Abstract
Protein–protein interactions (PPIs) contribute to the onset and/or progression of several diseases, especially cancer, and this discovery has paved the way for considering disruption of the PPIs as an attractive anti-tumor strategy. In this regard, simple and efficient biophysical methods for detecting the interaction of the inhibitors with the protein counterpart are still in high demand. Herein, we describe a convenient NMR method for the screening of putative PPI inhibitors based on the use of “hot peptides” (HOPPI-NMR). As a case study, HOPPI-NMR was successful applied to the well-known p53/MDM2 system. Our outcomes highlight the main advantages of the method, including the use of a small amount of unlabeled proteins, the minimization of the risk of protein aggregation, and the ability to identify weak binders. The last leaves open the possibility for application of HOPPI-NMR in tandem with fragment-based drug discovery as a valid strategy for the identification of novel chemotypes acting as PPI inhibitors.
Over the past two decades, knowledge about the protein–protein interaction (PPI) network, the so-called interactome, has greatly expanded,1 driving exhaustive investigations of this cellular machinery at a molecular level. The interactome modulates a plethora of physiological processes, and it is now well-established that the aberrant expression and/or regulation of numerous PPIs is directly correlated with the onset and development of specific human diseases, especially cancer.2 Hence, PPIs have gained tremendous attention, and substantial effort has been invested in developing PPI inhibitors to be investigated as potential therapeutics.3 In this light, a structural characterization that defines the minimal requirements in the PPI interface is highly needed, albeit challenging. Although most of the PPIs are driven by large and flat surface areas, which often get exposed upon conformational changes, it is possible to identify “hot spots” that are essential for the binding.4 These generally involve bulky amino acids such as tyrosine, arginine, and tryptophan, which bind in small pockets across the interface and contribute to the major part of the binding energy. Interestingly, in some cases PPIs are directed by a continuous binding epitope at the protein–protein interface, the so-called “hot segment”, which makes such a PPI a potential druggable candidate.4 Notably, isolated peptides encompassing the “hot segment” often maintain the capability to bind the counterpart protein, resulting in “hot segment” peptide and partner protein complexes with various stabilities.
The interaction of short peptides derived from hot segments (hereafter defined “hot peptides”) with the partner protein can be monitored by various biophysical methods (nuclear magnetic resonance (NMR), surface plasmon resonance (SPR), fluorescence spectroscopy, etc.), which are routinely employed for ligand/fragment screening.5 Out of the available screening techniques, NMR has emerged as a powerful tool thanks to its high versatility and to the additional wealth of structural knowledge it can provide.5
Herein we describe an advantageous method (Figure 1) for the screening of putative PPI inhibitors based on the use of short peptides along with protein- or ligand-based NMR techniques: HOt-peptide-based determination of PPI inhibitors by NMR (HOPPI-NMR). Briefly, a hot-peptide replaces one of the two protein partners of the PPI couple (protein-B in the example). The interaction of the hot-peptide with protein-A (PPI partner of protein-B) is detected by protein- and/or ligand-based NMR techniques. Next, competition experiments with known PPI inhibitors are carried out to validate the method and to gain both qualitative and quantitative information about the ability of an inhibitor to displace the hot-peptide and, therefore, indirectly the protein-B from the complex with protein-A.
Figure 1.
Schematic illustration of the proposed method.
The first step (Figure 1) involves identifying the interacting segments of the proteins in their complex state and then replacing one of them (protein-B in the example) with a short peptide (hot-peptide) endowed with an amino acid composition derived from the hot segment of the same protein.
In this regard, the numerous studies aimed at developing new peptides or peptide analogs as PPI inhibitors provide a large database of potential hot-peptides suitable for our study.6 In Table 1, a non-exhaustive list of pharmacologically interesting PPIs is reported for which a hot-peptide binder is already available.
