Abstract
Bispecific T-cell engaging antibodies are constructs engineered to bind to two different antigens, one to a tumor-specific target and the other to CD3-positive T cells or natural killer (NK) cells. Blinatumomab engages CD19 and CD3, performing effective serial lysis. The clinical development program in acute lymphoblastic leukemia (ALL) includes clinical trials in relapsed or refractory (R/R) patients and in B-cell precursor (BCP) ALL patients with measurable residual disease. Several trials are currently being conducted in de novo BCP-ALL, either in induction, consolidation, or before or after hematopoietic stem cell transplant. Combination with other targeted therapies or with other immunotherapeutic approaches are also underway. Several strategies are aimed to optimize the use of blinatumomab either by overcoming the mechanisms of resistance (e.g. inhibition of PD-1/PD-L1) or by improvements in the route of application, among others.
Keywords: acute lymphoblastic leukemia, B-cell precursor, bispecific antibodies, blinatumomab, review
Introduction
With the current treatment of acute lymphoblastic leukemia (ALL), cure is achieved in 90% of children and 40–50% of adults. The main reasons for failure are resistant disease (RD) and relapse. The frequencies of both events in children are less than 5% and 10–15%, respectively, whereas in adults 5–10% of patients are refractory to initial therapy, and an additional 30–60% of patients relapse despite aggressive consolidation and maintenance chemotherapy regimens, including allogeneic hematopoietic stem cell transplant (alloHSCT). New complete response (CR) can be attained in 20–40% of patients, but these remissions are, in general, of short duration despite the subsequent realization of alloHSCT. Prolonged disease-free survival (DFS) and cure are observed in around 10–15% of relapsed patients (Table 1).1–7 A study including 1706 adult patients with R/R B-cell precursor (BCP) ALL from several European and North American groups reported 3-year survival rates of only 10%.7 Predictors for outcomes include: patient age, duration of first remission, response to initial salvage therapy, and ability to undergo alloHSCT. For patients who are candidates for salvage therapy, the choice of regimens considers patient age and comorbidities, disease characteristics (e.g. immunophenotype, genetic characteristics, extramedullary involvement, among others), type of prior therapy, and duration of prior remission, among others.8 Although some new chemotherapeutic drugs have been approved for relapsed or refractory (R/R) patients (e.g. clofarabine, nelarabine, vincristine liposome sulfate), the most promising results have been achieved with immunotherapy [monoclonal antibodies (MoAb) and cellular therapy].
Table 1.
Results of representative studies of salvage chemotherapy in R/R ALL.
| Study | Thomas et al.1 | Fielding et al.2 | Oriol et al.4 | Gokbuget et al.5 | Kozlowski et al.6 |
|---|---|---|---|---|---|
| n | 314 | 609 | 263 | 547 | 71 |
| CR (%) | 31 | NA | 45 | 42 | 52 |
| Early death (%) | 21 | NA | 17 | NA | 4 |
| Refractory (%) | 49 | NA | 38 | NA | 44 |
| HSCT in ⩾CR2 (%) | NA | 25 | 30 | 75 | 62 |
| CR duration (median) | 6 months | NA | 6 months | NA | NA |
| OS (median) | 5 months | 24 weeks | 4.5 months | 8.4 months | 9 months |
| OS probability | 3% (5 years) | 7% (5 years) | 10% (5 years) | 24% (3 years) | 15% (5 years) |
| Prognostic factors | Age < 40 years CR1>1 year No blasts in PB |
Age < 20 years CR1 >2 years |
Age < 30 years CR1 >2 years | Age < 25 years CR1>1.5 year Response to 1st/2nd salvage |
Age < 35 years CR1 >1.5 year Response to 1st salvage |
ALL, acute lymphoblastic leukemia; CR, complete response; CR1, first complete response; CR2, second complete response; HSCT, hematopoietic stem cell transplant; N, number of patients; NA, not available; OS, overall survival; R/R, relapsed/refractory.
Unconjugated and antibody-drug conjugated MoAb in ALL
There are three types of MoAb constructs used in ALL therapy: unconjugated, antibody-drug conjugated (ADC), also known as immunoconjugated, and bispecific.9 The latter type are the subject of this review.
Rituximab, combined with front-line standard chemotherapy, has demonstrated to improve event-free survival (EFS) in one prospective randomized study from the GRAALL intergroup.10 Rituximab has also proven to improve the results of treatment of newly diagnosed patients with Burkitt’s lymphoma or leukemia.11
Epratuzumab, a naked humanized anti-CD22 MoAb has been explored in combination with clofarabine and high dose cytarabine in adult patients with relapsed disease with a response rate of 52%.12 Inotuzumab ozogamicin (InO), a humanized anti-CD22 MoAb conjugated to calicheamicin, has been approved to treat patients with R/R CD22-positive BCP-ALL on the basis of the randomized INO-VATE study, which showed significantly better response rate and overall survival (OS) compared with standard-of-care rescue therapy.13 Liver-related adverse events were more common in the InO group, with an 11% incidence of hepatic sinusoidal obstruction syndrome (SOS) versus 1% in the standard-therapy group. The concurrent combination of attenuated chemotherapy and InO showed promising results in patients with R/R ALL and could represent a step ahead in treating these patients. Furthermore, results from a phase II trial with reduced doses of InO in combination with the so-called mini hyperCVD chemotherapy schedule in patients aged 60 years or older with newly diagnosed ALL showed a 85% CR rate and an estimated progression-free survival of 59% at 2 years, with a median OS not reached.14
Chimeric antigen receptor T-cells
Chimeric antigen receptor T (CAR-T) cells targeting the CD19 antigen have generated highly promising results in children and adults with R/R ALL (Table 2).15–21 Overall response rates ranged from 67% to 97% in patients who were actually infused, with complete measurable residual disease (MRD) response achieved in the vast majority of responders. From the results of the global multi-institutional ELIANA trial,20 tisagenlecleucel was approved by both United States (US) and European Union (EU) agencies to treat patients aged 1–25 years with BCP-ALL in second relapse or in relapse after HSCT. Cytokine release syndrome (CRS) and neurotoxicity are common, and sometimes severe, with CAR-T as compared with bispecific MoAb such as blinatumomab, likely because of massive induced CAR-T expansion/activation and endothelial activation.22 Many issues still need to be elucidated, including CAR-T composition, CAR-T persistence, the role of prior allogeneic HSCT and disease burden at infusion time on CAR-T efficacy, and the mechanisms of resistance, among others. Genetically engineered “off-the-shell” allogeneic CAR-T, aiming to increase the applicability and rapidity of the procedure, are under clinical development. Due to a relatively high incidence of CD19-negative ALL recurrence, strategies combining CD19 CAR T with immune checkpoint inhibitors, or simultaneous CD19 and CD22 targeting using bispecific or bicistronic CAR-T, are also being investigated.
Table 2.
