Abstract

The ability of phenolic compounds from Morus nigra to modulate sarco-endoplasmic Ca2+-ATPase (SERCA1) activity was analyzed. Enzyme activity decrease correlated with the binding energy of agents to SERCA1. Results from theoretical and experimental approaches were coherent in identifying binding sites to SERCA1. Albanol A inhibited SERCA1 by immersion in the luminal gate at the site of Ca2+ release. Kuwanon U exerted an inhibitory effect by preventing ATP binding in the cytosolic region of SERCA1, and this was associated with conformational alterations. On the basis of similarities of SERCA isoforms, the viability of beta-cells containing SERCA2b was analyzed. Both correlation of viability and negative correlation of SERCA2b expression with SERCA1 activity were found for agents with the highest binding energy to SERCA1. The compounds studied may regulate viability and apoptosis of pancreatic beta-cells via modulation of SERCA activity. Novel pharmacological interventions in diabetes may be realized via compounds restoring ER calcium levels.
Keywords: SERCA activity, pancreatic beta-cells, viability, apoptosis, phenolic compounds, Morus nigra
Intracellular calcium plays the role of critical coordinator for many cellular events. A number of Ca2+ handling enzymes, proteins, channels and transporters located in the plasma membrane and in organelles regulate tightly intracellular Ca2+ concentrations generating precise calcium signals.1 A crucial role in maintaining calcium homeostasis via accumulating released Ca2+ back into SR/ER is played by the enzyme Ca2+-ATPase pump (SERCA).
The Ca2+ level reaches a concentration of 1 mM in extracellular space, while in the intracellular space it is maintained around the value of 100 nM.2 ER stress and cell death is evoked in cells without this gradient. Dysfunction of SERCA caused by altered SERCA expression and activity results in cell impairment associated with numerous diseases with abnormal cell calcium transport, including diabetes.1,3,4 Skeletal muscle insulin resistance in type 2 diabetes is the primary defect before beta-cell injury.5 Calcium homeostasis dysfunction induces disturbance of skeletal contractility.
In the diabetic muscle, in vitro and in vivo, a relationship between muscle fatigue and failure of the Ca2+ handling system was observed.6 Impaired SERCA1 resulted in skeletal muscle dysfunction in type II diabetes.7 There is a 75% or more homology between proteins from the SERCA1, SERCA2, and SERCA3 families.8 Three different SERCA isoforms are expressed by beta-cells, where SERCA2b is in prevalence and represents the key regulator of ER Ca2+ transport.1,9 In recent studies on pancreatic islets under diabetic conditions a significant reduction of SERCA2b expression has been reported.1,10 Increased basal cytosolic Ca2+ levels, decreased insulin secretion, and reduced beta-cell proliferation simultaneously with elevation of beta-cell ER stress and death resulted from SERCA2b deficiency.11
Drugs maintaining calcium levels of ER represent one of the novel therapeutic targets for treatment of redox diseases. Pharmacologic restoration of ER calcium levels can prevent ER stress resulting in ER calcium depletion and beta-cell death.12 Regulation of ER calcium levels by small molecules may prevent or induce death of cells by binding to SERCA, thus modulating its function.10,11,13,14 SERCA activators are summarized in a review by Chemaly et al.(1) SERCA activity represents control crossway for cell survival or death.
Binding of small molecules to SERCA may induce also negative regulation resulting in lowering SERCA activity or expression.15−20 Natural polyphenolic compounds are able to specifically modulate the Ca2+ pumping activity21 by binding to SERCA. Thapsigargin, cyclopiazonic acid, and curcumin, known selective SERCA pump inhibitors, have been proposed and used as anticancer drugs.22,23 Bilmen et al.24 previously indicated a relationship between SERCA activity decrease and apoptosis with respect to curcumin. Curcumin, a compound with antioxidant and anticancerogenic properties,25 was able to activate apoptosis26 and to affect a number of other cellular processes regulated by Ca2+.
It was previously found that phenolic compounds used for prevention and treatment of chronic diseases, including diabetes, are present in the leaves and root bark of Morus nigra.(27) We have recently reported the isolation and chemical characterization of seven phenolic compounds from the root bark of M. nigra.28 In the current study, these compounds were tested for their activity on SERCA1 in noncellular system and for their effect on viability and SERCA2 expression in pancreatic beta-cells. The present work was focused on finding a correlation between the binding of phenolic agents to SERCA1 resulting in its activity modulation in noncellular systems and SERCA2 expression in cellular systems of pancreatic beta-cells, possibly playing a role in modulation of viability, apoptosis, and insulin secretion.
Results and Discussion
Skeletal muscle disturbance associated with diabetes and observed before beta-cell injury was mediated by calcium homeostasis dysfunction via impaired SERCA1.7 A set of structurally diverse phenolic compounds, isolated from Morus nigra, were selected to study their possible effect on SERCA1. It may be worth mentioning that, while at first sight they may seem very different from the other compounds, the skeleton of the Diels–Alder adducts albanol A and B contains building blocks closely related to the other compounds, i.e. an arylbenzofuran (as in moracins P and O), and a 2′,4′-disubstituted flavan fragment (related to the flavanones kuwanon E and U).
