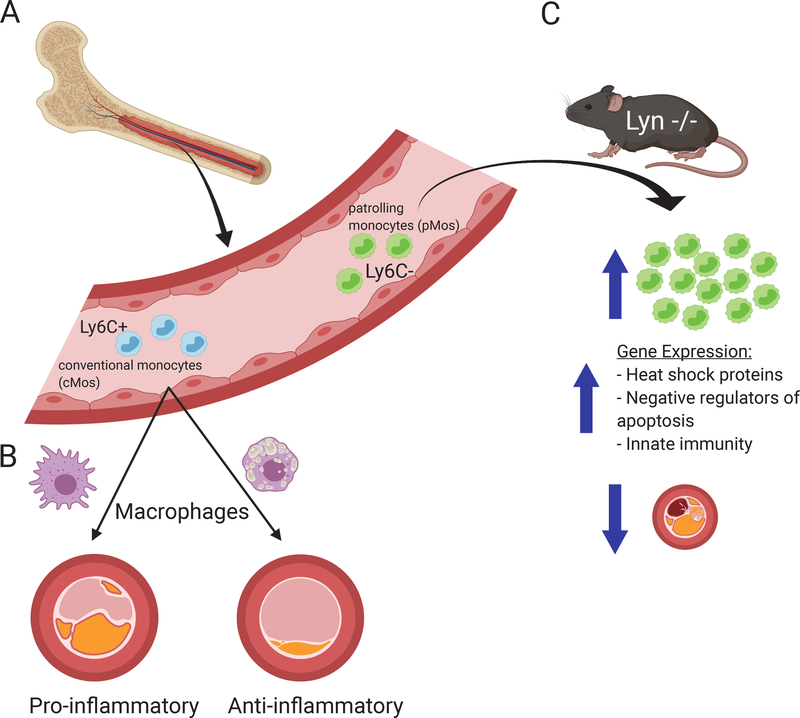
Monocytes contribute critically to the initiation, progression, and thrombus formation stages of atherosclerosis and myocardial infarction.1 Early arterial injury triggers an innate immune response, which promotes the entry of bone-marrow derived monocytes into the intima (Figure 1A).2 Risk factors for atherosclerosis that boost this recruitment of mononuclear phagocytes include hypercholesterolemia and the newly identified clonal hematopoiesis of indeterminate potential (CHIP).3 Infiltrating monocytes in the intima progress to lesional macrophages, and can proliferate in the intima where they promote plaque progression (Figure 1B). Monocyte and macrophage subsets secrete mediators such as cytokines and chemokines within the plaque and can also respond to systemic stimuli such as circulating pathogen-associated molecular patterns or to remote trauma such as myocardial infarction.4 At the same time, a class of resolving macrophages appear to promote plaque stabilization through efferocytosis, collagen production, and TGF-beta production.5 These disparate monocyte/macrophage roles suggest the presence of multiple subpopulations with distinct genetic markers and functions. Single-cell methods thus hold great promise for better understanding monocyte populations in the vasculature.
Figure 1.
Monocyte and macrophage subsets contribute to the initiation, progression, and thrombus formation stages of atherosclerosis. (A) Bone marrow-derived monocytes in circulation are classified as conventional (cMos) and patrolling (pMos) monocytes. During atherogenesis, the Ly6C+ cMos subpopulation infiltrates the intima, whereas the Ly6C- pMos subpopulation secretes inflammatory cytokines while patrolling the endothelium. (B) In the intima, pro-inflammatory macrophages contribute to plaque progression, while anti-inflammatory macrophages aid in plaque regression. (C) In the Roberts et al. study, genetic ablation of Lyn in mice leads to the expansion of pMos subpopulation only. The Lyn-deficient pMos have increased expression of genes that play a role in heat shock protein response, apoptosis inhibition, and innate immunity, compared to WT pMos. The Lyn-deficient mice are also protected against atherosclerosis.
Single-cell analysis of inflammatory cells in the context of atherosclerosis has quickly accelerated with innovations in droplet-based technology and computational analysis.6 Prior efforts in defining cell populations relied on the use of fluorescence-conjugated antibodies in conjunction with microscopy and flow cytometry. Droplet-based single-cell analysis allows for the transcriptional analysis of thousands of cells, which elucidates function in a way that surface markers cannot capture. The application of this technology to vascular disease has identified distinct endothelial cell populations and activated vascular smooth muscle cells associated with atherosclerosis.7, 8 Single-cell proteomic methods can now complement transcriptional analysis. Though it lacks high-throughput capabilities, single-cell mass cytometry (CyTOF) can interrogate expression of up to 40 proteins simultaneously in single cells using isotope-conjugated antibodies for mass spectroscopy analysis.
