Abstract
Purpose of review
Immune-mediated adverse drug reactions (IM-ADRs) are many times more common in HIV-infected patients. Usual offending drugs include antiretroviral and antiinfectives, but the burden of specific drug IM-ADRs is population-specific; changing as new and fixed dose combinations enter the market, and drug-resistance patterns demand. This review considers recent literature on epidemiology, mechanisms, clinical management and prevention of IM-ADRs amongst persons living with HIV/AIDS.
Recent findings
Epidemiological studies continue to describe high rates of delayed hypersensitivity to known offenders, as well as similar reactions in preexposure prophylaxis. IM-ADRs to oral and injectable integrase strand transfer inhibitors are reported with expanding use. The clinical spectrum and management of IM-ADRs occurring in HIV-infected populations is similar to uninfected; with exceptions such as a recently described severe delayed efavirenz DILI with high mortality. Furthermore, the context can be unique, such as the lower than expected mortality in a Stevens–Johnson syndrome and toxic epidermal necrolysis (SJS/TEN) cohort from a HIV/TB high burden setting. Programmatic data showing the near complete elimination of Abacavir drug hypersensitivity syndrome following implementation of HLA-B57:01 screening is a stellar example of how prevention is possible with mechanistic insight.
Summary
IM-ADRs remain a challenge in persons living with HIV. The complexities posed by polypharmacy, overlapping drug toxicities, drug interactions, overlap of IM-ADRs with other diseases, limited alternative drugs, and vulnerable patients with advanced immunosuppression with high mortality, necessitate increased use of drug provocation testing, treat-through and desensitization strategies. There is an urgent need for improved diagnostics and predictive biomarkers for prevention, or to guide treat-through, rechallenge and desensitization approaches.
Keywords: drug hypersensitivity, HIV, immune-mediated adverse drug reactions
INTRODUCTION
In 2017, an estimated 36.9 million people were living with the human immunodeficiency virus (HIV); 25.7 million in Africa [1]. In endemic settings, HIV and related comorbidities top the disease burdens; in the process altering the clinical presentation of common diseases, the incidence of previously rare diseases, and the pattern of drug use. Adverse drug reactions (ADRs) are common and deadly in HIV/TB (tuberculosis) endemic countries; a recent Ethiopian study attributing 10% of admissions, and 13% of deaths to ADRs [2]. Immune-mediated ADRs (IM-ADRs), predominantly delayed hypersensitivity sub-types, are up to 100-fold more common in persons with HIV [3]. We have recently comprehensively detailed IM-ADR phenotypes in a recent Lancet review on antibiotic allergy; all these phenotypes are reported in the context of HIV [4]. This review discusses the changing epidemiology of offending drugs and IM-ADR phenotypes, the limited mechanistic understanding, and the complexities of managing IM-ADR in the context of HIV that often necessitate novel approaches.
EPIDEMIOLOGY OF DRUG HYPERSENSITIVITY: AN EVOLVING LANDSCAPE
The continual development, optimization and early initiation of highly active antiretroviral therapy (HAART) has made HIV infection a treatable chronic disease in many high-income countries; with the treatment frontier focused on drug resistance and preexposure prophylaxis. Lower middle-income countries, particularly in Africa, that form the epicentre of the epidemic, are falling well below the 90 : 90 : 90 UNAIDS targets of HIV diagnosis and HAART initiation [5]. In these countries, patients continue to present with advanced immunosuppression and multiple comorbidities including opportunistic infections, particularly tuberculosis; malignancies, metabolic disorders; and end-organ damage [6]. The wide disease spectrum and associated infections combined with country-specific markets and accessibility, determine a changing landscape of drug exposures and associated patterns of hypersensitivity.
There are more than 100 drugs that have been reported to cause IM-ADRs in HIV, most commonly cutaneous adverse drug reactions (CADR). Table 1 lists the common offending drugs, dividing them into two broad groups – HAART and antiinfective agents used to treat comorbid infections. Wherever possible, the commonest individual offending agent in a particular drug class is listed, and prevalence data for specific hypersensitivity phenotypes are provided.
Table 1.
Drugs associated with hypersensitivity reactions in HIV
| A. | Drug class | Drug(s) in class | Commonest offending drugs for IM-ADRs | Types of reactions and estimated incidence |
|---|---|---|---|---|
| Antiretrovirals | NRTI | Tenofovir | Abacavir | Abacavir hypersensitivity reactions (2.3–9% – population dependent) [80–83] |
| Abacavir | ||||
| Emtricitabine | ||||
| Lamivudine | ||||
| Didanosine | ||||
| Stavudine | ||||
| Zidovudine | ||||
| Tenofovir | CADR – including MPE, photoallergic dermatitis, LDE 5–7% [84–86,20] | |||
| NNRTI | Nevirapine | Nevirapine | CADR: 15–32% | |
| Efavirenz | DRESS: 5% | |||
| Rilpivirine | SJS/TEN: 0.3–10% [8,9,20,87] | |||
| Etravirine | ||||
| Efavirenz | CADR 6% [9,66] | |||
| SCAR 0.1% [88] | ||||
| PI | Lopinavir | Fosamprenavir | CADR, SCAR 3–8% [84,89] | |
| Atazanavir | ||||
| Ritonavir | ||||
| Saquinavir | ||||
| Darunavir | ||||
| Fosamprenavir | ||||
| Tipranavir | ||||
| Indinavir | ||||
| Integrase strand transfer inhibitors | Dolutegravir | Dolutegravir | DHS, DILI 1% [17] | |
| Raltegravir | ||||
| Elvitegravir | ||||
| Bictegravir | ||||
| Fusion or CCR5 inhibitor | Maraviroc Enfuvirtide | Enfuvirtide | CADR <1% [89] | |
| Fixed dose combinations | Tenofovir+emtricitabine | Tenofovir+emtricitabine | DHS, rare, case report [90] | |
| Tenofovir+emtricitabine +efavirenz | ||||
| Tenofovir+lamivudine+efavirenz | ||||
| Tenofovir+emtricitabine+cobicistat+elvitegravir | ||||
| Tenofovir +emtricitabine + efavirenz | CADR, unknown, case reports [80,91] |
| B. | Antiinfectives commonly used in HIV | ||
|---|---|---|---|
| Drugs | Common drugs causing increased IM-ADRs in HIV | Type of reactions and estimated incidence | |
| Antibacterials | Beta-lactam antibiotics | Cotrimoxazole (TMP-SMX) | CADR – 10.9% [27] |
| Macrolides | SCAR – mainly DRESS/DHS, FDE, SJS/TEN [24–26] | ||
| Quinolones | |||
| Cotrimoxazole | |||
| (TMP/SMX) (PJP) | |||
| Dapsone | |||
| Dapsone | SCAR – mainly DRESS/DHS 1.4% [92,93] | ||
| Antifungals | Amphotericin | Fluconazole | FDE, DRESS, SJS/TEN – unknown, mainly case reports [20,94,95] |
| Azoles | |||
| Allylamines | |||
| Griseofulvin | |||
| echinocandins | |||
| Antimycobacterials | First-line | Standard anti-TB treatment | CADR, AIN – adjusted hazard ratio of 8 in HIV [74,96–99] |
| Rifampicin | |||
| Isoniazid | |||
| Pyrazinamide | |||
| Ethambutol | |||
| Second-line/DR-TB | |||
| Fluroquinolones | |||
| Kanamycin/Capreomycin/Amikacin | |||
| Bedaquiline | |||
| Ethionamide | |||
| Rifampicin | CADR, SCAR, DILI, AIN, immediate reactions [100,101] | ||
| Isoniazid | CADR, SCAR, DILI mainly case series [102,103] | ||
| Pyrazinamide | CADR 2.8%, SCAR, DILI [20,104] | ||
| Ethambutol | SCAR – SJS/TEN, DRESS [92,102,105–107] | ||
| Fixed dose combinations (Rifafour) | Mainly case reports [108] | ||
| Fluoroquinolones Ethionamide Kanamycin/Amikacin | SCAR ~20% [99] | ||
AIN, acute interstitial nephritis; CADR, cutaneous adverse drug reactions; DHS, drug hypersensitivity syndrome; DILI, drug-induced liver injury; DRESS, drug reaction with eosinophilia and systemic symptoms; FDE, fixed drug eruption; LDE, lichenoid drug eruption; MPE, maculopapular eruption; NNRTI, nonnucleoside reverse-transcriptase inhibitors; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; SCAR, severe cutaneous adverse reactions; SJS/TEN, Stevens–Johnson syndrome and toxic epidermal necrolysis; TMP-SMX, trimethoprim sulfamethoxazole (cotrimoxazole).
