Abstract
Poorer adherence to medication is normal in adolescence and is one of a range of risk-taking behaviours common during a developmental stage that encompasses enormous cognitive, physical, sexual, social and emotional change. For adolescents living with human immunodeficiency virus (HIV) infection, poor adherence to antiretroviral therapy (ART) confers two significant challenges: poor health, but also the specific additional burden of onward transmission to partners. Late adolescence (15–19 years) is the only age group where HIV-associated mortality is rising, driven by poor adherence to ART and lack of access to second-line therapy, particularly amongst surviving perinatally infected young people. A previous lack of well-powered randomised multimodal behavioural ART adherence interventions specifically targeting adolescents is now being addressed and ongoing studies registered to ClinicalTrials.gov are described in the context of previous data. Accepting that despite enhanced support, some adolescents will continue to struggle with adherence, we must address how best to use existing ART agents to reduce mortality and allow adolescents the time to mature into adult life. Single-tablet regimens with a high genetic barrier to resistance based on integrase inhibitors and boosted protease inhibitors exist, but global access, in resource limited settings of young people living with HIV reside, is limited. Pragmatically, such regimens tolerate the intermittent adherence so characteristic of adolescence, preserving immune function, without the rapid evolution of resistance. The potential role of long-acting injectable ART, specifically cabotegravir and rilpivirine, is discussed and future strategies including ultra-long-acting drug-delivery systems and broadly neutralising monoclonal antibodies explored.
Keywords: adherence, adolescents, antiretroviral therapy, HIV, viral suppression
Introduction
Globally, an estimated 1.8 million children aged up to 14 years and 5 million young persons aged 15–25 years live with human immunodeficiency virus (HIV), the majority in low- and middle-income settings.1 Improved access to antiretroviral therapy (ART) has resulted in increasing numbers of perinatally infected children (PaHIV) entering adolescence and transitioning into adult care where they join their behaviourally infected (BHIV) peers.2 However, whilst ART has dramatically improved survival for all people living with HIV, late adolescence (15–19 years) is the only age group where HIV-associated mortality continues to rise, driven by the survivors of the perinatal epidemic and in stark contrast to all other age groups where mortality continues to fall.3 In both resourced and resource-limited settings, retention in care and rates of viral suppression are lower in adolescents when compared with adults or younger children.4–7
Whilst the period of transition from paediatric to adult HIV services provides a particular risk for disengagement from care, in settings where transition occurs in late adolescence, rates of viral suppression begin to decline whilst still in paediatric services and cannot solely be attributable to transition of care.8 Poorer adherence to therapy during adolescence is by no means limited to HIV and is almost universal for those growing up with chronic diseases of childhood.2 For example, glycaemic control in type 1 diabetics starts to decline from 10 years of age, continues to decline through to 18 years of age with evidence of subsequent improvement in early adult life and has been linked to cognitive and psychosocial maturity, with poorer control associated with problems of executive functioning, particularly impulse control.9–11 Executive functioning, the complex cognitive processing that allows for reasoned decision making, develops through frontal lobe maturation from early adolescence and continues into the third decade. Poorer executive functioning, reported in adolescents living with PaHIV, particularly those with a prior CDC C diagnosis, has been associated with increased rates of risk-taking behaviours including suboptimal adherence to medication.12–14 Although the challenges of negotiating many of the normal developmental tasks of adolescence are similar for those living with chronic diseases of childhood, few disease states share the added burdens of stigma and the impact of the perinatal epidemic within families including parental and sibling loss, poverty and migration. Negotiating one’s sexuality with a sexually transmissible disease before having ever had sex and the subsequent considerations around disclosure to each sexual partner potentially adds a further layer of complexity.15,16 The knowledge that undetectable equals untransmittable may act as a motivator for improving adherence for adolescents who previously had to negotiate HIV status disclosure to HIV-negative partners, and information to access pre and post exposure prophylaxis in the event of condom failure, irrespective of plasma viral load.17,18 In this context, multimodal adherence interventions, behavioural, antiretroviral and the potential for long-acting (LA) agents are explored.
Behavioural interventions
Barriers to ART adherence can be patient related (suboptimal self-management skills, lack of family/social support, HIV stigma, lack of knowledge, mental health issues, drug use, poverty), regimen related (pill number, size, frequency, tolerability, drug–drug interactions), healthcare related (lack of youth friendly services and staff, drug stock outs, transportation, insurance, clinic location and opening hours) and societal (poverty, lack of collaboration between organisations, stigma).19–21 Recent reviews of interventions to improve ART adherence in adolescents highlight the potential benefits of community group and individual adherence support, youth friendly services and eHealth, but emphasise the small number of studies that include adolescents, inadequate sample sizes without appropriate comparison groups, use of surrogate measures of adherence rather than plasma HIV viral load and frequently inadequate length of follow-up post intervention required to assess sustainability.22–25 The impact of cash transfers, both conditional and unconditional, and of multimodal economic strengthening interventions to improve child and adolescent health have been explored from pre-conception through to adolescence.26–29 Aims at improving maternal–infant outcomes, relieving poverty, retention in education, improving mental health and psychosocial well-being and reducing sexual risk including HIV acquisition effects have been mixed. In ART adherence, small early pilot studies of cash incentives showed promise, and a cluster randomised study in over 700 Ugandan 10–16 year olds showed a significant improvement in viral suppression in those receiving a multimodal savings led economic empowerment intervention, although modest.30,31 The need for rigorously evaluated multimodal ART adherence intervention studies that encompass the adolescent within the family and the wider community is being addressed; studies registered on ClinicalTrials.gov and recruiting adolescents in June 2019 are summarised in Table 1. The breadth of ongoing studies, both in terms of intervention and geographical location highlight a global research response aiming for equality, where viral suppression rates for adolescents approach those of adult populations.
Table 1.
