Abstract
Background:
Traditionally believed to be an integral part of multiple myeloma (MM) treatment, the role of hematopoietic stem-cell transplantation (HSCT) is being challenged. As such, we sought to evaluate the impact of HSCT in the era of novel agents.
Methods:
A multicenter, retrospective, longitudinal cohort study was carried out between January 2016 and December 2018. A total of 55 patients who received VTD (bortezomib-thalidomide-dexamethasone) as first-line treatment and KRd (carfilzomib-lenalidomide-dexamethasone) as second-line treatment were analyzed for outcomes.
Results:
The enrolled patients were divided into Group 1, defined as those who continued KRd treatment until progression (n = 41), versus Group 2, defined as those who underwent HSCT after a certain number of cycles of KRd (n = 14). Both groups showed a generally favorable response to KRd, with overall response rate (ORR) of 87.9% and clinical benefit rate of 92.8% after a median of seven cycles in Group 1, and ORR 92.8% and clinical benefit rate 100% after median of five cycles in Group 2. However, significantly poorer progression-free survival (PFS) (p = 0.004) was observed in Group 1 (median 12 months) compared with Group 2 (median not reached). Multivariate analyses identified HSCT after KRd as potential risk factors associated with PFS. Also, in Group 1, bortezomib refractoriness was associated with significantly shorter PFS compared with those who were responsive (median 12 months versus 14 months, respectively, p = 0.039).
Conclusions:
In conclusion, even with the advent of novel agents, HSCT still remains a valuable treatment modality with additive efficacy.
Keywords: hematopoietic stem-cell transplantation, multiple myeloma, novel therapy
Introduction
Multiple myeloma (MM) represents a considerable clinical challenge as the number of patients continues to rise and the treatment landscape is constantly evolving.1 More than 30 years after its introduction, autologous stem-cell transplantation (ASCT) remains the standard for newly diagnosed MM.2–4 Even so, due to inevitable relapses and failure to produce overall survival (OS) benefits, the procedure is often a topic of debate, and some experts suggest delaying ASCT until relapse or progression.2 Furthermore, with the advent of novel agents, the very necessity for ASCT is being challenged. While SWOG S0777 showed comparable progression-free survival (PFS) and OS between VRD (bortezomib-lenalidomide-dexamethasone) versus ASCT,5 IFM2009 reported conflicting results and showed ASCT to be associated with longer PFS and improved complete remission (CR) rate compared with VRD only.6
In attempts to establish the current status of hematopoietic stem-cell transplantation (HSCT) for MM treatment in the era of novel agents, we compared the outcomes of patients undergoing second-line KRd (carfilzomib-lenalidomide-dexamethasone) after first-line VTD. A Korean population was selected for this study, because Korea has a single public medical insurance system that covers approximately 98% of the overall Korean population, and the range of coverage is strictly controlled.7 Thus, the general MM treatment algorithm is relatively uniform throughout the population.
Patients and methods
Study design and subjects
This was a multicenter, retrospective, longitudinal cohort study of MM patients over 18 years old. The study period was set between January 2016 and December 2018. HSCT-eligible patients, defined as those under the age of 65 years according to the national insurance coverage restrictions, who received VTD as first-line treatment and KRd as second-line treatment were included (Figure 1). Patients were treated with 28-day cycles of KRd: carfilzomib 20/27 mg/m2 (days 1-2, 8-9, 15-16), lenalidomide 25 mg/day (days 1-21), and dexamethasone (40 mg/week). A total of 55 patients were identified and their medical records were reviewed for demographics, disease characteristics, response to treatment, adverse events, and survival outcomes. For analyses, the patients were divided into Group 1, defined as those who continued KRd treatment until progression, versus Group 2, defined as those who underwent HSCT after a certain number of cycles of KRd.
Figure 1.
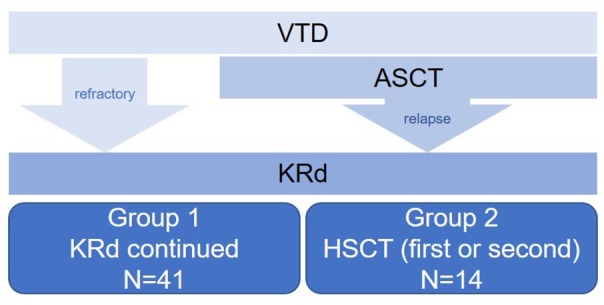
CONSORT diagram.
