Abstract
Background
Isocitrate dehydrogenase (IDH) mutation and 1p/19q-codeletion are oncogenetic alterations with a positive prognostic value for diffuse gliomas, especially grade II and III. Some studies have suggested differences in biological behavior as reflected by radiological characteristics. In this paper, the literature regarding radiological characteristics in grade II and III glioma subtypes was systematically evaluated and a meta-analysis was performed.
Methods
Studies that addressed the relationship between conventional radiological characteristics and IDH mutations and/or 1p/19q-codeletions in newly diagnosed, grade II and III gliomas of adult patients were included. The “3-group analysis” compared radiological characteristics between the WHO 2016 glioma subtypes (IDH-mutant astrocytoma, IDH-wildtype astrocytoma, and oligodendroglioma), and the “2-group analysis” compared radiological characteristics between 1p/19q-codeleted gliomas and 1p/19q-intact gliomas.
Results
Fourteen studies (3-group analysis: 670 cases, 2-group analysis: 1042 cases) were included. IDH-mutated astrocytomas showed more often sharp borders and less frequently contrast enhancement compared to IDH-wildtype astrocytomas. 1p/19q-codeleted gliomas had less frequently sharp borders, but showed a heterogeneous aspect, calcification, cysts, and edema more frequently. For the 1p/19q-codeleted gliomas, a sensitivity of 96% was found for heterogeneity and a specificity of 88.1% for calcification.
Conclusions
Significant differences in conventional radiological characteristics exist between the WHO 2016 glioma subtypes, which may reflect differences in biological behavior. However, the diagnostic value of the independent radiological characteristics is insufficient to reliably predict the molecular genetic subtype.
Keywords: astrocytoma, IDH mutation, MRI, oligodendroglioma, 1p/19q-codeletion
Key Points.
Conventional MRI characteristics differ significantly between glioma subtypes.
The diagnostic value of conventional MRI is insufficient to differentiate between subtypes.
Importance of the Study.
Since the WHO 2016 classification, gliomas are classified by their genetic status. IDH mutations and 1p/19q-codeletions are oncogenetic alterations with a positive prognostic value for diffuse gliomas, in particular, grade II and III. Multiple studies demonstrated differences in radiological characteristics between the glioma subtypes. This study systematically reviewed the relationship between conventional radiological characteristics and glioma subtypes. A meta-analysis was performed, which indeed demonstrates significant differences in several, but not all, radiological characteristics between glioma subtypes. These radiological differences may reflect differences in biological behavior and (partially) explain the prognostic variability. Furthermore, it seems that the diagnostic value of single radiological characteristics is insufficient to reliably differentiate distinct glioma subtypes. This meta-analysis is an important basis for further studies regarding the preoperative prediction of molecular glioma subtypes.
Grade II and III diffuse gliomas comprise a substantial part of the primary brain tumors and are known for their heterogenic behavior and prognosis. The genetic profile appears to be a factor of important prognostic value.1 Two mutations correlating with a better prognosis are the isocitrate dehydrogenase (IDH) mutation and the 1p/19q-codeletion.2 Since the 2016 World Health Organization (WHO) Classification of Tumors of the Central Nervous System, grade II and III gliomas are classified by the presence or absence of these mutations3; gliomas that are both IDH-mutated (IDHmut) and 1p/19q-codeleted are now defined as oligodendrogliomas (ODGs), whereas astrocytomas (ACTs) are 1p/19q-intact and either IDHmut or IDH wildtype (IDHwt). Median survival is most favorable in the ODGs, followed by IDHmut ACTs, with the worst prognosis in IDHwt ACTs.
The reasons for the variability in the prognosis of these glioma subtypes are not fully understood. Several studies demonstrated greater responsiveness for chemoradiation in 1p/19q-codeleted ODGs.4–7 The anatomical location and the extent of resection may be intermediate factors as well; a recent review by our group demonstrated a frontal lobe preference for IDHmut gliomas, which may partially explain the better resectability.8
Apart from differences in resectability, molecular subtypes may also influence prognosis by differences in biological behavior, as reflected by radiological differences. Multiple studies found differences in conventional magnetic resonance imaging (MRI) characteristics, such as tumor border and heterogeneity, between the genetically distinct gliomas (Figure 1).9–31 These radiological differences may reflect the differences in prognosis in terms of resectability (eg, a frontal tumor with sharp borders vs a deep-seated tumor with ill-defined borders), but also in terms of biological behavior. As more information becomes available on the radiological characteristics of the genetically distinct gliomas, the question arises how accurate conventional radiology is in differentiating between glioma subtypes.
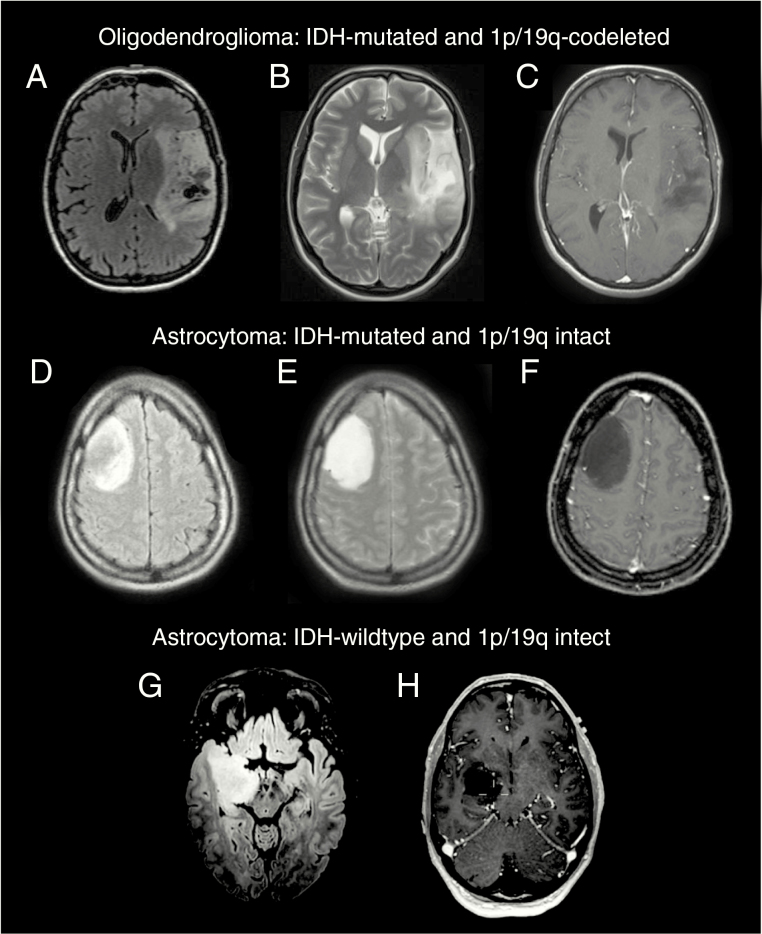
Fig. 1.
Typical MR images of WHO 2016 glioma subtypes. Top: Oligodendroglioma with ill-defined borders, heterogeneity, and cysts particularly on fluid-attenuated inversion recovery (FLAIR) (A) but also visible on T2 (B). No contrast enhancement (C). Center: A large but well-defined isocitrate dehydrogenase (IDH)-mutated astrocytoma in the right frontal lobe, no cysts or edema on FLAIR or T2 (D and E), homogenous and no contrast uptake (F). Bottom: IDH-wildtype astrocytoma, deeply seated in the right hemisphere, ill-defined margins on FLAIR and invading the midbrain (G), no contrast enhancement (H).
