Abstract
Characterizing the effects of force fields generated by cells on proliferation, migration, and differentiation processes is challenging due to limited availability of non-destructive imaging modalities. Here, we integrate a new real-time traction stress imaging modality, Hilbert phase dynamometry (HPD), with spatial light interference microscopy (SLIM) for simultaneous monitoring of cell growth during differentiation processes. HPD uses holographic principles to extract displacement fields from chemically patterned fluorescent grid on deformable substrates. This is converted into forces by solving an elasticity inverse problem. Since HPD uses the epi-fluorescence channel of an inverted microscope, cellular behavior can be concurrently studied in transmission with SLIM. We studied the differentiation of mesenchymal stem cells and found that cells undergoing osteogenesis and adipogenesis exerted larger and more dynamic stresses than their precursors, with MSCs developing the smallest forces and growth rates. Thus, we develop a powerful means to study mechanotransduction during dynamic processes where the matrix provides context to guide cells towards a physiological or pathological outcome.
INTRODUCTION
Cells and their microenvironment interact through a complex combination of pushing and pulling forces that in turn affect processes such as cell adhesion, migration, proliferation and differentiation 1. The cytoskeletal network, which is made up by filamentous actin, intermediate filaments, and microtubules, mediates the transmission of forces within the cell 2. The cytoskeletal elements bind to focal adhesion (FA) proteins at the cell membrane, that in turn mediate interactions with the extra-cellular matrix (ECM) through cell surface integrins. The mechanical forces generated at these interfaces influence biochemical signaling, in a process termed mechanotransduction, which has important implications on cell fate, and downstream tissue form and function 3. A focal adhesion, thus, serves as both the point at which the cell may exert forces on its surroundings and as the cell’s window into mechanical changes occurring in the surrounding tissue, leading to adaptive changes within the cell. Understanding mechanotransduction is especially critical in cancer cell biology, where processes such as cell lamellipodia extension, migration, and angiogenesis, are mediated through interactions with the surrounding ECM and can dramatically influence the aggressiveness and metastatic potential of the cancer 4–7. In addition to pathological processes, during normal development, mechanochemical signals regulate stem cell lineage determination, where the ECM provides context to guide the integration of multiple cues from the microenvironment. Stem cells show a degree of lineage plasticity and can shift their state through interrogation of ECM mechanics and composition. The mechanical interactions between stem cells and their environment influences proliferation, migration and differentiation, towards the regulation of wound healing, tissue morphogenesis and homeostasis 8–11.
In recent years, significant progress has been reported in the development of quantitative techniques to measure cell generated forces (see Ref. 12 for a recent review). These methods can be broadly classified into two categories: active, i.e., based on measuring the response of the cell to application of external forces, and passive, built on measuring the substrate deformation due to intrinsic, cell-generated forces. Common methods within the first category are atomic force microscopy, optical tweezers, and magnetic tweezers 13–16. However, these methods suffer from the limitations in spatial sampling and restrictive thresholds for the measurable forces. Traction force microscopy techniques that have gained the most widespread adoption employ micropatterned pillars, textured substrates, and coated fluorescent beads. Traction forces from micropillar arrays are calculated from the bending of soft pillars of known mechanical properties 17,18. However, by restricting the cell adhesion cites to the locations of the micropatterned pillars, this technique is not an ideal representation of 2D cell culture. A digital holography based technique that also uses pillars to measure traction forces has been developed, however a more valuable application of this technique lies in studying the assembly and internal cell behavior around the adhesion sites19. Substrate deformation measured from textured PDMS substrates are an alternative method 20. This technique requires florescent tagging of the focal adhesion sites within the cell. A similar method looks at the displacement of topographically patterned dots, however the method has multiple shortcomings from computational assumptions including limited spatial resolution, and the requirement that one observe single cells, or, at least, cells with high degree of spatial separation 21. Another established approach involves the incorporation of fluorescently tagged beads within an elastic substrate to study the traction of multiple cells 22,23. However, this method requires the removal of cells from the substrate surface in order to obtain the initial configuration of the incorporated beads, which is a tedious and error-prone process. Recently, other fluorescence based approaches includes confocal traction force microscopy which relies on nanodrip-printed monocrystalline array of fluorescent quantum dots 24 or super-resolved traction force microscopy (STFM) which uses stimulated emission depletion (STED) microscopy to measure displacement of fluorescent beads25. These approaches alleviate some of the previous limitations, but at the same time rely on specialized, high-precision substrate preparation, require expensive setups, and retain the need for fluorescent particles. Holographic traction force microscopy measures traction force without the need of fluorescent partials yet still requires non-fluorescent beads embedded within polyacrylamide hydrogels thus retaining the need for tracking individual particles26.