ASCT, autologous stem-cell transplantation; HSCT, hematopoietic stem-cell transplantation; KRd, carfilzomib-lenalidomide-dexamethasone; VTD, bortezomib-lenalidomide-dexamethasone.
This study was conducted according to the Declaration of Helsinki and was approved by the Institutional Review Board of each participating hospitals. The informed consent was waived in light of the retrospective nature of the study and the anonymity of the subjects. This study was supported by the Korean Multiple Myeloma Working Party (KMMWP, Protocol number KMM1908). All authors had access to the study data and reviewed and approved this study.
Definitions and statistical analysis
The response to KRd was evaluated according to the International Myeloma Working Group response criteria.8 Adverse events (AE) were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03). Refractory status was defined as disease progression on, or within, 60 days of the last treatment. Differences between groups were assessed using a Student’s t test or one-way analysis of variance for continuous variables, and Pearson chi-square test for categorical variables, as indicated. PFS curves were estimated using the Kaplan-Meier method. PFS was defined as the duration from the start of KRd to disease progression or death. If patients survived without death or progression, survival was censored at the latest date of follow up when no death or progression was confirmed, and data available up to October 2019 were used. Univariate and multivariate proportional hazards regression models were used to identify independent risk factors of PFS by means of log-rank tests and Cox proportional hazards models, respectively. A stepwise backward procedure was used to construct a set of independent predictors of each end point. All predictors achieving a p value below 0.05 were considered, and sequentially removed if the p value in the multiple model was above 0.05. The tested variables include age, sex, cytogenetic profile, International Staging System (ISS), bortezomib refractoriness, time to second line treatment, performance status at KRd, and laboratory findings at KRd. All data were analyzed using the Statistical Package for the Social Sciences software (IBM® SPSS® Statistics, version 22.0). p values of < 0.05 were considered statistically significant.
Results
Patient characteristics
As shown in Tables 1 and 2, there were 41 patients in Group 1 and 14 patients in Group 2. There were no significant differences between the two groups with regards to age at diagnosis, sex, or stage. There were seven patients who were refractory to first-line VTD. Two of these patients did not undergo HSCT during their entire clinical course: one patient had highly aggressive disease and expired within 6 months of diagnosis, whereas for the other patient poor mobilization hindered the transplant process and this patient, too, expired within a year of diagnosis. One patient with stable disease underwent ASCT (patient #7, Table 2) per attending physician’s choice as she did not show adequate response to VTD or KRd.
Table 1.
Baseline characteristics of 55 enrolled patients.
| n, % | All n = 55 |
Group 1 (KRd-continued, n = 41) |
Group 2 (KRd-HSCT, n = 14) |
p |
|---|---|---|---|---|
| Age, years (median, range) | 58 (39–65) | 58 (39–65) | 58.5 (47–67) | 0.938 |
| Sex, male | 32 (58.2) | 23 (56.1) | 9 (64.3) | 0.592 |
| Cytogenetic profile | ||||
| t(4;14) | 9/38 (23.7) | 7/29 (24.1) | 2/9 (22.2) | 1.000 |
| del17p | 4/39 (10.3) | 4/30 (13.3) | 0/9 (0) | 0.556 |