Therefore, in this paper, we systematically evaluate the existing literature regarding radiological characteristics in subtypes of grade II–III diffuse gliomas. The central research question is both etiological and diagnostic in nature. The etiological question aims at the correlation between glioma subtypes and their conventional radiological characteristics, reflecting biological behavior. The diagnostic question aims at the diagnostic potential of conventional radiological characteristics in differentiating between glioma subtypes.
Methods
This systematic review and meta-analysis are based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.32 A protocol for the search strategy and inclusion and exclusion criteria for selecting literature were constructed in advance. Study selection, data extraction, and quality assessment were performed by one author (D.I.v.L.) and reviewed by the second author (K.M.v.B.).
Information sources: A systematic search on PubMed, EMBASE, and Cochrane was performed and updated to November 14, 2019. The search aimed for all publications describing the correlation between the molecular features of diffuse gliomas (WHO grade II–III) and their radiological characteristics. The search consisted of 3 building blocks: (1) “glioma” (and synonyms of these terms) AND (2) “molecular” OR “genetic” OR “IDH” OR “1p/19q” (and synonyms of these terms) AND (3) “radiology” OR “MRI” (and synonyms of these terms). All terms were searched for in title/abstract. The full electronic search strategy can be found in Supplementary 1.
Study selection: The following inclusion criteria were applied: (1) studies including patients (≥18 years) with a newly diagnosed, histopathologically confirmed WHO grade II or grade III diffuse glioma and demonstrating the results of these gliomas separately from grade IV gliomas, (2) IDH status and/or 1p/19q status confirmed, (3) MRI performed before treatment, and (4) the relationship between the gliomas’ molecular status and qualitative conventional radiological characteristics was addressed. It was decided to focus on IDH mutations and 1p/19q-codeletions in grade II and grade III gliomas, as these mutations are invaluable for the contemporary classification of gliomas. Also, other mutations were reported significantly less frequently. We focused on grade II–III gliomas and excluded grade I and grade IV gliomas to ensure sufficient homogeneity of the study domain. Grade I gliomas are biologically very distinct from diffuse gliomas of grade II–IV. Although the biological and clinical characteristics of grade IV gliomas partially overlap with those of grade II–III gliomas, particularly when matching for IDH status, the differences in clinical presentation and prognosis set the grade IV tumors apart. Case reports, animal studies, studies with overlapping patient populations, and studies written in a language other than English, Dutch, French, German, or Spanish were excluded. Reference lists of the included studies were scanned for additional reports.
Study Characteristics
Data were extracted on number of cases, mean or median age, percentage male, duration of patient enrolment, study design, WHO grade and type of glioma, number of IDHmut and IDHwt gliomas, and number of 1p/19q-codeleted and 1p/19q-intact gliomas. Several other factors important for the quality assessment were also extracted and are discussed below.
Quality assessment: The quality of the included studies was assessed by a scoring model that was developed in advance and was based on several factors influencing bias (Supplementary 2). These factors were study size, inclusion of biopsies, molecular testing technique, MRI technique, the MRI investigators’ experience (ie, neuroradiologist, neurosurgeon, or other professional), blinding of the MRI investigator, and strategy for interobserver variability. Each quality item was allocated a number of points based on the possible amount of bias it could cause—the lower the bias, the higher the number of points. The maximum score was 36. Studies with 18 points or more were regarded as “low risk of bias” and included for further analysis, studies below 18 points were regarded as “high risk of bias” and discarded.
Data synthesis and analysis: Data regarding tumor borders, contrast enhancement, edema, heterogeneity, cysts, and calcification were extracted from the included studies. The radiological characteristics that were not presented in a dichotomized manner were dichotomized (yes/no) in order to perform the meta-analysis (Supplementary 3).
Based on the available data 2 separate analyses were conducted:
The “3-group analysis” is a comparison of the radiological characteristics between the grade II and III glioma subtypes based on the WHO 2016 classification: ODGs versus IDHmut ACTs versus IDHwt ACTs.
The “2-group analysis” is a comparison of the radiological characteristics between grade II and III gliomas based on 1p/19q status only (as this analysis also includes the literature from before the introduction of the IDH-mutation status as a prognostic factor in 200833).
Two-by-three and two-by-two tables were constructed, and sensitivity and specificity were calculated for the different radiological characteristics in the 2-group analysis. Differences in radiological characteristics were assessed by means of the Pearson’s Chi-square test, with a significance threshold set at P = .05.
Results
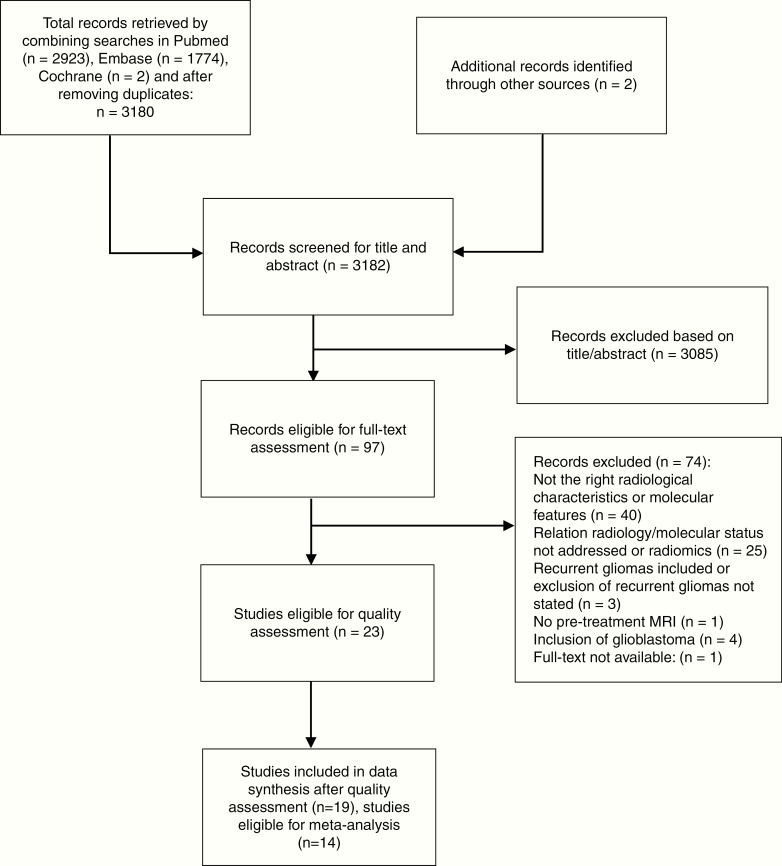
Study selection: A flow chart of the electronic search and selection process can be found in Figure 2. Ultimately, 23 publications were selected,9–31 of which 14 (after quality assessment) were eligible for the meta-analysis.9,10,13,16,17,21–27,29,31Study characteristics: All 23 studies were retrospective cohort studies (Table 1). The mean age of included patients varied from 36.5 to 58 years and 43.2–67.5% were male.
Fig. 2.
Flow chart representing the search and selection process.
Table 1.
