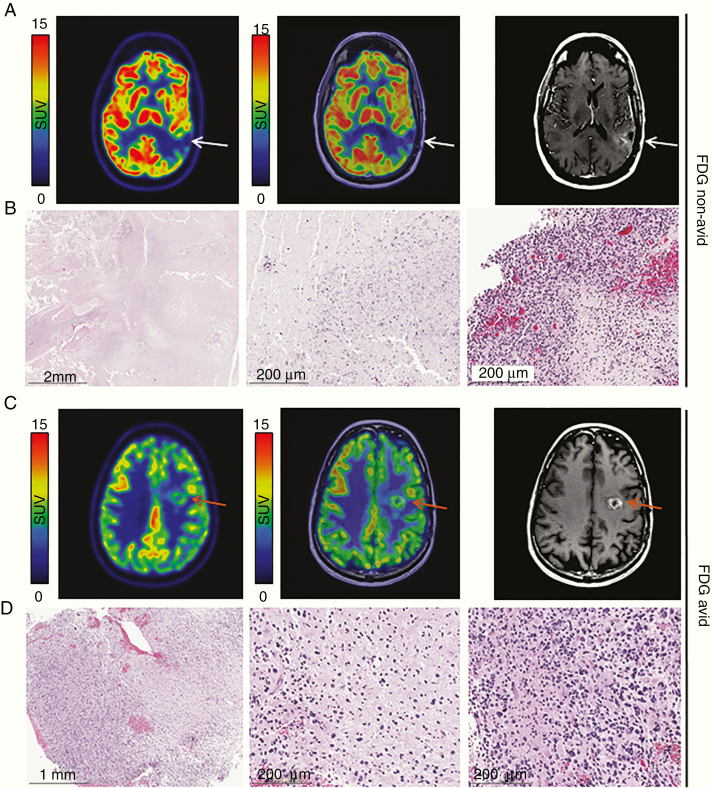
Seven patients (22.6%) had tissue available for histopathologic evaluation within 3 months of FDG PET, either due to re-resection (n = 6) or autopsy (n = 1). Of these patients, 1 (14.3%) had been categorized as non-avid and 6 (85.7%) as avid. There was strong concordance between the histopathologic findings and FDG avidity in these patients: the one non-avid lesion demonstrated predominantly necrosis with minute foci of residual malignant glioma (Figure 3A and B) while conversely a representative avid lesion showed a predominantly viable tumor with minimal necrosis (Figure 3C and D). The additional 5 avid lesions were also reported as being comprised of predominantly tumors with varying amounts of necrosis.
Figure 3.
A histopathologic correlate of FDG non-avid and FDG avid lesions. (A) Axial PET (left) and PET/MR (middle) of an FDG non-avid lesion demonstrate no distinct uptake in the left parietal region (white arrow) which enhances on post-contrast T1-weighted MRI (right). (B) On H&E, the FDG non-avid specimen consists almost entirely of necrotic tissue (left), with microcalcification, apoptotic debris (middle), and residual malignant glioma (right) focally present. (C) Axial PET (left) and PET/MR (middle) of an FDG avid lesion demonstrate focal uptake in the left frontal region (orange arrow) which enhances on post-contrast T1-weighted MRI (right). (D) On H&E, the FDG avid specimen contains minimal necrosis, consisting of predominately viable appearing tumor (left) in both highly cellular regions (right) and areas of infiltrating tumor (middle).
