Abstract
Background:
Ventricular septal flattening, frequently present in pulmonary hypertension (PH), can be quantified using eccentricity index (EI). EI has not been evaluated by concurrent echocardiography and cardiac catheterization, and traditionally does not account for post-systolic septal flattening, often seen in PH. We evaluated left ventricular (LV) shape, including a novel measure of maximal EI (EIM) to account for post-systolic septal flattening, to establish the relationship with concurrent invasive hemodynamics.
Methods:
Echocardiography was performed at 2 institutions in 78 pediatric pulmonary hypertension patients during cardiac catheterization, and in 78 matched controls. From mid-papillary parasternal short-axis views, EI and right-to-left ventricular diameter ratio (RV:LV) were assessed.
Results:
EI and RV:LV measures were significantly increased in PH compared to controls. Shape measures correlated with invasive hemodynamics and PH outcome measures (PH-related hospitalization, functional class, medical therapy escalation, and brain natriuretic peptide). End-systolic EI of 1.16 best identified the presence of PH, while an EIM of 1.42 and 1.94 best identified half-systemic and systemic PH, respectively. An EIM of 1.27 was associated with an odds ratio of 16.16 (95% CI 6.62-39.46) for PH-related hospitalization or escalation of therapy.
Conclusions:
Using simultaneous echocardiography and catheterization in the largest study population to date, we demonstrate that EI and RV:LV ratio correlate with invasive hemodynamics and outcomes measures, and EI can accurately define those with clinically important PH. These measures strengthen the ability of echocardiography to identify and follow pediatric PH patients, especially in the absence of methods to quantify RV systolic pressures.
Keywords: Pulmonary hypertension, eccentricity index, septal flattening, pediatrics, echocardiography, cardiac catheterization, Imaging, Congenital Heart Disease
Introduction
Pulmonary hypertension (PH) is a progressive disease with substantial morbidity and mortality.1–3 Although cardiac catheterization remains the gold standard to diagnose PH, associated significant risks typically dictate that PH be routinely followed by echocardiography.3, 4 Tricuspid regurgitation (TR) peak velocity by echocardiography can estimate right ventricular (RV) systolic pressure (though may over- or underestimate).5 However, TR is not interpretable in about 30-54% of pediatric PH echocardiograms.5–9 When PH cannot be quantified in such settings, subjective means, like end-systolic ventricular septal flattening, are utilized to qualitatively grade PH. As the ratio of right-to-left ventricular (LV) pressure increases, septal curvature typically flattens and may even reverse curvature. However, significant variability exists with subjective assessment of septal flattening; eccentricity index (EI) quantifies septal flattening, reducing interobserver variability.8 Although first introduced by Ryan et al. in 1985, echocardiographic EI has never been fully validated with simultaneous invasive hemodynamic assessment, limiting our understanding of how EI relates to invasive hemodynamics, and thus its applicability in identifying and managing PH.10
To address this gap, we assessed EI at end-systole (EIS) and end-diastole (EID) during concurrent echocardiography and cardiac catheterization in a large pediatric PH population. Importantly, neither EID nor EIS account for prominent early diastolic leftward septal displacement, often seen in PH as a result of systolic and diastolic timing differences between the RV and LV.11, 12 To evaluate this phenomenon, a novel maximal EI (EIM) was assessed. We also evaluated the RV:LV diameter ratio at end-systole and end-diastole, which have also not been evaluated in a large cohort with simultaneous echocardiogram and cardiac catheterization.13 We hypothesized that EI and RV:LV diameter ratio measures would be related to worsening cardiopulmonary hemodynamics in pediatric PH, and accurately identify patients with clinically-significant PH.
Methods
The data that support the findings in this study are available from the corresponding author upon reasonable request.
Study Population
Between November 1, 2008 and April 25, 2016 at Children’s Hospital Colorado (CHCO) and the Hospital for Sick Children (SickKids) in Toronto, we prospectively performed simultaneous transthoracic echocardiography in children and adolescents during clinically-indicated right-heart catheterization for initial evaluation of suspected PH or routine follow-up of previously documented pre-capillary PH (resting mean pulmonary artery pressure [MPAP]≥25 mmHg and pulmonary capillary wedge pressure [PCWP] ≤ 15 mmHg at catheterization).14 We previously published systolic & diastolic strain results in a portion of these patients & controls.11, 15 The study was approved by the Institutional Review Board at both institutions. Informed consent was obtained for all patients.
We excluded conditions that might directly impact LV function aside from PH, including single ventricle physiology, active pacing, cardiomyopathies, heart transplant, (branch) pulmonary artery stenosis, uncontrolled systemic hypertension, ventricular septal defect occlusion devices, any left-sided obstructive lesion, PCWP >15 mmHg, or lack of raw echocardiography data preventing analysis. Ten patients were excluded, leaving 80 in our final cohort (63 from CHCO, 17 from SickKids); 2 CHCO patients were found to not have PH on initial diagnostic catheterization and were thus excluded from comparisons of PH patients versus controls, but included in correlative data between echocardiographic measures and invasive hemodynamics. Some previously diagnosed (by catheterization) PH patients were well-controlled on PH therapy, with MPAP<25 mmHg; these patients were still included in the PH cohort for comparisons with controls. No patient had a bundle branch block on electrocardiogram.
Right-Heart Catheterization
Under general anesthesia, a right-heart catheterization was performed by individuals blinded to echocardiographic measures. Cardiac index was measured by thermodilution (if no shunting) or calculated using the modified Fick equation (if shunt present); pulmonary and systemic blood flow were documented. We measured pressures in the right atrium, RV, pulmonary artery, left atrium (and/or PCWP), and femoral (systemic) artery. We calculated systemic and indexed pulmonary vascular resistances.
Echocardiography
During the baseline condition of cardiac catheterization, transthoracic echocardiography images were obtained from parasternal short-axis views at LV mid-papillary muscle window, using a General Electric Vivid 7 or E9 system (General Electric Healthcare, USA). Measurements were made on EchoPAC (General Electric Healthcare, USA) by individuals blinded to clinical and catheterization data, and averaged over 3-5 cardiac cycles to account for beat-to-beat variability. EIS and EID were calculated similar to the method described by Ryan et al., as the ratio of LV diameter parallel to the septum divided by diameter perpendicular to the septum (Figure 1).10 However, we measured the diameter from compact myocardium rather than endocardium (as demonstrated in figures from the original Ryan et al. manuscript) to avoid trabeculations confounding LV shape analysis.