Table 1. Peptide Segments Involved in PPIs.
| PPI | peptide sequence | KD (μM) |
|---|---|---|
| menin/MLL1a | R6WRFPARPGTTGGGGGGGRR25 | 0.08212 |
| MTIP/MyoAa | N799IPSLLRVQAHIRKKMVAQ818 | 0.08513 |
| β-catenin/axina | E467NPESILDEHVQRVM481 | 514 |
| Rab6a/Rab6IP1a | D900DEKEQFLYHLLSFNAV916 | 2015 |
| ERα/NR-2Aa | H687KILHRLLQDS697 | 2.516 |
| KCTD11/Cullin3a | N49SGLSFEELYRNAYTMVLHK68 | 0.49717 |
| β-adaptin/β-arr.a | D383DDIVFEDFARQRLKGMKDD402 | 2.118 |
| Bcl-XL/Baka | G72QVGRQLAIIGDDINR87 | 0.34019 |
| Bcl-XL/Baxa | K57KLSECLKRIGDELDS72 | 1319 |
| MDM2/p53a | Q16ETFSDLWKLLP27 | 0.06020 |
| MDM2/p53a | F19SDLWKLL26 | 0.8021 |
| MDM2/p53a | F19SDLWKL25 | 15021 |
Peptides are fragments of the indicated protein.
In the second step, the hot-peptide/protein-A complex is analyzed by protein- and/or ligand-based NMR techniques, which are suitable methods to study the interactions of relatively small molecule (as peptides) with macromolecular receptors like proteins. Particularly, saturation transfer difference NMR (STD-NMR)7 and WaterLOGSY-NMR (WL-NMR)8 are extremely useful for high-throughput screening due to their fast acquisition time, usually 5–10 min, allowing an extremely fast readout. Both STD-NMR and WL-NMR observe ligand resonances and employ magnetization transfer by the nuclear Overhauser effect (NOE). Notably, these techniques do not entail isotopic labeling of the protein and require little protein consumption (typical concentrations are 20–10 μM), coping with the in vitro protein aggregation problem common to many techniques.9 There is no upper limit size for the receptor, which can even be located on a cell surface.10 Since ligands with very high affinities (usually in the nM range) have low off-rates from the complex and could score as non-binders in ligand-based methods, a hot-peptide having a dissociation constant (KD) in the low μM range should be preferred as a probe, allowing both STD- and WL-NMR analysis to detect the occurring interactions.
In the third step, putative PPI inhibitors are tested by competition experiments. The displacement of the hot-peptide from protein-A by the tested compounds can be registered by heteronuclear single-quantum coherence (HSQC, protein-based) or STD- and/or WL-NMR (ligand-based) spectra. Considering that the hot-peptides mimic the protein-B interacting surface with protein-A, we can assume that compounds disrupting the hot-peptide/protein-A complex will also be able to block the interaction between the protein-A/protein-B complex, thus allowing us to screen and identify novel PPIs inhibitors. Eventually, quantitative binding information can also be extracted from titration experiments.11
As a case study, the well-known MDM2/p53 protein complex was chosen since this PPI is one of the most widely studied for its implications in several cancer types.22 Development of inhibitors to disrupt this interaction has been the object of intensive pharmaceutical efforts for anti-cancer therapies.23−25 Crystallographic studies demonstrated that a short segment of the N-terminal region of the protein p53 interacts with MDM2, forming an amphipathic α-helix, with Phe19, Trp23, and Leu26 being crucial interacting residues.26 Several structure–activity relationship studies on peptides encompassing the N-terminal region of p53 have been carried out.20,21,27 Three peptides (Table 1) were selected as potential hot-peptides in our study, having different KD’s for MDM2: p53[16-27] (KD = 0.060 μM),20 p53[19-26] (KD = 0.80 μM),21 and p53[19-25] (KD = 150 μM).21 They were synthesized by applying an ultrasound-assisted solid-phase peptide synthesis protocol (Supporting Information).28 Analytical data and 1H NMR assignments are reported in Figures S1–S3 and Tables S1–S3.