Here, we present a multimodal microscopy-based approach that monitors cell growth using spatial light interference microscopy (SLIM) in transmission simultaneously with cell generated traction force monitoring in epi-fluorescence mode through a novel technique we developed called Hilbert phase dynamometry (HPD). SLIM27–30 performs mass measurements through quantitative phase imaging (QPI) of live mesenchymal stem cells in unperturbed culture under three different conditions: no treatment, adipogenic differentiation and osteogenic differentiation. QPI is an emerging field of label-free imaging that has found important applications in biomedicine 31. Phase sensitive methods have been applied before to studying dynamics in cellular systems32–34. Among other applications, studying cell growth has perhaps the broadest potential ramifications as it addresses this “long-standing question in biology”35. The label-free nature of SLIM measurements in the transmission channel of a conventional microscope allows for using the epi-fluorescence channel for force measurements using HPD. HPD measures forces exerted by cells in real-time, over extended periods of time. The cells are grown on flat, deformable substrates with a customized 2D fluorescent adhesion protein grid patterned at sub-cellular resolution. The in-plane displacement field is continuously measured with high spatial and temporal resolution. The adherent cell-induced strain field is contained in the 2D phase map of the complex analytical signal associated with the periodic grid. The key principle of HPD is rooted in calculation of displacement fields using the principles of phase reconstruction used in off-axis holography, as developed by Leith and Upatnieks in the 1960s’ 36 (for a review on phase reconstruction and imaging, see also 31). From the displacement field, we solve the inverse elasticity problem and extract a traction force vector field. Since the fluorescent grid is patterned for uniform sampling of the substrate, HPD eliminates the need for tracking individual particles. It can also monitor cell behavior continuously, without removing the cells from culture. Thus, through synchronized channel switching on the microscope, we are able to simultaneously measure cell growth and traction forces using SLIM and HPD respectively during stem cell differentiation. We used mesenchymal stem cells (MSCs) as a model since it is an adherent adult stem cell line that shows high responsivity to ECM properties22,37–39. Our results show that cells undergoing differentiation, osteogenesis and adipogenesis, exerted larger and more dynamic stresses than their precursor. Additionally, MSCs exert the smallest forces and have the lowest growth rates compared to their differentiated progeny. Thus, by using integrated HPD-SLIM system, we uncover the relationship between MSC generated traction, growth, and differentiation.
RESULTS
1. Technique development: Hilbert Phase Dynamometry (HPD)
Hilbert phase dynamometry relies on the extraction of displacement maps of adhesion proteins on a deformable substrate using holographic principles and solving the inverse elasticity problem. The preparation of substrates for retrieval of cell-induced deformation fields and thus, HPD-based force calculations, is illustrated in Figure 1. First, the 10kPa stiffness polyacrylamide (PA) gel is chemically activated and stamped with FITC-conjugated adhesion protein, to create a 9 μm period grid in both x and y directions (see Fig. 1 A–B, details on the gel preparation in Materials and Methods and Supplemental Information Section S3). The substrate is then uniformly exposed to non-fluorescent fibronectin to ensure homogeneous cell adhesion to the substrate (Fig. 1C). In this way FITC conjugated and non-FITC conjugated adhesion proteins are placed in alternating intervals, such that the cells do not sense the grid and, upon traction, generate bending of the substrate in both directions. The cells were seeded and allowed to settle on the substrate for an hour before imaging in epi-fluorescence mode for extraction of displacement maps (Fig. 1D).
Figure 1. Engineering of polyacrylamide hydrogels for traction force measurements using Hilbert Phase Dynamometry.
(A) Polyacrylamide hydrogels are activated with hydrazine hydrate to form hydrazide groups for conjugation of extracellular matrix proteins. (B) A fluorescent grid of 9μm spacing containing a mixture of 25μg/ml fibronectin and 25 μg/ml FITC-conjugated fibrinogen is stamped onto the activated hydrogel using a PDMS stamp. (C) Blank PDMS stamp with 25 μg/ml fibronectin is used to fill the hydrogel with non fluorescent adhesion proteins. (D) Cells are seeded onto the gel with uniform distribution of adhesion of proteins, and allowed to attach before measurements.
1.1. Calculation of displacement maps
The deformations in the substrate are measured from the phase of the 2D periodic signal associated with the fluorescence measurements of the grid (Fig 2A). The image of the 9μm periodicity fluorescent protein grid used in this study is shown in Fig. 2B and the absolute value of its Fourier transform is shown in Fig. 2C. Because the grid is not perfectly sinusoidal in shape, the Fourier transform of its image generates multiple orders along each direction laterally (x, y directions). However, if we only retain the first orders in both x and y directions, the analysis is equivalent to that of a perfect sinusoidal grid. The signal of interest along each direction has the form
| [1] |
where Rx,y are the sinusoidal fluorescence intensities (real-value of the signals) along x and y, ϕx,y(x,y) the respective phases that incorporate the displacement information, and β the spatial frequency of the grid, β=2π/9 rad/μm (See Supplemental Section 1 for more details). We apply a spatial frequency filter that selects the first order in the x and y direction as shown, in Figs. 2D and 2E, respectively. Inverse Fourier transform of the signals in Figs. 2D and 2E results in complex signals, namely the complex analytic signals associated with Rx,y. The concept of the complex analytic signal associated with a real optical field was exploited early on by Gabor40 and served as foundation for his development of holography41. These two complex signals, one for each direction, are derived from the fluorescent protein grid image via the following expressions
| [2a] |
| [2b] |
where P stands for principal value integral. The sequence of performing a Fourier transform of the real signal, spatial filtering, followed by inverse Fourier transform back to the spatial domain, is a Hilbert transform 42, which is captured in the HPD acronym. The argument of each signal provides the deformation of the grid at each point in the field of view along each direction, ϕx,y = arg(zx,y). The phase maps associated with the grid in Fig. 2B are shown in Figs. 2F and 2G, respectively. This phase information, in radians, is converted into spatial displacement, u, in microns, by noting that 2π radians corresponds to a displacement of a grid period, namely,
| [3] |
where Λ is the period, Λ = 9 μm. The minimum change in displacement was estimated as the phase noise in the time lapse images, which yielded a value of 0.23 rad in phase. Thus, the sensitivity for displacement measurements using this technique is 0.33 microns.
