Abstract
Pursuing our effort for developing effective inhibitors of the cancer-related hCA IX isoform, here we describe the synthesis of novel benzofuran-based carboxylic acid derivatives, featuring the benzoic (9a–f) or hippuric (11a,b) acid moieties linked to 2-methylbenzofuran or 5-bromobenzofuran tails via an ureido linker. The target carboxylic acids were evaluated for the potential inhibitory action against hCAs I, II, IX, and XII. Superiorly, benzofuran-containing carboxylic acid derivatives 9b, 9e, and 9f acted as submicromolar hCA IX inhibitors with KIs = 0.91, 0.79, and 0.56 μM, respectively, with selective inhibitory profile against the target hCA IX over the off-target isoforms hCA I and II (SIs: 2 to >63 and 4–47, respectively). Compounds 9b, 9e, and 9f were examined for their antiproliferative action against human breast cancer (MCF-7 and MDA-MB-231) cell lines. In particular, 9e displayed promising antiproliferative (IC50 = 2.52 ± 0.39 μM), cell cycle disturbance, and pro-apoptotic actions in MDA-MB-231 cells.
Keywords: Anticancer, benzofurans, carbonic anhydrases, carboxylic acids, synthesis
Carbonic anhydrases (CAs, EC 4.2.1.1) are considered the most widespread metalloenzymes present in living organisms that play a vital role in catalyzing the efficacious interconversion between carbon dioxide and bicarbonate.1 Such a simple CA-catalyzed reaction is crucial for diverse physiological and pathological events associated with pH and CO2 homeostasis, electrolyte secretion, tumorigenicity, and others.2 Up to now, 15 diverse human (h) CA isoforms (α-CAs) have been described and identified. Among these, 12 isoforms only are catalytically active with distinct kinetic properties, tissue distributions, and subcellular localizations; cytosolic (I, II, III, VII, and XIII), mitochondrial (VA and VB), secreted (VI), and membrane-bound (IV, IX, XII, and XIV).3
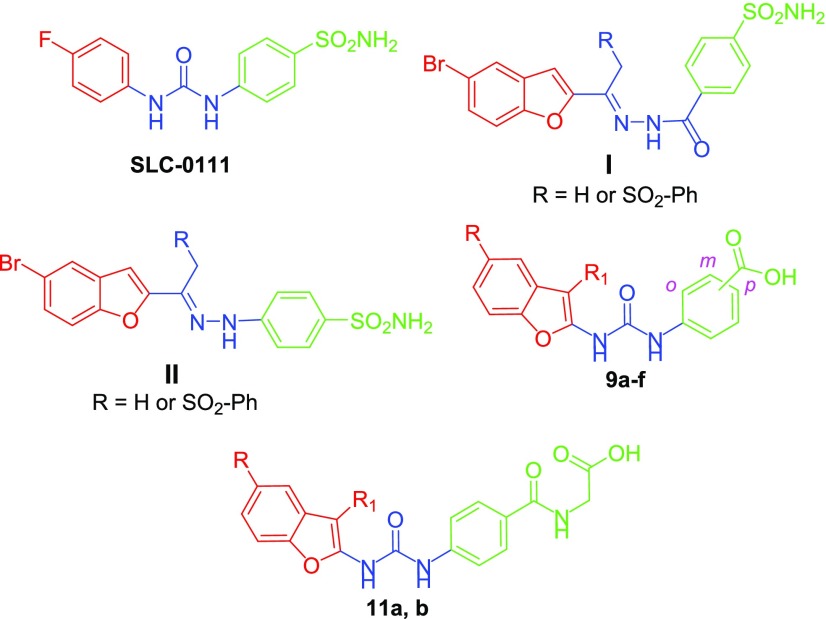
During the last decades it was well-established that modulators of these metalloenzymes represent an important class of therapeutics such as diuretics,4 antiepileptics,5 antiglaucoma agents,6 and anticancer agents.7,8 Moreover hCA IX isoform, not considerably expressed in most human normal tissues, is up-regulated in the hypoxic tumors upon induction via HIF-1α and thus considered as a crucial element in tumor cells proliferation, invasiveness, survival, and metastasis.8 Accordingly, selective inhibition of hCA IX emerged out as a valuable therapeutic approach for targeting and treatment of different human malignancies.8 SLC-0111 (Figure 1) is a front-runner carbonic anhydrase inhibitor that is currently in Phase II clinical trials for management of advanced hypoxic tumors, with a preferential hCA IX inhibitory action.9
Figure 1.
Structures for SLC-0111 and CAIs I and II and target benzofuran-based carboxylic acid derivatives 9a–f and 11a, b.