Main results of CD19 CAR T studies on ALL.
| Author, reference | Institution | Costimulatory domain | Age (median, range) | Infused N | ORR % | CRS, % | Neurotoxicity, % | OS |
|---|---|---|---|---|---|---|---|---|
| Maude et al.15 | UPenn | 4-1BB | 14 years (5–60) | 30 | 90% | 100% (severe, 27%) | 43% | 78% at 6 months |
| Davila et al.16 | MSKCC | CD28 | 50 years (NA) | 16 | 88% | severe, 44% | Gr 3/4, 25% | NA |
| Lee et al.17 | NCI | CD28 | 15 years (5–27) | 21 | 67% | 76% (Gr 3/4, 29%) | 29% (Gr 3/4, 5%) | 52% at 12 months |
| Turtle et al.18 | FHCRC | 4-1BB | 40 years (20–73) | 30 | 93% | 83% | 50% (Gr 3/4, 50%) | NA |
| Gardner et al.19 | SCRI | 4-1BB | 12 years (1–25) | 43 | 93% | 93% (Gr 3/4, 23%) | 49% (Gr 3/4, 21%) | 69.5% at 12 months |
| Maude et al.20 | Novartis | 4-1BB | 11 years (3–23) | 751 | 81% | 77% | 40% (Gr 3/4, 13%) | 76% at 12 months |
| Park et al.21 | MSKCC | CD28 | 44 years (23–74) | 532 | 83% | 85% (Gr 3/4, 26%) | 48% (Gr 3/4, 42%) | median, 12.5 months |
Screened: 92; 2Screened: 75.
ALL, acute lymphoblastic leukemia; CAR-T, chimeric antigen receptor T; CRS, cytokine release syndrome; FHCRC, Fred Hutchinson Cancer Research Center; Gr, grade; MSKCC, Memorial Sloan Kettering Cancer Center; NCI, National Cancer Institute; ORR, overall response rate; OS, overall survival; SCRI, Seattle Children’s Research Institute; UPenn, University of Pennsylvania.
Bispecific T-cell engaging MoAb
Bispecific MoAb are engineered to bind to two different antigens or two different epitopes on the same antigen. They are used mostly to redirect the cytotoxic potential of immune effector cells [T-cells or natural killer (NK) cells] to the destruction of tumor cells.23 The redirection of T-cells to tumors is not restricted by major histocompatibility complex, thus obviating the need for antigen recognition by the T-cell receptor. There are two main types of bispecific MoAb: CD3 bispecific formats and CD16 (NK) bispecific formats. Currently there are more than 100 different formats, including bispecific antibody T-cell engagers (BiTE), bispecific antibody-armed activated T-cells (BAT), dual-affinity re-targeting bispecific antibodies (DART), tetravalent bispecific tandem diabodies (TandAb), T-cell dependent bispecific antibody (TDB) and trifunctional antibodies (Trifab or Triomab), among others.24–26 This review focuses only on BiTE, and specifically blinatumomab, the only BiTE MoAb approved for treating BCP-ALL.
Blinatumomab for the treatment of ALL
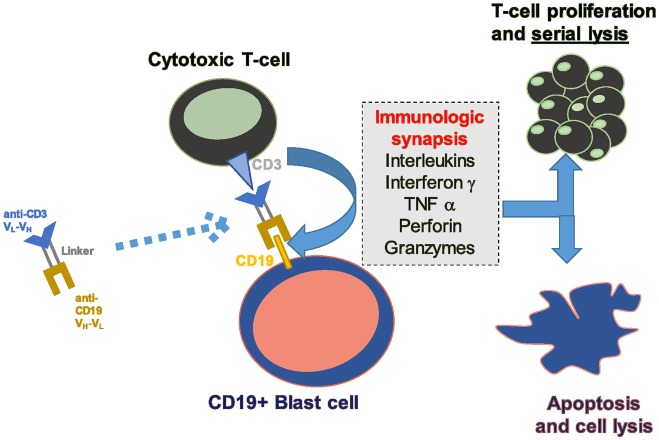
BiTE MoAb are built by joining two single-chain Fv fragments: one targeting a neoplastic cell and the other targeting CD3.27 Blinatumomab is the first bispecific MoAb, anti CD19-Anti CD3, that allows CD3-positive T-lymphocytes to eliminate CD19-positive B-lymphocytes, including malignant lymphoid B-cells from lymphomas and BCP-ALL blasts, being either Philadelphia (Ph) chromosome-positive or not.28 As a result of a preferential affinity to CD19, CD3 T-cells can move from one B-cell to another, resulting in serial lysis through a perforin/granzyme-mediated mechanism (Figure 1).29
Figure 1.
Mechanism of action of blinatumomab.
CD, cluster of differentiation; TNF, tumor necrosis factor.
The clinical development program of blinatumomab included phase II studies in patients with R/R ALL, with and without Ph chromosome in adults and in children, a phase III trial comparing blinatumomab as single drug versus standard-of-care (SOC) rescue chemotherapy in adults with R/R Ph-negative ALL, and a phase II study in adult patients with Ph-negative ALL in MRD-positive status (Table 3).30,31 As a result of these studies, blinatumomab is the first T-cell engager molecule approved by both the US Food and Drug Administration (FDA) and European Medical Agency (EMA) for treatment of adult and pediatric patients with R/R and MRD-positive BCP ALL.
Table 3.
Main results of clinical trials with blinatumomab in ALL.
| Type of ALL | Ph-positive, R/R adults | Ph-negative, R/R adults | Positive MRD, adults | Ph-negative, R/R, children | Ph-negative, R/R, children | ||
|---|---|---|---|---|---|---|---|
| Study | Pivotal phase II (ALCANTARA) | First phase II | Confirmatory phase II | phase III TOWER |
phase II BLAST |
phase I/II Study 205 |
phase II expanded access (RIALTO) |
| Patients (N) | 45 | 36 | 189 | 271 | 116 | 70 | 98 |
| CR/CRh (%) | 36 | 69 | 43 | 45 | NA | 39 | 60 |
| MRD level <0.01% | 88 | 88 | 82 | 76 | 78 | 54 | 48 |
| OS, median, months | 7.1 | 9.8 | 6.1 | 7.7 | 36.5 | 7.5 | 13 |
ALL, acute lymphoblastic leukemia; CR, complete remission; CRh, complete remission with incomplete hematologic recovery; MRD, measurable residual disease; OS, overall survival; R/R, relapsed or refractory.
