Abstract
Introduction:
Endometrial stromal sarcomas (ESSs) are rare and characterized by translocations t(7;17)(p15;q11.2) and t(10;17)(q22;p13), resulting in JAZF1-SUZ12 and YWHAE-FAM22 gene fusions used for defining low-grade (LG-ESS) and high-grade (HG-ESS) tumours.
Aim:
The objective of the study was to characterize ESSs using immunohistochemical and molecular markers.
Material and Methods:
Patients diagnosed as having ESSs between January 2014 and December 2018 were included in the study. The slides were reviewed along with a panel of immunohistochemical markers, CD10, cyclin D1, oestrogen receptor (ER) and progesterone receptor (PR), Ki67, and vimentin and classified according to World Health Organization (2014) criteria into LG-ESS, HG-ESS, and undifferentiated uterine sarcoma (UUS). Molecular characterization was performed by fluorescence in situ hybridization using relevant probes.
Results:
Over a 4-year period, 552 cases of endometrial malignancies were reported, 10 of which were ESS (1.8%). Of these, 5 were LG-ESS, 3 HG-ESS, and 2 UUS. CD10 was 100% sensitive and 75% specific for LG-ESS. Oestrogen receptor and PR were 100% specific but less sensitive (80%) for LG-ESS. Forty per cent (2/5) of LG-ESS demonstrated JAZF1-SUZ12 gene rearrangement. All 3 cases of HG-ESS showed diffuse strong cyclin D1 (>70% nuclei) positivity and were negative for cluster differentiation 10, ER, and PR and demonstrated YWHAE gene rearrangement. None of the UUS cases demonstrated this gene rearrangement.
Conclusion:
Endometrial stromal sarcomas are rare tumours (1.8% in this study). JAZF1-SUZ12 and YWHAE-FAM22 gene rearrangement helps in accurate characterization of ESS and can be used as diagnostic tools especially when the diagnosis is unclear or difficult. Cyclin D1 can be used as an adjuvant immunomarker for YWHAE gene–rearranged HG-ESS.
Keywords: Endometrial stromal sarcoma, JAZF1-SUZ12 gene rearrangement, YWHAE-FAM22 gene rearrangement, cyclin D1, CD10, ER, PR
Introduction
Endometrial stromal sarcomas (ESSs) are rare and account for 0.2% to 1.5% of all uterine malignancies with a prevalence less than 1 to 9 per 1 000 000.1,2 During the past 2.5 decades, the classification of ESSs was modified several times.3 As per the 2014 World Health Organization (WHO) classification, they were classified into 4 categories: benign endometrial stromal nodules (ESNs), low-grade endometrial stromal sarcomas (LG-ESSs), high-grade endometrial stromal sarcomas (HG-ESSs), and undifferentiated uterine sarcomas (UUSs).3 The diagnosis of these tumours by light microscopy is complicated by the presence of a number of variant forms, including smooth muscle differentiation, glandular and epithelial differentiation, and sex-cord differentiation.4 As the prognosis and the 5-year survival rate of LG-ESSs and HG-ESSs are drastically different, the precise distinction between these entities is clinically very important.5
Low-grade endometrial stromal sarcoma is an indolent tumour characterized by densely cellular tumour sheets of ovoid cells with hyperchromatic nuclei and little cytoplasm, resembling endometrial stroma.6 It needs to be differentiated from ESN, uterine cellular leiomyoma (UCL), uterine leiomyosarcoma (ULMS), and adenosarcoma.5 High-grade endometrial stromal sarcoma has intermediate prognosis and is characterized by densely cellular tumour with variable admixture of high-grade round cell elements and low-grade spindle cell elements.7 The round cells show irregular hyperchromatic nuclei and scant cytoplasm with necrosis and a high mitotic index.7 Undifferentiated uterine sarcoma is a high-grade tumour that lacks any features of normal endometrial stroma exhibiting marked nuclear pleomorphism and high mitotic activity.5 The typical immunoprofile of LG-ESS is the expression of cluster differentiation 10 (CD10), oestrogen receptor (ER), and progesterone receptor (PR), whereas diffuse and strong expression of cytoplasmic cyclin D1 in high-grade round cell elements, with negative CD10, ER, and PR expression, characterizes the HG-ESS. Undifferentiated uterine sarcoma shows no specific immunohistochemical profile exhibiting variable vimentin or CD10 expression.5
Endometrial stromal sarcomas are genetically heterogeneous group of malignancies.8 The JAZF1-SUZ12 gene fusion is the most frequent and seems to be the cytogenetic hallmark of ESN and LG-ESS. It is likely to become a specific diagnostic tool, especially in borderline cases.6 It might be useful for differential diagnosis between LG-ESSs and smooth muscle tumours of the uterus, where the latter are always negative for fusion.9 Other genetic abnormalities that characterize LG-ESSs less often include the translocations t(6;7)(p21;p15) and t(7;10)(p15;p11), resulting in JAZF1/PHF1 and JAZF1/EPC1, respectively. Variants of PHF1 are known in Endometrial stromal tumours (ESS) and include EPC1 at 10p11 and MEAF6 at 1p34.3. Rare chromosomal abnormalities noted in Endometrial stromal sarcomas (ESS) include der(22)t(X;22)(p11;q13) and t(X;17)(p11;q21), resulting in ZC3H7B/BCOR and MBTD1/CXorf67 fusions, respectively. All these abnormalities are mutually exclusive.8,10 High-grade endometrial stromal sarcoma is characterized by the recently described translocation t(10;17)(q22;p13), resulting in YWHAE-FAM22 (presently NUTM2A/B) gene fusion10 and is useful as a differential diagnostic tool between LG-ESSs and HG-ESSs.8,10,11 Differentiation between LG-ESS and HG-ESS is important as these are considered as 2 distinct clinical entities with different treatment protocols.12
Endometrial stromal sarcomas are uncommon tumours, and most studies consist of a limited case series.6,13-17 The current study aims to characterize them on molecular basis.