Preliminarily, we validated that the selected peptides interacted with protein-A, resembling the binding site of the hot-segment of the full protein-B (Figures 2 and S4–S7), by acquiring the 2D 1H–15N HSQC spectrum of the MDM2 N-terminal domain (residues 1–112) alone (blue) and after the addition of peptides (green spectra) p53[19-26] (Figures 2a and S4), p53[19-25] (Figures 2b and S5), and p53[16-27] (Figures 2c and S6). As expected, residues that are mainly affected by all peptides are within the known binding site of MDM2 for p53 interaction (see Figure S8),29 indicating that the binding is specific even for the weak ligand p53[19-25]. According to the peptides’ KD values, the p53[19-25] complex with MDM2 is in a fast exchange regime, while p53[19-26] and p53[16-27] are in a slow exchange regime on the NMR time scale.
Figure 2.
(a–c) Selected regions of 2D 1H–15N HSQC spectra of the MDM2 N-terminal domain alone (blue spectra), after the addition of peptides (green spectra) p53[19-26] (a), p53[19-25] (b), p53[16-27] (c), and nutlin-3a to the complexes (red spectra). (d) Selected regions of 2D 1H–15N HSQC spectra of the MDM2 N-terminal domain alone (blue spectra) and after addition of nutlin-3a (red spectra). Full 1H–15N HSQC spectra are reported in the Supporting Information (Figures S4–S7).
Next, to evaluate the possibility for screening applications by protein-based NMR, nutlin-3a, a well-known inhibitor of the MDM2/p53 interaction, was added to the preformed peptide/MDM2 complexes and the HSQC spectra were reacquired (Figure 2a–c, red spectra).
For comparison purposes, the HSQC spectrum of the complex MDM2/nutlin-3a without peptides was also registered (Figures 2d and S7). The HSQC spectra registered after the addition of nutlin-3a to the peptide/MDM2 complexes are almost perfectly superimposable with that of MDM2/nutlin-3a, thus showing the displacement of the preformed peptide/MDM2 complex by the nutlin-3a and the effective application of the protein-based NMR procedure as a screening test.
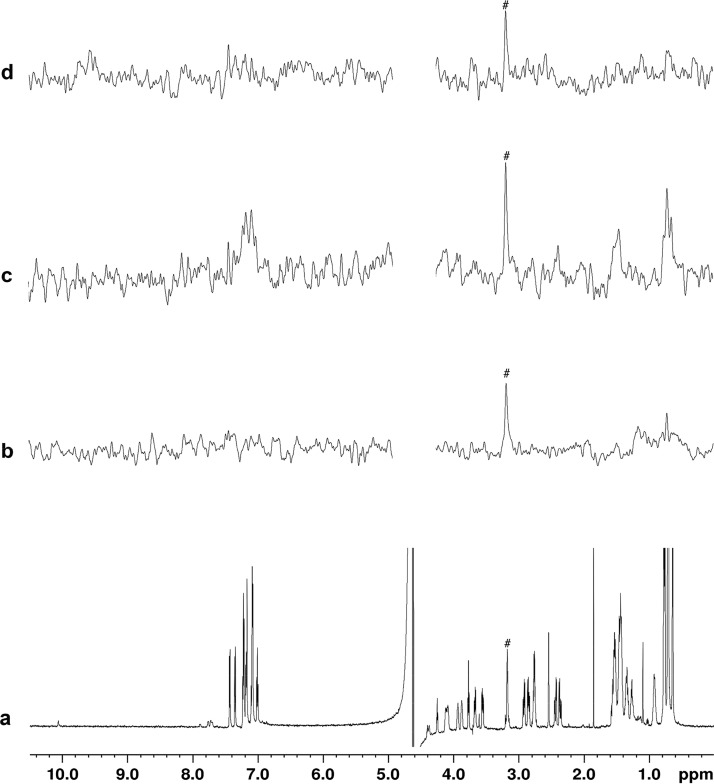
Next, the NMR study of the complex also by ligand-based STD and WL experiments (Figures 3, 4, and S9–S12) was carried out with peptides alone (1.0 mM) and in the presence of MDM2 (0.020 mM). The potential use of the techniques for inhibitor screening was then assessed by adding nutlin-3a as positive control (0.100 mM). Positive signals could be observed in the STD-NMR spectra of the three peptides mixed with MDM2 (Figures 3c, S9c, and S10c), including the tight binder p53[16-27], all showing higher signal-to-noise ratios compared to the peptide alone (Figures 3b, S9b, and S10b), proving that the interaction occurs. After the addition of nutlin-3a for the competition experiments, aromatic