Abstract
The medial cortico-striatal-thalamo-cortical (CSTC) motor circuit is a core system that exerts control over interval timing and action. A common network generates these behaviors possibly owing to cellular coding of temporal and non-temporal information, which in turn promotes reconfiguration of functional connectivity in accord with behavioral goals. At the neuroanatomical level, support for flexible CSTC reconfiguration comes from studies of temporal illusions demonstrating that this system calibrates the experience of time through functional interactions with various context-sensitive brain regions. Revelations that CSTC effective connectivity is pivotal for context-dependent facets of voluntary actions, namely action planning, complement its role in predictive processes such as timing. These observations suggest that the CSTC is positioned to represent high-level information about ‘what to do’ and ‘when to do it’ by dynamically reconfiguring effective connectivity as circumstances arise.
Introduction
Research into the neural mechanisms of temporal processing and voluntary action have proceeded independent of one another, even though time is a basic facet of action representation that comes into play when anticipating and guiding the timing of movements. This review seeks to integrate recent findings across these areas by focusing on a core system that exerts control over interval timing and voluntary actions, namely the supplementary and presupplementary motor areas (SMA/preSMA), the striatum, and the thalamus, otherwise referred to as the cortico-striatal-thalamo-cortical (CSTC) system (Figure 1, bottom). We begin by discussing neurophysiological mechanisms by which this system governs interval timing and may also permit reconfiguration of CSTC functional interactions for the control of action. Indeed, CSTC interactions are conditional on the behavioral goal, which is highlighted by the discovery that different neural architectures are specialized for relative and absolute forms of timing. We then consider emerging studies of temporal illusions, which demonstrate that CSTC interactions with various brain regions alter perceived duration in a contextually-sensitive manner. This revelation is a powerful illustration of the system’s capacity for flexibly calibrating time and by extension, modulating context-dependent facets of voluntary action. Here, we discuss the pivotal role of the CSTC system in planning actions, which complements its role in relative timing.
Figure 1.
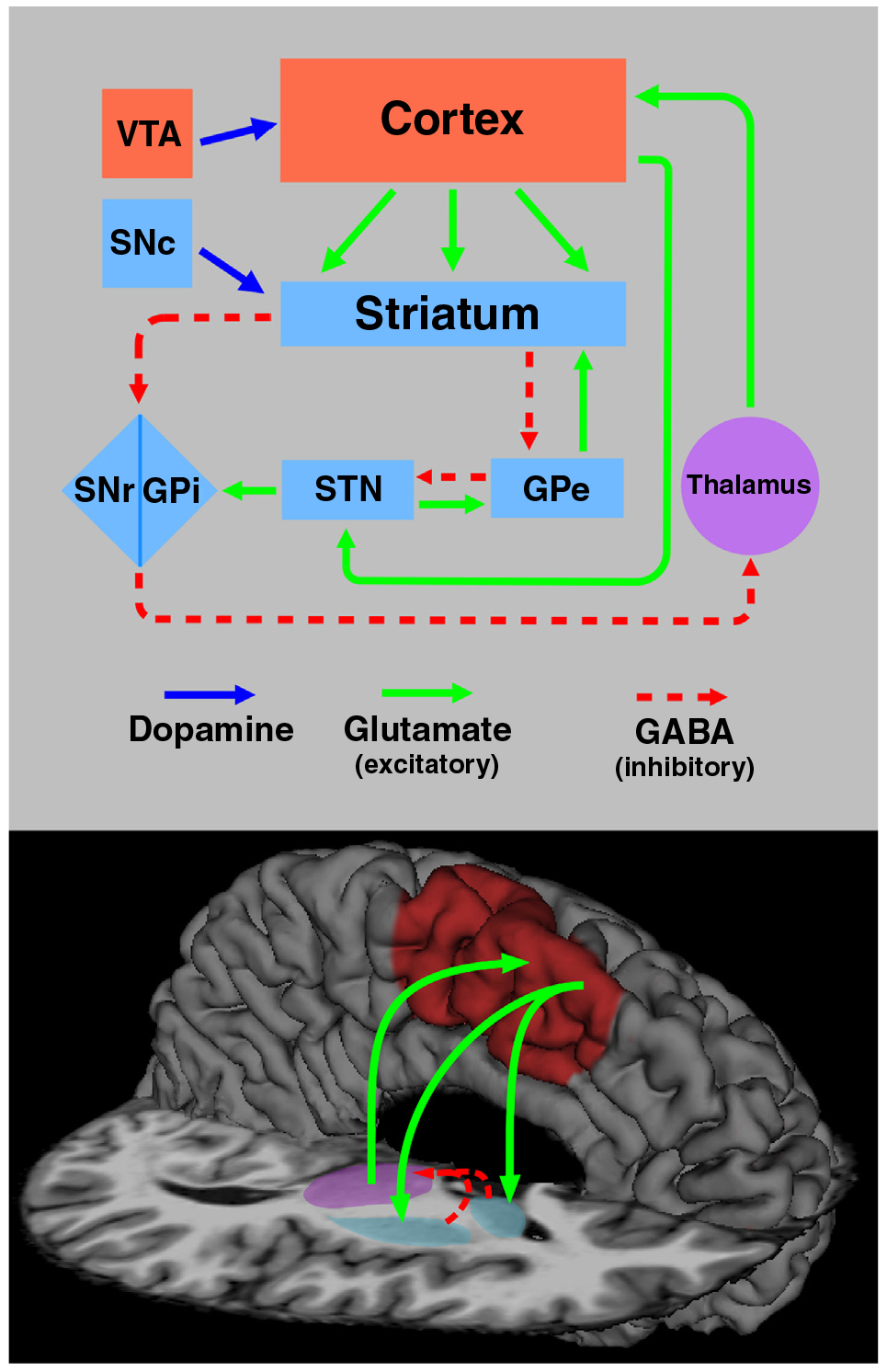
Neural substrates of interval timing and action planning. (Top) An illustration of cortical-basal ganglia and neurotransmitter systems that are the cornerstone for interval timing and action representation. Elements of basal ganglia and cortical networks are respectively highlighted in light blue and red boxes. Multiple functionally-segregated circuits link the cortex and basal ganglia, which enables the control of timing and actions in a context-sensitive manner. The nigrostriatal dopamine pathway connects the substantia nigra pars compacta (SNc) to the dorsal striatum and is part of the motor circuit. The mesocortical dopamine pathway connects the ventral tegmental area (VTA) to the cortex, particularly the frontal lobes. Excitatory (glutamate) and inhibitory (GABA) projections are designated by green and red dashed arrows, respectively. (Bottom) A core system that governs both interval timing and action planning is the medial corticostriatal thalamo-cortical (CSTC) motor circuit, which consists of the SMA and preSMA (red), the striatum (blue), and the thalamus (purple).
CSTC system and dopamine neurotransmission in interval timing
