Abstract
Objectives:
The objective of this study was to assess the prevalence of ultrasound (US) abnormalities and association with clinical parameters in rheumatoid arthritis (RA) clinical remission.
Methods:
Patients with established RA in clinical remission (DAS28-CRP < 2.4) taking conventional synthetic disease-modifying anti-rheumatic drugs were recruited as part of the Biomarkers of Remission in Rheumatoid Arthritis (BioRRA) Study. In addition, patients from the Newcastle Early Arthritis Clinic (NEAC) with early active RA (DAS28-CRP > 2.4) or seronegative non-inflammatory arthralgia (NIA) were studied as positive and negative controls, respectively. The association between individual dependent variables (synovial power Doppler and greyscale, tenosynovial greyscale, and erosions) and clinical parameters was assessed by multivariate ordinal logistic regression, with adjustment for multiple testing.
Results:
A total of 294 patients were included: 66 RA in remission, 146 active RA, and 82 NIA. Within the active RA group, significant associations were observed between swollen joint count and higher total synovial greyscale score (OR 1.17 95% CI 1.08–1.26, p < 0.001) and higher total synovial power Doppler score (OR 1.20, 95% CI 1.12–1.30, p < 0.001). No significant associations were observed for the NIA group. In the RA remission group, US abnormalities were frequently observed and comparable for both DAS28-CRP and 2011 ACR/EULAR Boolean remission, with no significant association with clinical parameters identified.
Conclusion:
We observed widespread subclinical US findings in RA patients in clinical remission, even when remission is defined using the stringent ACR/EULAR Boolean criteria. In contrast to active disease, synovial power Doppler failed to show significant association with any of the clinical parameters in RA remission. Our results suggest that clinical and US examinations are non-overlapping in evaluating RA remission, challenging the proposition of US-driven management strategies in this setting.
Keywords: greyscale, power Doppler, remission, rheumatoid arthritis, synovitis, ultrasound
Introduction
Rheumatoid arthritis (RA) is treated with disease-modifying anti-rheumatic drugs (DMARDs) which are escalated until a predefined target of disease activity is achieved, ideally disease remission.1 Currently, remission is defined clinically through the use of composite clinical scores such as the disease activity score in 28 joints (DAS28),2 and, more recently, the 2011 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) RA remission criteria.3 Nevertheless, some patients who achieve clinical remission can still accumulate joint erosions,4 suggesting a degree of subclinical synovitis that is captured imprecisely by clinical assessment alone.
The past two decades have witnessed an increased use of musculoskeletal ultrasound (US) in the diagnosis and management of RA. Active synovitis, visible as power Doppler (PD) change on US imaging, is predictive of future arthritis flare and joint erosion.4 However, the extent to which US parameters are associated with the ‘depth’ of remission – especially fulfilment of ACR/EULAR remission criteria – remains uncertain.
In this study, we aimed to assess the prevalence of US-defined abnormalities, and their association with clinical measures of disease activity, for patients with RA in clinical remission.
Methods
Patient recruitment
Patients with established RA were recruited as part of a study of DMARD cessation – the Biomarkers of Remission in Rheumatoid Arthritis (BioRRA) study.5 Patients were eligible if they were diagnosed with RA at least 12 months prior to assessment, and if they were treated with single or combination therapy using conventional synthetic DMARDs: methotrexate, sulfasalazine and/or hydroxychloroquine. Pregnant women, patients who had received glucocorticoids in the past 3 months and patients who had received any other DMARD in the past 6 months (12 months for leflunomide owing to its enterohepatic recirculation) were excluded.