Discussion
Prognostication of recurrent GBM in the setting of chronic antiangiogenic therapy remains a pervasive and clinically relevant challenge.22 The present study demonstrates an important association between FDG PET avidity and OS from the time of concern for progression in recurrent GBM patients on bevacizumab therapy, suggesting its potential utility as an adjunct tool in assessing prognosis in this setting. Qualitative lesion analysis showed that patients with non-avid lesions had a median OS twice that of patients with avid lesions, with a univariable HR of 2.97. Additionally, increased quantitative PET parameters SUVmax, SUVpeak, TLG, and TNR-WM were also significantly associated with inferior OS. The association of FDG avidity and SUVmax with inferior OS was confirmed in a multivariable analysis, though it should be noted that the constraint of our small sample size may impact the reliability of this assessment. Conversely, MTV showed no significant association with OS. This is in agreement with a recent systematic review and meta-analysis assessing the prognostic value of PET parameters in glioma.23 It is important to note that accurate determination of MTV in brain tumors is complicated by the technical challenge of delineating tumor boundaries due to the high physiologic uptake of FDG in gray matter. We adapted a semiautomated approach to determine tumor volume to address this issue. An SUVmax threshold that optimally stratified patients by OS was identified. This may contribute to more broad generalizability of our findings, as SUVmax is the most commonly used quantitative PET parameter. Importantly, histopathological correlation when available was concordant with FDG avidity in every sample. This correlation reinforces the accuracy of FDG avidity as a possible indicator of viable tumor in the setting of bevacizumab therapy.
Recent studies exploring the role of PET imaging in tumor recurrence have focused on amino acid tracers, most commonly [11C]-methyl-methionine (MET), O-(2-[18F]-fluoroethyl)-l-tyrosine (FET), and 3,4-dihydroxy-6-[18F]-fluoro-L-phenylalanine (FDOPA).19,24 Such tracers have lower background uptake in normal brain tissue than FDG and so are thought to have enhanced ability to reliably identify tumor tissue. A previous study by Harris et al.25 demonstrated that FET and FDOPA uptake metrics were correlated with 3-month PFS and 6-month OS in GBM patients treated with bevacizumab. However, only FDOPA parametric response maps were significantly predictive of OS. A similar study by Beppu et al.26 investigated the utility of MET PET in this setting and found MET uptake at 8 weeks to be predictive of PFS. However, PFS is a surrogate outcome known to be disconnected from OS in the setting of bevacizumab use, and this study did not include an analysis of OS. Furthermore, both studies performed PET imaging within 2 months of bevacizumab initiation and so are unable to comment on the role of PET imaging as a biomarker in the setting of prolonged bevacizumab use. Finally, none of these amino acid tracers are FDA-approved for use in brain tumors,27 which limits their widespread use in the United States.
A few prior studies have suggested the potential prognostic utility of FDG PET in the setting of antiangiogenic therapy in malignant gliomas. FDG avidity at 4 weeks (as measured by SUVmedian) was predictive of worse OS in a study of bevacizumab monotherapy for recurrent anaplastic glioma.28 In recurrent high-grade glioma patients treated with bevacizumab and irinotecan, FDG uptake within 6 weeks of treatment initiation was the most powerful predictor of PFS and OS of all variables tested in multivariate analysis.29 The authors were also able to establish objective cutoffs, with SUVmax more than 7 and tumor-to-normal contralateral brain ratio more than 1.348 correlated with decreased OS in both univariate and multivariate analyses. While these results suggest a prognostic value of early FDG PET in this patient population, they do not inform the utility of FDG PET performed after prolonged antiangiogenic therapy. In an exploratory imaging analysis, Omuro et al.16 demonstrated that FDG avidity at 6 months was associated with worse OS. However, this phase II clinical trial enrolled newly diagnosed GBM patients and initiated bevacizumab as part of upfront therapy, in stark contrast to its current clinical use primarily at relapse. To our knowledge, the present study is the first evaluation of the prognostic value of FDG PET including recurrent GBM patients on prolonged antiangiogenic therapy.
There are several potential limitations to the current study. First, it is based on retrospective data, and as such there is inevitable variability in several patient characteristics, such as the duration of bevacizumab therapy prior to PET scan. Second, the sample size of 31 with only 7 FDG non-avid patients is relatively small which limits the power of our statistical modeling, though it is similar to the overall sample size of the prospective PET studies referenced above. Finally, this is a single-center study and thus may not capture the heterogeneity that would be seen in a multicenter study. Our results should be confirmed with a prospective study in a larger, more consistently defined patient cohort.