A defining quality of purposeful behaviors is time and rhythm, which support the fluency, coordination, and organization of actions into abstract groupings [1]. Timing processes also optimize the prediction of when to act, drawing upon past experiences of temporal regularities in events or responses to them, which seemingly capture attention automatically. There is growing consensus across species that the striatum and dopamine (DA) neurotransmission (Figure 1, top) exert control over interval timing in the range of milliseconds to several seconds ([2–5]; for reviews see [6••,7,8]). This is compatible with impaired timing in basal-ganglia disorders such as Parkinson’s disease (PD) [9•,10••,11–13] and prodromal Huntington’s disease (prHD) [14,15]. However, psychophysiological features of interval timing, such as the scalar property, emerge through striatal interactions with the cerebral cortex. According to the striatal beat-frequency model, medium spiny neurons of the dorsal striatum sense temporal patterns by serving as coincidence detectors of activity engaged by cortical neurons, which comprise the ‘clock signal’ ([16]; for reviews see [6••,17•]). One activity pattern is the oscillatory firing patterns of cortical neurons, which are thought to be synchronized to the onset of relevant stimuli via DA release from the substantia nigra pars compacta [18]. There is growing consensus that a key cortical component of interval timing is the medial prefrontal cortex, namely the SMA and preSMA, which complete the medial CSTC motor circuit (Figure 1, bottom). Although SMA function is not well understood, it is routinely engaged during timing [19–25] and may support the accumulation and maintenance of temporal information [21].
Another important finding is that the CSTC system governs relative (e.g., longer of two intervals) rather than absolute (e.g., 500 ms) or implicit forms of timing, in which temporal regularities in events or motor responses can be used to achieve non-temporal goals. For example, when beat rhythms (relative timing) are compared to non-beat rhythms (absolute timing), striatal activity was higher [26] and effective connectivity was stronger between the putamen and SMA [27]. Thus, the CSTC system is pivotal for predicting and generating rhythms. These findings agree with cell recordings in animals showing that temporal tuning in the striatum rescaled when intervals were expanded or contracted [28••]. By contrast, when irregular sequences of clicks were compared to regular sequences, activation was greater in the olivocerebellar system [29].
The prospect of distinct networks for relative and absolute timing is compatible with observations of increased cerebellar activity and effective connectivity in PD during interval timing [10••,30,31•], possibly signifying compensation for CSTC dysfunction. Indeed, temporal and musical cueing therapies for improving gait in PD also improved performance on timing tasks [11,12,32], partly owing to increased metabolism in cerebellar–temporal– parietal regions [33]. The benefits conferred by cueing therapies on movement further suggest that motor disturbances in PD are partly linked to deficient internal timing, possibly due to CSTC coding of temporal and non-temporal information. In fact, cellular recordings in animals indicate that the SMA and putamen not only display chronotopy during timing, but also represent multiple information streams (e.g., time passed, remaining time for an action, serial order, sensorimotor state of organism) [28••,34,35••,36–39], which may enable the reconfiguration of functional interactions in accord with behavioral goals [40•]. These observations suggest that the CSTC is well placed to represent higher-level information about actions and their timing. Voluntary actions may also rely on the same oscillatory processes as proposed for interval timing [16,17•], yet differ in some measure with respect to the information streams that code features and reconfigure CSTC interactions for goal-directed action [40•].