Figure 2. Schematic illustration of the process to reconstruct the lateral displacement map (X and Y displacements).
(A) The traction force f exerted by the cell on the substrate can be retrieved by measuring the resulting substrate deformation u. (B) The raw fluorescence image of the engineered hydrogel with 9μm spacing fluorescent adhesion protein grid (C) Fourier transform of the fluorescence image of the hydrogel (D-E) Zooming into the central region of the Fourier transform shows well separated orders in X and Y due to the periodicity of the grid. The spectrum is band pass filtered over the regions shown. (F-G) Inverse Fourier transform of selected orders in D, E generates the phase shift maps of displacements along the x and y axes.
1.2. Calculation of force fields
The force field is extracted from the deformation map by solving a linear Cerruti-type inverse problem (Fig. 2A and see Materials and Methods and Supplementary Section 2 for details) 43. Due to the linearity of the problem, the 2D displacement field u at position x and a disk distributed force of density f applied at an arbitrary position x’ are related via a simple matrix vector multiplication:
| [4] |
In Eq. 4, the 2×2 matrix Gdisk is a response (or transfer) function that describes the displacement response of the substrate, and is obtained by solving a Cerruti type problem 43 (see Figs. 3A–C and Supplemental Section 2 for details on the derivation). We used disk distributed forces to represent the traction forces applied by the cell at focal adhesion (FA) sites. If one were given the locations of all FAs and the tractions that they produce, the surface displacements are then computed from the sum of the displacements associated with each FA separately, as shown in Eq. (4). Given a measured displacement map of size N × N, and a grid of hypothetical FAs position of size M × M (with M ≤ N), the problem of finding the forces exerted by the cell on these FAs can be written as the following linear system:
| [5] |
Figure 3. Calculation of force field from lateral displacement maps.
(A) Quantitative phase image of a cell (adipocyte) on polyacrylamide hydrogel coated with 2D fluorescent fibronectin grid at 9μm periodicity at the beginning of the experiment. (B) X displacement map calculated from the phase map of the fluorescent grid deformations. (C) Y displacement map calculated from the phase map of the fluorescent grid deformations. (D) Cell traction force calculated from the X, Y displacement maps by solving an inverse linear Cerruti-type elasticity problem is overlaid on the image of the cell. N=3, 43 images collected from each distinct sample with SLIM system. This experiment was replicated three times.
In Eq. 5, represents the summed response functions between multiple displacements and forces position. Finally, the force field F is computed by inverting the system above using a least square approximation, which concludes the HPD procedure. Figure 3D shows the force field calculated by HPD overlaid on the image of the cell. Supplemental movies S1–6 illustrate the work-flow for real-time calculations of force fields from the displacement maps for several cells.
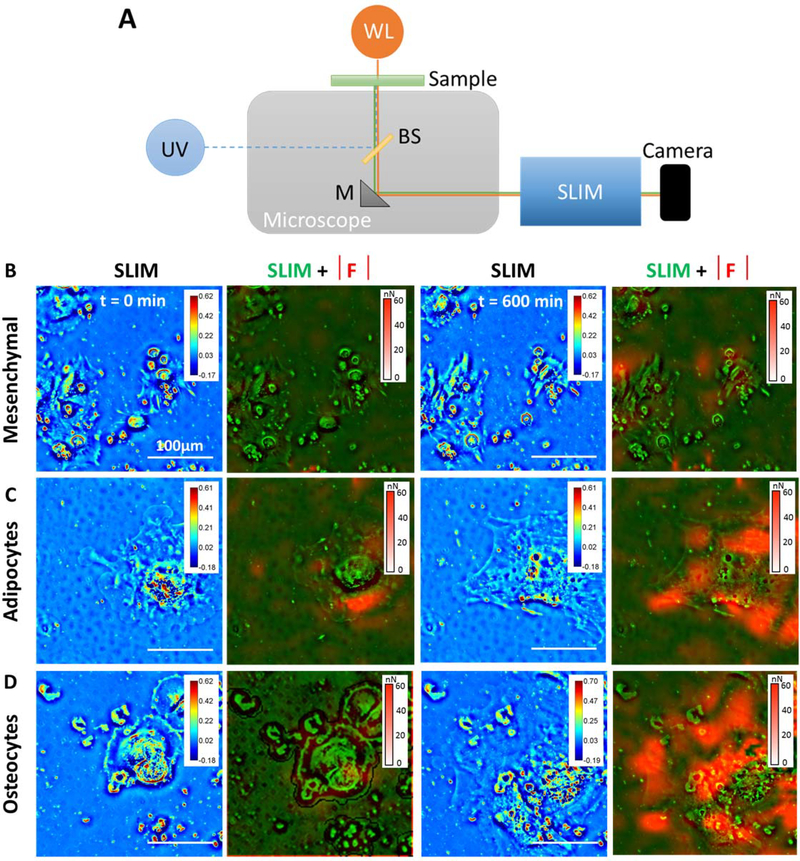
2. Microscopy: Synchronized cell mass and force measurements
The experimental setup for simultaneous measurement of cell mass and cell-induced traction forces, by software-based synchronization of SLIM and HPD are shown in Figure 4A. The measurements were performed with an inverted microscope, outfitted with both a SLIM module (Cell Vista SLIM Pro, Phi Optics, Inc.), and an epi-fluorescence optical train (see Fig. 4A and Materials and Methods for further information on the optics)44. The epi-fluorescence channel provides images of the FITC-conjugated adhesion protein grid, while SLIM renders quantitative phase images in trans-illumination, which can be further analyzed in terms of cell dry mass density 30. SLIM exploits the intrinsic refractive index contrast in live cells and is therefore, label-free enabling cell imaging over many hours without negative effects on cell viability. The SLIM and fluorescence channels are overlaid at the pixel level because of the common optical train used for measurements and hence, there is no need for computationally expensive registration algorithms.