IM-ADR to HAART is well recognized with the highest prevalence being CADRs to nonnucleoside reverse transcriptase inhibitors (NNRTIs). These occur in ~15% of patients starting treatment, and a significant portion are severe cutaneous adverse drug reactions (SCAR), most commonly epidermal necrolysis and drug rash with eosinophilia and systemic symptoms (DRESS); as well as drug-induced liver injury (DILI) to nevirapine [7–9]. CADRs to NRTIs, such as tenofovir and emtricitabine are most recently described in large postexposure prophylaxis cohorts, when used in two-drug fixed dose combination [10]. In contrast, abacavir hypersensitivity reaction has been largely eliminated in populations where HLA-testing preprescription has been implemented, but still occurs in African populations, but at a low frequency. The low prevalence of the allele in Africans makes testing not to be cost-effective [11]. Increased use of integrase strand transfer inhibitors, and their availability in fixed dose combinations will mean increased exposure to this class of agents. IM-ADRs – mostly CADRs, drug hypersensitivity syndrome (DHS) and case reports of drug-induced liver injury have been described with this class of drugs [12,13]. An interesting DHS, associated with marked elevation in inflammatory cytokines and markers, developed in a small healthy volunteer study evaluating a combination of dolutegravir with isoniazid/rifapentine for anti-TB prophylaxis and associated with high isoniazid plasma concentrations [14]. Larger studies with dolutegravir report only a low incidence of IM-ADRs [15–17]. High rates of injection site reactions, which may be immune-mediated, have also recently been reported with the use of a suspension form of cabotegravir, given as an intramuscular depot preparation [18]. There is an increased number and use of fixed dose combination HAART to improve adherence. Our clinic has several cases of CADRs, and even more severe DRESS, to fixed dose combination (FDC) preparations; where patients tolerate all individual drugs not as an FDC or an alternative FDC preparation, making excipients the likely offending agents [19]. Two earlier reviews have discussed rare reactions to other HAART drug classes, such as protease inhibitors, for example, fosamprenavir, fusion inhibitors and the CCR5 inhibitor maraviroc [20,21].
Antiinfective agents used to treat comorbid infections are the other common offending drugs causing IM-ADRs in HIV-infected patients, with the overall prevalence reflecting drug use and disease burdens. IM-ADRs to the first-line antituberculosis drugs (FLTDs) – rifampicin, isoniazid, pyrazinamide and ethambutol, are the commonest given the high prevalence of comorbid TB in HIV endemic settings. A full spectrum of particularly delayed hypersensitivity reactions to FLTDs and second-line anti-TB drugs is listed in Table 1 and has recently been comprehensively reviewed [22]. Isoniazid, rifampicin and pyrazinamide are the commonest offending agents for both SCAR and drug-induced liver injury (DILI) [22]. A recent cohort of 307 Thai adults with HIV/TB co-infection reported CADRs as the commonest IM-ADRs, occurring at an incidence rate of 0.41 events/person-year [23]. Cotrimoxazole, used in large amounts for prophylaxis and treatment of Pneumocystis, is a well known cause of IM-ADR, with DRESS/drug hypersensitivity syndrome, SJS/TEN, and fixed drug eruption often reported [24–26]. In a recent Ethiopian study, ~10% of patients discontinued therapy because of cotrimoxazole allergy [27]. Our clinic has begun to see fewer cases of cotrimoxazole IM-ADRs as the HIV treatment guidelines have changed, with initiation of HAART at diagnosis irrespective of CD4 cell count, and greater ART coverage resulting in a substantial drop in the usage of the drug [28]. Although infrequent, cross-reactivity has been reported between structurally related drugs including cotrimoxazole and dapsone [29]; efavirenz and nevirapine [30,31]; emtricitabine and lamivudine [32]; and darunavir and cotrimoxazole [33].
MECHANISMS OF DRUG HYPERSENSITIVITY
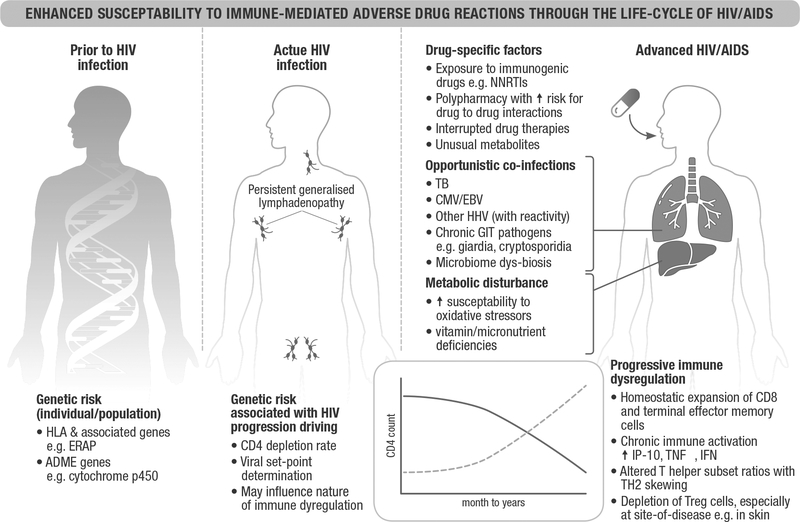
The current understanding of many drug-IM-ADR is limited, and the details behind why HIV infection causes an increased susceptibility to drug reactions is likewise unclear. Figure 1 outlines the likely contributors, both with respect to pre-HIV genetic risk, and the dynamics of immunologic, metabolic (both disease-related and drug-related), pharmacologic and infective factors along the course of HIV infection. It is thought to be multifactorial, an interplay between metabolic, immunologic, host and viral factors. Persistent injury of HIV-infected cells, immune dysregulation, increased oxidative stress, depletion of immunoregulatory cells and consumption of protective antioxidant molecules are thought to predispose to excessive ‘danger signals’ resulting in a cascade of immune responses, cytokine release and hypersensitivity reactions [3,34–36].
FIGURE 1.