Context-sensitive interactions of the CSTC calibrate perceived time
The proposal that the CSTC system flexibly alters interactions with various brain centers contingent on the situation is compatible with demonstrations that internal states of an organism, past experiences, and stimulus properties can distort the experience of time. Unraveling the mechanisms by which the CSTC system calibrates perceived duration is important because it also has implications for understanding how this system controls voluntary action, which is also context dependent [41–43]. It has long been known that emotionally aversive, larger magnitude, or intense stimuli are perceived as lasting longer than their physical duration, whereas duration is underestimated when stimuli are repeated, high probability, or non-salient (for a review see [44]). Although the neurobehavioral mechanisms underlying distortions in perceived duration remain debated, there is consensus that attention and arousal can speed up or slow down timing [45–47]. Emerging research also suggests that CSTC regions calibrate time via context-sensitive interactions with various brain centers.
One compelling demonstration of the striatum’s role in calibrating time comes from an fMRI study of time dilation in an emotionally arousing situation. Participants judged the duration of aversive-neutral pictures or two neutral pictures, and after imaging memory for the pictures was tested [48•]. Recognition memory was better for aversive pictures that were incorrectly timed (overestimated) than for incorrectly timed neutral pictures. Moreover, activation was greater for incorrectly than correctly timed pictures in the amygdala, striatum, medial frontal cortex, and a region of the ventral attention network (Figure 2), the anterior insula, which is routinely engaged during timing [21,49,50]. Interestingly, better recognition of aversive pictures was correlated with greater insula and putamen activation on incorrectly, but not correctly timed trials. These results suggested that arousal of the ventral attention system by emotionally aversive pictures may accelerate the accumulation of activity into the striatum, which in turn compromises timing accuracy yet improves memory.
Figure 2.
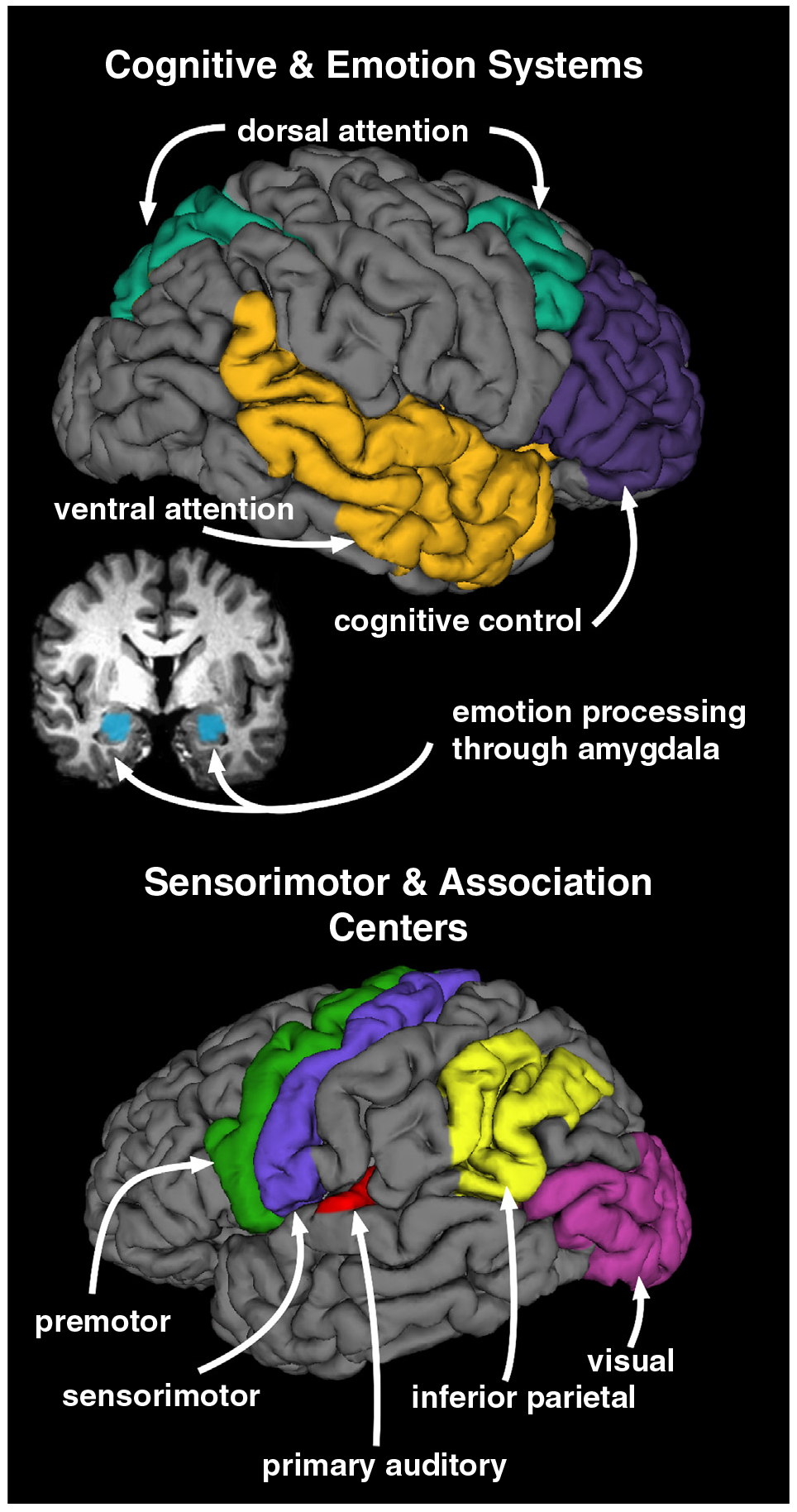
Brain systems that display context-dependent interactions with the CSTC system. The CSTC system is thought to shape the experience of time through interactions with other brain centers that are relevant to a situation. Thus, by extension these functional interactions may also come into play during action planning. (Top) Cognitive and emotion-based systems interact with the CSTC system during interval timing in a contextually-sensitive manner. Lateral and coronal views display cognitive and emotion processing centers known to exhibit effective connectivity or co-activate with the CSTC system during interval timing. CSTC functional interactions with of these regions are thought to bring about distortions in perceived duration. These regions include frontal cognitive-control or executive processing centers (purple), dorsal (green) and ventral (gold) attention networks, and an emotion-processing hub, the amygdala (blue; coronal view). (Bottom) Sensorimotor and association centers also interact with the CSTC system during interval timing and for the purpose of action planning. These centers include the premotor (green), sensorimotor (blue), inferior parietal (yellow), primary auditory (red), and visual (fuchsia) cortices.