For comparison, DMARD-naïve patients attending the Newcastle Early Arthritis Clinic (NEAC) undergoing clinical and ultrasonographic evaluation at first presentation with active RA (DAS28-CRP > 2.4) or seronegative non-inflammatory arthralgia (NIA) were studied as positive and negative controls respectively.6
Procedures
Clinical and ultrasonographic assessment of patients was performed as previously described.5,6 All patients underwent clinical examination, with remission defined as a disease activity score in 28 joints (DAS28-CRP) < 2.4.7 Where levels of C-reactive protein (CRP) were below the detection limit of the local laboratory (<5 mg/L), a value of zero was used for the purposes of DAS28-CRP calculation. A 7-joint US scan was performed (dominant wrist, 2nd/3rd metacarpophalangeal and proximal interphalangeal joints, and 2nd/5th metacarpophalangeal joints) to quantify erosions, PD and greyscale (GS) change according to the US7 protocol of Backhaus et al.8 The US operator was blinded to the clinical disease activity scores. Erosions and tenosynovial GS (TGS) were graded as present (1) or absent (0). Synovial/tenosynovial PD (SPD/TPD) and synovial GS (SGS) were graded using a 4-point (0–3) semi-quantitative scale and summed to create total scores as previously described.8 All abovementioned ultrasonographic parameters were recorded for the BioRRA cohort, whereas data were not available for tenosynovial findings for the NEAC cohort.
Statistical analysis
The association between total US scores and clinical parameters was assessed using multivariate ordinal logistic regression analyses with each individual US parameter as the dependent variable. Clinical parameters potentially correlated with US measures were selected, namely: sex, age, disease duration, smoking history, alcohol intake, rheumatoid factor and anti-citrullinated peptide antibody positivity, ACR/EULAR Boolean remission, Health Assessment Questionnaire Disability Index (HAQ-DI) score,9 erythrocyte sedimentation rate (ESR), and the individual components of the DAS28-CRP score. Correction for multiple testing was performed using a Benjamini–Hochberg procedure, with statistical significance defined using a significance threshold of <0.05. All statistical analysis was performed in the R statistical environment (R Core Team, version 3.3.2) using the ‘ordinal’ package.10
The BioRRA and NEAC studies were approved by the North East – Tyne & Wear South Research Ethics Committee (BioRRA: 14/NE/1042; NEAC: 12/NE/0251). Informed written consent was obtained from all participants in both studies.
Results
Patient characteristics
In total, 66, 146 and 82 patients were included within RA remission (BioRRA), active RA (NEAC) and NIA (NEAC) groups respectively (Table 1). There was no significant difference in sex or age of participants in the RA groups, although the NIA group were significantly younger with a higher proportion of females. Total SGS scores were comparable between remission and active RA, whereas total PDS score was significantly higher in the active RA group. Erosions were less commonly observed in the active RA group in keeping with the shorter disease duration. As expected, the prevalence of US-defined abnormalities was uniformly low in the NIA group.
Table 1.
Characteristics of patients included within the analysis.
| Demographic | RA remission (BioRRA) (n = 66) |
Active RA (NEAC) (n = 146) |
Seronegative non-inflammatory arthralgia (NEAC) (n = 82) |
||
|---|---|---|---|---|---|
| Value | p | Value | p | ||
| Female: n (%) | 38 (58) | 87 (60) | 0.88 | 68 (95) | <0.01 |
| Age: median (IQR) [range] | 66 (56–71) [35–82] | 59 (53–70) [17–88] | 0.07 | 39 (32–50) [20–80] | <0.01 |
| Years since symptom onset: median (IQR) [range] | 6 (4–12) [1–40] | 0 (0–0) [0–1] | <0.01 | 0.5 (0–1) [0–4] | <0.01 |
| RhF positive: n (%) | 40 (61) | 87 (56) | 1.00 | n/a | |