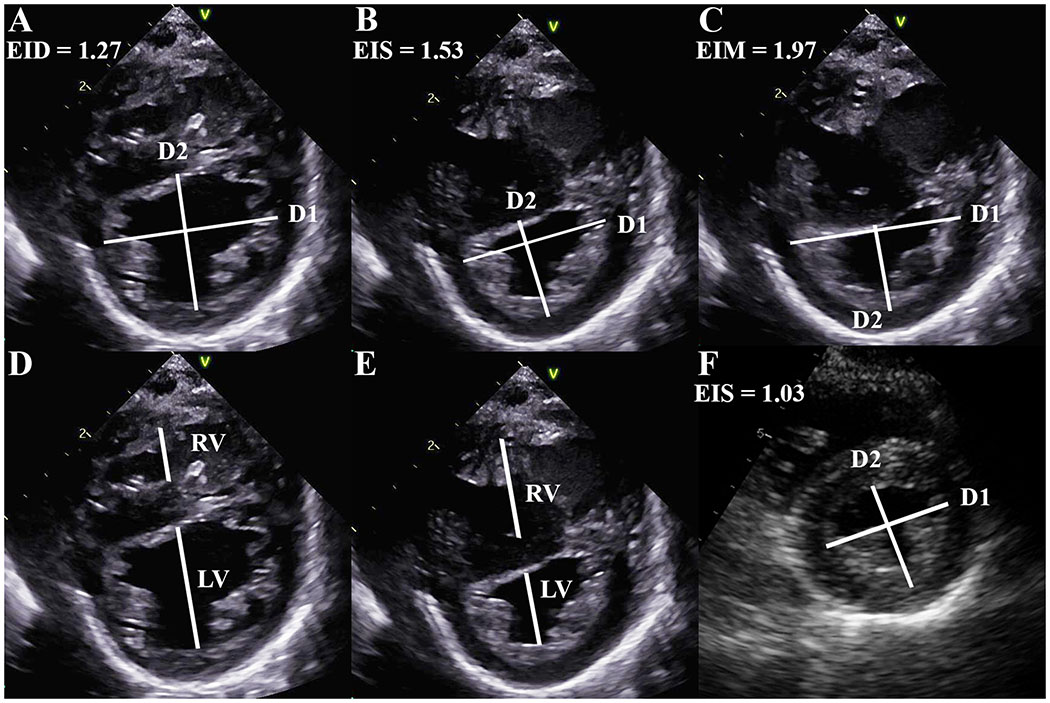
Figure 1. Left ventricular shape in pulmonary hypertension.
Eccentricity index, the ratio of the diameters (from compact myocardium) parallel (D1) and perpendicular (D2) to the ventricular septum, as measured at end-diastole (A, value 1.27), end-systole (B, value 1.53), and maximum septal displacement (C, value 1.97). Right:left ventricular diameter ratio at end-diastole (D) and end-systole (E). Maximal (and end-systolic) eccentricity index in control patient (F, value 1.03).
In PH, systole and diastole between the RV and LV can occur at different times, with RV diastolic inflow significantly delayed beyond LV inflow due to prolonged systole and/or isovolumic relaxation; this delay is related to increased afterload.11 Thus, end-systole and end-diastole can be different for each ventricle in PH, leading to confusion. In addition, variable electrocardiogram signals (onset of QRS, peak R wave, etc.) have been used to define the onset of systole. To simplify, we used the contractile pattern of the LV to define end-diastole and end-systole. End-diastole was defined as the frame immediately prior to LV contraction rather than by QRS on the electrocardiogram signal, to allow completion of LV filling due to atrial systole which may be missed due to electromechanical delay. End-systole was defined as the final frame of free-wall contraction, immediately prior to outward motion (relaxation) of the LV free-wall. In early LV diastole (outward motion of the free-wall), while the RV is still under increased pressure due to PH, there is often more profound leftward septal displacement.16 EIM, a new application of EI, captures this phenomenon and was calculated at the maximal period of septal flattening or leftward displacement. The point where the largest parallel:perpendicular diameter ratio was present was visualized and measured for EIM (Figure 1). If there was no additional septal displacement after end-systole, then EIM=EIS (present in 8 PH patients). The RV:LV ratio was calculated as described by Jone et al. (Figure 1).13
Control Population
Comparable measurements were obtained from the echocardiograms of 78 age- (within 12 months), gender-, and institution-matched healthy volunteers or children undergoing evaluation for murmur, chest pain, palpitations, syncope, or family history of CHD, with a normal echocardiogram (61 at CHCO, 17 at SickKids); one female PH patient was unable to be gender-matched, and so was matched with an age- and institution-matched male control.14 We excluded those with documented arrhythmias, chemotherapy exposure, family history of cardiomyopathy or bicuspid aortic valve, systemic illness, or documented/suspected genetic abnormality.17 No control patients underwent catheterization.
Outcomes Measures
Between November 1, 2008 and November 1, 2018, we documented death or placement in hospice care, heart and/or lung transplant or being listed for transplant, PH-related hospital admission, the need to escalate PH therapy based on the results of the cardiac catheterization, six-minute walk distance (6-MWD) if obtained within 6 weeks of catheterization, and World Health Organization (WHO) functional classification if defined within 6 months of catheterization. Brain natriuretic peptide (BNP) and N-terminal proBNP (NT-proBNP) were obtained during the cardiac catheterization in CHCO patients.
Reproducibility
A single observer (D.B.) analyzed all echocardiograms for all patients. To assess inter- and intraobserver variability of the novel EIM measure, eccentricity indices were measured in 20% of all echocardiograms by a second observer (C.C.), and by D.B. again, at least 6 months apart.
Statistical Analysis
Data were not normally distributed by the Shapiro-Wilk test. Continuous data are presented as median with interquartile range unless otherwise noted, and categorical data as frequencies (%). Comparative analysis was performed using Wilcoxon Mann-Whitney and Chi-square testing as appropriate. Matched data was analyzed for differences using Wilcoxon signed rank testing. Exact methods were utilized as necessary. Spearman correlation coefficients were used to assess relationships between echocardiographic LV shape parameters and invasive hemodynamics. Logistic regression was used to evaluate the relationship between left ventricular shape measures and dichotomous outcomes, including death, transplant, PH hospitalization, and need for therapy escalation. Cut-off sensitivity and specificity values for EI to predict PH using Youden’s index, and area under the curve (AUC) with 95% confidence intervals, were generated from receiver operator characteristic (ROC) curves. Threshold values were included in logistic regression analyses to determine ability to predict PH-related outcomes. A P value of <0.05 was considered statistically significant. All analyses were performed using SAS software, version 9.3 (SAS Corporation, Cary, NC).