Currently recruiting ART adherence intervention studies including adolescents living with HIV; June 2019.
| Study title | Country | Study type and intervention | n | Age of participants | Primary outcome* | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|---|
| Youth Engagement Study (YES): Intervention to increase HIV treatment engagement and adherence for young people living with HIV | US | Randomised mHealth Stigma-motivational-decision intervention Interactive versus passive texting |
400 | 16–35 | VL suppression | NCT03665532 |
| Technology Based Community Health Nursing to Improve cART adherence and Virologic Suppression in Youth living with HIV (Tech2check) | US | Randomised versus SOC Technology-enhanced community health nursing intervention |
120 | 12–25 | VL suppression | NCT03600103 |
| Adherence Interventions for HIV Youth via Text & Cell Phone - Sequential Multiple Assignment Randomized Trial (SMART) | US | Randomised – sequential Cell phone versus SMS support With versus without incentives |
190 | 15–24 | VL suppression | NCT03535337 |
| Triggered Escalating Real-time Adherence (TERA) Intervention | US | Randomised versus SOC Electronic dose monitor |
120 | 13–24 | VL suppression | NCT03292432 |
| FANMI: Community Cohort Care for HIV-Infected Adolescent Girls in Haiti | Haiti | Randomised versus SOC Community peer group |
160 | 16–23 | Retention VL suppression* |
NCT03286504 |
| Providing unique support for health (PUSH) Study for young black men who have sex with men (YBMSM) | US | Randomised versus SOC Mobile-enhanced engagement intervention |
465 | 15–24 | VL suppression | NCT03194477 |
| Connecting Youth and Young adults to optimise ART Adherence: YouTHrive efficacy trial | US | Randomised YouTHrive intervention versus weekly information only emails |
300 | 15–24 | VL suppression | NCT03149757 |
| Stepped care for youth living with HIV | US | Randomised eHealth enhanced SOC versus Stepped care: eSOC ± online peer support ± coaching | 220 | 12–24 | VL suppression | NCT03109431 |
| Positive Steps to enhanced problem solving skills (STEPS) | US | Randomised versus SOC STEPS; daily 2-way SMS ± CBT |
192 | 16–29 | VL suppression Self-report |
NCT03092531 |
| Motivational Enhancement system for Adherence (MESA) for youth starting ART | US | Randomised versus SOC 2 session computer delivered motivational intervention MESA |
200 | 16–24 | VL suppression Hair ART level Self-report |
NCT02761746 |
| Comparing the effectiveness of 2 alcohol + adherence interventions for HIV+ Youth | US | Randomised home versus clinic 4-session motivational enhancement therapy |
400 | 16–24 | VL suppression Alcohol use |
NCT01969461 |
| Virological treatment failure and drug resistance in HIV-infected Kenyan children (RESPECT) | Kenya | Single group assignment versus SOC MEMS cap monitoring |
685 | 4–19 | Viral Resistance Adherence MEMS* VL Suppression* |
NCT03120065 |
| Suubi4her: A combination intervention addressing HIV risk behaviours among older adolescent girls transitioning into adulthood in Uganda | Uganda | Randomised versus SOC Youth development account (YDA) YDA + multiple family Groups |
1260 | 14–17 | STI acquisition VL suppression* |
NCT03307226 |
| VITAL Start: Brief facility-based video intervention | Malawi | Randomised versus SOC Video-based pre-ART counselling in pregnant women |
892 | 16+ | VL suppression and retention | NCT03654898 |
| Impact of HIV Drug Resistance Testing, and Subsequent Change to an Individualized therapy in Tanzania | Tanzania | Randomised Resistance testing individualised ART versus standard second line |
1250 | 0.1–99 | VL suppression | NCT03557021 |
| A Prospective Cohort Study Evaluating a Psychosocial Programme for Adolescents Living with HIV and their caregivers in Botswana | Botswana | Observational Community support clubs ± Residential camps |
506 | 10–19 | VL suppression | NCT03571555 |
| Measuring and Monitoring adherence to ART With Pill Ingestible Sensor System | US | Randomised versus SOC Proteus digital health feedback (PDHF) system |
165 | 17+ | Adherence measured by sensor | NCT02797262 |
| Using Social Media to Improve ART Retention and Treatment (SMART) Outcomes Among Youth Living with HIV (YLHIV) in Nigeria - The Youth SMART Study | Nigeria | Randomised versus SOC Facilitated online support group |
500 | 15–22 | Retention Self-report* |
NCT03516318 |
| HIV Awal (Early) Testing and Treatment Indonesia Project in key populations: Intervention phase | Indonesia | Non-randomised multi-component includes SMS text reminders Motivational interviewing |
1000 | 16–50 | VL suppression | NCT03659253 |
| Village based versus Clinic-based ART Care – a cluster randomized controlled trial in Lesotho (VIBRA) | Lesotho | Randomised versus SOC Village based ART refill versus clinic based |
262 | 10+ | VL suppression | NCT03630549 |
| Reaching 90% HIV Suppression: The Role of Point-of-Care (POC) Viral Load Monitoring in Nigeria | Nigeria | Randomised versus SOC POC VL testing |
794 | 0–99 | VL suppression | NCT03533868 |
| PROvideMInor-friendly SErvices for Integrated TB/HIV Care in Lesotho Study (PROMISE Study) | Lesotho | Randomised versus SOC Minor-friendly TB/HIV services |
641 | 15+ | ART initiation VL suppression* Self-report* |
NCT03537872 |
| Dolutegravir, Darunavir/Ritonavir and Optimized NRTI Recycling as a Third-line Antiretroviral Regimen in Cambodia | Cambodia | Single group assignment | 54 | 15+ | VL suppression | NCT03602690 |
| Strategy for maintenance of HIV suppression with once daily integrase inhibitor + darunavir/ritonavir (DRV/r) in children (SMILE) | Multisite Global |
Randomised versus SOC Suppressed switch to DRV/r + integrase |
300 | 6–17 | VL failure | NCT02383108 |
ART, antiretroviral therapy; cART, combination antiretroviral therapy; CBT, cognitive behavioural therapy; HIV, human immunodeficiency virus; MEMS, medication electronic monitoring system; NRTI, nucleoside reverse transcriptase inhibitor; SMS, short messaging service; SOC, standard of care; VL, viral load.