| ACPA positive: n (%) | 38 (58) | 81 (55) | 0.88 | n/a | |
| RhF or ACPA positive: n (%) | 47 (71) | 97 (66) | 0.53 | n/a | |
| RhF and ACPA positive: n (%) | 31 (47) | 71 (49) | 0.88 | n/a | |
| Total SGS score: median (IQR) [range] | 5 (3–6) [1–10] | 5 (2–7) [0–14] | 0.85 | 1 (0–2) [0–9] | <0.01 |
| Total SPD score: median (IQR) [range] | 0 (0–1) [0–7] | 3 (1–5) [0–12] | <0.01 | 0 (0–0) [0–4] | 0.07 |
| Total TGS score: median (IQR) [range] | 0 (0–1) [0–3] | nr | nr | ||
| Total TPD score: median (IQR) [range] | 0 (0–0) [0–5] | nr | nr | ||
| Number of joints with erosion: median (IQR) [range] | 1 (0–2) [0–5] | 0 (0–0) [0–1] | <0.01 | 0 (0–0) [0–0] | <0.01 |
| Swollen (28) joint count: median (IQR) [range] | 0 (0–0) [0–2] | 2 (1–6) [0–24] | <0.01 | 0 (0–0) [0–4] | 0.75 |
| Tender (28) joint count: median (IQR) [range] | 0 (0–0) [0–2] | 6 (3–10) [0–26] | <0.01 | 5 (3–9) [0–24] | <0.01 |
| Patient VAS (mm): median (IQR) [range] | 5 (1–13) [0–35] | 51 (31–75) [0–100] | <0.01 | 60 (40–75) [8–100] | <0.01 |
| CRP in mg/L: median (IQR) [range] | 0 (0–0) [0–13] | 12 (5–28) [0–203] | <0.01 | 0 (0–8) [0–156] | <0.01 |
| ESR in mm/hr: median (IQR) [range] | 9 (5–17) [1–77]* | 27 (13–42) [1–122] | <0.01 | 9 (5–22) [2–73] | 0.57 |
| DAS28-CRP: median (IQR) [range] | 1.09 (0.99–1.59) [0.96–2.34] | 4.34 (3.51–5.29) [2.50–7.51] | <0.01 | n/a | |
| ACR/EULAR Boolean remission: n (%) | 40 (61) | 0 (0) | n/a | n/a | |
| Total DMARDs since diagnosis: median [range] | 2 [1–4] | 0 [0–0] | <0.01 | n/a | |
| Current methotrexate use: n (%) | 55 [83%] | n/a | n/a | ||
P values are presented for comparison with RA remission group (continuous/ordinal data: Mann–Whitney U test; categorical data: Fisher’s exact text).
One patient had an elevated ESR of 77 at baseline due to hypergammaglobulinaemia from secondary Sjögren’s syndrome.
ACPA, anti-citrullinated peptide antibody; ACR, American College of Rheumatology; BioRRA, Biomarkers of Remission in Rheumatoid Arthritis study; CRP, C-reactive protein; DAS28-CRP, disease activity score in 28 joints with C-reactive protein; DMARD, disease-modifying anti-rheumatic drug; ESR, erythrocyte sedimentation rate; EULAR, European League Against Rheumatism; IQR, interquartile range; n/a, not applicable; NEAC, Newcastle Early Arthritis Clinic; nr, not recorded; RhF, rheumatoid factor; SGS, synovial greyscale; SPD, synovial power Doppler; TGS, tenosynovial greyscale; TPD, tenosynovial power Doppler; VAS, visual analogue score.
US-defined abnormalities are common in RA remission
US-defined abnormalities, particularly SGS, were common in RA remission and were equally present in patients who did or did not satisfy clinical remission regardless of whether this was defined by DAS28-CRP < 2.4 (n = 66) or ACR/EULAR Boolean criteria (n = 40) (Table 2). There were insufficient occurrences of tendon PD to permit further analysis (2/66 [3%] patients).
Table 2.
The prevalence of US abnormalities in the RA remission group was equivalent for both DAS28-CRP and ACR/EULAR Boolean remission. Statistical significance of difference in observations between those who did and did not satisfy ACR/EULAR Boolean remission is calculated by Fisher’s exact test.
| Criterion | US parameter | Remission definition |
p | |
|---|---|---|---|---|
| DAS28-CRP < 2.4 (n = 66) |
ACR/EULAR Boolean (n = 40) |
|||
| n (%) patients with total score ⩾1 | SGS | 66 (100) | 40 (100) | n/a |
| SPD | 17 (26) | 10 (25) | >0.99 | |
| TGS | 29 (44) | 17 (43) | 0.80 | |
| Erosions | 45 (68) | 25 (63) | 0.28 | |
| n (%) patients with any individual joint score ⩾2 | SGS | 48 (73) | 27 (68) | 0.27 |
| SPD | 8 (12) | 6 (15) | 0.46 | |
ACR, American College of Rheumatology; CRP, C-reactive protein; DAS28, disease activity score in 28 joints; EULAR, European League Against Rheumatism; RA, rheumatoid arthritis; SGS, synovial greyscale; SPD, synovial power Doppler; TGS, tenosynovial greyscale; US, ultrasound.