and methyl signal STD intensities of peptides p53[19-26] and p53[19-25] were significantly reduced (Figures 3d and S9d, and Table 2). Residual signals observed in the STD spectrum of p53[19-25] (Figure S9d) can be due to a very efficient saturation transfer to the pool of free ligand deriving from a relatively high off-rate value, predictable for this weak binder. In contrast, p53[16-27] signal intensities were almost unchanged (Figure S10d and Table 2). This is probably due to the high affinity of this peptide for MDM2 (KD = 0.060 μM), which would prevent its displacement by nutlin-3a at the employed concentration (0.100 mM). In this regard, a relatively high peptide concentration (1.0 mM) was chosen to allow short STD acquisition times (few minutes), while 0.100 mM was found to be the solubility limit of nutlin-3a in PBS. Since poor water solubility is common for drug-like compounds,30 the described test conditions are expected to be found in many experimental cases. Thus, peptides p53[19-26] and p53[19-25] (but not p53[16-27]) are suited for HOPPI screening by STD-NMR.
Figure 3.
1H NMR (a) and STD spectra of 1.0 mM p53[19-26] alone (b), in the presence of 0.020 mM MDM2 solution (c), and after the addition of 0.100 mM nutlin-3a (d). Buffer impurity is marked with a hash symbol.
Figure 4.
1H NMR (a) and WL spectra of 1.0 mM p53[16-27] alone (b), in the presence of 0.020 mM MDM2 solution (c), and after the addition of 0.100 mM of nutlin-3a (d). Stars indicate the position of exchangeable proton signals. Buffer impurity is marked with a hash symbol.
Table 2. Variation of STD Effect, STDI/STD0 %, after the Addition of Nutlin-3aa.
| peptide | signal (ppm) | % |
|---|---|---|
| p53[19-26] | aromatic (7.09) | 36 |
| aliphatic (0.71) | 38 | |
| p53[19-25] | aromatic (7.11) | 33 |
| aliphatic (0.72) | 46 | |
| p53[16-27] | aromatic (7.09) | 103 |
| aliphatic (0.71) | 104 | |
Reported as STDI after the addition of nutlin-3a to the peptide/MDM2 complex divided by the STD0 of the complex alone.
In WL spectra, signal intensities of aromatic and methyl protons significantly change from peptide alone to the spectrum of the complex with MDM2 for p53[19-26] (Figure S11b,c), and they invert from negative to positive phase after the MDM2 addition for both p53[19-25] (Figure S12b,c) and p53[16-27] (Figure 4b,c), which is diagnostic of interaction in all cases.
After the addition of nutlin-3a, the same signals reduced their absolute intensities, roughly coming back to the free state situation for p53[19-26] (Figure S11d) and p53[19-25] (Figure S12d), which is diagnostic of displacement. In the case of p53[16-27], aromatic and aliphatic signal intensities remained almost unchanged, confirming the result obtained with STD.