| t(14;16) | 1/38 (2.6) | 1/29 (3.4) | 0/9 (0) | 1.000 |
| del13q | 12/37 (32.4) | 12/27 (44.4) | 0/10 (0) | 0.015 |
| amp1q21 | 13/31 (41.9) | 10/22 (45.5) | 3/9 (33.3) | 0.696 |
| ISS I/II/III | 2(3.6)/35(63.6)/18(32.7) | 1(2.4)/27(65.9)/13(31.7) | 1(7.1)/8(57.1)/5(35.7) | 0.667 |
| R-ISS I/II/III/missing | 0/19(34.5)/22(40.0)/14(25.5) | 0/13(31.7)/18(43.9)/10(24.4) | 0/6(42.9)/4(28.6)/4(28.6) | 0.588 |
| First line treatment (VTD) | ||||
| No. of cycles (median, range) | 4 (1–9) | 4 (2–6) | 4 (1–9) | 0.923 |
| Refractory | 7 (12.7) | 2 (4.9) | 5 (35.7) | 0.009 |
| Time to 2nd line tx, months (median, range) | 19 (1–38) | 19 (2–36) | 11.5 (1–38) | 0.245 |
| Second line treatment (KRd) | ||||
| Performance status | ||||
| ECOG 0–1 | 44 (80.0) | 33 (80.5) | 11 (78.6) | 1.000 |
| ECOG ⩾2 | 11 (20.0) | 8 (19.5) | 3 (21.4) | |
| Lab findings at KRd start (mean ± standard deviation) | ||||
| WBC (103/l) | 4.3 (3.1) | 4.5 (3.3) | 3.7 (2.4) | 0.346 |
| Hb (g/dl) | 11.1 (2.1) | 11.1 (2.2) | 10.8 (1.9) | 0.623 |
| Platelet (109/l) | 163.8 (80.4) | 157.0 (82.2) | 183.7 (74.3) | 0.288 |
| Creatinine (mg/dl) | 1.1 (1.4) | 0.9 (0.3) | 1.8 (2.7) | 0.041 |
| Best response to KRd | ||||
| sCR | 6 (10.9) | 5 (12.2) | 1 (7.1) | 0.529 |
| CR | 20 (36.4) | 15 (36.6) | 5 (35.7) | |
| VGPR | 8 (14.5) | 4 (9.8) | 4 (28.6) | |
| PR | 15 (27.3) | 12 (29.3) | 3 (21.4) | |
| SD | 3 (5.5) | 2 (4.9) | 1 (7.1) | |
| PD | 3 (5.5) | 3 (7.3) | 0 | |
| No. of cycles (median, range) | 7 (1–24) | 7 (1–24) | 5 (3–10) | 0.031 |
CR, complete remission; ECOG, Eastern Cooperative Oncology Group; Hb, hemoglobin; HSCT, hematopoietic stem-cell transplantation; ISS, International Staging System; KRd, cafilzomib-lenalidomide-dexamethasone; PD, progressive disease; PR, partial response; R-ISS, revised International Staging System; sCR, stringent complete remission; SD, stable disease; VGPR, very good partial response; VTD, bortezomib-thalidomide-dexamethasone; WBC, white blood cell count.
Table 2.
Detailed information of the 14 patients in Group 2.
| Age/Sex | Type | ISS | R-ISS | High risk1 | Treatment sequence | VTD to KRd (months) | Best response to KRd | Progression after HSCT | PFS (months) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 65/M | IgG kappa | 3 | N/A | N/A | VTD#2(refractory)->KRd#5->ASCT | 2 | CR | No | 8 |
| 2 | 55/M | IgA kappa | 2 | 3 | Yes | VTD#6->ASCT->KRd#5->2nd ASCT | 38 | VGPR | No | 8 |
| 3 | 55/M | Non-secretory | 2 | 2 | No | VTD#4->ASCT->KRd#8->2nd ASCT | 23 | CR | No | 9 |
| 4 | 59/F | IgG lambda | 3 | N/A | N/A | VTD#42->KRd#4->ASCT | 7 | PR | No | 13 |
| 5 | 53/M | Kappa | 3 | 3 | Yes | VTD#1(refractory)->KRd#4->ASCT | 1 | CR | Yes, re-KRd | 15 |
| 6 | 59/M | Kappa | 3 | 3 | No | VTD#5->ASCT->KRd#4->2nd ASCT | 28 | VGPR | No | 17 |
| 7 | 53/F | Non-secretory | 1 | N/A | N/A | VTD#33->KRd#5->ASCT | 5 | SD | No | 18 |
| 8 | 59/F | IgG kappa | 2 | 2 | No | VTD#3(refractory)->KRd#5->ASCT | 4 | PR | No | 8 |
| 9 | 54/F | Lambda | 2 | 2 | No | VTD#5->ASCT->KRd#4->alloSCT | 29 | CR | No | 15 |
| 10 | 60/M | IgG kappa | 3 | 3 | Yes | VTD#6->ASCT->KRd#4->alloSCT | 17 | CR | No | 12 |
| 11 | 61/M | IgG lambda | 2 | N/A | N/A | VTD#5(refractory)->KRd#10->ASCT | 7 | CR | No | 19 |
| 12 | 47/F | IgG lambda | 2 | 2 | No | VTD#4->ASCT->KRd#8->alloSCT | 24 | VGPR | No | 11 |
| 13 | 58/M | IgD lambda | 2 | 2 | No | VTD#4(refractory)->KRd#8->ASCT | 3 | sCR | No | 13 |
| 14 | 65/M | IgG kappa | 2 | 2 | No | VTD#9->KRd#3->ASCT | 16 | VGPR | No | 14 |
High-risk patients refers to those with del(17p), t(4;14), or t(14;16).