Study and Patient Characteristics of the Included Studies Before Quality Assessment
| Authors (Year) | No. of Cases | Mean Age in Years (Range or SD) | % Male | Patient Enrolment (in Years) | Study Design | Type of Glioma and Quantity | WHO Grade (and WHO Year) | IDHmut ACT vs IDHwt ACT vs ODG (%) | 1p/19q-Codel vs 1p/19q Intact (%) |
|---|---|---|---|---|---|---|---|---|---|
| Castet et al. (2019) | 72 | 42.0 (±13.9) | 63.6 | 16 | Retrospective cohort | Astrocytoma (n = 56), oligodendroglioma (n = 16) | II (2016) | 30 (41.7) vs 26 (36.1) vs 16 (22.2) | 16 (22.2) vs 56 (77.8) |
| Hyare et al. (2019) | 120 | Median: IDHwt 54, IDHmut 37 | 60.0 | 5 | Retrospective cohort | Astrocytoma (n = 120) | II/III (2016) | 68 (56.7) vs 52 (43.3) vs 0 (0) | NA |
| Kanazawa et al. (2019) | 45 | 46 (23–81) | 55.6 | NA | Retrospective cohort and validation cohort | Astrocytoma (n = 6), oligodendroglioma (n = 30), oligoastrocytoma (n = 9) | II/III (2007 or earlier) | NA | 29 (64.4) vs 16 (35.6) |
| Villanueva-Meyer et al. (2018) | 100 | Median: IDHwt 58, IDHmut 41 | NA | 4 | Retrospective cohort | Astrocytoma, oligodendroglioma, oligoastrocytoma (n = 100) | II (2007 or earlier) | NA | NA |
| Yamauchi et al. (2018) | 101 | Median: IDHwt 54, IDHmut 38, 1p/19-codel 40 | 63.4 | 15 | Retrospective cohort | Astrocytoma (n = 66), oligodendroglioma (n = 35) | II/III (2016) | 36 (35.6) vs 30 (29.7) vs 35 (34.6) | 35 (34.7) vs 66 (65.3) |
| Park et al. (2018) | 175 | 44.6 (±12.9) | 54.3 | 9 | Retrospective cohort | Astrocytoma (n = 127), oligodendroglioma (n = 48) | II/III (2016) | 54 (30.9) vs 73 (41.7) vs 48 (27.4) | 48 (27.4) vs 127 (72.6) |
| Delfanti et al. (2017) | 40 | NA | 67.5 | 7 | Retrospective cohort | Astrocytoma (n = 28), oligodendroglioma (n = 12) | II/III (2016) | 15 (37.5) vs 13 (32.5) vs 12 (30.0) | 12 (30) vs 28 (70) |
| Johnson et al. (2017) | 148 | 42.2 (±12.7) | 58.1 | NA | Retrospective cohort | Oligodendroglioma (n = 42), oligoastrocytoma (n = 106) | II/III (2007) | NA | 90 (60.8) vs 58 (39.2) |
| Darlix et al. (2017) | 196 | Median: 36.8 (17.3–67.1) | 55.6 | 7 | Retrospective cohort | Astrocytoma (n = 125), oligodendroglioma (n = 73) | II (2016) | 91 (46.4) vs 34 (17.3) vs 71 (36.2) | 71 (36.2) vs 125 (63.8) |
| Xing et al. (2017) | 42 | 41.8 (±16.0) | 61.9 | 2 | Retrospective cohort | Astrocytoma (n = 42) | II/III (2016) | 17 (40.5) vs 25 (59.5) vs 0 (0) | NA |
| Xiong et al. (2016) | 84 | 41.5 (24–60) | 47.6 | 2 | Retrospective cohort | Oligodendroglioma (n = 51), oligoastrocytoma (n = 33) | II/III (2007 or earlier) | NA | 60 (71.4) vs 24 (28.6) |
| Sonoda et al. (2015) | 122 | NA | NA | NA | Retrospective cohort | Astrocytoma (n = 66), oligodendroglioma (n = 40), oligoastrocytoma (n = 16) | III (2007 or earlier) | NA | 30 (24.6) vs 92 (75.4) |
| Wasserman et al. (2015) | 37 | 48 (20–81) | 43.2 | 2 | Retrospective cohort | Astrocytoma (n = 28), oligoastrocytoma (n = 9) | III (2007) | NA | 0 (0) vs 37 (100) |
| Wang et al. (2015) | 216 | Median: 44 (18–87) | 62.5 | 3 | Retrospective cohort | Astrocytoma (n = 57), oligodendroglioma (n = 44), oligoastrocytoma (n = 115) | III (2007 or earlier) | NA | NA |
| Nishiyama et al. (2014) | 55 | 47 (20–80) | 47.3 | 11 | Retrospective cohort | Astrocytoma (n = 19), oligodendroglioma (n = 13), oligoastrocytoma (n = 33) | II/III (2000 and 2007) | NA | 24 (43.6) vs 31 (66.4) |
| Reyes-Botero et al. (2014) | 50 | Median: 48 (24–78) | 62.0 | NA | Retrospective cohort | Oligodendroglioma (n = 50) | III (2007 or earlier) | NA | 39 (78.0) vs 11 (22.0) |
| Qi et al. (2014) | 193 | Median: 36.5(18–72) | 56.5 | 4 | Retrospective cohort | Astrocytoma (n = 193) | II/III (2007) | NA | NA |
| Sankar et al. (2012) | 100 | 42.6 (±12.7) | 55.0 | 9 | Retrospective and prospective cohort | Oligodendroglioma (n = 100) | II/III (2007 or earlier) | NA | 13 (13.0) vs 87 (87.0) |
| Kim et al. (2011) | 56 | 40 (20–62) | 57.1 | 13 | Retrospective cohort | Oligodendroglioma (n = 49), oligoastrocytoma (n = 7) | III (2000) | NA | 39 (69.6) vs 17 (30.4) |
| Metellus et al. (2010) | 47 | 41 (± 13.2) | 46.8 | 6 | Retrospective cohort | Astrocytoma (n = 7), oligodendroglioma (n = 18), oligoastrocytoma (n = 22) | II (2007) | NA | 17 (36.2) vs 30 (63.8) |
| Sherman et al. (2010) | 104 | 42.1 | 61.5 | 5 | Retrospective cohort | Oligodendroglioma (n = 65), oligoastrocytoma (n = 39) | II/III (2007 or earlier) | NA | 44 (42.3) vs 60 (57.7) |
| Jenkinson et al. (2006) | 62 | Median: 44 | 50.0 | 6 | Retrospective cohort | Oligodendroglioma (n = 23), oligoastrocytoma (n = 41) | II/III (2000) | NA | 30 (48.4) vs 32 (51.6) |
| Meygesi et al. (2004) | 40 | Median: 40 (24–82) | 52.5 | NA | Retrospective cohort | Oligodendroglioma (n = 40) | II/III (2000) | NA | 18 (47.4) vs 20 (52.6) |
Codel, codeleted; IDHmut ACT, isocitrate dehydrogenase-mutated astrocytoma; IDHwt ACT, isocitrate dehydrogenase-wildtype astrocytoma; NA, not available; SD, standard deviation; WHO, World Health Organization.