The exchangeable proton signals remained almost unvaried for the peptide alone, the peptide in the presence of MDM2, and after the addition of nutlin-3a for p53[19-26] (Figure S11b–d) and p53[19-25] (Figure S12b–d), which indicates that chemical exchange between excited bulk water and peptide labile protons predominates in all conditions. In contrast, the intensities of the exchangeable proton signals of p53[16-27] were significantly increased when the peptide was mixed with MDM2 (Figure 4b,c) and reduced at roughly the starting situation after the addition of nutlin-3a (Figure 4d). Hence, in p53[16-27], transfer of magnetization via intermolecular NOE and spin diffusion mediated by the protein is effectively working. Thus, the experimentally observed variation of the WL signal intensities of the labile protons allowed us, in the case of p53[16-27], to monitor the presence of an inhibitor of the complex. Although the peculiar behavior of the exchangeable protons of peptide p53[16-27] in WL spectra requires further investigations, the obtained results open the possibility of using even high-affinity peptides within the ligand-based HOPPI-NMR assay.
Once a PPI inhibitor has been identified throughout the above procedure, it is also possible to determine its dissociation constant (KI) from the observed ratio of the STD signals intensity before (STD0) and after the addition of the inhibitor (STDI), i.e., STDI/STD0 (Table 2). Calculations from competitive binding experiments have been reported for competitive binding assays31 and NMR studies.11,32,33 Equations 1–3 reported in Chart 1 allow us to calculate the dissociation constants, where [E0] is the total protein (MDM2) concentration, [L0] is the total peptide concentration, [I0] is the total nutlin-3a concentration, KD is the dissociation constant of the peptide (Table 1), [EI] is the inhibitor/MDM2 complex concentration, and [EL] is the peptide/MDM2 complex concentration.
Chart 1.
From the observed STDI/STD0 ratio expressed as a fraction, the dissociation constant of the competitive inhibitor (KI) can be calculated according to the equations in Chart 1. In particular, numerical solution of eqs 1–3 gives KI = 43 nM for nutlin-3a (see Supporting Information for details), reasonably in accordance with the IC50 = 90 nM reported in literature.25
Albeit preliminary, our results pointed out several advantages of the HOPPI-NMR method. First, it minimizes the potential problems related with the protein expression, purification, solubility, and aggregation. For instance, in our case the replacement of p53 by a hot peptide made it possible to overcome the solubility and self-aggregation issues typical of p53, which often represent the main limits during the development of new potential disrupters of p53/MDM2. In fact, the HOPPI method can, in principle, be used in combination with any other screening technique (SPR, fluorescence spectroscopy, etc.) maintaining this important benefit. Second, protein/peptide complex affinity can be tuned by properly selecting the peptide length (i.e., different KD), and thus a low-affinity peptide can be suitable for the detection of weak inhibitors (initial hits). Thus, peptides endowed with low affinity for the target, such as p53[19-25] that forms a complex with MDM2 400-fold weaker than the p53/MDM2 complex (KD = 0.4 μM),34 could be employed to detect even very weak inhibitors in the screening of compound and fragment libraries. In this context, the combination of HOPPI-NMR and fragment-based drug discovery (FBDD)35 methods would be a straightforward way to boost the discovery of PPI inhibitors, allowing researchers to detect also small fragments tested in FBDD which often display weak binding; hence, HOPPI-NMR emerges as a suitable tool to reliably and efficiently detect such weak binding. Third, the method allows for the application of ligand-based NMR techniques, which are endowed with intrinsic advantages, including no isotopic labeling of the receptor, little amount of receptor required, and no upper limit size for the receptor.
In conclusion, we described an efficient and simple method for the screening of PPI inhibitors (HOPPI-NMR), in which one of the two interacting proteins is replaced by a short peptide (hot-peptide). The replacement, among other benefits, allows the application of fast, low-consuming, ligand-based NMR techniques for the investigation of unlabeled samples. We envisage that the appropriate choice of hot-peptides will enable the discovery of hit compounds with weak binding affinity, potentially useful in FBDD investigations, providing new opportunities for the highly expanding field of medicinal chemistry devoted to the identification of effective PPI inhibitors.
Acknowledgments
This work was supported by a grant from Regione Campania – POR Campania FESR 2014/2020 “Combattere la resistenza tumorale: piattaforma integrata multidisciplinare per un approccio tecnologico innovativo alle oncoterapie – Campania Oncoterapia” (Project n. B61G18000470007).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.9b00620.