Enhanced susceptibility to immune-mediated adverse drug reactions through the life-cycle of HIV/AIDS. Several potential mechanisms may explain the increased susceptibility to IM-ADRs through HIV/AIDS life cycle. Prior to infection, individuals or populations may carry specific HLA or other pharmacogenomic risk alleles to particular drugs. Host factors determining HIV control may influence the dynamics of immune dysregulation. Upon infection and as disease progresses, CD4+ T cells are depleted, with homeostatic expansion of CD8+ T cells, particularly functional memory CD8+ T cells. Marked immune hyperactivation characterized by excessive levels of inflammatory cytokines, such as type I interferons (IFN-α) and suppressive cytokines, IL-10 and changes in the expression levels of IFN-γ induced chemokines (IP-10). Another influence includes altered immunoregulatory pathways, which results in the lack of counterbalance against cytotoxic immune responses. Reactivation of comorbid herpes viral infections, such as CMV, EBV, of HHV6 and HHV8 may occur during worsening immunosuppression; viral reactivation has a known association with DRESS. HIV can alter redox balance and potentially increase the production and accumulation of toxic reactive metabolites. Overall, during chronic infection, uncontrolled immune activation, loss of CD4+ T cells and Tregs and unopposed CD8+ cytotoxic T-cell responses likely promotes the enhanced susceptibility to IM-ADRs in HIV/AIDS patients. The precise balance of these signals may determine the severity of these reactions in different stages of infection. ADME, absorption, distribution, metabolism and elimination; CMV, cytomegalovirus; DRESS, drug reaction with eosinophilia and systemic symptoms; EBV, Epstein–Barr virus; HHVs, human herpes viruses; IFN-α, interferon-alpha; IFN-γ, interferon-gamma; IM-ADR, immune-mediated adverse drug reaction; IP-10, interferon gamma-induced protein 10; TB, tuberculosis; TH2, T-helper 2; TNF, tumor necrosis factor; Treg, regulatory T cells.
Genetic predisposition, particularly HLA risk, is important for several delayed IM-ADRs and has been reviewed recently for a number of drugs relevant to HIV, including the antiretrovirals abacavir, nevirapine and certain antiinfectives, for example, dapsone [37]. These genetic risk factors are critical determinants of certain IM-ADRs prior to HIV infection. Interestingly in our clinic (unpublished cases), there are instances where, following immune reconstitution with HAART, a prior offending drug causing IM-ADRs could be tolerated without recurrence of drug hypersensitivity. This illustrates the importance of HIV-related immune status in IM-ADR aetiology. In fact, even in IM-ADRs with a clear HLA risk allele, the positive predictive values are low (<10%), meaning other additional factors are necessary for a reaction. The progression of HIV infection is characterized by: loss of CD4 T cells; expansion of CD8 T cells; and chronic immune activation. Immune activation is associated with an increase in the production of IP-10 and MIG (chemokines induced by interferon-γ), TNF-α, IL-6, IFN-α and IL-10, and recent findings suggest polymorphisms in cytokine genes could influence susceptibility to efavirenz hypersensitivity [38,39]. In addition, immune activation and an increase in interferon-gamma levels may increase drug presentation through the upregulation of HLA molecules and other co-stimulatory molecules on both professional and nonprofessional APCs [40–44]. HAART initiation has been linked to increased CD28 (a costimulatory molecule required for T-cell activation and optimal responsiveness) expression on HIV-specific CD8+ T cells, with enhanced survival and response to antigen stimulation [45,46]. Furthermore, the loss of Tregs – a CD4+ T-cell subset may aggravate the ability to counterbalance cytotoxic CD8 cells, and this has been suggested in the skin for SCAR [47,48]. One study using mouse models to study the role of Tregs in pathogenesis of Stevens–Johnson syndrome and toxic epidermal necrolysis (SJS/TEN) found when CD4+ regulatory T cells are deficient, anti-OVA cytotoxic T cells killed OVA-expressing keratinocytes. In patients, the functional defects of Tregs are seen, but restored after the resolution of disease [49]. Another study found a decrease in the number of skin-directed CD4+ T cells and an increase in the ratio of CD8+ to CD4+ T cells in HIV-infected patients with TEN compared with noninfected individuals. They concluded, the loss of skin-protective CD4+CD25+ regulatory T cells in HIV-infected patients increases risk of developing severe drug reactions [48].
HIV-infected individuals have an increased susceptibility to oxidative stress, which has been proposed to increase exposure to toxic drug metabolites and potentially cause IM-ADRs. Glutathione, a cellular antioxidant responsible for detoxifying toxic reactive metabolites and preventing tissue damage is depleted in HIV/AIDS [50]. This results in higher risk of SMX-induced hypersensitivity reactions HIV/AIDS patients than in noninfected controls [51]. A cohort of HIV/AIDS patients on SMX, showed that a polymorphism in glutamate cysteine ligase catalytic subunit (GCLC - a rate limiting enzyme involved in glutathione biosynthesis), is significantly associated with SMX-induced hypersensitivity reactions [52]. An in-vitro study also supported the direct role of toxic reactive sulfamethoxazole metabolites on HIV-infected cell injury and necrotic cell death. The study alluded cell injury and death may provide danger signals and as a result, T cells are sensitized initiating immune responses and cytokine release eliciting hypersensitivity reactions. HIV-infected cells with GCLC knockdown were more susceptible to reactive metabolites induced cytotoxic responses, which were suppressed by addition of glutathione [50,53]. However, a more recent study in a macaque model of SMX hypersensitivity found antioxidants, proinflammatory mediators and SMX metabolites were not predictors of drug-induced immune responses [54■■]. Two randomized trials found the use of N-acetylcysteine (an inducer of glutathione production) could not prevent SMX hypersensitivity reactions in patients with HIV [55,56]. These studies call into question the importance of increased oxidative stress as a risk factor for susceptibility to drug hypersensitivity reactions in HIV-infected patients. Genetic variation in drug metabolizing enzymes with higher drug exposure and toxicity appear important for risk to certain IM-ADRs. Phillips et al. [57] found no association between the slow metabolizing genotype CYP2B6 and nevirapine hepatoxicity. In contrast, slow metabolizer genotypes for CYP2B6 correlate with increased plasma levels of nevirapine and increased risk for class I HLA-restricted cutaneous nevirapine hypersensitivity reactions [58■■]. There are CYP2B6 slow metabolizer single nucleotide polymorphisms (SNPs) that have also been associated with higher efavirenz plasma concentrations and adverse events [59].
SPECTRUM, CLINICAL PRESENTATIONS AND OUTCOMES
All phenotypes of IM-ADRs have been reported in HIV-infected persons, although CADRs and DILI are the commonest. The spectrum of CADR seen in HIV is wide, ranging from mild and transient morbilliform eruptions to severe life-threatening forms. The latency periods and clinical presentations (morphology and distribution) of CADR does not seem to be different between HIV-infected and uninfected patients; despite different incidence rates [60■]. We recently reviewed our cohort of SJS/TEN cases (n = 184, HIV-infected n = 142) and noted no difference in the incidence or severity of hepatitis between HIV-infected and uninfected cases [61]. We then examined overall mortality; and at only 3.3%, this was significantly lower than predicted by SCORTEN and reported for other SJS/TEN cohorts [62■]. Interestingly, the lower mortality was not confined to HIV co-infected patients, and thus other factors such as a younger median age (<40 years), pharmacological properties of specific drugs, for example, nevirapine or anti-TB drugs, or comorbid diseases, such as TB and HIV may be responsible [62■]. Certain interesting hypersensitivity phenotypes have occurred in the context of HIV and HAART, such as the abacavir hypersensitivity reaction and more recently the reaction to dolutegravir in healthy volunteers [14,63]. Abacavir hypersensitivity reaction has a predominance of systemic features, such as fever, constitutional symptoms, gastrointestinal disturbances, and respiratory symptoms, with rash reported in 70% of cases and often only a late symptom [64,65]. Efavirenz has been associated with a photo-distributed annular erythema without systemic features [66].