Materials and Methods
The study was approved by the scientific review board and the institutional ethics committee. Ten patients diagnosed as having ESS on morphology and/or immunohistochemistry (IHC) during the period January 2014 to December 2018 were included. The demographic, clinical, and radiological details were collected from the medical records. The details of the type of specimen (curettage and hysterectomy) and gross examination with respect to location, size, extension of the tumour, and necrosis were noted from the archives of the Department of Pathology at our institute. Haematoxylin and eosin–stained slides were subtyped and staged as per the 2014 WHO classification and TNM staging of uterine sarcomas.5
Immunohistochemistry was performed wherever indicated with a panel of markers as per the standard protocol on tissue sections of 3 to 4 µm thickness. Sections were dried at 60°C in oven for 30 minutes and stained using the Leica BOND-III automated system. The list of antibodies used was ready-to-use CD10 (56C6), Vimentin (V9), and PR (PgR636), and concentrate versions were used in 1:100 dilution of cyclin D1 (EP12) and Ki 67 (MIB1) and 1:40 dilution of ER (EP1).
The sensitivity and specificity of CD10, ER, and PR for the diagnosis of ESSs were calculated.
Sections with viable tumour and appropriate tumour morphology were identified by the pathologist. Three- to four-micrometer formalin-fixed paraffin-embedded (FFPE) sections were mounted on poly-l-lysine slides and baked at 60°C overnight. The sections were deparaffinized in xylene for 30 minutes (3×) and dehydrated in absolute ethanol for10 minutes (2×). The tissue was treated with 0.2 N HCL for 20 minutes and washed with distilled water and 2× saline sodium citrate. Sections were next pretreated in freshly prepared pretreatment solution (1 M sodium thiocyanate) prewarmed to 80°C for 30 minutes and digested in preheated protease solution (Pepsin, Sigma P6887-5G, 25 mg/50 mL distilled water) at 37 C to break the cell membrane and remove proteins. The sections were dehydrated in ethanol.
Molecular characterization of tissue was done by fluorescence in situ hybridization (FISH) using 2 ready-to-use probes procured from CytoTest Inc., Rockville, MD, USA. CytoTest JAZF1-SUZ12 Dual Fusion/Translocation FISH Probes which target chromosome bands 7p15.2 and 17q11.2, respectively, were used to detect rearrangements involving the human JAZF1 and SUZ12 genes, on LG-ESS specimens. The 3′ and 5′ JAZF1 probes were labelled in CytoOrange and the 3′ and 5′ SUZ12 probes were labelled in CytoGreen. CytoTest YWHAE Breakapart FISH Probe Kit was the second probe set used to detect rearrangements in the human YWHAE gene located on chromosome band 17p13.3 on HG-ESS and UUS specimens. The probe mixture consisted of a 5′ FISH Probe for sequences centromeric to YWHAE breakpoint labelled in CytoGreen and a YWHAE 3′ end-telomeric FISH Probe labelled in CytoOrange.
Ten microliters of undiluted probe was applied to the target area and sections were codenatured at 75°C for 10 minutes and hybridized overnight at 37°C in ThermoBrite (Abbot Inc., Westwood: MA, USA). Following post-hybridization wash and counterstaining with diamino-phenyl-indole, the signals were visualized using an Olympus BX41 Fluorescence Microscope. Fifty morphologically intact and non-overlapping nuclei were scored.