In conclusion, FDG PET is a promising prognostic imaging tool in recurrent GBM patients on prolonged antiangiogenic therapy and may serve as an adjunct to MRI for prognostication.
Supplementary Material
Acknowledgments
We thank Joanne Chin (Memorial Sloan Kettering Cancer Center) for her expert editorial support.
Funding
This study was partially supported by the National Institutes of Health Cancer Center Support Grant (P30 CA08748) and the National Institutes of Health/National Cancer Institute Paul Calabresi Career Development Award for Clinical Oncology (K12 CA184746 to S.K.).
Conflict of interest statement. R.J.Y. has received consulting and/or travel support from Agios, Puma, Icon, and NordicNeuroLab. The other authors of this study declare that they have no competing interests.
Authorship Statement
Study conception and design: M.S.G., S.K., T.K., and R.J.K. Study implementation: M.S.G., S.K., T.B., K.D., and M.P.D. Analysis and interpretation of data: M.S.G., S.K., S.M.L., Z.Z., M.P.D., T.K., and R.J.Y. Drafting of the manuscript and critical revision: M.S.G., S.K., T.B., K.D., S.M.L., Z.Z., M.P.D., T.K., and R.J.Y.
References
- 1. Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009;22(6):633–638. [DOI] [PubMed] [Google Scholar]
- 2. Wick W, Gorlia T, Bendszus M, et al. . Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 3. Wen PY, Macdonald DR, Reardon DA, et al. . Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 4. Ahluwalia MS, Wen PY. Antiangiogenic therapy for patients with glioblastoma: current challenges in imaging and future directions. Expert Rev Anticancer Ther. 2011;11(5):653–656. [DOI] [PubMed] [Google Scholar]
- 5. Essock-Burns E, Lupo JM, Cha S, et al. . Assessment of perfusion MRI-derived parameters in evaluating and predicting response to antiangiogenic therapy in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13(1):119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmainda KM, Zhang Z, Prah M, et al. . Dynamic susceptibility contrast MRI measures of relative cerebral blood volume as a prognostic marker for overall survival in recurrent glioblastoma: results from the ACRIN 6677/RTOG 0625 multicenter trial. Neuro Oncol. 2015;17(8):1148–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seligman L, Kovanlikaya I, Pisapia DJ, et al. . Integrated PET-MRI for glioma surveillance: perfusion-metabolism discordance rate and association with molecular profiling. AJR Am J Roentgenol. 2019;212(4):883–891. [DOI] [PubMed] [Google Scholar]
- 8. Beppu T, Sato Y, Sasaki T, et al. . Comparisons between PET with 11C-methyl-L-methionine and arterial spin labeling perfusion imaging in recurrent glioblastomas treated with bevacizumab. Clin Nucl Med. 2019;44(3):186–193. [DOI] [PubMed] [Google Scholar]
- 9. Barajas RF Jr, Butowski NA, Phillips JJ, et al. . The development of reduced diffusion following bevacizumab therapy identifies regions of recurrent disease in patients with high-grade glioma. Acad Radiol. 2016;23(9):1073–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pope WB, Qiao XJ, Kim HJ, et al. . Apparent diffusion coefficient histogram analysis stratifies progression-free and overall survival in patients with recurrent GBM treated with bevacizumab: a multi-center study. J Neurooncol. 