Results
Patient Characteristics
Patient characteristics and hemodynamic data are presented in Table 1. In total, 78 PH patients and 78 controls were compared. Idiopathic PH was present in 38% (30/78). Most PH patients were female (52/78, 67%), and born with congenital heart disease (46/78, 59%); a patent foramen ovale was included for the purposes of identifying the potential for shunting, though was not used in classifying PH. Some patients had multiple defects. Many patients still had a shunt at the time of catheterization (34/78, 44%), though median pulmonary:systemic flow ratio was 1.00 (1.00-1.00). Most PH patients were on at least one PH therapy (72/78, 92%). All patients had systemic saturations >90%. PH etiology according to current PH classification is documented in Table 1.18
Table 1.
Patient Characteristics
| Pulmonary Hypertension (n = 78) | Controls (n = 78) | P Value | |
|---|---|---|---|
| Age, yr (range) | 12.0 (0-23) | 11.5 (0-23) | 0.98 |
| Female, n (%) | 52 (66.7%) | 51 (65.4%) | 0.87 |
| Weight, kg | 31.7(15.8-49.5) | 31.4 (18.8-54.8) | 0.53 |
| Height, cm | 134.0 (105.0-160.0) | 138.0 (107.0-162.0) | 0.52 |
| Body Surface Area, m2 | 1.11 (0.68-1.55) | 1.10 (0.74-1.59) | 0.62 |
| Congenital Heart Disease, n (%) | 45 (57.7%) | … | … |
| Atrial Septal Defect, Patent Foramen Ovale | 34 (43.6%) | … | … |
| Ventricular Septal Defect | 8 (10.3%) | … | … |
| Patent Ductus Arteriosus | 12 (15.4%) | … | … |
| Other | 5 (6.4%) | … | … |
| Shunt Present at Catheterization, n (%) | 34 (43.6%) | … | … |
| Pulmonary Hypertension Classification | |||
| Group 1 | 55 (70.5%) | ||
| Idiopathic Pulmonary Arterial Hypertension | 30 (38.5%) | … | … |
| Group II | 0 (0%) | ||
| Group III | 6 (7.7%) | ||
| Group IV | 2 (2.6%) | ||
| Group V | 15 (19.2%) | ||
| World Health Organization Class, n (%) | |||
| Not Available | 18 (23.1%) | … | … |
| I | 24 (30.8%) | … | … |
| II | 26 (33.3%) | … | … |
| III | 9 (11.5%) | … | … |
| IV | 1 (1.3%) | … | … |
| Pulmonary Hypertension Medical Therapy | 72 (92.3%) | … | … |
| Mean Pulmonary Artery Pressure, mm Hg | 31.0 (24.5-43.0) | … | … |
| Mean Pulmonary/Systemic Pressure Ratio | 0.58 (0.39-0.72) | … | … |
| Mean Left Atrial Pressure, mm Hg | 9.0 (7.0-10.0) | … | … |
| Mean Right Atrial Pressure, mm Hg | 7.0 (5.0-8.0) | … | … |
| Cardiac Index, L/min/m2 | 3.25 (2.83-3.77) | … | … |
| Pulmonary/Systemic Flow ratio (Qp:Qs) | 1.00 (1.00-1.00) | … | … |
| Indexed Pulmonary Vascular Resistance, WU·m2 | 6.20 (3.94-10.55) | … | … |
| Pulmonary/Systemic Vascular Resistance Ratio | 0.38 (0.25-0.61) | … | … |
Presented as n (%) or median (interquartile range), unless otherwise noted.
Echocardiographic Measures
LV echocardiographic measures are presented in Table 2. LV EID (1.17 [1.08-1.30] vs 1.00 [0.94-1.06], P<0.0001), EIS (1.27 [1.10-1.49] vs 0.99 [0.97-1.04], P<0.0001), and EIM (1.46 [1.23-1.70] vs 1.03 [0.99-1.09], P<0.0001) were all significantly increased in PH compared to controls (respectively), as were end-diastolic (0.51 [0.44-0.71] vs 0.33 [0.25-0.43], P<0.0001) and end-systolic RV:LV ratios (0.81 [0.58-1.19] vs 0.51 [0.32-0.63], P<0.0001) (respectively).
Table 2.
Left Ventricular Shape Measures
| Pulmonary Hypertension (n = 78) | Controls (n = 78) | P Value | |
|---|---|---|---|
| Diastolic Eccentricity Index | 1.17 (1.08-1.30) | 1.00 (0.94-1.06) | <0.0001 |
| Systolic Eccentricity Index | 1.27 (1.10-1.50) | 1.00 (0.97-1.04) | <0.0001 |
| Maximum Eccentricity Index | 1.46 (1.23-1.70) | 1.04 (0.99-1.09) | <0.0001 |
| Diastolic Right:Left Ventricular Ratio | 0.51 (0.44-0.71) | 0.33 (0.25-0.43) | <0.0001 |
| Systolic Right:Left Ventricular Ratio | 0.81 (0.59-1.18) | 0.51 (0.32-0.63) | <0.0001 |
Presented as median (interquartile range).