Distortions in perceived duration also arise in settings void of emotional undercurrent, wherein temporal information from different senses is commonly paired, namely audition and vision. Auditory signals are perceived as lasting longer than visual signals of the same duration when they are compared together [51•,52], owing to the dominance of audition, which presumably captures and sustains attention during timing more automatically than visual stimuli ([47]; for an alternative interpretation see [53•]). Thus, time is overestimated (dilated) when the duration of an auditory (A) comparison interval is judged relative to a visual (V) standard interval (VA) and underestimated (compressed) when a V comparison interval is judged relative to an A standard (AV). One study reported that audiovisual timing was mediated by the CSTC, ventral attention (insula), and sensory-association systems (temporal-occipital) (Figure 2) [51•]. Frontal ‘cognitive-control’ centers (Figure 2) also exhibited greater activation when time was compressed (AV) than dilated (VA), consistent with the greater controlled attention demands of timing visual information, which if not sustained, leads to a loss in pulses from the pacemaker–accumulator mechanism [46]. Converging support for these findings comes from an fMRI study of audiovisual timing in PD [10••]. When timing emphasized controlled attention (AV), time compression was markedly exaggerated in patients relative to control participants, whereas when attention was more easily sustained (VA), time was dilated in both groups, but less so for patients. Importantly, abnormal CSTC and anterior insula effective connectivity in PD differed for the two audiovisual conditions. In the AV condition, effective connectivity of the CSTC and anterior insula was notably weakened in PD with distributed frontal cognitive-control centers and the ventral and dorsal attention networks (Figure 2), consistent with the considerable attentional demands of the condition. In the VA condition, however, effective connectivity of the striatum with frontal cortex and the SMA was stronger in PD relative to controls, possibly signifying compensation when attention to timing was less taxing. Regardless of the condition, effective connectivity of the cerebellum with frontal cortex was stronger in PD relative to controls, suggesting compensation in absolute timing systems [30,31•].
These discoveries are persuasive examples of the CSTC system’s capacity for shaping perceived duration by integrating signals from contextually relevant systems involved in cognitive control, attention, emotion and sensory processing, which can also come into play during voluntary action. As such, reconfiguration of CSTC interactions with context-dependent brain networks may be a mechanism by which this system governs ‘what to do’ and ‘when to do’ it as a situation arises.
Configuration of CSTC system for action planning
Although it has long been known that voluntary actions are supported by the CSTC system, its specific functional role has received relatively less attention. Nonetheless, there is growing consensus that the CSTC system governs action planning, which is compatible with this system’s role in predictive processes such as beat prediction, which depends on a representation of the structure of events to predict and guide behavior [26].
Indirect evidence for the centrality of the CSTC system in action planning comes from neuroimaging studies contrasting movements performed in the presence of sensory information that guides performance (externally guided) with movements performed from memory without external cues (internally generated). Early studies reported greater activation of the CSTC during internally-generated than externally-guided action [42,43], whereas frontoparietal and cerebellar activations were greater during externally-guided action [43]. Importantly, the different avenues for motor control were also distinguished by the type of functional interactions among the same set of brain regions. Stronger interactions were found amongst the SMA, putamen, and thalamus during internally-generated movement, whereas interactions amongst the cerebellum, premotor cortex, and sensorimotor cortex were stronger during externally-guided movement [54].