Quality Assessment
Ten studies included tumor biopsies as well as resections, in 12 studies the inclusion of biopsies was not addressed, and 1 study excluded biopsies (Table 2). Six studies used 1 MRI magnet strength, 9 studies used both 1.5 T and 3 T MRI, and 8 studies did not address MRI magnet strength. The method of imaging analysis was considerably diverse: it was performed by 1 to 4 analysts, with the level of experience varying from neuroradiologists and neurosurgeons to “unmentioned.” Three studies did not mention whether there was any form of blinding. Interobserver variability was commonly handled by consensus between observers. Three studies tested interobserver variability with kappa-values (values from 0.375 to 1.0)26,28,31 and 1 study computed an intraclass correlation (with high coefficients from 0.83 to 0.95).14 Nine studies used immunohistochemistry and/or fluorescence in situ hybridization combined with genomic sequencing for the genetic testing, 14 studies used only 1 technique. After applying the quality assessment scoring model (Supplementary 4) to the selected studies, 19 studies had a “low risk of bias” and were included in the final analysis.
Table 2.
Study Characteristics of the Included Studies Focused on the MRI Characteristics and Factors Important for the Quality Assessment, Sorted by Quality Assessment Score
| No. | Authors (Year) | MRI Characteristics | Molecular Testing Standard | Resection and/or Biopsy Included | MRI Magnet Strength | Imaging Analysis | MRI Assessors Blinded | Handling of Interobserver Variability | Quality Assessment Score (0–36) | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Villanueva-Meyer et al. (2018) | Tumor border, contrast enhancement, edema, cysts | Immunohistochemistry + genomic sequencing | Resection and biopsy included | 3 T | 2 neuroradiologists | Yes | Interobserver variability tested | 36 | Low |
| 2 | Xing et al. (2017) | Tumor border, contrast enhancement, edema, heterogeneity | Immunohistochemistry + genomic sequencing | Resection and biopsy included | 3 T | 2 neuroradiologists | Yes | Final decision by third senior neuroradiologist | 33 | Low |
| 3 | Metellus et al. (2010) | Contrast enhancement, heterogeneity | Genomic sequencing + FISH | Resection and biopsy included | 1.5 T | 2 neuroradiologists + 1 neurosurgeon | Yes | Agreement by consensus | 31 | Low |
| 4 | Park et al. (2018) | Tumor border, contrast enhancement, edema, cysts | Immunohistochemistry + FISH + genomic sequencing | NA | 3 T | 2 neuroradiologists | Yes | Interobserver variability tested | 30 | Low |
| 5 | Delfanti et al. (2017) | Tumor border, contrast enhancement | Immunohistochemistry + genomic sequencing + FISH | Resection and biopsy included | 1.5 T and 3 T | 2 neuroradiologists | Yes | Agreement by consensus | 30 | Low |
| 6 | Sankar et al. (2012) | Contrast enhancement | FISH | Resection and biopsy included | NA | 2 trained observers | Yes | Interobserver variability tested | 30 | Low |
| 7 | Hyare et al. (2019) | Contrast enhancement, edema, cysts | Immunohistochemistry + genomic sequencing | NA | NA | 2 neuroradiologists | Yes | Interobserver variability tested | 28 | Low |
| 8 | Xiong et al. (2016) | Tumor border, contrast enhancement, edema | Immunohistochemistry + FISH + genomic sequencing | NA | 3 T | 2 neuroradiologists | Yes | Final decision by third senior neuroradiologist | 28 | Low |
| 9 | Wang et al. (2015) | Contrast enhancement | Genomic sequencing | NA | 3 T | 2 neuroradiologists | Yes | Final decision by third senior neuroradiologist | 27 | Low |
| 10 | Jenkinson et al. (2006) | Tumor border, contrast enhancement, heterogeneity | Genomic sequencing | Resection and biopsy included | NA | 1 neuroradiologist + 1 neurosurgeon | Yes | Agreement by consensus | 27 | Low |
| 11 | Qi et al. (2014) | Tumor border, contrast enhancement, edema, heterogeneity | Genomic sequencing | Resection and biopsy included | NA | 2 neuroradiologist + 1 neurosurgeon | Yes | NA | 27 | Low |
| 12 | Reyes-Botero et al. (2014) | Tumor border, contrast enhancement, heterogeneity, cysts | Genomic sequencing | Resection and biopsy included | 1.5 T and 3 T | 2 neuroradiologists + 2 neurologists | Yes | NA | 26 | Low |
| 13 | Johnson et al. (2017) | Tumor border, contrast enhancement, cysts | Genomic sequencing + FISH | NA | 1.5 T and 3 T | 2 neuroradiologists | Yes | Agreement by consensus | 24 | Low |
| 14 | Kanazawa et al. (2019) | Tumor border, contrast enhancement, heterogeneity | Genomic sequencing | NA | 1.5 T and 3 T | 2 neuroradiologists + 2 neurosurgeons | Yes | Agreement by consensus (3 out of 4) | 24 | Low |
| 15 | Yamauchi et al. (2018) | Tumor border, contrast enhancement, heterogeneity, cysts | Genomic sequencing | Resection and biopsy included | 1.5 T and 3 T | 2 authors (function unknown) | Yes | Agreement by consensus | 24 | Low |
| 16 | Nishiyama et al. (2014) | Contrast enhancement | Genomic sequencing | NA | NA | 1 neuroradiologist + 1 neurosurgeon | Yes | Agreement by consensus | 21 | Low |
| 17 | Megyesi et al. (2004) | Tumor border, contrast enhancement, heterogeneity | Genomic sequencing | NA | NA | 1 neuroradiologist + 2 neurosurgeons | Yes | Agreement by consensus | 21 | Low |
| 18 | Darlix et al. (2017) | Tumor border | Genomic sequencing | NA | 1.5 T and 3 T | 1 trained investigator | Yes | Imaging software used | 18 | Low |
| 19 | Kim et al. (2012) | Tumor border, contrast enhancement, edema, heterogeneity, cysts | FISH | NA | 1.5T and 3 T | 1 neuroradiologist + 1 neurosurgeon | Yes | NA | 18 | Low |
| 20 | Sherman et al. (2010) | Tumor border, contrast enhancement, heterogeneity | FISH | NA | NA | 1 neuroradiologist | Yes | NA | 17 | High |
| 21 | Wasserman et al. (2015) | Tumor border, contrast enhancement, edema, heterogeneity, cysts | Immunohistochemistry + FISH | Resection and biopsy included | 1.5 T and 3 T | 1 neuroradiologist | NA | NA | 13 | High |
| 22 | Cartet et al. (2019) | Contrast enhancement | Immunohistochemistry + genomic sequencing + FISH | Resection included, biopsy excluded | NA | 2 investigators (function unknown) + 1 neurooncologist | NA | Final decision by senior neurooncologist | 10 | High |
| 23 | Sonoda et al. (2015) | Tumor border, contrast enhancement | Genomic sequencing | NA | 1.5 T and 3 T | NA | NA | NA | 7 | High |
FISH, fluorescence in situ hybridization; MRI, magnetic resonance imaging; NA, not available.
Results of Systematic Analysis
Of the 19 studies that remained after the quality assessment, 10 studies reported on both IDH status and 1p/19q status, 5 studies reported only on IDH status, and 4 studies reported only on 1p/19q status (Table 1 and Supplementary 5). Twelve studies were included in the 2-group analysis as 2 studies did not present data that directly addressed the relationship between 1p/19q status and radiological characteristics.12,14 Only 6 studies reported radiological characteristics for IDHmut ACTs, IDHwt ACTs, and ODGs separately (of which 2 studies only presented ACTs22,31) and were thus included in the 3-group analysis.