Strong association of US and clinical parameters in active RA but not in NIA
Multivariate ordinal logistic regression was performed for each US variable within each patient group. Firstly, the association between clinical and US parameters were assessed for active RA and NIA as a measure of the face validity of the scan protocol. In active RA, swollen joint count strongly associated with both SGS [odds ratio (OR) 1.17, 95% confidence interval (CI) 1.08–1.26, adjusted p < 0.01] and SPD (OR 1.20, 95% CI 1.12–1.30, adjusted p < 0.01). In contrast, no significant associations were observed between swollen joint count and SGS or SPD in the NIA group.
Lack of association of US and clinical parameters in RA remission
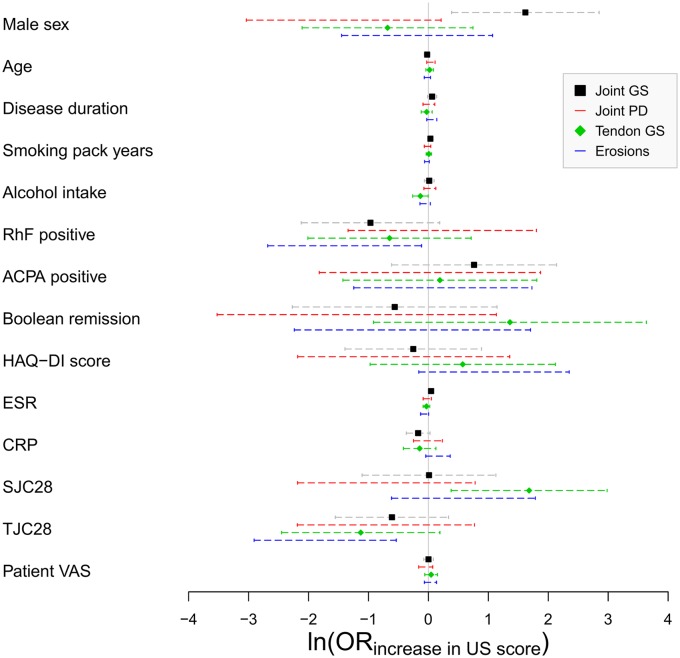
The association of clinical and US parameters was then assessed within the RA remission group (Figure 1). Six variable-score associations were statistically significant at an unadjusted p < 0.05 threshold: male sex and ESR versus total SGS score; swollen joint count and alcohol intake versus total TGS score; and tender joint count and rheumatoid factor positivity versus total erosion score (Table 3). However, none of these associations were robust to multiple test correction. Of note, no significant associations were observed between total SPD score and any of the clinical variables.
Figure 1.
Association between clinical and US parameters in the RA remission group as assessed by multivariate logistic regression. The (ln(OR) for increase in total US score for each clinical parameter is shown, with error bars indicating the 95% CI. An ln(OR) of zero indicates no association, shown by the vertical line. Adapted with permission from Baker et al.11
ACPA, anti-citrullinated peptide antibody; CI, confidence interval; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HAQ-DI, Health Assessment Questionnaire Disability Index; ln(OR), log-transformed odds ratio; RA, rheumatoid arthritis; RhF, rheumatoid factor; SJC28, swollen 28 joint count; TJC, tender 28 joint count; US, ultrasound; VAS, visual analogue score.
Table 3.