Liver toxicity and DILI is a common ADR in HIV-infected patients. HIV-infected patients have multiple causes and risk factors for both liver enzyme abnormalities and immune-mediated DILI including comorbid infections; alcoholism; nutritional abnormalities; and the need for multiple drugs, many metabolized through the liver and with induction/inhibition effects on cytochrome p450 enzyme systems, and/or other complex interactions [22,67]. In the majority of instances, DILI patterns are similar in HIV-infected versus uninfected patients, for example, anti-TB drugs; incidence, however, is dramatically increased, with patients on HAART having a 10-fold increased risk of anti-TB drug DILI [68]. Unique clinical and histological phenotypes are described with important clinical implications. For instance, three clinicopathological patterns of efavirenz DILI have been described in a South African cohort; with one having a very delayed onset, submassive necrosis, and poor outcomes [69■]. Although for the most part the patterns of IM-ADRs in HIV-infected and uninfected patients are similar, there remains a paucity of data from endemic populations.
COMPLEXITIES IN MANAGEMENT AND METHODS TO COMBAT
HIV-infected patients not only have an increased susceptibility to drug hypersensitivity but are vulnerable for multiple additional reasons: multiple comorbidities including infections, malignancies, metabolic and noncommunicable disease; polypharmacy with attendant drug–drug interactions and nonimmune ADRs, and under-resourced health systems in high-burden settings. There are also specific problems pertaining to HIV-related drug hypersensitivity: a predisposition to multiple drug hypersensitivity; a lack of validated diagnostic tests, especially in African populations; inconsistent phenotyping and very limited capacity in LMICs for site-of-disease sampling, for example, skin or liver biopsy; and no predictive or prognostic biomarkers. These intersecting vulnerabilities and obstacles to care have led to a focus on supportive treatment strategies and several innovative approaches to drug provocation and desensitization even in severe phenotypes.
Diagnosis – use of drug provocation testing in severe phenotypes
There is consensus that drug provocation test (DPT), defined as a controlled administration of a drug in order to diagnose a drug hypersensitivity reaction, is the gold standard in establishing drug causality. DPT, if performed for severe delayed IM-ADRs, should only occur once the acute clinical picture has settled to improve safety, this is often 4–6 weeks from initial presentation. DPT carries a risk of potentially life-threatening recurrence of the reaction and should only be performed in the absence of alternative well tolerated and effective drugs; in most SCAR and severe DILI, it is contraindicated [70]. In comparison, patch tests, skin prick test and intradermal tests are relatively low-risk methods of identifying the offending drug in IM-ADR, because of only a moderate re-exposure to the offending drug. These are most useful in cases of multiple drug exposure. Their specificity and sensitivity depend on the phenotype of IM-ADR and the offending drug. For example, patch tests have a sensitivity as high as 64% in DRESS whereas in SJS/TEN, this is only up to 30%; they also have low sensitivity in single-organ hypersensitivity, for example, DILI without cutaneous involvement [70]. Patch tests are reliably positive as early as 4 weeks after abacavir hypersensitivity reactions [70]. HLA-B*5701 testing has a 100% negative predictive value for patch-test-confirmed abacavir hypersensitivity reactions that is generalizable across White and Black populations [71]. In contrast, none of the patients who developed cotrimoxazole-associated CADR in a small series had a positive patch test. Patch tests and IDT have some utility in identifying cross-reactivity when CADR occurs across a class of drugs, for example, nevirapine and efavirenz [70,72]. In-vitro testing methods, such as ELISpot and lymphocyte proliferation testing remain in the research domain at present, but unquestionably, in certain drug-IM-ADR combinations may be useful diagnostic tools to prevent unnecessary DPT [73■■].
HIV has forced clinicians to challenge some of these testing paradigms, given the severity of disease and limited range of drug therapies. For instance, in our studies of anti-TB drug SCAR, we have found that more than 90% of HIV-infected patients developed systemic reactions to patch testing, with reactions including rash, fevers, eosinophilia, and transaminitis [74]; and sequential full dose drug provocation testing with FLTDs is well tolerated and offers significantly greater sensitivity in identifying the causative drug than patch testing [75]. Our experience, similar to studies of FLTD-induced DILI, is that in a proportion of patients all four FLTDs can be successfully reintroduced in patients presenting with IM-ADRs [76]. It must be stressed again that any DPT for SCAR should be performed in the absence of any alternative drugs and a serious illness with extreme caution under close supervision with expert input.
Supported treat through, slow reintroduction or desensitization
In most settings, HIV clinicians are comfortable treating through mild CADRs without systemic features and mild liver enzyme abnormalities (in the early phases of treatment) with close monitoring. IM-ADRs with more severe cutaneous manifestations, for example, oedema, indurated erythema, epidermal necrolysis or significant liver enzymes derangements necessitate treatment cessation. However, for certain drugs, even more severe phenotypes can be treated-through. We retrospectively reviewed factors associated with successful treat-through or reintroduction in patients hospitalized for efavirenz cutaneous and DRESS reactions. We noted that post 2013, when efavirenz was introduced in an FDC and the incentive to continue therapy increased, our success with treat-through or reintroduction increased from 10 to 70%, including success even in patients with induration erythema, oedema, eosinophilia and mild hepatitis. The patients were managed with supportive care and topical corticosteroids [77■].
Low starting dose and slow escalation is a well established strategy to reduce certain IM-ADRs and has been used with success to reduce nevirapine and lamotrigine-associated CADRs. Desensitization has also been used successfully in a number of HIV-associated IM-ADRs, often even in preference to DPT, especially when the offending drug is easily identified on history. Cotrimoxazole reactions are perhaps the best known, with several available desensitization protocols [78], and a Cochrane meta-analysis suggesting that desensitization was more successful than rechallenge in preventing cotrimoxazole discontinuation, and in reducing overall drug-related adverse effects [79]. Desensitization has also been successfully attempted in several drugs shown in Table 1 as referenced in previous reviews [20,21]. Despite guidance and expert opinion, that desensitization is contraindicated in severe CADR with multisystem involvement, attempts have been made to desensitize patients to FLTDs given the implications of not reintroducing in HIV–TB co-infection [109–111]. Thong et al. [110] tailored a desensitization regimen based on severity; a multistep protocol starting at 1 : 6000 or a single daily step increase starting at 1 : 3 of therapeutic dose for severe and mild to moderate CADRs respectively. Eleven patients were included, and 23/25 procedures were tolerated, with no severe reactions [110]. Siripassorn et al. performed 19 desensitization procedures in patients, including SJS/TEN (n = 4) and DRESS (n = 1); the starting dose was 1 : 100 000 of the therapeutic dose and it took 14 days to reach full dose. The success rate was lower in severe CADR than mild reactions (62.5 vs. 86.7%). No difference in outcomes were seen whether pre-medication was given or not [111].
PREVENTION
The abacavir success story
Precision medicine that allows for prevention of severe IM-ADR should be the target for all drug therapy. The success of HLA-B57 : 01 testing to eliminate abacavir DHS is an exemplar of this model. A recent meta-analysis by Stainsby et al. [11] found that post-2008 and the implementation of HLA-B57 : 01 testing prior to abacavir prescribing, there has been near elimination of abacavir DHS. At present there is no other HIV-relevant drug IM-ADRs with genetic markers as favorable as abacavir; although the negative predictive value of HLA C04 : 01 for nevirapine SJS/TEN in African populations appears close to 100% [112], but positive predictive values without other additional genetic markers would make the number needed to test not cost-effective. Ongoing efforts are required to discover new biomarkers for prediction and prevention.