Materials, synthetic protocol, analytical data for all peptides, NMR data, and STD- and WL-NMR spectra, including Figures S1–S12 and Tables S1–S3 (PDF)
Author Contributions
‡ D.B. and S.D.M. contributed equally.
The authors declare no competing financial interest.
Dedication
Dedicated to the memory of Prof. Maurizio Botta.
Supplementary Material
References
- Rolland T.; Taşan M.; Charloteaux B.; Pevzner S. J.; Zhong Q.; Sahni N.; Yi S.; Lemmens I.; Fontanillo C.; Mosca R.; et al. A Proteome-Scale Map of the Human Interactome Network. Cell 2014, 159, 1212–1226. 10.1016/j.cell.2014.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A. A.; Khuri F. R.; Fu H. Targeting Protein-Protein Interactions as an Anticancer Strategy. Trends Pharmacol. Sci. 2013, 34, 393–400. 10.1016/j.tips.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milroy L. G.; Grossmann T. N.; Hennig S.; Brunsveld L.; Ottmann C. Modulators of Protein-Protein Interactions. Chem. Rev. 2014, 114, 4695–4748. 10.1021/cr400698c. [DOI] [PubMed] [Google Scholar]
- London N.; Raveh B.; Schueler-Furman O. Druggable Protein-Protein Interactions - from Hot Spots to Hot Segments. Curr. Opin. Chem. Biol. 2013, 17, 952–959. 10.1016/j.cbpa.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Dalvit C. NMR Methods in Fragment Screening: Theory and a Comparison with Other Biophysical Techniques. Drug Discovery Today 2009, 14, 1051–1057. 10.1016/j.drudis.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Helmer D.; Schmitz K.. Peptides and Peptide Analogs to Inhibit Protein-Protein Interactions. In Protein Targeting Compounds; Böldicke T., Ed.; Advances in Experimental Medicine and Biology917; Springer, 2016; pp 147–183. 10.1007/978-3-319-32805-8_8. [DOI] [PubMed] [Google Scholar]
- Mayer M.; Meyer B. Characterization of Ligand Binding by Saturation Transfer Difference NMR Spectroscopy. Angew. Chem., Int. Ed. 1999, 38, 1784–1788. . [DOI] [PubMed] [Google Scholar]
- Dalvit C.; Fogliatto G. P.; Stewart A.; Veronesi M.; Stockman B. WaterLOGSY as a Method for Primary NMR Screening: Practical Aspects and Range of Applicability. J. Biomol. NMR 2001, 21 (4), 349–359. 10.1023/A:1013302231549. [DOI] [PubMed] [Google Scholar]
- Meyer B.; Peters T. NMR Spectroscopy Techniques for Screening and Identifying Ligand Binding to Protein Receptors. Angew. Chem., Int. Ed. 2003, 42, 864–890. 10.1002/anie.200390233. [DOI] [PubMed] [Google Scholar]
- Brancaccio D.; Diana D.; Di Maro S.; Di Leva F. S.; Tomassi S.; Fattorusso R.; Russo L.; Scala S.; Trotta A. M.; Portella L.; et al. Ligand-Based NMR Study of C-X-C Chemokine Receptor Type 4 (CXCR4)-Ligand Interactions on Living Cancer Cells. J. Med. Chem. 2018, 61, 2910–2923. 10.1021/acs.jmedchem.7b01830. [DOI] [PubMed] [Google Scholar]
- Wang Y.-S.; Liu D.; Wyss D. F. Competition STD NMR for the Detection of High-Affinity Ligands and NMR-Based Screening. Magn. Reson. Chem. 2004, 42, 485–489. 10.1002/mrc.1381. [DOI] [PubMed] [Google Scholar]