Phase II and related studies with blinatumomab as a single drug in R/R and MRD-positive ALL
Studies in relapsed or refractory Ph-negative ALL
The first exploratory phase II study (ClinicalTrials.gov identifier: NCT01209286) on blinatumomab in patients with R/R BCP-ALL was performed in 36 patients by the German Multicenter Adult Acute Lymphoblastic Leukemia (GMALL) group.32 This study included a search for optimal dose schedule, and found an initial dose of 5 μg/m2/day for 7 days followed by 15 μg/m2/day for the subsequent 3 weeks as the schedule associated with the lowest rate of adverse events (AE). In addition, debulking with dexamethasone and cyclophosphamide was recommended in patients with a high number of blasts before initiating blinatumomab therapy. The rate of CR and CR with incomplete hematologic recovery (CRi) was 69%, with median OS of 9 months,32 with longer survival for patients achieving MRD-negative status.33
A subsequent pivotal global multicenter phase II study (ClinicalTrials.gov identifier: NCT01466179),34 included 189 adult patients with R/R, Ph-negative ALL. Patients with poorer characteristics than that of the previous trial were included: higher disease burden [>10% bone marrow (BM) blast cells, refractoriness (failure to frontline therapy), CR duration <12 months], or cases beyond first relapse. The blinatumomab schedule consisted of 9 μg/day for the first 8 days and 28 μg/day thereafter for 3 weeks for the first cycle, followed by 28 μg/day for subsequent cycles, with a 14-day interval between cycles. One or two cycles were scheduled as induction followed by 1–3 cycles as consolidation. The rate of CR or CR with incomplete hematologic recovery (CRh) was 43%, being achieved by cycle 1 in 79% of responders. After the first two cycles, 82% of responders achieved an MRD level <10–4. MRD responders showed a significantly improved OS (11.5 months versus 6.7 months for non-responders). A subsequent alloHSCT was performed in 40% of CR/CRh patients. Based on the results of this trial, blinatumomab received accelerated US FDA approval for the treatment of Ph–negative R/R B-ALL in December 2014.
A comparison of the results of blinatumomab therapy in elderly patients from the two aforementioned studies (⩾65 years, n = 36) with results in younger adults (<65 years, n = 225)35 showed a similar CR/CRh and MRD response rate after two cycles (56% versus 46%, respectively, for CR/CRh and 60% versus 70%, respectively, for MRD response). Similarly, relapse-free survival (RFS) and OS were not significantly different (median RFS 7.4 months for both groups; median OS 5.5 versus 7.6 months, respectively), despite a higher frequency of alloHSCT in younger adults (59% versus 15%). The tolerability was similar for both groups of patients, although older adults showed more grade ⩾3 neurologic events (28% versus 13%).
A subset analysis was performed for patients with BCP-ALL from the global phase II trial who had relapsed following alloHSCT (n = 64).36 The CR/CRh rate was 45% (29 of 64), with molecular response in 22 of 29 (complete MRD response in 19 out of 22). The median RFS was 7.4 months for patients who achieved CR/CRh in the first two cycles, and the median OS was 8.5 months. The tolerability of blinatumomab did not differ from that of the remaining patients. Interestingly, seven patients experienced grade 3 or less graft-versus-host disease (GVHD) during the study, none of which resulted in discontinuation of blinatumomab or hospitalization.
As achievement of MRD response is a major goal for ALL patients in CR, an analysis of molecular response in CR/CRh patients treated within the global phase II study was performed.37 MRD response after 2 blinatumomab cycles was achieved in 83% (75/90) of patients in CR/CRh. This was translated into improved OS and RFS in patients who achieved MRD response (medians, 20.6 versus 9.0 months, respectively for OS and 12.5 versus 2.3 months, respectively for RFS). Thus, achieving MRD response can be used as a prognostic factor for blinatumomab treatment in R/R ALL.
A long-term follow-up analysis combining patients from the exploratory GMALL study and those of the global phase II study is being conducted to evaluate the OS and the characteristics of patients with long-term survival. In addition, a retrospective observational study (NEUF) including 253 patients treated with blinatumomab in the expanded access program between January 2014 and December 2016 in five European countries was recently concluded and presented in abstract form.38,39 Within two cycles of blinatumomab, 54 (51%) patients achieved CR/CRh, of whom 91% (49/54) had CR, and 85% of CR patients achieved molecular response. One-third of patients proceeded to alloHSCT. Outcomes were better in patients with hematologic response compared with those without and in patients with MRD response compared with those without. Overall, these results are widely consistent with published results from the global phase II clinical trial and confirm the effectiveness of blinatumomab in a real-world setting.
A phase I/II clinical trial in R/R pediatric ALL (ClinicalTrials.gov identifier: NCT01471782) established the dose of blinatumomab of 5 μg/m2/day for 7 days followed by 15 μg/m2/day for the remaining 3 weeks of the first and subsequent cycles.40 The 70 patients included in the phase II trial received the complete dosage. After the first two cycles, 27 patients (39%) achieved CR, with 14 (52%) becoming MRD negative. The frequency of side effects was similar to that observed in previous trials in adults. This trial provided evidence of the efficacy and tolerability of blinatumomab also in children with R/R ALL. The final results of this trial were published in 2018.41 The median OS was 7.5 months, with a 2-year OS probability of 25%, and 31% of patients (22 of 70) were alive. Failure to respond (n = 30) and relapse (n = 15) were the major causes of death. OS was longer in patients with complete MRD response (14.6 months versus 5.7 months for non-MRD responders).
An open label, multicenter, expanded access study (RIALTO trial, ClinicalTrials.gov identifier: NCT02187354) was initiated in 2014 for pediatric patients with R/R BCP-ALL.42 Blinatumomab was scheduled for up to 5 cycles, although patients could proceed to alloHSCT once CR had been achieved. At the last data cutoff, 37 out of 98 patients were under study. The most frequent grade ⩾3 AE (the main objective of the study) were cytopenias (31%), infections (16%), raised liver enzymes (12%), neurologic events (5%), CRS (2%), and tumor lysis syndrome (2%). None of the nine fatal AE was related to blinatumomab. The CR/CRh rate was 60%, and 27 out of 59 patients who achieved CR within two cycles received alloHSCT. The median OS was 13 months (median follow up 12.2 months for all 98 patients), disease progression being the main cause of death (32 of 38 patients). Data from the RIALTO study confirm the effectiveness and tolerability of blinatumomab in children with R/R BCP ALL.
Studies in R/R Philadelphia chromosome-positive ALL
A multicenter phase II trial (ALCANTARA, ClinicalTrials.gov identifier: NCT02000427) was conducted to assess the efficacy and tolerability of blinatumomab in patients with R/R Ph-positive ALL. Adult patients with Ph-positive ALL who had relapsed after or were refractory to at least one second-generation or later tyrosine kinase inhibitors (TKI) or were intolerant to second-generation or later TKI and intolerant or refractory to imatinib were included.43 Out of 45 patients, 16 (36%) achieved CR/CRh during the first two cycles. Notably, CR/CRh was attained in 4/10 patients with the T315I mutation; 88% of CR/CRh patients achieved complete molecular response, and 44% of patients in CR/CRh underwent alloHSCT. The median RFS and OS were 6.7 and 7.1 months, respectively. From these results it seems clear that the efficacy (and safety) of blinatumomab are similar in R/R patients with Ph-negative or -positive ALL. This prompted FDA approval of blinatumomab for R/R Ph-positive ALL in July 2017.