In this issue, Roberts et al. use multiple single-cell methods to demonstrate that deficiency in the Tyrosine-protein kinase Lyn (Lyn) skews monocytes towards the patrolling (pMos) phenotype in multiple tissues and limits atherosclerosis in mice (Figure 1C). Lyn is a member of the Src family of tyrosine kinases (SFK) and a well-established negative regulator of myeloid cell development and function.9 This study determined that loss of Lyn resulted in increased BM-derived monocyte survival in vitro and increased circulating pMos half-life in vivo. The expansion of pMos was age dependent, and seen in multiple tissues including blood, BM, spleen, and aorta. Though BM progenitors did not differ, cMos progenitors expanded in the spleen to suggest extramedullary hematopoiesis. With scRNA-seq and CyTOF the authors identify a gene expression signature for pMos that increases in the absence of Lyn.
This study is one of many recent applications of single-cell technology to understand monocyte heterogeneity. Using the massively parallel single-cell RNA sequencing (MARS-seq) technique, a recent study also identified Ly6C+ (cMos) and Ly6C- (pMos) monocytes in naïve mice.10 Roberts et al. could characterize better gene expression and heterogeneity within these populations because droplet-based single-cell RNA-seq allows analysis of a large number of cells compared with plate-based single cell that requires sorting for a population of interest. It would be of interest to expand this analysis to the monocytes that enter the plaque at the single cell level, as was recently done for monocyte-derived plaque macrophages defined by CX3CR1 expression.11 This latter study revealed that macrophages in plaque progression have more discernable subsets compared to macrophages involved in plaque regression. Whether the cellular heterogeneity within the cMos population reported by Roberts et al. persists in this Cx3CR1+ population or if the macrophages adopt new cellular identities after extravasation into the tissue’s inflammatory environment merits consideration in future studies.
In humans, circulating monocytes fall into similar subpopulations analogous to cMos (classical), pMos (non-classical), and intMos (intermediate) based on CD14 and CD16 surface marker expression.12 Single cell RNA-seq and CyTOF analysis of these populations reveals different expression profiles compared with the mouse data—suggesting that human monocytes have functional differences from those of mice or exhibit as yet uncharacterized further heterogeneity. For example, a recent CyTOF analysis of human monocytes identified eight distinct monocyte populations: four classical, three non-classical, and one intermediate monocyte population in healthy human blood.13 Interestingly, one subset of CD9+ non-classical monocytes appeared analogous to a pMos population in mice. When the patrolling properties of mouse Ly6C-/CD9+ monocytes were studied in a mouse femoral artery, only the CD9+ subset of monocytes could bind platelets. While the expanded population of pMos in the Roberts et al. study protected against atherosclerosis, these pMos did not display functional differences in oxLDL uptake or cell adhesion as seen in the earlier study. Functional assessment of pMos subsets will help to elucidate further their roles in atherosclerosis.
The human disease-relevance of the monocyte heterogeneity identified in mice is one avenue for further investigation.14 It remains unknown if the transcriptional signature of pMos in mice correlate with one or more monocyte subpopulation in humans. Identifying this protective population in humans with single-cell technology will be much more difficult given the heterogeneity between samples. It also remains unclear if the expansion of pMos are the sole explanation for the protective effects of Lyn deficiency. Although the authors show expansion of the pMos population in Lyn−/− mice, their single-cell data also demonstrate extensive transcriptional differences Lyn−/− pMos compared to WT. The protective effects of Lyn deficiency may extend beyond pMos expansion, and could include multiple other mechanisms.
Despite these limitations, Roberts et al. demonstrate the power of single-cell analysis to identify subpopulation changes in health and disease. Evidence conflicts regarding the association of circulating monocyte numbers with CVD in epidemiologic studies.15 New data shows that CHIP mutations, which may drive monocyte heterogeneity, confer increased risk of atherosclerosis and may also modestly affect total circulating monocyte numbers. Studies that make use of scRNA-seq can advance characterization of the transcriptional and functional heterogeneity that operates during atherosclerosis. We already can glean that the current labels we use for cell populations merely scratch the surface of cellular diversity, and certainly monocytes will be no different. Studies such as this one advance the field by making use of high-dimensional data to identify a protective population of cells that may be harnessed for the prevention and treatment of cardiovascular disease.