The patient refused further treatment after four cycles of VTD due to side effects. She was put on chemo-holiday but progressed after 3 months from the last delivery of VTD.
The change in treatment was per physician’s decision, as the patient showed only SD response after three cycles of VTD.
alloSCT, allogeneic stem-cell transplantation; ASCT, autologous stem-cell transplantation; CR, complete remission; F, female; HSCT, hematopoietic stem-cell transplantation; ISS, International Staging System; KRd, cafilzomib-lenalidomide-dexamethasone; M, male; N/A, not available; PFS, progression-free survival; PR, partial response; R-ISS, Revised International Staging System; sCR, stringent complete remission; SD, stable disease; VGPR, very good partial response; VTD, bortezomib-thalidomide-dexamethasone.
Both groups showed generally favorable response to KRd, with overall response rate (ORR) of 87.9% and clinical benefit rate of 92.8% after a median of seven cycles in Group 1, and ORR 92.8% and clinical benefit rate 100% after a median of five cycles in Group 2.
Survival
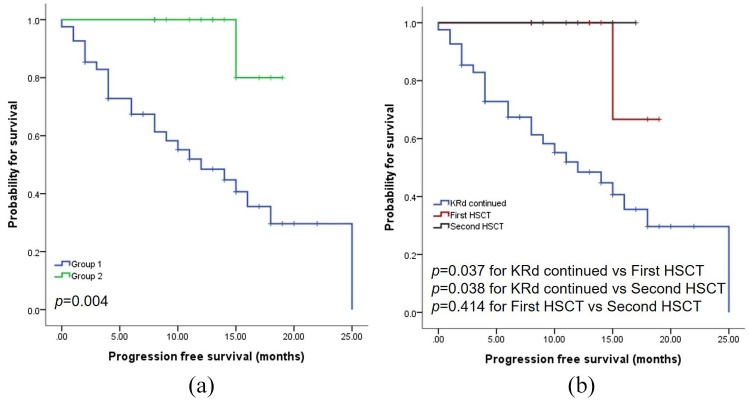
The median follow-up period was 29 months (range 6–52 months) and median PFS 10 months (range 0–25). As shown in Figure 2a, significantly poorer PFS (p = 0.004) was observed in Group 1 (median 12 months) compared with Group 2 (median not reached). Multivariate analyses identified HSCT after KRd and Hb at the start of KRd treatment as potential risk factors associated with PFS (Table 3).
Figure 2.
(a) PFS according to HSCT.(b) PFS between patients receiving first HSCT versus second HSCT in Group 2.
HSCT, hematopoietic stem-cell transplantation; KRd, carfilzomib-lenalidomide-dexamethasone; PFS, progression-free survival.
Table 3.
Multivariate analyses for PFS.
| Parameters | Univariate |
Multivariate |
||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Group 2 versus Group 1 | 0.096 (0.013–0.711) | 0.022 | 0.067 (0.009–0.501) | 0.009 |
| ECOG at KRd ⩾2 versus 0–1 | 2.433 (1.043–5.721) | 0.040 | 1.402 (0.468–4.202) | 0.546 |
| Hb at KRd < 10g/dl versus No | 3.035 (1.357–6.789) | 0.007 | 3.573 (1.253–10.186) | 0.017 |
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; Hb, hemoglobin; HR, hazard ratio; KRd, carfilzomib-lenalidomide-dexamethasone; PFS, progression-free survival.