An observational retrospective study performed in patients with R/R Ph-positive ALL compared outcomes of patients treated with blinatumomab in the phase II trial with those of historical control patients with similar characteristics treated with the SOC in Italy and Spain.44 Propensity score analysis (PSA) was used to compare outcomes. The rate of CR/CRh was 36% for blinatumomab and 25% for SOC. OS favored blinatumomab over SOC, with a hazard ratio of 0.77 [95% confidence interval (CI), 0.61–0.96].
Studies in MRD-positive ALL
A step ahead in the development program of blinatumomab included the evaluation of activity in patients with MRD-positive ALL in either first morphologic CR or in relapsed ALL. It is of note, however, that the first clinical trial with blinatumomab (ClinicalTrials.gov identifier: NCT00560794) in ALL was conducted by the GMALL group in a small group of adults with BCP ALL with molecular failure (n = 15) or relapse (n = 5) after consolidation of front-line therapy.45 The rate of molecular CR (80%, 16/20 evaluable patients) was similar to that attained in cases of R/R ALL. Interestingly, an extended follow up of this study showed no differences in survival for patients who received alloHSCT (n = 9) or not (n = 11).46
The most interesting results from blinatumomab in MRD-positive status came from the BLAST trial (ClinicalTrials.gov identifier: NCT01207388), a multicenter, phase II study conducted to evaluate the efficacy, safety, and tolerability of blinatumomab in adult patients with MRD-positive BCP-ALL.47 This trial included patients in CR after ⩾3 intensive chemotherapy treatments and with MRD ⩾10–3. The primary endpoint was complete MRD response (MRD negativity) after one cycle. The trial accrued 116 patients, 65% of whom were in first CR. Complete MRD response was attained in 91/113 evaluable patients (78%), being achieved after the first cycle in 82. The complete MRD rates did not differ according to the level of MRD positivity and CR status. The medians of RFS and OS for the whole series were 18.9 and 36.5 months, respectively (median follow up of 30 months). This compares favorably with the approximately 6–7 months observed in trials with R/R ALL.1–7 Not surprisingly, outcomes were better in patients treated in first CR (median EFS of 24.6 months versus 11 months for patients in second or later CR). A significantly longer OS was achieved for patients who attained MRD response after one cycle (median 35.2 months versus 7.1 months for those without molecular response). The rate of alloHSCT performed was 67% (74 patients, 55 in first CR and 19 in second CR), whereas 36 patients continued with the full blinatumomab schedule. Although evaluation of the impact of alloHSCT after blinatumomab treatment was not an objective of the BLAST trial, it is of note that no differences were observed in RFS according to transplantation in MRD-responsive patients treated in first CR, whereas the RFS probability was better for MRD-negative patients with second or later CR who underwent alloHSCT. The tolerability of blinatumomab was similar to that of previous studies. A recent update of these data with a median follow-up of 59.8 months, confirmed the median OS of 36.5 months. Interestingly, the median OS was not reached in patients with complete MRD response versus 12.5 months in MRD non-responders. The proportion of survivors was 40% in transplanted and 33% in non-transplanted patients. Based on the aforementioned data, the regulatory authorities in the US and Europe extended the marketing authorization for blinatumomab to MRD-positive BCP, Ph-negative ALL. A study aimed to compare blinatumomab with historic SOC treatments for MRD positive disease in adults with Ph-negative, BCP-ALL is underway.
Phase III and related studies with blinatumomab as a single drug in R/R ALL
A phase III randomized trial (TOWER, ClinicalTrials.gov identifier: NCT02013167) compared blinatumomab with conventional SOC salvage chemotherapy, with either of the following schedules: FLAG (fludarabine + high-dose cytarabine arabinoside ± anthracycline), high-dose ARA-C, high-dose ARA-C ± anthracycline, high-dose methotrexate-based regimen or clofarabine based regimens) at a 2:1 ratio in adult patients with R/R, Ph-negative ALL.48 The inclusion criteria, as well as the blinatumomab dosing, were similar to those from the aforementioned phase II global trial, and the primary endpoint was OS. A total of 271 patients received blinatumomab and 134 received SOC therapy. Significant differences in favor of blinatumomab were observed for CR/CRh rate (44% versus 25%), MRD response (76% versus 48%), and OS (median 7.7 versus 4.0 months), the latter being more evident in patients receiving blinatumomab as first salvage therapy (median OS of 11.1 versus 5.5 months).49 When the data were censored at the time of alloHSCT (performed in 24% of patients from both groups), the medians of OS were 6.9 versus 3.9 months. On the basis of the results of the TOWER study, blinatumomab was approved to treat patients with R/R, Ph-negative BCP-ALL.
A recently published study analyzed the outcomes of patients in the TOWER trial who underwent alloHSCT after treatment with blinatumomab (n = 65) or SOC (n = 32). No differences in survival benefit were found.50 In addition, no survival benefit of alloHSCT versus no alloHSCT was observed in patients who achieved CR/CRh with blinatumomab. As expected, the best outcomes were observed in patients with MRD remission who received blinatumomab as first salvage treatment regardless of subsequent performance of alloHSCT.
The impact of blinatumomab on health-related quality of life (HRQL) as measured by the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire QLQ-C30 was assessed in the phase III TOWER study.51 Patients treated with blinatumomab (n = 247) showed better HRQL across all QLQ-C30 subscales than those receiving chemotherapy (n = 95).
The Childrens Oncology Group conducted a randomized phase III trial comparing blinatumomab versus chemotherapy as post-reinduction therapy in children and adolescent and young adults (AYA) up to 30 years with high- and intermediate-risk BCP, Philadelphia chromosome-negative ALL in first relapse. The first results of this trial have recently been published in abstract form.52 After receiving one block of reinduction chemotherapy, patients were randomized to receive either two additional intensive reinduction cycles of chemotherapy or four cycles of blinatumomab. After randomization, both groups proceeded to alloHSCT. With a median follow up of 1.4 years, the 2-year DFS (the main objective of the trial) was 41% for the chemotherapy arm (n = 103 patients) versus 59.3% for the blinatumomab arm (n = 105 patients) (p = 0.05), and the 2-year OS was 59.2% versus 79.4% (p = 0.005). The MRD response (<0.01%) was also better in the blinatumomab arm (79% versus 21%, p < 0.0001), and the rate of alloHSCT was higher in patients receiving blinatumomab (73% versus 45%, p < 0.0001). Fewer and less severe toxicities were also observed in patients from the blinatumomab arm. Taken together, these data showed that blinatumomab was superior to standard chemotherapy as post-reinduction consolidation prior to alloHSCT in this specific group of patients.
Clinical trials with blinatumomab in newly diagnosed ALL
Table 4 shows the main clinical trials with blinatumomab in patients with newly diagnosed ALL. Most evaluated the use of blinatumomab during consolidation in newly diagnosed patients with MRD positive after induction, while others incorporated blinatumomab into first-line therapy regardless of MRD status. Other approaches include the use of blinatumomab as prephase therapy of ALL, the preemptive use of blinatumomab after alloHSCT, or the combination of blinatumomab with other targeted therapies. In Ph-positive ALL, studies with sequential application of TKI and blinatumomab are underway.