Figure 4. Simultaneous measurements of cell growth using Spatial Light Interference Microscopy (SLIM) and traction force using Hilbert Phase Dynamometry (HPD).
(A) An inverted microscope with a common optical train is used for measurement of SLIM in transmission mode and displacement maps for HPD in the epi-fluorescence mode. (Row B) Mesenchymal stem cells imaged using SLIM and traction force (red overlay) calculated using HPD from the epi-fluorescence image of the same field of view overlaid on the phase image (green) at t = 0min and t=600 mins. (Row C) MSC differentiated into adipocytes imaged using SLIM and traction force (red overlay) calculated using HPD from the epi-fluorescence image of the same field of view overlaid on the phase image (green) at t = 0min and t=600 mins. (Row D) MSC differentiated into osteocytes imaged using SLIM and traction force (red overlay) calculated using HPD from the epi-fluorescence image of the same field of view overlaid on the phase image (green) at t = 0min and t=600 mins. N=3, 43 images collected from each distinct sample with SLIM system. This experiment was replicated three times.
The microscope can switch between the fluorescence and SLIM channels in 0.7 s, which makes it particularly appealing for studying traction and cell growth simultaneously. This multi-modal setup can be programmed to scan large fields of view in the lateral direction (x-y), acquire depth scans (z-stack), as well as acquire images over variable time lines (seconds to days) at pre-specified intervals (fraction of seconds to hours).
3. Application: Interaction between traction forces and growth during mesenchymal stem cell differentiation
We cultured bone marrow derived mesenchymal stem cells (MSCs), and then subjected them to media containing soluble supplements supporting adipogenesis or osteogenesis for 1 week (see Materials and Methods for details on the differentiation process). We chose to study MSCs because these cells are a promising avenue for autologous therapy, and no technique to date has been able to relate cell-matrix traction and growth during lineage specification. Figure 4 demonstrates that displacement and dry mass density maps can be obtained simultaneously and quantitatively by our method.
Previous reports have shown changes in cytoskeleton structure and contractility in MSCs undergoing osteogenesis or adipogenesis 45, which could lead to changes in focal adhesions and traction force 46. In order to test the sensitivity of our system, we seeded patterned hydrogels with MSCs that were exposed to either basal, osteogenic, or adipogenic media for one to two weeks. To confirm differentiation of MSCs, cells were stained after one week in differentiation media with Oil Red O to confirm adipogenesis and with alkaline phosphatase to confirm osteogenesis. Figure 4 illustrates the concomitant measurements of traction and SLIM acquired over 10 hours, with a temporal sampling of 15 minutes. There are clear morphological differences between the three cell types. In particular, the MSCs are significantly smaller in size. The overlays between SLIM and the magnitude of the force field show that the forces applied by the MSCs are the smallest. The results also indicate that the forces exerted by the cells become stronger with time.
Histological staining of MSCs exposed to the different media formulations demonstrates the appearance of alkaline phosphatase in the osteogenic conditions and accumulation of lipid droplets in the adipogenic conditions (Figure 5C). Supplemental movies S7–S9 illustrate the real-time overlay between the cell mass measurements using SLIM and force measurements using HPD in MSCs, adipocytes and osteoblasts. The histogram of all the measured forces (Figure 5A) indicate that the mesenchymal stem cells apply the lowest mean force and also display the narrowest spread in force magnitude (22.9±17.1nN). The largest mean tractions are produced by the adipocytes (51.5±39.2nN) followed by cells undergoing osteogenesis (36.3±30.4nN). At the same time, the lowest dry mass growth was shown by the MSCs and the highest by the osteoblasts (Figure 5B).
Figure 5. Cell lineage specific simultaneous traction force and growth calculations.
(A) Histograms showing the distributions of cell traction forces associated with mesenchymal stem cells (black), adipocytes (red), and osteoblasts (purple), across the entire experiment. The average magnitude of force exerted by adipocytes was the highest and that of mesenchymal stem cells was the lowest. Differentiated cells demonstrated higher traction force magnitude and dynamic force distribution. N=3, 43 images collected from each distinct sample with SLIM. This experiment was replicated three times. (B) Growth curves displaying the relative cell mass of mesenchymal stem cells, adipocytes and osteoblasts longitudinally through the experiment. Differentiated cells had higher growth rates than mesenchymal stem cells. N=3, 43 images collected from each distinct sample with SLIM. This experiment was replicated three times.(C) At the end of the experiment, cells were stained with Oil Red O+ (red), an adipogenic marker, and ALP+ (purple), a marker for osteogenesis, to verify lineage-specific cell differentiation. N=3, image collected from distinct samples with phase contrast microscope. This experiment was replicated three times. Plots shown represent average of three distinct samples. Error bars indicate standard deviation.