The six clinical variables associated with US parameters in the RA remission group at an unadjusted p < 0.05 significance threshold. Corresponding adjusted p-values after Benjamini-Hochberg correction are also shown.
| US score | Clinical parameter | OR increase in US score | 95% CI | Unadjusted p | Adjusted p |
|---|---|---|---|---|---|
| SGS | Male sex | 5.04 | 1.47–17.26 | 0.01 | 0.14 |
| ESR (mm/h) |
1.05 | 1.00–1.09 | 0.04 | 0.24 | |
| TGS | SJC28 | 5.37 | 1.46–19.72 | 0.01 | 0.16 |
| Alcohol intake (units/week) |
0.88 | 0.77–1.00 | 0.04 | 0.31 | |
| Erosion | TJC28 | 0.18 | 0.05–0.58 | <0.01 | 0.06 |
| RhF positive | 0.25 | 0.07–0.89 | 0.03 | 0.23 |
ESR, erythrocyte sedimentation rate; OR, odds ratio; RA, rheumatoid arthritis; RhF, rheumatoid factor; SGS, synovial greyscale; SJC28, swollen joint count (28 joints); SPD, synovial power Doppler; TGS, tenosynovial greyscale; TJC28, tender joint count (28 joints); US, ultrasound.
Discussion
The past three decades have witnessed an exponential increase in the use of musculoskeletal US in the management of RA, especially as a diagnostic aid to supplement clinical examination. Strong evidence links US-defined abnormalities, in particular SPD, with active synovitis in the setting of active disease, including correlations with histological measures of inflammation in synovial tissue,12–14 Th17 cells in synovial fluid,15 and production of pro-inflammatory cytokines in ex vivo culture of synovial tissue.16 Furthermore, SPD is highly responsive to changes in disease activity,17 and responds quickly to treatment initiation.18 As such, US evaluation is broadly accepted as providing additional discriminatory value in the detection of synovitis in symptomatic individuals, especially in seronegative disease.19
In contrast, the role of US examination in the setting of RA remission remains uncertain, in particular the clinical significance of US-defined abnormalities in asymptomatic individuals. In this cross-sectional analysis, we demonstrate a high prevalence of musculoskeletal US abnormalities (especially SGS) in the setting of established RA remission, regardless of whether this is defined by DAS28-CRP or ACR/EULAR Boolean criteria. In a meta-analysis of 19 studies including 1369 patients in clinical remission,20 similarly high levels of SGS (74–86%) and combined SGS/SPD (32–44%) were observed across a range of clinical remission criteria and scan protocols. Furthermore, previous studies have shown the presence of US abnormalities at considerable levels even in healthy subjects,21 thus making it difficult to confidently ascribe clinically relevant thresholds for low-grade ultrasonographic findings.
Several previous studies have shown a degree of association, albeit modest, between low clinical disease activity scores and the absence of SPD;22,23 however, no such association has been observed in other studies.24,25 In our study, we first observe strong associations between swollen joint counts and SGS/SPD in active disease but not in non-inflammatory arthralgia, supporting the validity of the US7 protocol. We then demonstrate a lack of significant association between clinical and US parameters in the setting of RA remission.
Our most striking observation is the complete lack of association in RA remission between SPD and any of the clinical parameters assessed in our study, even without multiple test correction. Evidence exists to suggest that the presence of SPD, even in the setting of clinical remission, can represent ongoing subclinical synovitis. In this context, SPD has been shown to correlate with future arthritis flare,4,26 future bone erosions and immunohistochemical markers of synovial inflammation.4,27 Despite these observations, however, treatment strategies aimed at achieving ultrasonographic definitions of remission have so far failed to show superiority over standard clinical management,28,29 at the expense of increased adverse effects and treatment cost.30 Our study adds further evidence to support a lack of association between ultrasonographic and clinical measures of disease activity in the context of disease remission – a crucial disconnect which suggests a plausible explanation for the futility of US-defined treatment targets studied thus far.