Carboxylic acids are among the most versatile CA inhibitor (CAI) chemotypes.10 They are capable of interacting with the CAs through a variety of inhibition mechanisms, such as coordination to the metal ion likely as carboxylate anions,11 anchoring to the zinc-bound water/hydroxide ion,12 occluding the entrance of the carbonic anhydrase active site cavity,13 and inhibiting CAs binding out of the active site.14
Recently, Abdelrahman et al. developed a novel series of benzofuran-based CAIs exhibiting the zinc anchoring sulfonamide group connected to a benzofuran tail through hydrazido and hydrazino spacers, compounds I and II (Figure 1).15 The arylsulfonehydrazone derivatives exerted effective and selective inhibitory activities toward target hCA IX and XII isoforms over off-target hCA I and II isoforms.
Pursuing our endeavor toward developing efficient inhibitors for the cancer-related isoform hCA IX,16−20 here we present the design and synthesis of novel benzofuran-based carboxylic acid derivatives, featuring the benzoic acid (9a–f) or hippuric acid (11a, b) moieties linked to 2-methylbenzofuran or 5-bromobenzofuran tails via an ureido linker (Figure 1).
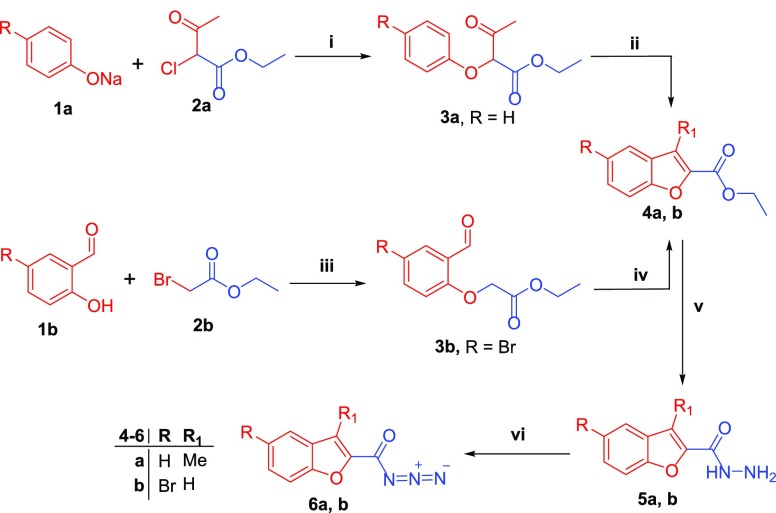
Herein reported benzofuran-based carboxylic acid derivatives (9a–f and 11a, b) were synthesized following the procedures outlined in Schemes 1 and 2. The ethyl benzofuran-2-carboxylate derivatives 4a, b were generated through a cyclocondensation of intermediates 3a, b yielded by an o-alkylation reaction for sodium phenolate 1a and 2-hydroxybenzaldehyde 1b with ethyl 2-chloroacetoacetate 2a and ethyl bromoacetate 2b, respectively.21,22 Thereafter, hydrazinolysis of ethyl benzofuran-2-carboxylates 4a, b was performed via their refluxing with an excess of 99% hydrazine hydrate in ethyl alcohol to yield benzofuran-2-carbohydrazides 5a, b, respectively.
Scheme 1.
Reagents and conditions: (i) Dry toluene, reflux 4 h, 91%; (ii) H2SO4, stirring 2 h (0–5 °C), 84%; (iii) NaH, DMF, stirring 2.5 h at 0 °C, 80%; (iv) (a) EtONa, EtOH, reflux 3 h, (b) EtOH, H2SO4, reflux 4 h, 72%; (v) 99% NH2NH2·H2O, EtOH, reflux 4 h, 79–84%; (vi) NaNO2, AcOH, stirring 1 h (0 °C) then 1.5 h (r.t.), 76-81%.
Scheme 2.
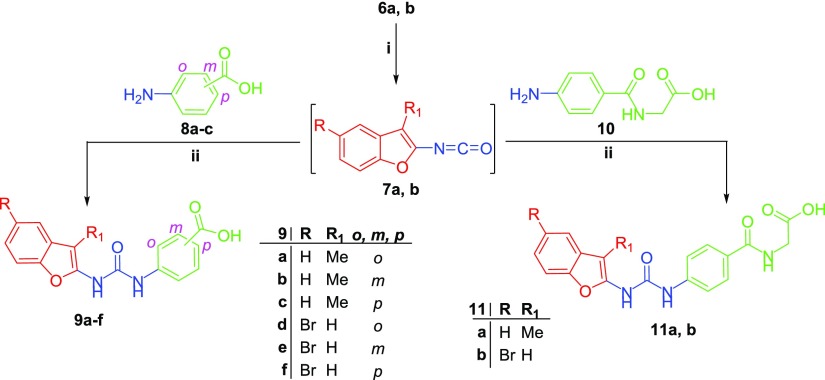
Reagents and conditions: (i) Dry xylene, reflux 1 h, 95%; (ii) Dry xylene, reflux 5 h, 68-85%.