Table 4.
Selected clinical trials, active or in development, with blinatumomab (Blin) in de novo or R/R pediatric and adult ALL.1
| ClinicalTrials.gov identifier | Schedule | Planned n pts | Age | Status |
|---|---|---|---|---|
| De novo ALL, Ph-positive | ||||
| NCT03263572 | Blin and ponatinib | 60 | ⩾60 | Active |
| NCT02744768 | Dasatinib, prednisone and Blin | 80 | ⩾18 | Active |
| NCT02143414 | Blin and CHT or Blin and dasatinib | 44 | ⩾65 | Active |
| De novo ALL. Ph-negative | ||||
| NCT02877303 | Blin and HyperCVAD | 60 | ⩾14 | Active |
| NCT02458014 | Blin in MRD-positive ALL | 40 | ⩾18 | Active |
| NCT03109093 | Blin in MRD-positive ALL | 60 | ⩾18 | Active |
| NCT03541083 | Blin prephase and CHT | 80 | 18–70 | Active |
| NCT03480438 | Blin and CHT | 50 | 56–74 | In preparation |
| NCT03523429 | CHT and Blin in consolidation in HR ALL | 38 | 18–55 | Active |
| NCT03709719 | Blin in HR ALL | 95 | 18–59 | Active |
| NCT03643276 | CHT and Blin in consolidation (randomized) | 5000 | <18 | Active |
| NCT02003222 | CHT versus Blin in consolidation | 509 | 30–70 | Active |
| NCT03367299 | Sequential CHT and Blin | 149 | 18–65 | Active |
| NCT03117751 | CHT and Blin or others | 1000 | <18 | Active |
| NCT03114865 | Blin maintenance after alloHSCT | 12 | ⩾18 | Active |
| NCT03751709 | Blin and haploidentical HSCT | 10 | ⩾18 | Active |
| NCT02807883 | Blin maintenance after alloHSCT | 30 | 18–70 | Active |
| NCT02877303 | Blin and inotuzumab | 64 | ⩾18 | Active, also for R/R ALL |
| NCT01371630 | CHT, inotuzumab followed by Blin | 256 | 56–74 | Active |
| Relapsed/refractory ALL | ||||
| NCT03518112 | CHT and Blin | 44 | ⩾18 | Active |
| NCT02101853 | CHT versus Blin (randomized) | 598 | 1–30 | Active |
| NCT02393859 | CHT versus Bin (randomized) | 202 | <18 | Active |
| NCT02997761 | Blin and ibrutinib | 20 | ⩾18 | Active |
| NCT03628053 | Blin or inotuzumab versus tisagenlecleucel | 220 | ⩾18 | In preparation |
| NCT03160079 | Blin and pembrolizumab | 24 | ⩾18 | Active |
| NCT02879695 | Blin and nivolumab w/o ipilimumab | 30 | ⩾16 | Active |
ClinicalTrials.gov, accessed 15 October 2019.
ALL, acute lymphoblastic leukemia; alloHSCT, allogeneic hematopoietic stem cell transplantation; Blin, blinatumomab; CHT, chemotherapy; HR ALL, high-risk ALL; NCT, National Clinical Trial; pts, patients; R/R, relapsed/refractory; w/o, with/without.
There is scarce published information on the results of these studies. In one trial, young adults with BCP, Ph-negative ALL received the hyper-CVAD regimen (four cycles) as induction, followed by four consecutive cycles of blinatumomab as consolidation and a maintenance phase. Although the number of evaluable patients was low (n = 14) and the follow up short, this regimen has proven to be tolerable and effective.53 In patients older than 65 years, preliminary results of the sequential application of blinatumomab and dose-reduced chemotherapy have been published in abstract form.54 The blinatumomab schedule consisted of one to two cycles of induction until the achievement of CR, followed by three additional consolidation cycles and 18 months of intensified maintenance therapy. The rate of CR/CRi in 29 eligible patients (median age 75 years) was 66%, and the treatment was tolerable without deaths in induction. In a small retrospective study, blinatumomab was administered to children with BCP ALL with MRD-positive status before alloHSCT. A MRD-negative status was achieved after one cycle in most of the children. Blinatumomab was well tolerated and no transplant-related mortality was observed in the first 100 days after alloHSCT.55 The preliminary results of a multicenter phase II trial with chemotherapy-free induction-consolidation trial (D-ALBA, GIMEMA LAL2116, ClinicalTrials.gov identifier: NCT02744768) based on the combination of dasatinib with blinatumomab in newly diagnosed Ph-positive ALL patients aged 18 years or older have recently been reported in abstract form.55 Dasatinib (140 mg/day) was administered as induction for 85 days, combined with steroids during the first month. The consolidation treatment consisted of dasatinib combined with blinatumomab (minimum two cycles, but possible administration of three additional cycles according to response and medical decision). The subsequent therapy was given at medical discretion. At the primary time point (end of the second cycle of blinatumomab), 27/47 patients (56%) presented molecular response (the main objective of the trial). Interestingly, the rate of molecular responses increased after subsequent cycles of blinatumomab (66% and 80% after the third and fourth cycle, respectively). With a median follow up of 10 months, 12 patients received alloHSCT and six patients experienced relapse, with projected 12-months OS and DFS of 94% and 88%, respectively. Six out seven ABL mutations detected before blinatumomab were not detected after blinatumomab therapy. Thus, this ongoing chemotherapy-free protocol for adult Ph+ ALL patients combining targeted therapy and immunotherapy shows good feasibility and promising rates of molecular responses and survival.
Toxicity of blinatumomab
The most frequent grade ⩾3 AE observed with blinatumomab include febrile neutropenia, infection, and hematological toxicities.34,40,42,47,48 Liver enzymes may show a transient increase as well but usually do not require infusion interruptions. Lymphopenia, as well as a decrease of immunoglobulin levels, may be observed with prolonged infusion, but generally do not require immunoglobulin supplementation. The two most important specific AE are CRS and neurotoxicity.
CRS is also observed with other immunotherapies involving T lymphocytes such as CAR T-cells, although the frequency and intensity are considerably lower with blinatumomab. With pre-phase treatment in patients with high leukemia burden, dose step, and pretreatment with dexamethasone, the incidence of CRS is less than 5%.34,48 Blinatumomab must be discontinued if clinical manifestations of grade ⩾3 CRS appear. Anti-IL-6 treatment with tocilizumab or siltuximab is rarely needed.