Search criteria in ClinicalTrials.gov accessed June 2019: HIV; Adherence; Youth; Children.
Selected currently recruiting studies that included participants aged 10–17 years.
Outcomes measures are primary: viral load suppression (HIC) or adherence assessment and/or viral suppression included in secondary outcomes (LMIC).
Antiretroviral therapy
Accepting that adolescence is a particularly challenging time for adherence, how best can we employ existing ART regimens to support young people living with HIV into adulthood? Ideally a regimen would be well tolerated with a high genetic barrier to resistance, treat hepatitis B co-infection and have minimal drug–drug interactions, particularly with hormonal contraception and Mycobacterium tuberculosis therapy. Regimens with medium- and long-term toxicities including mood/neuropsychiatric, metabolic/lipodystrophy/weight gain, bone and renal would be avoided. Historic paediatric first line regimens based on the non-nucleoside reverse transcriptase inhibitors (NNRTI) efavirenz (EFV) and nevirapine and the older protease inhibitors (PI) such as nelfinavir resulted in high rates of triple class HIV-1 associated resistance mutations as PaHIV adolescents entered adult care.32,33 The subsequent arrival of newer agents, both within class, once-daily boosted PIs darunavir (DRV) and atazanavir and newer class integrase strand inhibitors (INSTI) dolutegravir (DTG) and bictegravir (BIC), offer convenient once-daily dosing, and increasingly when combined with a dual nucleoside backbone, as single-tablet regimens (STRs) with a high genetic barrier to resistance. A recent meta-analysis suggests that STRs are associated with improved adherence when compared with multi-tablet regimens.34
DTG, with a dual nucleoside backbone, is recommended as a first-line ART in guidelines globally and is currently licensed from 6 years old.35 In treatment-naïve adults, DTG is superior to EFV, LPV and raltegravir (RAL) in terms of viral suppression, CD4 recovery, treatment discontinuation and a lower potential for drug–drug interactions.35 DTG has a high genetic barrier to resistance; in treatment naïve studies, while rates of virological failure were low, integrase resistance was remarkably absent.36
BIC, as yet unlicensed for those under 18, demonstrates non-inferiority to DTG independent of nucleoside backbone in adults with early data suggesting a comparably high genetic barrier to resistance.37–40 Adolescent studies are ongoing with adult fixed dose combination (FDC) dosing of BIC, emtricitabine and tenofovir alafenamide (B/F/TAF) from 12 years and 35 kg with a favourable interim analysis in a stable adolescent switch study.41
Second-generation INSTIs offer robust STRs with favourable safety and tolerability profiles and a very low risk of emergent resistance in the presence of virological failure, ideal for adolescents where intermittent adherence is predictable and maintaining immune function; reducing HIV-associated morbidity and mortality is sometimes a more realistic goal. World Health Organization (WHO) recommendations for DTG based ART as first-line therapy for all age groups reflect the advantages of DTG versus EFV-based ART in terms of tolerability and accumulation of resistance.35 However concerns emerged in May 2018 of a potential increased risk of neural tube defects in infants born to women who conceived on DTG in an observational cohort from Botswana.42 This association has not yet been reported in other cohorts and a public health approach suggests a substantial benefit switching to DTG-based regimens, including for adolescents and women of child bearing age in sub-Saharan Africa,43,44 particularly in the context of rising levels of NNRTI resistant virus.45 Certainly, the individual cost for a young woman switching from a well-tolerated single-tablet DTG containing regimen to a boosted PI (bPI) in the first trimester of pregnancy and subsequent viral rebound is documented.46 Adolescence is the peak age of first pregnancy globally and, hence, for young women living with HIV, the DTG concerns in pregnancy presented impediment to global switch however more recent data supports the use of DTG in women of child bearing potential.
Transmitted drug resistance (TDR) is of concern, potentially for recently infected young adults living with BHIV but more significantly for perinatally infected infants, although reassuringly to date, TDR impacting on integrase remains extremely infrequent.47–49 Global rates of perinatal transmission have declined by 35% since 2010, however worrying rates of pre-treatment drug resistance are reported in infants: 30% in an Argentinian cohort with and without exposure to ART for prevention of mother to child transmission; 5% to PIs, 9% to NRTIs and 18% to NNRTIs.50,51 Such infants have treatment options limited to regimens based around boosted lopinavir and RAL.52 Whether the global role out of DTG based ART for women of child-bearing age will result in a rise in TDR to INSTIs in infants requires surveillance as such mutations will impact on RAL and on DTG when infant formulations of the latter become available.