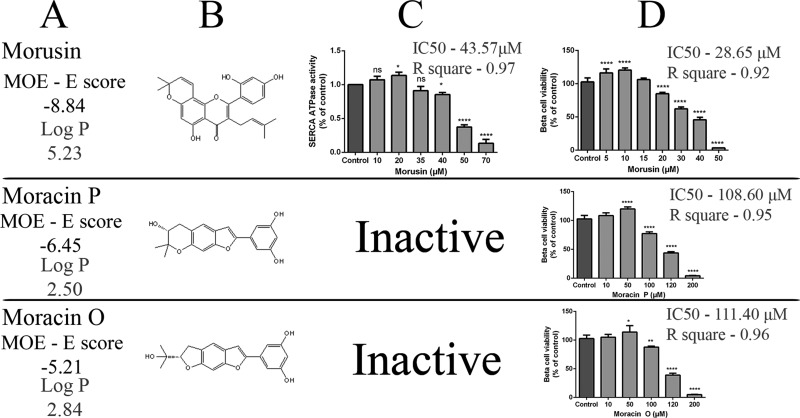
Skeletal muscle SERCA1 activity decrease induced by phenolic compounds isolated from Morus nigra correlated according to molecular docking with energy of interaction to SERCA1 (Figures 1, 2 and 5). Albanols A and B reduced SERCA1 activity most effectively. On the other hand, moracins, compounds with the weakest binding energy to SERCA1, were without any inhibitory effects (Figure 1 and Figure 2). This may also be connected to the significantly lower octanol/water partition coefficients (log P) of moracin O (2.8) and P (2.5) as compared with those of the other compounds (5.2–5.8). All compounds tested exerted similar hormetic effects. In each experiment, changes in SERCA1 activity and viability were observed in the same concentration range of treatment, which may support the significant role of SERCA in regulation of beta-cell viability. In all experiments not pure SERCA1 but rather SR vesicles containing SERCA1 were used. Of the total content of proteins in SR skeletal muscle, SERCA1 represents 70–80%,29 and therefore posttranslational and conformational changes of SR proteins may be considered to be changes of this enzyme.30,31
Figure 1.
Interaction of phenolic compounds (albanols and kuwanons) with SERCA1 and viability of INS-1E beta-cells. (A) Trivial names of compounds, molecular docking scores and partition coefficients (log P). Results of E score function and log P were obtained from MOE software. (B) Chemical structures of compounds. (C) SERCA1 activity measured by the NADH-coupled enzyme assay. SR vesicles (1 mg prot./ml) were incubated with phenolic compounds (10–70 μM) for 2 min at 37 °C, pH 7.2. The reaction rate was determined by measurement of the absorbance at 340 nm and 37 °C. *p < 0.05, **p < 0.01, ****p < 0.0001 mean significant changes of Ca2+-ATPase activity influenced by individual compounds compared to control not treated samples. (D) Viability of INS-1E beta-cells in the presence of phenolic compounds. The cells (5 × 104 cells per well) were preincubated for 24 h with or without different concentrations of individual phenolic compounds (5–100 μM) prior to MTT. The solubilized formazan was recorded at 570 nm with a microplate reader. **p < 0.01, ***p < 0.001, and ****p < 0.0001 mean significant changes of beta cell viability in the presence of individual compounds in comparison to not influenced controls.
Figure 2.
Interaction of morusin and moracin P and O with SERCA1 and viability of INS-1E beta-cells. (A) Trivial names of compounds, molecular docking scores, and partition coefficients (log P). Results of E score function and log P obtained from MOE software. (B) Chemical structures of compounds. (C) SERCA1 activity measured by the NADH-coupled enzyme assay. SR vesicles (1 mg prot./ml) were incubated with phenolic compounds (10–70 μM) for 2 min at 37 °C, pH 7.2. The reaction rate was determined by measurement of the absorbance at 340 nm and 37 °C. *p < 0.05, ****p < 0.0001 mean significant changes of Ca2+-ATPase activity influenced by individual compounds compared to control untreated samples. (D) Viability of INS-1E beta-cells in the presence of phenolic compounds. The cells (5 × 104 cells per well) were preincubated for 24 h with or without different concentrations of individual phenolic compounds (5–200 μM) prior to MTT. The solubilized formazan was recorded at 570 nm with a microplate reader. ****p < 0.0001 means significant changes of beta cell viability in the presence of individual compounds in comparison to untreated controls. .
Figure 5.