Other studies seeking to directly unravel the neural mechanisms of action planning separated activation associated with the premovement period of planning a sequence and the movement period when the action is performed. One study also manipulated sequence complexity [41], which increases planning difficulty. CSTC and premotor cortex activation increased with sequence complexity, more so during the premovement than the movement phase of internally-generated sequences [41]. Notably, only basal ganglia activation increased with sequence complexity during the premovement, but not the movement phase, suggesting a specific role of the striatum in integrating information for action plans. Other studies using similar methods also endorsed a CSTC role in planning [55,56,57•], sometimes jointly with the substantia nigra [55], suggesting dopaminergic gating of motor sequences. Impaired motor planning and an abnormal reliance upon external cues to sequence or initiate actions in PD and prHD [58–62,63•,64,65] provide converging support for CSTC centrality in action planning. More generally, weakened CSTC effective connectivity is observed in PD during voluntary actions [63•] and self-initiated movements [61], whereas strengthened connectivity of the lateral premotor systems [66] or the cerebellar-thalamocortical system is found in PD, especially during externally guided action [67].
Interestingly, the CSTC and the cerebellar systems’ respective roles in internally-generated and externally-guided movement parallel their control of relative and absolute timing. One speculation is that action planning exploits relative timing mechanisms, whereas online motor-control engages absolute or implicit timing processes, which predict sensory consequences of actions and rapidly fine tune movement on the basis of an efferent copy of sensorimotor information.
Conclusions
Altogether, interval timing and action planning appear to originate from the CSTC system. However, evidence for flexible, context-dependent CSTC interactions is only beginning to emerge. CSTC connectivity as modulated by timing versus action planning has yet to be studied, but would address basic questions about the nature of this system’s interactions with the brain for different behavioral goals. The influence of cognitive demands on connectivity will be especially important as investigations move toward studying more complicated forms of timing (e.g., multiple events) and action planning (e.g., complex actions, larger repertoires of behaviors). These lines of inquiry are highly relevant to unraveling mechanisms of pathological timing and action in basal ganglia disorders, and may provide insight into cognitive-training approaches that target brain networks capable of compensating for neuronal dysfunction.
Acknowledgements
The authors would like to thank Gabriel Castillo for his technical support in preparation of the figure. This work was supported by grants to DLH from the Department of Veterans Affairs (CX000146) and the National Institute for Neurological Disease and Stroke (U01NS082083).
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
•of special interest
••of outstanding interest
- 1.Povel D-J, Collard R: Structural factors in patterned finger tapping. Acta Psychol 1982, 52:107–123. [DOI] [PubMed] [Google Scholar]
- 2.Parker KL, Chen KH, Kingyon JR, Cavanagh JF, Narayanan NS: D1-dependent 4 Hz oscillations and ramping activity in rodent medial frontal cortex during interval timing. J Neurosci 2014, 34:16774–16783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lake JI, Meck WH: Differential effects of amphetamine and haloperidol on temporal reproduction: dopaminergic regulation of attention and clock speed. Neuropsychologia 2013, 51:284–292. [DOI] [PubMed] [Google Scholar]
- 4.Wiener M, Lohoff FW, Coslett HB: Double dissociation of dopamine genes and timing in humans. J Cogn Neurosci 2011, 23:2811–2821. [DOI] [PubMed] [Google Scholar]
- 5.Meck WH, Cheng RK, MacDonald CJ, Gainetdinov RR, Caron MG, Cevik MO: Gene-dose dependent effects of methamphetamine on interval timing in dopamine-transporter knockout mice. Neuropharmacology 2012, 62:1221–1229. [DOI] [PubMed] [Google Scholar]
- 6.••.Merchant H, Harrington DL, Meck WH: Neural basis of the perception and estimation of time. Annu Rev Neurosci 2013, 36:313–336. [DOI] [PubMed] [Google Scholar]; A comprehensive review and analysis is provided about advances in knowledge about the neural and the behavioral mechanisms of interval timing. The review spans research across species and different levels of analyses, and considers prominent neurophysiological models that capture the psychophysiological and neuroanatomical properties of timing networks.
- 7.Jones CR, Jahanshahi M: Dopamine modulates striato-frontal functioning during temporal processing. Front Integr Neurosci 2011, 5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allman MJ, Meck WH: Pathophysiological distortions in time perception and timed performance. Brain 2012, 135:656–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.•.Harrington DL, Castillo GN, Greenberg PA, Song DD, Lessig S, Lee RR, Rao SM: Neurobehavioral mechanisms of temporal processing deficits in Parkinson’s disease. PLoS ONE 2011, 6:e17461. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study deconstructs the neural mechanisms underlying time perception deficits in PD by separating the time courses of activation during the encoding and decision-making phases of the task. The findings reveal abnormal activation of the striatum, a working memory network, and memory systems in one or both of these phases. Dopamine therapy in PD was also found to influence the effective connectivity of the striatum.
- 10.••.Harrington DL, Castillo GN, Reed JD, Song DD, Litvan I, Lee RR: Dissociation of neural mechanisms for intersensory timing deficits in Parkinson’s disease. Timing Time Percept 2014, 2:145–168. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrates that time perception deficits in PD are well characterized by two context-dependent patterns of CSTC and anterior insula effective connectivity, which are dissociated by the presumed attentional demands of two audiovisual-timing conditions that produced underestimations or overestimations of time. Effective connectivity disturbances exhibited excellent sensitivity and specificity in distinguishing between control participants and PD patients without clinically significant cognitive impairment.