In Group 2, there were patients undergoing HSCT for the first (n = 8) and for the second (n = 6) time (Table 2). Whether HSCT was being performed for the first (p = 0.037) or second (p = 0.038) time, HSCT was associated with significantly longer survival compared with KRd only (Figure 2b).
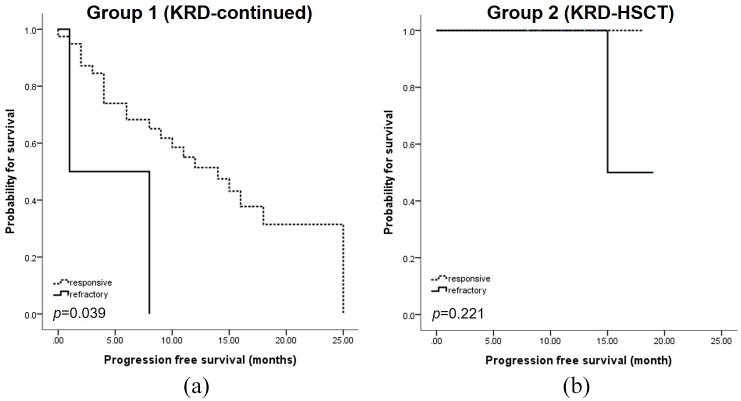
In Group 1, bortezomib refractoriness was associated with significantly shorter PFS compared with those who were responsive (median 12 months versus 14 months, respectively, p = 0.039) (Figure 3a). In Group 2, there were no differences in PFS according to bortezomib response (Figure 3b).
Figure 3.
Bortezomib response and outcomes. (a) PFS according to bortezomib refractoriness in Group 1.
(b) PFS according to bortezomib refractoriness in Group 2.KRd, carfilzomib-lenalidomide-dexamethasone; PFS, progression-free survival.
This PFS difference did not translate into differences in OS (Figure 4). More specifically, though there was a trend towards better OS in Group 2, the difference did not reach statistical significance (p = 0.061).
Figure 4.
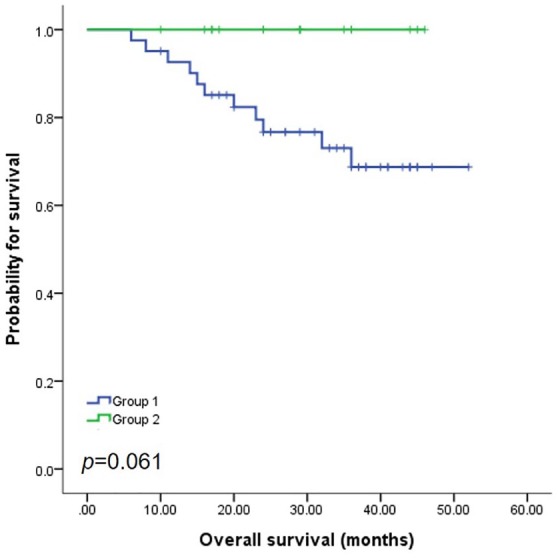
Overall survival.
Details of HSCT
Among the six patients who underwent second HSCT, three underwent allogeneic stem-cell transplantation (alloSCT). For these patients, neutrophil engraftment was defined as an absolute neutrophil count (ANC) > 0.5 × 109/L on three consecutive measurements while platelet recovery was defined as two consecutive measurements of 20.0 × 109/L without transfusion. Graft-versus-host-disease (GVHD) grading was performed according to the standard criteria.9 The specifics of the transplantation are illustrated in Table 4. All three patients received cells from full-matched donors after undergoing reduced intensity conditioning. The mean days to both neutrophil engraftment and platelet recovery was 11 days.
Table 4.