(A) Dependence of SERCA1 ATPase activity on binding energy (MOE-E score) of phenolic compounds to SERCA1. Moracin P and O with lowest binding energy are not involved as they were without any effect on enzyme activity. (B) Dependence of beta-cell viability on binding energy (MOE-E score) of phenolic compounds to SERCA1. All compounds included. (C) Dependence of beta-cell viability on SERCA ATPase activity. Compounds with highest binding energy to SERCA1 (albanols and kuwanons) are involved. (D) Dependence of early apoptotic cells on SERCA2b expression. Compounds with highest binding energy to SERCA1 (albanols and kuwanons) are involved. (E) Dependence of SERCA2b expression on SERCA1 ATPase activity. Compounds with highest binding energy to SERCA1 (albanols and kuwanons) are involved.
Sites of compound binding to SERCA1 were analyzed experimentally (using fluorescence marker FITC) by conformationally altered nucleotide binding site32 situated in the cytosolic region. Intrinsic fluorescence of Trp residues were a marker to analyze alterations of transmembrane part of SERCA1.
Kuwanon E and U, morusin and moracin P and R significantly decreased FITC fluorescence, indicating their binding at or near the ATP binding site and their simultaneous interaction with the transmembrane domain of SERCA1. Albanol A and B did not exert conformational alterations in either the cytosolic or transmembrane domain of SERCA1. As these compounds appeared to be the most powerful inhibitors with intensive binding energy to SERCA1, the structure of the albanol–SERCA complex was studied by molecular docking in greater detail. The results indicated the immersion of albanol A in the luminal gate at the Ca2+ release site in the ER lumen (Figure 3).
Figure 3.
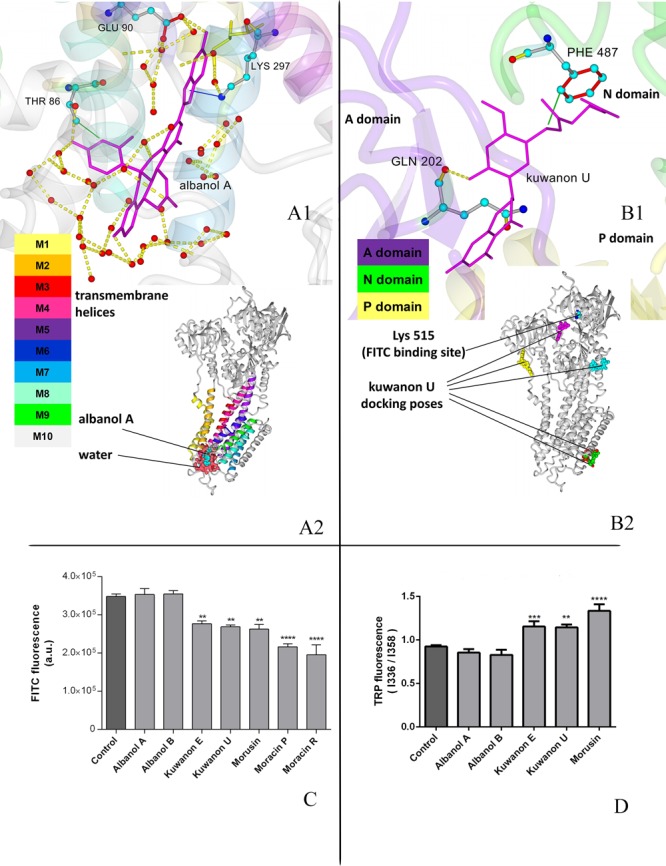
Binding of phenolic compounds to SERCA1, theoretical and experimental approach. (A) Optimal position for albanol A obtained by molecular modeling, detailed and global view. (A1) Detailed image with intermolecular interactions; albanol A–stick model in magenta, important residues–ball and stick models in atom coloring, small red circles–water oxygens. Green line–hydrophobic interaction; yellow dashed line–hydrogen bond. (A2) Global view on SERCA with albanol A (ball and stick model, atom coloring) and water molecules near luminal gate (surface model, dark pink). (B) Optimal position for kuwanon U obtained by molecular modeling, detailed and global view. (B1) Detailed image with intermolecular interactions; kuwanon U–stick model in magenta, important residues–stick models in atom coloring, small red circle–water oxygen. Green line–hydrophobic interaction; yellow dashed line–hydrogen bond. (B2) Global view on SERCA with the first five docking poses for kuwanon U (unicolor ball models) and Lys515 (atom color ball model). (C) Conformational changes of SERCA1 mediated by phenolic compounds in the cytosolic region of SERCA1. SR (20 mg/mL protein) was incubated for 30 min with agents at concentrations corresponding to IC50. FITC label was used as fluorescent label. **p < 0.01 and ****p < 0.0001 mean significant decrease of FITC fluorescence as compared to control not influenced samples. (D) Conformational changes of SERCA1 mediated by phenolic compounds in transmembrane region of SERCA1. SR (1 mg protein/mL) was incubated with individual polyphenolic compounds at concentrations corresponding to IC50 for 30 min at room temperature. The emission spectra corresponding to Trp residues were collected on a spectrofluorometer, Excitation was performed at 295 nm. **p < 0.01, ***P < 0.001 and ****p < 0.0001 mean significant increase of Trp fluorescence in samples influenced by individual compounds in comparison to control not influenced samples. .