There was a wide range of reported frequencies per radiological characteristic in both the 3-group analysis and the 2-group analysis (Supplementary 6, 7, and 8). How the included studies defined and presented the radiological characteristics can be found in Supplementary 3. Tumor border was mostly classified as sharp or indistinct on T2/FLAIR. Contrast enhancement was often classified as absent or present and some studies reported enhancing proportions or enhancement patterns. For the detection of edema, T2/FLAIR and the VASARI MRI feature set were used. Heterogeneity was commonly classified as mixed intensity signals on either T1 of T2/FLAIR. Cysts were often classified as absent or present. Three out of 4 studies detected calcification on computed tomography (CT) and 1 study did not clearly state which modality was used.
Meta-analysis: A total of 670 cases for the 3-group analysis and 1042 cases for the 2-group analysis were included in the meta-analysis.
Three-Group Analysis
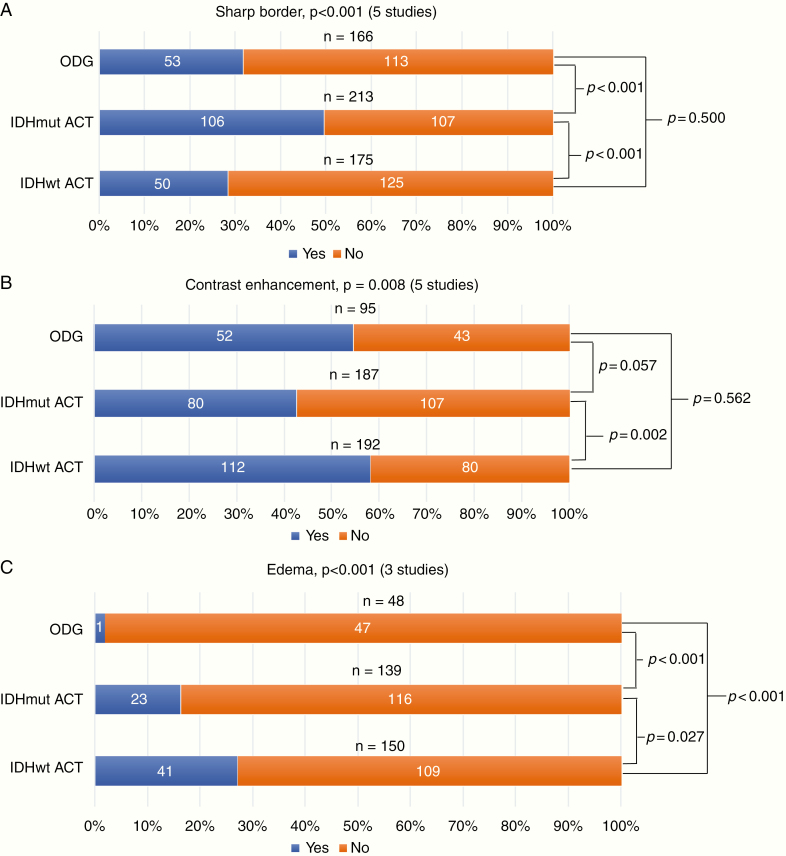
IDHmut ACTs had significantly more often sharp borders than IDHwt ACTs and ODGs (50% vs 29% vs 32%, respectively, P < .001, Figure 3A and Supplementary 7). IDHmut ACTs were less less frequently contrast enhanced than both IDHwt ACTs (43% vs 58%, respectively, P = .002, Figure 3B), but not compared to ODGs (43% vs 55%, respectively, P = .057). Edema was seen significantly more frequently in IDHwt ACTs compared to IDHmut ACTs and ODG (27% vs 17% vs 2%, respectively, P < .001, Figure 3C). Both IDHmut ACTs and IDHwt ACTs were significantly less frequently heterogeneous than ODGs (47% vs 60% vs 94%, respectively, P < .001, Figure 3D), but no significant difference was found between IDHmut ACTs and IDHwt ACTs (P = .181). Cysts were seen significantly more often in ODGs than IDHwt ACTs (29% vs 11%, P < .001, Figure 3E), but no significant difference between ODGs and IDHmut ACTs could be demonstrated (29% vs 18%, P = .060). No significant differences in cysts were seen between IDHmut ACTs and IDHwt ACTs either (P = .065).
Fig. 3.
The outcome of the 3-group meta-analysis, stratified for IDH and 1p/19q status, for (A) sharp border, (B) contrast enhancement, (C) edema, (D) heterogeneity and (E) cysts. P-values (acquired by the Pearson’s Chi-square test) and the included number of studies are presented per radiological characteristic. A total number of cases per glioma subtype are presented above the bars. The faded bars represent the data of 1 study, as no more data were available for this analysis. IDHmut ACT, isocitrate dehydrogenase-mutated astrocytoma; IDHwt ACT, isocitrate dehydrogenase-wildtype astrocytoma; ODG, oligodendroglioma.
Two-Group Analysis
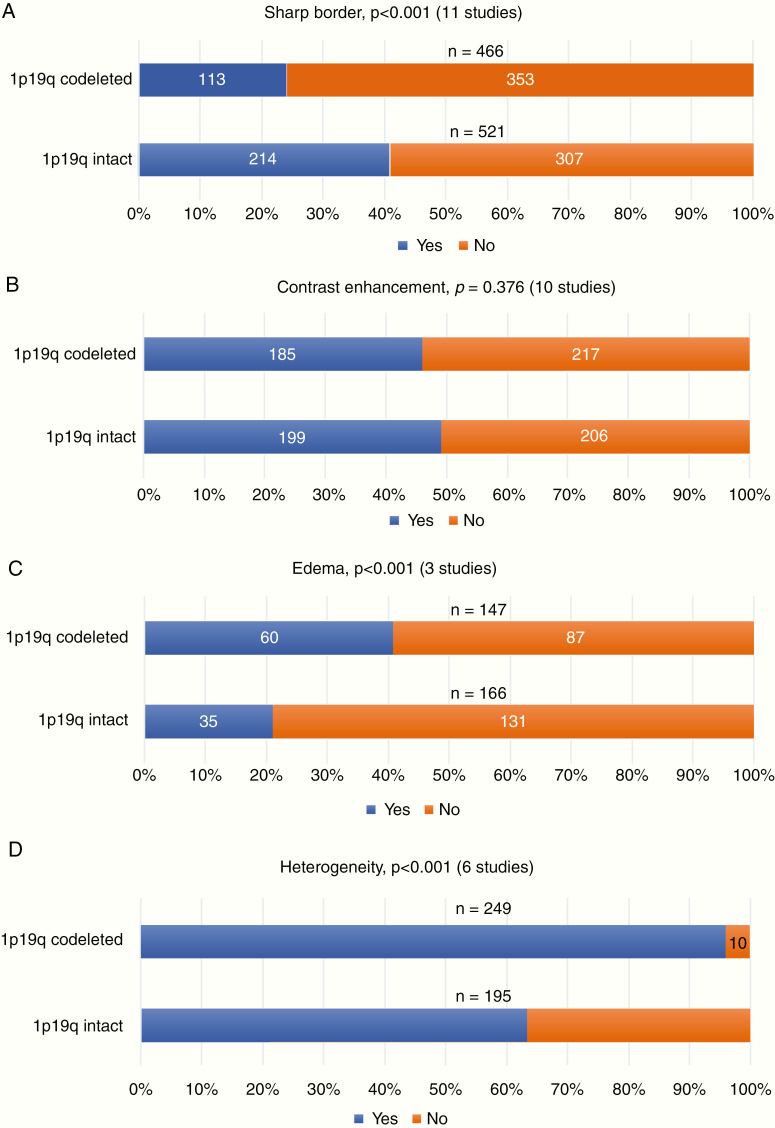
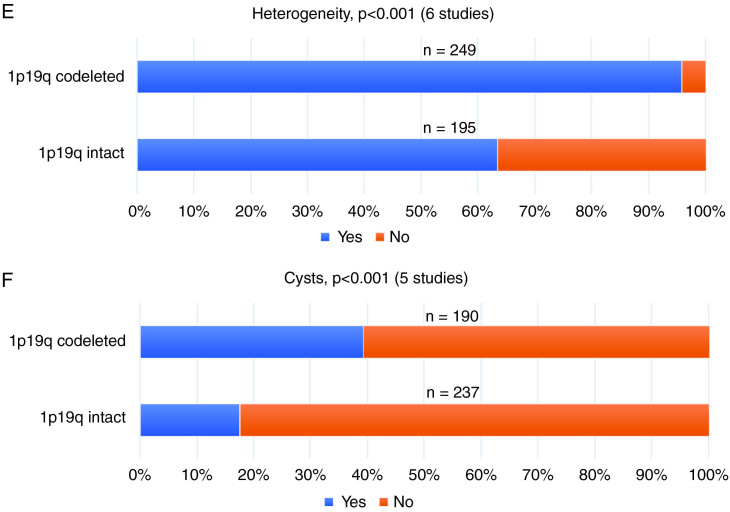
1p/19q-codeleted gliomas, when compared to 1p/19q-intact tumors, were characterized by a significantly lower frequency of sharp borders (24% vs 41%, Figure 4A), but higher rates of edema (41% vs 21%, Figure 4C), a heterogeneous aspect (96% vs 64%, Figure 4D), cysts (39% vs 18%, Figure 4E), and calcification (51% vs 9%, Figure 4F) (all P < 0.001) (Figure 4 and Supplementary 8). No significant difference was found for contrast enhancement (46% [1p/19q-codeleted] vs 49% [1p/19q-intact], P = .376, Figure 4B).
Fig. 4.
The outcome of the 2-group meta-analysis, stratified for 1p/19q status, for (A) sharp border, (B) contrast enhancement, (C) edema, (D) heterogeneity, (E) cysts and (F) calcification. P-values (acquired by the Pearson’s Chi-square test) and the included number of studies are presented per radiological characteristic. A total number of cases are presented above the bars.
Sensitivity and Specificity
For the 2-group analysis, a sensitivity of 96.0% (95% confidence interval [CI], 92.7–98.1%) for heterogeneity and specificities of 82.3% (95% CI, 76.8–86.9%) and 90.8% (95% CI, 84.4–95.1%) for cysts and calcification, respectively, were found for the diagnosis of ODG (Supplementary 8). All other values did not exceed 80%.
Discussion
Main Findings
This meta-analysis demonstrates that radiological characteristics, as visible on conventional MRI and CT, are significantly different between the 3 WHO 2016 gliomas subtypes (IDHmut ACTs, IDHwt ACTs, and ODGs). IDHmut ACTs have more frequently sharp borders and are less often contrast-enhancing than IDHwt ACTs. Compared to 1p/19q-intact gliomas, 1p/19q-codeleted gliomas display a significantly lower rate of sharp borders and a higher rate of edema, heterogeneity, cysts, and calcification. For the diagnosis of ODG, high sensitivity was found for heterogeneity (96.0%) and a high specificity was found for calcification (90.8%).