Interpretation of the 2 probes varied. Interphase nuclei with JAZF1-SUZ12 rearrangement had 2 orange (O)/green (G) fusion signal, whereas cells negative for the rearrangement had 2 green and 2 red signal patterns. The cutoff was >5%.10,18 Interphase nuclei having YWHAE-FAM22 fusion had 1 green, 1 orange, and 1 fusion signal pattern, the cutoff being 30%.7,19 Nuclei negative for the rearrangement had 2 fusion signal patterns.
Results
There were 552 cases of endometrial malignancies over a 4-year period (January 2014 to December 2018), of which 40 endometrial mesenchymal tumours (7.2%) were reported. These included 17 (42.5%) leiomyosarcomas, 13 (32.5%) carcinosarcomas, and 10 (25%) ESSs. Ten patients diagnosed as having ESS and satisfying inclusion criteria were included in the study. Seventy per cent of the patients with ESS presented at >45 years of age (median = 64), 8 (80%) presented with abnormal uterine bleeding, and 20% with mass per abdomen. Morphological diagnosis of LG-ESS was rendered in 5 (50%), HG-ESS in 3 (30%), and UUS in 2 (20%) patients (Table 1).
Table 1.
Clinicopathological details of patients diagnosed endometrial stromal sarcoma (N = 10).
| LGE-SS | HGE-SS | UUS | ||
|---|---|---|---|---|
| Age: | <45 years to | 1 (20%) | 2 (66.6%) | – |
| >45 years | 4 (80%) | 1 (33.3%) | 2 (100%) | |
| Presentation: | Abnormal uterine bleeding | 4 (80%) | 3 (100%) | 1 (50%) |
| Mass per abdomen | 1 (20%) | – | 1 (50%) | |
| Procedure: | TAH + BSO | 4 (80%) | 3 (100%) | 2 (100%) |
| TAH + BSO + anterior exenteration | 1 (20%) | – | ||
| Morphology | Tongue-like growth pattern, uniform nuclei, spiralling arterioles | Infiltrative border, uniform small round cells | Infiltrative border with necrosis, pleomorphic cells | |
| IHC | CD10: Positive | 5/5 (100%) | 0/3 (0%) | 1/2 (50%) |
| ER: Positive | 4/5 (83.3%) | 0/3 (0%) | 0/2 (0%) | |
| PR: Positive | 4/5 (83.3%) | 0/3 (0%) | 0/2 (0%) | |
| Cyclin D1: positive | 0/5 | 3/3 (100%) | 0/2 (0%) | |
| FISH – JAZF1-SUZ12 | Positive | 2/5(40%) | ND | ND |
| FISH – YWHAE-FAM22 | Positive | ND | 3/3 (100%) | 0/2 (0%) |
Abbreviations: CD10, cluster differentiation 10; ER, oestrogen receptor; HGESS: high-grade endometrial stromal sarcoma; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; LGESS: low-grade endometrial stromal sarcoma; ND, not done; PR, progesterone receptor; TAH + BSO, total abdominal hysterectomy with bilateral salpingo-oophorectomy; UUS, undifferentiated uterine sarcoma.
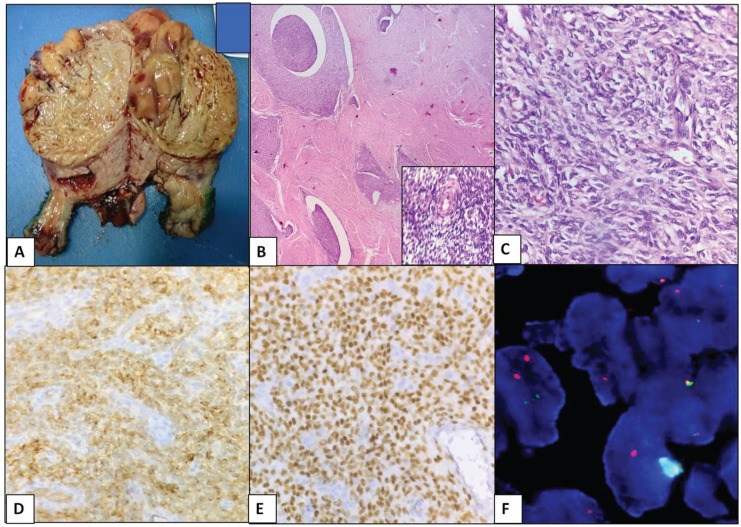
Four of 5 (80%) patients with LG-ESS were above the age of 45 years. The tumours were grey white, polypoidal masses with size varying from 3 to 10 cm. The tumour was extending up to bladder peritoneum in 1 of the ESS patients. Pathological staging of tumour was available in 4 patients, 2 in stage 1B, and 1 each in stages IIIC and IV. Morphologically, the tumour cells resembled those of normal proliferative-phase endometrium with tongue-like pattern of invasion into the myometrium and lympho-vascular spaces. All the 5 tumours displayed expression of CD10, whereas ER and PR were positive in 4 (80%) of 5 specimens. Ki67 index varied between 10% and 30%; however, cyclin D1 was negative in all the cases, confirming the morphological diagnosis (Figure 1). Molecular assessment for JAZF1-SUZ12 indicated that 2 (40%) of 5 were positive for the rearrangement. One patient had a dual fusion pattern in 50% of cells and the other patient harboured a single fusion pattern in 22% of cells (Figure 1), confirming the diagnosis of LG-ESS (Table 1). In 3 cases, lack of hybridization signals led to inconclusive results. The same has been attributed to possible preanalytical factors (which were blocks that were more than 2 years old)
Figure 1.