In treatment-experienced patients, more representative of the current cohort of perinatally infected adolescents, DTG was superior to RAL in terms of viral suppression and fewer treatment-emergent INSTI resistance mutations when added to an optimised background.53 In highly treatment experienced adults with INSTI resistance due to prior RAL/elvitegravir exposure, twice-daily DTG with an optimised regimen achieved viral suppression in almost two thirds.54 In treatment-experienced adolescents, DTG appears safe, well tolerated and with an optimised background, achieved viral suppression rates of 44–66% whilst acknowledging the challenges of established adherence issues.55,56 One adolescent acquired treatment-emergent INSTI resistance.56 For adolescents with dual class resistance living in resourced settings, the introduction of the first bPI STR – DRV/cobisistat/F/TAF in combination with DTG – offers the potential of a potent triple class regimen with a high genetic barrier to resistance in only two pills once daily.57 An analysis of seven adult studies of once-daily DRV reported treatment-emergent DRV resistance of <0.1%.58
For adolescents struggling with adherence, even with the potential risk of further DR developing, the risks associated with stopping ART completely far outweigh the benefits of retention on some ART due to poorer immunological outcomes and should be avoided.59 The pill size – and for those with access only to lopinavir, pill number and frequency – and gastrointestinal toxicity associated with bPIs has been a challenge for many adolescents who have failed first-line NNRTI-based therapy. In the Dawning study, adults failing first-line NNRTI-based therapy (n = 627) were randomised to either DTG or ritonavir boosted lopinavir (LPV/r) with an investigator led choice of dual nucleoside backbone, one of which was fully active on resistance testing.60 At week 48, 84% in the DTG group achieved viral suppression compared with 70% on LPV/r [adjusted difference 13.8%; 95% confidence interval (CI) 7.3–20.3: p < 0.0001]. More grade 2–4 drug-related adverse events (14% LPV/r versus 4% DTG) were reported with LPV/r, driven by gastrointestinal disorders. Whilst LPV/r is virologically inferior to DRV/r and a higher pill burden, the option of DTG as second line therapy in combination with F/TAF is attractive, currently in resourced settings, as two small pills, generally well tolerated and increasingly used in our adolescent practice as part of a multimodal approach to supporting adherence61,62 (Figure 1). The recent interim analysis of the Gilead 4030 switch study suggests that even with high levels of archived NRTI resistance, adults suppressed on DTG and emtricitabine and tenofovir alafenamide (F/TAF) or emtricitabine and tenofovir disoproxil fumarate (F/TDF) randomised 1:1 switch to B/F/TAF maintained very high rates of viral suppression, a second-generation option for a small single pill with a high genetic barrier to resistance, although further data of this strategy in second line is required.63
Figure 1.
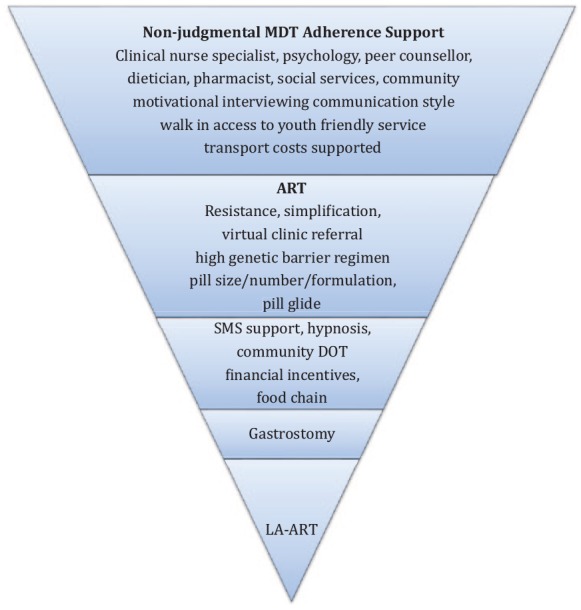
900 Clinic schematic approach to individualised patient support with established poor adherence to ART.
ART, antiretroviral therapy; DOT, directly observed therapy; LA-ART, long acting antiretroviral therapy; MDT, multidisciplinary team; SMS, short message service.
WHO guidance recommends DTG in combination with an optimised NRTI backbone as the preferred second line regimen for adolescents and children with approved DTG dosing.17 Previous ‘holding’ regimens such as lamivudine monotherapy following first-line failure are associated with immunological decline and highlight the urgent need for global access to second-line therapy.64,65 Yet despite two decades of marked improvement in both access to ART and in pill burden, frequency, toxicity and tolerability, some continue to struggle with daily oral medication and perhaps LA formulations offer a welcome alternative, being used with some success in therapeutic areas such as contraception and mental health.
LA injectable formulations for the treatment of HIV-1
At least 15 LA ART agents are being investigated in preclinical or advanced clinical trials, both in prevention of HIV acquisition and in treatment of established infection.66 For HIV treatment, the NNRTI rilpivirine (RPV), already licensed as a once-daily oral agent (FDC with F/TDF, F/TAF or as dual maintenance therapy with DTG) is currently in phase III clinical trials as an injectable nanosuspension. LATTE2, a phase IIb study of safety, efficacy and tolerability of RPV-LA combined with the long acting INSTI cabotegravir (CAB) as maintenance therapy in adults achieving viral suppression on oral CAB/lamivudine (3TC) and abacavir (ABC).67 LA-RPV/CAB given either four or eight weekly intervals was as effective as oral CAB/3TC/ABC, was safe and generally well tolerated, with injection site reactions, moderate in 15%, rarely leading to discontinuation (<1%). Recent data presented from the subsequent ongoing phase III studies, ATLAS and FLAIR, injectable LA-RPV/CAB administered every 4 weeks demonstrated non-inferiority to oral triple therapy and whilst rates of virological failure were low, treatment emergent INSTI resistance was reported.68,69 Participants aged 18 and older were on suppressive ART without history of virological failure (ATLAS) and treatment naïve (FLAIR). A dose-finding study of oral and LA-CAB and for LA-RPV is currently recruiting in virally suppressed adolescents age 12–17 years weighing at least 35 kg.70 Whilst awaiting licensing, LA-RPV/CAB is available on compassionate basis and individual case reports of viral suppression in adolescents/young adults with a history of poor adherence are encouraging and includes those with limited prior resistance in reverse transcriptase.71 The long-acting therapy to improve treatment success in early life (LATITUDE) study aims to examine this strategy in more detail, randomising adults age 18 and over with a prior history of poor adherence and/or loss to follow up, but without NNRTI or INSTI resistance, to