- 11.Benoit CE, Dalla BS, Farrugia N, Obrig H, Mainka S, Kotz SA: Musically cued gait-training improves both perceptual and motor timing in Parkinson’s disease. Front Hum Neurosci 2014, 8:494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grahn JA, Brett M: Impairment of beat-based rhythm discrimination in Parkinson’s disease. Cortex 2009, 45:54–61. [DOI] [PubMed] [Google Scholar]
- 13.Jones CR, Malone TJ, Dirnberger G, Edwards M, Jahanshahi M: Basal ganglia, dopamine and temporal processing: performance on three timing tasks on and off medication in Parkinson’s disease. Brain Cogn 2008, 68:30–41. [DOI] [PubMed] [Google Scholar]
- 14.Rowe KC, Paulsen JS, Langbehn DR, Duff K, Beglinger LJ, Wang C, O’Rourke JJ, Stout JC, Moser DJ: Self-paced timing detects and tracks change in prodromal Huntington disease. Neuropsychology 2010, 24:435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paulsen JS, Long JD, Ross CA, Harrington DL, Erwin CJ, Williams JK, Westervelt HJ, Johnson HJ, Aylward EH, Zhang Y, Bockholt HJ, Barker RA: Prediction of manifest Huntington’s disease with clinical and imaging measures: a prospective observational study. Lancet Neurol 2014, 13:1193–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matell MS, Meck WH: Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Cogn Brain Res 2004, 21:139–170. [DOI] [PubMed] [Google Scholar]
- 17.•.Gu BM, van RH, Meck WH: Oscillatory multiplexing of neural population codes for interval timing and working memory. Neurosci Biobehav Rev 2015, 48:160–185. [DOI] [PubMed] [Google Scholar]; Explores neurobiological theories of interval timing and working memory from behavioral, anatomical, pharmacological, and neurophysiological research. A hybrid model is put forth to explain how interval timing and working memory can originate from the same oscillatory processes in prefrontal–striatal–hippocampal circuits.
- 18.Jahanshahi M, Jones CR, Dirnberger G, Frith CD: The substantia nigra pars compacta and temporal processing. J Neurosci 2006, 26:12266–12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao SM, Mayer AR, Harrington DL: The evolution of brain activation during temporal processing. Nat Neurosci 2001, 4:317–323. [DOI] [PubMed] [Google Scholar]
- 20.Wiener M, Turkeltaub P, Coslett HB: The image of time: a voxel-wise meta-analysis. NeuroImage 2010, 49:1728–1740. [DOI] [PubMed] [Google Scholar]
- 21.Harrington DL, Zimbelman JL, Hinton SC, Rao SM: Neural modulation of temporal encoding, maintenance, and decision processes. Cereb Cortex 2010, 20:1274–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coull JT, Nazarian B, Vidal F: Timing, storage, and comparison of stimulus duration engage discrete anatomical components of a perceptual timing network. J Cogn Neurosci 2008, 20:2185–2197. [DOI] [PubMed] [Google Scholar]
- 23.Harrington DL, Boyd LA, Mayer AR, Sheltraw DM, Lee RR, Huang M, Rao SM: Neural representation of interval encoding and decision making. Cogn Brain Res 2004, 21:193–205. [DOI] [PubMed] [Google Scholar]
- 24.Macar F, Anton JL, Bonnet M, Vidal F: Timing functions of the supplementary motor area: an event-related fMRI study. Cogn Brain Res 2004, 21:206–215. [DOI] [PubMed] [Google Scholar]
- 25.Schwartze M, Rothermich K, Kotz SA: Functional dissociation of pre-SMA and SMA-proper in temporal processing. NeuroImage 2012, 60:290–298. [DOI] [PubMed] [Google Scholar]
- 26.Grahn JA, Rowe JB: Finding and feeling the musical beat: striatal dissociations between detection and prediction of regularity. Cereb Cortex 2013, 23:913–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grahn JA, Rowe JB: Feeling the beat: premotor and striatal interactions in musicians and nonmusicians during beat perception. J Neurosci 2009, 29:7540–7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.••.Mello GB, Soares S, Paton JJ: A scalable population code for time in the striatum. Curr Biol 2015, 25:1113–1122. [DOI] [PubMed] [Google Scholar]; Shows that the firing dynamics of neurons in the rat striatum encode time over tens of seconds and represent the interaction between time and the organism’s ongoing sensorimotor state. Importantly, neuronal responses rescaled in time when intervals changed, indicating that the striatum encodes relative time.
- 29.Teki S, Grube M, Kumar S, Griffiths TD: Distinct neural substrates of duration-based and beat-based auditory timing. J Neurosci 2011, 31:3805–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu H, Sternad D, Corcos DM, Vaillancourt DE: Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. NeuroImage 2007, 35:222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.•.Jahanshahi M, Jones CR, Zijlmans J, Katzenschlager R, Lee L, Quinn N, Frith CD, Lees AJ: Dopaminergic modulation of striato-frontal connectivity during motor timing in Parkinson’s disease. Brain 2010, 133:727–745. [DOI] [PubMed] [Google Scholar]; Shows that CSTC effective connectivity in PD patients during motor timing is altered by dopamine neurotransmission. The study also reports increased cerebellar connectivity in patients OFF versus ON medication, suggestive of compensation by this system.