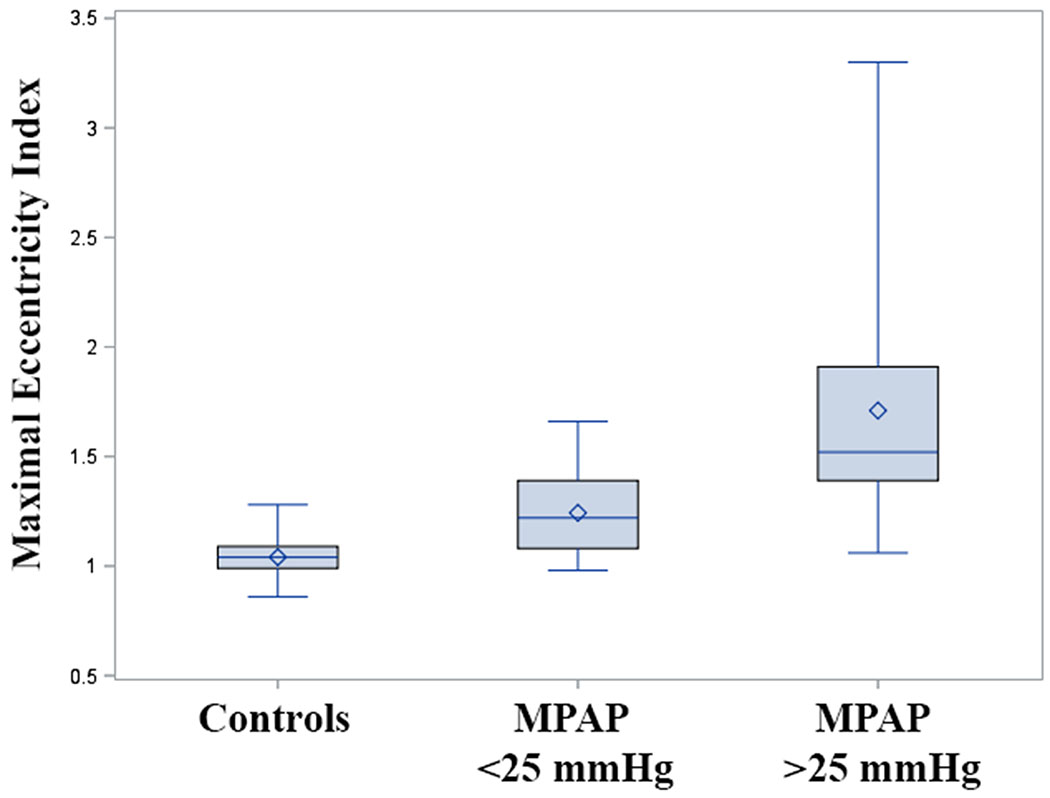
EIM was significantly different between controls, well-controlled PH patients (mean pulmonary artery pressure <25 mmHg), and poorly controlled PH (pressure ≥25 mmHg) (Figure 2).
Figure 2. Maximal eccentricity index in controls, well-controlled PH, and poorly-controlled pulmonary hypertension.
Box plot graph of maximal eccentricity index in control, well-controlled (MPAP<25mmHg) and poorly-controlled pulmonary hypertension (MPAP>25mmHg). Overall group difference P<0.0001; pairwise difference between all groups P<0.0001. Bar = median; diamond = mean. MPAP = mean pulmonary artery pressure.
LV Shape and Invasive Hemodynamic Relationships
Correlations between LV shape and invasive hemodynamic measures are presented in Table 3. All shape measures demonstrated moderate or strong correlation with invasive hemodynamics; EIM had the strongest correlation with most invasive hemodynamics.
Table 3.
Relationship Between Left Ventricular Shape and Invasive Hemodynamics
| Shape Measure | MPAP | P Value | MPAP/MAP | P Value | PVRi | P Value | PVR/SVR | P Value |
|---|---|---|---|---|---|---|---|---|
| Diastolic EI | 0.52 (0.33-0.67) | <0.0001 | 0.52 (0.32-0.67) | <0.0001 | 0.69 (0.54-0.79) | <0.0001 | 0.52 (0.32-0.67) | <0.0001 |
| Systolic EI | 0.62 (0.46-0.74) | <0.0001 | 0.66 (0.51-0.77) | <0.0001 | 0.64 (0.48-0.76) | <0.0001 | 0.64 (0.48-0.76) | <0.0001 |
| Maximal EI | 0.66 (0.50-0.77) | <0.0001 | 0.69 (0.54-0.79) | <0.0001 | 0.68 (0.53-0.78) | <0.0001 | 0.66 (0.50-0.77) | <0.0001 |
| Diastolic RV:LV Ratio | 0.51 (0.32-0.66) | <0.0001 | 0.50 (0.30-0.65) | <0.0001 | 0.68 (0.53-0.78) | <0.0001 | 0.50 (0.30-0.66) | <0.0001 |
| Systolic RV:LV Ratio | 0.59 (0.42-0.72) | <0.0001 | 0.60 (0.43-0.73) | <0.0001 | 0.61 (0.44-0.74) | <0.0001 | 0.63 (0.47-0.76) | <0.0001 |
Presented as correlation coefficient (95% confidence interval).
EI = eccentricity index; LV = left ventricle; MAP = mean (systemic) arterial pressure; MPAP = mean pulmonary arterial pressure; PVRi = indexed pulmonary vascular resistance; RV = right ventricle; SVR = systemic vascular resistance.