FUTURE CHALLENGES AND CONCLUSION
A considerable number of drugs are required to treat both HIV and associated comorbidities. Drug resistance is an ongoing battlefront, with need of complex multidrug regimens and new drug development. Treatment-limiting IM-ADRs in HIV are thus problematic not only because they are frequent and cause significant morbidity and mortality, but for the therapeutic limitations placed on vulnerable patients needing polypharmacy. Fortunately, we have discovered that the majority of reactions are benign and treatment interruption is often unnecessary; or alternatively well established desensitization protocols are available and effective. When interruption is warranted; the need for continuing therapy should be established, the benefits vs. risks of treatment interruption assessed; the available diagnostics and safety of rechallenge explored, and the option of desensitization considered with expert help. Future research efforts need to focus on the changing landscape of relevant drugs, identifying and validating predictive biomarkers and in-vitro diagnostics in high-burden populations.
KEY POINTS.
Immune-mediated adverse drug reactions (IM-ADRs) are increased up to 100-fold in persons living with HIV.
Delayed hypersensitivity reactions involving the skin – mild-to-severe cutaneous drug reactions (CADR) and drug-induced liver injury (DILI) are commonest, and key culprit drugs are antiretrovirals, for example, NNRTIs and antiinfectives used to treat comorbid infections, such as cotrimoxazole or anti-TB therapy.
The majority of hypersensitivity reactions, including mild CADR and liver function abnormalities are benign and can be safely treated through with close monitoring and expert guidance.
The management of IM-ADRs in HIV-infected persons is similar to that in the general population. However, when treatment cessation is warranted; the need for continuing therapy should be established, the benefits vs. risks of treatment interruption assessed, the available diagnostics performed, and if possible safe drug provocation testing performed, and the option of desensitization considered.
Acknowledgements
We acknowledge Karen Adamson for the graphics of Fig. 1.
Financial support and sponsorship
J.P. receives financial support from National Institutes of Health, award K43TW011178-02; the European Developing Clinical Trials Partnership (EDCTP) and the SA National Research Foundation (NRF). P.C. receives a PhD fellowship from the Fogarty HATTP program, funded by the National Institutes of Health, award D43 TW010559-01. R.L. receives financial support from the SA Medical Research Council (MRC). No sponsorship was received for the writing of this manuscript.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.UNAIDS, Miles to go - Global report and update. UNAIDS, Editor. 2018: Geneva, Switzerland. [Google Scholar]
- 2.Angamo MT, Chalmers L, Curtain CM, et al. Mortality from adverse drug reaction-related hospitalizations in south-west Ethiopia: a cross-sectional study. J Clin Pharm Ther 2018; 43:790–798. [DOI] [PubMed] [Google Scholar]
- 3.Coopman SA, Johnson RA, Platt R, Stern RS. Cutaneous disease and drug reactions in HIV infection. N Engl J Med 1993; 328:1670–1674. [DOI] [PubMed] [Google Scholar]
- 4.Blumenthal KG, Peter JG, Trubiano JA, Phillips EJ. Antibiotic allergy. Lancet 2019; 393:183–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bain LE, Nkoke C, Noubiap JJN. UNAIDS 90-90-90 targets to end the AIDS epidemic by 2020 are not realistic: comment on ‘Can the UNAIDS 90-90-90 target be achieved? A systematic analysis of national HIV treatment cascades’. BMJ Glob Health 2017; 2:e000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coughlan R, Cameron S. Key data from the 17th International Workshop on Co-morbidities and Adverse Drug Reactions in HIV. Antivir Ther 2016; 21:75–89. [DOI] [PubMed] [Google Scholar]
- 7.Wu PY, Cheng CY, Liu CE, et al. Multicenter study of skin rashes and hepatotoxicity in antiretroviral-naive HIV-positive patients receiving nonnucleoside reverse-transcriptase inhibitor plus nucleoside reverse-transcriptase inhibitors in Taiwan. PLoS One 2017; 12:e0171596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart A, Lehloenya R, Boulle A, et al. Severe antiretroviral-associated skin reactions in South African patients: a case series and case-control analysis. Pharmacoepidemiol Drug Saf 2016; 25:1313–1319. [DOI] [PubMed] [Google Scholar]
- 9.Sarfo FS, Sarfo MA, Norman B. Incidence and determinants of nevirapine and efavirenz-related skin rashes in West Africans: nevirapine’s epitaph? PLoS One 2014; 9:e94854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bekker LG, Roux S, Sebastien E, et al. Daily and nondaily preexposure prophylaxis in African women (HPTN 067/ADAPT Cape Town Trial): a randomised, open-label, phase 2 trial. Lancet HIV 2018; 5:e68–e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stainsby CM, Perger TM, Vannappagari V, et al. Abacavir hypersensitivity reaction reporting rates during a decade of HLA-B 5701 screening as a risk-mitigation measure. Pharmacotherapy 2019; 39:40–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B, Abbott L, Childs K, et al. Dolutegravir-induced liver injury leading to sub-acute liver failure requiring transplantation: a case report and review of literature. Int J STD AIDS 2018; 29:414–417. [DOI] [PubMed] [Google Scholar]
- 13.Thomas M, Hopkins C, Duffy E, et al. Association of the HLA-B 53:01 Allele With Drug Reaction With Eosinophilia and Systemic Symptoms (DRESS) Syndrome during treatment of HIV infection with raltegravir. Clin Infect Dis 2017; 64:1198–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks KM, George JM, Pau AK, et al. Cytokine-mediated systemic adverse drug reactions in a drug-drug interaction study of dolutegravir with once-weekly isoniazid and rifapentine. Clin Infect Dis 2018; 67:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nyaku AN, Zheng L, Gulick RM, et al. Dolutegravir plus lamivudine for initial treatment of HIV-1-infected participants with HIV-1 RNA <500 000 copies/ml: week 48 outcomes from ACTG 5353. J Antimicrob Chemother 2019. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taiwo BO, Zheng L, Stefanescu A, et al. ACTG A5353: a pilot study of dolutegravir plus lamivudine for initial treatment of human immunodeficiency virus-1 (HIV-1)-infected participants with HIV-1 RNA <500000 copies/ml. Clin Infect Dis 2018; 66:1689–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walmsley SL, Baumgarten A, Berenguer J, et al. , SINGLE Investigators. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013; 369:1807–1818. [DOI] [PubMed] [Google Scholar]
- 18.Landovitz RJ, Li S, Grinsztejn B, et al. Safety, tolerability, and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected individuals: HPTN 077, a phase 2a randomized controlled trial. PLoS Med 2018; 15:e1002690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehloenya RJ, Isaacs T, Dlamini S, Muloiwa R. Cutaneous adverse drug reactions caused by FDCAs - we need to characterise and manage them urgently. S Afr Med J 2013; 103:815. [DOI] [PubMed] [Google Scholar]
- 20.Yunihastuti E, Widhani A, Karjadi TH. Drug hypersensitivity in human immunodeficiency virus-infected patient: challenging diagnosis and management. Asia Pac Allergy 2014; 4:54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips E, Mallal S. Drug hypersensitivity in HIV. Curr Opin Allergy Clin Immunol 2007; 7:324–330. [DOI] [PubMed] [Google Scholar]