There are some limitations to our study. We used a limited 7-joint scan protocol, and thus it is possible that significant US abnormalities were missed in other joints. Indeed, a strategy targeting US to symptomatic joints, and interpreting imaging within the context of the individual patient history and examination findings, may be more discriminatory for active disease though would lack the reproducibility of the validated, blinded and systematic US7 scan protocol used in this study. Corroboration of independent assessment by two ultrasonographers, as opposed to a single scan per patient, may have improved the accuracy of US assessments in this study, but was not feasible due to limited study resources. Furthermore, our study population is relatively small, and infrequent occurrences and incomplete recording of tenosynovial abnormalities restricted analysis of these variables. Differing composition of the NEAC versus BioRRA cohorts – namely shorter symptom duration and DMARD-naïve status in the active RA group, and younger age and increased number of females in the NIA group – may limit the generalisability of scan findings to the established remission group. Similarly, our remission group comprised exclusively patients with established disease treated with conventional synthetic DMARDs, and thus our findings may not directly translate to patients with early disease or those receiving biologic therapy. Finally, the lack of a time criterion in our remission definition prevents further analysis of ultrasonographic findings in cross-sectional versus sustained disease remission.
In summary, we observe substantial levels of US abnormalities in established RA clinical remission, with no significant association with clinical parameters. Most strikingly SPD, which portends a poor prognosis, failed to show association with any of the clinical parameters. Our results suggest that clinical and US examinations are non-overlapping in RA remission, challenging the proposition of US-driven management strategies in this setting.
Acknowledgments
We thank all of the patients who participated in this study, and all of the rheumatology health professionals who referred patients. Ultrasound data from this study were previously presented at the EULAR 2016 and 2018 Congresses,11,31 and in a PhD Thesis (Newcastle University) by KFB. The research was supported by the National Institute for Health Research Newcastle Biomedical Research Centre based at Newcastle Hospitals National Health Service (NHS) Foundation Trust and Newcastle University, the Research into Inflammatory Arthritis Centre Versus Arthritis, and the European Union/European Federation of Pharmaceutical Industries and Associations (EU/EFPIA) Innovative Medicines Initiative 2 Joint Undertaking RTCure. The views expressed are those of the authors and not necessarily those of the NHS, the National Institute for Health Research (NIHR) or the Department of Health and Social Care.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by a Wellcome Trust Translational Medicine and Therapeutics Clinical PhD Fellowship (102595/Z/13/A to KFB), and by a National Institute for Health Research (NIHR) Infrastructure Doctoral Traineeship Award from the Newcastle NIHR Biomedical Research Centre (BH136167/PD0045 to KFB). KFB is funded by a NIHR Clinical Lectureship (CL-2017-01-004).
Conflict of interest statement: KFB, JDI, and AGP are named as inventors on a patent application by Newcastle University relating to the prediction of drug-free remission in rheumatoid arthritis. BT, DWL and AS have no conflicts of interest to declare.
ORCID iD: Kenneth F. Baker  https://orcid.org/0000-0002-6735-2911
https://orcid.org/0000-0002-6735-2911
Contributor Information
Kenneth F. Baker, Musculoskeletal Research Group, Translational and Clinical Research Institute, Newcastle University, Newcastle upon Tyne, UK Musculoskeletal Unit, Freeman Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, UK.
Ben Thompson, Musculoskeletal Research Group, Translational and Clinical Research Institute, Newcastle University, Newcastle upon Tyne, UK; Musculoskeletal Unit, Freeman Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, UK.
Dennis W. Lendrem, Musculoskeletal Research Group, Translational and Clinical Research Institute, Newcastle University, Newcastle upon Tyne, UK Musculoskeletal Unit, Freeman Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, UK.
Adam Scadeng, Musculoskeletal Unit, Freeman Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, UK.
Arthur G. Pratt, Musculoskeletal Research Group, Translational and Clinical Research Institute, Newcastle University, Newcastle upon Tyne, UK Musculoskeletal Unit, Freeman Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, UK.
John D. Isaacs, Musculoskeletal Research Group, Translational and Clinical Research Institute, Faculty of Medical Sciences, Newcastle University, William Leech Building, Framlington Place, Newcastle upon Tyne, NE2 4HH, UK; Musculoskeletal Unit, Freeman Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust, Newcastle upon Tyne, UK.