Intrinsic fluorescence of Trp residues may be a marker of conformational alterations in the transmembrane domain of SERCA1.33 Twelve Trp residues of SERCA1 are situated in its transmembrane region, one is present in the cytosolic domain.34 In the presence of phenolic compounds conformational alterations in the transmembrane domain of SERCA1 were measured by utilizing different fluorescence maxima of Trp in cytosolic and membrane environment with fluorescence intensities at 358 and 336 nm, respectively.
Kuwanon E and U, as well as morusin, evoked conformational changes in the SERCA1 transmembrane part, as suggested by the increase of fluorescence intensity ratio I358 nm/I336 nm of Trp, which may be caused by shifting Trp residues to the cytosolic area. Moracin P and O interfered with measurement of Trp fluorescence. Kuwanon U, as a representant of compounds with the ability to induce conformational alterations in both regions of SERCA1, cytosolic and membrane, was analyzed in greater detail by molecular docking. This study confirmed binding of kuwanon U in both regions. The inhibitory mechanism of kuwanon U is probably related to the occupation of residues Phe487 and Gln202 in the cytosolic region which may prevent ATP binding.
A close similarity of SERCA1 with its SERCA families was found in beta-cells, where SERCA2b is abundant and represents the key regulator of Ca2+ transport.1,9 Thus, we supposed that these isoforms may analogically interact with phenolic agents resulting in modulation of calcium homeostasis. Actually, the ability of compounds to decrease beta-cell viability (for agents with highest binding energy to SERCA1, albanols and kuwanons) was in correlation with their efficiency to reduce SERCA1 activity (Figure 1 and Figure 5). Morusin exerted a stronger effect in decreasing beta-cell viability than expected from its efficiency to inhibit SERCA (Figure 2). This can be caused by the capability of morusin to induce cell viability decrease and apoptosis by other mechanisms, for example through inactivating STAT3 signaling.35
Expression of SERCA2b in beta cells for albanols and kuwanons was in negative correlation with SERCA1 activity (Figure 4 and Figure 5). Increased expression of SERCA2b was induced by the compounds with the most favorable binding energy to SERCA1 (Albanol A and B), which may be an adaptation mechanism to SERCA1 inhibition. Ca2+-ATPases are involved in insulin response. Loss of ability to release insulin after glucose stimulation was in correlation with inhibition of SERCA activity induced by agents playing an important role in calcium homeostasis (Figure 4).
Figure 4.

SERCA2 expression, apoptosis, and insulin release in INS-1E beta cells in the presence of phenolic compounds. (A) Expression of SERCA2b. Cells were incubated for 24 h with individual compounds at concentrations corresponding to IC50 of cell viability normalized to β-actin in %, average of 3–6 experiments. *p < 0.05 and ****p < 0.0001 represent significant changes of SERCA2b expression compared with control not influenced samples. Densitometric quantification was performed using ImageJ (National Institute of Health, USA) software. (B) SERCA2b and β-actin expression. Blot displays one representative experiment of each compound. (C) Apoptosis of INS-1E beta cells. Apoptosis induced by phenolic compounds was evaluated after incubation with individual compounds at concentrations corresponding to IC50 of cell viability. Apoptotic changes were detected using the FITC Annexin V/propidium iodide kit (Invitrogen) by flow cytometry. ××××p < 0.0001 means significant decrease of living cells influenced by individual compounds compared with control not influenced beta cells, ++++p < 0.0001 means significant increase of early apoptotic cells influenced by individual compounds compared with that of controls not influenced by compounds. °°p < 0.01, °°°p < 0.001, and °°°°p < 0.0001 mean increase of late apoptotic cells influenced by individual compounds compared with that of controls, not influenced by compounds. (D) Insulin release in INS-1E beta cells. Cells were preincubated for 24 h with individual compounds in concentrations coresponding to IC50 of cell viability and insulin release was indicated in glucose free and glucose supplemented (16.7 mM) KRBH buffer. *p < 0.05 and ****p < 0.0001 mean significant decline of insulin release by Glu-stimulated cells in the presence of individual compounds in comparison to that of control cells not influenced by agents.
Conclusions
Phenolic compounds from Morus nigra exerted binding interaction to SERCA1 from skeletal muscles. Sites of interaction were determined by both theoretical and experimental studies. On the basis of this interaction, regulation of SERCA1 activity was found. Analogically, affinity of these agents to SERCA2b as main regulator of calcium homeostasis in beta-cells may be supposed. Phenolic compounds with high binding energy to SERCA may modulate viability, apoptosis, and insulin release.
Experimental Procedures
Details of Methods are included in the Supporting Information.
Isolation of Phenolic Compounds from Morus Nigra
Methanol extract of Morus nigra root bark was used for isolation of phenolic compounds. A multistep chromatographic procedure, utilizing OCC, FC, and HPLC was used for isolation. Silica gel, octadecyl-silica, or polyamide gel was used as stationary phase for OCC and FC. HRMS and 2D NMR techniques were used to fully charaterize compounds, as reported before.28
SERCA Activity Measurement
The NADH-coupled enzyme assay outlined by Warren et al.(36) was used to measure SERCA activity of SR isolated from fast-twitch skeletal muscle of a New Zealand female rabbit.