Neurologic AE are also common with CAR T and blinatumomab.56 Their clinical spectrum is highly variable, ranging from mild disorders such as tremor, headache, and dizziness, to severe symptoms of encephalopathy and seizures. This variability makes early detection extremely important, as dexamethasone can be useful to reverse these early symptoms. However, if neurologic AE develop during blinatumomab infusion, treatment must be interrupted (most are quickly reversible after interruption) and re-initiated at a lower dose when they have disappeared.31,56 If they reappear after dose reduction, permanent discontinuation of blinatumomab is mandatory. The pathogenic mechanisms of neurologic AE are incompletely known, but the main suspected mechanism is an alteration of the blood–brain barrier as a consequence of endothelial activation of blood vessels caused by the adhesion of activated T-cells to the endothelium, followed by extravasation of these T-cells with cytokine release by extravasated T-cells in the brain.57
Mechanisms of resistance to blinatumomab
In R/R BCP ALL, several trials showed a lower rate of CR/CRh in patients with a higher degree of BM blast infiltration, that is, >50%. High serum lactate dehydrogenase levels at relapse were also found to be associated with resistance to blinatumomab.58 The outcome with blinatumomab in the R/R setting is also better in less advanced stages of disease,49 with the presence of additional mutations conferring resistance mechanisms as the disease advances being the most probable reason. A history of prior extramedullary ALL and current extramedullary ALL are also predictors of lower response rates, with 40% of relapses being located in the extramedullary compartment.59 This could be explained by the lower accessibility of the immune system to some sanctuary extramedullary sites such as the central nervous system and others.
In the MRD setting, no pretreatment factor was associated with inferior response, although long-term outcome was poorer in patients in a more advanced disease stage (i.e. later CR compared with first CR), as mentioned before.47
Some immunologic factors could provide insights into the mechanisms of resistance to blinatumomab. A study aimed at identifying biomarkers for clinical outcomes of patients from the TOWER trial showed that a lower percentage of CD45+ CD3– CD19+ B-cells was predictive of survival in the blinatumomab group, whereas a higher percentage of CD45+ CD3+ CD8+ T-cells was predictive of hematologic remission in the blinatumomab group. A higher percentage of CD3+ T-cells was prognostic of MRD response in both groups. No biomarker prognostic or predictive for grade ⩾3 neurologic events, infections, or cytokine release syndrome was identified.60 Another study showed that a higher rate of T-cell expansion as well as a higher percentage of CD3-positive T-cells and lower levels of regulatory T-cells (Treg) were observed in long-term survivors after blinatumomab treatment.58 The reduction in the number of Treg with the systematic use of cyclophosphamide in the preparative regimen before blinatumomab administration could bypass this resistance mechanism.61 A reduced level of cytotoxic CD8+ T-cells was also correlated with non-response to blinatumomab.62 PD-L1 expression by lymphoblasts may inhibit T-cell function through the PD-1 receptor and contribute to resistance to blinatumomab.62 Inhibition of PD-1/PD-L1 with the concurrent administration of immune checkpoint inhibitors might increase the efficacy and reduce the resistance to blinatumomab,63 as observed in in vitro studies, as well as was shown in a patient refractory to blinatumomab in whom the combination of blinatumomab and pembrolizumab markedly reduced leukemic burden.62 These observations have led to the development of clinical trials combining blinatumomab with nivolumab, ipilimumab, orpembrolizumab in R/R ALL patients (Table 4).
CD19-negative relapses are observed in around 10% of patients treated with blinatumomab.64 The selective pressure to CD19 may induce clonal selection of CD19-negative cells by CD19 loss due to different mechanisms. Among these mechanisms, loss of the extracellular domain of CD19,65 conformational changes of the extracellular domain of CD19 due to mutations, and intracellular accumulation of CD19 are the most important.66 Different strategies being used to avoid this important problem include sequential combination with other MoAb such as the ADC anti-CD22 InO (Table 4), combination with TKI in Ph-positive ALL, or the sequential treatment with CAR T-cells directed against other antigens (e.g. CD22), among others.
In cases of BCP ALL with a very immature phenotype, such as KTMA-rearranged, relapses can be associated with a lineage shift to acute myeloid leukemia,67 this fact likely being due to the expansion of CD19-negative clones after clearance of the CD19-positive dominant clone.
Conclusion
Bispecific T-cell engaging MoAb constitute a very promising type of immunotherapy for hematologic malignancies and solid tumors. Among them, blinatumomab is the most developed, and is currently approved for clinical use in R/R and MRD-positive BCP ALL in children and adults. Although results in the R/R setting are superior to those of SOC chemotherapy, they are not satisfactory. Attempts to overcome resistance mechanisms are underway. As the best results have been achieved in patients with low tumor load (e.g. MRD-positive ALL), blinatumomab is currently investigated as first-line therapy, integrated in a multi-drug or multi-target treatment backbone. Several questions must be addressed in this setting, such as the inclusion of blinatumomab in the ALL therapeutic schedule (e.g. in induction, consolidation, or both), the intensity of concomitant or sequential chemotherapy (full dose or dose-reduced), combined use with targeted therapies (TKI and others) or with other immunotherapeutic approaches (InO or CAR T-cells),68 the systematic need for alloHSCT for patients successfully treated at the MRD level or its preemptive or prophylactic use after transplantation, among others.
The optimization of blinatumomab (and of bispecific MoAb in general) can take place in several directions, such as the identification of reliable predictive biomarkers and the use of strategies to overcome mechanisms of resistance, as discussed previously. Improvement in the current route of application (4-week intravenous continuous infusion) with the development of compounds with a prolonged half-life with other routes of administration (such as subcutaneous, ClinicalTrials.gov identifier: NCT02961881) would improve the flexibility of treatment. Finally, continuous development and improvement in the design of bispecific or trispecific MoAb will constitute a step forwards in the treatment of ALL and other hematologic neoplasms and solid tumors.69
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: JM Ribera declares the following conflicts of interest: Pfizer: speaker and advisory boards honoraria, clinical trials; AMGEN: speaker and advisory boards honoraria, research support, clinical trials; Shire: speaker and advisory boards honoraria; Ariad: speaker and advisory boards honoraria, clinical trials. The remaining authors declare no conflicts of interest.
ORCID iD: Jose-Maria Ribera  https://orcid.org/0000-0003-1042-6024
https://orcid.org/0000-0003-1042-6024
Contributor Information
Jose-Maria Ribera, Clinical Hematology Department, ICO-Hospital Germans Trias I Pujol, Josep Carreras Research Institute, Universitat Autònoma de Barcelona, C/ Canyet, s/n, Badalona, 08916, Spain.
Eulalia Genescà, Clinical Hematology Department, ICO-Hospital Germans Trias i Pujol, Josep Carreras Research Institute, Universitat Autònoma de Barcelona, Badalona, Spain.
Jordi Ribera, Clinical Hematology Department, ICO-Hospital Germans Trias i Pujol, Josep Carreras Research Institute, Universitat Autònoma de Barcelona, Badalona, Spain.