Low-grade endometrial stromal sarcoma. (A) Gross photo: well-circumscribed, yellow to tan fleshy cut surface. (B) Islands of tumour cells with tongue-like growth pattern without stromal response (H&E, ×100). Inset – delicate spiralling network of arterioles (haematoxylin and eosin [H&E], ×400). (C) Uniform small cells with minimal atypia (H&E, ×400). (D) Diffuse strong positive for CD10 (×400). (E) Diffuse positive for ER (×400). (F) JAZF1-SUZ12 dual fusion probe detecting t(7;17)(p15;q11.2) rearrangement. CD10 indicates cluster differentiation 10; ER, oestrogen receptor.
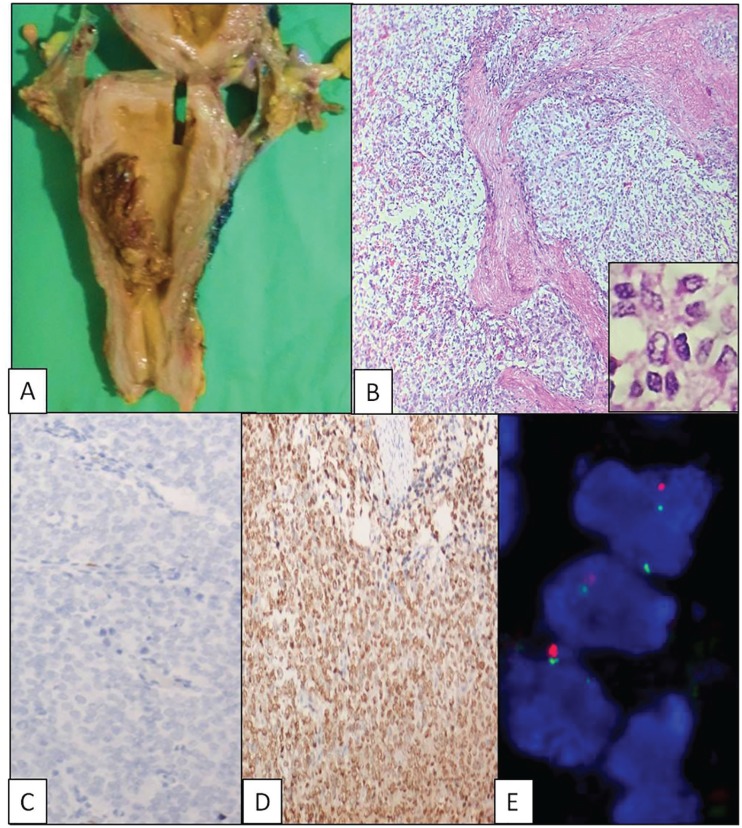
Two (66.6%) of the 3 HG-ESS patients were below 45 years of age. The tumour size varied from 2- to 11-cm grey white, polypoidal masses and confined to the endometrial cavity. Pathological staging of tumour was available in 2 patients, and both were in stage 1 (A and B). All the 3 tumours had a destructive growth pattern composed of cells which were predominantly round cells having scant eosinophilic cytoplasm and irregular nuclear contours with vesicular chromatin. Immunohistochemistry was uniform in all 3 cases: diffuse strong positive for cyclin D1 (100%) and negative for CD10, ER, and PR (Figure 2 and Table 1). Assessment of YWHAE-FAM22 breakapart translocation demonstrated all 3 (100%) cases to be positive for rearrangement; 1 case had 3 clones of cells, with predominant pattern being 1G1O1F (1 green, 1 orange, and 1 fusion) in 34% of cells and other patterns being 1F1G and 1F1O. Overall positivity rate was 82%. The second case had 2 clones, one with a typical breakapart signal pattern in 52% and the other being 1F1G signal pattern. The positivity rate was 68%. The third case was positive in 40% of cells, with 1F1O pattern predominantly suggesting a break and loss of 5′ centromeric sequence (Figure 2 and Table 1). The molecular findings confirmed the diagnosis of HG-ESS in all the 3 cases.
Figure 2.