LV Shape to Determine the Presence of PH
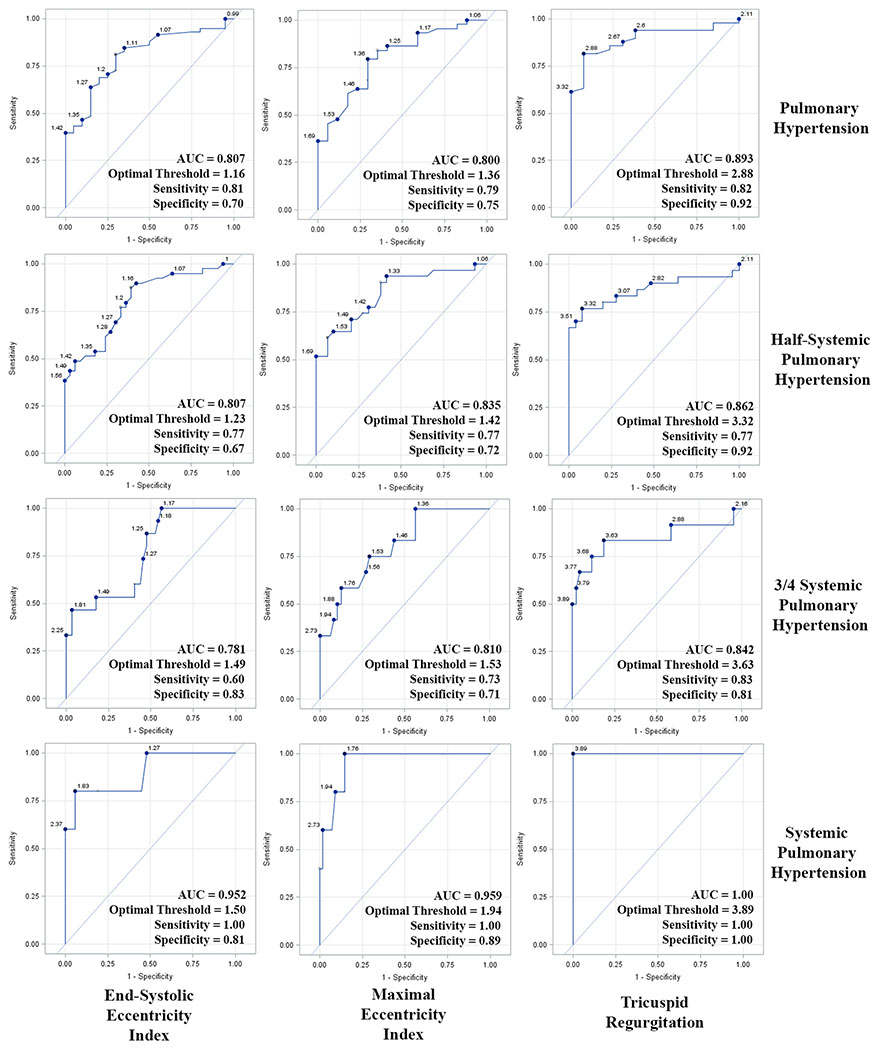
ROC curves to determine the optimal EI threshold values to define clinically important PH were generated (Figure 3). EIS (1.16, AUC=0.807 [0.705-0.910]) and EIM (1.36, AUC=0.800 [0.719-0.923]) had similar AUC’s to define PH (MPAP >25 mmHg). EIM had the largest AUC to define half-systemic (1.42, AUC=0.835 [0.742-0.927]), three-quarter-systemic (1.53, AUC=0.810 [0.694-0.927]), and systemic PH (1.94, AUC=0.959 [0.906-1.000]). When applied to the entire cohort, an EIM of 1.36 had an odds ratio of 9.00 (95% CI 2.80-28.96, P=0.0002) to predict PH (MPAP>25 mmHg); a value of 1.42 had an odds ratio of 8.00 (95% CI 2.80-22.82, P=0.0001) to predict half-systemic PH.
Figure 3. Receiver operator characteristic curves to define clinically important pulmonary hypertension.
Receiver operator characteristic curves to determine optimal end-systolic and maximal eccentricity index and tricuspid regurgitation values to define pulmonary hypertension, half-systemic, three-quarter systemic, and systemic pulmonary hypertension. AUC = area under the curve.
TR was not measurable in 17 PH patients (21.8%); all 6 patients with systemic PH had a quantifiable TR jet. Figure 3 demonstrates the ROC curves for TR to define PH, half-systemic PH, and systemic PH; the AUC was slightly larger than that of EIM for all 3 levels of PH.
LV Shape and Outcome Measures Relationships
Relationships between LV shape and outcome measures are presented in Table 4. There were 3 deaths (3.8%), and 3 patients status-post or actively listed for heart/lung transplant (3.8%); LV shape measures did not significantly relate to these rare events. All shape measures related to PH-related hospital admissions (34 patients; 66 total admissions), BNP, NT-proBNP and WHO functional class; only systolic shape measures correlated with the need to escalate therapy (22 patients required escalation of therapy). WHO functional class demonstrated the strongest correlation with LV shape measures.
Table 4.
Relationship Between Left Ventricular Shape and Outcome Measures
| Outcome Measure | Diastolic Eccentricity Index | P Value | Systolic Eccentricity Index | P Value | Maximum Eccentricity Index | P Value | Diastolic RV:LV Ratio | P Value | Systolic RV:LV Ratio | P Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Death | 3.49 (0.06-212.56) | 0.55 | 1.41 (0.08-25.68) | 0.81 | 1.35 (0.19-9.86) | 0.77 | 7.04 (0.31-236.85) | 0.28 | 2.91 (0.55-15.50) | 0.21 |
| Transplant | 0.011 (0-999.99) | 0.39 | 0.31 (0.01-58.95) | 0.66 | 0.83 (0.05-14.45) | 0.90 | 0.08 (0-212.34) | 0.53 | 0.74 (0.05-12.93) | 0.84 |
| PH Hospitalization | 17.77 (1.63-193.84) | 0.0183 | 15.31 (2.73-85.95) | 0.0019 | 5.57 (1.74-17.81) | 0.0038 | 11.14 (1.44-86.26) | 0.0209 | 3.99 (1.39-11.40) | 0.0099 |
| Therapy Escalation | 8.80 (0.59-131.96) | 0.12 | 4.32 (0.96-19.53) | 0.06 | 5.62 (1.42-22.24) | 0.0140 | 12.27 (0.83-181.80) | 0.07 | 3.99 (1.13-14.14) | 0.0321 |
| 6MWD | −0.37 (−0.62 - −0.05) | 0.0264 | −0.24 (−0.53-0.10) | 0.16 | −0.29 (−0.57-0.04) | 0.08 | −0.16 (−0.46-0.18) | 0.35 | −0.14 (−0.45-0.19) | 0.41 |
| BNP | 0.50 (0.27-0.67) | <0.0001 | 0.29 (0.04-0.51) | 0.0259 | 0.29 (0.03-0.52) | 0.0275 | 0.45 (0.22-0.64) | 0.0003 | 0.38 (0.13-0.58) | 0.0040 |
| NT-Pro BNP | 0.50 (0.27-0.67) | <0.0001 | 0.32 (0.07-0.54) | 0.0138 | 0.35 (0.10-0.56) | 0.0074 | 0.49 (0.26-0.67) | <0.0001 | 0.43 (0.19-0.63) | 0.0008 |
| WHO class | 0.45 (0.23-0.63) | 0.0002 | 0.58 (0.38-0.72) | <0.0001 | 0.62 (0.43-0.75) | <0.0001 | 0.49 (0.27-0.66) | <0.0001 | 0.63 (0.45-0.76) | <0.0001 |
Death, Transplant, PH Hospitalization, and Therapy Escalation were evaluated using logistic regression. Data are presented as odds ratio (95% confidence interval).
6MWD, BNP, pro-BNP, and WHO class were evaluated using Spearman correlation coefficients. Data are presented as correlation coefficient (95% confidence interval).