- 22.Nagarajan S, Whitaker P. Management of adverse reactions to first-line tuberculosis antibiotics. Curr Opin Allergy Clin Immunol 2018; 18:333–341. [DOI] [PubMed] [Google Scholar]
- 23.Boonyagars L, Hirunwiwatkul P, Hurst CP. CD4 count and risk of antituberculosis drug-associated cutaneous reactions in HIV-infected Thai patients. Int J Tuberc Lung Dis 2017; 21:338–344. [DOI] [PubMed] [Google Scholar]
- 24.Mockenhaupt M, Viboud C, Dunant A, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol 2008; 128:35–44. [DOI] [PubMed] [Google Scholar]
- 25.Hiransuthikul A, Rattananupong T, Klaewsongkram J, et al. Drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms (DIHS/DRESS): 11 years retrospective study in Thailand. Allergol Int 2016; 65:432–438. [DOI] [PubMed] [Google Scholar]
- 26.Kouotou EA, Nansseu JR, Ngono VN, et al. Prevalence and clinical profile of drug eruptions among antiretroviral therapy-exposed HIV infected people in Yaounde, Cameroon. Dermatol Res Pract 2017; 2017:6216193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sisay M, Bute D, Edessa D, et al. Appropriateness of cotrimoxazole prophylactic therapy among HIV/AIDS patients in public hospitals in Eastern Ethiopia: a retrospective evaluation of clinical practice. Front Pharmacol 2018; 9:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy RA, Sunpath H, Kuritzkes DR. Antiretroviral therapy-associated toxicities in the resource-poor world: the challenge of a limited formulary. J Infect Dis 2007; 196:S449–S456. [DOI] [PubMed] [Google Scholar]
- 29.Holtzer CD, Flaherty JF Jr, Coleman RL. Cross-reactivity in HIV-infected patients switched from trimethoprim-sulfamethoxazole to dapsone. Pharmacotherapy 1998; 18:831–835. [PubMed] [Google Scholar]
- 30.Soriano V, Dona C, Barreirov P, González-Lahoz J. Is there cross-toxicity between nevirapine and efavirenz in subjects developing rash? AIDS 2000; 14:1672–1673. [DOI] [PubMed] [Google Scholar]
- 31.Manosuthi W, Thongyen S, Chumpathat N, et al. Incidence and risk factors of rash associated with efavirenz in HIV-infected patients with preceding nevirapine-associated rash. HIV Med 2006; 7:378–382. [DOI] [PubMed] [Google Scholar]
- 32.Suarez-Lorenzo I, Castillo-Sainz R, Cárden-Santana MA, Carrillo-Díaz T. Severe reaction to emtricitabine and lamiduvine: evidence of cross-reactivity. Contact Dermatitis 2016; 74:253–254. [DOI] [PubMed] [Google Scholar]
- 33.Chung WH, Chang WC, Stocker SL, et al. Insights into the poor prognosis of allopurinol-induced severe cutaneous adverse reactions: the impact of renal insufficiency, high plasma levels of oxypurinol and granulysin. Ann Rheum Dis 2015; 74:2157–2164. [DOI] [PubMed] [Google Scholar]
- 34.Rieder MJ, Krause R, Bird IA, Dekaban GA. Toxicity of sulfonamide-reactive metabolites in HIV-infected, HTLV-infected, and noninfected cells. J Acquir Immune Defic Syndr Hum Retrovirol 1995; 8:134–140. [PubMed] [Google Scholar]
- 35.Smith KJ, Skelton HG, Yeager J, et al. Increased drug reactions in HIV-1-positive patients: a possible explanation based on patterns of immune dysregulation seen in HIV-1 disease. The Military Medical Consortium for the Advancement of Retroviral Research (MMCARR). Clin Exp Dermatol 1997; 22:118–123. [PubMed] [Google Scholar]
- 36.Naisbitt DJ, Farrell J, Gordon SF, et al. Covalent binding of the nitroso metabolite of sulfamethoxazole leads to toxicity and major histocompatibility complex-restricted antigen presentation. Mol Pharmacol 2002; 62: 628–637. [DOI] [PubMed] [Google Scholar]
- 37.Karnes JH, Miller MA, White KD, et al. Applications of Immunopharmaco-genomics: predicting, preventing, and understanding immune-mediated adverse drug reactions. Annu Rev Pharmacol Toxicol 2019; 59:463–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Espindola MS, Lima LJ, Soares LS, et al. Dysregulated immune activation in second-line HAART HIVþ patients is similar to that of untreated patients. PLoS One 2015; 10:e0145261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Oliveira Rodrigues R, Helena Barem Rabenhorst S, Germano de Carvalho P, et al. Association of IL10, IL4, IFNG, and CTLA4 gene polymorphisms with efavirenz hypersensitivity reaction in patients infected with human immunodeficiency virus. Jpn J Infect Dis 2017; 70:430–436. [DOI] [PubMed] [Google Scholar]
- 40.Roff S, Song E, Yamamoto J. The significance of interferon-g in HIV-1 pathogenesis, therapy, and prophylaxis. Front Immunol 2014; 4:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pichler WJ. Deciphering the immune pathomechanism of cutaneous drug reactions. Allergy 2002; 57(Suppl 72):34–36. [DOI] [PubMed] [Google Scholar]
- 42.Pichler WJ, Naisbitt DJ, Park BK. Immune pathomechanism of drug hypersensitivity reactions. J Allergy Clin Immunol 2011; 127(3 Suppl):S74–S81. [DOI] [PubMed] [Google Scholar]
- 43.Chang CH, Furue M, Tamaki K. Selective regulation of ICAM-1 and major histocompatibility complex class I and II molecule expression on epidermal Langerhans cells by some of the cytokines released by keratinocytes and T cells. Eur J Immunol 1994; 24:2889–2895. [DOI] [PubMed] [Google Scholar]
- 44.Raval A, Puri N, Rath PC, Saxena RK. Cytokine regulation of expression of class I MHC antigens. Exp Mol Med 1998; 30:1–13. [DOI] [PubMed] [Google Scholar]
- 45.Hayes TL, Asmuth DM, Critchfield JW, et al. Impact of highly active antiretroviral therapy initiation on CD4(+) T-cell repopulation in duodenal and rectal mucosa. Aids 2013; 27:867–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parish ST, Wu JE, Effros RB. Sustained CD28 expression delays multiple features of replicative senescence in human CD8 T lymphocytes. J Clin Immunol 2010; 30:798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takahashi R, Kano Y, Yamazaki Y, et al. Defective regulatory T cells in patients with severe drug eruptions: timing of the dysfunction is associated with the pathological phenotype and outcome. J Immunol 2009; 182:8071–8079. [DOI] [PubMed] [Google Scholar]
- 48.Yang C, Mosam A, Mankahla A, et al. HIV infection predisposes skin to toxic epidermal necrolysis via depletion of skin-directed CD4(+) T cells. J Am Acad Dermatol 2014; 70:1096–1102. [DOI] [PubMed] [Google Scholar]
- 49.Rozieres A, et al. Role of T cells in nonimmediate allergic drug reactions. Curr Opin Allergy Clin Immunol 2009; 9:305–310. [DOI] [PubMed] [Google Scholar]
- 50.Lavergne SN, Kurian JR, Bajad SU, et al. Roles of endogenous ascorbate and glutathione in the cellular reduction and cytotoxicity of sulfamethoxazolenitroso. Toxicology 2006; 222:25–36. [DOI] [PubMed] [Google Scholar]
- 51.Choi J, Liu RM, Kundu RK, et al. Molecular mechanism of decreased glutathione content in human immunodeficiency virus type 1 Tat-transgenic mice. J Biol Chem 2000; 275:3693–3698. [DOI] [PubMed] [Google Scholar]
- 52.Wang D, Curtisv FA. Papp AC, et al. Polymorphism in glutamate cysteine ligase catalytic subunit (GCLC) is associated with sulfamethoxazole-induced hypersensitivity in HIV/AIDS patients. BMC Med Genomics 2012; 5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hosomi H, Akai S, Minami K, et al. An in vitro drug-induced hepatotoxicity screening system using CYP3A4-expressing and gamma-glutamylcysteine synthetase knockdown cells. Toxicol In Vitro 2010; 24:1032–1038. [DOI] [PubMed] [Google Scholar]
- 54■■.Wong YY, Rakaszv EG, Gasper DJ, et al. Immunogenicity of trimethoprim/sulfamethoxazole in a macaque model of HIV infection. Toxicology 2016; 368–369:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fascinating primate study attempting to dissect the relative importance of toxic metabolites and immune contribtuions to cotrimoxazole hypersensitivity.