Details of alloSCT.
| Age1/ sex | Pre-alloSCT state | Donor/sex | HLA match | Conditioning | GVHD prophylaxis | Infused CD34+ cells (×106/kg) | Days to neutrophil engraft | Days to plt recovery | Acute GVHD | Chronic GVHD | Infection cx during alloSCT | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 57/F | CR | Unrelated/F | 8/8 | FluMel(RIC) | Tacro+MTX | 4.74 | 10 | 9 | None | None | No | CR, alive |
| 61/M | CR | Sibling/M | 8/8 | BuFlu(RIC) | CsA+MTX | 1.41 | 12 | 15 | None | Oral/Skin | No | CR, alive |
| 49/F | VGPR | Sibling/F | 8/8 | TBI(RIC) | ATG+CsA | 5.10 | 11 | 9 | None | Oral | No | CR, alive |
Age at alloSCT.
alloSCT, allogeneic stem-cell transplantation; ATG, antithymocyte globulin; BuFlu, busulfan and fludarabine; CR, complete remission; CSA, cyclosporine; cx, complications; F, female; FluMel, fludarabine and melphalan; GVHD, graft-versus-host-disease; HLA, human leukocyte antigens; M, male; MTX, methotrexate; plt, platelet; RIC, reduced intensity conditioning; Tacro, tacrolimus; TBI, total body irradiation; VGPR, very good partial response.
Different immunosuppressants were used as GVHD prophylaxis in each case (Table 4), but there were no cases of acute GVHD. Two eventually developed chronic GVHD. One patient developed score 2 oral and skin chronic GVHD, which responded well to steroids, and resolved without lasting sequelae. This patient remained in CR at the time of data collection. The other patient showed score 1 oral chronic GVHD without symptoms that was found incidentally on routine physical examination at D100 from alloSCT. Since the patient had no symptoms, this patient was not treated and she too remained in CR at the time of data collection.
There were no cases of hepatic sinusoidal obstruction syndrome or infection complications during transplantation, and no one developed post-transplant lymphoproliferative disorders at the time of data collection.
Adverse events
During KRd, there were three patients who required hemodialysis due to acute renal failure, two of which were associated with tumor lysis syndrome. There were three cases with grade 1 heart failure: two of them had underlying hypertension and one had no medical history. All three recovered with cessation of carfilzomib, but carfilzomib was discontinued per physician’s decision. All three continued on with lenalidomide plus dexamethasone, with one patient ultimately receiving autoSCT. None progressed at the time of data collection. There was one case of grade 2 heart failure. This patient had a history of non-ST elevation myocardial infarction, but, with the cessation of carfilzomib, he recovered fully and carfilzomib was re-administered. There were no cases of ischemic heart disease development or cerebrovascular events.
There were 12 cases of documented infection, including one mycobacterium tuberculosis infection, two influenza type A infection with pneumonia, one pneumocystis jirovecii pneumonia, one disseminated fungal infection, and one Stenotrophomonas maltophilia bacteremia. No new safety issues were raised.
Discussion
In the era of novel agents where chemotherapeutic agents alone can induce more than satisfactory responses,10–12 it becomes questionable whether HSCT has any additive value at all.13 With this question in mind, we conducted this study and, based on the results, the short answer to the question seems to be yes. Despite harboring similar responses to KRd, patients who underwent HSCT after KRd (Group 2) showed significantly longer survival compared with those who received only KRd (Group 1) (median PFS not reached versus 12 months, respectively, p = 0.004). Although the difference in PFS did not extend to significant OS gain (Figure 4), there were no deaths in Group 2, suggesting, if given more observational time, possibly we will also see an improvement in OS.
When Group 1 was further dissected, we saw that bortezomib refractory patients were associated with especially short PFS, suggesting the necessity of earlier HSCT incorporation in this subgroup of patients. Both the IFM2009 and EMN02/HO95 trials have proven the benefits of upfront ASCT.6,14 Even in patients who cannot undergo upfront ASCT due to age, progression, or comorbidities, late transplantation seems to secure similar OS.6,15 Thus, ASCT can improve outcomes whether performed as a first line or as a rescue treatment. The bortezomib-refractory patients in Group 1 represents those who had never undergone ASCT. As such, rescue ASCT in this population could lead to better PFS regardless of KRd response.