either LA-RPV/CAB or oral standard of care, which may exclude many young people with previous failure on first line therapy.72 All require a 20-week oral lead in period and to have achieved a viral load <400 copies/ml prior to randomisation. It remains to be seen how adolescents struggling with oral medication will achieve viral suppression prior to maintenance therapy with LA injectables and subsequently adhere to visits every 4 weeks and injection tolerability. Extending the injection interval to 8 weeks is being explored in an extension of the LATTE study and would be favourable to both patients and providers.73 Injection-site reactions are common and whilst tolerated within clinical trials, a selected population willing to both participate in a clinical trial and opt for one encompassing injections within a real-world setting in an adolescent population is untried. An additional challenge for the implementation of injectable ART amongst this hard to reach group is the requirement to attend injection visits. For those not attending repeat dosing visits, discontinuation of LA-ART results in subtherapeutic plasma levels of CAB and RPV that decline over time with the risk of treatment emergent resistance.74 Virological failure in the setting of LA-CAB/RPV has resulted in treatment emergent INSTI and NNRTI resistance, albeit infrequently in clinical trials, but leading to future treatment option challenges.67–69 Owing to their long half-lives, measurable levels of LA-CAB and LA-RPV have been reported in some individuals a year after cessation and current guidance is for a transition to oral ART for those deciding to stop LA-ART. This may be a challenge in an adolescent population who chose LA-ART because of challenges raised by adherence to daily oral ART.4 Perhaps lessons can be learnt from other LA agents. Discontinuations of the contraceptive injectable depot medoxyprogesterone acetate (Depot Provera) are greater than 40% at 12 months.75 Evidence suggest rates of continuation can be improved through self versus provider administration (73% versus 45%; incidence rate ratio of 0.40 (95% CI 0.31–0.51; p < 0.0001), improving local community access, adherence counselling and text reminders.76–78 The recently published ECHO study of the impact of long-acting contraceptives and HIV acquisitions, highlights the very real need for closer integration of reproductive health and HIV services, both for prevention and treatment.79 Implementation studies focusing on reducing subsequent discontinuation of LA- ART as treatment for HIV-1 should run in parallel with their introduction outside of clinical trials. Whilst lessons learnt from, and linkage to, LA contraceptive services may improve outcomes for young women, whether this is translatable to young men is unknown, however early data on the use of LA- ART antipsychotics in adolescents (80% male) suggests improvement in adherence and clinical outcome measures.80 Even with injection intervals extended to 8 weeks delivery and managing cessation, this will remain a challenge for both adherence and healthcare provision in many settings and longer-acting agents are required, perhaps comparable with the contraceptive implant or intrauterine system.81 Ultra-LA drug-delivery systems that deliver ART but are removable have considerable potential advantages, particularly in reducing the risk of acquiring resistance mutations on cessation and are currently being developed in animal models.82,83
Future strategies
Alternative non-ART approaches to controlling viral replication have a potentially important role for optimal management of adolescents living with HIV. HIV-specific broadly neutralising monoclonal antibodies (bNAbs) have been shown in animal models to have the potential to both prevent viral acquisition, to suppress viral replication and to delay viral rebound following treatment interruption.84,85 Phase I clinical trials suggest that potent bNAbs to HIV-positive participants with bNAb sensitive viral reservoirs given in combination can both maintain viral suppression post ART interruption and in untreated patients reduce viraemia in selected patients with an antibody-sensitive virus.86,87 Whilst bNAbs are under investigation in adult adherent cohorts as a potential HIV ‘cure’ approach, they may have a key alternative value as a mode of viral control off daily oral ART amongst vulnerable youth who struggle to adhere to current therapies. The latter raises the important question as to the potential future role of combination bNAbs infusions for adolescents unable to adhere to ART, or with multi-class ART drug-resistant virus, potentially in conjunction with LA-ART where appropriate.
Conclusion
PaHIV entering adolescence and transitioning to adult care continue to face significant challenges, including sustained adherence to ART in all settings. Encouragingly the data gap of well-powered controlled studies of multimodal interventions to support retention and adherence is being addressed. However once-daily STRs with a high genetic barrier to resistance are already available; the challenge is rapid global access for a vulnerable hard to reach population. Encouragingly if (ultra-)LA-ART prove safe and effective, the potential for combining with implantable contraceptives would offer a truly youth-friendly future for those living with HIV and as pre-exposure prophylaxis for their uninfected peers.
Footnotes
Author contributions: CF conceived the article and wrote the initial draft. SA and SF commented on, edited and contributed to the final draft.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Caroline Foster  https://orcid.org/0000-0001-8278-8564
https://orcid.org/0000-0001-8278-8564
Contributor Information
Caroline Foster, The 900 Clinic, Imperial College Healthcare NHS Trust, London W2 1NY, UK.
Sara Ayers, Imperial College Healthcare NHS Trust, London, UK.
Sarah Fidler, Imperial College Healthcare NHS Trust, London, UK.
References
- 1. World Health Organization. HIV and youth 2018, https://www.who.int/maternal_child_adolescent/topics/adolescence/hiv/en/ (accessed January 2019).