6MWD = 6-minute walk distance; BNP = brain natriuretic peptide; LV = left ventricle; NT-Pro BNP = N-terminal pro-BNP; PH = pulmonary hypertension; RV = right ventricle; WHO = world health organization.
An EIM of 1.52 was associated with an odds ratio of 4.10 (95% CI 1.60-10.54, P=0.0034) for PH-related hospitalization; an EIM of 1.70 was associated with an odds ratio of 5.83 (95% CI 1.66-20.52, P=0.0060) for escalation of medical therapy. An EIM of 1.27 was associated with an odds ratio of 16.16 (95% CI 6.62-39.46, P<0.0001) for either PH-related hospitalization or escalation of medical therapy.
Reproducibility
Intraobserver intraclass correlation coefficients (95% confidence interval) were as follows: EID 0.900 (0.840-0.938); EIS 0.945 (0.911-0.966); EIM 0.988 (0.980-0.993). Interobserver intraclass correlation coefficients (95% confidence interval) were as follows: EID 0.840 (0.749-0.900); EIS 0.948 (0.916-0.966); EIM 0.974 (0.957-0.984).
Discussion
Using simultaneous echocardiography and cardiac catheterization in the largest PH cohort to evaluate EI to date, we demonstrated that pediatric PH patients have abnormal maximal, end-systolic and end-diastolic EI and RV:LV diameter ratios, which are related to invasive cardiopulmonary hemodynamics, and are capable of defining clinically-important PH. All LV shape measures correlated with invasive hemodynamics, confirming the relationship with worsening PH, though EIM demonstrated stronger correlations for most measures than EIS or EID, highlighting the importance of a measure that accounts for timing abnormalities between the LV and RV in severe PH.11, 16 Diastolic shape measures correlated better with pulmonary vascular resistance and markers of atrial stretch, as was seen by Ploegstra et al; systolic shape measures correlated better with all other hemodynamics, PH-related admissions, the need to escalate therapy, and WHO functional class, similar to findings by Jone et al.7, 13 Although shape measures did not correlate with death or transplant, the paucity of these events in our study may account for lack of statistical significance.
Septal flattening and EI have been assessed in numerous PH studies, but the common limitation in all is that echocardiography and cardiac catheterization were performed hours, days, weeks, or even months apart, often under very different conditions, making data difficult to relate.5–7, 10, 19–23 Some don’t have cardiac catheterization data at all.8, 24, 25 Ours is the first study to evaluate numerous LV shape measures – including EI – with simultaneous cardiac catheterization and echocardiography, allowing for direct relationships to be defined for clinical use. One study evaluated EID with concurrent invasive hemodynamics during lung transplant, and demonstrated strong correlation with MPAP.26
When present, TR velocity is widely used to estimate pulmonary pressures in PH, though may over- or underestimate pulmonary pressures.27 Though a larger percentage of our patients had an interpretable TR Doppler than many other PH studies, perhaps related to a catheter across the tricuspid valve, 21.8% of patients still lacked an interpretable Doppler. EIM had similar ability to TR velocity to define clinically important PH, suggesting EIM could potentially identify PH in patients without a quantifiable TR jet; this needs to be validated.
Peak septal displacement in PH often occurs during early LV diastole, while the RV is still under higher pressure (prolonged ejection, post-systolic myocardial contraction, or isovolumic relaxation). EIM captures this phenomenon and had a stronger relationship with invasive hemodynamics than EIS. An EIS of 1.16, and EIM of 1.42, 1.53 and 1.94 yielded the largest AUC to define PH, half-, three-quarter-, and systemic PH, respectively; these values must be validated before clinical use. All patients with systemic PH had an EIM greater than 1.94. Importantly, EIM is significantly different between controls, well- and poorly-controlled PH patients, suggesting EIM could potentially differentiate PH patients on therapy with normal pulmonary artery pressures, from healthy individuals. That EIM better relates to invasive hemodynamics than EIS is notable given recent recommendations by pediatric PH expert guidelines to include EIS in evaluation of PH.28 EIM also potentially meets the call for establishing new, easy echocardiographic measures as metrics of clinical PH severity put forth by PH expert consensus groups and the 6th World Symposium on PH; EIM was highly reproducible as well.18, 28
There has been inconsistency in where EI is measured (compact myocardium versus endocardial border), and at what point in the cardiac cycle, which is problematic given RV and LV systolic and diastolic timing events often differ in PH (tricuspid inflow is significantly delayed compared to mitral inflow).11 Some have defined end-diastole as the frame before mitral valve closure, and end-systole as the frame before mitral valve opening.29 Others use the QRS signal to define end-diastole (some at the onset, and others at the peak of the R wave), which may not account for electromechanical delay, potentially excluding the full contribution of atrial systole to LV filling.5–8, 10, 19–21, 30–32 Some define end-systole as the smallest LV cavity size, which may not actually be at end-systole, but rather early LV diastole with post-systolic septal displacement.5–8, 10, 19–21, 25, 30–32 For clarity, we defined EID as the frame prior to initiation of LV contraction, EIS as the frame prior to relaxation of the free-wall, and EIM as the frame with maximal leftward septal displacement and largest parallel:perpendicular diameter ratio (be it at end-systole or early LV diastole). We thus deviated from prior methods of measuring EI to reduce heterogeneity, and better separate potentially clinically important events.
Subjective assessment of septal flattening (mild, moderate, severe) is likely more common in many echocardiography labs than quantification using EI, though has poor interobserver agreement, not seen with EI.8 When the relationship with hemodynamics and ability to identify PH are also considered, it suggests a benefit to adopting EI in a clinical setting to reduce variability and identify PH, especially in patients without interpretable TR jet.3
Limitations
We note that the definition of pulmonary hypertension changed after the study period presented here. In late 2018, the criteria for PH changed from MPAP 25 mmHg to 20 mmhg.18 Though this may affect patients that meet PH criteria, it would not affect the relationship of EI to invasive hemodynamics. Similarly, any restructuring of PH classification/groupings over the study period does not alter the comparison of echocardiographic shape measures to invasive hemodynamics. The heterogeneous study population (PH etiology, presence of congenital heart disease and/or shunts at the time of catheterization) may reduce generalizability to other populations, but accurately reflects the pediatric PH population. Selection bias was possible given PH patients were referred to a tertiary care center, though there was a mixture of well- and poorly-controlled patients. Similarly, many controls presented for evaluation of a cardiac concern, though were found to have a normal exam and echocardiogram. We attempted consistent catheterization protocols between sites, and though fluid-filled catheters were used at both, Millar catheters were used only in Toronto, where oxygen consumption was measured by mass spectrometry compared to estimation by LaFarge at CHCO. As these were clinically-indicated cardiac catheterizations in potentially sick pediatric patients, the anesthetic regimen was not dictated in the protocol, though all patients were under general anesthesia. This could potentially limit comparisons to unsedated controls, though strengthens the relationship with hemodynamics, which has been lacking in prior studies. WHO functional class was not available in all patients, but there were no significant differences in mean pulmonary artery pressures or eccentricity indices between those with and without WHO functional class documented. Lastly, EI is a relatively simplistic measure of the complex relationship between the RV and LV; another method of tracking ventricular shape throughout the cardiac cycle may better relate to invasive hemodynamics and identify PH.