References
- 1. Thomas DA, Kantarjian H, Smith TL, et al. Primary refractory and relapsed adult acute lymphoblastic leukemia: characteristics, treatment results, and prognosis with salvage therapy. Cancer 1999; 86: 1216–1230. [DOI] [PubMed] [Google Scholar]
- 2. Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 2007; 109: 944–50. [DOI] [PubMed] [Google Scholar]
- 3. Tavernier E, Boiron JM, Huguet F, et al. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia 2007; 21: 1907–1914. [DOI] [PubMed] [Google Scholar]
- 4. Oriol A, Vives S, Hernández-Rivas JM, et al. Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA Study Group. Haematologica 2010; 95: 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gökbuget N, Stanze D, Beck J, et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood 2012; 120: 2032–2041. [DOI] [PubMed] [Google Scholar]
- 6. Kozlowski P, Åström M, Ahlberg L, et al. High curability via intensive reinduction chemotherapy and stem cell transplantation in young adults with relapsed acute lymphoblastic leukemia in Sweden 2003-2007. Haematologica 2012; 97: 1414–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gökbuget N, Dombret H, Ribera JM, et al. International reference analysis of outcomes in adults with B-precursor PH-negative relapsed/refractory acute lymphoblastic leukemia. Haematologica 2016; 101: 1524–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frey N, Luger S. How I treat adults with relapsed or refractory Philadelphia chromosome negative acute lymphoblastic leukemia. Blood 2015; 126: 589–596. [DOI] [PubMed] [Google Scholar]
- 9. Jabbour E, O’Brien S, Ravandi F, et al. Monoclonal antibodies in acute lymphoblastic leukemia. Blood 2015; 125: 4010–4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maury S, Chevret S, Thomas X, et al. Rituximab in B-lineage adult acute lymphoblastic leukemia. N Engl J Med 2016; 375: 1044–1053. [DOI] [PubMed] [Google Scholar]
- 11. Ribrag V, Koscielny S, Bosq J, et al. Rituximab and dose-dense chemotherapy for adults with Burkitt’s lymphoma: a randomised, controlled, open-label, phase 3 trial. Lancet 2016; 387: 2402–2411. [DOI] [PubMed] [Google Scholar]
- 12. Advani AS, McDonough S, Coutre S, et al. SWOG S0910: a phase 2 trial of clofarabine/cytarabine/epratuzumab for relapsed/refractory acute lymphocytic leukaemia. Br J Haematol 2014; 165: 504–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzuma bozogamicin versus strandard therapy for acute lymphoblastic leukemia. N Engl J Med 2016; 375: 740–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kantarjian H, Ravandi F, Short NJ, et al. Inotuzuma bozogamicin in combination with low-intensity chemotherapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukemia: a single-arm, phase 2 study. Lancet Oncol 2018; 19: 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371: 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of a 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014; 6: 224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblactic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015; 385: 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016; 126: 2123–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 2017; 129: 3322–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018; 378: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park JH, Rivière I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med 2018; 378: 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gust J, Hay KA, Hanafi LA, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov 2017; 4: 1404–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Suurs FV, Lub-de Hooge MN, de Vries EGE, et al. A review of bispecific antibodies and antibody constructs in oncology and clinical challenges. Pharmacol Ther 2019; 201: 103–119. [DOI] [PubMed] [Google Scholar]
- 24. Foster LH, Lum LG. Treatment of hematological malignancies with T cell redirected bispecific antibodies: current status and future needs. Expert Opin Biol Ther 2019; 19: 707–720. [DOI] [PubMed] [Google Scholar]
- 25. Duell J, Lammers PE, Djuretic I, et al. Bispecific antibodies in the treatment of hematologic malignancies. Clin Pharmacol Ther 2019; 106: 781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Einsele H, Rasche L, Topp MS, et al. The use of bispecific antibodies to optimize the outcome of patients with acute leukemia, lymphoma and multiple myeloma after SCT. Bone Marrow Transplant 2019; 54(Suppl. 2): 721–726. [DOI] [PubMed] [Google Scholar]
- 27. Dahlen E, Veitonmaki N, Norlen P. Bispecific antibodies in cancer immunotherapy. Ther Adv Vaccines Immunother 2018; 6: 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 2008; 321: 974–977. [DOI] [PubMed] [Google Scholar]
- 29. Hoffmann P, Hofmeister R, Brischwein K, et al. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int J Cancer 2005; 115: 98–104. [DOI] [PubMed] [Google Scholar]
- 30. Ribera JM. Efficacy and safety of bispecific T-cell engager blinatumomab and the potential to improve leukemia-free survival in B-cell acute lymphoblastic leukemia. Expert Rev Hematol 2017; 10: 1057–1067. [DOI] [PubMed] [Google Scholar]
- 31. Wilke AC, Gökbuget N. Clinical applications and safety evaluation of the new CD19 specific T-cell engager antibody construct blinatumomab. Expert Opin Drug Saf 2017; 16: 1191–1202. [DOI] [PubMed] [Google Scholar]
- 32. Topp MS, Gökbuget N, Zugmaier G, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol 2014; 32: 4134–4140. [DOI] [PubMed] [Google Scholar]
- 33. Zugmaier G, Gökbuget N, Klinger M, et al. Long-term survival and T-cell kinetics in relapsed/refractory ALL patients who achieved MRD response after blinatumomab treatment. Blood 2015; 126: 2578–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol 2015; 16: 57–66. [DOI] [PubMed] [Google Scholar]
- 35. Kantarjian HM, Stein AS, Bargou RC, et al. Blinatumomab treatment of older adults with relapsed/refractory B-precursor acute lymphoblastic leukemia: results from 2 phase 2 studies. Cancer 2016; 122: 2178–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stein AS, Kantarjian H, Gökbuget N, et al. Blinatumomab for acute lymphoblastic leukemia relapse after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2019; 25: 1498–1504. [DOI] [PubMed] [Google Scholar]
- 37. Gökbuget N, Kantarjian HM, Brüggemann M, et al. Molecular response with blinatumomab in relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Blood Adv 2019; 3: 3033–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boissel N, Ribera JM, Chiaretti S, et al. Treatment of adults with relapsed/refractory Philadelphia chromosome negative acute lymphoblastic leukemia with blinatumomab in a real-world setting: results from the Neuf study. Blood 2019; 134(Suppl. 1): abstract 2627. [Google Scholar]
- 39. Boissel N, Bassan R, Ribera JM, et al. Treatment of adults with minimal residual disease (MRD) positive acute lymphoblastic leukemia with blinatumomab in a real-world setting: results from the Neuf Study. Blood 2019; 134(Suppl. 1): abstract 2624. [Google Scholar]
- 40. von Stackelberg A, Locatelli F, Zugmaier G, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol 2016; 34: 4381–4389. [DOI] [PubMed] [Google Scholar]