- 2. Foster C, Fidler S. Optimising HIV transition services for young adults. Curr Opin Infect Dis 2018; 31: 33–38. [DOI] [PubMed] [Google Scholar]
- 3. Slogrove AL, Mahy M, Armstrong A, et al. Living and dying to be counted: what we know about the epidemiology of the global adolescent HIV epidemic. J Int AIDS Soc 2017; 20: 474–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Enane L, Vreeman R, Foster C. Retention and adherence: the global challenge for adolescents and young adults living with HIV. Curr Opin HIV AIDS 2018; 13: 212–219. [DOI] [PubMed] [Google Scholar]
- 5. Zanoni BC, Archary M, Buchan S, et al. Systematic review and meta-analysis of the adolescent HIV continuum of care in South Africa: the cresting wave. BMJ Glob Health 2016; 1: e000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zanoni BC, Mayer KH. The adolescent and young adult HIV cascade of care in the United States: exaggerated health disparities. AIDS Patient Care STDS 2014; 28: 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lally MA, van den Berg JJ, Westfall AO, et al. HIV continuum of care for youth in the United States. J Acquir Immune Defic Syndr 2018; 77: 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Judd A, Collins J, Parrott F, et al. Growing up with perinatal HIV: changes in clinical outcomes before and after transfer to adult care in the UK. J Int AIDS Soc 2017; 20(Suppl. 3): 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clements MA, Foster NC, Maahs DM, et al. Hemoglobin A1c (HbA1c) changes over time among adolescent and young adult participants in the T1D exchange clinic registry. Pediatr Diabetes 2016; 17: 327–336. [DOI] [PubMed] [Google Scholar]
- 10. Broadley MM, White MJ, Andrew B. A systematic review and meta-analysis of executive function performance in type 1 diabetes mellitus. Psychosom Med 2017; 79: 684–696. [DOI] [PubMed] [Google Scholar]
- 11. Silva K, Miller VA. The role of cognitive and psychosocial maturity in type 1 diabetes management. J Adolesc Health 2019; 64: 622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenberg M, Pettifor A, Duta M, et al. Executive function associated with sexual risk in young South African women: findings from the HPTN 068 cohort. PLoS One 2018; 13: e0195217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nichols SL, Chernoff MC, Malee KM, et al. Executive functioning in children and adolescents with perinatal HIV infection and perinatal HIV exposure. J Pediatric Infect Dis Soc 2016; 5(Suppl. 1): S15–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Judd A, Le Prevost M, Melvin D, et al. Cognitive function in young persons with and without perinatal HIV in the AALPHI cohort in england: role of non-HIV-related factors. Clin Infect Dis 2016; 63: 1380–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Judd A, Foster C, Thompson LC, et al. Sexual health of young people with perinatal HIV and HIV negative young people in England. PLoS One 2018; 13: e0205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abrams EJ, Mellins CA, Bucek A, et al. Behavioral health and adult milestones in young adults with perinatal HIV infection or exposure. Pediatrics 2018; 142: pii: e20180938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greenhalgh C, Evangeli M, Frize G, et al. Intimate relationships in young adults with perinatally acquired HIV: a qualitative study of strategies used to manage HIV disclosure. AIDS Care 2016; 28: 283–288. [DOI] [PubMed] [Google Scholar]
- 18. Rodger AJ, Cambiano V, Bruun T, et al; PARTNER Study Group. Sexual activity without condoms and risk of hiv transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA 2016; 316: 171–181. [DOI] [PubMed] [Google Scholar]
- 19. Mesic A, Halim N, MacLeod W, et al. Facilitators and barriers to adherence to antiretroviral therapy and retention in care among adolescents living with HIV/AIDS in Zambia: a mixed methods study. AIDS Behav 2019; 23: 2618–2628. [DOI] [PubMed] [Google Scholar]
- 20. Ammon N, Mason S, Corkery JM. Factors impacting antiretroviral therapy adherence among human immunodeficiency virus-positive adolescents in Sub-Saharan Africa: a systematic review. Public Health 2018; 157: 20–31. [DOI] [PubMed] [Google Scholar]
- 21. Kim SH, McDonald S, Foster C, et al. Importance of self-motivation and social support in medication adherence in HIV-infected adolescents in the United Kingdom and Ireland: a multicentre HYPNet study. AIDS Patient Care STDS 2015; 29: 354–364. [DOI] [PubMed] [Google Scholar]
- 22. Ridgeway K, Dulli LS, Murray KR, et al. Interventions to improve antiretroviral therapy adherence among adolescents in low- and middle-income countries: a systematic review of the literature. PLoS One 2018; 13: e0189770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reif LK, McNairy ML, Lamb MR, et al. Youth-friendly services and differentiated models of care are needed to improve outcomes for young people living with HIV. Curr Opin HIV AIDS 2018; 13: 249–256. [DOI] [PubMed] [Google Scholar]
- 24. Mulawa MI, LeGrand S, Hightow-Weidman LB. eHealth to enhance treatment adherence among youth living with HIV. Curr HIV/AIDS Rep 2018; 15: 336–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Casale M, Carlqvist A, Cluver L. Recent interventions to improve retention in HIV care and adherence to antiretroviral treatment among adolescents and youth: a systematic review. AIDS Patient Care STDS 2019; 33: 237–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barker M, Dombrowski SU, Colbourn T, et al. Intervention strategies to improve nutrition and health behaviours before conception. Lancet 2018; 391: 1853–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Owusu-Addo E, Renzaho AMN, Smith BJ. The impact of cash transfers on social determinants of health and health inequalities in Sub-Saharan Africa: a systematic review. Health Policy Plan 2018; 33: 675–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pega F, Liu SY, Walter S, et al. Unconditional cash transfers for reducing poverty and vulnerabilities: effect on use of health services and health outcomes in low- and middle-income countries. Cochrane Database Syst Rev 2017; 11: CD011135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hosek S, Pettifor A. HIV prevention interventions for adolescents. Curr HIV/AIDS Rep 2019; 16: 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Foster C, McDonald S, Frize G, et al. “Payment by Results”–financial incentives and motivational interviewing, adherence interventions in young adults with perinatally acquired HIV-1 infection: a pilot program. AIDS Patient Care STDS 2014; 28: 28–32. [DOI] [PubMed] [Google Scholar]
- 31. Bermudez LG, Ssewamala FM, Neilands TB, et al. Does economic strengthening improve viral suppression among adolescents living with HIV? Results from a cluster randomized trial in Uganda. AIDS Behav 2018; 22: 3763–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Collins IJ, Foster C, Tostevin A, et al. Clinical status of adolescents with perinatal HIV at transfer to adult care in the UK/Ireland. Clin Infect Dis 2017; 64: 1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Judd A, Lodwick R, Noguera-Julian A, et al. Higher rates of triple-class virological failure in perinatally HIV-infected teenagers compared with heterosexually infected young adults in Europe. HIV Med 2017; 18: 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Altice F, Evuarherhe O, Shina S, et al. Adherence to HIV treatment regimens: systematic literature review and meta-analysis. Patient Prefer Adherence 2019; 13: 475–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. World Health Organization. Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV. WHO interim guidance, https://apps.who.int/iris/bitstream/handle/10665/277395/WHO-CDS-HIV-18.51-eng.pdf?ua=1 (2018, accessed 1 June 2019).