References
- 1. Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017; 76: 960–977. [DOI] [PubMed] [Google Scholar]
- 2. Prevoo ML, van ’t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995; 38: 44–48. [DOI] [PubMed] [Google Scholar]
- 3. Felson DT, Smolen JS, Wells G, et al. American college of rheumatology/European league against rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis 2011; 70: 404. [DOI] [PubMed] [Google Scholar]
- 4. Han J, Geng Y, Deng X, et al. Subclinical synovitis assessed by ultrasound predicts flare and progressive bone erosion in rheumatoid arthritis patients with clinical remission: a systematic review and metaanalysis. J Rheumatol 2016; 43: 2010–2018. [DOI] [PubMed] [Google Scholar]
- 5. Baker KF, Skelton AJ, Lendrem DW, et al. Predicting drug-free remission in rheumatoid arthritis: a prospective interventional cohort study. J Autoimmun 2019; 105: 102298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iqbal K, Lendrem DW, Hargreaves B, et al. Routine musculoskeletal ultrasound findings impact diagnostic decisions maximally in autoantibody-seronegative early arthritis patients. Rheumatology 2019; 58: 1268–1273. [DOI] [PubMed] [Google Scholar]
- 7. Fleischmann R, van der Heijde D, Koenig AS, et al. How much does disease activity score in 28 joints ESR and CRP calculations underestimate disease activity compared with the simplified disease activity index? Ann Rheum Dis 2015; 74: 1132–1137. [DOI] [PubMed] [Google Scholar]
- 8. Backhaus M, Ohrndorf S, Kellner H, et al. Evaluation of a novel 7-joint ultrasound score in daily rheumatologic practice: a pilot project. Arthritis Rheum 2009; 61: 1194–1201. [DOI] [PubMed] [Google Scholar]
- 9. Bruce B, Fries JF. The Stanford Health Assessment Questionnaire: dimensions and practical applications. Health Qual Life Outcomes 2003; 1: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Christensen RHB. Ordinal - Regression models for ordinal data. R package version 2015.6-28, http://www.cran.r-project.org/package=ordinal (2015, accessed 25 October 2019).
- 11. Baker KF, Thompson B, Lendrem D, et al. SAT0631 Lack of association between clinical and ultrasound measures of disease activity in rheumatoid arthritis clinical remission: a cross-sectional analysis. Ann Rheum Dis 2018; 77: 1167. [Google Scholar]
- 12. Takase K, Ohno S, Takeno M, et al. Simultaneous evaluation of long-lasting knee synovitis in patients undergoing arthroplasty by power Doppler ultrasonography and contrast-enhanced MRI in comparison with histopathology. Clin Exp Rheumatol 2012; 30: 85–92. [PubMed] [Google Scholar]
- 13. Andersen M, Ellegaard K, Hebsgaard JB, et al. Ultrasound colour Doppler is associated with synovial pathology in biopsies from hand joints in rheumatoid arthritis patients: a cross-sectional study. Ann Rheum Dis 2014; 73: 678–683. [DOI] [PubMed] [Google Scholar]
- 14. Abe A, Ishikawa H, Nakazono K, et al. A comparison of the ultrasonography images of the joints of patients with rheumatoid arthritis and the corresponding synovial histological findings. Mod Rheumatol 2016; 26: 534–539. [DOI] [PubMed] [Google Scholar]
- 15. Gullick NJ, Evans HG, Church LD, et al. Linking power Doppler ultrasound to the presence of th17 cells in the rheumatoid arthritis joint. PLoS One 2010; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andersen M, Boesen M, Ellegaard K, et al. Synovial explant inflammatory mediator production corresponds to rheumatoid arthritis imaging hallmarks: a cross-sectional study. Arthritis Res Ther 2014; 16: R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Naredo E, Collado P, Cruz A, et al. Longitudinal power Doppler ultrasonographic assessment of joint inflammatory activity in early rheumatoid arthritis: predictive value in disease activity and radiologic progression. Arthritis Rheum 2007; 57: 116–124. [DOI] [PubMed] [Google Scholar]