- 32.Bella SD, Benoit CE, Farrugia N, Schwartze M, Kotz SA: Effects of musically cued gait training in Parkinson’s disease: beyond a motor benefit. Ann N Y Acad Sci 2015, 1337:77–85. [DOI] [PubMed] [Google Scholar]
- 33.del Olmo MF, Arias P, Furio MC, Pozo MA, Cudeiro J: Evaluation of the effect of training using auditory stimulation on rhythmic movement in Parkinsonian patients — a combined motor and [18F]-FDG PET study. Parkinsonism Relat Disord 2006, 12:155–164. [DOI] [PubMed] [Google Scholar]
- 34.Merchant H, Zarco W, Perez O, Prado L, Bartolo R: Measuring time with different neural chronometers during a synchronization-continuation task. Proc Natl Acad Sci U S A 2011, 108:19784–19789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.••.Crowe DA, Zarco W, Bartolo R, Merchant H: Dynamic representation of the temporal and sequential structure of rhythmic movements in the primate medial premotor cortex. J Neurosci 2014, 34:11972–11983. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrates that cells in the Rhesus monkey medial premotor cortex encode duration and serial order in rapid succession during the production of rhythmic movements.
- 36.Merchant H, Perez O, Bartolo R, Mendez JC, Mendoza G, Gamez J, Yc K, Prado L: Sensorimotor neural dynamics during isochronous tapping in the medial premotor cortex of the macaque. Eur J Neurosci 2015, 41:586–602. [DOI] [PubMed] [Google Scholar]
- 37.Matell MS, Meck WH, Nicolelis MA: Interval timing and the encoding of signal duration by ensembles of cortical and striatal neurons. Behav Neurosci 2003, 117:760–773. [DOI] [PubMed] [Google Scholar]
- 38.Matell MS, Shea-Brown E, Gooch C, Wilson AG, Rinzel J: A heterogeneous population code for elapsed time in rat medial agranular cortex. Behav Neurosci 2011, 125:54–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merchant H, Perez O, Zarco W, Gamez J: Interval tuning in the primate medial premotor cortex as a general timing mechanism. J Neurosci 2013, 33:9082–9096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.•.Akam T, Kullmann DM: Oscillatory multiplexing of population codes for selective communication in the mammalian brain. Nat Rev Neurosci 2014, 15:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]; An opinion paper that considers the role of multiplexing, whereby multiple information streams share a common neural substrate and enable flexible reconfiguration of connectivity among brain regions.
- 41.Elsinger CL, Harrington DL, Rao SM: From preparation to online control: reappraisal of neural circuitry mediating internally generated and externally guided actions. NeuroImage 2006, 31:1177–1187. [DOI] [PubMed] [Google Scholar]
- 42.Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ: Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain 2000, 123:1216–1228. [DOI] [PubMed] [Google Scholar]
- 43.Debaere F, Wenderoth N, Sunaert S, van HP, Swinnen SP: Internal vs external generation of movements: differential neural pathways involved in bimanual coordination performed in the presence or absence of augmented visual feedback. NeuroImage 2003, 19:764–776. [DOI] [PubMed] [Google Scholar]
- 44.Eagleman DM: Human time perception and its illusions. Curr Opin Neurobiol 2008, 18:131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mella N, Conty L, Pouthas V: The role of physiological arousal in time perception: psychophysiological evidence from an emotion regulation paradigm. Brain Cogn 2011, 75:182–187. [DOI] [PubMed] [Google Scholar]
- 46.Lustig C, Meck WH: Modality differences in timing and temporal memory throughout the lifespan. Brain Cogn 2011, 77:298–303. [DOI] [PubMed] [Google Scholar]
- 47.Berry AS, Li X, Lin Z, Lustig C: Shared and distinct factors driving attention and temporal processing across modalities. Acta Psychol (Amst) 2014, 147:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.••.Dirnberger G, Hesselmann G, Roiser JP, Preminger S, Jahanshahi M, Paz R: Give it time: neural evidence for distorted time perception and enhanced memory encoding in emotional situations. NeuroImage 2012, 63:591–599. [DOI] [PubMed] [Google Scholar]; Provides persuasive evidence that the overestimation of time (dilation) by emotionally aversive pictures increases arousal of the ventral attention systems (anterior insula), which may accelerate the accumulation of activity into the striatum. Memory was also better for aversive pictures that were incorrectly (overestimated), but not correctly timed.
- 49.Wittmann M, Simmons AN, Aron JL, Paulus MP: Accumulation of neural activity in the posterior insula encodes the passage of time. Neuropsychologia 2010, 48:3110–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wittmann M, van Wassenhove V, Craig AD, Paulus MP: The neural substrates of subjective time dilation. Front Hum Neurosci 2010, 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.•.Harrington DL, Castillo GN, Fong CH, Reed JD: Neural underpinnings of distortions in the experience of time across senses. Front Integr Neurosci 2011, 5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrates that distortions in audivisual timing (overestimation and underestimation) are related to differential activation and effective connectivity of contextually-sensitive regions (frontal cognitive-control, ventral attention, memory, and sensory-association centers). Striatal activation and functional connectivity also differed between intersensory and unimodal timing conditions.