The benzofuran-2-carbonyl azides 6a,b were provided by stirring of benzofuran-2-carbohydrazides 5a,b with sodium nitrite (NaNO2) in ice-cold acetic acid (Scheme 1), which was subsequently subjected to Curtius rearrangement via stirring under reflux temperature in dry xylene to furnish the corresponding key intermediates isocyanatobenzofuran derivatives 7a,b (Scheme 2).23 Finally, the target benzofuran-based carboxylic acids 9a–f and 11a,b were obtained through addition of aminobenzoic acids 8a–c or para-aminohippuric acid 10 to the hot stirred solution of isocyanatobenzofurans 7a,b in dry xylene, with moderate yields (68–85%) (Scheme 2).
Elucidation of the structures for the newly synthesized benzofuran-based carboxylic acids (9a–f and 11a,b) were supported by the elemental and spectral data which was consistent with the postulated structures
All the prepared benzofuran-based carboxylic acid derivatives 9a–f and 11a, b were assessed for their potential inhibitory actions against the cytosolic hCA I and II, in addition to the trans membrane cancer-related hCA IX and XII isoforms, by the use of an instrument of applied photophysics stopped-flow.24 Thereafter, the antiproliferative activities for the best efficient and selective hCA IX inhibitors in this study were screened toward two breast cancer (MCF-7 and MDA-MB-231) cell lines. Furthermore, the impact of benzofuran-based benzoic acid derivative 9e on the distribution of the cell cycle phases in breast cancer MDA-MB-231 cell line was assessed, in addition to assessment of its ability to induce the early and late apoptosis via AnnexinV-FITC/PI binding assay.
Inhibition data for the tested hCA isoforms (I, II, IX, and XII) are displayed in Table 1.
Table 1. Inhibition Data (KI’s) of hCAs I, II, IX, and XII with Carboxylic Acids 9a–f and 11a,b and AAZ as Reference Inhibitor by a Stopped-Flow CO2 Hydrase Assay24.
|
KI (μM) |
|||||||
|---|---|---|---|---|---|---|---|
| Cmp | R | R1 | o/m/p | CA I | CA II | CA IX | CA XII |
| 9a | H | CH3 | o | >100 | 7.9 | 1.6 | 3.4 |
| 9b | H | CH3 | m | 32.8 | 10.1 | 0.91 | 2.2 |
| 9c | H | CH3 | p | 4.5 | 3.1 | 5.1 | 0.88 |
| 9d | Br | H | o | >100 | 4.1 | 5.1 | 8.0 |
| 9e | Br | H | m | 33.2 | 37.0 | 0.79 | 2.3 |
| 9f | Br | H | p | 20.5 | 7.2 | 0.56 | 1.6 |
| 11a | H | CH3 | 64.7 | 25.8 | 35.7 | 2.7 | |
| 11b | Br | H | >100 | 67.1 | 19.0 | 10.1 | |
| AAZ | 0.25 | 0.01 | 0.02 | 0.006 | |||
Mean data from three different assays. SD: standard deviations ranged from ±5% to ±10% of the indicated KI values.
The slow cytosolic isoform hCA I (mainly considered as an off-target isoform when CAIs are developed as a potential anticancer agents) was moderately or weakly inhibited by five of the investigated benzofuran-based carboxylic acids: 9b, 9c, 9e, 9f, and 11a which showed KI’s spanning in the range of 4.5 and 64.7 μM, whereas benzofuran derivatives 9a, 9d, and 11b could not inhibit the cytosolic isoform hCA I up to 100 μM.
It is worth stressing that grafting carboxylic acid functionality at the ortho-position in both 2-methylbenzofuran (9a) and 5-bromobenzofuran (9d) scaffolds resulted in a diminished hCA I inhibitory activity. Moreover, replacement of the benzoic acid moiety with the hippuric acid one led to a significant worsening (for the 2-methylbenzofuran scaffold; 11a: KI = 64.7 μM) or led to an abolished (for the 5-bromobenzofuran scaffold; 11b: KI > 100 μM) inhibitory activity against hCA I.