Another issue to address is the value of the second HSCT. Although the role of second transplantation in relapsed MM remains controversial,16–18 the six patients who underwent second HSCT in our cohort did very well, with no transplant-related mortality or relapses. This further accentuates the synergistic role of HSCT to triple novel agent-based therapy. Interestingly, of six HSCTs, three were allogeneic (Table 4). It is true that alloSCT is not used widely due to treatment-related toxicity, but, in the absence of readily available chimeric antigen receptor (CAR) T-cell therapy, it is still a potentially curative option. In all three cases, the attending physician deemed that the risk of disease progression outweighed the allogeneic transplant-related risks. All three cases were from full matched donors, and reduced intensity conditioning was used. Although the follow-up period is short, there were no reports of intractable acute GVHD or chronic GVHD.
Lastly, since recurrent exposure to lenalidomide can lead to poor mobilization, the timing of cell collection is important. Although there are no guidelines on this, consensus is that collection should be done between the second and fourth cycles of lenalidomide, and that stem-cell reserve is significantly altered beyond six cycles.19–21 For Group 2 patients, the median cycle of KRd was five (range 3–10), and cell collection was carried out within 3–5 cycles of KRd in all cases. There was one unfortunate case in Group 1, who progressed after three cycles of VTD (best response to VTD being stable disease) and was subsequently put on KRd with the intention to go into transplant. However, after seven cycles of KRd, collection failed on two separate occasions (first with cyclophosphamide and second with plerixafor and G-CSF), thus she could not be transplanted. Based on our data and experience, we suggest meticulous calculation of the cell collection timing if the patient does not have excess cryopreserved cells from the first ASCT, preferably before five cycles of lenalidomide is delivered.
One obvious limitation of this study is the small number of patients included. Since the insurance coverage of carfilzomib as second-line started in Korea only in 2018, longer follow up will be necessary for analyses of a larger number of patients. Other limitations include the short follow-up period and the limited availability of cytogenetic profiles. Although there were no differences in the percentage of higher risk myeloma between the two groups, and cytogenetic profile was not associated with PFS on multivariate analyses, there were insufficient patients to evaluate the impact of risk stratification on treatment responses. However, these limitations do not diminish the importance of our findings, which can be readily incorporated into real-world practice.
Conclusion
This real-world data provides insights into the current status of HSCT in MM treatment. HSCT should remain the standard of treatment for MM patients, as it is clearly synergistic to triple novel agent-based therapy and can improve treatment outcomes. With multiple monoclonal antibodies and CAR T-cell therapy in the picture, it will be interesting to observe how the status of HSCT changes for MM treatment in the near future.
Footnotes
Authors’ note: The results of this study have been submitted to the 45th Japanese Society of Myeloma Annual Meeting, Tokyo, Japan.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Research Foundation of Korea (NRF; grant number NRF-2016R1A5A1011974).
Conflict of interest statement: The author(s) declare that there is no conflict of interest.
ORCID iD: Ja Min Byun  https://orcid.org/0000-0003-1780-5553
https://orcid.org/0000-0003-1780-5553
Contributor Information
Ja Min Byun, Department of Internal Medicine, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea; Cancer Research Institute, Seoul National University, Seoul, Korea.
Sung-Soo Yoon, Department of Internal Medicine, Seoul National University Hospital, 101, Daehak-ro, Jongro-gu, Seoul 03080, Republic of Korea; Cancer Research Institute, Seoul National University, Seoul, Korea; Center for Medical Innovation, Biomedical Research Institute, Seoul National University Hospital, Seoul, Korea.
Youngil Koh, Department of Internal Medicine, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea; Cancer Research Institute, Seoul National University, Seoul, Korea.
Chang-Ki Min, Department of Internal Medicine, Seoul St. Mary’s Hospital, Seoul, Korea.
Jae Hoon Lee, Department of Internal Medicine, Gachon University Gil Hospital, Incheon, Korea.
Jaemin Jo, Department of Internal Medicine, Jeju National University Hospital, Jeju, Korea.