DISCUSSION
Understanding cell mechanotransduction is important for discerning matrix structure-cell function relationships underlying health and disease. Despite the crucial role of mechanochemical signaling in phenomena such as cell migration, proliferation, and differentiation, measuring the cell-generated forces at the interface with the extracellular matrix remains challenging. An ideal method would provide continuous, non-destructive images of the force field applied by cells, over broad spatial and temporal scales while also allowing the study of other native cell behavior. Our combined approach of SLIM and HPD enables simultaneous measurement of changes in cell mass and dynamic traction in real time during the initial stages of lineage specification. This advance provides the first technique where dynamic interactions of cells and their matrix can be queried during cell and tissue level processes in situ. Such studies can be conducted over a time period of up to a few days. While some studies have shown that certain cell lines might be vulnerable to blue light exposures over extended time periods, other studies have been able to measure robust cell growth over extended time periods30,47,48. This might be a confounding factor that could be addressed in future light tolerance studies for specific cell lines and using that information to adjust interval times between measurements. Another imaging technique was recently shown to detect adhesion points between cells and the substrate49,50. Results from such modalities are complementary to SLIM-HPD approach as they can inform grid periodicity for more accurate traction force measurements specific to the cell type of interest.
Recent studies using beaded polyacrylamide hydrogels for traction force microscopy have demonstrated that the magnitude of traction forces are higher when the gels are conjugated with fibronectin, as opposed to laminin or collagen 22,51. Therefore, we conjugated fibronectin in patterned grids for our combined SLIM-HPD studies on cellular behavior during differentiation processes. Cells exposed to osteogenic supplements, including ascorbic acid, β-glycerophosphate, and dexamethasone, exerted higher traction stress over time compared to MSCs cultured under standard growth media. Cells exposed to adipogenic supplements, including indomethacin, insulin, dexamethasone, and isobutylmethylxanthine, exerted significantly higher traction stress compared to both MSCs in growth media and those undergoing osteogenesis. Previous work has demonstrated increased traction stress exerted by cells on microposts during the initial stages of adipogenesis and osteogenesis compared to MSCs 46, thus supporting our observations of increased and more dynamic traction forces during cell differentiation. Fu et. al. observed an initial spike in traction forces followed by a rapid decay to basal levels for cells undergoing adipogenesis (over 7 days; micropost arrays)46. However, our study showed that cells undergoing adipogenesis exerted higher average traction compared to cells undergoing osteogenesis and those under basal conditions. We attribute this variance to differences in experimental conditions: we trypsinized MSCs after 1 week in differentiating media, followed by a transfer to patterned substrates for HPD and SLIM measurements under normal media conditions. We performed our analysis in the absence of hormones to aid the unambiguous assessment of cell generated force as a function of cell state. We believe that the observed differences in cell traction force is related to the evolution of mechanosensing machinery and the actomyosin network that has previously been shown to accompany specification to adipocyte and osteoblast lineages 52
Previous studies have demonstrated enhanced proliferation rates in MSCs undergoing differentiation, with the supplements dexamethasone and ascorbic acid playing a clear role in this phenomenon 53,54. Multipotent MSCs are known to show a degree of quiescence with low division rates when cultured in niche-mimetic conditions 38,54,55. We observed enhanced cell dry mass growth with adipogenesis > osteogenesis > basal conditions, which is consistent with known relationships between differentiation and proliferation. By simultaneously quantitating traction force using HPD and measuring cell growth with SLIM, we demonstrate the superior reporting capability of this multi-modal imaging approach. In our present iteration of this technique, we measure in-plane displacements due to the 2D nature of the fluorescence grid and single focal plane measurements. In principle, this technique can be extended for measurement of out-of-plane displacement by using a 3D fluorescence grid.
This multi-modal SLIM-HPD approach enables in situ tracking of relationships between extracellular motif recognition, force transduction, and specific bioactivities including growth and differentiation. Thus, we anticipate that our technique will improve understanding of mechanotransduction, especially the interplay between growth and motility.
Materials & Methods
Gel Preparation
10kPA polyacryamide hydrogels were fabricated by mixing 5% polyacrylamide (Sigma Aldrich) and 0.15% bis-acylamide (Sigma Aldrich) as previously described (Tse & Engler, 2010). 0.1% Ammonium Persulfate (APS, Sigma Aldrich) and 0.1% Tetramethylenediamine (TEMED, Sigma Aldrich) was added to the acrylamide mixture and pipetted onto a hydrophobically treated glass slide (Fisher). An amino-silanized glass cover slip was then flipped onto the solution and allowed to incubate for 20 minutes. Gels were then lifted off and immersed in 55% hydrazine hydrate (Fisher) for two hours to convert amide groups to reactive hydrazide groups, followed by immersion in 5% glacial acetic acid for one hour. Gel stiffness was confirmed with AFM as previously described 56. In order to prepare the substrates for protein patterning and cell adhesion, hydrazine hydrate was used to convert PAAm amide groups to reactive hydrazide groups allowing for the conjugation of ECM proteins via coupling of aldehyde groups formed after oxidation with sodium periodate (Fig. 1A).