Inhibition of the most physiologically relevant cytosolic isoform hCA II ranged from moderate to weak, with KI values in the range of 3.1–67.1 μM. In particular benzofuran derivatives 9a, 9c, 9d, and 9f were the best herein reported hCA II inhibitors with single-digit micromolar inhibitory activity (KI’s = 7.9, 3.1, 4.1, and 7.2 μM, respectively) (Table 1). It is worth stressing that meta-substituted 2-methylbenzofuran derivative (9b) exhibited a slightly reduced inhibitory efficacy (KI = 10.1 μM) than its ortho-substituted (9a: KI = 7.9 μM) and para-substituted (9c: KI = 3.1 μM) analogues; likewise, the meta-substituted 5-bromobenzofuran derivative (9e) showed a weaker hCA II inhibitory activity (KI = 37.0 μM) than both ortho-substituted (9d: KI = 4.1 μM) and para-substituted (9f: KI = 7.2 μM) counterparts. Furthermore, incorporation of the hippuric acid moiety resulted in a decreased activity for both 2-methylbenzofuran and 5-bromobenzofuran scaffolds (11a and 11b: KI’s = 25.8 and 67.1 μM, respectively) in comparison to their benzoic acid-containing analogues (9c and 9f: KI’s = 3.1 and 7.2 μM, respectively) (Table 1). These structure–activity relationships (SARs) highlighted that appending ortho- and para-benzoic acids is more advantageous for hCA II inhibitory action than incorporation of meta-benzoic acid or hippuric acid.
The obtained KI values pointed out that the main antitumor target isoform hCA IX was effectively inhibited by the here reported benzofuran-based derivatives decorated with benzoic acid moiety 9a–f with KI’s ranging between 0.56 and 5.1 μM, whereas hCA IX was moderately affected by the benzofuran-based carboxylic acids decorated with hippuric acid moiety (11a and 11b) with KI’s values equal to 35.7 and 19.0 μM, respectively. Superiorly, benzofuran-based carboxylic acids 9b, 9e, and 9f emerged as submicromolar hCA IX inhibitors with KI’s = 0.91, 0.79, and 0.56 μM, respectively.
It is noteworthy to mention that the order of activities within the 2-methylbenzofuran-based regioisomers 9a–c toward hCA IX was decreased in the order of meta isomer (9b) > ortho isomer (9a) > para isomer (9c), whereas the order of hCA IX inhibitory activities for the 5-bromobenzofuran-based regioisomers 9d–f was decreased in the order para isomer (9f) > meta isomer (9e) > ortho isomer (9d).
The second cancer-related target CA isoform examined here is hCA XII. As can be seen from the results presented in Table 1, hCA XII was efficiently inhibited by the herein reported benzofuran-based acids with KI’s in the range of 0.88–3.4 μM, aside from benzofuran derivatives 9d and 11b, whose potency was raised at slightly higher concentration (KIs = 8.0 and 10.1 μM, respectively). The 2-methylbenzofuran-based derivative 9c, with KI equal to 0.88 μM, is the only submicromolar CA inhibitor identified toward hCA XII isoform in this study (Table 1). Remarkably, the deduced SAR suggested that utilization of the 2-methylbenzofuran scaffold elicited an enhancement of effectiveness toward hCA XII for both benzoic acid-containing derivatives 9a–c (KIs = 3.4, 2.2, and 0.88 μM, respectively) and hippuric acid-containing derivative 11a (KI = 2.7 μM) in comparison to their corresponding 5-bromobenzofuran counterparts 9d–f (KIs = 8.0, 2.3, and 1.6 μM, respectively) and 11b (KI = 10.1 μM). With regard to the impact of regioisomerism, the order of potencies within the 2-methylbenzofuran-based regioisomers 9a–c and 5-bromobenzofuran-based regioisomers 9d–f against hCA XII was lowered in the following order: para isomers > meta isomers > ortho isomers.
Concerning the target/off-target CAs selectivity indexes (SIs) of action for target benzofuran-based carboxylic acid derivatives (9a–f and 11a,b), all compounds, except 9c, exhibited adequate selectivity profiles for hCA IX over hCA I (SI: 2 to >63). In addition, compounds 9a, 9b, 9e, 9f, and 11b displayed remarkable II/IX inhibitory specificity with SIs spanning the range of 4–47 (Table 2).