Labeling of SERCA1 in SR Vesicles Using FITC
Fluorescent probe FITC (fluorescein-5′-isothiocyanate) was used to measure alterations in the cytosolic region of SERCA1.37 Vesicles of SR were at first preincubated with individual compounds for 1 h. Protein concentration of 12.5 mg/mL was used and concentrations of individual compounds corresponded to their IC50.
Intrinsic Tryptophan Fluorescence
Incubation (30 min at 37 °C) of SR vesicles (1 mg protein/mL) with phenolic compounds at concentrations of IC50 was performed. Spectrofluorometer (Fluoromax-4, HORIBA, USA) was used to measure fluorescence of Trp residues. Excitation at 295 nm was used; local emission maxima were 336 and 358.
Culturing of Beta-Cells
Pancretic beta-cells, line INS-1E were cultured in RPMI 1640 (11 mM glucose, Sigma-Aldrich) with additional supplements as specified in the Supporting Information. Cells were kindly provided from University of Geneva, (Prof. Claes Wollheim). After incubation of cells with individual compounds (concentrations of IC50) for 24 h, lysis in ice cold lysis buffer was performed. To homogenize the cells, the lysate was passed through a syringe needle. Before using cells, the lysates were kept on ice (10 min) and centrifuged (10 min at 20 160 RCF). Until further use the lysates were kept at −30 °C.
Separation of Proteins
SDS-PAGE with a mini-PROTEAN II electrophoresis cell (Bio-Rad, DE) was used to separate cell lysates (15 μg/lane). SERCA2 and β-actin bands were identified using related antibodies as indicated in the Supporting Information.
Cytotoxicity Assay (MTT)
As an indicator of cell damage the MTT (Sigma-Aldrich) reduction assay was used and performed according to a standard protocol. A microplate reader (Infinite M200, Tecan, Switzerland) was used to record absorbance at 570 nm.
Detection of Apoptosis
Apoptotic alterations were detected by FITC Annexin V/propidium iodide kit (Invitrogen) by flow cytometry after incubation for 24 h with individual phenolic compounds.
Release of Insulin
To measure secretory responses to glucose, the INS-1E cells were preincubated with individual compounds for 24 h and afterward were washed with glucose-free KRBH buffer. The cells were incubated for 30 min at 37 °C at first in glucose-free KRBH buffer and then under the same conditions in KRBH buffer supplemented with 16.7 mM glucose. To detect insulin secretion by RIA kit (Mercodia, Sweden) using rat insulin as standard, the supernatants were collected.
Docking Study
Structures of derivatives were built and optimized in Spartan software using the MMFF94 force field.38 We used the structure with pdb code 3w5c from the Protein Data Bank (http://www.rcsb.org; PDB ID 3w5c). Molecular Operating Environment (MOE 2019.01) modeling program was performed to study docking.39 The protein was prepared by the QuickPrep module, which includes a structure preparation, protonation with Protonate3D method under pH = 7.4, neutralization, and refining the structure to 0.1 kcal/mol per amino acid. In the framework of the Dock module we set a Triangle Matcher for the placement method, London dG score and 30 poses. The subsequent refinment was performed with an Induced Fit method, with GBVI/WSA dG score and 5 poses used. The MMFF94 force field was used for the docking protocol. The structure of albanol A docked in SERCA1 was then studied in detail. The complex was additionally optimized in YASARA software using AMBER14 force field and a standard optimization protocol em_run.mcr, which consists of the steepest gradient optimization, molecular dynamics and simulated annealing.40
Statistics
One way ANOVA by the Bonferroni test was performed to statistical analysis. Minimally three independent experiments including individual samples measured 2–3 times were used to calculate mean ± SD. The following statistical significances were set: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Acknowledgments
The present work was financially supported by COST Action CM1407; Slovak National grant APVV-15-0455; National Research, Development and Innovation Office, Hungary (NKFIH; K119770); the Ministry of Human Capacities, Hungary grant 20391-3/2018/FEKUSTRAT; and the EU-funded Hungarian grant EFOP-3.6.1-16-2016-00008. The authors thank Lucia Rackova for critical comments.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00047.