Conclusions
Using simultaneous echocardiography and catheterization in the largest study population to date, we demonstrate that EI and RV:LV ratio correlate with invasive hemodynamics and outcomes measures, and can accurately define those with PH (EIS=1.16), half-systemic PH (EIM=1.42) and systemic PH (EIM=1.94). EIM and other shape measures strengthen the ability of echocardiography to identify and follow pediatric PH patients, especially in the absence of adequate Doppler measures to quantify RV systolic pressures.
Clinical Perspective.
We evaluated left ventricular shape measures in children and young adults with pulmonary hypertension by echocardiography performed simultaneously with cardiac catheterization, to allow direct comparisons between invasive hemodynamics and measures of left ventricular shape. In addition to the evaluation of traditional end-diastolic and end-systolic eccentricity indices, we evaluated a new measure: maximal eccentricity index. This novel measure quantifies and accounts for the substantial post-systolic septal flattening frequently seen in pulmonary hypertension. Although all eccentricity indices, as well as right:left ventricular diameter ratio, were abnormal in pulmonary hypertension patients and were related to invasive hemodynamics, maximal eccentricity index was the most deranged in pulmonary hypertension. In addition, it was able to differentiate healthy controls, well-controlled, and poorly controlled pulmonary hypertension patients. Notably, it was able to define clinically important pulmonary hypertension in patients nearly as well as the tricuspid regurgitation jet velocity. This is important given the frequency with which the tricuspid regurgitation jet cannot be measured in patients with pulmonary hypertension. As such, it is possible this simple, highly reproducible, echocardiographic measure can identify pulmonary hypertension in patients without other methods of quantifying right ventricular or pulmonary artery pressures. Further validation of these measures is necessary before clinical implementation. Lastly, left ventricular shape measures were shown to be related to pulmonary hypertension outcomes measures. Thus, left ventricular shape measures add to the utility of echocardiography to assess and follow pulmonary hypertension in a clinical setting.
Acknowledgements
We would like to thank Courtney Cassidy, RDCS for her work in data collection and participation in interobserver variability measurements.
Sources of Funding
NIH/NCATS Colorado CTSA Grant Numer UL1 TR002535; Jayden deLuca Foundation, Eagle, Idaho.
Abbreviations:
- PH
Pulmonary hypertension
- LV
left ventricle
- RV
right ventricle
- EI
eccentricity index
- EID
end-diastolic eccentricity index
- EIS
end-systolic eccentricity index
- EIM
maximal eccentricity index
- TR
tricuspid regurgitation
- CHCO
Children’s Hospital Colorado
- MPAP
mean pulmonary artery pressure
- PCWP
pulmonary capillary wedge pressure
- BNP
brain natriuretic peptide
- NT-proBNP
N-terminal pro brain natriuretic peptide
- 6-MWD
6-minute walk distance
- WHO
World Health Organization
- ROC
receiver operator characteristic
- AUC
area under the curve
Footnotes
Disclosures
No pertinent disclosures.
References
- 1.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–9. [DOI] [PubMed] [Google Scholar]
- 2.Ivy DD, Abman SH, Barst RJ, Berger RM, Bonnet D, Fleming TR, Haworth SG, Raj JU, Rosenzweig EB, Schulze Neick I, et al. Pediatric pulmonary hypertension. J Am Coll Cardiol. 2013;62:D117–26. [DOI] [PubMed] [Google Scholar]
- 3.Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, Hanna BD, Rosenzweig EB, Raj JU, Cornfield D, et al. Pediatric Pulmonary Hypertension: Guidelines From the American Heart Association and American Thoracic Society. Circulation. 2015;132:2037–99. [DOI] [PubMed] [Google Scholar]
- 4.Kreutzer J Catastrophic Adverse Events During Cardiac Catheterization in Pediatric Pulmonary Hypertension May Not Be So Rare. J Am Coll Cardiol. 2015;66:1270–2. [DOI] [PubMed] [Google Scholar]
- 5.Amsallem M, Sternbach JM, Adigopula S, Kobayashi Y, Vu TA, Zamanian R, Liang D, Dhillon G, Schnittger I, McConnell MV, et al. Addressing the Controversy of Estimating Pulmonary Arterial Pressure by Echocardiography. J Am Soc Echocardiogr. 2016;29:93–102. [DOI] [PubMed] [Google Scholar]
- 6.Averin K, Michelfelder E, Sticka J, Cash M, Hirsch R. Changes in Ventricular Geometry Predict Severity of Right Ventricular Hypertension. Pediatr Cardiol. 2016;37:575–81. [DOI] [PubMed] [Google Scholar]
- 7.Ploegstra MJ, Roofthooft MT, Douwes JM, Bartelds B, Elzenga NJ, van de Weerd D, Hillege HL, Berger RM. Echocardiography in pediatric pulmonary arterial hypertension: early study on assessing disease severity and predicting outcome. Circ Cardiovasc Imaging. 2015;8:e000878. [DOI] [PubMed] [Google Scholar]
- 8.Abraham S, Weismann CG. Left Ventricular End-Systolic Eccentricity Index for Assessment of Pulmonary Hypertension in Infants. Echocardiography. 2016;33:910–5. [DOI] [PubMed] [Google Scholar]
- 9.Mourani PM, Sontag MK, Younoszai A, Ivy DD, Abman SH. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics. 2008;121:317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan T, Petrovic O, Dillon JC, Feigenbaum H, Conley MJ, Armstrong WF. An echocardiographic index for separation of right ventricular volume and pressure overload. J Am Coll Cardiol. 1985;5:918–27. [DOI] [PubMed] [Google Scholar]