Table 2. Selectivity Index for Inhibition of Isoforms hCA IX and XII over hCA I and II, for Carboxylic Acids 9a–f and 11a,b.
| Cmp | I/IX | II/IX | I/XII | II/XII |
|---|---|---|---|---|
| 9a | >63 | 5 | >29 | 2 |
| 9b | 36 | 11 | 15 | 5 |
| 9c | 5 | 4 | ||
| 9d | 20 | 13 | ||
| 9e | 42 | 47 | 14 | 16 |
| 9f | 37 | 13 | 13 | 5 |
| 11a | 2 | 24 | 10 | |
| 11b | 5 | 4 | 10 | 7 |
Benzofuran-based carboxylic acids 9b, 9e, and 9f displayed effective and selective inhibitory actions toward the cancer-associated hCA IX/XII isoforms over the off-target isoforms CA I and II (Tables 1 and 2). Therefore, the three benzofuran derivatives have been chosen to be tested for their possible in vitro antiproliferative actions toward the breast cancer (MCF-7 and MDA-MB-231) cell lines, using the SRB colorimetric reduction method as reported by Skehan et al.25 The results from this assay have been expressed as IC50 values and presented in Table 3.
Table 3. Anti-proliferative Activities of Benzofuran-Based Carboxylic Acids 9b, 9e, and 9f against Two Breast Cancer Cell Lines: MCF-7 and MDA-MB-231.
| IC50 (μM)a |
||
|---|---|---|
| Cmp | MCF-7 | MDA-MB-231 |
| 9b | NAb | 37.60 ± 1.86 |
| 9e | 14.91 ± 1.04 | 2.52 ± 0.39 |
| 9f | 19.70 ± 2.06 | 11.50 ± 1.05 |
| Dox | 1.43 ± 0.12 | 2.36 ± 0.18 |
IC50 values are the mean ± SD of three separate experiments.
NA: Derivatives possessing IC50 value >100 μM.
The obtained IC50 values from the SRB analysis (Table 3) indicated that the tested benzofuran derivatives 9b, 9e, and 9f were more effective toward MDA-MB-231 (IC50 values = 37.60 ± 1.86, 2.52 ± 0.39, and 11.50 ± 1.05 μM, respectively) than MCF-7 cells (IC50 values = >100 μM, 14.91 ± 1.04 μM, and 19.70 ± 2.06 μM, respectively). Superiorly, the 5-bromobenzofuran-based derivative 9e was the most effective antiproliferative agent toward MDA-MB-231 cells with IC50 value = 2.52 ± 0.39 μM, with a comparable potency to Doxorubicin (Dox), the reference drug (IC50 = 2.36 ± 0.18 μM).
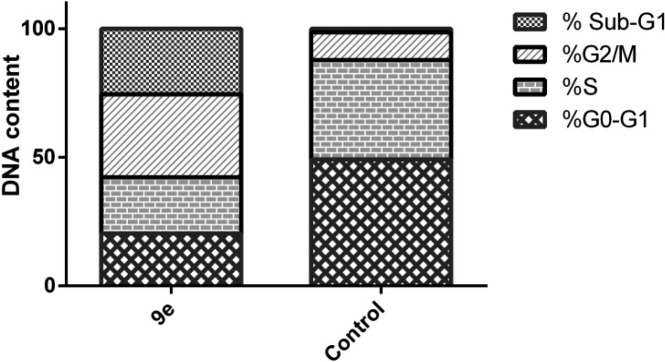
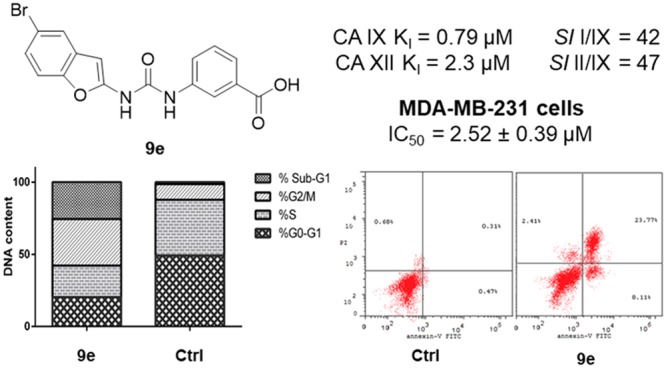
The promising anticancer effect of benzofuran-based carboxylic acid derivative 9e toward MDA-MB-231 breast cancer cell line (Table 3) motivated a further examination for its growth inhibitory activity. The effect of carboxylic acid derivative 9e on cell cycle phases distribution, upon incubation with breast cancer MDA-MB-231 cells for 24 h at its IC50 concentration (2.52 μM), was assessed by the use of a DNA flow cytometry assay (Figure 2).
Figure 2.
Effect of benzofuran-based carboxylic acid 9e on the phases of cell cycle in breast cancer MDA-MB-231cells.