2012;108(3):491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grossmann P, Narayan V, Chang K, et al. . Quantitative imaging biomarkers for risk stratification of patients with recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2017;19(12): 1688–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kickingereder P, Götz M, Muschelli J, et al. . Large-scale radiomic profiling of recurrent glioblastoma identifies an imaging predictor for stratifying anti-angiogenic treatment response. Clin Cancer Res. 2016;22(23):5765–5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schwarzenberg J, Czernin J, Cloughesy TF, et al. . Treatment response evaluation using 18F-FDOPA PET in patients with recurrent malignant glioma on bevacizumab therapy. Clin Cancer Res. 2014;20(13):3550–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galldiks N, Rapp M, Stoffels G, et al. . Response assessment of bevacizumab in patients with recurrent malignant glioma using [18F]Fluoroethyl-L-tyrosine PET in comparison to MRI. Eur J Nucl Med Mol Imaging. 2013;40(1):22–33. [DOI] [PubMed] [Google Scholar]
- 15. Hutterer M, Nowosielski M, Putzer D, et al. . O-(2-18F-fluoroethyl)-L-tyrosine PET predicts failure of antiangiogenic treatment in patients with recurrent high-grade glioma. J Nucl Med. 2011;52(6):856–864. [DOI] [PubMed] [Google Scholar]
- 16. Omuro A, Beal K, Gutin P, et al. . Phase II study of bevacizumab, temozolomide, and hypofractionated stereotactic radiotherapy for newly diagnosed glioblastoma. Clin Cancer Res. 2014;20(19):5023–5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Law I, Albert NL, Arbizu J, et al. . Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2019;46(3):540–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Erdi YE, Mawlawi O, Larson SM, et al. . Segmentation of lung lesion volume by adaptive positron emission tomography image thresholding. Cancer. 1997;80(12 Suppl):2505–2509. [DOI] [PubMed] [Google Scholar]
- 19. Albert NL, Weller M, Suchorska B, et al. . Response assessment in neuro-oncology working group and European association for neuro-oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18(9):1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller R, Siegmund D. Maximally selected chi square statistics. Biometrics. 1982;38(4):1011–1016. [Google Scholar]
- 21. Brandes AA, Franceschi E, Tosoni A, et al. . MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26(13):2192–2197. [DOI] [PubMed] [Google Scholar]
- 22. Hutterer M, Hattingen E, Palm C, Proescholdt MA, Hau P. Current standards and new concepts in MRI and PET response assessment of antiangiogenic therapies in high-grade glioma patients. Neuro Oncol. 2015;17(6):784–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Q, Gao X, Wei G, Qiu C, Qu H, Zhou X. Prognostic value of MTV, SUVmax and the T/N ratio of PET/CT in patients with glioma: a systematic review and meta-analysis. J Cancer. 2019;10(7):1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Langen KJ, Watts C. Neuro-oncology: amino acid PET for brain tumours—ready for the clinic? Nat Rev Neurol. 2016;12(7):375–376. [DOI] [PubMed] [Google Scholar]
- 25. Harris RJ, Cloughesy TF, Pope WB, et al. . 18F-FDOPA and 18F-FLT positron emission tomography parametric response maps predict response in recurrent malignant gliomas treated with bevacizumab. Neuro Oncol. 2012;14(8):1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beppu T, Terasaki K, Sasaki T, et al. . MRI and 11C-methyl-L-methionine PET differentiate bevacizumab true responders after initiating therapy for recurrent glioblastoma. Clin Nucl Med. 2016;41(11): 852–857. [DOI] [PubMed] [Google Scholar]
- 27. Clarke BN. PET Radiopharmaceuticals: what’s new, what’s reimbursed, and what’s next? J Nucl Med Technol. 2018;46(1):12–16. [DOI] [PubMed] [Google Scholar]
- 28. Kreisl TN, Zhang W, Odia Y, et al. . A phase II trial of single-agent bevacizumab in patients with recurrent anaplastic glioma. Neuro Oncol. 2011;13(10):1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Colavolpe C, Chinot O, Metellus P, et al. . FDG-PET predicts survival in recurrent high-grade gliomas treated with bevacizumab and irinotecan. Neuro Oncol. 2012;14(5):649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.