- 41. Gore L, Locatelli F, Zugmaier G, et al. Survival after blinatumomab treatment in pediatric patients with relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Blood Cancer J 2018; 8: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Locatelli F, Zugmaier G, Bader P, et al. B cell precursor acute lymphoblastic leukemia (r/r ALL) treated with blinatumomab: rialto an open-label, multicenter, expanded access study. Blood 2018; 132(Suppl. 1): 1375. [Google Scholar]
- 43. Martinelli G, Boissel N, Chevallier P, et al. Complete hematologic and molecular response in adult patients with relapsed/refractory Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia following treatment with blinatumomab: results from a phase II, single-arm, multicenter study. J Clin Oncol 2017; 35: 1795–1802. [DOI] [PubMed] [Google Scholar]
- 44. Rambaldi A, Ribera JM, Kantarjian HM, et al. Blinatumomab compared with standard of care for the treatment of adult patients with relapsed/refractory Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia. Cancer. Epub ahead of print 18 October 2019. DOI: 10.1002/cncr.32558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Topp MS, Kufer P, Gökbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 2011; 29: 2493–2498. [DOI] [PubMed] [Google Scholar]
- 46. Gökbuget N, Zugmaier G, Klinger M, et al. Long-term relapse-free survival in a phase 2 study of blinatumomab for the treatment of patients with minimal residual disease in B-lineage acute lymphoblastic leukemia. Haematologica 2017; 102: e132–e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gökbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood 2018; 131: 1522–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med 2017; 376: 836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dombret H, Topp MS, Schuh AC, et al. Blinatumomab versus chemotherapy in first salvage or in later salvage for B-cell precursor acute lymphoblastic leukemia. Leuk Lymphoma 2019; 60: 2214–2222. [DOI] [PubMed] [Google Scholar]
- 50. Jabbour EJ, Gökbuget N, Kantarjian HM, et al. Transplantation in adults with relapsed/refractory acute lymphoblastic leukemia who are treated with blinatumomab from a phase 3 study. Cancer. Epub ahead of print 21 August 2019. DOI: 10.1002/cncr.32335. [DOI] [PubMed] [Google Scholar]
- 51. Topp MS, Zimmerman Z, Cannell P, et al. Health-related quality of life in adults with relapsed/refractory acute lymphoblastic leukemia treated with blinatumomab. Blood 2018; 131: 2906–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brown PA, Ji L, Xu X, et al. A Randomized phase 3 trial of blinatumomab vs. chemotherapy as post-reinduction therapy in high and intermediate risk (HR/IR) first relapse of B-Acute lymphoblastic leukemia (B-ALL) in children and adolescents/young adults (AYAs) demonstrates superior efficacy and tolerability of blinatumomab: a report from children’s oncology group study AALL1331. Blood 2019; 134(Suppl. 2): abstract LBA-1. [Google Scholar]
- 53. Richard-Carpentier G, Kantarjian HM, Short NJ, et al. A phase II study of the hyper-CVAD regimen in sequential combination with blinatumomab as frontline therapy for adults with B-cell acute lymphoblastic leukemia(B-ALL). Blood 2018; 132(Suppl. 1): 32. [Google Scholar]
- 54. Advani AS, Moseley A, O’Dwyer KM, et al. Results of SWOG 1318: a phase 2 trial of blinatumomab followed by pomp (prednisone, vincristine, methotrexate, 6-mercaptopurine) maintenance in elderly patients with newly diagnosed Philadelphia chromosome negative B-cell acute lymphoblastic leukemia. Blood 2018; 132(Suppl. 1): 33. [Google Scholar]
- 55. Chiaretti S, Bassan R, Vitale A, et al. Dasatinib-blinatumomab combination for the front-line treatment of adult Ph+ ALL patients. Updated results of the gimema LAL2116 D-Alba trial. Blood 2019; 134(Suppl. 1): abstract 740. [Google Scholar]
- 56. Stein AS, Schiller G, Benjamin R, et al. Neurologic adverse events in patients with relapsed/refractory acute lymphoblastic leukemia treated with blinatumomab: management and mitigating factors. Ann Hematol 2019; 98: 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Klinger M, Zugmaier G, Nägele V, et al. Adhesion of T cells to endothelial cells facilitates blinatumomab-associated neurologic adverse events. Cancer Res. Epub ahead of print 29 October 2019. DOI: 10.1158/0008-5472.CAN-19-1131. [DOI] [PubMed] [Google Scholar]
- 58. Duell J, Dittrich M, Bedke T, et al. Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody blinatumomab in patients with B-precursor ALL. Leukemia 2017; 31: 2181–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Aldoss I, Song J, Stiller T, et al. Correlates of resistance and relapse during blinatumomab therapy for relapsed/refractory acute lymphoblastic leukemia. Am J Hematol 2017; 92: 858–865. [DOI] [PubMed] [Google Scholar]
- 60. Wei A, Ribera JM, Martinelli G, et al. Baseline biomarkers influencing clinical outcomes in adults with relapsed/refractory B-precursor acute lymphoblastic leukemia (R/R ALL) treated with blinatumomab versus standard-of-care chemotherapy (SOC) from a randomized phase 3 study. Blood 2017; 130(Suppl. 1): 2556. [Google Scholar]
- 61. Le DT, Jaffee EM. Regulatory T-cell modulation using cyclophosphamide in vaccine approaches: a current perspective. Cancer Res 2012; 72: 3439–3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Feucht J, Kayser S, Gorodezki D, et al. T-cell responses against CD19+ pediatric acute lymphoblastic leukemia mediated by bispecific T-cell engager (BiTE) are regulated contrarily by PD-L1 and CD80/CD86 on leukemic blasts. Oncotarget 2016; 7: 76902–76919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kobold S, Pantelyushin S, Rataj F, et al. Rationale for combining bispecific T cell activating antibodies with checkpoint blockade for cancer therapy. Front Oncol 2018; 8: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jabbour E, Düll J, Yilmaz M, et al. Outcome of patients with relapsed/refractory acute lymphoblastic leukemia after blinatumomab failure: no change in the level of CD19 expression. Am J Hematol 2018; 93: 371–374. [DOI] [PubMed] [Google Scholar]
- 65. Sotillo E, Barrett DM, Black KL, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov 2015; 5: 1282–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Braig F, Brandt A, Goebeler M, et al. Resistance to anti-CD19/CD3 BiTE in acute lymphoblastic leukemia may be mediated by disrupted CD19 membrane trafficking. Blood 2017; 129: 100–104. [DOI] [PubMed] [Google Scholar]
- 67. Gardner R, Wu D, Cherian S, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood 2016; 127: 2406–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jabbour EJ, Sasaki K, Ravandi F, et al. Inotuzumab ozogamicin in combination with low-intensity chemotherapy (mini-HCVD) with or without blinatumomab versus standard intensive chemotherapy (HCVAD) as frontline therapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukemia: a propensity score analysis. Cancer 2019; 125: 2579–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Duell J, Lukic DS, Karg M, et al. Functionally defective T cells after chemotherapy of B-cell malignancies can be activated by the tetravalent bispecific CD19/CD3 antibody AFM11. J Immunother 2019; 42: 180–188. [DOI] [PubMed] [Google Scholar]