- 11.Burkett DA, Slorach C, Patel SS, Redington AN, Ivy DD, Mertens L, Younoszai AK, Friedberg MK. Impact of Pulmonary Hemodynamics and Ventricular Interdependence on Left Ventricular Diastolic Function in Children With Pulmonary Hypertension. Circ Cardiovasc Imaging. 2016;9:e004612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alkon J, Humpl T, Manlhiot C, McCrindle BW, Reyes JT, Friedberg MK. Usefulness of the right ventricular systolic to diastolic duration ratio to predict functional capacity and survival in children with pulmonary arterial hypertension. Am J Cardiol. 2010;106:430–6. [DOI] [PubMed] [Google Scholar]
- 13.Jone PN, Hinzman J, Wagner BD, Ivy DD, Younoszai A. Right ventricular to left ventricular diameter ratio at end-systole in evaluating outcomes in children with pulmonary hypertension. J Am Soc Echocardiogr. 2014;27:172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30:2493–537. [DOI] [PubMed] [Google Scholar]
- 15.Burkett DA, Slorach C, Patel SS, Redington AN, Ivy DD, Mertens L, Younoszai AK, Friedberg MK. Left Ventricular Myocardial Function in Children With Pulmonary Hypertension: Relation to Right Ventricular Performance and Hemodynamics. Circ Cardiovasc Imaging. 2015;8:e003260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedberg MK. Imaging Right-Left Ventricular Interactions. JACC Cardiovasc Imaging. 2018;11:755–771. [DOI] [PubMed] [Google Scholar]
- 17.Biner S, Rafique AM, Ray I, Cuk O, Siegel RJ, Tolstrup K. Aortopathy is prevalent in relatives of bicuspid aortic valve patients. J Am Coll Cardiol. 2009;53:2288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenzweig EB, Abman SH, Adatia I, Beghetti M, Bonnet D, Haworth S, Ivy DD, Berger RMF. Paediatric pulmonary arterial hypertension: updates on definition, classification, diagnostics and management. Eur Respir J. 2019;53:1801916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raymond RJ, Hinderliter AL, Willis PW, Ralph D, Caldwell EJ, Williams W, Ettinger NA, Hill NS, Summer WR, de Boisblanc B, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol. 2002;39:1214–9. [DOI] [PubMed] [Google Scholar]
- 20.Haddad F, Guihaire J, Skhiri M, Denault AY, Mercier O, Al-Halabi S, Vrtovec B, Fadel E, Zamanian RT, Schnittger I. Septal curvature is marker of hemodynamic, anatomical, and electromechanical ventricular interdependence in patients with pulmonary arterial hypertension. Echocardiography. 2014;31:699–707. [DOI] [PubMed] [Google Scholar]
- 21.Kassem E, Humpl T, Friedberg MK. Prognostic significance of 2-dimensional, M-mode, and Doppler echo indices of right ventricular function in children with pulmonary arterial hypertension. Am Heart J. 2013;165:1024–31. [DOI] [PubMed] [Google Scholar]
- 22.Schena M, Clini E, Errera D, Quadri A. Echo-Doppler evaluation of left ventricular impairment in chronic cor pulmonale. Chest. 1996;109:1446–51. [DOI] [PubMed] [Google Scholar]
- 23.Mori S, Nakatani S, Kanzaki H, Yamagata K, Take Y, Matsuura Y, Kyotani S, Nakanishi N, Kitakaze M. Patterns of the interventricular septal motion can predict conditions of patients with pulmonary hypertension. J Am Soc Echocardiogr. 2008;21:386–93. [DOI] [PubMed] [Google Scholar]
- 24.Puwanant S, Park M, Popovic ZB, Tang WH, Farha S, George D, Sharp J, Puntawangkoon J, Loyd JE, Erzurum SC, et al. Ventricular geometry, strain, and rotational mechanics in pulmonary hypertension. Circulation. 2010;121:259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson T, McCracken CE, Slesnick T, Kanaan U, Border WL, Sachdeva R. Quantitative Assessment of Ventricular Septal Contour for Estimation of Right Ventricular Pressure. Echocardiography. 2016;33:444–9; quiz 443. [DOI] [PubMed] [Google Scholar]
- 26.Katz WE, Gasior TA, Quinlan JJ, Lazar JM, Firestone L, Griffith BP, Gorcsan J 3rd. Immediate effects of lung transplantation on right ventricular morphology and function in patients with variable degrees of pulmonary hypertension. J Am Coll Cardiol. 1996;27:384–91. [DOI] [PubMed] [Google Scholar]
- 27.Groh GK, Levy PT, Holland MR, Murphy JJ, Sekarski TJ, Myers CL, Hartman DP, Roiger RD, Singh GK. Doppler echocardiography inaccurately estimates right ventricular pressure in children with elevated right heart pressure. J Am Soc Echocardiogr. 2014;27:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansmann G, Koestenberger M, Alastalo TP, Apitz C, Austin ED, Bonnet D, Budts W, D’Alto M, Gatzoulis MA, Hasan BS, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: The European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. 2019;38:879–901. [DOI] [PubMed] [Google Scholar]
- 29.King ME, Braun H, Goldblatt A, Liberthson R, Weyman AE. Interventricular septal configuration as a predictor of right ventricular systolic hypertension in children: a cross-sectional echocardiographic study. Circulation. 1983;68:68–75. [DOI] [PubMed] [Google Scholar]
- 30.Lammers AE, Haworth SG, Riley G, Maslin K, Diller GP, Marek J. Value of tissue Doppler echocardiography in children with pulmonary hypertension. J Am Soc Echocardiogr. 2012;25:504–10. [DOI] [PubMed] [Google Scholar]
- 31.McCrary AW, Malowitz JR, Hornick CP, Hill KD, Cotten CM, Tatum GH, Barker PC. Differences in Eccentricity Index and Systolic-Diastolic Ratio in Extremely Low-Birth-Weight Infants with Bronchopulmonary Dysplasia at Risk of Pulmonary Hypertension. Am J Perinatol. 2016;33:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aggarwal S, Natarajan G. Echocardiographic correlates of persistent pulmonary hypertension of the newborn. Early Hum Dev. 2015;91:285–9. [DOI] [PubMed] [Google Scholar]