High-grade endometrial stromal sarcoma. (A) Gross photo: polypoidal mass. (B) Infiltrative growth with invasion into myometrium (haematoxylin and eosin [H&E], ×100). Inset – non-cohesive uniform round cells (H&E, ×400). (C) CD10 negative (×400). (D) Diffuse cyclin D1 positive (×400). (E) YWHAE-FAM22 breakapart probe detecting t(10;17)(q22; p13) translocation. CD10 indicates cluster differentiation 10.
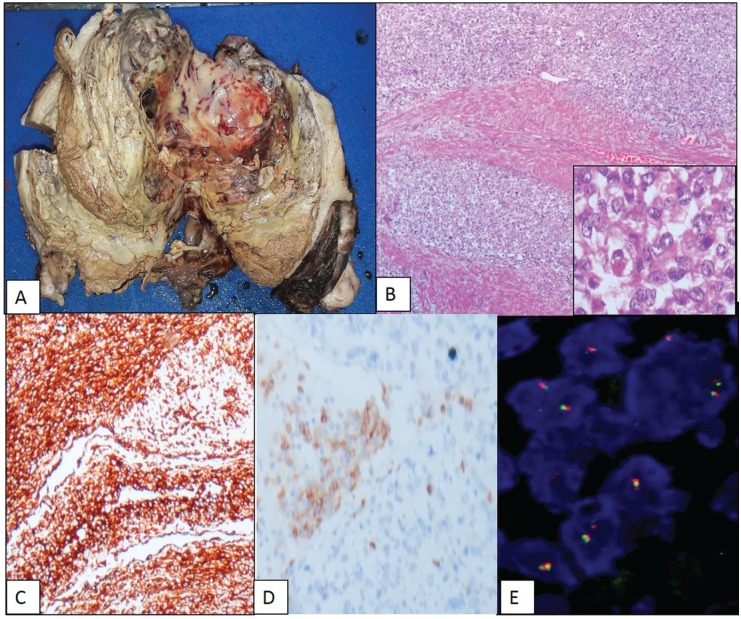
Two patients with UUS presented at >45 years of age (100%), one with abnormal uterine bleeding and the other with mass per abdomen. Both underwent hysterectomy, and grossly the tumours were localized to the uterus (stages IA and IB), with size varying between 8 and 11 cm. The tumours were widely infiltrative in sheets with large areas of necrosis and haemorrhage. The cells were pleomorphic and spindle shaped with large vesicular nuclei and scant cytoplasm. Mitoses was >10/10 hpf. One of these tumours showed extensive myxoid change. Immunohistochemically, the tumours lacked the expression of cyclin D1, ER, and PR. CD10 was focally positive in one, and both expressed vimentin. Fluorescence in situ hybridization assessment for YWHAE-FAM22 rearrangement was negative and the diagnosis was consistent with UUS (Figure 3 and Table 1).
Figure 3.
Undifferentiated uterine sarcoma. (A) Gross photo: large intracavitary fleshy polypoidal mass with haemorrhage and necrosis. (B) Diffuse infiltration into myometrium (haematoxylin and eosin, ×100). (C) Diffuse vimentin positive (×400). (D) Focal CD10 positive (×400). (E) Negative for YWHAE-FAM22 gene translocation. CD10 indicates cluster differentiation 10.
Discussion
In this study, clinicopathological and immunohistochemical subtyping along with the molecular characterisation of ESS using FISH was done.
Endometrial stromal sarcomas are mesenchymal neoplasms composed of cells that morphologically resemble proliferative-phase endometrial stroma. In a large series of 105 patients diagnosed with ESS, 72 were LG-ESS, 31 HG-ESS, and 2 unclassified.12 Results of this study also indicate that LG-ESS was the commonest subtype, followed by 3 (30%) HG-ESS and 2 (20%) UUS.
Degree of differentiation, stage of the tumour, and evaluation of the interphase between the proliferation and surrounding endometrium (tongue-like or infiltrative) are the important predictors of the behaviour.6 Low-grade endometrial stromal sarcomas frequently have been reported in younger women, either asymptomatic or present in early stage with abnormal uterine bleeding. They behave as indolent tumours and were associated with a favourable prognosis.5,20 In this study, most (80%) of the LG-ESSs presented with abnormal uterine bleeding but at higher stage. Morphology of the tumours showed typical histology with well-differentiated endometrial stromal cells exhibiting only mild nuclear atypia and characteristically invading the lympho-vascular spaces of the myometrium. Leath et al12 observed that 68% the patients with LG-ESS had disease confined to the uterine corpus or cervix, compared with 39% in HG-ESS.