Acknowledgments
Funding
RMG is supported by NHLBI DP2HL15243, R03HL148483, and K08HL128810. VLK is supported by T32 HL007604. PL is supported by the National Heart, Lung, and Blood Institute (R01HL080472 and 1R01HL134892), the American Heart Association (18CSA34080399), and the RRM Charitable Fund.
Disclosures
RMG has received research support from Bayer. PL is an unpaid consultant to, or involved in clinical trials for Amgen, AstraZeneca, Esperion Therapeutics, Ionis Pharmaceuticals, Kowa Pharmaceuticals, Novartis, Pfizer, Sanofi-Regeneron, and XBiotech, Inc. PL is a member of scientific advisory board for Amgen, Corvidia Therapeutics, DalCor Pharmaceuticals, IFM Therapeutics, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Novartis, and XBiotech, Inc. Dr. Libby serves on the Board of XBiotech, Inc. Dr. Libby’s laboratory has received research funding in the last 2 years from Novartis.
References
- 1.Ghattas A, Griffiths HR, Devitt A, Lip GY and Shantsila E. Monocytes in coronary artery disease and atherosclerosis: where are we now? J Am Coll Cardiol. 2013;62:1541–51. [DOI] [PubMed] [Google Scholar]
- 2.Swirski FK, Robbins CS and Nahrendorf M. Development and Function of Arterial and Cardiac Macrophages. Trends Immunol. 2016;37:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med. 2017;377:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins C, Iwamoto Y, Thompson B, Carlson AL, Heidt T,. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabas I and Lichtman AH. Monocyte-Macrophages and T Cellset al in Atherosclerosis. Immunity. 2017;47:621–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regev A, Teichmann SA, Lander ES, Amit I, Benoist C, Birney E, Bodenmiller B, Campbell P, Carninci P, Clatworthy M, et al. The Human Cell Atlas. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalluri AS, Vellarikkal SK, Edelman ER, Nguyen L, Subramanian A, Ellinor PT, Regev A, Kathiresan S and Gupta RM. Single-Cell Analysis of the Normal Mouse Aorta Reveals Functionally Distinct Endothelial Cell Populations. Circulation. 2019;140:147–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wirka RC, Wagh D, Paik DT, Pjanic M, Nguyen T, Miller CL, Kundu R, Nagao M, Coller J, Koyano TK,. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by singleet al-cell analysis. Nat Med. 2019;25:1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts ME, Barvalia M, Silva JAFD, Cederberg R, Chu W, Wong A, Tai DC, Chen S, Matos I, Priatel JJ, et al. Deep Phenotyping by Mass Cytometry and Single Cell RNA-Sequencing Reveals LYN Regulated Signaling Profiles Underlying Monocyte Subset Heterogeneity and Lifespan. Circ Res. 2020; 126:xxx–xxx. [DOI] [PubMed] [Google Scholar]
- 10.Mildner A, Schönheit J, Giladi A, David E, Lara-Astiaso D, Lorenzo-Vivas E, Paul F, Chappell-Maor L, Priller J, Leutz A, et al. Genomic Characterization of Murine Monocytes Reveals C/EBPβ Transcription Factor Dependence of Ly6C− Cells. Immunity. 2017;46:849–862.e7. [DOI] [PubMed] [Google Scholar]
- 11.Lin JD, Nishi H, Poles J, Niu X, Mccauley C, Rahman K, Brown EJ, Yeung ST, Vozhilla N, Weinstock A, et al. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villani A-C, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, Griesbeck M, Butler A, Zheng S, Lazo S, et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. 2017;356:eaah4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamers Anouk AJ, Dinh Huy Q, Thomas Graham D, Marcovecchio P, Blatchley A, Nakao Catherine S, Kim C, McSkimming C, Taylor Angela M, et al. Human Monocyte Heterogeneity as Revealed by High-Dimensional Mass Cytometry. Arteriosclerosis, Thrombosis, and Vascular Biology. 2019;39:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murine Libby P. “Model” Monotheism: An Iconoclast at the Altar of Mouse. Circ Res. 2015;117:921–5. [DOI] [PubMed] [Google Scholar]
- 15.Meeuwsen JAL, de Vries JJ, van Duijvenvoorde A, van der Velden S, van der Laan SW, van Koeverden ID, van de Weg SM, de Borst GJ, de Winther MPJ, Kuiper J, et al. Circulating CD14(+)CD16(−) classical monocytes do not associate with a vulnerable plaque phenotype, and do not predict secondary events in severe atherosclerotic patients. J Mol Cell Cardiol. 2019. [DOI] [PubMed] [Google Scholar]