Gel Patterning
A patterned master of photoresist (SU-8, Microchem) was created via UV light through a laser printed mask. Polydimethysiloxane (PDMS, Polysciences, Inc) was then polymerized on top of the master to create a stamp with 9 μm spaced grids, such that FITC conjugated and non-FITC conjugated adhesion proteins were placed in alternating 9 μm intervals along both the X and Y axes. In principle, there is no limitation on grid periodicity provided that it can be resolved by the imaging system. A mixture of 25μg/ml of fibronectin and 25 μg/ml of FITC conjugated fibrinogen was incubated with Sodium Periodate (Sigma Aldrich) for 20 min to yield free aldehydes. This incubation took place on top of the patterned PDMS stamp for 30 mins, air dried and then applied to the surface of the hydrogel, which had been dried in room temperature for 40 minutes (Fig. 1B). Next, 25 μg/ml fibronectin on a blank PDMS stamp was applied onto the hydrogel, following the same procedures as the previous step (Fig. 1C). By following these procedures, we obtained a uniform distribution of adhesion proteins on the gel surface for attachment of cell with periodic regions displaying fluorescence signal. The coverslip with the gel was then glued to the bottom of a glass-bottom cell culture dish (MatTek) at two points using tissue adhesion glue (Liquid bandage, CVS).
Cell Culture and Staining
Human mesenchymal stem cells (MSC, Lonza) were allowed to grow until they reached 70% confluency and then seeded onto a 6-well plate to initiate differentiation processes. The cells were cultured in MSC growth media (low glucose DMEM, 10% FBS, 5% Pen/Strep, Gibco), adipogenic media (Lonza), or osteogenic media (Lonza) for one week. Adipogenic media was rotated between induction and maintenance every 3 days. Cells were then lifted off the substrate with 0.25% trypsin (Sigma Aldrich) and seeded onto glass-bottom dishes (Fig. 1D) and imaged using the multi-modal SLIM system. At the end of imaging, to confirm cell lineage, the cells were fixed with 4% paraformaldehyde (PFA) for 20 minutes and incubated in 60% isopropanol for 5 min followed by immersion in Oil Red O working solution (3:2; 300 mg/mL Oil Red O in isopropanol:DI water, Sigma Aldrich) for 10 min and then BCIP/NBT (Sigma Aldrich) for 10min (Fig 5C).
Multi-modal SLIM/ Fluorescence Imaging System
Spatial light interference microscopy (Cell Vista SLIM Pro, Phi Optics, Inc.) is a QPI system that operates as an add-on module to an existing commercial phase contrast microscope 27,44,57. The back focal plane of the phase contrast objective is projected onto a liquid crystal phase modulator, where programmable phase rings introduce 3 additional phase shifts, in increments of π/2, between the scattered and un-scattered light transmitted through the sample. The phase is computed in real-time using the corresponding intensity images. Using software developed in-house, the imaging modality can be switched between phase and various fluorescence channels. Thus, we are able to obtain quantitative phase images for cell mass measurements and FITC images for measurement of the deformation fields within the same field of view.
Mesenchymal stem cells, adipocytes and osteocytes placed on deformable substrates with fluorescent protein grids were imaged using the phase and FITC module on the SLIM system using a 20X/0.45NA objective. The cells were imaged for 12 hours at 15 minute intervals. Typically, 6–8 fields of view were selected from each plate for imaging. The FITC image was taken at the plane of focus for the protein stamp, while the SLIM data were recorded as z-stacks with 2 frames above and below the plane of focus. This ensures longitudinal integration of dry mass of the cell along its entire thickness. The maximum phase projection through the SLIM z-stack at each time point accounts for changes in cell structure during longitudinal studies. Cell mass was calculated from the phase image using the following relationship, as described in detail in 48,58:
| [5] |
where λ is the central wavelength of the light source, φ(x,y) is the phase value of the corresponding pixel and γ = 0.2ml/g, is the refractive index increment of protein 59. Since the mass from multiple cells are averaged for analysis, the mass of each cell at each time point was calculated relative to the first time point (relative cell mass). With this procedure, we eliminate the possibility of a few cells with larger mass dominating the mean mass measurements and therefore, growth trends.
Code Availability
The code used in this study is available from the corresponding author upon reasonable request.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary Material
AKNOWLEGEMENT
This work was supported by National Science Foundation (NSF) Grants CBET-0939511 STC, DBI 14-50962 EAGER, IIP-1353368 (to G.P.), DMR-1309188 (to A.J.L.), 1454616 CAR (to K.K.), and National Institutes of Health, HL12175 (to K.K.). Y.L. was supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE – 1144245.