Biological Behavior
The radiological differences between IDHmut and IDHwt ACTs presumably reflect the more aggressive biological behavior of IDHwt ACTs, as both contrast enhancement and indistinct borders are seen more frequently in IDHwt ACTs, and are believed to indicate invasiveness.9,10,15,34,35 This may explain why IDH mutations are an independent positive prognostic marker.18,28 Furthermore, sharp borders18,23 and frontal anatomical location8 may contribute to better resectability, a larger extent of resection, and thus better survival.
Among the IDH-mutated tumors, the ODGs also frequently feature radiological characteristics which could possibly reflect more aggressive biological behavior. For example, Lee et al.36 demonstrated that high proportions of edema affect progression-free and overall survival negatively, and Zhou et al.37 demonstrated longer progression-free survival and overall survival for gliomas with smooth nonenhancing margins. Moreover, heterogeneity is considered to be a result of (micro)calcification, intratumoral hemorrhage, necrosis, and degeneration13,27 and these factors might complicate surgery. Considering the presumably “aggressive” appearance of ODGs on MRI, the relatively favorable prognosis of ODGs may seem contradictive. The factor that is generally believed to attribute to this good prognosis is their susceptibility for chemoradiation.4–7
Diagnostic Properties of (Conventional) MRI Characteristics
Several studies proposed the possibility of conventional MRI replacing biopsies for the noninvasive assessment of the mutational status of gliomas (especially in the absence of genetic testing),10,19,25 but the results of this meta-analysis show that differentiation solely based on independent conventional radiological characteristics is not possible. The only characteristics that hold reasonable diagnostic value on their own are heterogeneity and calcification for the diagnosis of 1p/19q-codeletion. Unfortunately, there is insufficient literature for a meta-analysis on the combination of MRI characteristics, which may yield higher predictive values. For example, 2 studies found positive predictive values of 91–100% for detecting 1p/19q-codeletions after combining calcification and surface localization.17,29 Other studies found differences in MR diffusion, perfusion, and spectroscopy between genetic subtypes, which could all contribute to the noninvasive assessment of gliomas.21,38–43 Especially the diagnostic performance of 2-hydroxy-glutarate magnetic resonance spectroscopy has shown promising results; a recent meta-analysis found a sensitivity and specificity of 95% and 91%, respectively, for detecting IDH mutations in grade II–IV gliomas.44 Moreover, the field of radiology is evolving, and radiomics and machine learning as diagnostic tools are demonstrating promising results with sensitivities of 81–97% and specificities of 77–100%.45–49 These new techniques are, however, not yet incorporated and readily applicable in daily practice. Further study on the robustness of standardized radiomics protocols is needed.
Growing Importance of Imaging
In light of the new WHO 2016 classification of gliomas, all previous studies regarding prognostic factors should be reevaluated. Several studies have already confirmed the positive effect of gross total resection on overall survival for IDHmut ACTs,50–53 but it also seems that a small residue in ODGs does not have the same negative impact on survival as in IDHmut ACTs.51 Future lines of research should finally answer the question whether the surgical strategy for a diffuse glioma is dependent on the molecular subtype. If this is the case, then the preoperative prediction of a molecular subtype with advanced imaging becomes increasingly important.
Limitations
This review and meta-analysis are subject to several limitations. First, in the 2-group analysis on the effects of 1p/19q status, some studies only included ODGs and oligoastrocytomas based on the WHO 2007 criteria (thus excluding the histologically pure ACTs), which could have led to selection bias (Supplementary 9). This factor was not included in the quality assessment as it could not be applied to all studies.