The immunoprofile of ER, PR, and CD10 expression was generally considered indicative of LG-ESS.2 However, none of these markers were reported to be sufficiently sensitive and specific to aid in accurate diagnosis.21 All the 5 cases of LG-ESS in this study were positive for CD10, whereas ER and PR were positive in 4 cases (83.3%). The positivity of ER and/or PR suggests a potential role for hormonal treatment in the treatment of LG-ESS.20 Gonadotropin-releasing hormone (GnRH) is involved in oestrogen synthesis and exerts its action through its interaction with 2 receptors, GnRH-R I and GnRH-R II. Hence, immunohistochemical detection of the receptors may enable identification of tumour cells with an autocrine regulatory potential. Gonadotropin-releasing hormone and its agonists have an inhibitory effect on the growth of the tumours and may have therapeutic implication22 Although initially considered as a specific immunomarker of LG-ESS, CD10 positivity ranged from 75% to 100% of ESS, 0% to 60% of ULMS and about 90% in the sarcomatous component of adenosarcoma.21,23 Conversely, smooth muscle differentiation can also be found in 45% of LG-ESS.21 Hence, a panel of markers may be needed. Hwang et al21 also suggested that the combination of CD10+/ER+/PR+, and caldesmon might be useful in distinguishing LG-ESS from ULMS. In this study, 5 of 5 LG-ESSs, 1 case of UUS and none of the HG-ESSs expressed CD10. Hence, CD10 was 100% sensitive and 90% specific for LG-ESS. The ER and PR were not expressed in any HG-ESS; hence, it is 100% specific but less sensitive (80%) for LG-ESS.
The most common cytogenetic abnormality described in LG-ESS is a recurrent translocation involving chromosomes 7 and 17,t(7;17)(p15;q11.2), which results in a fusion between JAZF1 and SUZ12.3 It has been described in 75% of ESN, 45% to 50% of LG-ESS and 15% of HG-ESS, and 33% of UUS with ER and PR positivity.13,6,21 Earlier studies demonstrated that this fusion has been shown to be associated with classic histologic type of LG-ESS and not in other histologic variations.3,24 However, this translocation is not reported in any other uterine sarcomas and thus helps to differentiate LG-ESS from other CD10-positive tumours. Sato et al25 showed the rearrangement in primary extra-uterine ESS and emphasized its utility in the diagnosis of extrauterine ESS.
A dual colour dual fusion probe specific for the common translocation t(7;17)(p15;q11.2) was used in this study. Only 2 (40%) of the 5 LG-ESSs showed JAZF1-SUZ12 gene rearrangement. The percentage positivity is in concordance with other similar studies.2,3 Variations in the incidence of JAZF1-SUZ12 positivity have been attributed to (1) the method of tissue collection and/or preservation, (2) JAZF1-SUZ12 detection methods/probes used, and (3) ethnic considerations.3
High-grade endometrial stromal sarcomas are described to be rare tumours with features and prognosis intermediate between LG-ESS and UUS and patients’ age ranging from 28 to 67 years (mean = 50 years). In this study, 66.6% of patients were aged >45 years and the most common presentation was abnormal uterine bleeding. Tumours were grossly confined to the uterus (stage IB). Morphologically, all the cases were composed of atypical cells resembling endometrial stromal cells, but lacking the degree of pleomorphism required for the diagnosis of UUS. Mitoses >10/10 HPF and necrosis were noted. All the cases showed diffuse strong cyclin D1 (>70% nuclei) positivity and were negative for CD10, ER, and PR. This immunoprofile was documented in earlier studies.7,11,19 It was reported that cyclin D1 immunoreactivity was not demonstrated in UUS and LG-ESS. The immunoprofile correlated with the specific molecular genetic fusion – YWHAE-FAM22. This translocation has been demonstrated to be highly specific and not found in any other gynaecologic and non-gynaecologic neoplasms except for clear cell sarcoma of the kidney.26 This study demonstrated YWHAE gene rearrangement in all the 3 cases (100%).
Undifferentiated uterine sarcomas comprise a very rare group of aggressive neoplasms and are diagnosed by exclusion of other high-grade uterus sarcomas. Patients are typically postmenopausal (mean age is 60 years) and have abnormal uterine bleeding or signs/symptoms secondary to extrauterine spread. Approximately 60% of patients present with high-stage disease (stage III/IV).5 The diagnosis of undifferentiated endometrial sarcoma is applied to tumours that exhibit myometrial invasion, severe nuclear pleomorphism, high mitotic activity, and/or tumour cell necrosis and those that lack smooth muscle or endometrial stromal differentiation.5 They show variable expression of CD10, smooth muscle markers, ER, and PR. Cyclin D1 is almost always negative.5 In this study, both the patients presented after 45 years with invasion >50% of the myometrium but confined to the uterus (stage IB). Morphologically, the tumour cells were pleomorphic, spindle-shaped cells with focal fascicular pattern. They exhibited high mitotic activity, areas of necrosis, and had infiltrative border into the myometrium. Immunohistochemically, there was variable expression. Vimentin was positive in both the cases. Both were negative for smooth muscle actin SMA, ER, and PR. CD10 was focal positive in 1 case. Both the tumours were negative for YWHAE gene rearrangements.