- 55.Walmsley SL, Khorasheh S, Singer J, Djurdjev O. A randomized trial of N-acetylcysteine for prevention of trimethoprim-sulfamethoxazole hypersensitivity reactions in Pneumocystis carinii pneumonia prophylaxis (CTN 057). Canadian HIV Trials Network 057 Study Group. J Acquir Immune Defic Syndr Hum Retrovirol 1998; 19:498–505. [DOI] [PubMed] [Google Scholar]
- 56.Akerlund B, Tynellv FE. Brattv FG, et al. N-acetylcysteine treatment and the risk of toxic reactions to trimethoprim-sulphamethoxazole in primary Pneumocystis carinii prophylaxis in HIV-infected patients. J Infect 1997; 35:143–147. [DOI] [PubMed] [Google Scholar]
- 57.Phillips E, Bartlett JA, Sanne I, et al. Associations between HLA-DRB1*0102, HLA-B*5801, and hepatotoxicity during initiation of nevirapine-containing regimens in South Africa. J Acquir Immune Defic Syndr 2013; 62:e55–e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58■■.Pavlos R, McKinnon EJ, Ostrov DA, et al. Shared peptide binding of HLA Class I and II alleles associate with cutaneous nevirapine hypersensitivity and identify novel risk alleles. Sci Rep 2017; 7:8653. [DOI] [PMC free article] [PubMed] [Google Scholar]; Important mechanistic study as a possible model to explain more than one predictive HLA typing for the same drug hypersensitivity reactions across different populations.
- 59.de Almeida TB, de Azevedo MCVM, Pinto JFDC, et al. Drug metabolism and transport gene polymorphisms and efavirenz adverse effects in Brazilian HIV-positive individuals. J Antimicrob Chemother 2018; 73: 2460–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60■.Lehloenya R, Peter J. Cutaneous adverse drug reaction in human immunodeficiency virus In: Shear NH, Dodiuk-Gad RP, editors. Advances in the diagnosis and managment of cutaneous adverse drug reactions. Springer Nature: Singapore; 2018. p. 197. [Google Scholar]; Further detailed information particular around the management of CADRs in HIV-infectedv patients.
- 61.Haitembu N, et al. Liver involvement complicating SJS/TEN in an HIV endemic setting. Clin Translat Allergy 2018; 8:10. [Google Scholar]
- 62■.Lehloenya RJ, Haitembu N, Basera W, Peter J. Lower-than-predicted mortality in a predominantly HIV-infected population with epidermal necrolysis regardless of HIV status: implications and challenges for interventional studies. J Allergy Clin Immunol Pract 2019; 7:1653–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]; Study showing low mortality with only supportive care in SJS/TEN from a cohort of high HIVv burden.
- 63.Phillips EJ, Wong GA, Kaul R, et al. Clinical and immunogenetic correlates of abacavir hypersensitivity. AIDS 2005; 19:979–981. [DOI] [PubMed] [Google Scholar]
- 64.Hernandez J, Williams V, Goodwin D, et al. The rate of hypersensitivity reactions to abacavir is similar in under-represented populations and incarcerated subjects. AIDS 2000v; 14:S69. [Google Scholar]
- 65.Hughes CA, Foisy MM, Dewhurst N, et al. Abacavir hypersensitivity reaction: an update. Ann Pharmacother 2008; 42:387–396. [DOI] [PubMed] [Google Scholar]
- 66.Isaacs T, Ngwanyav FMR. Dlamini S, Lehloenya RJ. Annular erythema and photosensitivity as manifestations of efavirenz-induced cutaneous reactions: a review of five consecutive cases. J Antimicrob Chemother 2013; 68: 2871–2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yew WW, Chang KC, Chan DP. Oxidative stress, first-line antituberculosis drug-induced hepatotoxicity. Antimicrob Agents Chemother 2018; 62:; pii: e01633–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yimer G, Gry M, Amogne W, et al. Evaluation of patterns of liver toxicity in patients on antiretroviral and antituberculosis drugs: a prospective four arm observational study in Ethiopian patients. PLoS One 2014; 9:e94271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69■.Sonderup MW, Maughan D, Gogela N, et al. Identification of a novel and severe pattern of efavirenz drug-induced liver injury in South Africa. AIDS 2016; 30:1483–1485. [DOI] [PubMed] [Google Scholar]; Description of three patterns of efavirenz DILI, with a novel pattern of severe DILI with usual onset several months after treatment onset and high mortality.
- 70.Phillips EJ, Bigliardi P, Bircher AJ, et al. Controversies in drug allergy: testing for delayed reactions. J Allergy Clin Immunol 2019; 143:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Phillips E, Mallal S. Successful translation of pharmacogenetics into the clinic: the abacavir example. Mol Diagn Ther 2009; 13:1–9. [DOI] [PubMed] [Google Scholar]
- 72.Mehta U, Maartens G. Is it safe to switch between efavirenz and nevirapine in the event of toxicity? Lancet Infect Dis 2007; 7:733–738. [DOI] [PubMed] [Google Scholar]
- 73■■.Konvinse KC, Phillips EJ, White KD, Trubiano JA. Old dog begging for new tricks: current practices and future directions in the diagnosis of delayed antimicrobial hypersensitivity. Curr Opin Infect Dis 2016; 29:561–576. [DOI] [PMC free article] [PubMed] [Google Scholar]; Good update on in-vitro diagnostics for delayed hypersensitivity reactions.
- 74.Lehloenya RJ, Todd G, Wallace J, et al. Diagnostic patch testing following tuberculosis-associated cutaneous adverse drug reactions induces systemic reactions in HIV-infected persons. Br J Dermatol 2016; 175:150–156. [DOI] [PubMed] [Google Scholar]
- 75.Lehloenya RJ, Todd G, Badri M, Dheda K. Outcomes of reintroducing antituberculosis drugs following cutaneous adverse drug reactions. Int J Tuberc Lung Dis 2011; 15:1649–1657. [DOI] [PubMed] [Google Scholar]
- 76.Sharma SK, Singla R, Sarda P, et al. Safety of 3 different reintroduction regimens of antituberculosis drugs after development of antituberculosis treatment-induced hepatotoxicity. Clin Infect Dis 2010; 50:833–839. [DOI] [PubMed] [Google Scholar]
- 77■.Isaacs T, Ngwanya RM, Dlamini S, et al. Treatment can be continued for mild cutaneous reactions associated with efavirenz. J Allergy Clin Immunol Pract 2018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]; Our recent study describing treat-through and succesful rechallenge even amongst patients hospitalized with efavirenz hypersensitivity.