Second, the techniques used to describe the MRI characteristics differed between the studies and not all studies defined and presented the radiological characteristics in a similar manner. This was overcome in the meta-analysis by uniformly dichotomizing the MRI characteristics (Supplementary 3) although one might argue that this is somewhat arbitrary. Third, not all studies clearly stated whether or not biopsies had been included. The exclusion of biopsies may lead to an underrepresentation of deep-seated and poorly delineated tumors, because these tumors are less likely to be resected. Although these study limitations are partly accounted for by the quality assessment, they may still have influenced the results. Fourth, although different entities, grade II and grade III diffuse gliomas were reported separately in only 2 of the included studies, and therefore a multiple regression analysis including grade could not be conducted.
Lastly, we summarized predictive values of the different radiological features by calculation of mean (compound) sensitivity and specificity. For an exact evaluation of the diagnostic test accuracy of different MRI features, a formal meta-analysis of diagnostic test accuracy, including summary ROC curves, is superior. However, for each individual feature, we found that the ranges and summary statistics of predictive value were not sufficiently high to replace current tissue diagnosis. Further efforts on improving radiological methods of tumor typing should therefore focus on combinations of features and new techniques, rather than single radiological features.
Conclusions
Subtypes of diffuse grade II–III gliomas, as defined in the WHO 2016 classification, differ significantly in radiological characteristics, which might reflect differences in biological behavior as a result of their genetic make-up. This may partly explain the heterogenic clinical outcome of these gliomas. Even though the absence of heterogeneity on MRI strongly suggests an 1p/19q-intact glioma and the presence of calcification strongly suggests an 1p/19q-codeleted glioma, the diagnostic values of single MRI characteristics are insufficient to reliably diagnose distinct molecular subtypes. Large-scale studies that simultaneously evaluate multiple radiological features, both conventional MRI and advanced techniques (MR-spectroscopy, diffusion, and perfusion), may improve predictive value through multivariable modeling. Developing technologies such as radiomics and machine learning are not yet applicable in the present clinical practice, but are indeed promising for even better prediction of the molecular genetic status of gliomas in the future. Such methods of noninvasive genotyping may prove valuable in the tailoring of surgical strategy and general treatment planning, ultimately improving the outlook for patients with diffuse glioma.
Funding
None.
Conflict of interest statement. None.
Authorship Statement
Study design: D.I.v.L. and K.M.v.B.; data acquisition: D.I.v.L. and K.M.v.B.; data analysis and synthesis: all authors; first draft of the manuscript: D.I.v.L. and K.M.v.B.; manuscript revision and approval of final version: all authors.
Supplementary Material
References
- 1. Brandner S, von Deimling A. Diagnostic, prognostic and predictive relevance of molecular markers in gliomas. Neuropathol Appl Neurobiol. 2015;41(6):694–720. [DOI] [PubMed] [Google Scholar]
- 2. Brat DJ, Verhaak RG, Aldape KD, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 4. Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol. 2013;31(3):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350. [DOI] [PubMed] [Google Scholar]
- 6. Cairncross JG, Wang M, Jenkins RB, et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol. 2014;32(8):783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van den Bent MJ, Erdem-Eraslan L, Idbaih A, et al. MGMT-STP27 methylation status as predictive marker for response to PCV in anaplastic oligodendrogliomas and oligoastrocytomas. A report from EORTC study 26951. Clin Cancer Res. 2013;19(19):5513–5522. [DOI] [PubMed] [Google Scholar]
- 8. de Leeuw BI, van Baarsen KM, Snijders TJ, et al. Interrelationships between molecular subtype, anatomical location, and extent of resection in diffuse glioma: a systematic review and meta-analysis. Neuro Oncol Adv. 2019;1(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Megyesi JF, Kachur E, Lee DH, et al. Imaging correlates of molecular signatures in oligodendrogliomas. Clin Cancer Res. 2004;10(13):4303–4306. [DOI] [PubMed] [Google Scholar]
- 10. Jenkinson MD, du Plessis DG, Smith TS, Joyce KA, Warnke PC, Walker C. Histological growth patterns and genotype in oligodendroglial tumours: correlation with MRI features. Brain. 2006;129(Pt 7):1884–1891. [DOI] [PubMed] [Google Scholar]
- 11. Sherman JH, Prevedello DM, Shah L, et al. MR imaging characteristics of oligodendroglial tumors with assessment of 1p/19q deletion status. Acta Neurochir (Wien). 2010;152(11):1827–1834. [DOI] [PubMed] [Google Scholar]
- 12. Metellus P, Coulibaly B, Colin C, et al. Absence of IDH mutation identifies a novel radiologic and molecular subtype of WHO grade II gliomas with dismal prognosis. Acta Neuropathol. 2010;120(6):719–729. [DOI] [PubMed] [Google Scholar]
- 13. Kim JW, Park CK, Park SH, et al. Relationship between radiological characteristics and combined 1p and 19q deletion in World Health Organization grade III oligodendroglial tumours. J Neurol Neurosurg Psychiatry. 2011;82(2):224–227. [DOI] [PubMed] [Google Scholar]
- 14. Sankar T, Moore NZ, Johnson J, et al. Magnetic resonance imaging volumetric assessment of the extent of contrast enhancement and resection in oligodendroglial tumors. J Neurosurg. 2012;116(6):1172–1181. [DOI] [PubMed] [Google Scholar]
- 15. Qi S, Yu L, Li H, et al. Isocitrate dehydrogenase mutation is associated with tumor location and magnetic resonance imaging characteristics in astrocytic neoplasms. Oncol Lett. 2014;7(6):1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reyes-Botero G, Dehais C, Idbaih A, et al. ; POLA Network Contrast enhancement in 1p/19q-codeleted anaplastic oligodendrogliomas is associated with 9p loss, genomic instability, and angiogenic gene expression. Neuro Oncol. 2014;16(5):662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nishiyama Y, Sasaki H, Nagahisa S, et al. Radiological features of supratentorial gliomas are associated with their genetic aberrations. Neurosurg Rev. 2014;37(2):299–300. [DOI] [PubMed] [Google Scholar]
- 18. Wang YY, Wang K, Li SW, et al. Patterns of tumor contrast enhancement predict the prognosis of anaplastic gliomas with IDH1 mutation. AJNR Am J Neuroradiol. 2015;36(11):2023–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wasserman JK, Nicholas G, Yaworski R, et al. Radiological and pathological features associated with IDH1-R132H mutation status and early mortality in newly diagnosed anaplastic astrocytic tumours. PLoS One. 2015;10(4):e0123890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sonoda Y, Shibahara I, Kawaguchi T, et al. Association between molecular alterations and tumor location and MRI characteristics in anaplastic gliomas. Brain Tumor Pathol. 2015;32(2):99–104. [DOI] [PubMed] [Google Scholar]