Hyunkyung Park, Department of Internal Medicine, Seoul National University Boramae Medical Center, Seoul, Korea.
Jiyun Lee, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
Ka-Won Kang, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea.
Yoojin Lee, Department of Hematology and Oncology, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea.
References
- 1. Lee JH, Lee DS, Lee JJ, et al. Multiple myeloma in Korea: past, present, and future perspectives. Experience of the Korean Multiple Myeloma Working Party. Int J Hematol 2010; 92: 52–57. [DOI] [PubMed] [Google Scholar]
- 2. Mohty M, Harousseau JL. Treatment of autologous stem cell transplant-eligible multiple myeloma patients: ten questions and answers. Haematologica 2014; 99: 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 2003; 348: 1875–1883. [DOI] [PubMed] [Google Scholar]
- 4. Koreth J, Cutler CS, Djulbegovic B, et al. High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: a systematic review and meta-analysis of randomized controlled trials. Biol Blood Marrow Transplant 2007; 13: 183–196. [DOI] [PubMed] [Google Scholar]
- 5. Durie BGM, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet 2017; 389: 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med 2017; 376: 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim DS. Introduction: health of the health care system in Korea. Soc Work Public Health 2010; 25: 127–141. [DOI] [PubMed] [Google Scholar]
- 8. Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood 2011; 117: 4691–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 1974; 18: 295–304. [DOI] [PubMed] [Google Scholar]
- 10. Barlogie B, van Rhee F, Shaughnessy JD, Jr, et al. Making progress in treating multiple myeloma with total therapies: issue of complete remission and more. Leukemia 2008; 22: 1633–1636. [DOI] [PubMed] [Google Scholar]
- 11. Paiva B, Martinez-Lopez J, Vidriales MB, et al. Comparison of immunofixation, serum free light chain, and immunophenotyping for response evaluation and prognostication in multiple myeloma. J Clin Oncol 2011; 29: 1627–1633. [DOI] [PubMed] [Google Scholar]
- 12. Ladetto M, Pagliano G, Ferrero S, et al. Major tumor shrinking and persistent molecular remissions after consolidation with bortezomib, thalidomide, and dexamethasone in patients with autografted myeloma. J Clin Oncol 2010; 28: 2077–2084. [DOI] [PubMed] [Google Scholar]
- 13. Al Hamed R, Bazarbachi AH, Malard F, et al. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J 2019; 9: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gambella M, Omede P, Spada S, et al. Minimal residual disease by flow cytometry and allelic-specific oligonucleotide real-time quantitative polymerase chain reaction in patients with myeloma receiving lenalidomide maintenance: a pooled analysis. Cancer 2019; 125: 750–760. [DOI] [PubMed] [Google Scholar]
- 15. Fermand JP, Ravaud P, Chevret S, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood 1998; 92: 3131–3136. [PubMed] [Google Scholar]
- 16. Hagen PA, Stiff P. The role of salvage second autologous hematopoietic cell transplantation in relapsed multiple myeloma. Biol Blood Marrow Transplant 2019; 25: e98–e107. [DOI] [PubMed] [Google Scholar]
- 17. Shah N, Ahmed F, Bashir Q, et al. Durable remission with salvage second autotransplants in patients with multiple myeloma. Cancer 2012; 118: 3549–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ziogas DC, Terpos E, Dimopoulos MA. When to recommend a second autograft in patients with relapsed myeloma? Leuk Lymphoma 2017; 58: 781–787. [DOI] [PubMed] [Google Scholar]
- 19. Giralt S, Costa L, Schriber J, et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant 2014; 20: 295–308. [DOI] [PubMed] [Google Scholar]
- 20. Shah EE, Young RP, Wong SW, et al. Impact of plerixafor use at different peripheral blood CD34+ thresholds on autologous stem cell collection in patients with multiple myeloma. Biol Blood Marrow Transplant. Epub ahead of print 27 November 2019. DOI: 10.1016/j.bbmt.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 21. Zheng G, He J, Cai Z, et al. A retrospective study of autologous stem cell mobilization by G-CSF in combination with chemotherapy in patients with multiple myeloma and lymphoma. Oncol Lett 2020; 19: 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]