REFERENCES
- 1.Boal DH Mechanics of the cell. (Cambridge University Press, 2002). [Google Scholar]
- 2.Wang N, Butler JP & Ingber DE Mechanotransduction across the cell surface and through the cytoskeleton. Science 260, 1124–1127 (1993). [DOI] [PubMed] [Google Scholar]
- 3.Johnson WA, Harley ACB & (eds.). Mechanobiology of cell-cell and cell-matrix interactions. (Springer, 2011). [Google Scholar]
- 4.Wolf K et al. Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J Cell Biol 201, 1069–1084, doi: 10.1083/jcb.201210152 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker KK et al. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. Faseb J 16, 1195–1204 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Stromblad S & Cheresh DA Cell adhesion and angiogenesis. Trends Cell Biol 6, 462–468 (1996). [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Abdeen AA, Wycislo KL, Fan TM & Kilian KA Interfacial geometry dictates cancer cell tumorigenicity. Nature Materials In press, doi:DOI: 10.1038/NMAT4610 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Yang JT, Rayburn H & Hynes RO Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development 121, 549–560 (1995). [DOI] [PubMed] [Google Scholar]
- 9.Juhasz I, Murphy GF, Yan HC, Herlyn M & Albelda SM Regulation of extracellular matrix proteins and integrin cell substratum adhesion receptors on epithelium during cutaneous human wound healing in vivo. Am J Pathol 143, 1458–1469 (1993). [PMC free article] [PubMed] [Google Scholar]
- 10.Frith JE, Mills RJ, Hudson JE & Cooper-White JJ Tailored integrin-extracellular matrix interactions to direct human mesenchymal stem cell differentiation. Stem Cells Dev 21, 2442–2456, doi: 10.1089/scd.2011.0615 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thannickal VJ et al. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem 278, 12384–12389, doi: 10.1074/jbc.M208544200 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Polacheck WJ & Chen CS Measuring cell-generated forces: a guide to the available tools. Nat Methods 13, 415–423, doi: 10.1038/nmeth.3834 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alonso JL & Goldmann WH Feeling the forces: atomic force microscopy in cell biology. Life Sci 72, 2553–2560, doi: 10.1016/S0024-3205(03)00165-6 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Veigel C & Schmidt CF Moving into the cell: single-molecule studies of molecular motors in complex environments. Nat Rev Mol Cell Bio 12, 163–176, doi: 10.1038/nrm3062 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Bausch AR, Moller W & Sackmann E Measurement of local viscoelasticity and forces in living cells by magnetic tweezers. Biophys J 76, 573–579, doi: 10.1016/S0006-3495(99)77225-5 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Style RW et al. Traction force microscopy in physics and biology. Soft Matter 10, 4047–4055, doi: 10.1039/c4sm00264d (2014). [DOI] [PubMed] [Google Scholar]
- 17.Schoen I, Hu W, Klotzsch E & Vogel V Probing cellular traction forces by micropillar arrays: contribution of substrate warping to pillar deflection. Nano Lett 10, 1823–1830, doi: 10.1021/nl100533c (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan JL et al. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc Natl Acad Sci U S A 100, 1484–1489, doi: 10.1073/pnas.0235407100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fusco S et al. Nanomechanics of a fibroblast suspended using point-like anchors reveal cytoskeleton formation. Rsc Adv 6, 24245–24249, doi: 10.1039/c5ra26305k (2016). [DOI] [Google Scholar]
- 20.Balaban NQ et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol 3, 466–472, doi: 10.1038/35074532 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Polio SR, Rothenberg KE, Stamenovic D & Smith ML A micropatterning and image processing approach to simplify measurement of cellular traction forces. Acta Biomater 8, 82–88, doi: 10.1016/j.actbio.2011.08.013 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, Abdeen AA, Tang X, Saif TA & Kilian KA Geometric guidance of integrin mediated traction stress during stem cell differentiation. Biomaterials 69, 174–183, doi: 10.1016/j.biomaterials.2015.08.005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang X, Tofangchi A, Anand SV & Saif TA A novel cell traction force microscopy to study multi-cellular system. PLoS Comput Biol 10, e1003631, doi: 10.1371/journal.pcbi.1003631 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergert M et al. Confocal reference free traction force microscopy. Nat Commun 7, 12814, doi: 10.1038/ncomms12814 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colin-York H et al. Super-Resolved Traction Force Microscopy (STFM). Nano Letters 16, 2633–2638, doi: 10.1021/acs.nanolett.6b00273 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makarchuk S, Beyer N, Gaiddon C, Grange W & Hébraud P Holographic Traction Force Microscopy. Scientific Reports 8, 3038, doi: 10.1038/s41598-018-21206-2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim T et al. White-light diffraction tomography of unlabeled live cells. Nat Photonics 8, 256–263, doi:Doi 10.1038/Nphoton.2013.350 (2014). [DOI] [Google Scholar]
- 28.Wang Z et al. Spatial light interference microscopy (SLIM). Optics Express 19, 1016 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z et al. Spatial light interference tomography (SLIT). Optics Express 19, 19907–19918 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mir M et al. Optical measurement of cycle-dependent cell growth. Proc. Nat. Acad. Sci 108, 13124 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Popescu G Quantitative phase imaging of cells and tissues. (McGraw-Hill, 2011). [Google Scholar]