Details of Experimental Procedures: SERCA activity measurement; Labeling of SERCA1 in SR vesicles using FITC; culturing of beta-cells; separation of proteins; cytotoxicity assay (MTT); release of insulin (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Chemaly E. R.; Troncone L.; Lebeche D. SERCA Control of Cell Death and Survival. Cell Calcium 2018, 69, 46–61. 10.1016/j.ceca.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R.; Marchi S.; Bonora M.; Aguiari P.; Bononi A.; De Stefani D.; Giorgi C.; Leo S.; Rimessi A.; Siviero R.; Zecchini E.; Pinton P. Ca(2+) Transfer from the ER to Mitochondria: When, How and Why. Biochim. Biophys. Acta, Bioenerg. 2009, 1787 (11), 1342–1351. 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano F. Wolfram Syndrome: Diagnosis, Management, and Treatment. Curr. Diabetes Rep. 2016, 16 (1), 1–8. 10.1007/s11892-015-0702-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Kaufman R. J. The Impact of the Unfolded Protein Response on Human Disease. J. Cell Biol. 2012, 197 (7), 857–867. 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A.; Tripathy D. Skeletal Muscle Insulin Resistance Is the Primary Defect in Type 2 Diabetes. Diabetes Care 2009, 32 (Suppl 2), S157–S163. 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshima H.; Poole D. C.; Kano Y. In Vivo Calcium Regulation in Diabetic Skeletal Muscle. Cell Calcium 2014, 56 (5), 381–389. 10.1016/j.ceca.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Safwat Y.; Yassin N.; Gamal El Din M.; Kassem L. Modulation of Skeletal Muscle Performance and SERCA by Exercise and Adiponectin Gene Therapy in Insulin-Resistant Rat. DNA Cell Biol. 2013, 32 (7), 378–385. 10.1089/dna.2012.1919. [DOI] [PubMed] [Google Scholar]
- Chemaly E. R.; Bobe R.; Adnot S.; Hajjar R. J.; Lipskaia L. Sarco (Endo)Plasmic Reticulum Calcium Atpases (SERCA) Isoforms in the Normal and Diseased Cardiac, Vascular and Skeletal Muscle. J. Cardiovasc. Dis. Diagn. 2013, 1 (3), 1–6. 10.4172/2329-9517.1000113. [DOI] [Google Scholar]
- Kono T.; Ahn G.; Moss D. R.; Gann L.; Zarain-Herzberg A.; Nishiki Y.; Fueger P. T.; Ogihara T.; Evans-Molina C. PPAR-γ Activation Restores Pancreatic Islet SERCA2 Levels and Prevents β-cell Dysfunction Under Conditions of Hyperglycemic and Cytokine Stress. Mol. Endocrinol. 2012, 26 (2), 257–71. 10.1210/me.2011-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.; Dahl R.; Hsieh W.; Shin A.; Zsebo K. M.; Buettner C.; Hajjar R. J.; Lebeche D. Small Molecular Allosteric Activator of the Sarco/Endoplasmic Reticulum Ca 2+-ATPase (SERCA) Attenuates Diabetes and Metabolic Disorders. J. Biol. Chem. 2016, 291 (10), 5185–5198. 10.1074/jbc.M115.705012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X.; Kono T.; Anderson-Baucum E. K.; Yamamoto W.; Gilon P.; Lebeche D.; Day R. N.; Shull G. E.; Evans-Molina C. SERCA2 Deficiency Impairs Pancreatic β-Cell Function in Response to Diet-Induced Obesity. Diabetes 2016, 65 (10), 3039–3052. 10.2337/db16-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T.; Mahadevan J.; Kanekura K.; Hara M.; Lu S.; Urano F. Calcium Efflux From the Endoplasmic Reticulum Leads to β-Cell Death. Endocrinology 2014, 155 (3), 758–68. 10.1210/en.2013-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardozo A. K.; Ortis F.; Storling J.; Feng Y.-M.; Rasschaert J.; Tonnesen M.; Van Eylen F.; Mandrup-Poulsen T.; Herchuelz A.; Eizirik D. L. Cytokines Downregulate the Sarcoendoplasmic Reticulum Pump Ca2+ ATPase 2b and Deplete Endoplasmic Reticulum Ca2+, Leading to Induction of Endoplasmic Reticulum Stress in Pancreatic Beta-Cells. Diabetes 2005, 54 (2), 452–61. 10.2337/diabetes.54.2.452. [DOI] [PubMed] [Google Scholar]