- 18. D’Agostino MA, Wakefield RJ, Berner-Hammer H, et al. Value of ultrasonography as a marker of early response to abatacept in patients with rheumatoid arthritis and an inadequate response to methotrexate: results from the APPRAISE study. Ann Rheum Dis 2016; 75: 1763–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Freeston JE, Wakefield RJ, Conaghan PG, et al. A diagnostic algorithm for persistence of very early inflammatory arthritis: the utility of power Doppler ultrasound when added to conventional assessment tools. Ann Rheum Dis 2010; 69: 417–419. [DOI] [PubMed] [Google Scholar]
- 20. Nguyen H, Ruyssen-Witrand A, Gandjbakhch F, et al. Prevalence of ultrasound-detected residual synovitis and risk of relapse and structural progression in rheumatoid arthritis patients in clinical remission: a systematic review and meta-analysis. Rheumatology 2014; 53: 2110–2118. [DOI] [PubMed] [Google Scholar]
- 21. Padovano I, Costantino F, Breban M, et al. Prevalence of ultrasound synovial inflammatory findings in healthy subjects. Ann Rheum Dis 2016; 75: 1819–1823. [DOI] [PubMed] [Google Scholar]
- 22. Nemoto T, Ogasawara M, Matsuki Y, et al. Can routine clinical measures predict ultrasound-determined synovitis and remission in rheumatoid arthritis patients? Clin Exp Rheumatol 2014; 32: 54–60. [PubMed] [Google Scholar]
- 23. Geng Y, Han J, Deng X, et al. Deep clinical remission: an optimised target in the management of rheumatoid arthritis? Experience from an ultrasonography study. Clin Exp Rheumatol 2016; 34: 581–586. [PubMed] [Google Scholar]
- 24. Horton SC, Tan AL, Freeston JE, et al. Discordance between the predictors of clinical and imaging remission in patients with early rheumatoid arthritis in clinical practice: implications for the use of ultrasound within a treatment-to-target strategy. Rheumatology 2016; 55: 1177–1187. [DOI] [PubMed] [Google Scholar]
- 25. van der Ven M, Kuijper TM, Gerards AH, et al. No clear association between ultrasound remission and health status in rheumatoid arthritis patients in clinical remission. Rheumatology 2017; 56: 1276–1281. [DOI] [PubMed] [Google Scholar]
- 26. Filippou G, Sakellariou G, Scire CA, et al. The predictive role of ultrasound-detected tenosynovitis and joint synovitis for flare in patients with rheumatoid arthritis in stable remission. Results of an Italian multicentre study of the Italian Society for Rheumatology Group for Ultrasound: the STARTER study. Ann Rheum Dis 2018; 77: 1283–1289. [DOI] [PubMed] [Google Scholar]
- 27. Ramirez J, Celis R, Usategui A, et al. Immunopathologic characterization of ultrasound-defined synovitis in rheumatoid arthritis patients in clinical remission. Arthritis Res Ther 2016; 18: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dale J, Stirling A, Zhang R, et al. Targeting ultrasound remission in early rheumatoid arthritis: the results of the TaSER study, a randomised clinical trial. Ann Rheum Dis 2016; 75: 1043–1050. [DOI] [PubMed] [Google Scholar]
- 29. Haavardsholm EA, Aga AB, Olsen IC, et al. Ultrasound in management of rheumatoid arthritis: ARCTIC randomised controlled strategy trial. BMJ 2016; 354: i4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aletaha D, Smolen JS. Achieving clinical remission for patients with rheumatoid arthritis. JAMA 2019; 321: 457–458. [DOI] [PubMed] [Google Scholar]
- 31. Baker KF, Thompson B, Lendrem DW, et al. AB0957 Ultrasound measures of synovitis are independent of clinical parameters in the setting of rheumatoid arthritis remission: a cross-sectional analysis. Ann Rheum Dis 2016; 75: 1228.1222–1228. [Google Scholar]