The assay outcomes revealed that MDA-MB-231 cancer cells treated with benzofuran-based acid derivative 9e were significantly arrested at the G2-M phase showing a cell population increase from 10.80% (for the control cells) to 32.30% (in 9e-treated MDA-MB-231 cells). In addition, the number of cells in the sub-G1 phase was strikingly increased from 1.43% (for the control cells) to 25.53% (in 9e-treated MDA-MB-231 cells) (Figure 2).
The AV/PI staining assay has been adopted in order to investigate the effect of benzofuran-based carboxylic acid derivative 9e on the early and late-apoptosis percentages in human breast MDA-MB-231 cancer cells (Figure 3, Table 4). The results of this assay pointed out that incubation of MDA-MB-231 cells with acid derivative 9e provoked apoptosis in such cells, evidenced by the significant increase in the percentages of apoptotic MDA-MB-231 cells for both the early apoptosis phase (from 0.47%, for the control, to 8.11%) and the late apoptosis phase (from 0.31%, for the control, to 23.77%) (Table 4).
Figure 3.
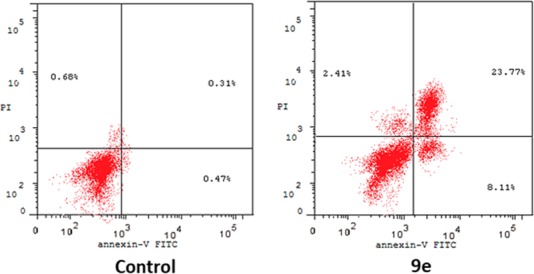
Influence of benzofuran-based carboxylic acid derivative 9e over the AV-FITC-positive staining percentages in breast cancer MDA-MB-231 cells. (Lower right: early apoptotic; upper right: late apoptotic; lower left: viable; upper left: necrotic).
Table 4. Distribution of Apoptotic MDA-MB-231 Cells after Incubation with Benzofuran Derivative 9e in the AV-FITC/PI Staining Assay.
| Cmp | Total % | Early Apoptosis | Late Apoptosis % | Necrosis |
|---|---|---|---|---|
| 9e | 34.29 | 8.11 | 23.77 | 2.41 |
| Ctrl | 1.46 | 0.47 | 0.31 | 0.68 |
In summary, we explored in this letter the design and synthesis for novel benzofuran-based carboxylic acid derivatives, featuring the benzoic (9a–f) or hippuric (11a, b) acid moieties linked to 2-methylbenzofuran or 5-bromobenzofuran tails via an ureido linker. All the target carboxylic acid derivatives were tested for their potential inhibitory action against four hCA isoforms (I, II, IX, and XII). The cancer-related hCA IX isoform was effectively affected by the all prepared benzofuran derivatives decorated with benzoic acid moiety 9a–f with KI’s ranging between 0.56 and 5.1 μM, Superiorly, compounds 9b, 9e, and 9f emerged as submicromolar hCA IX inhibitors with KI’s = 0.91, 0.79, and 0.56 μM, respectively. Moreover, all compounds, except 9d and 11b, inhibited hCA XII isoform with KI’s in the range 0.88–3.4 μM. Regarding the target/off-target CA SIs of action for the target benzofuran-based carboxylic acid derivatives, compounds 9b, 9e, and 9f showed good selective inhibitory action toward hCA IX over the off-target hCA I and II (SIs: 2 to >63 and 4–47, respectively). The concluded SAR revealed that replacement of the benzoic acid moiety with the hippuric acid one led to a worsening or abolishment of inhibitory activity against all the tested hCA isoforms, whereas utilizing of the 2-methylbenzofuran scaffold elicited an enhancement of effectiveness toward hCA XII for both benzoic and hippuric acid-containing derivatives in comparison to the corresponding 5-bromobenzofuran-based counterparts 9d–f. Moreover, compounds 9b, 9e, and 9f were tested for their potential growth inhibitory actions toward two human breast cancer (MCF-7 and MDA-MB-231) cell lines. In particular, 9e displayed the best antiproliferative action toward MDA-MB-231 cancer cells (IC50 = 2.52 ± 0.39 μM) that is comparable to Doxorubicin (IC50 = 2.36 ± 0.18 μM). Furthermore, 9e disrupted the cell cycle (through arrest of the G2-M stage and alteration of the Sub-G1 phase) and significantly boosted the percentage of AV-FITC positive MDA-MB-231 apoptotic cells (from 0.78 to 31.88%).
Glossary
Abbreviations
- CA
carbonic anhydrase
- AAZ
acetazolamide
- SRB
sulfo-rhodamine-B
- AV
annexinV
- PI
propidium iodide.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00094.
Synthetic procedures, compounds characterization, and in vitro kinetic method (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors extend their appreciation to the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University for funding this work through the Research Groups Program Grant No. RGP-1440-0025.