- 78.Dorn JM, Alpern M, McNulty C, Volcheck GW. Sulfonamide drug allergy. Curr Allergy Asthma Rep 2018; 18:38. [DOI] [PubMed] [Google Scholar]
- 79.Lin D, Li WK, Rieder MJ. Cotrimoxazole for prophylaxis or treatment of opportunistic infections of HIV/AIDS in patients with previous history of hypersensitivity to cotrimoxazole. Cochrane Database Syst Rev 2007; (2):CD005646. [DOI] [PubMed] [Google Scholar]
- 80.Borras-Blasco J, Navarro-Ruiz A, Borrás C, Casterá E. Adverse cutaneous reactions associated with the newest antiretroviral drugs in patients with human immunodeficiency virus infection. J Antimicrob Chemother 2008; 62:879–888. [DOI] [PubMed] [Google Scholar]
- 81.Ma JD, Lee KC, Kuo GM. HLA-B 5701 testing to predict abacavir hypersensitivity. PLoS Curr 2010; 2:RRN1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sulkowski MS, Thomas DL Mehta SH, et al. Hepatotoxicity associated with nevirapine or efavirenz-containing antiretroviral therapy: role of hepatitis C and B infections. Hepatology 2002; 35:182–189. [DOI] [PubMed] [Google Scholar]
- 83.Martin-Carbonero L, Núñez M, González-Lahoz J, Soriano V. Incidence of liver injury after beginning antiretroviral therapy with efavirenz or nevirapine. HIV Clin Trials 2003; 4:115–120. [DOI] [PubMed] [Google Scholar]
- 84.Woolley IJ, Veitch AJ, Harangozo CS, et al. Lichenoid drug eruption to tenofovir in an HIV/hepatitis B virus co-infected patient. AIDS 2004; 18:1857–1858. [DOI] [PubMed] [Google Scholar]
- 85.Lockhart SM, Rathbun RC, Stephens JR, et al. Cutaneous reactions with tenofovir disoproxil fumarate: a report of nine cases. AIDS 2007; 21:1370–1373. [DOI] [PubMed] [Google Scholar]
- 86.Verma R, Vasudevan B, Shankar S, et al. First reported case of tenofovir-induced photoallergic reaction. Indian J Pharmacol 2012; 44:651–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Budamakuntla L, Challa N, Basappa P, Puttappa C. A retrospective study of spectrum of nevirapine induced cutaneous drug reactions in HIV positive patients. J US-China Med Sci 2015; 12:85–89. [Google Scholar]
- 88.Colebunders R, Vanwolleghem T, Meurrens P, Moerman F. Efavirenz-associated Stevens-Johnson syndrome. Infection 2004; 32:306–307. [DOI] [PubMed] [Google Scholar]
- 89.Chaponda M, Pirmohamed M. Hypersensitivity reactions to HIV therapy. Br J Clin Pharmacol 2011; 71:659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sousa M, Cadinha S, Mota M, et al. Hypersensitivity to antiretroviral drugs a case report. Clin Translat Allergy 2014; 4(Suppl 3):104–1104. [DOI] [PubMed] [Google Scholar]
- 91.Lehloenya RJ, Isaacs T, Dlamini S, Muloiwa R. Cutaneous adverse drug reactions caused by FDCAs – we need to characterise and manage them urgently. S Afr Med J 2013; 103:815. [DOI] [PubMed] [Google Scholar]
- 92.Sánchez-Borges M, Thong B, Blanca M, et al. Hypersensitivity reactions to non beta-lactam antimicrobial agents, a statement of the WAO special committee on drug allergy. World Allergy Organ J 2013; 6:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lorenz M, Wozel G, Schmitt J. Hypersensitivity reactions to dapsone: a systematic review. Acta Derm Venereol 2012; 92:194–199. [DOI] [PubMed] [Google Scholar]
- 94.Rankin BT, Jariwala S. Graded challenge protocol for fluconazole hypersensitivity in a patient with cryptococcal pneumonitis. Ann Allergy Asthma Immunol 2012; 108:466. [DOI] [PubMed] [Google Scholar]
- 95.George J, Sharma A, Dixit R, et al. Toxic epidermal necrolysis caused by fluconazole in a patient with human immunodeficiency virus infection. J Pharmacol Pharmacother 2012; 3:276–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matono T, Nishijima T, Teruya K, et al. Substantially higher and earlier occurrence of anti-tuberculosis drug-related adverse reactions in HIV coinfected tuberculosis patients: a matched-cohort study. AIDS Patient Care STDS 2017; 31:455–462. [DOI] [PubMed] [Google Scholar]
- 97.Lehloenya RJ, Dheda K. Cutaneous adverse drug reactions to antituberculosis drugs: state of the art and into the future. Expert Rev Anti Infect Ther 2012; 10:475–486. [DOI] [PubMed] [Google Scholar]
- 98.Chang CH, Chen YF, Wu VC, et al. Acute kidney injury due to antituberculosis drugs: a five-year experience in an aging population. BMC Infect Dis 2014; 14:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lehloenya RJ, Wallace J, Todd G, Dheda K. Multiple drug hypersensitivity reactions to antituberculosis drugs: five cases in HIV-infected patients. Int J Tuberc Lung Dis 2012; 16:1260–1264. [DOI] [PubMed] [Google Scholar]
- 100.Girling DJ. Adverse reactions to rifampicin in antituberculosis regimens. J Antimicrob Chemother 1977; 3:115–132. [DOI] [PubMed] [Google Scholar]
- 101.Lehloenya RJ, Dlamini S, Muloiwa R, et al. Therapeutic trial of rifabutin after rifampicin-associated DRESS syndrome in tuberculosis-human immunodeficiency virus coinfected patients. Open Forum Infect Dis 2016; 3:ofw130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bakkum RS, Waard-Van Der Spek FB, Thio HB. Delayed-type hypersensitivity reaction to ethambutol and isoniazid. Contact Dermatitis 2002; 46:359. [DOI] [PubMed] [Google Scholar]
- 103.Viswanath BK, Ranka P, Ramanjanayalu M. Severe cutaneous adverse reactions due to isoniazid in a HIV positive patient. Indian J Lepr 2012; 84:227–232. [PubMed] [Google Scholar]
- 104.Tan WC, Ong CK, Kang SC, Razak MA. Two years review of cutaneous adverse drug reaction from first line antituberculous drugs. Med J Malaysia 2007; 62:143–146. [PubMed] [Google Scholar]
- 105.Surjapranata FJ, Rahaju NN. A case of Stevens-Johnson’s syndrome caused by ethambutol. Paediatr Indones 1979; 19:195–201. [PubMed] [Google Scholar]
- 106.Pegram PS Jr, Mountz JD, O’Bar PR. Ethambutol-induced toxic epidermal necrolysis. Arch Intern Med 1981; 141:1677–1678. [PubMed] [Google Scholar]
- 107.Wong PC, Yew WW, Wong CF, Choi HY. Ethambutol-induced pulmonary infiltrates with eosinophilia and skin involvement. Eur Respir J 1995; 8:866–868. [PubMed] [Google Scholar]
- 108.Lehloenya RJ, Kgokolo M. Clinical presentations of severe cutaneous drug reactions in HIV-infected Africans. Dermatol Clin 2014; 32:227–235. [DOI] [PubMed] [Google Scholar]
- 109.Scherer K, Brockow K, Aberer W, et al. , ENDA, the European Network on Drug Allergy and the EAACI Drug Allergy Interest Group. Desensitization in delayed drug hypersensitivity reactions – an EAACI position paper of the Drug Allergy Interest Group. Allergy 2013; 68:844–852. [DOI] [PubMed] [Google Scholar]
- 110.Thong BY, Chia FL, Tan SC, et al. A retrospective study on sequential desensitization-rechallenge for antituberculosis drug allergy. Asia Pac Allergy 2014; 4:156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Siripassorn K, Ruxrungtham K, Manosuthi W. Successful drug desensitization in patients with delayed-type allergic reactions to antituberculosis drugs. Int J Infect Dis 2018; 68:61–68. [DOI] [PubMed] [Google Scholar]
- 112.Carr DF, Bourgeois S, Chaponda M, et al. Genome-wide association study of nevirapine hypersensitivity in a sub-Saharan African HIV-infected population. J Antimicrob Chemother 2017; 72:1152–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]