Sciallis et al7 demonstrated YWHAE rearrangement only in 1 of the 3 morphological subsets (4/6 – 66%) of HG-ESS and subcategorized them on morphology and molecular grounds. Hoang et al27 proposed BCOR-rearranged ESS harbouring t(X;22)(p11.4;q13.2), a form of HG-ESS distinct from YWHAE-rearranged ESS in their study of 3 HG-ESSs. These tumours showed extensive myxoid change, focal fascicular architecture, and diffuse CD10 expression with limited Desmin or SMA staining. BCOR-rearranged ESS may also contribute to the differential diagnosis of myxoid uterine mesenchymal tumours.27 In this study, there was 1 sarcoma with myxoid change and lacked expression of any immunomarkers except vimentin and was reported as a UUS myxoid variant. Molecular studies for YWHAE-FAM22 were conducted and were negative. However, due to lack of other probes, we failed to conclude the specific gene rearrangement.
Fluorescence in situ hybridization is a robust technology. Availability of the probe and validation of the same in FFPE sections have enabled the detection of these rearrangements and thus helped in accurate characterization of these rare tumours. The genetic abnormalities were specific for the morphological grade and were mutually exclusive. Previous studies from India were case-based reports on small sample size. Fluorescence in situ hybridization is routinely being performed as a part of clinical theranostics in our laboratory. Detection of these rearrangements on large sample size is warranted across subcontinent as it has definitive prognostic and therapeutic implications.
This study characterizes ESS by clinicopathological, immunohistochemical, and molecular features. The limited sample size is due to the rarity of endometrial stromal sarcomas (1.8% of all endometrial malignancies in our study) even in a high-volume centre.
Conclusion
Endometrial stromal sarcomas constitute only a small subgroup of endometrial malignancies (1.8%). It is pertinent to subclassify ESSs in view of varied prognosis and management. CD10 had a sensitivity of 100% and specificity of 90%, and ER/PR showed a sensitivity and specificity of 80% and 100%, respectively, in the diagnosis of LG-ESS. Cyclin D1 expression is highly specific for YWHAE-FAM22 rearrangement in HG-ESS (100%). The molecular tests were useful in the differential diagnosis of uterine sarcomas. Fluorescence in situ hybridization using probes for JAZF1-SUZ12 and YWHAE-FAM22 is a useful complementary diagnostic tool to grade ESSs appropriately.
Acknowledgments
We thank Mr Ravinder, Mr Hussain and Mrs Padma for their technical support.
Footnotes
Author Contributions: SSM(Sudha) was responsible for concept and design of study, overseeing the experimental study including validation and interpretation and approval of the final manuscript. SS(Subbaraya) was responsible for conduct of the study, statistical analysis and drafting of the manuscript. SDG(Sandhya validated the FISH probes and interpreted the signals and did critical review of the manuscript.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Sneha Subbaraya  https://orcid.org/0000-0002-4560-3791
https://orcid.org/0000-0002-4560-3791
Sudha S Murthy  https://orcid.org/0000-0001-6406-7659
https://orcid.org/0000-0001-6406-7659
References
- 1. Benson C, Miah AB. Uterine sarcoma – current perspectives. Int J Womens Health. 2017;9:597-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chu PG, Arber DA, Weiss LM, Chang KL. Utility of CD10 in distinguishing between endometrial stromal sarcoma and uterine smooth muscle tumors: an immunohistochemical comparison of 34 cases. Mod Pathol. 2001;14:465-471. [DOI] [PubMed] [Google Scholar]
- 3. Hrzenjak A. JAZF1/SUZ12 gene fusion in endometrial stromal sarcomas. Orphanet J Rare Dis. 2016;11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stemme S, Ghaderi M, Carlson JW. Diagnosis of endometrial stromal tumors. Am J Clin Pathol. 2013;141:133-139. [DOI] [PubMed] [Google Scholar]
- 5. Mbatani N, Olawaiye AB, Prat J. Uterine sarcomas. Int J Gynecol Obs. 2018;143:51-58. [DOI] [PubMed] [Google Scholar]
- 6. Puliyath G, Nair MK. Endometrial stromal sarcoma: a review of the literature. Indian J Med Paediatr Oncol. 2012;33:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sciallis AP, Bedroske PP, Schoolmeester JK, et al. High-grade Endometrial Stromal Sarcomas. Am J Surg Pathol. 2014;38:1161-1172. [DOI] [PubMed] [Google Scholar]