- 52.Ulrich R, Nitschke J, Rammsayer T: Crossmodal temporal discrimination: assessing the predictions of a general pacemaker-counter model. Percept Psychophys 2006, 68:1140–1152. [DOI] [PubMed] [Google Scholar]
- 53.•.Hove MJ, Fairhurst MT, Kotz SA, Keller PE: Synchronizing with auditory and visual rhythms: an fMRI assessment of modality differences and modality appropriateness. NeuroImage 2013, 67:313–321. [DOI] [PubMed] [Google Scholar]; Challenges the common assumption that audition dominates vision in the context of timing. The results suggest that striatal activation depends on sensorimotor synchronization stability, rather than modality-specificity per se. Sensorimotor synchronization stability was manipulated for auditory and visual signals and shown to be associated with putamen activation.
- 54.Taniwaki T, Okayama A, Yoshiura T, Togao O, Nakamura Y, Yamasaki T, Ogata K, Shigeto H, Ohyagi Y, Kira J, Tobimatsu S: Functional network of the basal ganglia and cerebellar motor loops in vivo: different activation patterns between self-initiated and externally triggered movements. NeuroImage 2006, 31:745–753. [DOI] [PubMed] [Google Scholar]
- 55.Boecker H, Jankowski J, Ditter P, Scheef L: A role of the basal ganglia and midbrain nuclei for initiation of motor sequences. NeuroImage 2008, 39:1356–1369. [DOI] [PubMed] [Google Scholar]
- 56.Jankowski J, Scheef L, Huppe C, Boecker H: Distinct striatal regions for planning and executing novel and automated movement sequences. NeuroImage 2009, 44:1369–1379. [DOI] [PubMed] [Google Scholar]
- 57.•.Glover S, Wall MB, Smith AT: Distinct cortical networks support the planning and online control of reaching-to-grasp in humans. Eur J Neurosci 2012, 35:909–915. [DOI] [PubMed] [Google Scholar]; Provides recent evidence that planning of reach to grasp movements is uniquely modulated by the CSTC system together with regions of the parietal cortex. By contrast, the cerebellum uniquely governs the execution of movements along with the parietal and the sensorimotor cortices.
- 58.Harrington DL, Haaland KY: Sequencing in Parkinson’s disease: abnormalities in programming and controlling movement. Brain 1991, 114:99–115. [PubMed] [Google Scholar]
- 59.Chuma T, Faruque RM, Ikoma K, Mano Y: Motor learning of hands with auditory cue in patients with Parkinson’s disease. J Neural Transm 2006, 113:175–185. [DOI] [PubMed] [Google Scholar]
- 60.Nowak DA, Tisch S, Hariz M, Limousin P, Topka H, Rothwell JC: Sensory timing cues improve akinesia of grasping movements in Parkinson’s disease: a comparison to the effects of subthalamic nucleus stimulation. Mov Disord 2006, 21:166–172. [DOI] [PubMed] [Google Scholar]
- 61.Wu T, Wang L, Hallett M, Chen Y, Li K, Chan P: Effective connectivity of brain networks during self-initiated movement in Parkinson’s disease. NeuroImage 2011, 55:204–215. [DOI] [PubMed] [Google Scholar]
- 62.Michely J, Barbe MT, Hoffstaedter F, Timmermann L, Eickhoff SB, Fink GR, Grefkes C: Differential effects of dopaminergic medication on basic motor performance and executive functions in Parkinson’s disease. Neuropsychologia 2012, 50:2506–2514. [DOI] [PubMed] [Google Scholar]
- 63.•.Michely J, Volz LJ, Barbe MT, Hoffstaedter F, Viswanathan S, Timmermann L, Eickhoff SB, Fink GR, Grefkes C: Dopaminergic modulation of motor network dynamics in Parkinson’s disease. Brain 2015, 138:664–678. [DOI] [PMC free article] [PubMed] [Google Scholar]; Provides converging support for the CSTC system’s centralitiy in the control of voluntary actions in PD, showing that CSTC effective connectivity was weaker in patients OFF medications relative to healthy control participants.
- 64.Georgiou-Karistianis N, Long JD, Lourens SG, Stout JC, Mills JA, Paulsen JS: Movement sequencing in Huntington disease. World J Biol Psychiatry 2014, 15:459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ: Self-initiated versus externally triggered movements: 1. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain 1995, 118:913–933. [DOI] [PubMed] [Google Scholar]
- 66.Rowe JB, Hughes LE, Barker RA, Owen AM: Dynamic causal modelling of effective connectivity from fMRI: are results reproducible and sensitive to Parkinson’s disease and its treatment? NeuroImage 2010, 52:1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Palmer SJ, Li J, Wang ZJ, McKeown MJ: Joint amplitude and connectivity compensatory mechanisms in Parkinson’s disease. Neuroscience 2010, 166:1110–1118. [DOI] [PubMed] [Google Scholar]