- Zatyka M.; Da Silva Xavier G.; Bellomo E. A.; Leadbeater W.; Astuti D.; Smith J.; Michelangeli F.; Rutter G. A.; Barrett T. G. Sarco(endo)plasmic Reticulum ATPase is a Molecular Partner of Wolfram Syndrome 1 Protein, which Negatively Regulates its Expression. Hum. Mol. Genet. 2015, 24 (3), 814–827. 10.1093/hmg/ddu499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranias E. G.; Hajjar R. J. Modulation of Cardiac Contractility by the Phopholamban/SERCA2a Regulatome. Circ. Res. 2012, 110 (12), 1646–1660. 10.1161/CIRCRESAHA.111.259754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhupathy P.; Babu G. J.; Periasamy M. Sarcolipin and Phospholamban as Regulators of Cardiac Sarcoplasmic Reticulum Ca2+ ATPase. J. Mol. Cell. Cardiol. 2007, 42 (5), 903–11. 10.1016/j.yjmcc.2007.03.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. M.; Anderson K. M.; Chang C.-L.; Makarewich C. A.; Nelson B. R.; McAnally J. R.; Kasaragod P.; Shelton J. M.; Liou J.; Bassel-Duby R.; Olson E. N. A Micropeptide Encoded by a Putative Long Noncoding RNA Regulates Muscle Performance. Cell 2015, 160 (4), 595–606. 10.1016/j.cell.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho C.; Lee A.; Jeong D.; Oh J. G.; Chaanine A. H.; Kizana E.; Park W. J.; Hajjar R. J. SUMO1-Dependent Modulation of SERCA2a in Heart Failure. Nature 2011, 477 (7366), 601–605. 10.1038/nature10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. J.; McDonough P. M.; Swanson E.; Trost S. U.; Suzuki M.; Fukuda M.; Dillmann W. H. Diabetes and the Accompanying Hyperglycemia Impairs Cardiomyocyte Calcium Cycling through Increased Nuclear O-GlcNAcylation. J. Biol. Chem. 2003, 278 (45), 44230–7. 10.1074/jbc.M303810200. [DOI] [PubMed] [Google Scholar]
- Bidasee K. R.; Zhang Y.; Shao C. H.; Wang M.; Patel K. P.; Dincer U. D.; Besch H. R. Diabetes Increases Formation of Advanced Glycation End Products on Sarco(endo)plasmic Reticulum Ca2+-ATPase. Diabetes 2004, 53 (2), 463–473. 10.2337/diabetes.53.2.463. [DOI] [PubMed] [Google Scholar]
- Ogunbayo O. A.; Harris R. M.; Waring R. H.; Kirk C. J.; Michelangeli F. Inhibition of the Sarcoplasmic/Endoplasmic Reticulum Ca2+-ATPase by Flavonoids: a Quantitative Structure-Activity Relationship Study. IUBMB Life 2008, 60 (12), 853–8. 10.1002/iub.132. [DOI] [PubMed] [Google Scholar]
- Gu J. L.; Veerapanane D.; Rossi J.; Natarajan R.; Thomas L.; Nadler J. Ribozyme-Mediated Inhibition of Expression of Leukocyte-Type 12-Lipoxygenase in Porcine Aortic Vascular Smooth Muscle Cells. Circ. Res. 1995, 77 (1), 14–20. 10.1161/01.RES.77.1.14. [DOI] [PubMed] [Google Scholar]
- Bakhshi J.; Weinstein L.; Poksay K. S.; Nishinaga B.; Bredesen D. E.; Rao R. V. Coupling Endoplasmic Reticulum Stress to the Cell Death Program in Mouse Melanoma Cells: Effect of Curcumin. Apoptosis 2008, 13 (7), 904–14. 10.1007/s10495-008-0221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilmen J. G.; Khan S. Z.; Javed M. H.; Michelangeli F. Inhibition of the SERCA Ca2+ Pumps by Curcumin. Curcumin Putatively Stabilizes the Interaction Between the Nucleotide-Binding and Phosphorylation Domains in the Absence of ATP. Eur. J. Biochem. 2001, 268 (23), 6318–27. 10.1046/j.0014-2956.2001.02589.x. [DOI] [PubMed] [Google Scholar]
- Keiloff G. J.; Boone C. W.; Crowell J. A.; Steele V. E.; Lubet R. A.; Doody L. A.; Malone W. F.; Hawk E. T.; Sigman C. C. New Agents for Cancer Chemoprevention. J. Cell. Biochem. 1996, 26, 1–28. 10.1002/jcb.240630703. [DOI] [PubMed] [Google Scholar]
- Piwocka K.; Zabłocki K.; Wieckowski M. R.; Skierski J.; Feiga I.; Szopa J.; Drela N.; Wojtczak L.; Sikora E. A Novel Apoptosis-like Pathway, Independent of Mitochondria and Caspases, Induced by Curcumin in Human Lymphoblastoid T (Jurkat) Cells. Exp. Cell Res. 1999, 249 (2), 299–307. 10.1006/excr.1999.4480. [DOI] [PubMed] [Google Scholar]
- Zoofishan Z.; Hohmann J.; Hunyadi A. Phenolic Antioxidants of Morus nigra Roots, and Antitumor Potential of Morusin. Phytochem. Rev. 2018, 17 (5), 1031–1045. 10.1007/s11101-018-9565-1. [DOI] [Google Scholar]