- 36. Walmsley S, Baumgarten A, Berenguer J, et al. Brief report: dolutegravir plus abacavir/lamivudine for the treatment of HIV-1 infection in antiretroviral therapy-naive patients: week 96 and week 144 results from the SINGLE randomized clinical trial. J Acquir Immune Defic Syndr 2015; 70: 515–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stellbrink HJ, Arribas JR, Stephens JL, et al. Co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV 2019; 6: e364–e372. [DOI] [PubMed] [Google Scholar]
- 38. Wohl DA, Yazdanpanah Y, Baumgarten A, et al. Bictegravir combined with emtricitabine and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV 2019; 36: e355–e363. [DOI] [PubMed] [Google Scholar]
- 39. Sax PE, Pozniak A, Montes ML, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet 2017; 390: 2073–2082. [DOI] [PubMed] [Google Scholar]
- 40. Oliveira M, Ibanescu RI, Anstett K, et al. Selective resistance profiles emerging in patient-derived clinical isolates with cabotegravir, bictegravir, dolutegravir, and elvitegravir. Retrovirology 2018; 15: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ghaur A, Rodriguez C, Hellstrom E, et al. Bictegravir/FTC/TAF single tablet regimen in adolescents: 24 week results. Presented at Conference on Retroviruses ad Oppoutunistic Infections, 4-7 March 2018, Boston, MA Poster Abstract 844. [Google Scholar]
- 42. World Health Organization Statement on DTG. Potential safety issue affecting women living with HIV using dolutegravir at the time of conception, www.who.int/medicines/publications/drugalerts/Statement_on_DTG_18May_2018final.pdf (2018, accessed June 2019).
- 43. Zash R, Makhema J, Shapiro RL. Neural-tube defects with dolutegravir treatment from the time of conception. N Engl J Med 2018; 379: 979–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Phillips AN, Venter F, Havlir D, et al. Risks and benefits of dolutegravir-based antiretroviral drug regimens in Sub-Saharan Africa: a modelling study. Lancet HIV 2019; 6: e116–e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hunt GM, Ledwaba J, Salimo A, et al. Prevalence of HIV-1 drug resistance amongst newly diagnosed HIV- infected infants age 4-8 weeks, enrolled in three nationally representative PMTCT effectiveness surveys, South Africa 2010, 2011-12, 2012-13. BMC Infect Dis 2019; 19(Suppl 1): 787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Foster C, Fidler S, Lyall E, et al. Careful consideration when responding to new data: dolutegravir and pregnancy. J Virus Erad 2018; 4: 208. [PMC free article] [PubMed] [Google Scholar]
- 47. Rutstein SE, Chen JS, Nelson JAE, et al. High rates of transmitted NNRTI resistance among persons with acute HIV infection in Malawi: implications for first-line dolutegravir scale-up. AIDS Res Ther 2019; 16: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Spertilli Raffaelli C, Rossetti B, Paglicci L, et al. Impact of transmitted HIV-1 drug resistance on the efficacy of first-line antiretroviral therapy with two nucleos(t)ide reverse transcriptase inhibitors plus an integrase inhibitor or a protease inhibitor. J Antimicrob Chemother 2018; 73: 2480–2484. [DOI] [PubMed] [Google Scholar]
- 49. Inzaule SC, Jordan MR, Cournil A, et al. Increasing level of pretreatment HIV drug resisatnce and safety concerns for dolutegravir use in women of reproductive age. AIDS 2019; 33: 1797–1799. [DOI] [PubMed] [Google Scholar]
- 50. UNAIDS. Global HIV & AIDS statistics -2018 fact sheet, https://www.unaids.org/en/resources/fact-sheet (2018, accessed 1 June 2019).
- 51. Aulicino PC, Zapiola I, Kademian S, et al. Pre-treatment drug resistance and HIV-1 subtypes in infants from Argentina with and without exposure to antiretroviral drugs for prevention of mother-to-child transmission. J Antimicrob Chemother 2019; 74: 722–730. [DOI] [PubMed] [Google Scholar]
- 52. Paediatric European Network for Treatment of AIDS (PENTA). First and second line antiretroviral treatment guidelines, https://penta-id.org/hiv/treatment-guidelines/ (2019, accessed 28 June 2019).
- 53. Cahn P, Pozniak AL, Mingrone H, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 2013; 382: 700–708. [DOI] [PubMed] [Google Scholar]
- 54. Castagna A, Maggiolo F, Penco G, et al. Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J Infect Dis 2014; 210: 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Briand C, Dollfus C, Faye A, et al. Efficacy and tolerance of dolutegravir-based combined ART in perinatally HIV-1-infected adolescents: a French multicentre retrospective study. J Antimicrob Chemother 2017; 72: 837–843. [DOI] [PubMed] [Google Scholar]
- 56. Viani RM, Ruel T, Alvero C, et al. Long-term safety and efficacy of dolutegravir in treatment-experienced adolescents with human immunodeficiency virus infection: results of the IMPAACT P1093 study. J Pediatric Infect Dis Soc. Epub ahead of print 5 April 2019. DOI: 10.1093/jpids/piy139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. SYMTUZA®. Symtuza product insert: Patheon Inc., Mississauga, Canada, http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/SYMTUZA-pi.pdf (accessed 1 June 2019).