The authors declare no competing financial interest.
Supplementary Material
References
- Supuran C. T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. 10.1042/BCJ20160115. [DOI] [PubMed] [Google Scholar]
- Supuran C. T. Carbonic anhydrase activators. Future Med. Chem. 2018, 10, 561–573. 10.4155/fmc-2017-0223. [DOI] [PubMed] [Google Scholar]
- Supuran C. T. Carbonic anhydrases: from biomedical applications of the inhibitors and activators to biotechnological use for CO2 capture. J. Enzyme Inhib. Med. Chem. 2013, 28, 229–230. 10.3109/14756366.2013.761876. [DOI] [PubMed] [Google Scholar]
- Carta F.; Supuran C. T. Diuretics with carbonic anhydrase inhibitory action: a patent and literature review (2005–2013). Expert Opin. Ther. Pat. 2013, 23, 681–691. 10.1517/13543776.2013.780598. [DOI] [PubMed] [Google Scholar]
- Mishra C. B.; Kumari S.; Angeli A.; Monti S. M.; Buonanno M.; Tiwari M.; Supuran C. T. Discovery of benzenesulfonamides with potent human carbonic anhydrase inhibitory and effective anticonvulsant action: design, synthesis, and pharmacological assessment. J. Med. Chem. 2017, 60, 2456–2469. 10.1021/acs.jmedchem.6b01804. [DOI] [PubMed] [Google Scholar]
- Scozzafava A.; Supuran C. T.. Glaucoma and the applications of carbonic anhydrase inhibitors. In Carbonic Anhydrase: Mechanism, Regulation, Links to Disease, and Industrial Applications; Springer: Dordrecht, 2014; pp 349–359. [DOI] [PubMed] [Google Scholar]
- Supuran C. T. Carbonic anhydrase inhibitors as emerging agents for the treatment and imaging of hypoxic tumors. Expert Opin. Invest. Drugs 2018, 27, 963–970. 10.1080/13543784.2018.1548608. [DOI] [PubMed] [Google Scholar]
- Supuran C. T.; Alterio V.; Di Fiore A.; D’Ambrosio K.; Carta F.; Monti S. M.; De Simone G. Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: three for the price of one. Med. Res. Rev. 2018, 38, 1799–1836. 10.1002/med.21497. [DOI] [PubMed] [Google Scholar]
- Eldehna W. M.; Abo-Ashour M. F.; Berrino E.; Vullo D.; Ghabbour H. A.; Al-Rashood S. T.; Hassan G. S.; Alkahtani H. M.; Almehizia A. A.; Alharbi A.; Abdel-Aziz H. A.; Supuran C. T. SLC-0111 enaminone analogs, 3/4-(3-aryl-3-oxopropenyl) aminobenzenesulfonamides, as novel selective subnanomolar inhibitors of the tumor-associated carbonic anhydrase isoform IX. Bioorg. Chem. 2019, 83, 549–558. 10.1016/j.bioorg.2018.11.014. [DOI] [PubMed] [Google Scholar]
- Rotondi G.; Guglielmi P.; Carradori S.; Secci D.; De Monte C.; De Filippis B.; Maccallini C.; Amoroso R.; Cirilli R.; Akdemir A.; Angeli A. Design, synthesis and biological activity of selective hCAs inhibitors based on 2-(benzylsulfinyl) benzoic acid scaffold. J. Enzyme Inhib. Med. Chem. 2019, 34, 1400–1413. 10.1080/14756366.2019.1651315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocentini A.; Bonardi A.; Gratteri P.; Cerra B.; Gioiello A.; Supuran C. T. Steroids interfere with human carbonic anhydrase activity by using alternative binding mechanisms. J. Enzyme Inhib. Med. Chem. 2018, 33, 1453–1459. 10.1080/14756366.2018.1512597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. P.; Cohen S. M. Nucleophile recognition as an alternative inhibition mode for benzoic acid based carbonic anhydrase inhibitors. Chem. Commun. 2012, 48, 5259–5261. 10.1039/c2cc32013d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca A.; Temperini C.; Vu H.; Pham N. B.; Poulsen S. A.; Scozzafava A.; Quinn R. J.; Supuran C. T. Non-zinc mediated inhibition of carbonic anhydrases: coumarins are a new class of suicide inhibitors. J. Am. Chem. Soc. 2009, 131, 3057–3062. 10.1021/ja809683v. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio K.; Carradori S.; Monti S. M.; Buonanno M.; Secci D.; Vullo D.; Supuran C. T. Out of the active site binding pocket for carbonic anhydrase inhibitors. Chem. Commun. 2015, 51, 302–305. 10.1039/C4CC07320G. [DOI] [PubMed] [Google Scholar]