- 32.Chalut KJ, Ekpenyong AE, Clegg WL, Melhuish IC & Guck J Quantifying cellular differentiation by physical phenotype using digital holographic microscopy. Integr Biol-Uk 4, 280–284, doi: 10.1039/c2ib00129b (2012). [DOI] [PubMed] [Google Scholar]
- 33.Guck J et al. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys J 88, 3689–3698, doi: 10.1529/biophysj.104.045476 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miccio L et al. Particle tracking by full-field complex wavefront subtraction in digital holography microscopy. Lab on a chip 14, 1129–1134, doi: 10.1039/c3lc51104a (2014). [DOI] [PubMed] [Google Scholar]
- 35.Tzur A, Kafri R, LeBleu VS, Lahav G & Kirschner MW Cell Growth and Size Homeostasis in Proliferating Animal Cells. Science (New York, N.Y.) 325, 167–171, doi: 10.1126/science.1174294 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leith E & Upatnieks J Reconstructed wavefronts and communication theory. JOSA 52, 1123–1128 (1962). [Google Scholar]
- 37.Lee J, Abdeen AA & Kilian KA Rewiring mesenchymal stem cell lineage specification by switching the biophysical microenvironment. Sci Rep 4, 5188 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J, Abdeen AA, Kim AS & Kilian KA Influence of Biophysical Parameters on Maintaining the Mesenchymal Stem Cell Phenotype. ACS Biomater. Sci. Eng. 1, 218–226, doi: 10.1021/ab500003s (2015). [DOI] [PubMed] [Google Scholar]
- 39.Lee J, Abdeen AA, Zhang D & Kilian KA Directing stem cell fate on hydrogel substrates by controlling cell geometry, matrix mechanics and adhesion ligand composition. Biomaterials 34, 8140–8148, doi: 10.1016/j.biomaterials.2013.07.074 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Gabor D Theory of communication. J. Inst. Electr. Eng 93, 329 (1946). [Google Scholar]
- 41.Gabor D A new microscopic principle. Nature 161, 777 (1948). [DOI] [PubMed] [Google Scholar]
- 42.Ikeda T, Popescu G, Dasari RR & Feld MS Hilbert phase microscopy for investigating fast dynamics in transparent systems. Opt. Lett 30, 1165–1168 (2005). [DOI] [PubMed] [Google Scholar]
- 43.Cerruti V Ricerche intorno all’equilibrio de’corpi elastici isotropi: memoria. (coi tipi del Salviucci, 1882). [Google Scholar]
- 44.Kandel ME et al. Label-free tissue scanner for colorectal cancer screening. Journal of biomedical optics 22, 66016, doi: 10.1117/1.jbo.22.6.066016 (2017). [DOI] [PubMed] [Google Scholar]
- 45.MacQueen L, Sun Y & Simmons CA Mesenchymal stem cell mechanobiology and emerging experimental platforms. Journal of The Royal Society Interface 10, 20130179 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu J et al. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nature methods 7, 733–736 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calabuig A, Mugnano M, Miccio L, Grilli S & Ferraro P Investigating fibroblast cells under “safe” and “injurious” blue-light exposure by holographic microscopy. Journal of biophotonics 10, 919–927, doi: 10.1002/jbio.201500340 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Sridharan S, Mir M & Popescu G Simultaneous optical measurements of cell motility and growth. Biomed Opt Express 2, 2815–2820, doi: 10.1364/BOE.2.002815 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biagio M, Oriella G, Valentina M, Melania P & Pietro F Label free imaging of cell-substrate contacts by holographic total internal reflection microscopy. Journal of biophotonics 10, 1163–1170, doi:doi: 10.1002/jbio.201600177 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Mandracchia B, Pagliarulo V, Paturzo M & Ferraro P Surface Plasmon Resonance Imaging by Holographic Enhanced Mapping. Analytical Chemistry 87, 4124–4128, doi: 10.1021/acs.analchem.5b00095 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Lee J, Abdeen AA, Tang X, Saif TA & Kilian KA Matrix directed adipogenesis and neurogenesis of mesenchymal stem cells derived from adipose tissue and bone marrow. Acta Biomater, Ahead of Print, doi: 10.1016/j.actbio.2016.06.037 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Treiser MD et al. Cytoskeleton-based forecasting of stem cell lineage fates. Proc. Natl. Acad. Sci. U. S. A 107, 610–615, S610/611-S610/616, doi: 10.1073/pnas.0909597107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Atmani H, Chappard D & Basle MF Proliferation and differentiation of osteoblasts and adipocytes in rat bone marrow stromal cell cultures: Effects of dexamethasone and calcitriol. J. Cell. Biochem 89, 364–372, doi: 10.1002/jcb.10507 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Choi K-M et al. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. J. Biosci. Bioeng 105, 586–594, doi: 10.1263/jbb.105.586 (2008). [DOI] [PubMed] [Google Scholar]
- 55.Zhang D & Kilian KA The effect of mesenchymal stem cell shape on the maintenance of multipotency. Biomaterials 34, 3962–3969, doi: 10.1016/j.biomaterials.2013.02.029 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Lee J, Abdeen AA, Huang TH & Kilian KA Controlling cell geometry on substrates of variable stiffness can tune the degree of osteogenesis in human mesenchymal stem cells. Journal of the mechanical behavior of biomedical materials 38, 209–218 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Wang Z et al. Spatial light interference microscopy (SLIM). Opt Express 19, 1016–1026, doi: 10.1364/OE.19.001016 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mir M et al. Optical measurement of cycle-dependent cell growth. Proc Natl Acad Sci U S A 108, 13124–13129, doi: 10.1073/pnas.1100506108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barer R Determination of dry mass, thickness, solid and water concentration in living cells. Nature 172, 1097–1098 (1953). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.