- 21. Xiong J, Tan W, Wen J, et al. Combination of diffusion tensor imaging and conventional MRI correlates with isocitrate dehydrogenase ½ mutations but not 1p/19q genotyping in oligodendroglial tumours. Eur Radiol. 2016;26(6):1705–1715. [DOI] [PubMed] [Google Scholar]
- 22. Xing Z, Yang X, She D, et al. Noninvasive assessment of IDH mutational status in World Health Organization grade II and III astrocytomas using DWI and DSC-PWI combined with conventional MR imaging. Am J Neuroradiol. 2017;38(6):1134–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Darlix A, Deverdun J, Menjot de Champfleur N, et al. IDH mutation and 1p19q codeletion distinguish two radiological patterns of diffuse low-grade gliomas. J Neurooncol. 2017;133(1):37–45. [DOI] [PubMed] [Google Scholar]
- 24. Johnson DR, Diehn FE, Giannini C, et al. Genetically defined oligodendroglioma is characterized by indistinct tumor borders at MRI. AJNR Am J Neuroradiol. 2017;38(4):678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Delfanti RL, Piccioni DE, Handwerker J, et al. Imaging correlates for the 2016 update on WHO classification of grade II/III gliomas: implications for IDH, 1p/19q and ATRX status. J Neurooncol. 2017;135(3):601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park YW, Han K, Ahn SS, et al. Prediction of IDH1-mutation and 1p/19q-codeletion status using preoperative MR imaging phenotypes in lower grade gliomas. AJNR Am J Neuroradiol. 2018;39(1):37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamauchi T, Ohno M, Matsushita Y, et al. Radiological characteristics based on isocitrate dehydrogenase mutations and 1p/19q codeletion in grade II and III gliomas. Brain Tumor Pathol. 2018;35(3):148–158. [DOI] [PubMed] [Google Scholar]
- 28. Villanueva-Meyer JE, Wood MD, Choi BS, et al. MRI features and IDH mutational status of grade II diffuse gliomas: impact on diagnosis and prognosis. AJR Am J Roentgenol. 2018;210(3):621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kanazawa T, Fujiwara H, Takahashi H, et al. Imaging scoring systems for preoperative molecular diagnoses of lower-grade gliomas. Neurosurg Rev. 2019;42(2):433–441. [DOI] [PubMed] [Google Scholar]
- 30. Castet F, Alanya E, Vidal N, et al. Contrast-enhancement in supratentorial low-grade gliomas: a classic prognostic factor in the molecular age. J Neurooncol. 2019;143(3):515–523. [DOI] [PubMed] [Google Scholar]
- 31. Hyare H, Rice L, Thust S, et al. Modelling MR and clinical features in grade II/III astrocytomas to predict IDH mutation status. Eur J Radiol. 2019;114:120–127. [DOI] [PubMed] [Google Scholar]
- 32. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34. [DOI] [PubMed] [Google Scholar]
- 33. Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. White ML, Zhang Y, Kirby P, Ryken TC. Can tumor contrast enhancement be used as a criterion for differentiating tumor grades of oligodendrogliomas? AJNR Am J Neuroradiol. 2005;26(4):784–790. [PMC free article] [PubMed] [Google Scholar]
- 35. Chi AS, Sorensen AG, Jain RK, Batchelor TT. Angiogenesis as a therapeutic target in malignant gliomas. Oncologist. 2009;14(6):621–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee M, Han K, Ahn SS, et al. The added prognostic value of radiological phenotype combined with clinical features and molecular subtype in anaplastic gliomas. J Neurooncol. 2019;142(1):129–138. [DOI] [PubMed] [Google Scholar]
- 37. Zhou H, Vallières M, Bai HX, et al. MRI features predict survival and molecular markers in diffuse lower-grade gliomas. Neuro Oncol. 2017;19(6):862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carrillo JA, Lai A, Nghiemphu PL, et al. Relationship between tumor enhancement, edema, IDH1 mutational status, MGMT promoter methylation, and survival in glioblastoma. AJNR Am J Neuroradiol. 2012;33(7):1349–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leu K, Ott GA, Lai A, et al. Perfusion and diffusion MRI signatures in histologic and genetic subtypes of WHO grade II-III diffuse gliomas. J Neurooncol. 2017;134(1):177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tan WL, Xiong J, Huang WY, et al. Noninvasively detecting Isocitrate dehydrogenase 1 gene status in astrocytoma by dynamic susceptibility contrast MRI. J Magn Reson Imaging. 2017;45(2):492–499. [DOI] [PubMed] [Google Scholar]
- 41. Luks TL, McKnight TR, Jalbert LE, et al. Relationship of in vivo MR parameters to histopathological and molecular characteristics of newly diagnosed, nonenhancing lower-grade gliomas. Transl Oncol. 2018;11(4):941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Park YW, Han K, Ahn SS, et al. Whole-tumor histogram and texture analyses of dti for evaluation of IDH1-mutation and 1p/19q-codeletion status in World Health Organization grade II gliomas. AJNR Am J Neuroradiol. 2018;39(4):693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fellah S, Caudal D, De Paula AM, et al. Multimodal MR imaging (diffusion, perfusion, and spectroscopy): is it possible to distinguish oligodendroglial tumor grade and 1p/19q codeletion in the pretherapeutic diagnosis? AJNR Am J Neuroradiol. 2013;34(7):1326–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Suh CH, Kim HS, Jung SC, et al. 2-Hydroxyglutarate MR spectroscopy for prediction of isocitrate dehydrogenase mutant glioma: a systemic review and meta-analysis using individual patient data. Neuro Oncol. 2018;20(12):1573–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. De Looze C, Beausang A, Cryan J, et al. Machine learning: a useful radiological adjunct in determination of a newly diagnosed glioma’s grade and IDH status. J Neurooncol. 2018;139(2):491–499. [DOI] [PubMed] [Google Scholar]
- 46. Lu CF, Hsu FT, Hsieh KL, et al. Machine learning-based radiomics for molecular subtyping of gliomas. Clin Cancer Res. 2018;24(18):4429–4436. [DOI] [PubMed] [Google Scholar]
- 47. Mazurowski MA, Clark K, Czarnek NM, et al. Radiogenomics of lower-grade glioma: algorithmically-assessed tumor shape is associated with tumor genomic subtypes and patient outcomes in a multi-institutional study with The Cancer Genome Atlas data. J Neurooncol. 2017;133(1):27–35. [DOI] [PubMed] [Google Scholar]
- 48. Hong EK, Choi SH, Shin DJ, et al. Radiogenomics correlation between MR imaging features and major genetic profiles in glioblastoma. Eur Radiol. 2018;28(10):4350–4361. [DOI] [PubMed] [Google Scholar]
- 49. Yogananda CGB, Shah BR, Vejdani-Jahromi M, et al. A novel fully automated MRI-based deep learning method for classification of IDH mutation status in brain gliomas. Neuro Oncol. 2019;212(1):26–37. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50. Beiko J, Suki D, Hess KR, et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 2014;16(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wijnenga MMJ, French PJ, Dubbink HJ, et al. The impact of surgery in molecularly defined low-grade glioma: an integrated clinical, radiological, and molecular analysis. Neuro Oncol. 2018;20(1):103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kawaguchi T, Sonoda Y, Shibahara I, et al. Impact of gross total resection in patients with WHO grade III glioma harboring the IDH ½ mutation without the 1p/19q co-deletion. J Neurooncol. 2016;129(3):505–514. [DOI] [PubMed] [Google Scholar]
- 53. Patel SH, Bansal AG, Young EB, et al. Extent of surgical resection in lower-grade gliomas: differential impact based on molecular subtype. AJNR Am J Neuroradiol. 2019;40(7):1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.