- Abdelrahman M. A.; Eldehna W. M.; Nocentini A.; Ibrahim H. S.; Almahli H.; Abdel-Aziz H. A.; Abou-Seri S. M.; Supuran C. T. Novel benzofuran-based sulfonamides as selective carbonic anhydrases IX and XII inhibitors: Synthesis and in vitro biological evaluation. J. Enzyme Inhib. Med. Chem. 2020, 35, 298–305. 10.1080/14756366.2019.1697250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelrahman M. A.; Eldehna W. M.; Nocentini A.; Bua S.; Al-Rashood S. T.; Hassan G. S.; Bonardi A.; Almehizia A. A.; Alkahtani H. M.; Alharbi A.; Gratteri P.; Supuran C. T. Novel Diamide-Based Benzenesulfonamides as Selective Carbonic Anhydrase IX Inhibitors Endowed with Antitumor Activity: Synthesis, Biological Evaluation and In Silico Insights. Int. J. Mol. Sci. 2019, 20, 2484. 10.3390/ijms20102484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldehna W. M.; Abo-Ashour M. F.; Nocentini A.; El-Haggar R. S.; Bua S.; Bonardi A.; Al-Rashood S. T.; Hassan G. S.; Gratteri P.; Abdel-Aziz H. A.; Supuran C. T. Enhancement of the tail hydrophobic interactions within the carbonic anhydrase IX active site via structural extension: Design and synthesis of novel N-substituted isatins-SLC-0111 hybrids as carbonic anhydrase inhibitors and antitumor agents. Eur. J. Med. Chem. 2019, 162, 147–160. 10.1016/j.ejmech.2018.10.068. [DOI] [PubMed] [Google Scholar]
- Bua S.; Lomelino C. L.; Murray A. B.; Osman S. M.; Alothman Z. A.; Bozdag M.; Aziz H.A. A.; Eldehna W. M.; McKenna R.; Nocentini A.; Supuran C. T. A Sweet Combination”: Developing saccharin and acesulfame K structures for selectively targeting the tumor-associated carbonic anhydrases IX and XII. J. Med. Chem. 2020, 63, 321–333. 10.1021/acs.jmedchem.9b01669. [DOI] [PubMed] [Google Scholar]
- Said M. A.; Eldehna W. M.; Nocentini A.; Fahim S. H.; Bonardi A.; Elgazar A. A.; Kryštof V.; Soliman D. H.; Abdel-Aziz H. A.; Gratteri P.; Abou-Seri S. M.; Supuran C. T. Sulfonamide-based ring-fused analogues for CAN508 as novel carbonic anhydrase inhibitors endowed with antitumor activity: Design, synthesis, and in vitro biological evaluation. Eur. J. Med. Chem. 2020, 189, 112019. 10.1016/j.ejmech.2019.112019. [DOI] [PubMed] [Google Scholar]
- Eldehna W. M.; Abdelrahman M. A.; Nocentini A.; Bua S.; Al-Rashood S. T.; Hassan G. S.; Bonardi A.; Almehizia A. A.; Alkahtani H. M.; Alharbi A.; Gratteri P.; Supuran C. T. Synthesis, biological evaluation and in silico studies with 4-benzylidene-2-phenyl-5(4H)-imidazolone-based benzenesulfonamides as novel selective carbonic anhydrase IX inhibitors endowed with anticancer activity. Bioorg. Chem. 2019, 90, 103102. 10.1016/j.bioorg.2019.103102. [DOI] [PubMed] [Google Scholar]
- Werner R. B. 3-Methylcoumarone (benzofuran, 3-methyl-). Org. Synth. 1953, 33, 43–46. 10.15227/orgsyn.033.0043. [DOI] [Google Scholar]
- Yoo S. E.; Lee S. H.; Kim S. K.; Lee S. H. The conformation and activity relationship of benzofuran derivatives as angiotensin II receptor antagonists. Bioorg. Med. Chem. 1997, 5, 445–459. 10.1016/S0968-0896(96)00244-1. [DOI] [PubMed] [Google Scholar]
- El-Naggar M.; Almahli H.; Ibrahim H. S.; Eldehna W. M.; Abdel-Aziz H. A. Pyridine-ureas as potential anticancer agents: synthesis and in vitro biological evaluation. Molecules 2018, 23, 1459. 10.3390/molecules23061459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifah R. G. The carbon dioxide hydration activity of carbonic anhydrase I. Stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 1971, 246, 2561–2573. [PubMed] [Google Scholar]
- Skehan P.; Storeng R.; Scudiero D. New colorimetric cyto-toxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.