- Huang J.; Gurung B.; Wan B.; Matkar S.; Veniaminova N. A.; Wan K.; Merchant J. L.; Hua X.; Lei M. The Same Pocket in Menin Binds Both MLL and JUND but Has Opposite Effects on Transcription. Nature 2012, 482 (7386), 542–546. 10.1038/nature10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douse C. H.; Green J. L.; Salgado P. S.; Simpson P. J.; Thomas J. C.; Langsley G.; Holder A. A.; Tate E. W.; Cota E. Regulation of the Plasmodium Motor Complex: Phosphorylation of Myosin a Tail-Interacting Protein (MTIP) Loosens Its Grip on MyoA. J. Biol. Chem. 2012, 287 (44), 36968–36977. 10.1074/jbc.M112.379842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T. N.; Yeh J. T.-H.; Bowman B. R.; Chu Q.; Moellering R. E.; Verdine G. L. Inhibition of Oncogenic Wnt Signaling through Direct Targeting of -Catenin. Proc. Natl. Acad. Sci. U. S. A. 2012, 109 (44), 17942–17947. 10.1073/pnas.1208396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel J.; Cromm P. M.; Itzen A.; Goody R. S.; Grossmann T. N.; Waldmann H. Direct Targeting of Rab-GTPase-Effector Interactions. Angew. Chem., Int. Ed. 2014, 53 (9), 2498–2503. 10.1002/anie.201308568. [DOI] [PubMed] [Google Scholar]
- Phillips C.; Roberts L. R.; Schade M.; Bazin R.; Bent A.; Davies N. L.; Moore R.; Pannifer A. D.; Pickford A. R.; Prior S. H.; et al. Design and Structure of Stapled Peptides Binding. J. Am. Chem. Soc. 2011, 133, 9696–9699. 10.1021/ja202946k. [DOI] [PubMed] [Google Scholar]
- De Paola I.; Pirone L.; Palmieri M.; Balasco N.; Esposito L.; Russo L.; Mazzà D.; Marcotullio L.; Di; Di Gaetano S.; Malgieri G.; et al. Cullin3 - BTB Interface:A Novel Target for Stapled Peptides. PLoS One 2015, 10 (4), e0121149. 10.1371/journal.pone.0121149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevola L.; Martín-Quirõs A.; Eckelt K.; Camarero N.; Tosi S.; Llobet A.; Giralt E.; Gorostiza P. Light-Regulated Stapled Peptides to Inhibit Protein-Protein Interactions Involved in Clathrin-Mediated Endocytosis. Angew. Chem., Int. Ed. 2013, 52 (30), 7704–7708. 10.1002/anie.201303324. [DOI] [PubMed] [Google Scholar]
- Kelekar A.; Chang B. S.; Harlan J. E.; Fesik S. W.; Thompson C. B. Bad Is a BH3 Domain-Containing Protein That Forms an Inactivating Dimer with Bcl-XL. Mol. Cell. Biol. 1997, 17 (12), 7040–7046. 10.1128/MCB.17.12.7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa B.; Grillone A. F.; Salvetti A.; Rocchiccioli S.; Iacopetti P.; Daniele S.; Da Pozzo E.; Campiglia P.; Novellino E.; Martini C.; et al. An Antibody-Free Strategy for Screening Putative HDM2 Inhibitors Using Crude Bacterial Lysates Expressing GST-HDM2 Recombinant Protein. Drug Test. Anal. 2013, 5, 596–601. 10.1002/dta.1492. [DOI] [PubMed] [Google Scholar]
- Lai Z.; Auger K. R.; Manubay C. M.; Copeland R. A. Thermodynamics of P53 Binding to Hdm2(1–126): Effects of Phosphorylation and P53 Peptide Length. Arch. Biochem. Biophys. 2000, 381 (2), 278–284. 10.1006/abbi.2000.1998. [DOI] [PubMed] [Google Scholar]
- Sun Y. P53 and Its Downstream Proteins as Molecular Targets of Cancer. Mol. Carcinog. 2006, 45, 409–415. 10.1002/mc.20231. [DOI] [PubMed] [Google Scholar]
- Vassilev L. T. P53 Activation by Small Molecules: Application in Oncology. J. Med. Chem. 2005, 48, 4491–4499. 10.1021/jm058174k. [DOI] [PubMed] [Google Scholar]