- 8. Micci F, Gorunova L, Agostini A, et al. Cytogenetic and molecular profile of endometrial stromal sarcoma. Gen Chrom Cancer. 2016;55:834-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vijayan P, Ilias L, Ponniah A, Mohammed B. Low grade endometrial stromal sarcoma – A series of 4 cases and review of relevant literature. J Pathol Nepal. 2015;5:774-777. [Google Scholar]
- 10. Hodge JC, Bedroske PP, Pearce KE, Sukov WR. Molecular cytogenetic analysis of JAZF1, PHF1, and YWHAE in endometrial stromal tumors discovery of genetic complexity by fluorescence in situ hybridization. J Mol Diagn. 2016;18:516-526. [DOI] [PubMed] [Google Scholar]
- 11. Lee C-H, Ali RH, Rouzbahman M, et al. Cyclin D1 as a Diagnostic Immunomarker for Endometrial Stromal Sarcoma With YWHAE-FAM22 Rearrangement. Am J Surg Pathol. 2012;36:1562-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leath CA, III, Huh WK, Hyde J, Jr, et al. A multi-institutional review of outcomes of endometrial stromal sarcoma. Gynecol Oncol. 2007; 105: 630-634. [DOI] [PubMed] [Google Scholar]
- 13. Devi P, Himaja S, Manasa R, Atla B. Uterine low grade endometrial stromal sarcoma: a case report with review of literature. Int J Res Med Sci. 2015;3:2510-2513. [Google Scholar]
- 14. Sivakumari S, Rajaraman R, Subbiah S. Uterine sarcoma: the Indian scenario. Indian J Surg Oncol. 2015;6:232-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bhat S, Beigh AFS. histopathological study of endometrial stromal sarcomas. Int J Reprod Contracept Obs Gynecol. 2018;7:4891-4894. [Google Scholar]
- 16. Ramamoorthi S, Sinhasan S, Bhat R, Bupathy A. Endometrial stromal sarcoma: unexpected guest! Clin Cancer Investig J. 2018;7:241. [Google Scholar]
- 17. Nusrath S, Bafna S, Rajagopalan R, et al. Uterine sarcomas: experience from a tertiary cancer care center from India. Indian J Surg Oncol. 2019;10:342-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee CH, Hoang LN, Yip S, et al. Frequent expression of KIT in endometrial stromal sarcoma with YWHAE genetic rearrangement. Mod Pathol. 2014;27:751-757. [DOI] [PubMed] [Google Scholar]
- 19. Croce S, Hostein I, Ribeiro A, et al. YWHAE rearrangement identified by FISH and RT-PCR in endometrial stromal sarcomas: genetic and pathological correlations. Mod Pathol. 2013;26:1390-1400. [DOI] [PubMed] [Google Scholar]
- 20. Cui R, Yuan F, Wang Y, Li X, Zhang Z, Bai H. Clinicopathological characteristics and treatment strategies for patients with low-grade endometrial stromal sarcoma. Medicine (Baltimore). 2017;96:e6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hwang H, Matsuo K, Duncan K, et al. Immunohistochemical panel to differentiate endometrial stromal sarcoma, uterine leiomyosarcoma and leiomyoma: something old and something new. J Clin Pathol. 2015;68:710-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reich O, Nogales FF, Regauer S. Gonadotropin-releasing hormone receptor expression in endometrial stromal sarcomas: an immunohistochemical study. Mod Pathol. 2005;18:573-576. [DOI] [PubMed] [Google Scholar]
- 23. Nathenson MJ, Ravi V, Fleming N, Wang W-L, Conley A. Uterine adenosarcoma: a review. Curr Oncol Rep. 2016;18:68. [DOI] [PubMed] [Google Scholar]
- 24. Staats PN, Garcia JJ, Dias-Santagata DC, et al. Uterine tumors resembling ovarian sex cord tumors (UTROSCT) Lack the JAZF1-JJAZ1 translocation frequently seen in endometrial stromal tumors. Am J Surg Pathol. 2009;33:1206-1212. [DOI] [PubMed] [Google Scholar]
- 25. Sato K, Ueda Y, Sugaya J, Ozaki M, Hisaoka M, Katsuda S. Extrauterine endometrial stromal sarcoma with JAZF1/JJAZ1 fusion confirmed by RT-PCR and interphase FISH presenting as an inguinal tumor. Virchows Arch. 2007;450:349-353. [DOI] [PubMed] [Google Scholar]
- 26. Lee C-H, Marino-Enriquez A, Ou W, et al. The clinicopathologic features of YWHAE-FAM22 endometrial stromal sarcomas: a histologically high-grade and clinically aggressive tumor. Am J Surg Pathol. 2012;36:641-653. [DOI] [PubMed] [Google Scholar]
- 27. Hoang LN, Aneja A, Conlon N, et al. Novel high-grade endometrial stromal sarcoma: a morphologic mimicker of myxoid leiomyosarcoma. Am J Surg Pathol. 2017;41:12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]