- 58. Lathouwers E, Wong EY, Luo D, et al. HIV-1 resistance rarely observed in patients using darunavir once-daily regimens across clinical studies. HIV Clin Trials 2017; 18: 196–204. [DOI] [PubMed] [Google Scholar]
- 59. Fairlie L, Karalius B, Patel K, et al. CD4+ and viral load outcomes of antiretroviral therapy switch strategies after virologic failure of combination antiretroviral therapy in perinatally HIV-infected youth in the United States. AIDS 2015; 29: 2109–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Aboud M, Kaplan R, Lombaard J, et al. Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): an open-label, non-inferiority, phase 3b trial. Lancet Infect Dis 2019; 19: 253–264. [DOI] [PubMed] [Google Scholar]
- 61. Bánhegyi D, Katlama C, da Cunha CA, et al. Week 96 efficacy, virology and safety of darunavir/r versus lopinavir/r in treatment-experienced patients in TITAN. Curr HIV Res 2012; 10: 171–181. [DOI] [PubMed] [Google Scholar]
- 62. Foster C, Ayers S, McDonald S, et al. Post transition outcomes for young adults living with HIV:90:99:80. Presented at the 22nd International AIDS Conference 23-27 July 2018, Amsterdam, Holland. THPEB159. [Google Scholar]
- 63. Acosta R, Willkom M, Andreatta K, et al. High level of preexisiting NRTI resistance prior to switching to B/F/TAF:Study 4030. Presented at Conference on Retroviruses and Opportunistic Infections, 4–7 March 2019, Seattle, WA, Abstract 551. [Google Scholar]
- 64. Patten G, Schomaker M, Davies MA, et al. What should we do when HIV-positive children fail first-line combination antiretroviral therapy? A comparison of 4 ART management strategies. Pediatr Infect Dis J 2019; 38: 400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Patten G, Bernheimer J, Fairlie L, et al. Lamivudine monotherapy as a holding regimen for HIV-positive children. PLoS One 2018; 13: e0205455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Singh K, Sarafianos SG, Sönnerborg A. Long-acting anti-HIV drugs targeting HIV-1 reverse transcriptase and integrase. Pharmaceuticals (Basel) 2019; 12: pii: E62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 2017; 390: 1499–1510. [DOI] [PubMed] [Google Scholar]
- 68. Swindells S, Andrade-Villanueva JF, Gary J, et al. Long-acting cabotegravir+rilpivirine maintenance therapy: atlas week 48 results. Presented at Conference on Retroviruses and Opportunistic Infections, 4–7 March 2019, Seattle, WA Abstract 139. [Google Scholar]
- 69. Orkin C, Arastéh K, Hernández-Mora MC, et al. Long-acting cabotegravir + rilpivirine for HIV maintenance: flair week 48 results. Conference on Retroviruses and Opportunistic Infections, 4–7 March 2019, Seattle, WA: Abstract 140. [Google Scholar]
- 70. More Options for Children and Adolescents (MOCHA) Study. Oral and long-acting cabotegravir and long-acting rilpivirine in HIV-infected children and adolescents, https://aidsinfo.nih.gov/clinical-trials/details/NCT03497676. (accessed 3 June 2019).
- 71. Chiltern D, Mukela A, Ali A, et al. Long acting (LA) injectable ARVs; two case studies of compassionate access to LA cabotegravir and rilpivirine in young adults with perinatally acquired HIV-1 Presented at 25th Annual Conference of the British HIV Association (BHIVA), 2-5 April 2019, Bornemouth: Abstract P11. [Google Scholar]
- 72. ClinicalTrials.gov. The long-acting therapy to improve treatment success in daily life (LATITUDE) study, https://aidsinfo.nih.gov/clinical-trials/details/NCT03635788 (accessed 3 June 2019).
- 73. ClinicalTrials.gov. Phase 2b, open-label, multicenter, rollover study to assess antiviral activity and safety of long-acting cabotegravir (cab la) plus long-acting rilpivirine (RPV LA), administered every 2 months (Q2M), in HIV - positive subjects from the LATTE study, https://clinicaltrials.gov/ct2/show/NCT03639311?term=LONG+ACTING&cond=HIV&rank=1 (accessed 6 June 2019).
- 74. Margolis D, Boffito M. Long-acting antiviral agents for HIV treatment. Curr Opin HIV AIDS 2015; 10: 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gallo MF, Grimes DA, Lopez LM, et al. Combination injectable contraceptives for contraception. Cochrane Database Syst Rev 2013; 3: CD004568. [PubMed] [Google Scholar]
- 76. Burke HM, Chen M, Buluzi M, et al. Effect of self-administration versus provider-administered injection of subcutaneous depot medroxyprogesterone acetate on continuation rates in Malawi: a randomised controlled trial. Lancet Glob Health 2018; 6: e568–e578. [DOI] [PubMed] [Google Scholar]
- 77. Mack N, Crawford TJ, Guise JM, et al. Strategies to improve adherence and continuation of shorter-term hormonal methods of contraception. Cochrane Database Syst Rev 2019; 4: CD004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Buchanan CRM, Tomaszewski K, Chung SE, et al. Why didn’t you text me? Poststudy trends from the depotext trial. Clin Pediatr (Phila) 2018; 57: 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Evidence for Contraceptive Options and HIV Outcomes (ECHO) Trial Consortium. HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: a randomised, multicentre, open-label trial. Lancet 2019; 394: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lytle S, McVoy M, Sajatovic M. Long acting injectable antipsychotics in children and adolescents. J Child Adolesc Psychopharmacol 2017; 27: 2–9. [DOI] [PubMed] [Google Scholar]
- 81. Cohen R, Sheeder J, Teal SB. Predictors of discontinuation of long-acting reversible contraception before 30 months of use by adolescents and young women. J Adolesc Health 2019; 65: 295–302. [DOI] [PubMed] [Google Scholar]
- 82. Kovarova M, Benhabbour SR, Massud I, et al. Ultra-long-acting removable drug delivery system for HIV treatment and prevention. Nat Commun 2018; 9: 4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Flexner C. Antiretroviral implants for treatment and prevention of HIV infection. Curr Opin HIV AIDS 2018; 13: 374–380. [DOI] [PubMed] [Google Scholar]
- 84. Julg B, Barouch DH. Neutralizing antibodies for HIV-1 prevention. Curr Opin HIV AIDS 2019; 14: 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cohen YZ, Caskey M. Broadly neutralizing antibodies for treatment and prevention of HIV-1 infection. Curr Opin HIV AIDS 2018; 13: 366–373. [DOI] [PubMed] [Google Scholar]
- 86. Mendoza P, Gruell H, Nogueira L, et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 2018; 561: 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bar-On Y, Gruell H, Schoofs T, et al. Safety and antiviral activity of combination HIV-1 broadly neutralizing antibodies in viremic individuals. Nat Med 2018; 24: 1701–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]