- Zoofishan Z.; Kúsz N.; Csorba A.; Tóth G.; Hajagos-Tóth J.; Kothencz A.; Gáspár R.; Hunyadi A. Antispasmodic Activity of Prenylated Phenolic Compounds from the Root Bark of Morus nigra. Molecules 2019, 24 (13), 2497. 10.3390/molecules24132497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir G.; Menguy T.; Corre F.; Montigny C.; Pedersen P. A.; Thines D.; le Maire M.; Falson P. Overproduction in yeast and rapid and efficient purification of the rabbit SERCA1a Ca(2+)-ATPase. Biochim. Biophys. Acta, Biomembr. 2002, 1560 (1–2), 67–83. 10.1016/S0005-2736(01)00458-8. [DOI] [PubMed] [Google Scholar]
- Pessah I. N.; Kim K. H.; Feng W. Redox sensing properties of the ryanodine receptor complex. Front. Biosci., Landmark Ed. 2002, 7, 72–9. 10.2741/pessah. [DOI] [PubMed] [Google Scholar]
- Engelender S.; Wolosker H.; de Meis L. The Ca(2+)-ATPase isoforms of platelets are located in distinct functional Ca2+ pools and are uncoupled by a mechanism different from that of skeletal muscle Ca(2+)-ATPase. J. Biol. Chem. 1995, 270 (36), 21050–5. 10.1074/jbc.270.36.21050. [DOI] [PubMed] [Google Scholar]
- Chen B.; Jones T. E.; Bigelow D. J. The Nucleotide-Binding Site of the Sarcoplasmic Reticulum Ca-ATPase is Conformationally Altered in Aged Skeletal Muscle. Biochemistry 1999, 38 (45), 14887–96. 10.1021/bi991125n. [DOI] [PubMed] [Google Scholar]
- Munishkina L. A.; Fink A. L. Fluorescence as a Method to Reveal Structures and Membrane-Interactions of Amyloidogenic Proteins. Biochim. Biophys. Acta, Biomembr. 2007, 1768 (8), 1862–85. 10.1016/j.bbamem.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Suzuki H.; Kanazawa T. The Tryptophan Fluorescence Change upon Conformational Transition of the Phosphoenzyme Intermediate in Sarcoplasmic Reticulum Ca-ATPase Is Revealed in the Absence of K and the Presence of Lasalocid. J. Biol. Chem. 1995, 270 (7), 3089–3093. 10.1074/jbc.270.7.3089. [DOI] [PubMed] [Google Scholar]
- Lim S.-L.; Park S.-Y.; Kang S.; Park D.; Kim S.-H.; Um J.-Y.; Jang H.-J.; Lee J.-H.; Jeong C.-H.; Jang J.-H.; Ahn K. S.; Lee S.-G. Morusin Induces Cell Death Through Inactivating STAT3 Signaling in Prostate Cancer Cells. Am. J. Cancer Res. 2015, 5 (1), 289–99. [PMC free article] [PubMed] [Google Scholar]
- Warren G. B.; Toon P. A.; Birdsall N. J.; Lee A. G.; Metcalfe J. C. Reconstitution of a Calcium Pump Using Defined Membrane Components. Proc. Natl. Acad. Sci. U. S. A. 1974, 71 (3), 622–626. 10.1073/pnas.71.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froud R. J.; Lee A. G. A Model for the Phosphorylation of the Ca2++ Mg2+-Activated ATPase by Phosphate. Biochem. J. 1986, 237 (1), 207–15. 10.1042/bj2370207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y.; Molnar L. F.; Jung Y.; Kussmann J.; Ochsenfeld C.; Brown S. T.; Gilbert A. T. B.; Slipchenko L. V.; Levchenko S. V.; O’Neill D. P.; DiStasio R. A.; Lochan R. C.; Wang T.; Beran G. J. O.; Besley N. A.; Herbert J. M.; Lin C. Y.; Van Voorhis T.; Chien S. H.; Sodt A.; Steele R. P.; Rassolov V. A.; Maslen P. E.; Korambath P. P.; Adamson R. D.; Austin B.; Baker J.; Byrd E. F. C.; Dachsel H.; Doerksen R. J.; Dreuw A.; Dunietz B. D.; Dutoi A. D.; Furlani T. R.; Gwaltney S. R.; Heyden A.; Hirata S.; Hsu C.-P.; Kedziora G.; Khalliulin R. Z.; Klunzinger P.; Lee A. M.; Lee M. S.; Liang W.; Lotan I.; Nair N.; Peters B.; Proynov E. I.; Pieniazek P. A.; Rhee Y. M.; Ritchie J.; Rosta E.; Sherrill C. D.; Simmonett A. C.; Subotnik J. E.; Woodcock H. L.; Zhang W.; Bell A. T.; Chakraborty A. K.; Chipman D. M.; Keil F. J.; Warshel A.; Hehre W. J.; Schaefer H. F.; Kong J.; Krylov A. I.; Gill P. M. W.; Head-Gordon M. Advances in Methods and Algorithms in a Modern Quantum Chemistry Program Package. Phys. Chem. Chem. Phys. 2006, 8 (27), 3172–91. 10.1039/B517914A. [DOI] [PubMed] [Google Scholar]
- Vilar S.; Cozza G.; Moro S. Medicinal Chemistry and the Molecular Operating Environment (MOE): Application of QSAR and Molecular Docking to Drug Discovery. Curr. Top. Med. Chem. 2008, 8 (18), 1555–1572. 10.2174/156802608786786624. [DOI] [PubMed] [Google Scholar]
- Krieger E.; Vriend G. YASARA View - Molecular Graphics for All Devices - from Smartphones to Workstations. Bioinformatics 2014, 30 (20), 2981–2. 10.1093/bioinformatics/btu426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.