- Lu Y.; Nikolovska-Coleska Z.; Fang X.; Gao W.; Shangary S.; Qiu S.; Qin D.; Wang S. Discovery of a Nanomolar Inhibitor of the Human Murine Double Minute 2 (MDM2)-P53 Interaction through an Integrated, Virtual Database Screening Strategy. J. Med. Chem. 2006, 49, 3759–3762. 10.1021/jm060023+. [DOI] [PubMed] [Google Scholar]
- Vassilev L. T.; Vu B. T.; Graves B.; Carvajal D.; Podlaski F.; Filipovic Z.; Kong N.; Kammlott U.; Lukacs C.; Klein C.; et al. In Vivo Activation of the P53 Pathway by Small-Molecule Antagonists of MDM2. Science 2004, 303, 844–848. 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- Chen L.; Yin H.; Farooqi B.; Sebti S.; Hamilton A. D.; Chen J. Interaction and Activate P53. Mol. Cancer Ther. 2005, 4 (June), 1019–1025. 10.1158/1535-7163.MCT-04-0342. [DOI] [PubMed] [Google Scholar]
- Schon O.; Friedler A.; Bycroft M.; Freund S. M. V.; Fersht A. R. Molecular Mechanism of the Interaction between MDM2 and P53. J. Mol. Biol. 2002, 323, 491–501. 10.1016/S0022-2836(02)00852-5. [DOI] [PubMed] [Google Scholar]
- Merlino F.; Tomassi S.; Yousif A. M.; Messere A.; Marinelli L.; Grieco P.; Novellino E.; Cosconati S.; Di Maro S. Boosting Fmoc Solid-Phase Peptide Synthesis by Ultrasonication. Org. Lett. 2019, 21, 6378–6382. 10.1021/acs.orglett.9b02283. [DOI] [PubMed] [Google Scholar]
- Kussie P. H.; Gorina S.; Marechal V.; Elenbaas B.; Moreau J.; Levine A. J.; Pavletich N. P. Structure of the MDM2 Oncoprotein Bound to the P53 Tumor Suppressor Transactivation Domain. Science 1996, 274, 948–953. 10.1126/science.274.5289.948. [DOI] [PubMed] [Google Scholar]
- Lipinski C. A. Drug-like Properties and the Causes of Poor Solubility and Poor Permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. 10.1016/S1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- Yung-Chi C.; Prusoff W. H. Relationship between the Inhibition Constant (KI) and the Concentration of Inhibitor Which Causes 50 per Cent Inhibition (I50) of an Enzymatic Reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Dalvit C.; Fasolini M.; Flocco M.; Knapp S.; Pevarello P.; Veronesi M. NMR-Based Screening with Competition Water-Ligand Observed via Gradient Spectroscopy Experiments: Detection of High-Affinity Ligands. J. Med. Chem. 2002, 45, 2610–2614. 10.1021/jm011122k. [DOI] [PubMed] [Google Scholar]
- Meinecke R.; Meyer B. Determination of the Binding Specificity of an Integral Membrane Protein by Saturation Transfer Difference NMR: RGD Peptide Ligands Binding to Integrin AIIbβ3. J. Med. Chem. 2001, 44, 3059–3065. 10.1021/jm0109154. [DOI] [PubMed] [Google Scholar]
- Domenici F.; Frasconi M.; Mazzei F.; D’Orazi G.; Bizzarri A. R.; Cannistraro S. Azurin Modulates the Association of Mdm2 with P53: SPR Evidence from Interaction of the Full-Length Proteins. J. Mol. Recognit. 2011, 24, 707–714. 10.1002/jmr.1105. [DOI] [PubMed] [Google Scholar]
- Davis B. J.; Roughley S. D. Fragment-Based Lead Discovery. Annu. Rep. Med. Chem. 2017, 50, 371–439. 10.1016/bs.armc.2017.07.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.